ABSTRACT
Background
Interest in point-of-care ultrasound (POCUS) and lung ultrasound (LUS) is growing in the nephrology and dialysis field, and the number of nephrologists skilled in what is proving to be the “5th pillar of bedside physical examination” is increasing. Patients on hemodialysis (HD) are at high risk of contracting severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) and developing coronavirus disease 2019 (COVID-19) serious complications. Despite this, to our knowledge there are no studies to date that show the role of LUS in this setting, while there are many in the emergency room, where LUS proved to be an important tool, providing risk stratification and guiding management strategies and resource allocation. Therefore, it is not clear whether the usefulness and cut-offs of LUS highlighted in studies in the general population are reliable in dialysis, or whether variations, precautions and adjustments to this specific situation are necessary.
Methods
This was a 1-year monocentric prospective observational cohort study of 56 HD patients with COVID-19. Patients underwent a monitoring protocol that included at first evaluation bedside LUS, using a 12-scan scoring system, by the same nephrologist. All data were prospectively and systematically collected. Outcomes. hospitalization rate, combined outcome [non-invasive ventilation (NIV + death)], mortality. Descriptive variables are presented as medians (interquartile range), or percentage. Univariate and multivariate analysis, as well as Kaplan–Meier (K-M) survival curves, were carried out. P was fixed at .05.
Results
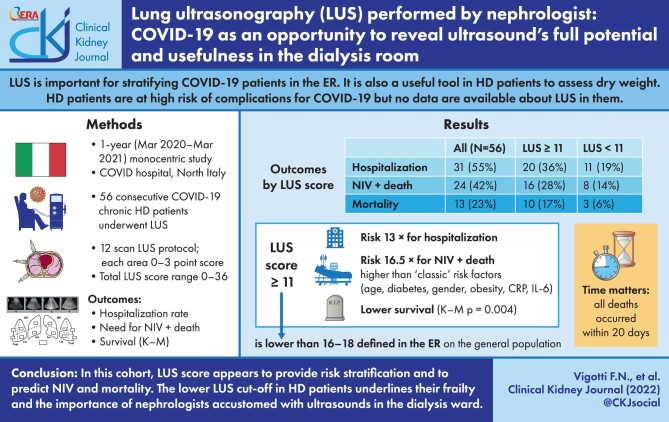
Median age was 78 years, 90% had at least one comorbidity (46% diabetics), 55% were hospitalized and 23% deaths. Median duration of disease was 23 days (14–34). A LUS score ≥11 represented a 13-fold risk of hospitalization, a 16.5-fold risk of combined outcome (NIV + death) vs risk factors such as age [odds ratio (OR) 1.6], diabetes (OR 1.2), male sex (OR 1.3) and obesity (OR 1.25), and a 7.7-fold risk of mortality. In the logistic regression, LUS score ≥11 is associated with the combined outcome with a hazard ratio (HR) of 6.1 vs inflammations indices such as CRP ≥9 mg/dL (HR 5.5) and interleukin-6 (IL-6) ≥62 pg/mL (HR 5.4). In K-M curves, survival drops significantly with LUS score above 11.
Conclusions
In our experience of COVID-19 HD patients, LUS appeared to be an effective and easy tool, predicting the need for NIV and mortality better than “classic” known COVID-19 risk factors such as age, diabetes, male sex and obesity, and even better than inflammations indices such as CRP and IL-6. These results are consistent with those of the studies in the emergency room setting, but with a lower LUS score cut-off (11 vs 16–18). This is probably due to the higher global frailty and peculiarity of HD population, and emphasizes how nephrologists should themselves use LUS and POCUS as a part of their everyday clinical practice, adapting it to the peculiarity of the HD ward.
Keywords: COVID-19, hemodialysis, lung ultrasound, LUS score, POCUS
Graphical Abstract
Graphical Abstract.
INTRODUCTION
In recent decades the interest in point-of-care ultrasound (POCUS) and lung ultrasound (LUS) has been growing in the nephrology field, as well as the number of nephrologists skilled in what has been defined as the “5th pillar of bedside physical examination” [1, 2].
LUS in particular has been increasingly introduced in dialysis wards, thanks to its easy handling, reliability and absence of ionizing radiations, to define dry weight and pulmonary congestion better than X-rays [3–5] together with the evaluation of the inferior vena cava in terms of diameter and collapsibility [6, 7].
In the coronavirus disease 2019 (COVID-19) pandemic, which has so far caused over 270 million infections and 5 million deaths worldwide [8], POCUS and in particular LUS, have proved to be important diagnostic tools in the emergency room, and showed to be very effective in identifying and stratifying COVID-19 critically ill patients [9].
In the emergency room experiences published so far, findings on LUS correlated with clinical course, similar to findings on high-resolution computed tomography (CT), provided prediction of patients’ outcomes and guided management strategies, triage and resource allocation [10–13].
Hemodialysis (HD) patients are at a greater risk of contracting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) than the general population, because of their high exposure and the impossibility of fully adhering to social distancing measures [6]. Furthermore, their frailty due to the presence of multiple comorbidities and their relative immune deregulation linked to the dialytic treatment [14, 15], puts them at risk of developing the most severe forms of COVID-19. As a matter of fact, epidemiological data show that mortality for COVID-19 in hemodialyzed patients is significantly higher than in the general population [16, 17].
These are the reasons why worldwide HD patients have been given vaccination priority since vaccines became available [18].
Because of their well-known frailty and their peculiar characteristic of being outpatients that assiduously frequent the hospital environment, it is of fundamental importance to promptly identify and isolate positive COVID-19 cases, to treat them in the best possible way, to prevent the spread of infection in dialysis rooms, and to find a reliable and practical way to prognostically stratifying them by the risk of developing complications requiring hospitalization or—if possible—maintain home management [19–21]. The ability to discriminate in advance between the possible different evolutions of the clinical course allows early identification and treatment of patients at greater risk, and the rationalization of hospitalization, and enables for safely encouraging care and management of the patient at home, optimizing the resources allocation and the humanization of care.
The importance of LUS in the diagnosis of oligosymptomatic COVID-19 patients on HD has recently been underlined in a two-cases report [22], showing that the interest of the nephrologist community in the matter is growing.
However, to our knowledge there are no studies to date about LUS in the stratification and assessment of COVID-19 severity in HD patients, and its role in predicting the outcomes. Therefore, it is not clear whether the usefulness and cut-offs of LUS highlighted in studies in the general population are reliable in dialysis, or whether variations, precautions and adjustments to this specific situation are necessary.
The aim of this study is to report the monocentric prospective experience of the systematic use of LUS in a cohort of HD patients affected by COVID-19, to evaluate its predictive value in estimating the severity of the disease, to determine whether there could be a specific warning cut-off different from the ones defined in the general population, and to encourage dialysis centres—where LUS and POCUS are not used—to equip themselves with ultrasounds and to train their medical staff, not only in the COVID-19 setting but also in the everyday clinical practice of the HD ward.
MATERIALS AND METHODS
We studied with LUS 56 out of all consecutive adult patients (N = 62) with COVID-19 admitted between 1 March 2020 and 1 March 2021 to the HD ward at Martini Hospital, Turin, a 200-bed urban medical centre.
All patients had a diagnosis of COVID-19 confirmed by a positive polymerase chain reaction assay for SARS-CoV-2 in a respiratory tract sample.
In our dialysis centre we organized an isolated dialysis room for patients diagnosed with COVID-19 disease with three beds and a dedicated nurse for the whole treatment; the room had an independent access and a filter zone, and was fully dedicated to COVID-19-positive patients.
Once they tested positive, patients started a prospective and specific monitoring protocol in addition to the routine HD care, which included:
each dialysis: interview about symptoms and recording of clinical parameters such as blood pressure, heart rate, body temperature and spO2;
once a week: hematological exams—blood cells count with leukocyte formula, D-dimer, fibrinogen, C-reactive protein (CRP), procalcitonine, lactate dehydrogenase, interleukin-6 (IL-6), ferritin, plus arterial hemogasanalysis for PaO2/FiO2 (P/F ratio) calculation on clinical indication;
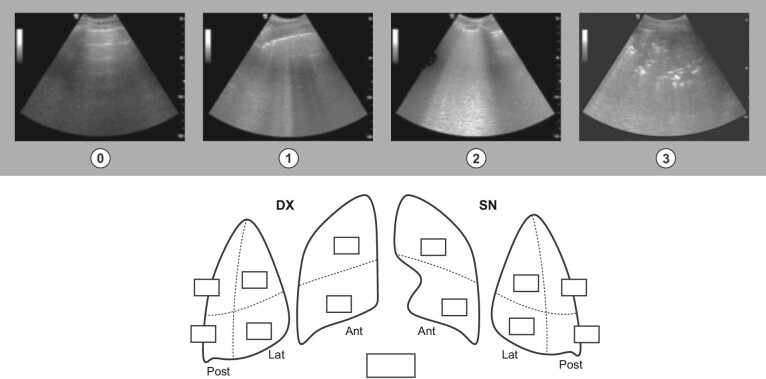
first dialysis (and subsequent ones on clinical need): performance of bedside LUS, using a 12-scan LUS protocol as defined in literature [23–25] combined with sub-xiphoid longitudinal inferior vena cava evaluation (diameter and collapsibility) [26].
LUS assessments were performed at the start and at the end of dialysis by one nephrologist with expertise in LUS recording and interpretation, using the same equipment (Mindray M5, convex probe 3.5 MHz, preset FAST ultrasound system), dedicated to and set aside in the COVID-19 HD room.
Six out of the 62 HD COVID-19 patients were not tested with LUS, and so were excluded from the analysis, because the nephrologist that performed all LUS was not present on shift at the time of their first dialysis in the COVID-19 room.
Demographic data, comorbid conditions, medications, and clinical and laboratory findings were recorded. Clinical follow-up was obtained by thrice a week review of all medical records until end of follow-up (recovery of the patient or death).
Ethical disclosure
This is an observational study that does not detach from the usual clinical practice of our dialysis center, and it does not involve the administration of experimental drugs or invasive maneuvers.
LUS method
Each LUS lasts between 2 and 3 min, with the patient supine or semi-supine. We adopted the LUS scoring system dividing the thorax in 12 regions (two anterior, two antero-lateral and two postero-lateral areas per side). Each area is assigned a score ranging from 0 to 3 depending on the ultrasound pattern, and the sum of all the areas represents the LUS score. Thus, LUS score ranges between 0 and 36 (Fig. 1).
Figure 1:
0 point: A-lines (normal reverberation artifacts of the pleural line that when accompanied by lung sliding correspond to normal aeration of the lung). 1 point: separated B-lines (hyperechoic lines vertical to the pleura line, arising from it and reaching the edge of the screen erasing A-lines, which represent reverberation artifacts through edematous interlobular septa or alveoli). 2 points: coalescent B-lines that correspond to severe lung aeration loss. 3 points: lung consolidation (segmentary/diffuse), pleural thickening (patchy/diffuse). https://www.simeu.it/w/download/get/0/Rapporto%20Prima%20Linea_Covid-19_ecografia.pdf/download/articoli/4031 (published under permission from G.A. Cibinel).
Statistical analysis
Data were prospectively and systematically collected. The size of the study cohort was determined by the number of COVID-19 patients admitted to our dialysis during the 1-year prospective observation.
Endpoints included: need for hospitalization; combined outcome of need for non-invasive ventilation (NIV) + death; all-cause mortality. Continuous variables were described as median and interquartile range (IQR) according to their non-normal distribution.
To compare independent groups we used the Mann–Whitney test. The difference between before-and-after observations was analyzed with paired Student or Wilcoxon test.
Some cut-off levels were defined with receiver operating characteristic (ROC) curves.
Categorical variables were presented as fractions and Pearson's χ2 test or, for small samples, Fisher's exact test was employed to compare groups. The odds ratios (OR) with 95% confidence intervals (CI) were used as a measure of relative risk.
Univariate survival analysis was performed by means of the Kaplan–Meier method with Log Rank test to compare strata. Risk factors whose independence was tested were examined in multivariate analysis using the logistic regression model.
Scatter and box plot were used to explore the relationship between variables visually.
Significance level for all tests was set at α < 0.05.
All statistical analyses were performed using SPSS (IBM Corp., released 2020; IBM SPSS Statistics for Windows, Version 28.0; IBM Corp., Armonk, NY, USA).
RESULTS
During the study period, 62 consecutive chronic HD patients with COVID-19 have been dialyzed in our center. Thirty-six patients were hospitalized and 26 were outpatients. Three patients (5%) started HD at the time of the diagnosis of COVID-19.
Of them, 56 patients (90%) underwent LUS evaluation and scoring and so were considered for the study. Baseline characteristics, symptoms, laboratory findings and outcomes of the cohort are shown in Tables 1–3.
Table 1:
Baseline characteristics.
| Characteristic (N = 56) | Median (IQR) or N (%) |
|---|---|
| Age (years) | 78 (66–84) |
| Dialytic age (months) | 30 (12–49) |
| Male gender | 40 (71) |
| Comorbidity | |
| Hypertension | 51 (91) |
| Cardiovascular disease | 39 (70) |
| Diabetes | 26 (46) |
| Obesity (BMI >30 kg/m2) | 10 (18) |
BMI: body mass index.
Table 3:
Outcomes of the global cohort.
| N = 56 | Median (IQR) or N (%) |
|---|---|
| Hospitalization | 31 (55) |
| Combined outcome (NIV + death) | 24 (43) |
| Death (all causes) | 13 (23) |
| Death directly due to COVID-19 | 8 (14) |
| Duration of disease (days)a | 23 (14–34) |
aExpressed as days between first positive RT-PCR swab and test negative or death.
Table 2:
Symptoms and laboratory findings.
| N = 56 | Median (IQR) or N (%) |
|---|---|
| Symptoms | |
| None | 12 (21) |
| Fever | 22 (39) |
| Dyspnea | 17 (30) |
| Gastrointestinal symptoms | 11 (20) |
| Cough | 9 (16) |
| Lipothymia | 9 (16) |
| Neurological symptoms | 5 (9) |
| Laboratory findings | |
| IL-6 (pg/mL) | 35.5 (22–94) |
| CRP (mg/dL) | 7.3 (2–14) |
| D-dimer | 2282 (1401–5583) |
| PaO2/FiO2 (P/F ratio) | 322 (131–420) |
| Lymphocytes/mm3 | 700 (420–960) |
At first we considered the rate of hospitalization for COVID-19, which was 31/56 patients (55%).
In the inpatients group, those with a LUS score ≥11 were significantly more than those in the outpatients group (64.5% and 12%, respectively). Therefore, COVID-19 HD patients with a LUS score ≥11 have a 13-fold greater risk of hospitalization than patients with a LUS score <11 (OR 13.3; P = .000). Of note, the decision whether to admit or not patients was made by the same medical team that follows dialysis patients, in a homogeneous way.
We then considered what correlation could there be between LUS score and a combined outcome of need for NIV + death. We compared LUS score with the other known “classic” risk factors for poor COVID-19 prognosis [27] such as age, diabetes, gender and obesity, and with laboratory parameters indicative of an important cytokine activation (CRP, IL-6); cut-off levels for continuous variables were defined on the basis of ROC curves.
The univariate analysis showed that patients with a LUS score ≥11 have a 16.5-fold risk of experiencing the combined outcome compared with those with lower LUS score. Also, the values of CRP and IL-6 showed a statistically significant impact on the outcome, but with an OR lower than that of the LUS score. Results are displayed in Table 4.
Table 4:
Univariate analysis—combined outcome (NIV + death).
| NO NIV + death, N (%) | NIV + death, N (%) | P | OR (95% CI) | |
|---|---|---|---|---|
| Age (≥78 years) | 17 (58.6) | 12 (41.4) | .41 | 1.6 (0.5–5) |
| Diabetes | 16 (61.5) | 10 (38.5) | .78 | 1.2 (0.4–3.7) |
| Male gender | 25 (62.5) | 15 (37.5) | .76 | 1.3 (0.3–4.5) |
| Obesity (BMI ≥30 kg/m2) | 6 (60) | 4 (40) | .73 | 1.25 (0.3–5) |
| CRP ≥9 mg/dL | 11 (42.3) | 15 (57.7) | .002 | 6.8 (1.9–23.4) |
| IL-6 ≥62 pg/mL | 7 (41.2) | 10 (58.8) | .001 | 11.9 (2.5–55.4) |
| LUS score ≥11 | 7 (30.4) | 16 (69.6) | .000 | 16.5 (4.2–65.3) |
BMI: body mass index.
We further evaluate the variables associated with death + NIV in a Logistic Regression model, and the LUS score was found to be the most relevant risk factor (OR = 6.1, 95% CI 1.0–38.5; P = .05), despite the small size of the sample (Table 5).
Table 5:
Logistic regression—combined outcome (NIV + death).
| P | HR | 95% CI | |
|---|---|---|---|
| CRP ≥9 mg/dL | .09 | 5.5 | 0.8–39.2 |
| IL-6 ≥62 pg/mL | .07 | 5.4 | 0.9–33.7 |
| LUS score ≥11 | .05 | 6.1 | 1–38.5 |
Of note, in our hospital COVID-19 protocol, patients were considered for therapy with tocilizumab when their CRP and their IL-6 were above 7.5 mg/dL and 70 pg/mL, respectively; these cut-off levels match very well with those defined with ROC curves and used in our analysis (9 mg/dL and 62 pg/mL).
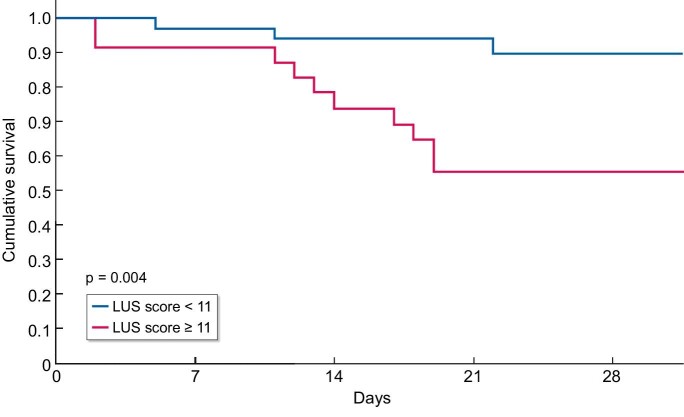
Finally, we performed a survival analysis. Of the 56 patients who underwent LUS there were 13 deaths; the difference in event rates between patients with LUS ≥11 and <11 was significant (Table 6). The LUS score was significantly associated with increased mortality: Kaplan–Meier curve showed lower survival with total LUS score ≥11 compared with LUS score <11 (P = .004) (Fig. 2).
Table 6:
Death rate of HD patients by LUS score.
| LUS score | N patients | N deaths | % deaths |
|---|---|---|---|
| <11 | 33 | 3 | 9 |
| ≥11 | 23 | 10 | 43 |
| All | 56 | 13 | 23 |
Figure 2:
Survival by LUS score.
DISCUSSION
In the nephrology and dialysis field, POCUS and LUS represent a way to revolutionize physical exam and the approach to the patient in general, as they have done in emergency medicine and critical care medicine, where nowadays ultrasounds are a core part of training for students and residents. Despite the suggestion that ultrasounds should be included in daily clinical practice also in nephrology [7, 28], dialysis centers that have adopted them as an integrated part of the management of HD patients are still few.
Moreover, during the 2 years of COVID-19 pandemic LUS has been widely used in the emergency ward to identify disease severity in terms of interstitial pneumonia involvement, and to guide management of patients. In this context, LUS showed to be a reliable imaging study, reducing the number of CT scans performed and being easily performed at bedside.
Outside the emergency ward, in outpatients like hemodialyzed ones, a dynamic disease like COVID-19 can be further challenging: in fact, one of the peculiarities of COVID-19 is that the patient can remain for a long time in an apparent clinical compensation, even in the presence of important pulmonary involvement, and then rapidly precipitate. For these reasons, a quick bedside imaging exam such as LUS in HD COVID-19 patients showed to be extremely helpful in distinguishing between mild and severe cases, following patients’ clinical status and deciding whether or not to hospitalize a patient.
One of the challenges of performing LUS in COVID-19 HD patients is the need to carry out a correct differential diagnosis between pulmonary involvement due to COVID-19 with pre-existing conditions that can cause a LUS pattern of interstitial involvement that is COVID-19 independent (such as heart failure, diastolic dysfunction, other lung diseases or the simple “overload” B lines, typical of dialysis).
In this sense, the coexistence of B lines together with other signs (an irregular pleural line, pleural and subpleural thickening, white lung prevailing in some districts in an asymmetrical way) may be more indicative of COVID-19.
The difficulty of differential diagnosis is exacerbated by the loss of lean mass and the need to redefine the dialysis ideal weight due to nausea, hyporexia, vomiting and diarrhea linked with COVID-19.
This condition occurred in 100% of the patients in our study. To adjust their dry weight, we used the inferior vena cava diameter and collapsibility, as suggested by other authors [6], together with the evaluation of suprahepatic veins and the presence/absence of atrial dilatation, all signs of volume overload.
We suggest to determine LUS score at the end of dialysis, after getting the patient to the correct dry weight, because it may help in clarifying the patterns, together with the knowledge of the medical history of the patients.
In any case, all pre-existing conditions of decompensation of the cardiopulmonary performance may affect LUS score and patient's general survival expectation in the same way, so a higher LUS score (whether it can be “purely” from COVID-19 or “mixed,” due to anamnestic cardiopulmonary disease and superimposed COVID-19) correctly allows one to estimate that the patient will have worse survival rate, and therefore appears worthy of hospitalization and more intensive observation and therapeutic intervention, rather than a patient with a lower LUS score.
The LUS score cut off of 11, defined in our dialysis COVID-19 cohort on the basis of the ROC curve, and showing a significative correlation with clinical course and short-term mortality, is lower than those of 16–18 defined by other authors in the emergency ward in the general population [11, 12].
This is probably due to the aforementioned higher global frailty of the HD population, in which even a lower lung involvement may have a significant impact on clinical evolution and survival [29].
In our opinion, the potential imprecision of using a paradigm developed in a different context on hemodialyzed patients emphasizes how nephrologists themselves should learn the use of clinical ultrasound, and carry out LUS in their patients, not only in the COVID-19 scenario but in the everyday practice, in order to be able to manage them in the best possible way.
Time matters: in our experience all deaths occurred within 20 days from the first positive test for SARS-CoV-2, thus underlining the importance of acting promptly in the diagnosis of the high-risk cases, to set the optimal therapy and try to change the course of the disease.
Limitations
First, ours is a single-center study. Outcome analyses in our study should be interpreted with caution due to the small number of patients. For some evaluation we used a combined outcome (death + NIV) in spite of mortality per se, due to the sample size.
Ultrasonography is by definition an operator-dependent method; in our study we eliminate the inter-observer variability as the exams were all performed by the same nephrologist.
The epidemiologic and clinical scenario of our experience, which dates from 2020 to 2021, has now changed, thanks to vaccines and to the fact that mild SARS-CoV-2 variants have emerged. But—beyond COVID-19 and its more or less aggressive forms on the lung—it is still important in our opinion that nephrologists are accustomed to and confident about how much ultrasounds can add in clinical decision making and global management of HD patients.
We adopted in this study the point of view of the management of COVID-19 HD patients because it has challenged us all very much in the last years, revealing all the potential of the use of POCUS in the dialysis ward.
Conclusions and clinical implications
Our study, in which a protocoled systematic LUS was used in 56 consecutive COVID-19 HD patients admitted to the COVID Hospital Martini in Turin, Northern Italy, shows that LUS score appeared to be an effective and easy tool, predicting the need for NIV and mortality better than “classic” known COVID-19 risk factors such as age, diabetes, male sex and obesity, and even better than inflammations indices as CRP and IL-6, and may aid risk stratification and clinical decision making.
These results are consistent with those of the studies in the emergency room setting, but with a lower LUS score cut off (11 vs 16–18). This is probably due to the higher global frailty and peculiarity of HD population, and emphasizes how nephrologists should themselves use LUS as a part of their everyday clinical practice, adapting it to the peculiarity of the HD ward.
In fact, the interpretation of patterns in HD patients may not always be so easy, so it is desirable that the nephrology team have operators adequately trained and accustomed to pulmonary ultrasound evaluation, dynamic of the inferior vena cava and POCUS in general.
The use of wireless probes can encourage the use of POCUS in the HD setting for ease of transport, disinfection and safety.
In summary, COVID-19 has provided an opportunity for many clinicians and nephrologists to approach LUS and discover its full potential, which can also be transferred—with a global POCUS approach—to other clinical conditions in everyday practice in the HD ward.
ACKNOWLEDGEMENTS
The authors thank all the staff of the hemodialysis department for the practical support and all the efforts made during the COVID-19 pandemic. Dr Federica Vigotti especially thanks Dr Antonio Marciello for teaching her the use of ultrasounds in dialysis.
Contributor Information
Federica N Vigotti, Nephrology and Dialysis, Martini Hospital, ASL Città di Torino, Turin, Italy.
Carlo Di Benedetto, University of Turin, Nephrology and Dialysis, San Giovanni Bosco Hospital, ASL Città di Torino, Turin, Italy.
Fabrizio Fop, University of Turin, Nephrology and Dialysis, Molinette Hospital, ASO Città della Salute e della Scienza, Turin, Italy.
Simona Bianco, Nephrology and Dialysis, Martini Hospital, ASL Città di Torino, Turin, Italy.
Donatella Bilucaglia, Nephrology and Dialysis, Martini Hospital, ASL Città di Torino, Turin, Italy.
Giulio Cesano, Nephrology and Dialysis, Martini Hospital, ASL Città di Torino, Turin, Italy.
CONFLICT OF INTEREST STATEMENT
None declared. The results presented in this paper have not been published previously in whole or part, except in abstract format.
AUTHORS’ CONTRIBUTIONS
F.N.V. was responsible for conceptualization, methodology and original draft preparation. F.N.V., S.B. and C.D.B. carried out bibliographic research. F.N.V., C.D.B. and D.B. carried out data recording. F.F. performed the statistical analysis. All authors contributed to clinical care of patients, commented on previous versions of the manuscript, and read and approved the final manuscript.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
REFERENCES
- 1. Omero-Gonzales G, Manrique J, Slon-Roblero MF.et al. PoCUS in nephrology: a new tool to improve our diagnostic skills. Clin Kidney J 2022;sfac203. 10.1093/ckj/sfac203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Narula J, Chandrashekhar Y, Braunwald E.. Time to add a fifth pillar to bedside physical examination: inspection, palpation, percussion, auscultation, and insonation. JAMA Cardiol 2018;3:346–50. 10.1001/jamacardio.2018.0001. [DOI] [PubMed] [Google Scholar]
- 3. Mallamaci F, Benedetto FA, Tripepi R.et al. Detection of pulmonary congestion by chest ultrasound in dialysis patients. JACC Cardiovasc Imaging 2010;3:586–94. 10.1016/j.jcmg.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 4. Donadio C, Bozzoli MDL, Colombini E.et al. Effective and timely evaluation of pulmonary congestion: qualitative comparison between lung ultrasound and thoracic bioelectrical impedance in maintenance hemodialysis patients. Medicine (Baltimore) 2015;94:e473. 10.1097/MD.0000000000000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Loutradis C, Papadopoulos CE, Sachpekidis V.et al. Lung ultrasound-guided dry weight assessment and echocardiographic measures in hypertensive hemodialysis patients: a randomized controlled study. Am J Kidney Dis 2020;75:11–20. 10.1053/j.ajkd.2019.07.025. [DOI] [PubMed] [Google Scholar]
- 6. Vieira ALS, Pazeli JM Júnior, Bastos MG. Role of point-of-care ultrasound during the COVID-19 pandemic: our recommendations in the management of dialytic patients. Ultrasound J 2020;12:30. 10.1186/s13089-020-00177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ross DW, Moses AA, Niyyar VD.. Point-of-care ultrasonography in nephrology comes of age. Clin Kidney J 2022;15:sfac160. 10.1093/ckj/sfac160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. https://covid19.who.int/ (5 January 2022. , date last accessed).
- 9. Pivetta E, Goffi A, Tizzani M.et al. Molinette MedUrg Group on Lung Ultrasound . Lung ultrasonography for the diagnosis of SARS-CoV-2 pneumonia in the emergency department. Ann Emerg Med 2021;77:385–94. 10.1016/j.annemergmed.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lichter Y, Topilsky Y, Taieb P.et al. Lung ultrasound predicts clinical course and outcomes in COVID-19 patients. Intensive Care Med 2020;46:1873–83. 10.1007/s00134-020-06212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Alencar JCG, Marchini JFM, Marino LO.et al. COVID U. S. P. Registry Team . Lung ultrasound score predicts outcomes in COVID-19 patients admitted to the emergency department. Ann Intensive Care 2021;11:6. 10.1186/s13613-020-00799-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Convissar DL, Gibson LE, Berra L.et al. Application of lung ultrasound during the COVID-19 pandemic: a narrative review. Anesth Analg 2020;131:345–50. 10.1213/ANE.0000000000004929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peng Q, Wang X, Zhang L.et al. Findings of lung ultrasonography of novel corona virus pneumonia during the 2019–2020 epidemic. Intensive Care Med 2020;46:849–50. 10.1007/s00134-020-05996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kato S, Chmielewski M, Honda H.et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol 2008;3:1526–33. 10.2215/CJN.00950208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vaziri ND, Pahl MV, Crum A.et al. Effect of uremia on structure and function of immune system. J Ren Nutr 2012;22:149–56. 10.1053/j.jrn.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alberici F, Delbarba E, Manenti C.et al. A report from the Brescia Renal COVID Task Force on the clinical characteristics and short-term outcome of hemodialysis patients with SARS-CoV-2 infection. Kidney Int 2020;98:20–6. 10.1016/j.kint.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Puchalska-Reglińska E, Dębska-Ślizień A, Biedunkiewicz B.et al. Extremely high mortality rates among hemodialysis patients with COVID-19 before the era of SARS-CoV-2 vaccination: results from a large database from the North of Poland. Pol Arch Intern Med 2021;131:643–8. 10.20452/pamw.16028. [DOI] [PubMed] [Google Scholar]
- 18. https://www.theisn.org/blog/2021/02/15/priority-covid-19- vaccination-for-dialysis-patients/ (5 January 2022, date last accessed).
- 19. Quintaliani G, Reboldi G, Di Napoli A.et al. Exposure to novel coronavirus in patients on renal replacement therapy during the exponential phase of COVID-19 pandemic: survey of the Italian Society of Nephrology. J Nephrol 2020;33:725–36. 10.1007/s40620-020-00794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hsu CM, Weiner DE.. COVID-19 in dialysis patients outlasting and outsmarting a pandemic. Kidney Int 2020;98:1402–4. 10.1016/j.kint.2020.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xiong F, Tang H, Liu L.et al. Clinical characteristics of and medical interventions for COVID-19 in hemodialysis patients in Wuhan, China. J Am Soc Nephrol 2020;31:1387–97. 10.1681/ASN.2020030354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Allinovi M, Laudicina S, Dallari L.et al. Lung ultrasound may help in the differential diagnosis of suspected oligosymptomatic COVID-19 patients on hemodialysis: a case report. Hemodial Int 2021;25:E48–52. 10.1111/hdi.12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gargani L, Volpicelli G.. How I do it: lung ultrasound. Cardiovasc Ultrasound 2014;12:25. 10.1186/1476-7120-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Soldati G, Smargiassi A, Inchingolo R.et al. Proposal for international standardization of the use of lung ultrasound for COVID-19 patients: a simple, quantitative, reproducible method. J Ultrasound Med 2020;39:1413–9. 10.1002/jum.15285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. https://www.simeu.it/w/download/get/0/Rapporto% 20Prima%20Linea_Covid-19_ecografia.pdf/download/articoli/4031 (5 January 2022, date last accessed).
- 26.Arun Thomas ET, Mohandas MK, George J. Comparison between clinical judgement and integrated lung and inferior vena cava ultrasonography for dry weight estimation in hemodialysis patients. Hemodial Int 2019;23:494–503. [DOI] [PubMed] [Google Scholar]
- 27. Tylicki L, Puchalska-Reglińska E, Tylicki P.et al. Predictors of mortality in hemodialyzed patients after SARS-CoV-2 infection. J Clin Med 2022;11:285. 10.3390/jcm11020285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mullangi S, Sozio SM, Segal P.et al. Point-of-care ultrasound education to improve care of dialysis patients. Semin Dial 2018;31:154–62. 10.1111/sdi.12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alfano G, Ferrari A, Magistroni R.et al. The frail world of haemodialysis patients in the COVID-19 pandemic era: a systematic scoping review. J Nephrol 2021;34:1387–403. 10.1007/s40620-021-01136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.