Abstract
The European Society of Cardiology 2021 guideline on cardiovascular (CV) disease (CVD) prevention in clinical practice has major implications for both CV risk screening and kidney health of interest to primary care physicians, cardiologists, nephrol-ogists, and other professionals involved in CVD prevention. The proposed CVD prevention strategies require as first step the categorization of individuals into those with established atherosclerotic CVD, diabetes, familiar hypercholesterolaemia, or chronic kidney disease (CKD), i.e. conditions that are already associated with a moderate to very-high CVD risk. This places CKD, defined as decreased kidney function or increased albuminuria as a starting step for CVD risk assessment. Thus, for adequate CVD risk assessment, patients with diabetes, familiar hypercholesterolaemia, or CKD should be identified by an initial laboratory assessment that requires not only serum to assess glucose, cholesterol, and creatinine to estimate the glomerular filtration rate, but also urine to assess albuminuria. The addition of albuminuria as an entry-level step in CVD risk assessment should change clinical practice as it differs from the current healthcare situation in which albuminuria is only assessed in persons already considered to be at high risk of CVD. A diagnosis of moderate of severe CKD requires a specific set of interventions to prevent CVD. Further research should address the optimal method for CV risk assessment that includes CKD assessment in the general population, i.e. whether this should remain opportunistic screening or whether systematic screening.
Keywords: GFR, albuminuria, cardiovascular risk, risk prediction, prevention
Introduction
The recent publication of the European Society of Cardiology (ESC) 2021 Guideline on cardiovascular (CV) disease (CVD) prevention in clinical practice introduces chronic kidney disease (CKD) risk categories that are based on the integration of estimated glomerular filtration rate (eGFR) and urinary albumin-to-creatinine ratio (UACR) data. Furthermore, this guideline indicates that assessment of eGFR and UACR (i.e. a urine analysis) are needed for correct patient categorization according to CVD risk that will guide preventive measures. This has major implications for CVD risk screening as well as kidney health, which is of interest to primary care physicians, cardiologists, nephrologists, and other professionals involved in CVD prevention. In this Position Paper, we first provide an overview of the concept and disease burden of CKD and of the key messages on CKD embedded in the 2021 ESC guideline and then provide a call for action to implement this guideline in order to improve the outcomes of patients with CKD.
The concept of chronic kidney disease
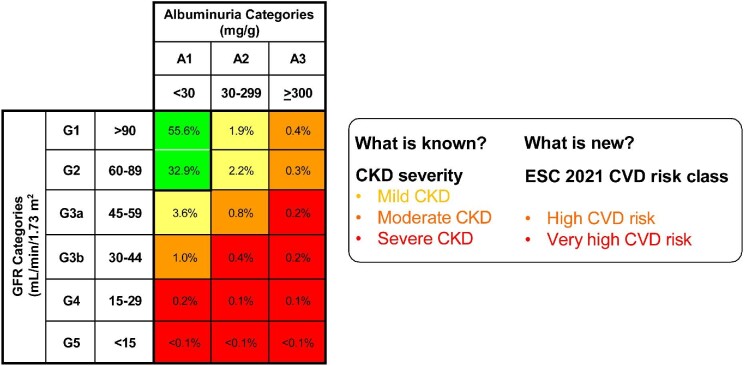
In the 2012 KDIGO (abbreviation for Kidney Disease: Improving Global Outcomes), the global organization developing clinical practice guidelines for nephrology, defined CKD as abnormalities of kidney structure or function present for ≥3 months, with implications for health [1, 2]. In clinical practice, the main diagnostic criteria for CKD are the presence of an eGFR below 60 mL/min/1.73 m2, and/or elevated albuminuria, i.e. UACR over 30 mg/g. Chronic kidney disease is staged according to six eGFR and three UACR categories (Figure 1), with higher categories being associated with increased risk of adverse outcomes including CKD progression, kidney failure, and fatal and non-fatal CVD. Combinations of specific eGFR and UACR cells are used to define three global risk classes: CKD with mildly increased risk, CKD with moderately increased risk, and CKD with severely increased risk (Figure 1).
Figure 1:
Translation of chronic kidney disease risk classes (as defined by Kidney Disease: Improving Global Outcomes in 2012) into cardiovascular disease risk classes as defined by the European society of cardiology in the 2021 guideline on cardiovascular disease prevention in clinical practice. Numbers within cells represent prevalence in the general population.
A key message from the 2012 KDIGO guideline was that a low eGFR is not a prerequisite for CKD diagnosis; for example, a person with normal eGFR can be diagnosed with CKD if albuminuria is elevated. This enables CKD identification at an early stage, and thus allows timely start of effective prevention of CKD progression towards kidney failure.
Current and future burden of chronic kidney disease
Globally, around 10–12% of the general population has CKD, implying that this involves 850 million persons [3]. Despite being largely preventable and treatable, the prevalence of CKD as well as mortality due to CKD are increasing [4]. The Global Burden of Disease study projects that worldwide by 2040, CKD will become the fifth most common cause of death [5]. The years of life lost due to CKD will double. This represents the fastest predicted increase among the major causes of death, after Alzheimer's disease [5]. Notably, the increasing burden of CKD may be magnified in countries with changing demography due to an ageing population, as is the case for several European countries. For example, it is projected that CKD will become the second leading cause of death in Spain before the end of the century [6].
KDIGO 2012 guideline and the concept of chronic kidney disease
Awareness of the concept of CKD, and of the possible consequences, is key to promoting both CV and kidney health. However, in routine clinical practice, the implementation of diagnosing CKD is far from desirable. For instance, in several countries screening for eGFR and albuminuria in patients at high risk for CKD lags seriously behind what is advised in guidelines [7]. Currently, the invisibility of CKD as a clinical diagnosis is a barrier to the implementation of CV risk mitigation strategies as CKD patients are not identified as such. In recent cohort studies, only 23–39% of patients with CKD actually had a CKD diagnosis registered in the electronic health records by their treating physician [8, 9]. This has consequences, as for instance patients lacking CKD diagnoses were more frequently prescribed nephrotoxic medications [8].
Notably, there is still confusion regarding the concept of CKD among some physicians. For instance, as recently as 2018, a seminal medical journal published a manuscript that referred to all patients with an eGFR above 60 mL/min/1.73 m2 as having CKD G1/G2, although in most albuminuria or structural kidney abnormalities were not assessed [2].
ESC 2021 guideline on cardiovascular disease prevention in clinical practice
In 2021, the ESC issued an update of its seminal guideline on CVD prevention in clinical practice [10]. This update was made in consensus with 12 medical societies, including the European Renal Association (ERA) [10]. As the prior 2016 edition, the 2021 guideline recognizes CKD, diagnosed as a low eGFR (<60 mL/min/1.73 m2), as a CV risk factor [11]. However, from the perspective of the ERA, this guide-line incorporates four main novelties:
The concept of moderate and severe CKD is expanded by incorporating albuminuria into the definition. Thus, moderate CKD is no longer limited to persons with an eGFR 30– 59 mL/min/1.73 m2 and severe CKD is no longer limited to an eGFR <30 mL/min/1.73 m2, as they were in 2016. Rather, moderate, and severe CKD now follow the KDIGO global risk classes to encompass a combination of eGFR and UACR values that is associated with high or very-high CVD risk (Figure 1). Thus, now CKD diagnosed by not only low eGFR, but also high UACR is acknowledged as a strong and independent risk factor for CVD. Although the 2016 guideline mentioned albuminuria, it did not integrate it into an integrated CV risk score.
Assessment of CKD, based on either elevated albuminuria (>30 mg/g) or low eGFR (<60 mL/min/1.73 m2), should be part of routine CV risk assessment (see below).
When CKD is present, not only an ACE inhibitor or an ARB, but also an SGLT2 inhibitor should be considered if nondiabetic and is recommended if the patient has Type 2 diabetes to prevent progression of CKD and CV events (Table 1). This advice was based on the results of the DAPA-CKD trial, in which patients with CKD and with or without Type 2 diabetes were included [12]. Recently, the EMPA-KIDNEY Trial, that included a similar patient population, was stopped early because of positive efficacy that met the study's pre-specified threshold for early termination [13]. This has strengthened the evidence supporting this advice.
Nomenclature for CKD classes is proposed, which is easier to understand than the original one by KDIGO. It is based on the associated overall risk of adverse health outcomes: mild CKD, moderate CKD (associated with high CV risk), and severe CKD (associated with very-high CV risk) (Figure 1). This simplified nomenclature may facilitate uptake of the CKD concept, and its incorporation into clinical diagnostic codes and risk assessment tools.
Table 1:
Interventions suggested or recommended for primary prevention in persons with chronic kidney disease according to the ESC 2021 guideline on cardiovascular disease prevention in clinical practice.
| In persons with moderate or severe CKD, there is no need to estimate 10-year fatal and non-fatal CVD risk with either SCORE2 or SCORE2-OP. The CVD risk is already high (moderate CKD) or very high (severe CKD), respectively, warranting preventive interventions according to the level of risk, such as: |
| • For all patients with CKD: |
| • Lifestyle advice including diet and appropriate weight. |
| • Blood pressure control, preferably by RAS inhibitors. |
| • For CKD patients with specific conditions: |
| • LDL-cholesterol reduction to target [LDL-C < 1.8 mmol/L (70 mg/dL) in high-risk patients and < 1.4 mmol/L (55 mg/dL) in very-high-risk patients]. |
| • RAS inhibition in case of severely elevated albuminuria, independent of blood pressure. |
| • SGLT2 inhibition for those with Type 2 diabetes, or with diabetic as well as non-diabetic kidney disease and an eGFR higher than 25 mL/min/1.73 m2, or with heart failure. |
| • In addition, for consideration to prevent CVD in specific patients (not part of the ESC guideline): (nonsteroidal) mineralocorticoid receptor antagonists and/or PCSK9 inhibitors. |
Implications for screening
The ESC 2021 guideline on CVD Prevention in Clinical Practice has major implications for both CV risk screening and kidney health, stating that not only blood pressure, cholesterol, and glucose should be assessed to estimate CV risk in an individual, but also albuminuria and eGFR. This goes beyond the traditional view that eGFR and UACR should only be assessed in populations at high risk for CKD, i.e. those with proven hypertension, proven diabetes, or a CVD history. In this regard, every healthcare system should promote that individuals should know their ABCDE profile regarding CV risk—i.e. albuminuria, blood pressure, cholesterol, diabetes status, and eGFR. This ABCDE profile should be considered along with other risk factors that are self-evident for the individual, such as obesity and smoking. Screening strategies should begin at an age at which absolute risk for outcomes is high enough to warrant preventive strategies, and that allows sufficient lead time for preventive approaches to be successful. The ESC 2021 guideline on CVD prevention in clinical practice supports considering opportunistic and even systematic CV risk assessment among the general population, in men >40 years of age and in women who are postmenopausal or >50 years of age, even when these subjects have no known atherosclerotic CVD risk factors (Class IIb, level C). As discussed above such screening should include eGFR and UACR, and could be repeated after 5 years, or sooner if risk was close to treatment thresholds (Class IIb, level C).
The design of a health check-up directed at prevention of CVD includes CKD awareness and should be adapted according to local resources and characteristics of the population. It may range from opportunistic screening by general practitioners, as done nowadays in most countries, to an extensive health check-up performed at healthcare centres, including a medical history, physical exam, and basic serum and urine biochemistry. From a CKD perspective, the future may even bring home-based urine screening for albuminuria. Such screening could be linked to currently existent colon cancer screening campaigns that in some countries are implemented around the assessment of occult faecal blood in a sample collected at home [14]. Addition of a urine sample will take advantage of an ongoing screening effort and would only add the cost of albuminuria assessment. This would identify target organ injury (i.e. kidney injury) that could result from various known or unknown pre-existing conditions, and thereby identify patients who require a more in-depth assessment and optimized therapy. Such a strategy is likely cost-effective to delay or prevent dialysis and CVD [15].
Emphasizing that screening for CVD risk factors should include CKD markers is timely for several reasons. First, because we can now estimate risk better. Adding albuminuria and eGFR to the SCORE2 and SCORE2-OP algorithms improves CVD risk prediction in subjects with CKD [16, 17] (see Supplementary material online, Figure S1). Precise information about prognosis may stimulate patients and physicians to start preventive treatments (Table 1). Second, because we can now treat patients better. In the last decade, various agents have demonstrated cardio- and renoprotection on top of standard-of-care including RAS inhibitors, GLP1 analogs, SGLT2 inhibitors, and the nonsteroidal MRA finerenone [12, 18–21]. Notwithstanding this progress in knowledge, it should also be noted that CKD patients have been excluded from several CVD trials [22]. For some interventions it is therefore still unresolved what their efficacy-safety ratio is in specifically subjects with CKD. Including subgroups of CKD patients in CVD trials yet to be started will therefore be important.
A call to action by the Council of the European Renal Association
The ESC emphasizes in their guideline that subjects with CKD are at high risk for CVD, and has given CKD a prominent place in CVD prevention. The guideline now requires several stages of dissemination and implementation into clinical practice. If correctly planned this may also generate novel information regarding the optimal implementation strategies:
Wide dissemination of the concept that assessment of serum creatinine (eGFR) and albuminuria (UACR) should be part of any evaluation of CV risk. The message should reach all key stakeholders: healthcare system payers, healthcare personnel, patients, and the general population. To achieve this goal, nephrologists should liaise with primary care physicians, CVD physicians, and cardiologists as key healthcare stakeholders. For instance, they could work out this concept in national guidelines for management of CKD and for CVD prevention, develop educational tools, and organize post-graduate medical education for the various specialties involved. To reach other healthcare personnel, healthcare system payers and the general population the organization of CKD awareness campaigns are indicated. The ERA has templates for such campaigns, for instance to be organized around World Kidney Day [23].
Europe-wide evaluation of optimal screening strategies to identify CKD. Given the differences in baseline risk for CVD and CKD, and the different healthcare systems, it is unlikely that a single strategy fits all and that the type of screening will depend on local financial resources and logistic possibilities. On a national level initiatives should be started how to best screen for CKD, as for instance the THOMAS study in the Netherlands that investigates the cost-effectiveness of two different ways of home-based albuminuria screening of the general population [24]. A prospective pan-European evaluation of different screening strategies may allow definition of the optimal strategy for local conditions. This could be achieved via the organization of an invitational conference on this topic to be organized by the ERA, KDIGO, and/or ESC.
Chronic kidney disease patients should be included in CVD trials yet to be started. Integration of cohorts into registries may generate pragmatic trials that test novel CV protective strategies identified through clinical trials from which CKD patients were excluded. Conversely, clinical trials focused on CKD populations should also have CV end points as CVD constitutes a major proportion of morbidity and mortality in this patient population.
In conclusion, the ESC 2021 guideline on CVD Prevention in Clinical Practice identifies albuminuria and eGFR as CV risk factors that need assessment. Ample dissemination of this guideline among primary care physicians and specialists offers the opportunity for an earlier diagnosis and treatment of CKD and is expected to improve both CV and kidney outcomes in affected subjects.
Conflict of interest statement
None declared.
Supplementary Material
APPENDIX
ERA Council
Alberto Ortiz (IIS-Fundacion Jimenez Diaz- UAM, Madrid, Spain.) (Department of Medicine, Universidad Autonoma de Madrid, Madrid, Spain), Christoph Wanner (Department of Internal Medicine I, University Hospital Würzburg, Würzburg, Germany.), Ron T. Gansevoort (Department of Nephrology, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.), Mario Cozzolino (Department of Health Sciences, University of Milan, Renal Division, ASST Santi Paolo e Carlo, Milan, Italy.), Danilo Fliser (Internal Medicine IV, Renal and Hypertensive Disease, University Medical Center, Homburg, Saar, German), Giovanni Gambaro (Division of Nephrology and Dialysis, Department of 295 Medicine, Ospedale Maggiore, Verona, Italy.), Albert Ong (Kidney Genetics Group, Academic Nephrology Unit, Department of Infection, Immunity and Cardiovascular Disease, University of Sheffield Medical School, Sheffield, UK), Alexander R. Rosenkranz (Department of Internal Medicine, Division of Nephrology, Medical University of Graz, Graz, Austria.), Ivan Rychlık (1st Department of Internal Medicine, Third Faculty of Medicine, Charles University, Faculty Hospital Krvskinohrady, Prague, Czech Republic.), Pantelis Sarafidis (Department of Nephrology, Hippokration Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece.), Roser Torra (Inherited Kidney Diseases Nephrology Department, Fundació Puigvert Instituto de Investigaciones Biomédicas Sant Pau, Universitat Autónoma de Barcelona, Barcelona, Spain. Redinren, Barcelona, Spain.), Serhan Tuglular (Division of Nephrology, Department of Internal Medicine, School of Medicine, Marmara University, Istanbul, Turkey.)
Contributor Information
Alberto Ortiz, IIS-Fundacion Jimenez Diaz-UAM, Madrid, Spain; Department of Medicine, Universidad Autonoma de Madrid, Madrid, Spain.
Christoph Wanner, Department of Internal Medicine I, University Hospital Würzburg, Würzburg, Germany.
Ron Gansevoort, Department of Nephrology, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
the ERA Council:
Alberto Ortiz, Christoph Wanner, Ron T Gansevoort, Mario Cozzolino, Danilo Fliser, Giovanni Gambaro, Albert Ong, Alexander R Rosenkranz, Ivan Rychlık, Pantelis Sarafidis, Roser Torra, and Serhan Tuglular
References
- 1. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1–150. [Google Scholar]
- 2. Perez-Gomez MV, Bartsch LA, Castillo-Rodriguez Eet al. Clarifying the concept of chronic kidney disease for non-nephrologists. Clin Kidney J 2019;12:258–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jager KJ, Kovesdy C, Langham Ret al. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Nephrol Dial Transplant 2019;34:1803–5. [DOI] [PubMed] [Google Scholar]
- 4. GBD Chronic Kidney Disease Collaboration . Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet 2020;395:709–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Foreman KJ, Marquez N, Dolgert Aet al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet 2018;392:2052–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shin JI, Chang AR, Grams MEet al. Gansevoort RT for the CKD Prognosis Consortium . Albuminuria testing in hypertension and diabetes: an individual-participant data meta-analysis in a global consortium. Hypertension 2021;78:1042–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ortiz A, Sanchez-Niño MD, Crespo-Barrio Met al. The Spanish society of nephrology (SENEFRO) commentary to the Spain GBD 2016 report: keeping chronic kidney disease out of sight of health authorities will only magnify the problem. Nefrologia 2019;39:29–34. [DOI] [PubMed] [Google Scholar]
- 8. Bosi A, Xu Y, Gasparini Aet al. Use of nephrotoxic medications in adults with chronic kidney disease: parallel cohort studies in Swedish and U.S. Routine care. Clin Kidney J 2021;15:442–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang HY, Ding GH, Lin Het al. Influence of doctors’ perception on the diagnostic status of chronic kidney disease: results from 976 409 individuals with electronic health records in China. Clin Kidney J 2021;14:2428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Visseren FLJ, Mach F, Smulders YMet al. ; ESC Scientific Document Group . 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur J Prev Cardiol 2022;29:5–115. [DOI] [PubMed] [Google Scholar]
- 11. Authors/Task Force Members , Piepoli MF, Hoes AW, Agewall Set al.2016 European guidelines on cardiovascular disease prevention in clinical practice: the sixth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts): developed with the special contribution of the European association for cardiovascular prevention & rehabilitation (EACPR). Eur J Prev Cardiol 2016;23:1–96. [DOI] [PubMed] [Google Scholar]
- 12. Heerspink HJL, Stefansson BV, Correa-Rotter Ret al. , DAPA-CKD Trial Committees and Investigators . Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–46. [DOI] [PubMed] [Google Scholar]
- 13. https://www.medscape.com/viewarticle/970434 . [Google Scholar]
- 14. Senore C, Basu P, Anttila Aet al. Performance of colorectal cancer screening in the European union member states: data from the second European screening report. Gut 2019;68:1232–44. [DOI] [PubMed] [Google Scholar]
- 15. Crews DC, Boulware LE, Gansevoort RTet al. Albuminuria: is it time to screen the general population? Adv Chronic Kidney Dis 2011;18:249–57. [DOI] [PubMed] [Google Scholar]
- 16. Matsushita K, Jassal SK, Sang Yet al. Incorporating kidney disease measures into cardiovascular risk prediction: development and validation in 9 million adults from 72 datasets. EClinicalMedicine 2020;27:100552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Matsushita K, Kaptoge S, Hageman SHJet al. Adding albuminuria and eGFR to the SCORE2 algorithms to improve CVD risk prediction in subjects with chronic kidney disease. Eur J Prev Cardiol 2022. doi: 10.1093/eurjpc/zwac176 [Google Scholar]
- 18. Pitt B, Filippatos G, Agarwal Ret al. FIGARO-DKD Investigators . Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med 2021;385:2252–63. [DOI] [PubMed] [Google Scholar]
- 19. Bakris GL, Agarwal R, Anker SDet al. FIDELIO-DKD Investigators . Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020;383:2219–29. [DOI] [PubMed] [Google Scholar]
- 20. Perkovic V, Jardine MJ, Neal Bet al. CREDENCE Trial Investigators . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–306. [DOI] [PubMed] [Google Scholar]
- 21. Sarafidis P, Ferro CJ, Morales Eet al. SGLT-2 inhibitors and GLP-1 receptor agonists for nephroprotection and cardioprotection in patients with diabetes mellitus and chronic kidney disease. A consensus statement by the EURECA-m and the DIABESITY working groups of the ERA-EDTA. Nephrol Dial Transplant 2019;34:208–30. [DOI] [PubMed] [Google Scholar]
- 22. Zoccali C, Blankestijn PJ, Bruchfeld Aet al. Children of a lesser god: exclusion of chronic kidney disease patients from clinical trials. Nephrol Dial Transplant 2019;34:1112–4. [DOI] [PubMed] [Google Scholar]
- 23. https://www.era-online.org/en/strongkidneys/european-awareness-campaign/ideasforactivities/ . [Google Scholar]
- 24. https://ClinicalTrials.gov/ct2/show/NCT04295889 . [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.