Abstract
A novel protease, hydrolyzing azocasein, was identified, purified, and characterized from the culture supernatant of the fish pathogen Yersinia ruckeri. Exoprotease production was detected at the end of the exponential growth phase and was temperature dependent. Activity was detected in peptone but not in Casamino Acid medium. Its synthesis appeared to be under catabolite repression and ammonium control. The protease was purified in a simple two-step procedure involving ammonium sulfate precipitation and ion-exchange chromatography. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis of the purified protein indicated an estimated molecular mass of 47 kDa. The protease had characteristics of a cold-adapted protein, i.e., it was more active in the range of 25 to 42°C and had an optimum activity at 37°C. The activation energy for the hydrolysis of azocasein was determined to be 15.53 kcal/mol, and the enzyme showed a rapid decrease in activity at 42°C. The enzyme had an optimum pH of around 8. Characterization of the protease showed that it required certain cations such as Mg2+ or Ca2+ for maximal activity and was inhibited by EDTA, 1,10-phenanthroline, and EGTA but not by phenylmethylsulfonyl fluoride. Two N-methyl-N-nitro-N-nitrosoguanidine mutants were isolated and analyzed; one did not show caseinolytic activity and lacked the 47-kDa protein, while the other was hyperproteolytic and produced increased amounts of the 47-kDa protein. Azocasein activity, SDS-PAGE, immunoblotting by using polyclonal anti-47-kDa-protease serum, and zymogram analyses showed that protease activity was present in 8 of 14 strains tested and that two Y. ruckeri groups could be established based on the presence or absence of the 47-kDa protease.
Yersinia ruckeri is known to be the etiological agent responsible for the enteric red mouth (ERM) disease of fish. ERM disease is spread throughout the world and affects mainly intensive aquaculture of trout and salmon. Disease outbreaks have a relationship with stress conditions, but little information is available about the virulence mechanisms involved in the disease progression. Several factors contribute to the pathogenic potential of this bacterium. Y. ruckeri produces protease, lipase, and hemolysin as extracellular factors which when injected into fish lead to the appearance of symptoms associated with pathogenicity (38). Furones et al. (15, 16) have shown that virulence of Y. ruckeri is related to the activity of a heat-sensitive factor present in cell extracts. Iron availability influences the virulence of many pathogens, and in Y. ruckeri several outer membrane proteins regulated by iron have also been identified (11, 37).
Bacterial proteases are mainly involved in providing peptide nutrients for the microorganism. However, the production of bacterial proteases could contribute to the pathogenesis of infections, and therefore they could be considered virulence factors. In fact, some authors regard proteases as the main virulence factors present among the extracellular factors. Although direct evidence revealing the molecular mechanisms by which bacterial proteases participate in the development of the pathology is still lacking, it has been suggested that proteolytic enzymes of fish pathogens, such as Aeromonas hydrophila (26), Vibrio anguillarum (33), Vibrio vulnificus (24), Aeromonas salmonicida (19, 39), Flexibacter columnaris (18), and Flexibacter psychrophilus (2), play an important role in causing massive tissue damage in the host, which may aid the establishment of infection. Most of the characterized proteases from fish pathogens are metalloproteases requiring zinc for enzymatic activity (19, 24, 32). For the pathogen Pseudomonas aeruginosa extensive evidence suggests that elastase, a metalloprotease, is required for maximal virulence (23, 35).
The effect of environmental conditions on the production of extracellular proteolytic enzymes could play an important role in the induction or repression of the enzyme by specific compounds. Production of extracellular proteases has been shown to be sensitive to repression by different carbohydrate and nitrogen sources (21, 27). Catabolic enzymes responded to both carbon control and nitrogen control in enteric bacteria (14, 17). In the bacteria A. hydrophila (34), A. salmonicida (9), and P. aeruginosa (23) protease production is influenced by carbon and nitrogen sources. Additionally, the temperature can influence the protease production, as occurs in A. hydrophila (31, 34).
In this paper, we report the finding of a protease with caseinolytic activity produced by Y. ruckeri strains from different origins. The protease was detected at the end of the exponential growth phase under different culture conditions, and it was repressed by the presence of NH4Cl as well as glucose and other sugars in the medium. It was purified and biochemically characterized. We also obtained mutants that showed either no caseinolytic activity or overproduction which will be useful for further studies of this protease as a virulence factor.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The origins of the Y. ruckeri strains are as follows: strains 146, 147, 148, 149, and 150 had been isolated during naturally occurring outbreaks of ERM disease at a Danish fish farm and were kindly provided by J. L. Larsen; strains A189, N190, R191, and C192 had been isolated during different outbreaks of the disease at a Spanish fish farm and were taxonomically characterized by I. Marquez; strains 35/85 and 13/86 were kindly provided by C. J. Rodgers; and strains 11.4, 11.29, and ATCC 29473 were from the CECT (Spanish Type Culture Collection).
Bacterial strains were routinely cultured on nutrient agar (NA) or nutrient broth (NB) (Difco) at 20°C. Growth curves of Y. ruckeri 150 and mutant strains derived from it, CS1 and E1, were obtained by monitoring the culture absorbance at 600 nm by using a Perkin-Elmer spectrophotometer at different incubation times. For this purpose, 250-ml flasks containing 50 ml of NB were each inoculated with 500 μl of a stationary-phase NB culture and incubated at 20°C and 250 rpm on a New Brunswick controlled environmental incubator shaker.
The effects of culture conditions on protease production were assayed by growing the microorganism in 1% peptone medium containing 2.9 mM K2HPO4 and 5 mM MgCl2 to which different carbohydrates (50 mM), NH4Cl (50 mM), or Casamino Acids (1%) was added. Growth was monitored as previously described, and protease activity was determined after 24 h of incubation. Determinations of the numbers of CFU per ml of culture were performed by plating 100-μl samples from serial 10-fold dilutions of the cell culture on NA.
Culture supernatants from different Y. ruckeri strains and mutants for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), zymograms, and protease immunodetection were obtained as follows. Fifty milliliters of NB medium contained in a 250-ml flask was inoculated with 500 μl of an overnight culture and incubated at 20°C in a shaker at 250 rpm for 24 h. Culture supernatants were collected by centrifugation (23,500 × g, 15 min) with a Kontron T-124 centrifuge. Aliquots of 20 ml were taken and dialyzed twice against 4 liters of distilled water for 12 h. Then, samples were freeze-dried and resuspended in 500 μl of 25 mM Tris-HCl, pH 7.6 (Tris buffer), distributed in 25-μl volumes, and stored at −20°C until used.
Assay of proteolytic activity and protein content.
Proteolytic activity was assayed by using azocasein (Sigma) as a substrate. Briefly, 120 μl of a suitable dilution of enzyme solution was added to 480 μl of azocasein (1%, wt/vol) in reaction buffer (Tris buffer containing MgCl2 [final concentration, 5 mM]), and the mixture was incubated at 30°C for 30 min. The reaction was terminated by adding 600 μl of 10% (vol/wt) trichloroacetic acid and left for 30 min on ice, followed by centrifugation at 15,000 × g, at 4°C for 10 min. Eight hundred microliters of the supernatant was neutralized by adding 200 μl of 1.8 N NaOH, and the absorbance at 420 nm (A420) was measured using a spectrophotometer (lambda 3A; Perkin-Elmer). One unit of enzyme activity was defined as the amount which yielded an increase in A420 of 0.01 in 30 min at 30°C.
The protein content of samples was estimated by the methods of Lowry et al. (30) by using bovine serum albumin as the standard.
Protease purification.
Among the different strains tested, Y. ruckeri 150 was chosen for further studies because it showed the highest hydrolysis activity on casein plates (NA supplemented with 1.25% skim milk powder; Oxoid).
Five milliliters of an overnight culture of Y. ruckeri 150 (per flask) was used to inoculate two 2-liter Erlenmeyer flasks containing 500 ml of NB. After 24 h of incubation at 20°C with agitation at 250 rpm, cells were harvested by centrifugation (23,500 × g for 15 min at 4°C), and the culture supernatant was used as the starting source for protease purification. All steps were carried out at 4°C.
(i) For ammonium sulfate precipitation, 328.5 g of ammonium sulfate was slowly added to 910 ml of a 4°C culture supernatant, to achieve 60% saturation. After 1 h, the precipitate was recovered by centrifugation (30,000 × g for 45 min), dissolved in Tris buffer, and dialyzed twice (for 16 h the first time and 4 h the second time) against 2 liters of Tris buffer.
(ii) For ion-exchange chromatography, the dialyzed material (13 ml) was loaded at a flow rate of 0.5 ml/min onto an anion-exchange column (DEAE-Sephacel; Pharmacia, Uppsala, Sweden) (3 by 10 cm) previously equilibrated with Tris buffer. The column was then washed with 250 ml of Tris buffer, and bound proteins were eluted with a 250-ml linear gradient of NaCl ranging from 0 to 0.5 M at a flow rate of 0.3 ml/min. Fractions (3.1 ml) were collected, and 60-μl aliquots were assayed for protease activity by using azocasein as a substrate. Positive fractions (range, 34 to 42 fractions) were pooled together, dialyzed overnight against 4 liters of 5 mM Tris buffer (volume recovered, 33 ml), lyophilized, resuspended in Tris buffer, and dialyzed again against the same buffer (volume recovered, 1.5 ml). Aliquots of the purified protein were stored at −20°C.
Characterization of the enzyme activity.
The caseinolytic activity was assayed at different pH values ranging from 4 to 11 by using azocasein as a substrate. The buffers used were the following: for pH 4, 25 mM piperazine; for pHs 6.1 and 6.7, 25 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)]; for pHs 7.0, 7.6, 8.1, 8.5, and 9.5, 25 mM Tris-HCl; and for pHs 10 and 11, 25 mM CAPS [3-(cyclohexylamino)-1-propanesulfonic acid]. For thermostability testing, the purified protein was incubated at 4, 12, 18, 25, 30, 37, 42, 47, and 55°C for 30 min in Tris buffer containing 5 mM MgCl2 and 1% azocasein. The activation energy (Ea) was determined from the slope (−Ea/R) of Arrhenius plots of ln k (k = 100 × enzyme units [EU]) against the reciprocal of the temperature. The effects of different ions (Ca2+, Mg2+, and K+) on protease thermostability were studied by incubating purified protein at 42°C in Tris buffer containing 10 μM to 5 mM ion concentrations for 0, 10, 20, and 30 min. Afterwards, the residual activities were assayed with 1% azocasein as the substrate at 30°C for 30 min.
For inhibition studies, pure protease was preincubated with different inhibitors for 10 min at room temperature in Tris buffer and then caseinolytic activity was assayed.
Electrophoresis and zymograms.
The method used for SDS-PAGE was essentially the one described by Laemmli (25). For zymogram analysis, sodium caseinate (1%) copolymerized with the gels was used. Samples were loaded into the gel without heating, and electrophoresis was performed at 4°C at a constant current of 25 mA. Following electrophoresis, gels were washed successively, twice with deionized water and then twice with Tris buffer, each for 30 min at 4°C. Then, gels were incubated overnight in Tris buffer containing 5 mM MgCl2 at room temperature. Finally, gels were stained with 0.1% Coomassie brilliant blue R 250 in 4:1:5 methanol/acetic acid/water (vol/vol/vol) and destained in the same solution without the dye, to reveal zones of substrate hydrolysis.
Preparation of antiserum and immunodetection.
A 2-ml mixture of equal parts of Freund complete adjuvant and purified protease (80 μg) was injected subcutaneously in 50-μl aliquots into a New Zealand White rabbit (2.5 kg). Twenty days after the injections, the rabbit was exsanguinated and serum was separated and stored in fractions. For immunodetection experiments, proteins were separated electrophoretically as described above and transferred onto nitrocellulose membranes (Hybond-P; Amersham) for 1 h at 50 volumes in 10 mM CAPS (pH 11) containing 20% methanol by using a Trans-blots cell (Bio-Rad). The nitrocellulose filters were then washed by incubation at room temperature in 20 mM Tris-HCl (pH 7.6)–137 mM NaCl containing 0.1% Tween 20 (TBS-T) for 10 min, and immunodetection was carried out by following the procedure for the ECL Western blotting system from Amersham (ECL kit) (RPN2108) by using rabbit antiserum raised against the protease (1:500) and anti-rabbit immunoglobulin G-peroxidase conjugate (Amersham) (1:2,500). Excess of ligand was washed away in TBS-T for 15 min, and detection of the proteins was performed according to the manufacturer’s instructions. Membranes were exposed for different times to Hyperfilm ECL (Amersham) until a suitable signal was obtained.
Mutagenesis.
Y. ruckeri 150 was cultured in 100 ml of NB in a 250-ml flask at 20°C, with constant agitation (250 rpm). Cells were harvested by centrifugation from 20-ml cultures in early stationary phase (16.5 × 108 cells/ml) and washed twice with Tris buffer. The pellets were then suspended in 5 ml of Tris buffer containing 1 mM EDTA and incubated for 30 min at room temperature. Cells were washed twice with Tris buffer, resuspended in 1 ml of the same buffer containing 100 μg of N-methyl-N′-nitro-N-nitrosoguanidine (NTG) and incubated for 90 min at room temperature. Under those conditions 99% cell mortality was achieved. Then, cells were washed twice with Tris buffer, resuspended in 1 ml of the same buffer, and smeared on NA plates supplemented with 1.25% (wt/vol) skim milk powder. After incubation at 20°C for 3 days, about 2,000 colonies were screened for mutants displaying both extremes of protease activity, i.e., deficient and hyperproducing mutants. Therefore, colonies with a major hydrolysis zone as well as those without a clear proteolytic one were selected and subcultured several times in order to obtain stable mutants.
RESULTS
Effects of culture conditions on protease production.
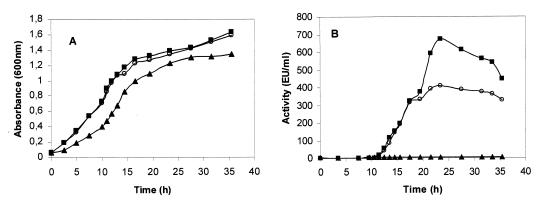
Caseinolytic activity in NB culture supernatants of Y. ruckeri 150 was detected in the late logarithmic growth phase (Fig. 1A and B). Approximately 12 h after the initiation of protease production activity reached a maximum, and this was followed by a slow but progressive decrease (Fig. 1B). Two mutant strains (CS1 and E1) of Y. ruckeri 150 were isolated after treatment with NTG. The mutant CS1 produced 1.5 to 2 times more protease activity than the parent strain, and the mutant strain E1 did not produce any detectable caseinolytic activity (Fig. 1B).
FIG. 1.
Production of caseinolytic protease during Y. ruckeri growth. Cells were grown in NB medium, and at different incubation times 120 μl of the cell-free supernatants was used to measure the caseinolytic activity, with azocasein used as a substrate. (A) Absorbance at 600 nm as a measure of cell density. (B) Caseinolytic activity. Symbols: ○, Y. ruckeri wild-type strain; ■, CS1 mutant; and ▴, E1 mutant.
Table 1 shows the results for protease production of strains grown in the presence of different nutrients. The highest levels of both protease production and growth were obtained when peptone medium was used. NB was also found to give good protease production, while growth in Casamino Acids resulted in poor growth and no detectable caseinolytic activity. Growth was significantly improved when peptone was supplemented with carbohydrates; however, the production of the caseinolytic activity was repressed. The greatest repression effect (approximately 95.5% repression) was observed when glucose or fructose was added to the culture medium. Glycerol, mannitol, and maltose also acted as potent production inhibitors, while the presence of lactose had no effect at all. Protease production was diminished when peptone was supplemented with NH4Cl. A 77.2% reduction was found when NH4Cl was present at a final concentration of 50 mM. One percent gelatin or 1% sodium caseinate was unable to support further growth of bacteria following 24 h of incubation. On the other hand, protease production initiation was independent of the medium composition (data not shown).
TABLE 1.
Effect of the medium composition on growth and caseinolytic protease productiona
| Nutrient | Cell growth (CFU [108]/ ml) | Protease (EU/ml) | Protease production (EU/ml)/(CFU [108]/ml) | Caseinolytic activity (%)b |
|---|---|---|---|---|
| None (peptone) | 25.7 | 507.6 | 19.7 | 100 |
| Lactose | 28.1 | 560.2 | 19.9 | 101.6 ± 2 |
| Galactose | 39.1 | 436.6 | 11.1 | 56.6 ± 3 |
| Sucrose | 30.3 | 446.5 | 14.7 | 74.6 ± 2 |
| Glycerol | 48.2 | 173.5 | 3.6 | 18.2 ± 1 |
| Mannitol | 62 | 151.9 | 2.4 | 12.3 ± 1 |
| Fructose | 63.8 | 52.3 | 0.8 | 4.1 ± 0 |
| Maltose | 71.6 | 383.5 | 5.3 | 27.1 ± 1 |
| Glucose | 63.2 | 59.8 | 0.9 | 4.7 ± 0 |
| Peptone + NH4Cl | 25.9 | 117 | 4.5 | 22.8 ± 1 |
| Casamino Acids | 15.1 | 0 | 0 | 0 ± 0 |
| Casamino Acids + NH4Cl | 14.8 | 0 | 0 | 0 ± 0 |
| NB | 17.5 | 382.6 | 21.8 | 110.8 ± 3 |
For nutrient studies cells were grown on 1% (wt/vol) peptone medium containing 5 mM MgCl2, 2.9 mM K2HPO4, and the indicated nutrient source (50 mM for each carbohydrate and NH4Cl; 1% (wt/vol) for glycerol, mannitol, and Casamino Acids). Samples were obtained at stationary growth phase (24 h), and caseinolytic activity was determined by using 120 μl of cell-free supernatant. Values are averages ± standard deviations for three independent experiments.
Percent caseinolytic activity in each medium expressed in relation to the activity in peptone medium (100%).
The effects of two different incubation temperatures (4 and 28°C) on protease production were evaluated. The microorganism was able to grow at 4°C in NB, and caseinolytic activity started after approximately 48 h of incubation, being maximum after 72 h and reaching 75% of the activity obtained at 20°C. The optimal growth temperature for Y. ruckeri is considered to be 28°C; however, at this temperature, no caseinolytic activity was detected at all.
Purification and biochemical properties of exocellular protease from Y. ruckeri.
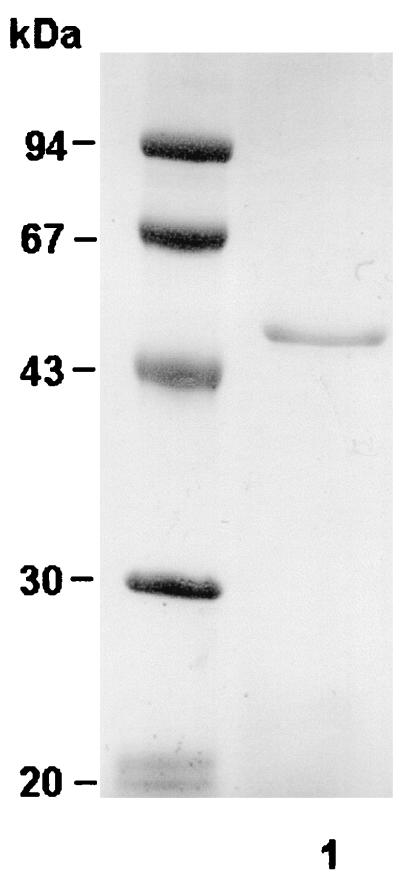
Y. ruckeri protease was purified from NB culture supernatant as indicated in Materials and Methods. The purification results are shown in Table 2. Ammonium sulfate precipitation followed by dialysis resulted in a 1.6-fold increase of specific activity. The protein was then adsorbed onto DEAE-Sephacel, and the caseinolytic activity was recovered as a sharp peak, with the greater proportion of proteolytic activity eluting at 0.27 M NaCl. The SDS-PAGE analysis of the molecular mass of the purified enzyme revealed a single band, of 47 kDa (Fig. 2). The process yielded about 80-fold purification.
TABLE 2.
Purification of Y. ruckeri 47-kDa caseinolytic protease
| Purification step | Total protein (mg) | Total activity (EU) | Sp act (EU/mg) | Purifi-cation (fold) | Yield (%) |
|---|---|---|---|---|---|
| Culture supernatant | 4,400 | 412,533 | 92.91 | 1 | 100 |
| Ammonium sulfate | 11.44 | 176,800 | 154.54 | 1.66 | 42.8 |
| DEAE-Sephacel | 1.12 | 8,580 | 7,633.45 | 82.15 | 2.07 |
FIG. 2.
SDS–12% PAGE of purified caseinolytic protease produced by Y. ruckeri. Protein (∼7.2 μg) pooled at the DEAE-Sephacel purification step was gel loaded and, after electrophoresis, stained with Coomassie brilliant blue R-250. Protein molecular mass markers (expressed in kilodaltons) are indicated on the left. Lane 1, Purified 47-kDa protein.
Detailed studies were carried out in order to characterize the Y. ruckeri extracellular caseinolytic protease. The proteolytic cleavage of azocasein was linear for at least 1 h under the conditions tested. Elastine Congo red was not degraded by the enzyme (data not shown). The maximum activity was exhibited at pH 8.1 under the assay conditions used, although the optimal conditions for activity were displayed at pHs ranging from 7.6 to 8.5 and from 6.1 to 9.5, with 24 and 35% relative activities, respectively. Very little activity was seen at or below pH 6.1, but a sharp increase occurred at pH 6.7 and enzyme activity decreased markedly at pH values above 8.6.
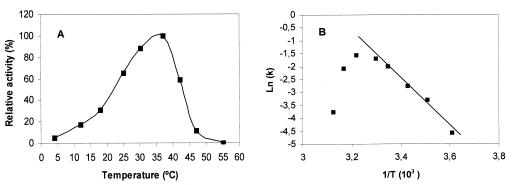
The optimum reaction temperature for the purified protease was 37°C (Fig. 3A). The enzyme remained active over a range of temperatures varying from 4 to 47°C, with approximately 65 and 58% relative activities at 25 and 42°C, respectively. From 42°C onwards, the activity declined sharply, and it was finally undetectable at 55°C. The enzyme became unstable at about 42°C, as can be deduced from the Arrhenius plot shown in Fig. 3B. The Ea for the hydrolysis of azocasein (15.53 ± 0.22 kcal/mol) in the range from 0 to 37°C was estimated from the linear portion of the Arrhenius plot (Fig. 3B). Total enzyme activity was lost after the incubation of the protein in Tris buffer for 10 min at 42°C. However, under the same incubation conditions, the enzyme remained active with 58 and 74% relative activities in the presence of 5 mM MgCl2 or CaCl2, respectively. This difference between the effects of the two ions on the protease activity at this temperature was maintained for at least 30 min of incubation. A smaller effect on thermostability was observed with 1 mM concentrations of the same ions, and no effect at all was detected below 0.5 mM. KCl had no effect on thermostability.
FIG. 3.
Effect of temperature on the activity of the 47-kDa protease. (A) The enzyme (∼2.1 μg) was incubated in 600 μl of Tris buffer containing 5 mM MgCl2 and 1% azocasein for 30 min at various temperatures, and caseinolytic activity was measured as described. The values obtained at 37°C were taken as 100%. Relative activities are the averages for two independent experiments. (B) Arrhenius plot showing thermal deactivation of the 47-kDa protease. The ln of specific activity (k) (100 × EU) was plotted against the reciprocal of absolute temperature (T).
The effects of different protease inhibitors and cations on the activity were also investigated (Table 3). The chelating agents EDTA and EGTA and the metalloprotease inhibitor 1,10-phenanthroline strongly reduced activity. The serine protease inhibitor phenylmethylsulfonyl fluoride failed to suppress activity, while dithiothreitol caused approximately 70% inhibition of activity.
TABLE 3.
Metal ion and inhibitor effects on the activity of the purified protease
| Compound (mM concn) | Caseinolytic activity (%)a |
|---|---|
| None | 100 |
| CaCl2 (1) | 124.2 ± 8 |
| CaCl2 (10) | 132.1 ± 10 |
| MgCl2 (1) | 122.1 ± 9 |
| MgCl2 (10) | 158.4 ± 6 |
| ZnCl2 (1) | 84.13 ± 7 |
| ZnCl2 (10) | 10.5 ± 3 |
| MnCl2 (1) | 94.3 ± 4 |
| MnCl2 (10) | 66.6 ± 3 |
| Phenylmethysulfonyl fluoride (1) | 100 ± 7 |
| 1,10-Phenanthroline (1) | 5.5 ± 1 |
| Dithiothreitol (1) | 28.4 ± 3 |
| EGTA (1) | 49.6 ± 4 |
| EDTA (1) | 6.6 ± 3 |
| EDTAb (5) | 5.1 ± 2 |
| EDTA (5) + CaCl2 (10) | 43.6 ± 6 |
| EDTA (5) + MgCl2 (10) | 55.7 ± 5 |
| EDTA (5) + CaCl2 (5) + MgCl2 (5) | 57.4 ± 6 |
Caseinolytic activity is expressed as the percentage of the control value (with no addition). The values are the averages ± standard deviations for two independent experiments. Purified protease (∼1.7 μg) was incubated in Tris buffer containing each cation. For inhibition studies the enzyme (∼1.7 μg) was preincubated with the inhibitor compound for 10 min at room temperature, and then caseinolytic activities were determined.
The effect of Ca2+ and Mg2+ on EDTA-inhibited protease was studied by incubating approximately 1.7 μg of enzyme in Tris buffer containing 5 mM EDTA for 10 min at room temperature. The reaction mixture was then made 10 mM with either CaCl2 or MgCl2 or with 5 mM concentrations of each of the two combined. Caseinolytic activity was then determined by incubation for 30 min at 30°C in the presence of 1% azocasein as the substrate.
On the other hand, protease activity was enhanced when CaCl2 and MgCl2 were added to the reaction mixture (Table 3). On the contrary, other cations, like Zn2+, had an inhibitory effect. The nature of the metal ion(s) required for caseinolytic activity was studied by using a reconstitution assay. As shown in Table 3, the protease was inhibited by EDTA (5 mM) and the activity was restored with metal ions included in CaCl2 or MgCl2 at a concentration of 10 mM or a mixture of both salts (5 mM CaCl2 with 5 mM MgCl2).
Caseinolytic activities of different Y. ruckeri strains and mutants.
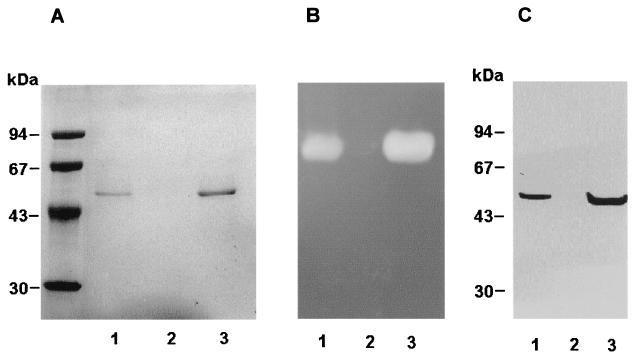
In order to discern if the caseinolytic activity is a constant characteristic in this bacterium, the presence of the enzyme as indicated by SDS-PAGE analysis, sodium caseinate zymograms, and quantitative azocasein assays was determined for 14 Y. ruckeri strains grown in NB. The culture supernatants of stationary-phase cultures of these strains had different caseinolytic activities as measured by the azocasein method. Thus, the strains 150, 148, 4319, 13/86, 149, A189, N190, E191, and C192 showed strong activity (Azo+), whereas negligible levels were found in the strains 146, 147, 955, 956 and 35/85 (Azo−) (data not shown). In order to establish a correlation between azocasein hydrolysis and the presence of a particular protein in the culture supernatant, SDS-PAGE or the 14 different strains was performed. Only those strains defined as Azo+ produced and excreted the 47-kDa protein, whereas Azo− strains lacked this protein (Fig. 4A). Furthermore, two different protein patterns, indicated as Azo+ and Azo− (lane 1 and lane 2, respectively, in Fig. 4A), were observed. The 47-kDa protein was the predominant protein in the strains with the Azo+ pattern, whereas Azo− strains presented two different major proteins of approximately 42 and 52 kDa. At the same time, zymograms obtained with sodium caseinate as the substrate showed a unique degradation area for Azo+ strains while no degradation was observed for cell-free supernatant obtained from Azo− strains (Fig. 4B). Immunoblot analysis using antibodies raised against the 47-kDa protein showed that it was present in the Azo+ strains and that there was no immunological reaction with any protein from the Azo− group, confirming that the 47-kDa protein was absent in this group of strains (Fig. 4C).
FIG. 4.
Analysis of the presence of the 47-kDa enzyme in cell-free supernatants of representative Azo+ (Y. ruckeri 150) and Azo− (Y. ruckeri 146) strains. Culture supernatants (20 ml) from cells grown on NB for 24 h were dialyzed, freeze-dried, and resuspended in 0.5 ml of Tris buffer (protein concentration, 50 μg/ml). Aliquots (10 to 40 μl) were used to analyze the presence of the 47-kDa protein by SDS–12% PAGE, (A) caseinolytic activity by zymograms by using 1% sodium caseinate (B) and the immunoblot probed with antibodies (1:500) raised against the 47-kDa protein (C). The details are described in the Materials and Methods section. Molecular mass markers (expressed in kilodaltons) are indicated on the left. Lanes 1, Azo+ strain (Y. ruckeri 150); lanes 2: Azo− strain (Y. ruckeri 146).
The results of analysis by SDS-PAGE of the extracellular proteins from the mutants CS1 and E1 are shown in Fig. 5A. The band corresponding to a molecular mass of 47 kDa is absent in the sample from the supernatant of E1, whereas a greater amount of protein appears at this level in the sample for the hyperproducing mutant CS1. Sodium caseinate zymograms (Fig. 5B) showed no casein degradation for E1 (Fig. 5B, lane 2) and a larger zone of hydrolysis for CS1 compared with that for the wild-type strain (Fig. 5B, lane 3 and lane 1, respectively). Protein detection using polyclonal antibodies proved the absence of the 47-kDa enzyme from mutant E1 and its overproduction in the mutant CS1 (Fig. 5C, lane 2 and lane 3, respectively).
FIG. 5.
Comparison of the 47-kDa protease production by the parental strain and production by the mutant (E1 and CS1) strains. The different strains were grown in NB; after 24 h of incubation 20 ml of each supernatant was dialyzed, freeze-dried, and resuspended in 0.5 ml of Tris buffer (protein concentration, 50 μg/ml), and 10- to 40-μl aliquots were used to analyze the presence of the 47-kDa protein by SDS–12% PAGE (A), caseinolytic activity by zymograms by using 1% sodium caseinate (B), and the immunoblot probed with antibodies (1:500) raised against the 47-kDa protein (C). Molecular mass markers (expressed in kilodaltons) are indicated on the left. Lanes 1, parent strain (Y. ruckeri 150); lanes 2, mutant strain E1; and lanes 3, mutant strain CS1.
DISCUSSION
Proteolytic activity was first detected in Y. ruckeri culture supernatants towards the end of the exponential growth phase. This phenomenon of protease synthesis occurring at the end of the exponential growth phase is a common feature of many bacteria, including Vibrio alginolyticus (29) and Clostridium sporogenes (1). By contrast, A. hydrophila (34), Vibrio cholerae (45), Vibrio strain SA1 (43), and Erwinia chrysanthemi (41) display proteolytic activity during the exponential growth phase.
The composition of the culture medium had no effect on the onset of protease production, including that for the hyperproteolytic strain CS1. However, protease synthesis seemed to be regulated in some way, as it occurred only after active growth was about to cease. Protease activity was absent in the mutant E1, suggesting that protease is not essential for growth.
The production of the 47-kDa protease was influenced by the composition of the culture medium: growth and protease production were optimum in peptone medium whereas activity was not detected when the microorganism was grown in the presence of Casamino Acids, suggesting that intact peptides are necessary in the induction process. A similar behavior has been observed for A. salmonicida (9), Aeromonas liquefaciens (9), Serratia marcescens (4), Vibrio species (12), and E. chrysanthemi (41). By contrast, in the case of A. hydrophila, peptone was the best medium for protease production, although growth was not too prolific (34). After 24 h of incubation, Y. ruckeri did not grow in the presence of sodium caseinate or gelatin. This fact could suggest that the basal level of protease was insufficient, during early exponential growth, to release enough peptides and that therefore there was a lack of induction of enzyme production and hence a lack of growth. Alternatively, low levels of particular amino acids in those proteins could explain the inability of bacteria to grow on these substrates.
Growth was increased when carbohydrates were present in the culture media, but they had negative effects on protease production. A similar catabolic repression mechanism for extracellular enzyme production has been described for V. alginolyticus (29), Pseudomonas maltophilia (3), and Staphylococcus aureus (44), suggesting that in the absence of the sugar (i.e., glucose) the protease plays a role in supplying peptides or amino acids as the carbon or energy source in addition to being the nitrogen source. Thus, protease synthesis may be repressed when the energy status of the cells is high. This kind of regulatory mechanism has been postulated for proteases of other pathogens like P. aeruginosa (42) and Vibrio strain SA1 (43). However, it should be considered that a study of the effect of carbohydrates on enzyme production may not reveal the in vivo situation, where amino acids, peptides, lipids, and host proteins are more likely to be the carbon and energy sources.
The presence of ammonium significantly decreased protease production. Thus, inhibition was found when 50 mM NH4Cl was present in the medium. This NH4+ effect was similar to that described for A. hydrophila (34), A. salmonicida (28), and Vibrio strain SA1 (43), giving evidence to support the idea that ammonium-specific repression is likely to be the explanation for the results found.
The inhibition of protease production observed at 28°C seems to be a specific effect not related to the growth rate. This kind of enzyme regulation dependent on temperature and independent of growth could indicate that protease production (as a putative virulence factor) in Y. ruckeri is a process adapted to the aquatic environmental conditions needed for infecting and survival in fish. Other enteropathogenic Yersinia species (Y. enterocolitica and Y. pseudotuberculosis) also express temperature-dependent proteins at 37°C but not at 26°C (7, 8). Among these proteins are the regulators or effectors of virulence which allow the expression at host temperature but not at room temperature.
The fact that azocasein protease activities were found in 8 of 14 strains of Y. ruckeri tested shows that this characteristic is not general in this species. The absence of the 47-kDa protein in the Azo− strains indicated that the protease does not occur in any of these strains. These results were confirmed by the zymogram and immunoblot analyses. There was also a correlation between the presence or absence of caseinolytic activity and the protein profile observed by SDS-PAGE. Thus, two different exocellular protein patterns with no apparent common components were found, each one corresponding with the Azo+ or Azo− characteristic, and all the strains analyzed in this work could be classified into one of them. Since all the strains with the exception of strain 956 (which was serotype 2) were serotype 1, the most virulent and common serotype, these two patterns had no relationship with serotype. Nevertheless, it is possible that the presence of this protease could be a factor that enhances virulence. On the other hand, the growth curve of E1, which lacked the protease, probably because it was affected at a regulatory level, showed a shallower slope than that for the wild-type strain. This could suggest that the presence of the protease means a better adaptation to competitive growth.
At this point, the role of this protease is unclear. Although it was not necessary for growth, it could digest proteins as a nutrient source and support in vivo proliferation from digested tissues. In addition to providing nutrients for the bacteria, the protease could have potential toxicity for fish as a virulence factor which helps the bacteria to produce ERM disease, as it seems to occur in other fish pathogens such as V. anguillarum (33) and A. salmonicida (19).
Ammonium sulfate precipitation followed by ion exchange was an effective procedure in increasing the specific activity of this protease by more than 7,633-fold and the recovery of protease activity was 2.07%. It also yielded a pure homogeneous protein as evidenced by detection of a single band by SDS-PAGE.
The profile of enzyme temperature dependence, having the maximum at 37°C, shows that this is a cold-adapted enzyme, like other enzymes from different bacteria, including lipases from Pseudomonas sp. (6), an amylase from a psychrophilic bacterium isolated from Japan Sea sediments (20), and subtilisin from Bacillus strain TA41 (10). Cold-adapted enzymes exhibit lower activation temperatures than their mesophilic counterparts but also exhibit an increased heat lability. The instability at temperatures of 42°C and above, together with the energy of activation (Ea = 15.53 ± 0.23 kcal/mol), defines this 47-kDa protease as a thermolabile enzyme similar to enzymes from other psychrotrophs, such as Moraxella (13) and Acinetobacter (5). The protease can be classified as an alkaline metalloprotease (optimum pH near 8) dependent on Mg2+ for its activity, since EDTA and 1,10-phenanthroline did inhibit the enzyme’s action. EGTA inhibited the enzyme activity by approximately 50%, and the presence of Ca2+ was shown to be more effective than that of Mg2+ in conferring thermostability. Thus, the protease seems to be dependent on Mg2+ for its activity and Ca2+ for its thermostability. In this way, for further enzyme purification MgCl2 (5 mM) might be included in the culture medium as well as in the purification buffers in order to increase the total activity and yield. CaCl2 should also be included for the final purification step in order to improve the enzyme stability. On the other hand, phenylmethylsulfonyl fluoride, which irreversibly and specifically reacts with serine residues in the active site, had no effect on protease activity. Furthermore, inhibition by dithiothreitol suggests that disulfide bonds could be important in maintaining the molecular conformation required for activity.
The pure protease is a valuable tool for testing the effect of the enzyme on fish tissues and also for studying the immunogenic ability of heat-denatured protease in the context of subsequent infections. Fifty percent lethal dose experiments, using Azo+ and Azo− strains, as well as the mutants, will be especially useful to directly test the role of the 47-kDa protease from Y. ruckeri in ERM disease.
ACKNOWLEDGMENTS
This work was supported by the Spanish DIGICYT (grant PB93-1080) and by a “colaboración” grant from the Spanish Ministerio de Educación y Ciencia to P. Secades.
We thank F. Uruburu, J. L. Larsen, I. Marquez, and C. J. Rodgers for kindly providing the different Y. ruckeri strains. We thank Ana de Lillo and Jesús F. Aparicio for critical reading and corrections of the manuscript. We also thank Santiago Cal for his extensive help and Ricardo Sánchez Cármenes for helpful advice about thermostability experiments.
REFERENCES
- 1.Allison C, Macfarlane G T. Regulation of protease production in Clostridium sporogenes. Appl Environ Microbiol. 1990;56:3485–3490. doi: 10.1128/aem.56.11.3485-3490.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertolini J M, Wakabayashi H, Watral V G, Whipple M J, Rohovec J S. Electrophoretic detection of proteases from selected strains of Flexibacter psychrophilus and assessment of their variability. J Aquat Anim Health. 1994;6:224–233. [Google Scholar]
- 3.Boethling R S. Regulation of extracellular protease secretion in Pseudomonas maltophilia. J Bacteriol. 1975;123:954–961. doi: 10.1128/jb.123.3.954-961.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun V, Schmitz G. Excretion of a protease by Serratia marcescens. Arch Microbiol. 1980;124:55–61. doi: 10.1007/BF00407028. [DOI] [PubMed] [Google Scholar]
- 5.Brenil C, Kushner J. Partial purification and characterization of lipase of a facultative psychrophilic bacterium (Acinetobacter O16) Can J Microbiol. 1975;21:434–441. doi: 10.1139/m75-062. [DOI] [PubMed] [Google Scholar]
- 6.Choo D-W, Kurihara T, Suzuki T, Soda K, Esaki N. A cold-adapted lipase of an Alaskan psychrotroph, Pseudomonas sp. strain B11-1: gene cloning and enzyme purification and characterization. Appl Environ Microbiol. 1998;64:486–491. doi: 10.1128/aem.64.2.486-491.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornelis G R. The Yersinia deadly kiss. J Bacteriol. 1998;180:5495–5504. doi: 10.1128/jb.180.21.5495-5504.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornelis G R, Boland A, Boyd A P, Genijen C, Iriarte M, Neyt C, Sory M-P, Stanier I. The virulence plasmid of Yersinia, an antihost genome. Microbiol Mol Biol Rev. 1998;62:1315–1352. doi: 10.1128/mmbr.62.4.1315-1352.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalhe H K. Regulation of the proteinase production in two strains of Aeromonas. Acta Pathol Microbiol Scand Sect B. 1971;79:739–746. doi: 10.1111/j.1699-0463.1971.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 10.Davail S, Feller G, Narinx E, Gerday C. Cold adaptation of proteins. J Biol Chem. 1994;269:17448–17453. [PubMed] [Google Scholar]
- 11.Davies R L. Yersinia ruckeri produces four iron-regulated outer membrane proteins but does not produce detectable siderophores. J Fish Dis. 1991;14:563–570. [Google Scholar]
- 12.Dreisbach J H, Merkel J R. Induction of collagenase production in Vibrio B-30. J Bacteriol. 1978;135:521–527. doi: 10.1128/jb.135.2.521-527.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feller G, Thiry M, Arpigny J L, Mergeay M, Gerday C. Lipases from psychrotrophic antarctic bacteria. FEMS Microbiol Lett. 1990;66:239–244. [Google Scholar]
- 14.Friedrich B, Magasanik B. Utilization of arginine by Klebsiella aerogenes. J Bacteriol. 1978;133:680–685. doi: 10.1128/jb.133.2.680-685.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furones M D, Gilpin M L, Munn C B. Culture media for the differentiation of isolates of Yersinia ruckeri based on detection of a virulence factor. J Appl Bacteriol. 1993;74:360–366. doi: 10.1111/j.1365-2672.1993.tb05139.x. [DOI] [PubMed] [Google Scholar]
- 16.Furones M D, Gilpin M L, Alderman D J, Munn C B. Virulence of Yersinia ruckeri serotype 1 strains is associated with a heat sensitive factor (HSF) in cell extracts. FEMS Microbiol Lett. 1990;66:339–344. doi: 10.1016/0378-1097(90)90309-e. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg R B, Bloom F R, Magasanik B. Regulation of histidinase synthesis in intergenic hybrids of enteric bacteria. J Bacteriol. 1976;127:114–119. doi: 10.1128/jb.127.1.114-119.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffin B R. Columnaris disease: recent advances in research. Aquaculture. 1987;13:48–50. [Google Scholar]
- 19.Gunnlaugsdottir B, Gudmundsdottir B K. Pathogenicity of atypical Aeromonas salmonicida in Atlantic salmon compared with protease production. J Appl Microbiol. 1997;83:543–541. doi: 10.1046/j.1365-2672.1997.00247.x. [DOI] [PubMed] [Google Scholar]
- 20.Hamamoto T, Russell N J. Psychrophiles. In: Horikoshi K, Grant W D, editors. Extremophiles. New York, N.Y: Wiley; 1988. pp. 1–21. [Google Scholar]
- 21.Haulon G W, Hodges N A, Russell A D. The influence of glucose, ammonium and magnesium availability on the production of protease and bacitracin by Bacillus licheniformis. J Gen Microbiol. 1982;128:845–851. doi: 10.1099/00221287-128-4-845. [DOI] [PubMed] [Google Scholar]
- 22.Howe T R, Iglewski B H. Isolation and characterization of alkaline protease deficient mutants of Pseudomonas aeruginosa in vitro and in a mouse eye model. Infect Immun. 1984;43:1058–1060. doi: 10.1128/iai.43.3.1058-1063.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen S E, Phillippe L, Teng Tseng J, Stemke G W, Campbell J N. Purification and characterization of exocellular proteases produced by a clinical isolate and a laboratory strain of Pseudomonas aeruginosa. Can J Microbiol. 1980;26:77–86. doi: 10.1139/m80-012. [DOI] [PubMed] [Google Scholar]
- 24.Kothary M H, Kreger A S. Production and partial characterization of an elastolytic protease of Vibrio vulnificus. Infect Immun. 1985;50:534–540. doi: 10.1128/iai.50.2.534-540.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 26.Leung K Y, Stevenson R M W. Tn5-induced protease-deficient strains of Aeromonas hydrophila with reduced virulence for fish. Infect Immun. 1988;56:2639–2644. doi: 10.1128/iai.56.10.2639-2644.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levisohn S, Aronson A I. Regulation of extracellular protease production in Bacillus cereus. J Bacteriol. 1967;93:1023–1030. doi: 10.1128/jb.93.3.1023-1030.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu P V, Hsieh M C. Inhibition of protease production of various bacteria by ammonium salts: its effect on toxin production and virulence. J Bacteriol. 1969;99:406–413. doi: 10.1128/jb.99.2.406-413.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long S, Mothibeli M A, Robb F T, Woods D R. Regulation of extracellular alkaline activity by histidine in a collagenolytic Vibrio alginolyticus strain. J Gen Microbiol. 1981;127:193–199. doi: 10.1099/00221287-127-1-193. [DOI] [PubMed] [Google Scholar]
- 30.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 31.Mateos D, Anguita J, Naharro G, Paniagua C. Influence of growth temperature on the production of extracellular virulence factors and pathogenicity of environmental and human strains of Aeromonas hydrophila. J Appl Bacteriol. 1993;74:111–118. doi: 10.1111/j.1365-2672.1993.tb03003.x. [DOI] [PubMed] [Google Scholar]
- 32.Milton D L, Norquist A, Wolf-Watz H. Cloning of a metalloprotease gene involved in the virulence mechanism of Vibrio anguillarum. J Bacteriol. 1992;174:7235–7244. doi: 10.1128/jb.174.22.7235-7244.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norqvist A, Norrman B, Wolf-Watz H. Identification and characterization of a zinc metalloprotease associated with invasion by the fish pathogen Vibrio anguillarum. Infect Immun. 1990;58:3731–3736. doi: 10.1128/iai.58.11.3731-3736.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Reilly T, Day F. Effects of culture conditions on protease production by Aeromonas hydrophila. Appl Environ Microbiol. 1983;45:1132–1135. doi: 10.1128/aem.45.3.1132-1135.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paulovski O R, Wretlind B. Invasiveness of Pseudomonas aeruginosa correlates with elastase production. Infect Immun. 1979;24:181–187. doi: 10.1128/iai.24.1.181-187.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pollock M R. Exoenzymes. In: Gunsalus I C, Stanier R Y, editors. The bacteria. Vol. 4. New York, N.Y: Academic Press. Inc.; 1963. pp. 121–178. [Google Scholar]
- 37.Ronalde J L, Conchas R F, Toranzo A. Evidence that Yersinia ruckeri possesses a high affinity iron uptake system. FEMS Microbiol Lett. 1991;80:121–126. doi: 10.1016/0378-1097(91)90581-t. [DOI] [PubMed] [Google Scholar]
- 38.Ronalde J L, Toranzo A E. Pathological activities of Yersinia ruckeri, the enteric red mouth (ERM) bacterium. FEMS Microbiol Lett. 1993;112:291–300. doi: 10.1111/j.1574-6968.1993.tb06465.x. [DOI] [PubMed] [Google Scholar]
- 39.Sakai D K. Loss of virulence in a protease-deficient mutant of Aeromonas salmonicida. Infect Immun. 1985;48:146–152. doi: 10.1128/iai.48.1.146-152.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stevenson R M W. Immunization with bacterial antigens: yersiniosis. Dev Biol Stand. 1997;90:117–124. Basel Karger. [PubMed] [Google Scholar]
- 41.Wandersman C, Andro T, Berthean I. Extracellular proteases in Erwinia chrysanthemi. J Gen Microbiol. 1986;132:899–906. [Google Scholar]
- 42.Whooley M A, O’Callaghan J A, McLonghlin A J. Effect of substrate on the regulation of exoprotease production by Pseudomonas aeruginosa ATCC 10145. J Gen Microbiol. 1983;129:981–988. doi: 10.1099/00221287-129-4-981. [DOI] [PubMed] [Google Scholar]
- 43.Wiersma M, Hansen T A, Harder W. Effect of environmental conditions on the production of two extracellular proteolytic enzymes by Vibrio SA1. Antonie Leeuwenhoek J Microbiol Serol. 1978;44:129–140. doi: 10.1007/BF00643216. [DOI] [PubMed] [Google Scholar]
- 44.Yoshikawa M, Matsuda F, Naka M, Murofushi E, Tsunematsu J. Pleiotropic alterations of activities of several toxins and enzymes in mutants of Staphylococcus aureus. J Bacteriol. 1974;119:117–122. doi: 10.1128/jb.119.1.117-122.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Young D B, Broadbent D A. Biochemical characterization of extracellular proteases from Vibrio cholerae. Infect Immun. 1982;37:875–883. doi: 10.1128/iai.37.3.875-883.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]