ABSTRACT
Background
Patients with chronic kidney disease (CKD) or kidney replacement therapy demonstrate lower antibody levels after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination compared with healthy controls. In a prospective cohort, we analysed the impact of immunosuppressive treatment and type of vaccine on antibody levels after three SARS-CoV-2 vaccinations.
Methods
Control subjects (n = 186), patients with CKD G4/5 (n = 400), dialysis patients (n = 480) and kidney transplant recipients (KTR) (n = 2468) were vaccinated with either mRNA-1273 (Moderna), BNT162b2 (Pfizer-BioNTech) or AZD1222 (Oxford/AstraZeneca) in the Dutch SARS-CoV-2 vaccination programme. Third vaccination data were available in a subgroup of patients (n = 1829). Blood samples and questionnaires were obtained 1 month after the second and third vaccination. Primary endpoint was the antibody level in relation to immunosuppressive treatment and type of vaccine. Secondary endpoint was occurrence of adverse events after vaccination.
Results
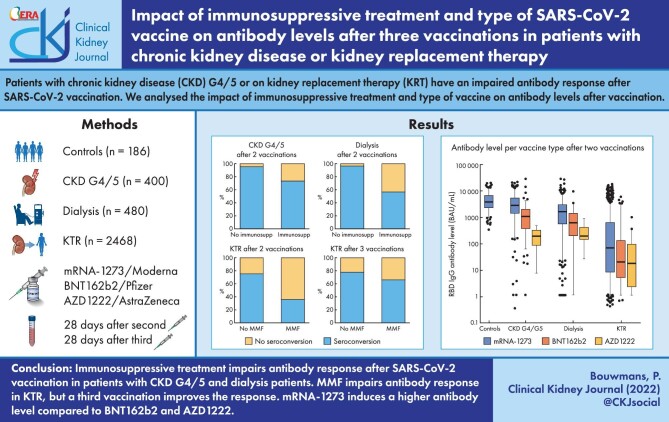
Antibody levels after two and three vaccinations were lower in patients with CKD G4/5 and dialysis patients with immunosuppressive treatment compared with patients without immunosuppressive treatment. After two vaccinations, we observed lower antibody levels in KTR using mycophenolate mofetil (MMF) compared with KTR not using MMF [20 binding antibody unit (BAU)/mL (3–113) vs 340 BAU/mL (50–1492), P < .001]. Seroconversion was observed in 35% of KTR using MMF, compared with 75% of KTR not using MMF. Of the KTR who used MMF and did not seroconvert, eventually 46% seroconverted after a third vaccination. mRNA-1273 induces higher antibody levels as well as a higher frequency of adverse events compared with BNT162b2 in all patient groups.
Conclusions
Immunosuppressive treatment adversely affects the antibody levels after SARS-CoV-2 vaccination in patients with CKD G4/5, dialysis patients and KTR. mRNA-1273 vaccine induces a higher antibody level and higher frequency of adverse events.
Keywords: antibody response, chronic kidney disease, dialysis, kidney transplantation, SARS-CoV-2 vaccination
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Patients with chronic kidney disease (CKD) or receiving kidney replacement therapy have a lower response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination and therefore remain at higher risk for coronavirus disease 2019 (COVID-19) [1–3]. It is well known that the use of immunosuppressive drugs, especially mycophenolate mofetil (MMF), severely affects the response to vaccination. While a third vaccination can enhance antibody levels in kidney transplant recipients (KTR) [4], this is only described in relatively small cohorts using MMF [5, 6]. Limited data are available on the impact of immunosuppressive treatment on the antibody levels in patients with CKD G4/5 and dialysis patients after SARS-CoV-2 vaccination [7–10].
Recent systematic reviews showed that the highest level of protection against symptomatic COVID-19 in the general population was reached with mRNA-based SARS-CoV-2 vaccines [11], although vector-based vaccines such as AZD1222 (Oxford/AstraZeneca) were also reported to be effective [12]. Of the two available mRNA-based vaccines, the mRNA-1273 (Moderna) vaccine is associated with higher antibody levels and lower rates of breakthrough infections than the BNT162b2 (Pfizer-BioNTech) vaccine in the general population [13, 14]. Higher antibody levels after mRNA-1273 in comparison with BNT162b2 have also been reported in patients with kidney disease, although differences in safety outcomes between these vaccines have not yet been reported [2, 3, 15–18].
In this prospective observational cohort study, we assessed the impact of immunosuppressive treatment and type of vaccine on antibody levels and safety outcomes after two and three SARS-CoV-2 vaccinations in patients with CKD G4/5, dialysis patients and KTR.
MATERIALS AND METHODS
Study design
In this study, we measured antibody levels and adverse events (AEs) in control subjects and patients with kidney disease after two and three vaccinations with either mRNA-1273 (Moderna), BNT162b2 (Pfizer-BioNTech) or AZD1222 (Oxford/AstraZeneca) in the Dutch SARS-CoV-2 vaccination programme [19–21]. Kidney patients were prioritized for the first two vaccinations in April–May 2021, and for the third vaccination in October–November 2021, except for patients with CKD G4/5 without immunosuppressive treatment, who were not prioritized for the third vaccination.
Study participants
Subjects were included for analysis from two different cohort studies of the REnal patients COVID-19 VACcination (RECOVAC) consortium. The main cohort is established from the Long-term Efcacy and Safety of SARS-CoV-2 vaccination (LESS CoV-2) study, of which the design has been registered in ClinicalTrials.gov (NCT04841785), and has previously been published [22]. In this study, patients with CKD G4/5 were recruited from the Santeon hospitals, a cooperation of seven non-university hospitals. Dialysis patients and KTR were recruited from all dialysis centres and hospitals in the Netherlands. Patients older than 80 years were not invited since they were prioritized in the vaccination campaign, and the timing of their second vaccination preceded the start of this study by more than 28 days. Subjects provided informed consent in writing or electronically, in accordance to the International Committee of Medical Journal Editors (ICMJE) recommendations. Ethical approval was obtained from the Medical Ethics Committee at the University Medical Center Groningen (2021/099).
We have additionally included control subjects, and patients with CKD G4/5 from the RECOVAC IR-study [1, 23]. Control subjects were eligible for inclusion if they were partners or siblings of participants with CKD G4/5, dialysis patients or KTR. Non-transplant subjects who used immunosuppressive drugs were excluded from participation. Participants were vaccinated twice with mRNA-1273, after which blood samples were collected at 28 days after the second vaccination.
Data collection
Blood samples were obtained by home based finger prick kits (Sanquin, Amsterdam, The Netherlands). Blood samples and questionnaires were collected at 28 days after the second and third vaccination. Questionnaires requested for information on patient characteristics, including previous COVID-19 and medication use, and AEs within 7 days after each vaccination. AEs were categorized in local AEs (pain or erythema at injection site and myalgia) or systemic AEs (fever, arthralgia, fatigue, headache and other). We asked participants whether they used corticosteroids, calcineurin inhibitors, MMF, mycophenolic acid, mTOR inhibitors or azathioprine. Mycophenolic acid was considered as MMF for further analysis.
Additional information on characteristics of dialysis patients and KTR was extracted from the Dutch Renal Registry (RENINE) and the Dutch Organ Transplant Registry (NOTR). Data on the use of immunosuppressive drugs in KTR was collected from the NOTR. Primary kidney disease was defined following the European Renal Association coding system [24].
For analysis, we included patients with complete information on demographics, vaccine type, date of SARS-CoV-2 vaccination, date of blood sample collection and successful measurement of antibody concentration. Patients were excluded if their blood was obtained <14 days or >56 days after the second or third vaccination, or if they were diagnosed with COVID-19 before blood collection.
Antibody measurement
We analysed blood samples for the presence of antibodies against the receptor binding domain (RBD) of the SARS-CoV-2 spike protein [immunoglobulin G (IgG) anti-RBD antibody] using an in-house anti-SARS-CoV-2 RBD IgG ELISA assay (Sanquin) [25]. We combined this assay with an in-house anti-SARS-CoV-2 nucleocapsid protein (NP) bridging ELISA to detect an antibody response due to previous COVID-19 infection. RBD IgG antibody levels are expressed in binding antibody unit (BAU)/mL [26]. The cut-off value for assessing seroconversion rates was set at ≥50 BAU/mL [27]. In addition, we used an arbitrary cut-off value of >1000 BAU/mL to assess the proportion of patients with high-level antibody response.
Statistical analysis
We assessed characteristics in patients who received at least two vaccinations, and in a subcohort of patients who received three vaccinations using Student's t-test for normally distributed data, Mann–Whitney U test for non-normally distributed data and Pearson's chi-squared test for categorical data. In addition, we compared characteristics between patients with data on third vaccination and those without data on third vaccination to assess potential selection bias.
Antibody levels between patient groups and vaccine types were compared using Wilcoxon rank-sum test. Additionally, antibody levels and seroconversion rates were stratified for use of immunosuppressive drugs (yes vs no) in patients with CKD G4/5 and dialysis patients, and for use of MMF (yes vs no) in KTR. Seroconversion rates were compared by Pearson's chi-squared test. In patients who were vaccinated three times, we assessed the change in antibody level compared with the antibody level after two vaccinations. We also compared antibody levels between three-vaccination schemes.
We analysed the association between type of vaccine and antibody levels after two vaccinations by multivariable linear regression analysis (BNT162b2 or AZD1222 compared with mRNA-1273). Primarily, we adjusted for age, sex and ethnicity. Additionally, we adjusted for variables that could be of influence on antibody levels. These variables were selected if they reached a statistically significant difference (α = 0.1) between patient groups receiving different vaccine types in univariate analysis. As a result, we adjusted for eGFR in patients with CKD G4/5 and KTR, and transplant type in KTR. We also adjusted for use of immunosuppressive drugs (yes vs no) in patients with CKD stages G4/5 and dialysis patients, and for type of immunosuppressive treatment in KTR.
AEs after each vaccination were compared using Pearson's chi-squared test. We analysed the association between type of vaccine and the occurrence of AEs after any of the first two vaccinations by using multivariable logistic regression analysis. We adjusted for variables in concordance with the previously mentioned regression analysis. Lastly, we show the occurrence of different AEs separately after each vaccination (e.g. pain at injection site, fever, myalgia, arthralgia, fatigue, headache, allergy and other).
RESULTS
Participant characteristics
After two SARS-CoV-2 vaccinations, 186 control subjects, 400 patients with CKD G4/5, 480 dialysis patients and 2468 KTR were enrolled in the study (Supplementary data, Fig. S1). The average age (±SD) was 59 ± 12 years in control subjects, 65 ± 11 years in patients with CKD G4/5, 65 ± 12 years in dialysis patients and 59 ± 13 years in KTR (Table 1). The proportion of males was 39% in the controls and approximately 60% in the three patient groups. The eGFR was 82 ± 18 mL/min/1.73 m2 in control subjects, 20 ± 9 mL/min/1.73 m2 in patients with CKD G4/5 and 51 ± 18 mL/min/1.73 m2 in KTR. The majority of the patients was of Caucasian origin. The most frequently administered vaccine was mRNA-1273 (CKD G4/5, 68%; dialysis, 86%; KTR, 93%), followed by BNT162b2 (CKD G4/5, 29%; dialysis, 11%; KTR, 5%) and AZD1222 (<5% in all groups). Immunosuppressive drugs were used by 22 of 400 (6%) of the patients with CKD G4/5, and 84 of 480 (18%) of the dialysis patients and all KTR. Only 36 KTR (1%) were transplanted within 6 months before vaccination.
Table 1:
Patient characteristics categorized by two or three SARS-CoV-2 vaccinations.
| Control | CKD G4/5 | Dialysis | KTR | ||||
|---|---|---|---|---|---|---|---|
| 2 vaccinations | 2 vaccinations | 3 vaccinations | 2 vaccinations | 3 vaccinations | 2 vaccinations | 3 vaccinations | |
| n = 186 | n = 400 | n = 40 | n = 480 | n = 242 | n = 2468 | n = 1547 | |
| Age (years) | 59 (12) | 65 (11) | 67 (9) | 65 (12) | 66 (10) | 59 (13) | 59 (12) |
| Sex, male, n (%) | 72 (39) | 241 (60) | 25 (63) | 294 (61) | 143 (59) | 1428 (60) | 860 (56) |
| Ethnicity, n (%) | |||||||
| Caucasian | 171 (92) | 359 (90) | 35 (88) | 413 (86) | 214 (88) | 2229 (90) | 1413 (91) |
| Non-Caucasian | 11 (6) | 31 (8) | 3 (8) | 52 (11) | 22 (9) | 184 (78) | 100 (6) |
| Unknown | 4 (2) | 10 (2) | 2 (5) | 15 (3) | 6 (2) | 55 (2) | 34 (2) |
| BMI (kg/m2), mean (SD) | 28 (5) | 29 (6) | 28 (7) | 27 (6) | 27 (6) | 26 (7) | 26 (7) |
| eGFR (mL/min/1.73 m2), mean (SD) | 82 (18) | 20 (9) | 22 (13) | 51 (18) | 50 (19) | ||
| Comorbidities, n (%) | |||||||
| Cardiovascular disease | 9 (5) | 76 (19) | 6 (15) | 124 (26) | 59 (24) | 293 (12) | 177 (11) |
| Peripheral vascular disease | 20 (8) | 5 (13) | 45 (9) | 26 (11) | 95 (4) | 55 (4) | |
| Heart failure | 3 (2) | 53 (13) | 6 (15) | 81 (17) | 45 (19) | 152 (6) | 83 (5) |
| Diabetes | 19 (10) | 123 (30) | 12 (30) | 156 (33) | 74 (31) | 540 (22) | 308 (20) |
| Hypertension | 53 (28) | 294 (74) | 26 (65) | 293 (61) | 147 (61) | 1553 (63) | 968 (63) |
| Cancer | 11 (6) | 33 (8) | 3 (8) | 41 (9) | 12 (5) | 71 (3) | 44 (3) |
| Stroke | 17 (7) | 4 (10) | 41 (9) | 21 (9) | 126 (5) | 82 (5) | |
| Dementia | 2 (1) | 1 (0) | 1 (0) | ||||
| Lung disease | 16 (9) | 45 (11) | 6 (15) | 71 (15) | 34 (14) | 166 (7) | 94 (6) |
| Liver cirrhosis | 4 (2) | 6 (1) | 4 (2) | 24 (1) | 13 (1) | ||
| HIV/aids | 4 (1) | 2 (1) | 6 (0) | 5 (0) | |||
| Primary kidney disease, n (%) | |||||||
| Diabetes | 76 (18) | 39 (18) | 120 (5) | 73 (6) | |||
| Hypertension | 110 (26) | 57 (26) | 156 (8) | 91 (7) | |||
| Glomerulonephritis | 51 (12) | 33 (15) | 406 (20) | 240 (19) | |||
| Interstitial nephritis | 37 (9) | 16 (7) | 151 (8) | 90 (7) | |||
| PCKD | 41 (10) | 25 (11) | 343 (17) | 240 (19) | |||
| Congenital/hereditary | 8 (2) | 1 (0) | 66 (3) | 42 (3) | |||
| Autoimmune disease | 38 (9) | 20 (9) | 101 (5) | 69 (6) | |||
| Other | 35 (8) | 17 (7) | 538 (27) | 339 (27) | |||
| Unknown | 34 (8) | 13 (6) | 112 (6) | 66 (5) | |||
| Dialysis modality, n (%) | |||||||
| Hemodialysis | 334 (70) | 166 (69) | |||||
| Peritoneal dialysis | 78 (16) | 42 (17) | |||||
| Unknown | 68 (14) | 34 (14) | |||||
| Dialysis vintage, median (IQR), months | 26 (11–50) | 24 (11–50) | |||||
| Previous transplantation, n (%) | |||||||
| Yes | 66 (14) | 31 (13) | |||||
| Time between transplantation and 2nd or 3rd vaccination, median (IQR), months | 92 (47–163) | 104 (57–171) | |||||
| Time between transplantation and 2nd or 3rd vaccination, n (%) | |||||||
| <6 months | 36 (1) | 2 (0) | |||||
| ≥6 months | 1913 (78) | 1210 (78) | |||||
| Unknown | 519 (21) | 335 (22) | |||||
| Type of transplant, n (%) | |||||||
| DBD | 430 (17) | 282 (18) | |||||
| DCD | 280 (11) | 172 (11) | |||||
| Living | 1239 (50) | 758 (49) | |||||
| Unknown | 519 (21) | 335 (22) | |||||
| Immunosuppressive treatment, n (%) | |||||||
| Yes | 22 (6) | 9 (23) | 84 (18) | 38 (16) | 1583 (64) | 964 (62) | |
| No | 378 (95) | 31 (78) | 396 (83) | 204 (84) | |||
| Unknown | 885 (36) | 583 (38) | |||||
| Type of immunosuppressive treatmenta, n (%) | |||||||
| Corticosteroids | 17 (4) | 6 (15) | 66 (14) | 31 (13) | 1145 (72) | 710 (74) | |
| CNIs | 5 (1) | 1 (3) | 39 (8) | 19 (8) | 1297 (82) | 781 (81) | |
| MMF | 3 (1) | 2 (5) | 14 (3) | 7 (3) | 1029 (65) | 609 (63) | |
| mTOR inhibitors | 1 (0) | 1 (3) | 2 (0) | 2 (1) | 116 (7) | 90 (9) | |
| Azathioprine | 5 (1) | 2 (5) | 3 (1) | 2 (1) | 166 (11) | 97 (10) | |
| Other | 0 (–) | 0 (–) | 0 (–) | 21 (1) | 10 (1) | ||
| Two-dose vaccination scheme, n (%) | |||||||
| mRNA-1273 | 186 (100) | 273 (68) | 411 (86) | 2297 (93) | |||
| BNT162b2 | 114 (29) | 52 (11) | 117 (5) | ||||
| AZD1222 | 13 (3) | 17 (4) | 54 (2) | ||||
| Three-dose vaccination scheme | |||||||
| 3× mRNA-1273 | 2 (5) | 16 (7) | 99 (6) | ||||
| 2× mRNA-1273, 1× BNT162b2 | 19 (48) | 177 (73) | 1280 (83) | ||||
| 3× BNT162b2 | 15 (38) | 28 (12) | 79 (5) | ||||
| Other | 4 (10) | 21 (9) | 89 (6) | ||||
| Time between vaccination and antibody measurement, days, mean (SD) | |||||||
| 2nd vaccination to 1st antibody measurement | 28 (1) | 32 (7) | 33 (10) | 38 (9) | 37 (8) | 33 (8) | 33 (7) |
| 3rd vaccination to 2nd antibody measurement | 37 (8) | 41 (8) | 42 (7) | ||||
| Time between 2nd and 3rd vaccination, days, mean (SD) | 172 (22) | 177 (19) | 178 (18) | ||||
Total numbers and % can vary because of missing values.
CNIs: calcineurin inhibitors; BMI: body mass index; eGFR: estimated glomerular filtration rate; DBD: donation after brain death; DCD: donation after circulatory death; mTOR inhibitors: mammalian target of rapamycin; PCKD: polycystic kidney disease.
A subcohort of 40 patients with CKD G4/5, 242 dialysis patients and 1547 KTR received a third SARS-CoV-2 vaccination followed by a second blood sample. In total, 1519 patients were excluded of which 73 patients had COVID-19 between their second and third vaccination (Supplementary data, Fig. S1). In this subcohort, 9 patients with CKD G4/5 (23%) and 38 dialysis patients (16%) used immunosuppressive drugs. Patients predominantly received BNT162b2 vaccine as their third vaccination (Table 1). Baseline characteristics of dialysis patients and KTR included for analysis after three vaccinations did not differ from dialysis patients and KTR who were excluded from analysis. CKD G4/5 patients included for analysis after three vaccinations were significantly older (67 ± 9 vs 64 ± 12 years) and more often used immunosuppressive drugs (23% vs 4%) as compared with CKD G4/5 patients who were excluded for analysis (Supplementary data, Table S1).
Antibody level after SARS-CoV-2 vaccination
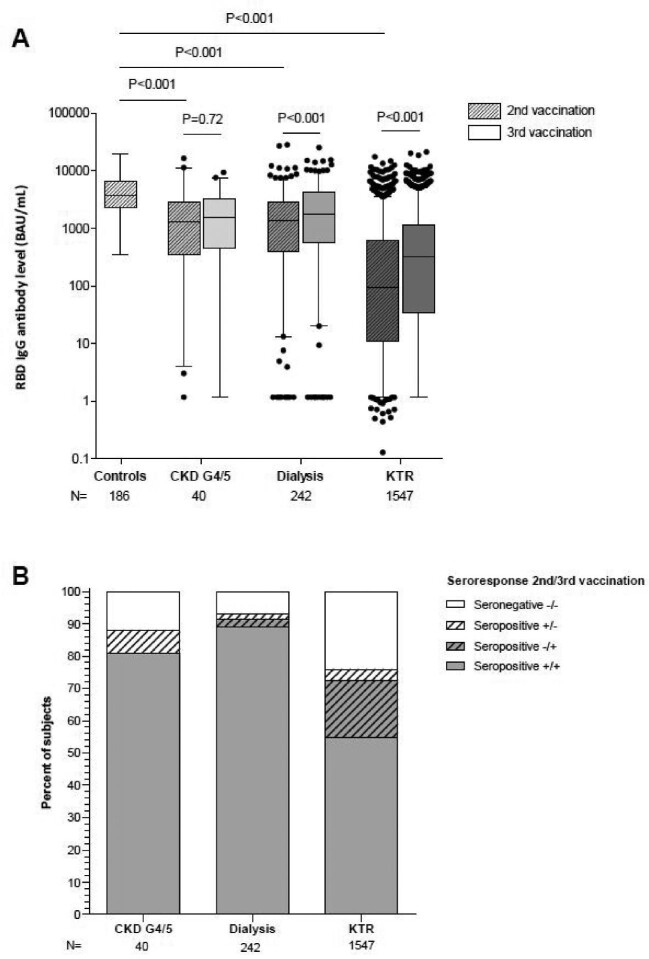
The median [interquartile range (IQR)] RBD IgG antibody level after two vaccinations was 3713 (2291–6451) BAU/mL in control subjects and all these subjects seroconverted. In comparison with control subjects, antibody levels and seroconversion rates were significantly lower in patients with CKD G4/5 [2097 (828–4077) BAU/mL and 96% seroconversion; P ≤ .001 and P = .006, respectively], in dialysis patients [1375 (431–2896) BAU/mL and 92% seroconversion; both P < .001] and in KTR [66 (8–573) BAU/mL and 49% seroconversion; both P < .001].
Antibody level and seroconversion rate did not increase in patients with CKD G4/5 after a third vaccination (Fig. 1A and B). In contrast, a rise in antibody levels after third vaccination was observed in both dialysis patients (P < .001) and KTR (P < .001). Seroconversion after a third vaccination was observed in 26% of dialysis patients and 43% of KTR who did not respond after two vaccinations. An antibody level >1000 BAU/mL after three vaccinations was induced in 55% of patients with CKD G4/5, 63% of dialysis patients and 25% of KTR (Table 2a and b).
Figure 1:
RBD IgG antibody levels (A) and seroconversion rates (B) after two and three SARS-CoV-2 vaccinations. These figures describe antibody levels and response rates in patients with CKD G4/5, dialysis patients and KTR who have data available on the first two vaccinations, and the third vaccination.
Table 2a:
RBD IgG antibody levels after two and three vaccinations in patients with CKD stages G4/5 and dialysis patients categorized by use of immunosuppressive drugs.
| 2 vaccinations | 3 vaccinations | |||||||
|---|---|---|---|---|---|---|---|---|
| Immunosuppressive treatment | Immunosuppressive treatment | |||||||
| All | No | Yes | P a | All | No | Yes | P a | |
| CKD G4/5, n (%) | 400 (100) | 378 (94) | 22 (6) | 40 (100) | 31 (78) | 9 (22) | ||
| RBD IgG Ab level (BAU/mL) | 2097 (828–4077) | 2186 (887–4160) | 1110 (34–2456) | .003 | 1551 (459–3225) | 1680 (631–3466) | 11 (3–739) | .01 |
| RBD IgG seroconversion rate, n (%) | 384 (96) | 368 (97) | 16 (73) | <.001 | 32 (80) | 29 (94) | 3 (33) | <.001 |
| RBD IgG antibody level >1000 BAU/mL, n (%) | 286 (72) | 275 (73) | 11 (50) | .02 | 22 (55) | 20 (65) | 2 (22) | .03 |
| Dialysis patients, n (%) | 480 (100) | 396 (83) | 84 (18) | 242 (100) | 204 (84) | 38 (16) | ||
| RBD IgG Ab level (BAU/mL) | 1375 (431–2896) | 1798 (667–3073) | 291 (29–748) | <.001 | 1727 (570–4254) | 2309 (867–4741) | 200 (9–1102) | <.001 |
| RBD IgG seroconversion rate, n (%) | 443 (92) | 386 (97) | 57 (68) | <.001 | 222 (92) | 199 (98) | 23 (61) | <.001 |
| RBD IgG antibody level >1000 BAU/mL, n (%) | 274 (57) | 260 (66) | 14 (17) | <.001 | 153 (63) | 143 (70) | 10 (26) | <.001 |
Note: Antibody levels, seroconversion rates, and rates of high-level antibody response (>1000 BAU/ml) after two and three SARS-CoV-2 vaccinations for all CKD G4/5 and dialysis patients, and according to the use of immunosuppressive drugs.
Ab: antibody.
aNot using immunosuppressive drugs versus using immunosuppressive drugs.
Table 2b:
RBD IgG antibody levels after two and three vaccinations in KTR categorized by immunosuppressive regimen with or without mycophenolate mofetil.
| 2 vaccinations | 3 vaccinations | |||||||
|---|---|---|---|---|---|---|---|---|
| MMF | MMF | |||||||
| All | No | Yes | P a | All | No | Yes | P a | |
| KTR, n (%) | 1583 (100) | 554 (35) | 1029 (65) | 964 (100) | 355 (37) | 609 (63) | ||
| RBD IgG Ab level (BAU/mL) | 66 (8–573) | 340 (50–1492) | 20 (3–113) | <.001 | 259 (26–1008) | 437 (74–1445) | 165 (16–791) | <.001 |
| RBD IgG seroconversion rate, n (%) | 780 (49) | 412 (75) | 365 (35) | <.001 | 675 (70) | 277 (78) | 398 (65) | <.001 |
| RBD IgG antibody level >1000 BAU/mL, n (%) | 263 (17) | 182 (33) | 81 (8) | <.001 | 244 (25) | 117 (33) | 127 (21) | <.001 |
Note: Antibody levels, seroconversion rates, and rates of high-level antibody response (>1000 BAU/ml) after two and three SARS-CoV-2 vaccinations for all KTR, and according to an immunosuppressive regimen with or without mycophenolate mofetil (MMF).
Ab: antibody.
aDifference between ‘MMF yes’ and ‘MMF no’.
The antibody levels and seroconversion rates in patients with CKD G4/5 and dialysis patients were lower in those with immunosuppressive treatment as compared with those without immunosuppressive treatment (Table 2a). No difference in change of antibody levels between second and third vaccination was observed in patients with CKD G4/5 and dialysis patients according to the use of immunosuppressive treatment (data not shown).
After two vaccinations, KTR who use MMF had lower antibody levels and a lower seroconversion rate compared with KTR without MMF [20 (3–113) BAU/mL vs 340 (50–1492) BAU/mL, and 35% vs 75%, respectively; P < .001; Table 2b]. The third vaccination resulted in a stronger increase in antibody level in KTR using MMF compared with KTR without MMF [+81 (0, +470) vs 0 (–20, +340) BAU/mL; P < .001]. In KTR using MMF who have not responded after two vaccinations, 164 KTR (46%) did seroconvert after a third vaccination. In KTR not using MMF, only 20 previously non-responding KTR (26%) seroconverted after a third vaccination.
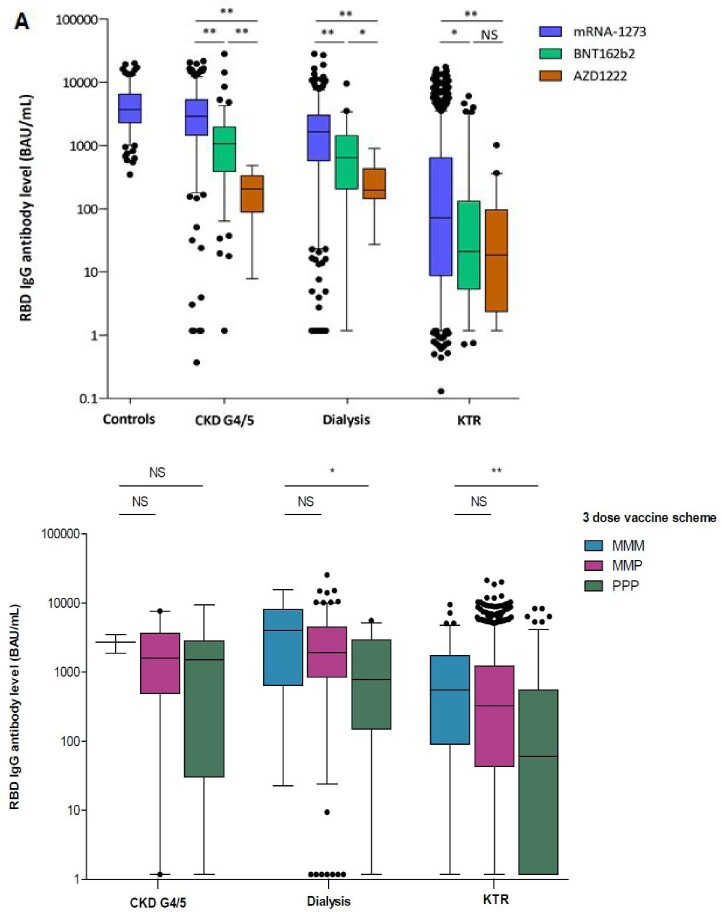
Two vaccinations with mRNA-1273 resulted in higher antibody levels in all three patient groups compared with two vaccinations with BNT162b2 or AZD1222 (P < .001, Fig. 2A). This finding was confirmed after multivariable linear regression analysis (Supplementary data, Table S2). We also observed higher antibody levels in vaccination schemes containing three vaccinations of mRNA-1273 compared with three vaccinations of BNT162b2 in dialysis patients and KTR (Fig. 2B).
Figure 2:
RBD IgG antibody levels after two vaccinations (A) and three vaccinations (B) in different patient groups categorized per vaccine type.*P ≤ .01, **P ≤ .001. MMM: mRNA-1273 3×; MMP: mRNA-1273 2×, BNT162b2; PPP: BNT162b2 3×.
Adverse events after SARS-CoV-2 vaccination
The frequency of any AE within the first 7 days after the second mRNA-1273 vaccination was lower in patients with CKD G4/5 (84%), dialysis patients (60%) and KTR (63%) in comparison with control subjects (94%, P ≤ .001, Table 3). More systemic and local AEs were reported after the second vaccination with mRNA-1273 in comparison with BNT162b2 in all three patient groups (P < .01, Supplementary data, Table S3). This could be confirmed after multivariable logistic regression analysis (Supplementary data, Table S4). No statistically significant differences in local or systemic AEs were observed between administration of BNT162b2 and mRNA-1273 after the third vaccination (Supplementary data, Table S3). The most frequently reported AE was pain at the injection site for all three vaccine types in all patient groups (Supplementary data, Table S5).
Table 3:
Any adverse events after each SARS-CoV-2 vaccination in control subjects, patients with CKD G4/5, dialysis patients and KTR.
| mRNA-1273 | BNT162b2 | AZD1222 | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 vaccination | 2 vaccinations | 3 vaccinations | 1 vaccination | 2 vaccinations | 3 vaccinations | 1 vaccination | 2 vaccinations | |
| Controls, n (%) | 166 (89) | 175 (94) | ||||||
| CKD G4/5, n (%) | 219 (80) | 229 (84) | 2 (100) | 46 (40) | 38 (33) | 16 (44) | 8 (62) | 4 (31) |
| Dialysis, n (%) | 254 (62) | 245 (60) | 8 (50) | 13 (25) | 11 (21) | 84 (39) | 8 (47) | 7 (41) |
| KTR, n (%) | 1723 (75) | 1455 (63) | 50 (50) | 45 (38) | 42 (36) | 599 (43) | 32 (59) | 17 (36) |
DISCUSSION
In the present study, we found that immunosuppressive treatment in patients with CKD G4/5 and dialysis patients, as well as MMF use in KTR, leads to lower antibody levels and seroconversion rates after three SARS-CoV-2 vaccinations. Remarkably, 46% of the KTR using MMF who did not respond to the first two vaccinations had seroconversion after a third vaccination. In addition, we observed that mRNA-1273 in comparison with BNT162b2 and AZD1222 induced higher antibody levels, which was accompanied by higher rates of short-term reported AEs.
To date, only two small series have described the effect of immunosuppressive treatment on the immune response after SARS-CoV-2 vaccination in patients with CKD G4/5. In one study, 36 patients with CKD G4/5 that were mainly treated with rituximab demonstrated lower antibody levels [2]. Another study in 18 patients with CKD G4/5 using immunosuppressive drugs also demonstrated lower antibody levels after inactivated whole-virus SARS-CoV-2 vaccination [10]. Our data show an adverse effect of immunosuppressive treatment on antibody level and seroconversion rate in the largest cohort of patients with CKD G4/5 being described so far. In dialysis patients, more data are available on the effect of immunosuppressive treatment on the immune response after SARS-CoV-2 vaccination. Several studies show lower antibody levels after two [3, 28] and three [7–9] vaccinations, as we could likewise observe in our cohort. Both CKD G4/5 and dialysis patients who receive immunosuppressive treatment and do not respond to vaccination may be at a persistent higher risk of a severe course of COVID-19.
The effect of immunosuppressive treatment on the immune response after SARS-CoV-2 vaccination in KTR has extensively been studied, albeit in much smaller cohorts than in our study [29]. We demonstrate a higher antibody level and seroconversion rate after the second as well as the third vaccination in KTR not using MMF compared with KTR using MMF. Of the KTR using MMF that did not respond after two vaccinations, eventually 46% seroconverted after a third vaccination. This is a higher response than in non-responding KTR without MMF of whom 26% eventually seroconverted after a third vaccination. This shows that repeated vaccination is an effective strategy to improve antibody levels in KTR, especially those using MMF.
The lower immune response in KTR using MMF raises the question of whether the response to SARS-CoV-2 vaccination in KTR can be optimized by temporary discontinuation of MMF [5, 30, 31]. One randomized controlled trial recently investigated withdrawal of MMF 1 week before and after a third or fourth vaccination in 103 previous non-responding KTR [32]. No difference in antibody response was found between the intervention and the placebo group. The authors argue that the withdrawal period of 2 weeks could have been too short to identify any differences. Similar studies with a longer withdrawal period of MMF or switch to another immunosuppressant agent should be performed to further investigate this issue.
The use of mRNA-1273 has previously been shown to yield higher antibody concentrations than BNT162b2 in the general population [11]. This has also been demonstrated in patients with kidney disease [2, 3, 15–17, 33–36], but a comparison between mRNA-1273 and AZD1222 has not been previously reported for patients with CKD G4/5 and KTR. In dialysis patients, conflicting results have been reported when comparing antibody levels after vector-based and mRNA-based SARS-CoV-2 vaccination [16, 18, 37, 38]. Differences in IgG antibody levels are thought to be of importance, since higher antibody levels are correlated with higher virus neutralization titres [39], and a higher protection against severe COVID-19 [40]. We could confirm higher antibody levels with mRNA-1273 vs BNT162b2 in a large cohort of patients with CKD G4/5, dialysis patients and KTR. It may be that a higher dose of mRNA in mRNA-1273 versus BNT162b2 is responsible for the difference in antibody levels. Due to the low representation of AZD1222 in our cohort, we cannot generalize our findings on vaccination with AZD1222 to other patients with CKD G4/5, dialysis patients and KTR.
As the COVID-19 pandemic further evolves with new variants of concern, currently used vaccines (targeted at the ancestral SARS-CoV-2 strain) will become less effective. Against the emerging Omicron variant, strongly reduced cross-neutralization was observed [41–44]. Nevertheless, a lower risk of severe disease after infection with this variant was described [45]. This is potentially due to inherent differences in viral properties between the Omicron and previously circulating variants. In addition, immunological mechanisms other than virus neutralization are also involved in cross-protection against severe disease. Examples are, beside functions of virus-specific T-cells, effector functions mediated by non-neutralizing antibodies such as antibody-dependent cellular cytotoxicity, phagocytosis and complement deposition.
We assume that the induction of higher level of antibody levels is a desirable outcome in these patients at high-risk of severe COVID-19. Our study demonstrates that a third vaccination induces antibody levels above 1000 BAU/mL in the majority of patients with CKD G4/5 and dialysis patients. However, only a minority of dialysis patients using immunosuppressive drugs and KTR have antibody levels above 1000 BAU/mL after the third vaccination. Recently, an antibody level above 1000 BAU/mL was shown to correlate with in vitro neutralization against the Omicron variant 28 days after vaccination [39]. Furthermore, high-level antibody response is associated with clinical protection against SARS-CoV-2 infection [46] and severe COVID-19 [47].
We observed a lower rate of AEs after BNT162b2 compared with mRNA-1273 in all patient groups. The reported AEs were mild and self-limiting. Given the vulnerability of the kidney patient groups for severe COVID-19, we consider the impact of higher AE rates of lesser importance than the beneficial immunogenicity of mRNA-1273.
The main strength of our study is the real-life representation of all high-risk patient groups with kidney disease. Recently, Quiroga et al. also described antibody responses in a cohort including high-risk patients with kidney disease after two and three vaccinations [36, 48]. In contrast to these studies, we report on the impact of immunosuppressive treatment in patients with CKD G4/5 and dialysis patients. Furthermore, we have stratified the regression analysis for the three subgroups of patients with kidney disease. Our cohort also contains the largest number of KTR so far, enabling us to perform detailed analysis on the impact of type of immunosuppressive drugs and vaccine type on antibody levels. We also performed NP antibody measurement to exclude previous COVID-19 in patients who have not self-reported a previous COVID-19 diagnosis. Doing so, we minimize the possibility of asymptomatic infections influencing our results. A specific strong feature of this study is the measurement of antibody levels by home-based fingerprick sampling of blood. This prevented additional workload for hospital workers and circumvented visits of patients to healthcare centres during the pandemic.
The study has some limitations. First, we complied with the Dutch vaccination programme, in which different vaccines were administered in different age groups. Nevertheless, our main findings remain unchanged after adjustment for age. Second, we did not measure neutralizing antibodies after vaccination. The antibody level, however, is correlated with neutralizing capacity after SARS-CoV-2 vaccination or infection [39, 40]. Therefore, we also expect our findings to apply on neutralizing capacity. Third, we lost a part of our initial cohort for the analysis after third vaccination. We found no differences between characteristics of dialysis patients and KTR with and without data on third vaccination, which suggests no indication of selection bias. In contrast, patients with CKD G4/5 with data on third vaccination had a higher percentage of immunosuppressive treatment at baseline compared with those without data on third vaccination. This is a direct result of the prioritization of only CKD G4/5 patients with immunosuppressive treatment in the Dutch vaccination programme. Lastly, we did not collect data on reason and duration of immunosuppressive treatment, nor the dose of immunosuppressive drugs. The importance of immunosuppressive treatment dosage was previously reported for corticosteroids [49] and MMF [50].
In conclusion, the antibody level after SARS-CoV-2 vaccination is adversely affected by immunosuppressive treatment in patients with CKD G4/5, dialysis patients and KTR. The mRNA-1273 vaccine yields the highest antibody level with an acceptable increase of AEs. Repetitive SARS-CoV-2 vaccination is an effective strategy to establish antibody response in dialysis patients and KTR who did not respond to previous SARS-CoV-2 vaccination, especially in KTR who use MMF.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank all RECOVAC Collaborators for their effort and intellectual contribution to the RECOVAC consortium. Names and affiliations of collaborative authors are listed in the Appendix.
APPENDIX
RECOVAC Collaborators: names and affiliations of collaborative authors:
Canisius Wilhelmina Ziekenhuis, Nijmegen, The Netherlands
• Rik C.G. ter Meulen,
• Jennifer Cheng;
Catharina Hospital, Eindhoven, The Netherlands
• Constantijn J.A.M. Konings,
• Vincent J.P. Peters;
Amsterdam University Medical Center, Amsterdam, The Netherlands
• Ester B.M. Remmerswaal,
• Sophie C. Frölke;
Center for Infectious Disease Control, National Institute for Public Health and the Environment, Bilthoven, The Netherlands
• Nynke Rots,
• Fiona van der Klis;
Dutch Kidney Patients Association (NVN), Bussum, The Netherlands
• Wanda S. Konijn,
• Anthony de Ronde,
• Hanneke J.P.M. Vervoort,
• Marion H.J. Braks;
Erasmus Medical Center, Erasmus MC Transplant Institute, Rotterdam, The Netherlands
• Marcia L. Kho,
• Carla C. Baan,
• Reshwan S.R.K. Malaha;
Martini Ziekenhuis, Groningen, The Netherlands
• Wilbert M.T. Janssen,
• Erik Til;
Medisch Spectrum Twente, Enschede, The Netherlands
• M. Zwerink,
• J. Niels Brinkman;
OLVG, Amsterdam, The Netherlands
• Carl Siegert,
• Hein R. Fritsen;
Maasstad Hospital, Rotterdam, The Netherlands
• L. den Biggelaar;
St Antonius Hospital, Utrecht, The Netherlands
• Willem Jan Bos,
• Manou Willems;
Radboud University Medical Center, Nijmegen, The Netherlands
• Renate G. van der Molen,
• Dimitri A. Diavatopoulos;
University Medical Center Groningen, Groningen, The Netherlands
• Debbie van Baarle.
Notes
A list of the RECOVAC Collaborators can be found in the Appendix.
Contributor Information
Pim Bouwmans, Department of Internal Medicine, Division of Nephrology, Maastricht University Medical Center, Maastricht, The Netherlands; CARIM School for Cardiovascular Diseases, University of Maastricht, Maastricht, The Netherlands.
A Lianne Messchendorp, Department of Nephrology, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
Céline Imhof, Department of Nephrology, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
Jan-Stephan F Sanders, Department of Nephrology, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
Luuk B Hilbrands, Department of Nephrology, Radboud University Medical Center, Nijmegen, The Netherlands.
Marlies E J Reinders, Department of Internal Medicine, Nephrology and Transplantation, Erasmus MC Transplant Institute, Erasmus Medical Center, Rotterdam, The Netherlands.
Priya Vart, Department of Internal Medicine, University Medical Center Groningen, Groningen, The Netherlands; Department of Clinical Pharmacy and Pharmacology, University Medical Center Groningen, Groningen, The Netherlands.
Frederike J Bemelman, Amsterdam UMC Location University of Amsterdam, Renal Transplant Unit, Meibergdreef 9, Amsterdam, The Netherlands; Amsterdam Institute for Infection and Immunity, Infectious Diseases, Amsterdam, The Netherlands.
Alferso C Abrahams, Department of Nephrology and Hypertension, University Medical Center Utrecht, Utrecht, The Netherlands.
René M A van den Dorpel, Department of Nephrology, Maasstad Hospital, Rotterdam, The Netherlands.
Marc A G J Ten Dam, Department of Internal Medicine, Canisius Wilhelmina Hospital, Nijmegen, The Netherlands.
Aiko P J de Vries, Department of Medicine, Division of Nephrology, Leiden University Medical Center, Leiden, The Netherlands; Leiden Transplant Center, Leiden, The Netherlands.
Theo Rispens, Department of Immunopathology, Sanquin Research, Amsterdam, The Netherlands; Landsteiner Laboratory, Amsterdam University Medical Center, University of Amsterdam, Amsterdam, The Netherlands.
Maurice Steenhuis, Department of Immunopathology, Sanquin Research, Amsterdam, The Netherlands; Landsteiner Laboratory, Amsterdam University Medical Center, University of Amsterdam, Amsterdam, The Netherlands.
Ron T Gansevoort, Department of Nephrology, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
Marc H Hemmelder, Department of Internal Medicine, Division of Nephrology, Maastricht University Medical Center, Maastricht, The Netherlands; CARIM School for Cardiovascular Diseases, University of Maastricht, Maastricht, The Netherlands.
the RECOVAC Collaborators:
Rik C G ter Meulen, Jennifer Cheng, Constantijn J A M Konings, Vincent J P Peters, Ester B M Remmerswaal, Sophie C Frölke, Nynke Rots, Fiona van der Klis, Wanda S Konijn, Anthony de Ronde, Hanneke J P M Vervoort, Marion H J Braks, Marcia L Kho, Carla C Baan, Reshwan S R K Malaha, Wilbert M T Janssen, Erik Til, M Zwerink, J Niels Brinkman, Carl Siegert, Hein R Fritsen, L den Biggelaar, Willem Jan Bos, Manou Willems, Renate G van der Molen, Dimitri A Diavatopoulos, and Debbie van Baarle
CONFLICT OF INTEREST STATEMENT
None declared.
AUTHORS’ CONTRIBUTIONS
P.B., A.L.M., J.-S.F.S., R.T.G., L.B.H. and M.H.H. drafted the manuscript. P.B., P.V., A.L.M. and M.H.H. were responsible for analysis. All authors provided intellectual content of critical importance to the study, and revised and approved the final manuscript. The RECOVAC Collaborators contributed to the design of the consortium and data collection.
FUNDING
The RECOVAC consortium received funding from ZonMw (10430072010002) and Nierstichting (21OP + 036).
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
REFERENCES
- 1. Sanders JF, Bemelman FJ, Messchendorp AL.et al. The RECOVAC Immune-Response study: the immunogenicity, tolerability, and safety of COVID-19 vaccination in patients with chronic kidney disease, on dialysis, or living with a kidney transplant. Transplantation 2021;106:821–34. 10.1097/tp.0000000000003983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Buchwinkler L, Solagna CA, Messner J.et al. Antibody response to mRNA vaccines against SARS-CoV-2 with chronic kidney disease, hemodialysis, and after kidney transplantation. J Clin Med 2021;11:148. 10.3390/jcm11010148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stumpf J, Siepmann T, Lindner T.et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg Health Eur 2021;9:100178. 10.1016/j.lanepe.2021.100178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kamar N, Abravanel F, Marion O.et al. Anti-SARS-CoV-2 spike protein and neutralizing antibodies at 1 and 3 months after three doses of SARS-CoV-2 vaccine in a large cohort of solid organ transplant patients. Am J Transplant 2022;22:1467–74. 10.1111/ajt.16950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yahav D, Rahamimov R, Mashraki T.et al. Immune response to third dose BNT162b2 COVID-19 vaccine among kidney transplant recipients-a prospective study. Transpl Int 2022;35:10204. 10.3389/ti.2022.10204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Massa F, Cremoni M, Gérard A.et al. Safety and cross-variant immunogenicity of a three-dose COVID-19 mRNA vaccine regimen in kidney transplant recipients. EBioMedicine 2021;73:103679. 10.1016/j.ebiom.2021.103679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Housset P, Kubab S, Pardon A.et al. Waning but persistent humoral response 6 months after the third dose of the mRNA BNT162b2 vaccine in hemodialysis and peritoneal dialysis patients. J Nephrol 2022;35:783–5. 10.1007/s40620-022-01276-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bensouna I, Caudwell V, Kubab S.et al. SARS-CoV-2 antibody response after a third dose of the BNT162b2 vaccine in patients receiving maintenance hemodialysis or peritoneal dialysis. Am J Kidney Dis 2022;79:185–92.e1. 10.1053/j.ajkd.2021.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benning L, Klein K, Morath C.et al. Neutralizing antibody activity against the B.1.617.2 (delta) variant before and after a third BNT162b2 vaccine dose in hemodialysis patients. Front Immunol 2022;13:840136. 10.3389/fimmu.2022.840136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang YM, Liu XZ, Lin MM.et al. Immunosuppression impaired the immunogenicity of inactivated SARS-CoV-2 vaccine in non-dialysis kidney disease patients. J Infect 2022;85:174–211. 10.1016/j.jinf.2022.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Naranbhai V, Garcia-Beltran WF, Chang CC.et al. Comparative immunogenicity and effectiveness of mRNA-1273, BNT162b2, and Ad26.COV2.S COVID-19 vaccines. J Infect Dis 2022;225:1141–50. 10.1093/infdis/jiab593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fiolet T, Kherabi Y, MacDonald CJ.et al. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect 2022;28:202–21. 10.1016/j.cmi.2021.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Steensels D, Pierlet N, Penders J.et al. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA 2021;326:1533–5. 10.1001/jama.2021.15125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dickerman BA, Gerlovin H, Madenci AL.et al. Comparative effectiveness of BNT162b2 and mRNA-1273 vaccines in U.S. veterans. N Engl J Med 2021;386:105–15. 10.1056/NEJMoa2115463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wijtvliet V, Ariën KK, Abrams S.et al. mRNA-1273 vaccine (Moderna): a better option than BNT162b2 (Pfizer) in kidney transplant recipients and dialysis patients? Nephrol Dial Transplant 2021;37:799–803. 10.1093/ndt/gfab352 [DOI] [PubMed] [Google Scholar]
- 16. Affeldt P, Koehler FC, Brensing KA.et al. Immune responses to SARS-CoV-2 infection and vaccination in dialysis patients and kidney transplant recipients. Microorganisms 2021;10:4. 10.3390/microorganisms10010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Van Praet J, Reynders M, De Bacquer D.et al. Predictors and dynamics of the humoral and cellular immune response to SARS-CoV-2 mRNA vaccines in hemodialysis patients: a multicenter observational study. J Am Soc Nephrol 2021;32:3208–20. 10.1681/asn.2021070908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meijers B, Goedgezelschap A, Peeters D.et al. Heterologous vs. homologous triple anti-COVID-19 vaccine regimens in patients on maintenance hemodialysis. Nephrol Dial Transplant 2022;37:1384–6. 10.1093/ndt/gfac033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Polack FP, Thomas SJ, Kitchin N.et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med 2020;383:2603–15. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Voysey M, Clemens SAC, Madhi SA.et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021;397:99–111. 10.1016/s0140-6736(20)32661-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baden LR, El Sahly HM, Essink B.et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403–16. 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bouwmans P, Messchendorp AL, Sanders JS.et al. Long-term efficacy and safety of SARS-CoV-2 vaccination in patients with chronic kidney disease, on dialysis or after kidney transplantation: a national prospective observational cohort study. BMC Nephrol 2022;23:55. 10.1186/s12882-022-02680-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kho MML, Reinders MEJ, Baan CC.et al. The RECOVAC IR study: the immune response and safety of the mRNA-1273 COVID-19 vaccine in patients with chronic kidney disease, on dialysis, or living with a kidney transplant - a prospective, controlled, multicenter observational cohort by the REnal patients COVID-19 VACcination (RECOVAC) consortium COVID-19 VACcination (RECOVAC) consortium. Nephrol Dial Transplant 2021;36:1761–1764. 10.1093/ndt/gfab186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. ERA-EDTA Registry . ERA-EDTA Registry Annual Report 2019. Amsterdam, The Netherlands: Amsterdam UMC, location AMC, Department of Medical Informatics, 2021. [Google Scholar]
- 25. Vogelzang EH, Loeff FC, Derksen NIL.et al. Development of a SARS-CoV-2 total antibody assay and the dynamics of antibody response over time in hospitalized and nonhospitalized patients with COVID-19. J Immunol 2020;205:3491–9. 10.4049/jimmunol.2000767 [DOI] [PubMed] [Google Scholar]
- 26. Infantino M, Pieri M, Nuccetelli M.et al. The WHO international standard for COVID-19 serological tests: towards harmonization of anti-spike assays. Int Immunopharmacol 2021;100:108095. 10.1016/j.intimp.2021.108095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Steenhuis M, van Mierlo G, Derksen NI.et al. Dynamics of antibodies to SARS-CoV-2 in convalescent plasma donors. Clin Transl Immunology 2021;10:e1285. 10.1002/cti2.1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Espi M, Charmetant X, Barba T.et al. The ROMANOV study found impaired humoral and cellular immune responses to SARS-CoV-2 mRNA vaccine in virus-unexposed patients receiving maintenance hemodialysis. Kidney Int 2021;100:928–36. 10.1016/j.kint.2021.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Manothummetha K, Chuleerarux N, Sanguankeo A.et al. Immunogenicity and risk factors associated with poor humoral immune response of SARS-CoV-2 vaccines in recipients of solid organ transplant: a systematic review and meta-analysis. JAMA Netw Open 2022;5:e226822. 10.1001/jamanetworkopen.2022.6822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Connolly CM, Chiang TP, Boyarsky BJ.et al. Temporary hold of mycophenolate augments humoral response to SARS-CoV-2 vaccination in patients with rheumatic and musculoskeletal diseases: a case series. Ann Rheum Dis 2022;81:293–5. 10.1136/annrheumdis-2021-221252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schrezenmeier E, Rincon-Arevalo H, Jens Aet al. Temporary antimetabolite treatment hold boosts SARS-CoV -2 vaccination-specific humoral and cellular immunity in kidney transplant recipients. JCI Insight 2022;7. 10.1172/jci.insight.157836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kho MML, Messchendorp AL, Frölke SCet al. Alternative strategies to increase the immunogenicity of COVID-19 vaccines in kidney transplant recipients not responding to two or three doses of an mRNA vaccine (RECOVAC): a randomized clinical trial. Lancet Infect Dis 2022;27:S1473–3099. doi: 10.1016/s1473-3099(22)00650-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Anand S, Montez-Rath ME, Han J.et al. SARS-CoV-2 vaccine antibody response and breakthrough infection in patients receiving dialysis. Ann Intern Med 2021;175:371–8. 10.7326/m21-4176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Correia AL, Leal R, Pimenta AC.et al. The type of SARS-CoV-2 vaccine influences serological response in kidney transplant recipients. Clin Transplant 2022;36:e14585. 10.1111/ctr.14585 [DOI] [PubMed] [Google Scholar]
- 35. Tylicki L, Dębska-Ślizień A, Muchlado M.et al. Boosting humoral immunity from mRNA COVID-19 vaccines in kidney transplant recipients. Vaccines (Basel) 2021;10:56. 10.3390/vaccines10010056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Quiroga B, Soler MJ, Ortiz A.et al. Safety and immediate humoral response of COVID-19 vaccines in chronic kidney disease patients: the SENCOVAC study. Nephrol Dial Transplant 2021;37:1868–78. 10.1093/ndt/gfab313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Billany RE, Selvaskandan H, Adenwalla SF.et al. Seroprevalence of antibody to S1 spike protein following vaccination against COVID-19 in patients receiving hemodialysis: a call to arms. Kidney Int 2021;99:1492–4. 10.1016/j.kint.2021.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carr EJ, Wu M, Harvey R.et al. Neutralising antibodies after COVID-19 vaccination in UK haemodialysis patients. Lancet 2021;398:1038–41. 10.1016/s0140-6736(21)01854-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sanders JSF, Messchendorp AL, de Vries RD.et al. Antibody and T-cell responses 6 months after coronavirus disease 2019 messenger RNA-1273 vaccination in patients with chronic kidney disease, on dialysis, or living with a kidney transplant. Clin Infect Dis 2022. 10.1093/cid/ciac557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Muir L, Jaffer A, Rees-Spear C.et al. Neutralizing antibody responses after SARS-CoV-2 infection in end-stage kidney disease and protection against reinfection. Kidney Int Rep 2021;6:1799–809. 10.1016/j.ekir.2021.03.902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Anft M, Blazquez-Navarro A, Frahnert M.et al. Inferior cellular and humoral immunity against Omicron and Delta variants of concern compared with SARS-CoV-2 wild type in hemodialysis patients immunized with 4 SARS-CoV-2 vaccine doses. Kidney Int 2022;102:207–8. 10.1016/j.kint.2022.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cinkilic O, Anft M, Blazquez-Navarro A.et al. Inferior humoral and sustained cellular immunity against wild-type and omicron variant of concern in hemodialysis patients immunized with 3 SARS-CoV-2 vaccine doses compared with 4 doses. Kidney Int 2022;101:1287–9. 10.1016/j.kint.2022.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Karaba AH, Johnston TS, Aytenfisu TY.et al. A fourth dose of COVID-19 vaccine does not induce neutralization of the omicron variant among solid organ transplant recipients with suboptimal vaccine response. Transplantation 2022;106:1440–4. 10.1097/tp.0000000000004140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Al Jurdi A, Gassen RB, Borges TJet al. Suboptimal antibody response against SARS-CoV-2 Omicron variant after third dose of mRNA vaccine in kidney transplant recipients. Kidney Int 2022;101:1282–6. 10.1016/j.kint.2022.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wolter N, Jassat W, Walaza S.et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet 2022;399:437–46. 10.1016/s0140-6736(22)00017-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Montez-Rath ME, Garcia P, Han J.et al. SARS-CoV-2 infection during the Omicron surge among patients receiving dialysis: the role of circulating receptor-binding domain antibodies and vaccine doses. J Am Soc Nephrol 2022;33:1832–9. 10.1681/asn.2022040504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Malahe SRK, Hoek RAS, Dalm V.et al. Clinical characteristics and outcome of immunocompromised patients with COVID-19 caused by the Omicron variant: a prospective observational study. Clin Infect Dis 2022. 10.1093/cid/ciac571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Quiroga B, Soler MJ, Ortiz A.et al. Humoral response to third dose of SARS-CoV-2 vaccines in the CKD spectrum. Clin J Am Soc Nephrol 2022;17:872–6. 10.2215/cjn.01770222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rabinowich L, Grupper A, Baruch R.et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol 2021;75:435–8. 10.1016/j.jhep.2021.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kantauskaite M, Müller L, Kolb T.et al. Intensity of mycophenolate mofetil treatment is associated with an impaired immune response to SARS-CoV-2 vaccination in kidney transplant recipients. Am J Transplant 2022;22:634–9. 10.1111/ajt.16851 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.