Abstract
Background
Immune checkpoint inhibitors (ICIs) are regarded as the most promising treatment for advanced-stage non-small cell lung cancer (aNSCLC). Unfortunately, there has been no unified accuracy biomarkers and systematic model specifically identified for prognostic and severe immune-related adverse events (irAEs). Our goal was to discover new biomarkers and develop a publicly accessible method of identifying patients who may maximize benefit from ICIs.
Methods
This retrospective study enrolled 138 aNSCLC patients receiving ICIs treatment. Progression-free survival (PFS) and severe irAEs were end-points. Data of demographic features, severe irAEs, and peripheral blood inflammatory-nutritional and immune indices before and after 1 or 2 cycles of ICIs were collected. Independent factors were selected by least absolute shrinkage and selection operator (LASSO) combined with multivariate analysis, and incorporated into nomogram construction. Internal validation was performed by applying area under curve (AUC), calibration plots, and decision curve.
Results
Three nomograms with great predictive accuracy and discriminatory power were constructed in this study. Among them, two nomograms based on combined inflammatory-nutritional biomarkers were constructed for PFS (1 year-PFS and 2 year-PFS) and severe irAEs respectively, and one nomogram was constructed for 1 year-PFS based on immune indices. ESCLL nomogram (based on ECOG PS, preSII, changeCAR, changeLYM and postLDH) was constructed to assess PFS (1-, 2-year-AUC = 0.893 [95% CI 0.837–0.950], 0.828 [95% CI 0.721–0.935]). AdNLA nomogram (based on age, change-dNLR, changeLMR and postALI) was constructed to predict the risk of severe irAEs (AUC = 0.762 [95% CI 0.670–0.854]). NKT-B nomogram (based on change-CD3+CD56+CD16+NKT-like cells and change-B cells) was constructed to assess PFS (1-year-AUC = 0.872 [95% CI 0.764–0.965]). Although immune indices could not be modeled for severe irAEs prediction due to limited data, we were the first to find CD3+CD56+CD16+NKT-like cells were not only correlated with PFS but also associated with severe irAEs, which have not been reported in the study of aNSCLC-ICIs. Furthermore, our study also discovered higher change-CD4+/CD8+ ratio was significantly associated with severe irAEs.
Conclusions
These three new nomograms proceeded from non-invasive and straightforward peripheral blood data may be useful for decisions-making. CD3+CD56+CD16+NKT-like cells were first discovered to be an important biomarker for treatment and severe irAEs, and play a vital role in distinguishing the therapy response and serious toxicity of ICIs.
Keywords: Immune, Adverse events, Lung cancer, Biomarkers, Nomogram
Introduction
Lung cancer is one of the deadliest cancers globally [1]. Non-small cell lung cancer (NSCLC) causes 76% of lung cancer cases [2, 3]. Immune checkpoint inhibitors (ICIs), targeting programmed-cell death 1 (PD-1) and programmed-cell death ligand 1 (PD-L1) have transformed the treatment landscape of advanced-stage NSCLC (aNSCLC) [4]. However, only 15–20% experience durable response, others displaying primary or secondary resistance [5, 6]. Beyond that, ICIs may even cause severe immune-related adverse events (irAEs), which often come with the recommendation of discontinuing all further immunotherapy [7, 8]. PD-L1 in tissue is being explored as a predictive biomarker for outcomes of PD-L1/PD-1 treatment in NSCLC, while there are some conflicting data from the predictive biomarkers of response in terms of the level of PD-L1 expression. Many challenges remain in the clinical use of PD-L1, including different companion diagnostic assays, geographical heterogeneity, insufficient tissue for dynamic monitoring, and high levels of inter-assay variability in terms of both performance and cut-off points [9].
Recently, there has been a growing interest in the field of peripheral blood to identify non-invasive, cost-effective, easily accessible, and promising prognostic biomarkers to predict prognostic and toxicity of ICIs [10]. Consequently, many peripheral blood inflammatory-nutritional indices such as albumin, lactate dehydrogenase (LDH), neutrophil-to-lymphocyte ratio (NLR), derived NLR (dNLR), and C-reactive protein-albumin ratio (CAR) were found to link with metabolic homeostasis and affect the survival outcomes of ICIs [11–17]. Moreover, lymphocyte subsets, including CD3+, CD4+ and CD8+ T cell subsets, natural killer (NK) cells, and B cells, comprise a major population of tumor-infiltrating immune indices in circulating and are also of potential value for early judgment of clinical efficacy in ICIs [18–21]. However, their predictive values were verified in different studies and remained controversial. Until now, there has been no unified accuracy and comprehensive biomarkers and systematic construction model specifically identified for prognostic effect and severe toxicity.
The development of easy-to-use clinical tools able to identify ICIs benefit patients, resistant patients, and severe irAEs patients represents an unmet clinical need. Therefore, to predict therapeutic prognostic and early severe toxicity recognition for decision-making in the palliative treatment of aNSCLC patients receiving ICIs, the goal of the present study was to discover new predictive biomarkers and develop novel nomograms of prognostic and severe irAEs of aNSCLC patients receiving ICIs. The predictive biomarkers in these nomograms were based on a comprehensive assessment of clinical features, peripheral blood inflammatory-nutritional, and immune indices, which were more convenient and feasible for clinical acquisition.
Methods
Patients recruitment
In this study, a total of 180 advanced aNSCLC patients receiving ICIs treatment were initially recruited from Peking University Shenzhen Hospital from December 2018 to December 2021. The follow-up period ended on August 1st, 2022. Following the inclusion and exclusion criteria, as bellowed, 138 patients receiving ICIs (including Pembrolizumab, Nivolumab, Camrelizumab and Sintilimab) were enrolled in the final analysis (Fig. 1). The inclusion criteria were as follows: (i) above 18 years of age receiving ICIs treatment, or ICIs-based combination chemotherapy; (ii) pathologically or cytologically diagnosed clinical stage IIIB or stage IV NSCLC; (iii) Eastern Cooperative Oncology Group performance status scores (ECOG PS) between 0 and 2; (iv) no recent infection, trauma and not in the use of immune-regulatory drugs. The exclusion criteria were as follows: (i) patients with other types of tumors within 5 years; (ii) absence of measurable lesions to evaluate response according to immune-modified response evaluation criteria in solid tumors (imRECIST) criteria [22].
Fig. 1.
Flow chart of patient enrollment
Data collection
Data were collected at pre-treatment (within 7 days prior to ICIs, presented as “pre”) and post-treatment (after 1 or 2 cycles ICIs, presented as “post”), and the percentage change between the values (presented as “change = [post–pre]/pre”) was calculated as follows: (i) demographic features and clinical manifestations: gender, age, body mass index (BMI, kg/m2), ECOG PS, smoking history, comorbidities, treatment types, treatment lines, histology, TNM staging, driver gene mutation (EGFR, ALK, MET, HER-2, K-RAS, ROS-1, BRAF,TP53, PIK3CA), PD-L1 expression, and tumor mutation burden (TMB) when available; (ii) Peripheral blood inflammatory-nutritional indices: white blood cell count (WBC, E + 9/L), neutrophil count (NEU, E + 9/L), absolute lymphocyte count (LYM, E + 9/L), monocyte count (MONO, E + 9/L), hemoglobin (HB, g/L), albumin (Alb, g/L), LDH (U/L), C-reactive protein (CRP, mg/L), IL-6 (pg/mL), neutrophil–lymphocyte ratio (NLR), derived NLR (dNLR): NEU/(WBC − NEU), platelet-lymphocyte ratio (PLR), lymphocyte-to-monocyte ratio (LMR), prognostic nutritional index (PNI): Alb (g/L) + 5 × LYM; advanced lung cancer inflammation index (ALI): BMI × Alb (g/dL)/NLR, C-reactive protein-albumin ratio (CAR), system inflammation response index (SIRI): NEU × MONO/LYM, systemic immune inflammation index (SII): platelet count × NEU/LYM. (iii) Peripheral blood immune indices—lymphocyte subsets detected by flow cytometry assay: total T cells (CD3+ cells, Cells/μL), CD4+ T cells (Cells/μL), CD8+ T cells (Cells/μL), CD4+/CD8+ ratio (%), CD3+CD56+CD16+ Natural killer T (NKT)-like cells (Cells/μL), B cells (CD3−CD19+ cells, Cells/μL) and NK cells (CD3−CD16+CD56+ cells, Cells/μL).
Follow-up and irAEs recognition
The follow-up on patients’ clinical outcomes was performed by referring to the clinic's attendance records and phone calls. According to imRECIST [22], those whose tumor growth increased at least 20% compared to pre-treatment were defined as progression diseases (PD), whereas decreased by 30% were defined as partial response (PR) or stable disease (SD). The primary endpoint of this study was progression-free survival (PFS), measured from treatment initiation to disease progression or last follow-up. The second endpoint was severe irAEs. Data of irAEs reported by physicians in every cycle of ICIs treatment was recorded. irAEs were typically referred to as a broad spectrum of manifestations involving different organ systems, including the skin, endocrine, pneumological, cardiovascular, gastrointestinal, hepatic, neurological, hematological, and other rare irAEs [23]. According to Common Terminology Criteria Adverse Events, “irAEs of any grade” were defined as occurring in any grade of one or more events; “severe irAEs” were defined as irAEs of grades 3–4 or irAEs leading to discontinuation of medication.
Statistical analysis
The statistical analysis was performed using R studio (version 2022.02.0; https://www.R-project.org), SPSS (version 25.0) and X-tile software version 3.6.1 (Yale University). Missing data but no more than 10% missing were imputed with multiple imputations by the chained equations (MICE) approach. Categorical variables were expressed as n (%). Continuous data are presented as mean (± SD) or median (IQR, interquartile range). The best cut-off value of all variables for binary classification of PFS was recognized by X-tile. For the establishment of nomogram, firstly, we used the least absolute shrinkage and selection operator (LASSO) regression analysis to select the most useful prognostic factors. The optimal values of the penalty parameter λ were determined through tenfold cross-validation. Variables with zero regression coefficient are excluded while variables with nonzero are most strongly associated with the response. Secondly, variables with statistical significance (P < 0.05) in multivariate COX or logistic regression analysis were selected for constructing the nomogram. R package ‘rms’ was used for establishing and applying the nomogram. And each variable had a weighted score calculated by their respective coefficients, and the sum of the risk scores was associated with PFS or severe irAEs. The performance of nomogram was evaluated by area under (AUC) receiver operating characteristic curve (ROC), and calibration plots with bootstrapping (1000 resamples). Decision curve analysis (DCA) was used to assess whether patients could benefit from ICIs based on the nomogram (1000 resamples bootstrapping in logistic regression). Patients using nomograms for the prediction of PFS were split into low-risk and high-risk groups according to the optimal cut-off value of model risk score. Kaplan–Meier method and log-rank tests were used to assess differences in PFS between the predicted high-risk and low-risk groups. All p-values were set at P < 0.05 based on the two-tailed test.
Results
Patients’ clinical characteristics
The baseline characteristics of 138 aNSCLC patients with ICIs treatment were listed in Table 1. Among them, 44.9% were above 65 years of age, 81.9% were males, 64.5% were smokers, and 75.4% had an ECOG PS score < 2. In terms of clinical pathology, the proportion of squamous cell carcinoma (33.3%) was less than that of adenocarcinoma (58.0%), and more than half of patients (60.1%) had distant metastasis before ICIs treatment onset. 49.3% were evaluated in PD-L1 expression and 9.4% are detected as negative. 23.9% were evaluated in TMB testing and 10.9% demonstrated TMB-High expression. What’s more, there were 15.9% of the patients showed driver gene mutations in our study, and 7.2% were EGFR-sensitive mutation positive. In addition, 37.7% received ICIs monotherapy and 21.7% had received three or more prior lines of therapy.
Table 1.
Clinical characteristics of 138 aNSCLC patients receiving ICIs treatment
| Clinical characteristics | Level | Overall (n = 138) |
|---|---|---|
| Gender (%) | Female | 25 (18.1) |
| Male | 113 (81.9) | |
| Age (%) | < 65 | 76 (55.1) |
| ≥ 65 | 62 (44.9) | |
| BMI (%) | < 24 | 38 (27.5) |
| ≥ 24 < 28 | 73 (52.9) | |
| ≥ 28 | 27 (19.6) | |
| Smoking (%) | No | 49 (35.5) |
| Yes | 89 (64.5) | |
| ECOG PS (%) | < 2 | 104 (75.4) |
| ≥ 2 | 34 (24.6) | |
| Distant metastasis (%) | No | 55 (39.9) |
| Yes | 83 (60.1) | |
| Histology (%) | Adenocarcinoma | 80 (58.0) |
| Squamous | 46 (33.3) | |
| Others | 12 (8.7) | |
| PD-L1 (%) | < 1% | 13 (9.4) |
| ≥ 1%, < 50% | 28 (20.3) | |
| ≥ 50% | 27 (19.6) | |
| Unknown | 70 (50.7) | |
| TMB (%) | TMB-high | 15 (10.9) |
| TMB-low | 18 (13.0) | |
| Unknown | 105 (76.1) | |
| Commodities (%) | Hypertension | 33 (23.9) |
| Diabetes | 13 (9.4) | |
| Coronary heart disease | 12 (8.7) | |
| COPD | 10 (7.2) | |
| Bronchiectasis | 14 (10.1) | |
| Latent tuberculosis | 6 (4.3) | |
| Hepatitis B | 11 (8.0) | |
| Thyroid nodules | 16 (11.6) | |
| Mutation types (%) | BRAF | 1 (0.7) |
| EGFR | 7 (5.1) | |
| EGFR + KARS | 2 (1.4) | |
| EGFR + TP53 | 1 (0.7) | |
| HER2 | 1 (0.7) | |
| HER2 + PIK3CA | 1 (0.7) | |
| KRAS | 2 (1.4) | |
| MET | 1 (0.7) | |
| PIK3CA | 1 (0.7) | |
| TP53 | 5 (3.6) | |
| No | 116 (84.1) | |
| Treatment types (%) | Chemoimmunotherapy | 86 (62.3) |
| Immunotherapy | 52 (37.7) | |
| Treatment lines (%) | First line | 83 (60.2) |
| Second line | 25 (18.1) | |
| ≥ 3 lines | 30 (21.7) | |
| irAEs of any grade (%) | Non-irAEs | 63 (45.7) |
| irAEs | 75 (54.3) | |
| severe irAEs (%) | Non-Grade3/4 | 99 (71.7) |
| Grade3/4 | 39 (28.3) | |
| irAEs number (%) | 0 | 65 (47.1) |
| 1 | 47 (34.1) | |
| 2 | 18 (13.0) | |
| 3 | 5 (3.6) | |
| ≥ 4 | 3 (2.2) | |
| irAEs types (%) | Thyroid disorders | 22 (15.9) |
| Skin rash | 23 (16.7) | |
| Diarrhea | 13 (9.4) | |
| Hepatitis | 19 (13.8) | |
| Renal | 7 (5.1) | |
| Pneumonitis | 19 (13.8) | |
| Cardiovascular | 8 (5.8) | |
| Neurotoxicity | 1 (0.7) | |
| Thrombocytopenia | 2 (1.4) | |
| PFS (month, median [IQR]) | 11.0 (3.55–21.23) | |
| PFS status (%) | Non-recurrence | 44 (37.6) |
| Recurrence | 73 (62.4) | |
| Best overall response (%) | PD + SD | 51 (43.6) |
| PR | 66 (56.4) |
aNSCLC advanced-stage non-small cell lung cancer, ICIs immune checkpoint inhibitors, irAEs immune-related adverse events, TMB tumor mutational burden, ECOG PS Eastern Cooperative Oncology Group performance status scores, COPD chronic obstructive pulmonary disease, PFS progression-free survival, PD progression diseases, PR partial response, SD stable disease
During follow-up, 117 patients were available for survival outcome, while the remaining 21 discontinued treatment due to severe irAEs. The median PFS was 11.0 months (IQR 3.55–21.23). In terms of irAEs in 138 aNSCLC patients, approximately half of patients (75, 54.3%) experienced at least one irAE and a smaller number of them (39, 28.3%) experienced severe irAEs reported in total. The most common irAEs involved skin rash (23, 16.7%), thyroid disorders (22, 15.9%), pneumonitis (19, 13.8%) and hepatitis (19, 13.8%), followed by the diarrhea (13, 9.4%), cardiovascular (8, 5.8%), renal (7, 5.1%), neurotoxicity (1, 0.7%), thrombocytopenia (2, 1.4%).
New biomarkers exploration and nomograms construction
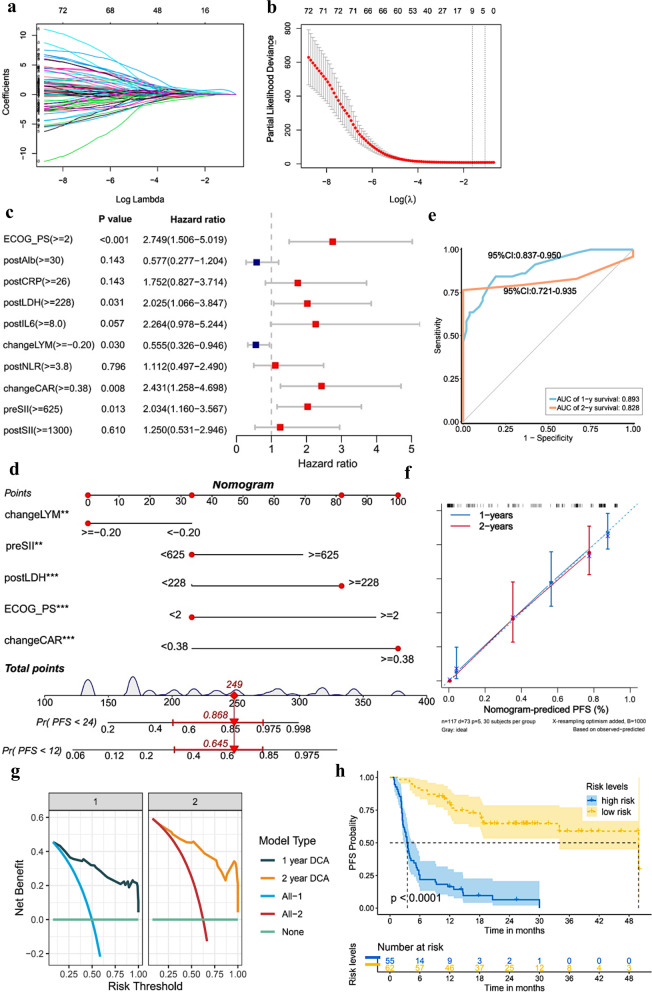
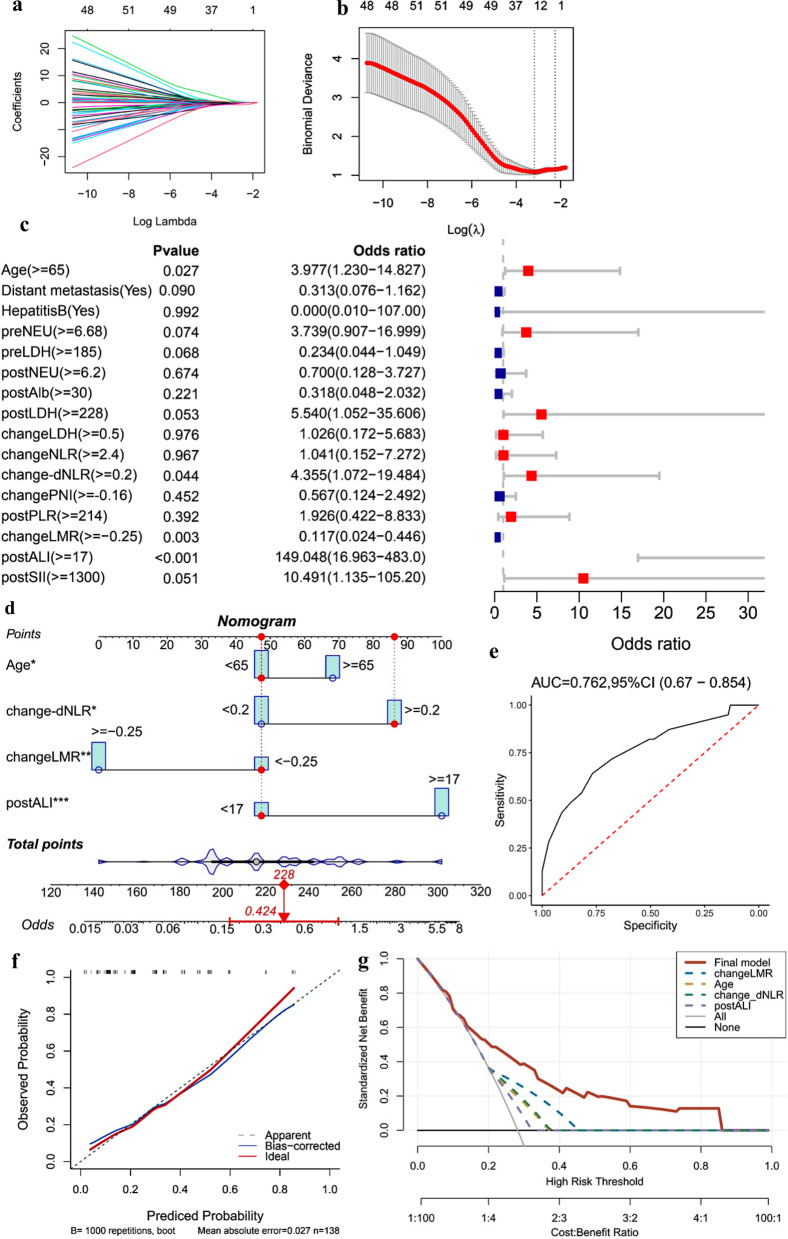
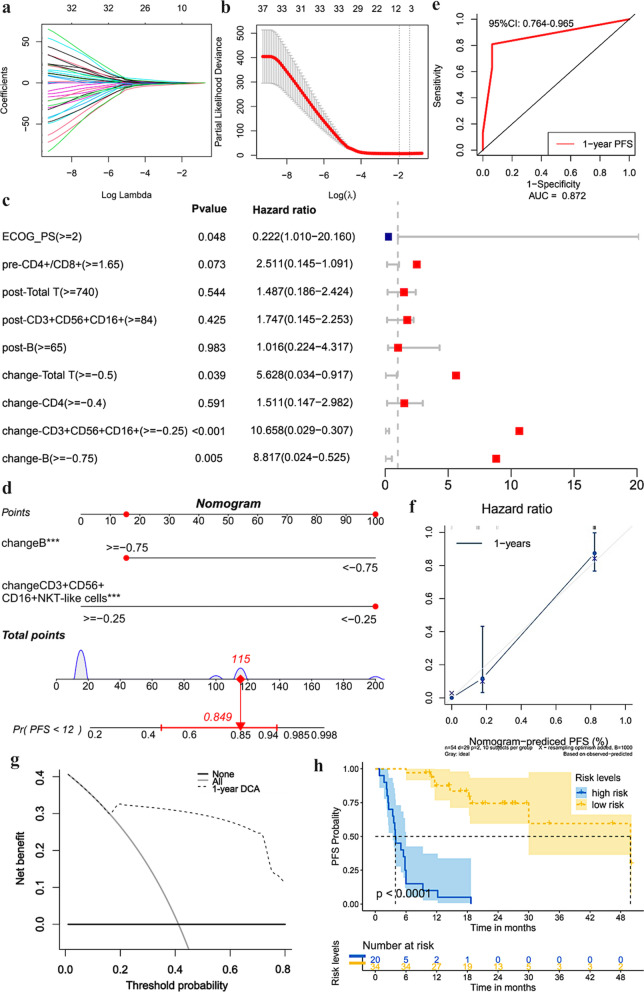
In this study, we constructed three nomograms for PFS and severe irAEs prediction (Figs. 2, 3, 4). Among them, two nomograms based on combined inflammatory-nutritional biomarkers were constructed for PFS (1y-PFS and 2y-PFS) and severe irAEs, and one nomogram was constructed for 1y-PFS based on immune indices. These nomograms were as follows: (i) ESCLL nomogram (based on ECOG PS, preSII, changeCAR, changeLYM and postLDH) was constructed to assess 1-, 2-year PFS probability. (ii) AdNLA nomogram (based on age, change-dNLR, changeLMR and postALI) was constructed to predict severe irAEs. (iii) NKT-B nomogram (based on change-CD3+CD56+CD16+NKT-like cells and change-B cells) was constructed to assess 1-year PFS probability.
Fig. 2.
Factors selection and nomogram construction based on peripheral-blood inflammatory-nutritional indices of PFS. a The LASSO coefficient profiles of candidate variables; b the optimal tuning parameter lambda λ in LASSO analysis selected with tenfold cross-validation. Dotted vertical line is set at the nonzero coefficients selected via tenfold cross-validation. The first vertical line equals the minimum error. The second vertical line shows cross-validated error within one standard error of the minimum; c forest plot of multivariate analysis of PFS. d “ESCLL” nomogram (based on ECOG PS, preSII, changeCAR, changeLYM and postLDH) was established to assess 1-year and 2-year PFS; Each factor corresponds to a specific point by drawing a vertical line from that variable to the points axis. After sum of the scores for each variable located on the Total Points axis. Finally, the sum represents the probability of 1-, 2-year survival by drawing straight down to the survival axis. (For example, an aNSCLC patient with changeLYM ≥ − 0.20, preSII < 625, postLDH ≥ 228, ECOG PS < 2 and changeCAR ≥ 0.38, the total score will be given by 249, corresponding to 1- and 2-year risks of progression of 0.645 and 0.868, respectively. The patient will accordingly have approximately 35.5% and 13.2% survival probabilities at 1 and 2 years, respectively) *P < 0.05; **P < 0.01; ***P < 0.001. e The ROC curve of the nomogram for 1-year, 2-year PFS; f calibration curves of the nomogram for 1-year, 2-year PFS; g decision curve analysis of the nomogram for 1-year, 2-year PFS; h Kaplan–Meier curves depicting PFS according to risk levels (cutpoint value: 1.37). Significant difference in survival of patients was observed between high and low risk score. ECOG PS, Eastern Cooperative Oncology Group Performance Status scores; preSII, pre-treatment systemic immune inflammation index; postLDH, post-treatment lactate dehydrogenase; changeLYM, change of absolute lymphocyte count; changeCAR, change of C-reactive protein-albumin ratio; ROC, receiver operator characteristic
Fig. 3.
Factors selection and nomogram construction based on peripheral-blood inflammatory-nutritional indices of severe irAEs. a The LASSO coefficient profiles of the candidate variables; b the optimal tuning parameter (lambda, λ) in the LASSO analysis selected with tenfold cross-validation via minimum criteria; c forest plot of multivariate analysis independent prognostic analysis of severe irAEs; d the “AdNLA” nomogram (based on age, change-dNLR, changeLMR and postALI) were constructed for severe irAEs prediction of aNSCLC patients receiving ICIs; e the ROC curve of the nomogram for severe irAEs; f calibration curves of the nomogram for severe irAEs; g decision curves analysis of the nomogram for severe irAEs. irAEs immune-related adverse events, change-dNLR change of derived-neutrophil–lymphocyte ratio, changeLMR change of lymphocyte-to-monocyte ratio, postALI post-treatment advanced lung cancer inflammation index
Fig. 4.
Factors selection and nomogram construction based on peripheral blood immune indices of PFS. a The LASSO coefficient profiles of the candidate variables; b the optimal tuning λ in the LASSO analysis selected with tenfold cross-validation via minimum criteria; c forest plot of multivariate analysis independent prognostic analysis of PFS. d “NKT-B” nomogram (based on change-CD3+CD56+CD16+NKT-like cells and change-B cells) was established to assess 1-year PFS of aNSCLC patients receiving ICIs; e the ROC curve of the nomogram for 1-year PFS; f calibration curves of the nomogram for 1-year PFS; g decision curve analysis of the nomogram for 1-year PFS; h Kaplan–Meier curves depicting PFS according to risk levels (cut-point value: − 4.1868)
“ESCLL” nomogram based on inflammatory-nutritional indices for PFS assessment
As shown in Fig. 2a–c, taking PFS as dependent variables, 10 variables (ECOG PS, postAlb, postCRP, postLDH, postIL6, changeLYM, postNLR, changeCAR, preSII and postSII) based on clinical features and inflammatory-nutritional indices with non-zero coefficient were obtained from LASSO regression and were further incorporated into the Cox multivariate analysis. Multivariate results showed that ECOG PS ≥ 2 (HR 2.749; 95% CI 1.506–5.019; P < 0.001), preSII ≥ 625 (HR 2.034; 95% CI 1.160–3.567; P = 0.013), changeCAR ≥ 0.38 (HR 2.431; 95% CI 1.258–4.698; P = 0.008), changeLYM ≥ − 0.20 (HR 0.555; 95% CI 0.326–0.946; P = 0.030) and postLDH ≥ 228 (HR 2.025; 95% CI 1.066–3.847; P = 0.031) were independent predictors affecting the prognosis of PFS in aNSCLC patients receiving ICIs. We further developed a model that includes 5 independent predictors—ESCLL—and displayed it as a nomogram to predict the 1- and 2-year PFS (1y-PFS, 2y-PFS) probabilities (Fig. 2d). A subsequent ROC analysis revealed that our prognostic nomogram had favorable discrimination, with 1- and 2-year AUC of 0.893 (95% CI 0.837–0.950) and 0.828 (95% CI 0.721–0.935), respectively (Fig. 2e). The calibration plot revealed that the 1- and 2-year survival probabilities predicted by ESCLL nomogram had an excellent agreement with the actual observations (Fig. 2f). The DCA curve demonstrated that ESCLL had a net benefit for 1- and 2-year PFS across threshold probabilities (Fig. 2g). The prognostic model risk score for each patient was computed according to coefficient generated from the COX regression: the model risk score formula = 1.3199 × ECOG PS + 0.7961 × preSII + 1.4788 × changeCAR − 0.7447 × changeLYM + 1.0738 × postLDH. All patients were then stratified into high-risk and low-risk groups using the optimal cutoff of ESCLL nomogram risk scores (cutoff = 1.37, P < 0.001). The Kaplan–Meier survival curve showed patients in the high-risk group exhibited a significantly poorer PFS (P < 0.001) (Fig. 2h).
“AdNLA” nomogram based on inflammatory-nutritional indices for severe irAEs prediction
Combined with the results from LASSO and multivariate logistic analysis, we confirmed 4 independent indicators, including age ≥ 65 (OR 3.977; 95% CI 1.230–14.827; P = 0.027), change-dNLR ≥ 0.2(OR 4.355; 95% CI 1.072–19.484; P = 0.044), changeLMR ≥ − 0.25(OR 0.117; 95% CI 0.024–0.446; P = 0.003) and postALI ≥ 17(OR 149.048; 95% CI 16.963–483.0; P < 0.001), for predicting the risk of severe irAEs (Fig. 3a–c). AdNLA nomogram based on the above predictors was constructed (Fig. 3d), exhibiting good model discrimination (AUC = 0.762, 95% CI 0.670–0.854) and calibration ability (Fig. 3e, f). The model risk score formula = 0.7480 × age + 1.3961 × change-dNLR − 1.7077 × changeLMR + 1.8928 × postALI. In addition, DCA showed across threshold probability, using AdNLA to predict severe irAEs incidence risk could be beneficial (Fig. 3g).
“NKT-B” nomogram based on immune indices for PFS assessment
57 patients had complete lymphocyte subsets data, of which 3 patients discontinued treatment due to severe irAEs. As shown in Fig. 4a–c, 9 variables (ECOG PS, pre-CD4+/CD8+ ratio, post-Total T cells, post-CD3+CD56+CD16+NKT-like cells, post-B cells, change-Total T cells, change-CD4+ T cells, change-CD3+CD56+CD16+NKT-like cells, and change-B cells) based on clinical features and immune indices with non-zero coefficient were obtained from LASSO regression and were further incorporated into the Cox multivariate analysis. Multivariate analysis showed that ECOG PS ≥ 2 (OR 0.222; 95% CI 1.010–20.160; P = 0.048), change-Total T cells ≥ − 0.5 (OR 5.628; 95% CI 0.034–0.917; P = 0.039), change-CD3+CD56+CD16+NKT-like cells ≥ − 0.25 (OR 10.658; 95% CI 0.029–0.307; P < 0.001) and change-B cells ≥ − 0.75 (OR 8.817; 95% CI 0.024–0.525; P = 0.005) were independent predictors associated with poor PFS. Notably, change-CD3+CD56+CD16+NKT-like cells had the highest OR, followed by change-B cells. Thus, we further selected change-CD3+CD56+CD16+NKT-like cells and change-B cells incorporated into new nomogram construction (namely NKT-B) to predict PFS probabilities (Fig. 4d). The model risk score formula = − 2.2680 × change-CD3+CD56+CD16+NKT-like cells − 1.9188 × change-B cells. NKT-B nomogram demonstrated great predictive accuracy (1-year-AUC = 0.872, 95% CI 0.764–0.965) and goodness of fit in the calibration plot (Fig. 4e, f). Additionally, DCA demonstrated that NKT-B had the highest net benefit for 1y-PFS across threshold probabilities (Fig. 4g). Kaplan–Meier survival curves showed that patients with high-risk scores exhibited significantly poor 1y-PFS (cutoff = − 4.1868, P < 0.001) (Fig. 4h).
Correlation of lymphocyte subsets with severe irAEs in aNSCLC with ICIs treatment
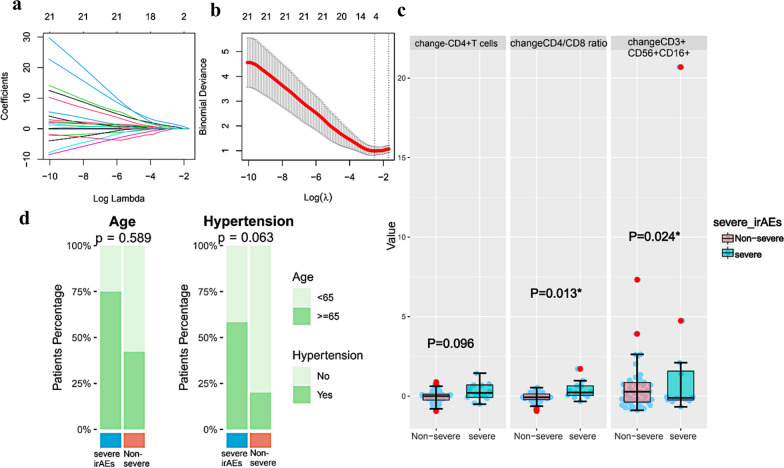
Since our sample size was limited in lymphocyte subsets detection, we chose to use those immune indices for association analysis instead of nomogram construction of severe irAEs. Variables retained by LASSO regression were shown in Fig. 5a, b. Multivariate analysis confirmed that changeCD3+CD56+CD16+NKT-like cells (P = 0.024) and changeCD4+/CD8+ ratio (P = 0.013) significantly were correlated with incidence of severe irAEs (Fig. 5c, d).
Fig. 5.
Factors selection and correlation analysis based on peripheral-blood immune indices of severe irAEs. a The LASSO coefficient profiles of the candidate variables; b the optimal tuning λ in the LASSO analysis selected with tenfold cross-validation via minimum criteria; c the development of severe irAEs corresponds to change-CD4+ T cells, change-CD4+/CD8+ ratio and change-CD3+CD56+CD16+NKT-like cells; d the development of severe irAEs corresponds to age and hypertension
Discussion
Identification of the biomarker set with maximal predictivity that is the easiest to apply in clinical practice to foresee ICIs benefit and serious toxicity is deeply needed in aNSCLC. While tissue PD-L1 expression is the most heavily studied biomarker, its definite role as a predictor of response to ICIs is more debated and problematic in geographical heterogeneity and insufficient tissue obtaining [24, 25]. As the noninvasive, low-cost, and reproducible clinical item, peripheral blood was used here as a source of common players of the inflammatory response and immunophenotypes critically implicated in PD-1/PD-L1 pathway [26]. Herein, we successfully established three new nomograms that demonstrate great predictive accuracy, including ESCLL nomograms for PFS (1y-PFS and 2y-PFS) and AdNLA nomograms for severe irAEs prediction respectively, based on integrated peripheral blood inflammatory-nutritional indices; in addition, NKT-B nomogram based on peripheral blood immune indices for 1y-PFS prediction of aNSCLC patients receiving ICIs. These noninvasive nomograms can help clinicians accurately predict outcomes, and identify those more likely to suffer toxicity from ICIs. Moreover, it was also notable that CD3+CD56+CD16+NKT-like cells, as special lymphocyte subset, could be a new potential biomarker of prognostic and severe irAEs. To our knowledge, that has not been reported in the study of aNSCLC ICIs.
In this study, it is noteworthy that higher postLDH and changeCAR were consistent predictors of PFS, which go beyond previous reports showing that LDH and CAR after ICIs treatment but not at baseline could be associated with poor prognostic [27–29]. Moreover, we also observed that poor ECOG PS, low changeLYM and high preSII were associated with worse PFS, which ties well with prior studies [30–33]. Another further novel finding was higher change-dNLR, postALI, and lower change-LMR could increase the risk of severe irAEs, giving new light on that early ICIs treatment could trigger circulating inflammation status and then even induce severe toxicity. Our results underscore the importance of the inflammation and nutrition status for aNSCLC patients with ICIs treatment [26]. However, most prior prognostic indices or models did not have a comprehensive analysis of peripheral inflammatory indices and may be affected by tumor-independent variables, such as infections or co-morbidities, partly limiting their clinical significance. Also of note, we further attempted to construct ESCLL nomogram incorporating ECOG PS, preSII, changeCAR, changeLYM and postLDH for 1y-PFS and 2y-PFS prediction. Intriguingly, it performs well, giving clearly better results than that of individuals and categorizing patients into high- and low-risk groups. Another key strength of our study was that AdNLA nomogram was developed, encompassing age, change-dNLR, changeLMR and postALI, and was able to early identify patients with severe irAEs. The power of this integrated model overcame that of individual scores. Our finding supports the notion that multiparametric strategies may improve our ability to anticipate the clinical response to ICIs and severe irAEs-specific early identification.
Moreover, considering that anti-tumor immunity is not only affected by inflammatory response but also in addition to facilitating activation of immune effector cells, we further screened the circulating lymphocyte subsets combined with clinical factors to provide immune profiles more representative of the multifaceted aspects of cancer immunity. Most notably, our observation is rather unique as it is, to our knowledge, the first to discover that change-CD3+CD56+CD16+NKT-like cells could be a new biomarker that was not only associated with favorable PFS but also a key discriminative feature of a group of patients with severe irAEs. Most prior studies have examined the immune profile of CD3+, CD4+, CD8+ T cells with yielded controversial results [34–38], but the importance of NKT-like cells was not investigated in ICIs-treated aNSCLC. Actually, NKT-like cells can express the surface markers of T cells (CD3+) and NK cells (CD56+ additional with CD16+) [39, 40]. Although NKT-like cells are poorly known, previous studies have proposed that the CD3+CD56+NKT-like cells may be a crucial effector for the antitumor activities in surgery-treated colon carcinoma, radiotherapy-treated gastric cancer, and ICIs-treated hepatocellular carcinoma patients [41, 42]. It was speculated that ICIs may trigger CD3+CD56+CD16+NKT-like cells to help screen benefit patient groups, however, this effect would be likely to indicate responses against self-antigens, which may lead to severe toxicity. What is also worth noticing is that high change-B cells group was linked to better PFS. Xia et al. [21] have reported that a high percentage of B cells before ICIs treatment positively impacted PFS in aNSCLC. To be noticed, our study not only highlighted the dynamic change of B cells but also focused on exploring the predictive value of absolute count. Moreover, it is shown that higher early change of CD4+/CD8+ ratio was significantly linked to severe irAEs, however, to our knowledge, has not been reported in the study of aNSCLC ICIs. Furthermore, we constructed a new immune-based nomogram NKT-B, which incorporated change-CD3+CD56+CD16+NKT-like cells and change-B cells to accurately predict 1y-PFS. We demonstrated that NKT-B outperformed each of the individual features alone and well in performance. As another noninvasive model previously published in CELL research, DIREct-On did not include peripheral blood inflammatory indices but pre-treatment CD8+ T cells and blood TMB, and early ctDNA change, which was associated with PFS in a retrospective study on 99 ICIs-treated aNSCLC patients [43]. It is particularly worth mentioning here that even though we did not replicate DIREct-On model, our results still suggested excellent performance (DIREct-On: AUC = 0.93; NKT-B: 0.872) and exploring new biomarkers, although this should be validated on a larger cohort.
Some limitations of the study should be noted. Firstly, this study is restricted to a one-center, retrospective, internal validated perspective. Future work might consider extending this study to a larger scale to externally validate. Secondly, due to the limited data on lymphocyte subsets, immune indices failed to be modeled for severe irAEs prediction and explored in conjunction with inflammatory-nutritional indices. Besides, tissue biopsy is not routinely performed in advanced patients, and thus PD-L1 expression has only been evaluated in small patient cohorts. Future studies could take into account additional data to present a more comprehensive and representative work. Thirdly, due to the time limitations, the overall survival was not reached which is a potentially important aspect that would add greater depth to the study.
Conclusions
The three brand-new nomograms derived from peripheral blood inflammatory-nutritional and immune indices, and clinical factors have utility for predicting response and serious toxicity to ICIs in aNSCLC. Given that the difficulty to date in developing robust predictive biomarkers for immunotherapy, noninvasive early identification proceeded from low-cost and straightforward peripheral blood data of response and toxicity assessment could help fill the unmet need of improving personalization. Notably, CD3+CD56+CD16+NKT-like cells could be a new biomarker that plays a vital role in distinguishing the survival and serious toxicity of ICIs. These preliminary results represent a significant step forward but warrant further research on a larger cohort.
Acknowledgements
The authors thank the Clinical Research Academy from Peking University Shenzhen Hospital for their support.
Abbreviations
- ICIs
Immune checkpoint inhibitors
- aNSCLC
Advanced-stage non-small cell lung cancer
- PD-1
Programmed-cell death 1
- PD-L1
Programmed-cell death ligand 1
- irAEs
Immune-related adverse events
- LASSO
Least absolute shrinkage and selection operator
- TMB
Tumor mutational burden
- ECOG PS
Eastern Cooperative Oncology Group performance status scores
- COPD
Chronic obstructive pulmonary disease
- PFS
Progression-free survival
- PD
Progression diseases
- PR
Partial response
- SD
Stable disease
- imRECIST
Immune-modified response evaluation criteria in solid tumors
- WBC
White blood cell count
- NEU
Neutrophil count
- LYM
Absolute lymphocyte count
- MONO
Monocyte count
- HB
Hemoglobin
- Alb
Albumin
- LDH
Lactate dehydrogenase
- CRP
C-reactive protein
- IL-6
Interleukin-6
- NLR
Neutrophil–lymphocyte ratio
- dNLR
Derived neutrophil–lymphocyte ratio
- PLR
Platelet-lymphocyte ratio
- LMR
Lymphocyte-to-monocyte ratio
- PNI
Prognostic nutritional index
- ALI
Advanced lung cancer inflammation index
- CAR
C-reactive protein-albumin ratio
- SIRI
System inflammation response index
- SII
Systemic immune inflammation index
- NK cells
Natural killer cells
- NKT
Natural killer T
- AUC
Area under curve
- ROC
Receiver operating characteristic curve
- DCA
Decision curve analysis
- MICE
Multiple imputations by the chained equations
- IQR
Interquartile range
Author contributions
LXW and XP designed and performed main experiments, analyzed data and wrote the manuscript; CX, LX, and ZC provided clinical data; CX and ZZH analyzed the data. FWY reviewed the data. XP and FWY conceived of the study, secured research funding, and supervised all aspects of the work. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81974460), Shenzhen Science and Technology Innovation Commission Foundation (Grant No. JCYJ20190809103203711), Shenzhen Science and Technology Innovation Commission Foundation (Grant No. JCYJ20210324105411031), Shenzhen High Level Hospital Construction Fund (LCYJ2021022, LCYJ2021008), Natural Science Foundation of Guangdong Province (2023A1515012460).
Availability of data and materials
The data generated in this study may be available upon reasonable request from the corresponding author.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Peking University Shenzhen Hospital and informed consent from all patients (Grant Number 2019-786).
Consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Weiyi Fang, Email: fangweiyi1975@163.com.
Ping Xu, Email: ping-xu@hotmail.com.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Forjaz G, Mooradian MJ, Meza R, Kong CY, Cronin KA, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383(7):640–649. doi: 10.1056/NEJMoa1916623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vansteenkiste J, Wauters E, Reymen B, Ackermann CJ, Peters S, De Ruysscher D. Current status of immune checkpoint inhibition in early-stage NSCLC. Ann Oncol. 2019;30(8):1244–1253. doi: 10.1093/annonc/mdz175. [DOI] [PubMed] [Google Scholar]
- 4.Doroshow DB, Sanmamed MF, Hastings K, Politi K, Rimm DL, Chen L, et al. Immunotherapy in non-small cell lung cancer: facts and hopes. Clin Cancer Res. 2019;25(15):4592–4602. doi: 10.1158/1078-0432.CCR-18-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gettinger SN, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33(18):2004–2012. doi: 10.1200/JCO.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei H, Jiang H, Song B. Role of medical imaging for immune checkpoint blockade therapy: from response assessment to prognosis prediction. Cancer Med. 2019;8(12):5399–5413. doi: 10.1002/cam4.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson JA, Schneider BJ, Brahmer J, Achufusi A, Armand P, Berkenstock MK, et al. Management of immunotherapy-related toxicities, version 1.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2022;20(4):387–405. doi: 10.6004/jnccn.2022.0020. [DOI] [PubMed] [Google Scholar]
- 9.Doroshow DB, Bhalla S, Beasley MB, Sholl LM, Kerr KM, Gnjatic S, et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol. 2021;18(6):345–362. doi: 10.1038/s41571-021-00473-5. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Z, Xie T, Qi C, Zhang X, Shen L, Peng Z. Peripheral blood biomarkers predictive of efficacy outcome and immune-related adverse events in advanced gastrointestinal cancers treated with checkpoint inhibitors. Cancers (Basel) 2022;14(15):3736. doi: 10.3390/cancers14153736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Z, Li Y, Yan X, Song Q, Wang G, Hu Y, et al. Pretreatment lactate dehydrogenase may predict outcome of advanced non small-cell lung cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Cancer Med. 2019;8(4):1467–1473. doi: 10.1002/cam4.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang A, Zhong G, Wang L, Cai R, Han R, Xu C, et al. Dynamic serum biomarkers to predict the efficacy of PD-1 in patients with nasopharyngeal carcinoma. Cancer Cell Int. 2021;21(1):518. doi: 10.1186/s12935-021-02217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Liu Y, Mi X, Shao M, Liu L. The prognostic value of prognostic nutritional index (PNI) and neutrophil to lymphocyte ratio (NLR) for advanced non-small cell lung cancer treated with platinum-based chemotherapeutics. Ann Palliat Med. 2020;9(3):967–978. doi: 10.21037/apm.2020.04.31. [DOI] [PubMed] [Google Scholar]
- 14.Shoji F, Takeoka H, Kozuma Y, Toyokawa G, Yamazaki K, Ichiki M, et al. Pretreatment prognostic nutritional index as a novel biomarker in non-small cell lung cancer patients treated with immune checkpoint inhibitors. Lung Cancer. 2019;136:45–51. doi: 10.1016/j.lungcan.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Klein F, von Muenchow L, Capoferri G, Heiler S, Alberti-Servera L, Rolink H, et al. Accumulation of multipotent hematopoietic progenitors in peripheral lymphoid organs of mice over-expressing interleukin-7 and Flt3-ligand. Front Immunol. 2018;9:2258. doi: 10.3389/fimmu.2018.02258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hardy-Werbin M, Rocha P, Arpi O, Taus A, Nonell L, Duran X, et al. Serum cytokine levels as predictive biomarkers of benefit from ipilimumab in small cell lung cancer. Oncoimmunology. 2019;8(6):e1593810. doi: 10.1080/2162402X.2019.1593810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drobni ZD, Zafar A, Zubiri L, Zlotoff DA, Alvi RM, Lee C, et al. Decreased absolute lymphocyte count and increased neutrophil/lymphocyte ratio with immune checkpoint inhibitor-associated myocarditis. J Am Heart Assoc. 2020;9(23):e018306. doi: 10.1161/JAHA.120.018306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim KH, Cho J, Ku BM, Koh J, Sun JM, Lee SH, et al. The first-week proliferative response of peripheral blood PD-1(+)CD8(+) T cells predicts the response to anti-PD-1 therapy in solid tumors. Clin Cancer Res. 2019;25(7):2144–2154. doi: 10.1158/1078-0432.CCR-18-1449. [DOI] [PubMed] [Google Scholar]
- 19.Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020;577(7791):561–565. doi: 10.1038/s41586-019-1914-8. [DOI] [PubMed] [Google Scholar]
- 20.Petitprez F, de Reynies A, Keung EZ, Chen TW, Sun CM, Calderaro J, et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature. 2020;577(7791):556–560. doi: 10.1038/s41586-019-1906-8. [DOI] [PubMed] [Google Scholar]
- 21.Xia L, Guo L, Kang J, Yang Y, Yao Y, Xia W, et al. Predictable roles of peripheral IgM memory B cells for the responses to anti-PD-1 monotherapy against advanced non-small cell lung cancer. Front Immunol. 2021;12:759217. doi: 10.3389/fimmu.2021.759217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodi FS, Ballinger M, Lyons B, Soria JC, Nishino M, Tabernero J, et al. Immune-modified response evaluation criteria in solid tumors (imRECIST): refining guidelines to assess the clinical benefit of cancer immunotherapy. J Clin Oncol. 2018;36(9):850–858. doi: 10.1200/JCO.2017.75.1644. [DOI] [PubMed] [Google Scholar]
- 23.Fiala O, Sorejs O, Sustr J, Kucera R, Topolcan O, Finek J. Immune-related adverse effects and outcome of patients with cancer treated with immune checkpoint inhibitors. Anticancer Res. 2020;40(3):1219–1227. doi: 10.21873/anticanres.14063. [DOI] [PubMed] [Google Scholar]
- 24.Buttner R, Gosney JR, Skov BG, Adam J, Motoi N, Bloom KJ, et al. Programmed death-ligand 1 immunohistochemistry testing: a review of analytical assays and clinical implementation in non-small-cell lung cancer. J Clin Oncol. 2017;35(34):3867–3876. doi: 10.1200/JCO.2017.74.7642. [DOI] [PubMed] [Google Scholar]
- 25.Cristescu R, Mogg R, Ayers M, Albright A, Murphy E, Yearley J, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science. 2018;362(6411):eaar3593. doi: 10.1126/science.aar3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rebuzzi SE, Leonetti A, Tiseo M, Facchinetti F. Advances in the prediction of long-term effectiveness of immune checkpoint blockers for non-small-cell lung cancer. Immunotherapy. 2019;11(12):993–1003. doi: 10.2217/imt-2019-0107. [DOI] [PubMed] [Google Scholar]
- 27.Lee DS, Park KR, Kim SJ, Chung MJ, Lee YH, Chang JH, et al. Serum lactate dehydrogenase levels at presentation in stage IV non-small cell lung cancer: predictive value of metastases and relation to survival outcomes. Tumour Biol. 2016;37(1):619–625. doi: 10.1007/s13277-015-3776-5. [DOI] [PubMed] [Google Scholar]
- 28.Alfranca YL, Garcia MEO, Rueda AG, Ballesteros PA, Rodriguez DR, Velasco MT. Blood biomarkers of response to immune checkpoint inhibitors in non-small cell lung cancer. J Clin Med. 2022;11(11):3245. doi: 10.3390/jcm11113245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim SR, Chun SH, Kim JR, Kim SY, Seo JY, Jung CK, et al. The implications of clinical risk factors, CAR index, and compositional changes of immune cells on hyperprogressive disease in non-small cell lung cancer patients receiving immunotherapy. BMC Cancer. 2021;21(1):19. doi: 10.1186/s12885-020-07727-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Passaro A, Spitaleri G, Gyawali B, de Marinis F. Immunotherapy in non-small-cell lung cancer patients with performance status 2: clinical decision making with scant evidence. J Clin Oncol. 2019;37(22):1863–1867. doi: 10.1200/JCO.18.02118. [DOI] [PubMed] [Google Scholar]
- 31.Yan Q, Ertao Z, Zhimei Z, Weigang D, Jianjun P, Jianhui C, et al. Systemic immune-inflammation index (SII): a more promising inflammation-based prognostic marker for patients with synchronic colorectal peritoneal carcinomatosis. J Cancer. 2020;11(18):5264–5272. doi: 10.7150/jca.46446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanizaki J, Haratani K, Hayashi H, Chiba Y, Nakamura Y, Yonesaka K, et al. Peripheral blood biomarkers associated with clinical outcome in non-small cell lung cancer patients treated with nivolumab. J Thorac Oncol. 2018;13(1):97–105. doi: 10.1016/j.jtho.2017.10.030. [DOI] [PubMed] [Google Scholar]
- 33.Botticelli A, Salati M, Di Pietro FR, Strigari L, Cerbelli B, Zizzari IG, et al. A nomogram to predict survival in non-small cell lung cancer patients treated with nivolumab. J Transl Med. 2019;17(1):99. doi: 10.1186/s12967-019-1847-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kagamu H, Kitano S, Yamaguchi O, Yoshimura K, Horimoto K, Kitazawa M, et al. CD4(+) T-cell immunity in the peripheral blood correlates with response to anti-PD-1 therapy. Cancer Immunol Res. 2020;8(3):334–344. doi: 10.1158/2326-6066.CIR-19-0574. [DOI] [PubMed] [Google Scholar]
- 35.Zhang N, Bevan MJ. CD8(+) T cells: foot soldiers of the immune system. Immunity. 2011;35(2):161–168. doi: 10.1016/j.immuni.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Subudhi SK, Aparicio A, Gao J, Zurita AJ, Araujo JC, Logothetis CJ, et al. Clonal expansion of CD8 T cells in the systemic circulation precedes development of ipilimumab-induced toxicities. Proc Natl Acad Sci USA. 2016;113(42):11919–11924. doi: 10.1073/pnas.1611421113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu K, Xia B, Zhang J, Li X, Yang S, Zhang M, et al. Positive correlation of peripheral CD8(+) T lymphocytes with immune-related adverse events and combinational prognostic value in advanced non-small cell lung cancer patients receiving immune checkpoint inhibitors. Cancers (Basel) 2022;14(15):3568. doi: 10.3390/cancers14153568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamphorst AO, Pillai RN, Yang S, Nasti TH, Akondy RS, Wieland A, et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci USA. 2017;114(19):4993–4998. doi: 10.1073/pnas.1705327114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114(10):1379–1388. doi: 10.1172/JCI200423594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ito K, Karasawa M, Kawano T, Akasaka T, Koseki H, Akutsu Y, et al. Involvement of decidual Valpha14 NKT cells in abortion. Proc Natl Acad Sci USA. 2000;97(2):740–744. doi: 10.1073/pnas.97.2.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romero-Olmedo AJ, Schulz AR, Huber M, Brehm CU, Chang HD, Chiarolla CM, et al. Deep phenotypical characterization of human CD3(+) CD56(+) T cells by mass cytometry. Eur J Immunol. 2021;51(3):672–681. doi: 10.1002/eji.202048941. [DOI] [PubMed] [Google Scholar]
- 42.Tao L, Wang S, Kang G, Jiang S, Yin W, Zong L, et al. PD-1 blockade improves the anti-tumor potency of exhausted CD3(+)CD56(+) NKT-like cells in patients with primary hepatocellular carcinoma. Oncoimmunology. 2021;10(1):2002068. doi: 10.1080/2162402X.2021.2002068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nabet BY, Esfahani MS, Moding EJ, Hamilton EG, Chabon JJ, Rizvi H, et al. Noninvasive early identification of therapeutic benefit from immune checkpoint inhibition. Cell. 2020;183(2):363–376.e13. doi: 10.1016/j.cell.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated in this study may be available upon reasonable request from the corresponding author.