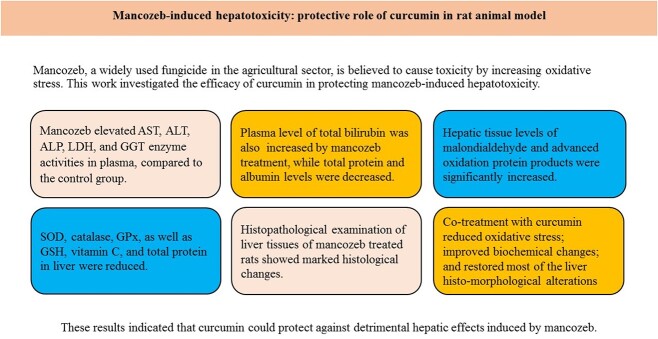
Abstract
Background
Mancozeb—a widely used fungicide in the agricultural sector—is believed to cause toxicity by increasing oxidative stress. This work investigated the efficacy of curcumin in protecting mancozeb-induced hepatotoxicity.
Materials and Methods
Mature Wistar rats were assigned into 4 equal groups: control, mancozeb (30 mg/kg/day, ip), curcumin (100 mg/kg/day, po), and mancozeb+curcumin. The experiment lasted for 10 days.
Results
Our results reported that mancozeb elevated aspartate transaminase, alanine transaminase, alkaline phosphatase, lactate dehydrogenase, gamma glutamyltranspeptidase enzyme activities, and total bilirubin level in plasma; and decreased total protein and albumin levels, compared with the control group (P < 0.05–0.001). Hepatic tissue levels of malondialdehyde, and advanced oxidation protein products were significantly increased; whereas activities of superoxide dismutase, catalase, glutathione peroxidase, as well as levels of reduced glutathione, vitamin C, and total protein were reduced (P < 0.05–0.001). Histopathological examination showed marked histological changes. Co-treatment with curcumin improved the antioxidant activity; reversed oxidative stress and biochemical changes; and restored most of the liver histo-morphological alterations; thus, attenuating the hepatic toxicities induced by mancozeb.
Conclusion
These results indicated that curcumin could protect against detrimental hepatic effects induced by mancozeb.
Keywords: curcumin, dithiocarbamates, hepatotoxicity, oxidative stress, antioxidants
Graphical abstract
Introduction
Curcumin (turmeric) is a popular spice derived from the underground stems or rhizomes of the plant, Curcuma longa Linn., which belongs to the ginger family, Zingiberaceae. The plant is indigenous to Southeast Asia and is predominantly cultivated in the tropical and subtropical countries. It is extensively grown commercially in India, which is the largest producer and exporter of curcuma globally.1 The active constituents of curcuma are known as curcuminoids, and curcumin is the principal component. Curcumin is a polyphenol compound (Fig. 1), and contributes to most of the activities of curcuma.2
Fig. 1.
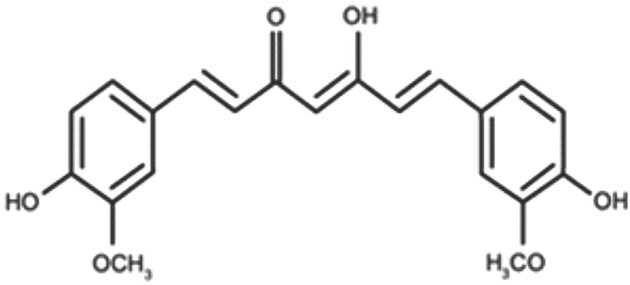
Chemical structure of curcumin.
Curcumin has great relevance in ethnomedicine. The plant has been widely used in Indian, Chinese, and other eastern Asian traditional medicine over a long period of time. It is locally prescribed to treat different disorders that affect the joints, skin, upper respiratory tract, digestive system, liver, and kidney.3 Animal studies have confirmed many of the ethnomedicinal applications of curcumin. Such studies have demonstrated the anti-inflammatory, wound healing, immunomodulatory, and analgesic effects, as well as its beneficial role in chronic renal failure, and degenerative diseases.4–6
Mancozeb is a fungicide belonging to the ethylene bis dithiocarbamate group. It is used on different agricultural products like crops, fruits, vegetables, nuts, etc. against fungal diseases.7 Mancozeb is one of the most effective and widely used pesticides in agriculture, it inactivates the sulfhydryl groups in amino acids and enzymes within fungal cells, and typically cause disruption of metabolic processes, and mitochondrial function.8 Because the fungicide requires repeated application for good protection of plant products, its potential for human and animal exposure is high.7 Besides accidental consumption of the fungicide, humans can be exposed to it via contaminated agricultural products, contaminated water, or food chain. Hazardous effects from consumption of mancozeb-contaminated agriculture products have been well documented in humans, including neurotoxicity, genotoxicity, and hematotoxic effects.9–11 Furthermore, mancozeb has been reported to cause liver toxicity in animals.12,13 Mancozeb is structurally related to maneb and zineb as they all belong to the ethylene bis dithiocarbamate group. Maneb contains manganese linked with ethylene bis dithiocarbamate units, zineb has zinc linked with ethylene bis dithiocarbamate units, whereas mancozeb is a manganese/zinc ethylene bis dithiocarbamate (i.e. made up of a mixture of maneb and zineb). Several studies have indicated that dithiocarbamate fungicides induce oxidative stress to cause damage to animal tissues and organs, including the liver,14,15 and mancozeb and maneb are known to exhibit similar toxicity profile.16,17
It is logical to consider that compounds with antioxidant properties may be useful to mitigate oxidative tissue damages induced by carbamate fungicides. Curcumin possesses potent antioxidant properties, which has been well documented.2,18,19 A recent systematic review and meta-analysis reported that curcuminoids supplementation would increase activities of superoxide dismutase (SOD) and catalase (CAT), as well as concentrations of glutathione peroxidase (GPx) and lipid peroxides in plasma or serum.20 Also, curcumin administration has been shown to attenuate drugs and chemicals induced oxidative stress in various organs in animals.20,21 To our knowledge, the effectiveness of curcumin to attenuate mancozeb toxicity in the liver has been sparsely studied. Thus, the present study investigated the hepatoprotective ability of curcumin by analyzing the oxidative and antioxidative biomarkers against mancozeb-induced hepatotoxicity effects in adult Wistar rats.
Materials and methods
Experimental animals
The study was performed on 24 healthy male Wistar rats aged ~12 weeks, weighing 180–200 g. Animals were obtained from the Animal House of our institution, housed in metal cages, and maintained under the same environmental conditions (12-h light and dark cycle, temperature of 27 ± 5 °C, fed with standard pellet diet, free access to water). Animals were handled in accordance with the principles of laboratory animal care,22 and all experimental procedures were approved by our institutional Research Ethics Committee (UPH/CHREC/APP/032/2019).
Experimental design
The rats were randomly distributed into 4 groups (n = 6 per group). Group I (control) animals, received dimethyl sulphoxide, DMSO (TNS Corporations, Mumbai, India) 50% v/v, 0.4 mL/day, intraperitoneally, ip. DMSO was used as solvent for curcumin powder. Group II received mancozeb (Sabero Organic Gujarat Ltd, Mumbai, India), 30 mg/kg/day, ip. Group III received curcumin (Puritans Pride Inc., Oakdale, NY 11769, United States), 100 mg/kg/day, by oral gavage. Group IV received mancozeb (30 mg/kg/day, ip) + curcumin (100 mg/kg/day, po). Administration was done for 10 days in all groups.
The dose used for mancozeb 30 mg/kg was chosen based on the earlier studies of Sefi et al.15 and Gautam and Kapoor23 who respectively reported that maneb and mancozeb caused organ toxicity at this dose in mice. We have also used in our preliminary experiment the dose of 30-mg/kg mancozeb with same route and duration of exposure and demonstrated hepatoxicity in Wistar rats. The given dose of curcumin (100 mg/kg) was also selected based on previous studies that confirmed curcumin is effective in protecting tissue damage at this dose.24–26 Moreover, Tung et al.25 have shown that 10-day administration of curcumin at the dose of 100 mg/kg and same oral route protected paracetamol-induced hepatoxicity in mice.
At the end of the treatment period (~24 h after administering the last dose), all rats were weighed and sacrificed under anesthesia. Blood was collected and transferred into heparinized tubes. Blood sample was centrifuged thereafter at 3,000 rpm for 15 min and plasma was stored at −80 °C for analysis of liver enzyme activities, and other biochemical parameters of liver function. The liver was carefully dissected out from each rat, weighed, and organ body weight ratio was determined. Livers were trimmed of fat prior to recording their weights. A part of the liver was then rinsed in ice cold 1.15% KCl solution, and homogenized in phosphate buffer (0.1 M PBS; pH 7.4). The homogenates were centrifuged and supernatants were stored at −80 °C to estimate the tissue levels of oxidative biomarkers. The remaining part was fixed in 10% neutral buffered formalin for histopathological study.
Liver biochemical parameters in plasma
Plasma activities of liver transaminase enzymes (alanine transaminase, ALT and aspartate transaminase, AST), alkaline phosphatase (ALP), gamma glutamyl transpeptidase (GGT), lactate dehydrogenase (LDH), and levels of total protein, albumin, and total bilirubin were assayed spectrophotometrically using commercially available diagnostic kits (Randox Laboratory Kits, United Kingdom) following previously described standard procedures.27–30
Oxidative biomarkers in liver homogenates
MDA, AOPP, and total protein
Malondialdehyde (MDA) content in liver was measured spectrophotometrically at 532 nm according to the method of Buege and Aust.31 Liver levels of advanced oxidation protein products (AOPP) were measured using the method described by Witko-Sarsat et al.32 Total protein content in liver homogenates was estimated according to the method of Lowry et al.27
Nonenzymatic antioxidants (GSH and vitamin C)
Reduced glutathione (GSH) content in liver homogenates was quantified at 412 nm according to the method described by Brehe and Burch.33 Liver ascorbic acid (vitamin C) content was determined using the dinitrophenylhydrazine method described by Angirekula et al.34
Enzymatic antioxidants (CAT, SOD, and GPx)
CAT activity in liver tissue was assayed colorimetrically at 480 nm and expressed as units per millilitre, using the method of Aebi.35 SOD activity was estimated at 480 nm using the method of Sun and Zigman.36 Glutathione peroxidase (GPx) activity was measured according to the method described by Flohe and Gunzler.37
Histological study
The liver tissues were removed from the 10% buffered formalin solution, washed, and dehydrated through series of graded ethanol solutions. They were then embedded in paraffin, blocked out, and thereafter sectioned at a thickness of 5 μm. The tissues were then stained with hematoxylin and eosin (H&E) and viewed under the light microscope. All alterations of histological features from normal were noted and photographed.
Statistical analysis
The results were analyzed by 1-way analysis of variance and Newman–Keuls test using the GraphPad Prism 5 Software (GraphPad Software Inc., San Diego, CA, United States), and values were expressed as means + SEM. P-values < 0.05 were considered significant.
Results
Liver weight
Absolute and relative weight of the liver were significantly increased (P < 0.01) in mancozeb-exposed rats by 30% and 24%, respectively compared with the control. In addition, liver weight (absolute or relative) in mancozeb + curcumin treated rats was lower (P < 0.05) than that of only mancozeb intoxicated rats. There was no significant change in absolute or relative liver weight of mancozeb + curcumin treated rats compared to the control. Furthermore, there were no differences between control and curcumin administered rats (Table 1).
Table 1.
Absolute and relative liver weights in control and experimental rats after 10 days treatment with mancozeb, mancozeb combined with curcumin, and curcumin alone.
| Parameters/treatments | Absolute liver weight (g) | Relative liver weight (g × 100) |
|---|---|---|
| Control | 6.82 ± 0.18 | 3.10 ± 0.14 |
| MZ | 8.89 ± 0.54a | 3.96 ± 0.20a |
| MZ + Cur | 7.23 ± 0.22b | 3.28 ± 0.13b |
| Cur | 6.77 ± 0.34 | 3.17 ± 0.09 |
Values are means ± SEM for 6 rats per group.
MZ, mancozeb; Cur, curcumin.
aDenotes P < 0.01: MZ versus control.
bDenotes P < 0.05: (MZ + Cur) versus MZ.
Liver biochemical parameters in plasma
ALT, AST, and ALP activities in the plasma of mancozeb-treated rats increased when compared with the control rats (P < 0.01, P < 0.05, and P < 0.001) as presented in Table 2. The activities of GGT and LDH were also increased (P < 0.001 and P < 0.01) in mancozeb-treated rats. In addition, significant increase in total bilirubin and reductions (P < 0.05) in total protein and albumin levels were observed in mancozeb-treated rats, compared with control (Table 2). The activities of ALT and ALP in the rats that received mancozeb together with curcumin were reduced (P < 0.01) as compared to rats treated with mancozeb alone. GGT and LDH activities in mancozeb + curcumin treated rats were also reduced as well as total bilirubin level, whereas total protein and albumin levels were increased (P < 0.05) compared with mancozeb group (Table 2). The plasma activities or levels of the above parameters in the mancozeb + curcumin treated rats did not significantly differ from control or curcumin groups. Furthermore, there were no differences between control and curcumin treated rats (Table 2).
Table 2.
Plasma activities of ALT, AST, ALP, LDH, GGT, and levels of total protein, albumin, and total bilirubin in control and experimental rats after 10 days treatment with mancozeb, mancozeb combined with curcumin, and curcumin alone.
| Parameters/treatments | Control | MZ | MZ + Cur | Cur |
|---|---|---|---|---|
| ALT (IU/L) | 23.67 ± 1.67 | 41.50 ± 3.66b | 23.00 ± 3.51e | 23.75 ± 1.75 |
| AST (IU/L) | 46.33 ± 10.73 | 92.33 ± 1.67a | 52.33 ± 10.91 | 53.00 ± 7.88 |
| ALP (IU/L) | 92.40 ± 8.07 | 216.30 ± 8.74c | 139.00 ± 22.87e | 89.00 ± 9.60 |
| LDH (IU/L) | 32.83 ± 3.11 | 49.00 ± 2.89b | 36.25 ± 1.80d | 34.07 ± 1.68 |
| GGT (IU/L) | 10.25 ± 0.72 | 24.75 ± 2.66c | 11.74 ± 0.78f | 11.33 ± 0.88 |
| Total protein (g/L) | 88.00 ± 2.65 | 68.75 ± 3.12a | 83.25 ± 2.84d | 84.25 ± 4.73 |
| Albumin (g/L) | 40.67 ± 2.03 | 33.33 ± 0.88a | 39.67 ± 0.88d | 40.00 ± 0.58 |
| Total bilirubin (μmol/L) | 6.33 ± 1.20 | 13.00 ± 0.41a | 6.25 ± 1.65d | 6.33 ± 1.45 |
Values are means ± SEM for 6 rats per group.
Abbreviations: MZ, mancozeb; Cur, curcumin; ALT, alanine transaminase; AST, aspartate transaminase; ALP, alkaline phosphatase; LDH, lactate dehydrogenase; and GGT, gamma glutamyl transpepdidase.
aDenotes P < 0.05: MZ versus control.
bDenotes P < 0.01: MZ versus control.
cDenotes P < 0.001: MZ versus control.
dDenotes P < 0.05: (MZ + Cur) versus MZ.
eDenotes P < 0.01: (MZ + Cur) versus MZ.
fDenotes P < 0.001: (MZ + Cur) versus MZ.
Oxidative biomarkers in liver tissue homogenates
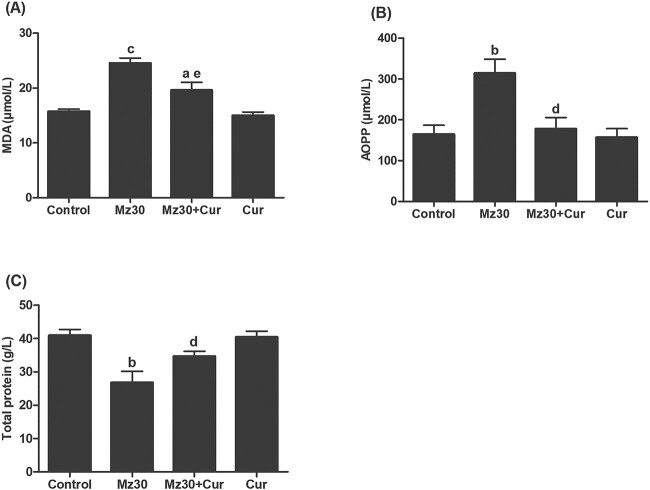
The levels of lipid peroxidation, protein oxidation, and protein content in the livers of control and experimental rats are illustrated in Fig. 2A–C. Lipid peroxidation product, malondialdehyde (MDA), and AOPP concentrations in liver tissue of mancozeb injected rats significantly increased (P < 0.001, P < 0.01), whereas total protein content decreased (P < 0.01) compared with control rats. In mancozeb + curcumin treated rats, MDA and AOPP levels were significantly decreased (P < 0.01, P < 0.05), and total protein was increased (P < 0.05) compared with rats treated with only mancozeb. In addition, the obtained values of these parameters were not significantly different when compared with the control or curcumin group.
Fig. 2.
Liver tissue homogenate levels of A) MDA, B) AOPP, and C) total protein in control and experimental rats after 10 days treatment with mancozeb, mancozeb combined with curcumin, and curcumin alone. Values are means ± SEM for 6 rats per group. aDenotes P < 0.05: (MZ + Cur) versus control; bdenotes P < 0.01: MZ versus control; cdenotes P < 0.001: MZ versus control; ddenotes P < 0.05: (MZ + Cur) versus MZ; edenotes P < 0.01: (MZ + Cur) versus MZ. Abbreviations: MZ, mancozeb; Cur, curcumin; MDA, malondialdehyde; AOPP, advanced oxidation protein product.
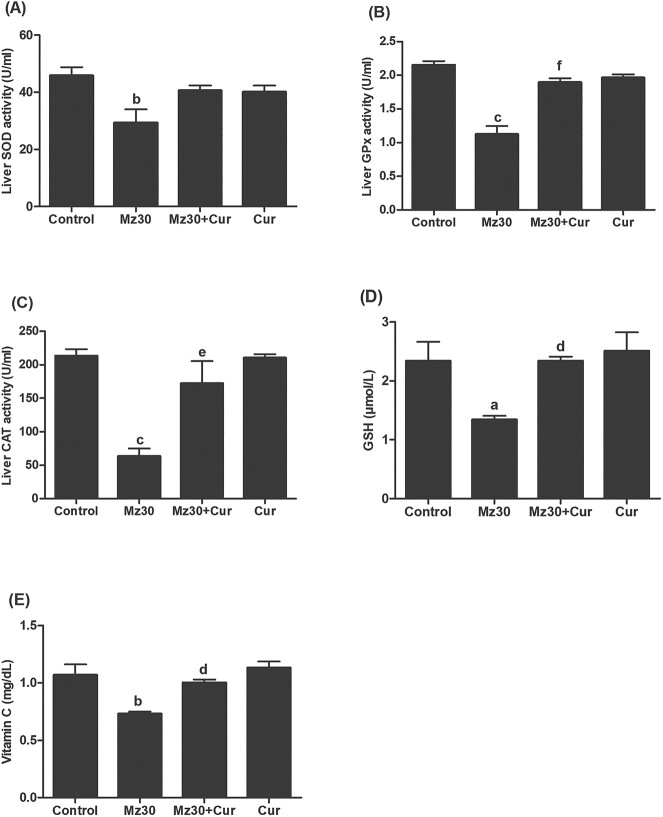
Furthermore, the activities of enzymatic antioxidants-SOD, GPx, and CAT, and nonenzymatic antioxidants (reduced glutathione and vitamin C) in the liver of control and experimental rats are illustrated in Fig 3A–C. SOD, GPx, and CAT activities in the liver of mancozeb-treated rats significantly decreased (P < 0.01, P < 0.001, and P < 0.001) compared with those of controls. The enzymes activities were increased in rats that received co-treatment of mancozeb with curcumin compared with the rats that received only mancozeb. The values obtained in the mancozeb + curcumin treated rats were not significantly different compared with the controls. In addition, the levels of reduced glutathione (GSH), and vitamin C were significantly decreased (P < 0.05 and P < 0.01) in mancozeb-treated rats as compared to those of the control rats (Fig. 3D and E). The levels of these nonenzymatic antioxidants were observed to be higher (P < 0.05) in rats that received mancozeb and curcumin together when compared with those that received only mancozeb, and the concentrations were comparable with control values. There were no differences in all the above mentioned parameters between control and curcumin administered rats.
Fig. 3.
Antioxidant activities and concentrations (A—SOD, B—GPx, C—CAT, D—GSH, and E—vitamin C) in the livers of control and experimental rats after 10 days treatment with mancozeb, mancozeb combined with curcumin, and curcumin alone. Values are means ± SEM for 6 rats per group. aDenotes P < 0.05: MZ versus control; bdenotes P < 0.01: MZ versus control; cdenotes P < 0.001: MZ versus control; ddenotes P < 0.05: (MZ + Cur) versus MZ; edenotes P < 0.01: (MZ + Cur) versus MZ; fdenotes P < 0.01: (MZ + Cur) versus MZ. Abbreviations: MZ, mancozeb; Cur, curcumin; SOD, superoxide dismutase; GPx, glutathione peroxidase; CAT, catalase.
Histopathological findings
Evaluation of liver sections from control and curcumin rats showed normal histo-architecture without any significant alteration (Fig. 4A and D). Histopathological changes in the form of vascular congestion, periportal inflammation, and presence of pyknotic nuclei were observed in rats that received mancozeb (Fig. 4B). Liver sections from rats treated with mancozeb and curcumin concurrently showed mild periportal inflammation (Fig. 4C).
Fig. 4.
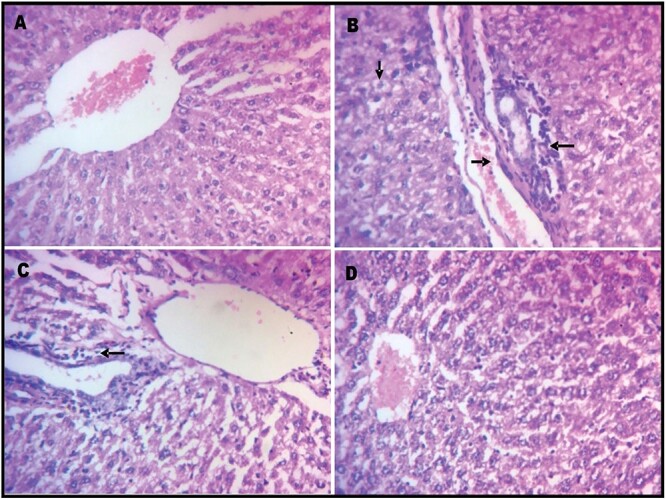
Liver photomicrographs of H&E sections of Wistar rats treated with mancozeb (30 mg/kg, ip) with or without curcumin (100 mg/kg, po) for 10 days, magnification: 400×. A) control group, B) group treated with mancozeb, C) mancozeb co-treated with curcumin group, and D) group treated with curcumin alone. Tissues stained with hematoxylin and eosin. Arrows indicate pathomorphological changes: vascular congestion (left arrow), pyknotic nuclei (down arrow), and periportal inflammation (right arrow).
Discussion
Mancozeb is widely used to control pests in agriculture, and its exposure to humans is common and has been associated with harmful effects.7 The protective role of curcumin in mancozeb-induced hepatotoxicity was examined in Wistar rats in this study. Curcumin, a polyphenolic flavoring agent, is an established antioxidant and has been reported to have protective effect against organ damage by different toxicants.20,21,38 This study reports the cytoprotective capacity of curcumin against mancozeb-induced alterations of biochemical profiles and liver injury in Wistar rats. Hepatoprotective effect of curcumin has been reported in an earlier study using higher oral dose level of 750mg/kg mancozeb,39 but the present study is more focused on the antioxidant capacity of curcumin.
The liver plays central role in detoxification of xenobiotics, which also makes the organ to be highly prone to injury. In a number of studies, pesticides have caused derangement of biochemical parameters and liver injury.15,40 From the results of the current study, 10 days exposure of mancozeb caused increase in the absolute and relative weight of treated rats, indicative of organ toxicity.41 This increase in liver weight that occurred after mancozeb exposure, could be as a result of the hepatic inflammation caused by mancozeb, as confirmed by our histological findings. Sefi et al.15 obtained similar result, where they observed increase in liver weight in mice after intraperitoneal exposure of maneb (which has similar toxicological profile as mancozeb) at same dose and duration that were used in the current study. Our result was also in agreement with the studies of Yahia et al.42 who reported increase in liver weight of Wistar rats treated orally for 8 weeks with 1,000mg/kg mancozeb, and Kacker et al.12 who reported that relative weight of liver increased in rats orally exposed to 1,000 and 1,500mg/kg mancozeb for 45 and 90 weeks with pathomorphological changes. Interestingly, the alteration in liver weight was prevented when mancozeb was administered together with curcumin in this study, demonstrating hepatoprotection.
In our present study, the histopathological changes observed in liver of mancozeb-exposed rats could have resulted from increased oxidative stress in this organ. Lipid peroxidation represents one of the most frequent reactions resulting from free radicals’ attack on biomolecules. In fact, different mechanisms have been suggested to explain pesticides-induced liver oxidative injury, including lipid peroxidation and damage to cell components and membrane integrity.43,44 From our results, the liver malondialdehyde (MDA) level, index of lipid peroxidation, increased in the mancozeb-treated rats. In addition, elevation in liver advanced oxidative protein products (AOPP) level was induced by mancozeb, suggesting breakdown of cellular proteins. This was reflected in the reduced liver protein content that was observed in the mancozeb-treated rats. These findings are supported by the results of Bailey et al.14 who reported that treatment of Caenorhabditis elegans with mancozeb resulted in increased reactive oxygen species (ROS) concentrations, including hydrogen peroxide (H2O2). Supplementation with curcumin ameliorated the increase in lipid peroxidation and protein oxidation levels. We suggest that the mechanism of this protection could be the inhibition of ROS generation by suppressing NADPH oxidase, which enhance H2O2 generation by mancozeb.45 The curcumin-induced cytoprotection and hepatoprotective effects observed in our study might also be ascribed to its direct antioxidant and free radical scavenging activity. This is corroborated by the findings of Messarah et al.46 who reported that curcumin inhibited oxidative damage (lipid peroxidation) of erythrocytes in rats induced by diazinon. Similar results have also been reported by Saber et al.39
Furthermore, we observed that administration of curcumin improved the reduction in vitamin C levels that was caused by mancozeb treatment. There are not available reports concerning vitamin C concentrations during mancozeb-induced organ toxicity. However, a previous study had reported a decrease in ascorbic acid levels in liver of mice treated with maneb, which corroborates our result.15 Vitamin C—an efficient scavenger of ROS—is a potent antioxidant. The antioxidant role of vitamin C is based on its reaction with peroxyl radicals in the aqueous phase, forming ascorbyl radical. Peroxyl radicals readily initiate peroxidation, so by trapping this radical, vitamin C protects biomembranes and lipoproteins from oxidative damage.47 Therefore, reduction in vitamin C levels could be a major contributing factor for increased production of lipid peroxidation products, like MDA and lipid hydroperoxides (LOOHs). In the present investigation, the decrease in vitamin C that was observed in the liver of mancozeb-treated rats may be indicative of increased oxidative stress, and resultant damage of the liver plasma membrane lipid bilayer arising from mancozeb intoxication. So, improvement of vitamin C levels by curcumin effectively protected mancozeb against generation of free radicals and lipid peroxidation in liver tissues animals that received mancozeb + curcumin. This finding is similar to the results reported by Sefi et al.15 who indicated attenuation of maneb-induced decrease in vitamin C level in the liver when vanillin, a phenolic compound was concurrently administered with maneb for 10 days in mice. Furthermore, GSH is the main thiol antioxidant that preserves and protects proteins against ROS, reactive nitrogen species (RNS), and electrophiles.48 GSH concentrations in treated Wistar rats decreased after exposure to mancozeb. This corroborated the findings of Grosicka-Maciag et al.49 who showed that GSH concentrations decreased in Chinese hamster V79 cells after maneb treatment. The depletion of GSH is suggestive of a deleterious effect of mancozeb on the antioxidant defense in the liver of the rats, and consistent with the generation of oxidative stress. This could also explain the elevated level of protein oxidation products and reduced protein content that was observed in the liver of mancozeb intoxicated rats. The present study showed that co-treatment of mancozeb with curcumin improved the GSH levels in the animals.
The activities of SOD, CAT, and GPx in the liver were also evaluated to assess the effect of mancozeb treatment on the hepatic enzymatic antioxidant status in the present work. Mancozeb exposure resulted in decrease in all the 3 antioxidant enzymes activities. SOD catalyzes the primary step of the defense mechanism in the antioxidant system against oxidative stress by catalyzing the dismutation of superoxide anion (O2−) to form H2O2. In all vertebrates, CAT and GPx act in a coordinated manner to neutralize the H2O2 that is produced.50,51 In the present study, the decrease of SOD activity observed in mancozeb group suggest an increased superoxide radical production and other ROS, which will result in oxidative damage. More so, the decreased CAT activity in mancozeb-treated rats indicated the presence of superoxide radical. This is because poor neutralization of H2O2 as a result of diminished CAT activity would increase inactivation of SOD by H2O2, whereas rapid inactivation of CAT would occur when SOD activity is low in the system. Our results were in agreement with the studies of Sefi et al.15 and Amara et al.52 who have observed a decrease in the activities of SOD, CAT, and GPx in the liver of adult mice exposed to maneb. The significant recovery of hepatic antioxidant enzyme activities by curcumin suggested its chemopreventive effect against mancozeb mediated hepatic injury. Besides increase in the activities of SOD, CAT, and GPx, the improvement of GSH, and vitamin C levels after curcumin treatment in the current study, demonstrated the antioxidant capacity and beneficial role of this polyphenolic flavor compound. This was not surprising although, as the antioxidant activity of curcumin and other structurally similar natural compounds have been well documented.6,53
Sufficient data have indicated that one of the cellular antioxidant mechanisms of curcumin is activation of nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway, which results in enhancement of nuclear translocation of Nrf2.54,55 The cellular mechanism underlying the antioxidant effect of curcumin may also involve its protective role of dysregulation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway as reported in earlier studies.56,57 Furthermore, curcumin is known to have potent anti-inflammatory property, which it can exhibit also via modulation of NF-κB mediated inflammatory signaling pathway.58,59 Some examples of hepatoprotective actions of curcumin mediated via Nrf2 are; prevention of lipid deposition in hepatocytes in ethanol-induced hepatic steatosis, improvement of renal autoghagy in experimental membranous nephropathy, etc.60,61 Similarly, examples of the anti-inflammatory properties of curcumin mediated via NF-κB include, down regulation of pro-inflammatory cytokines such as interleukin (IL)-1, IL-2, IL-6, IL-8, and tumor necrosis factor (TNF)-α.62 These actions contribute to its usefulness in the treatment of neurodegenerative diseases (e.g. Alzheimer’s disease) and other inflammatory disorders like ulcerative colitis.62,63
From the biochemical analysis data, AST, ALT, and ALP activities in the plasma of mancozeb-treated rats were increased, which may be as a consequence of loss of hepatocyte membrane integrity. Similarly, LDH, another biomarker of liver toxicity, was elevated in plasma by mancozeb. This is in agreement with the findings of Yahia et al.42 who have reported that this enzyme increased in serum of mancozeb-treated rats. The elevation of these enzymes could potentially be attributed to the hepatotoxicity of mancozeb, leading to an increase in permeability of liver membrane and a leakage of the enzymes into blood.64 In fact, changes in transaminase activities (AST and ALT) have long been associated with liver pathology.65,66 GGT is also a well-established serum marker for liver pathology.67 This enzyme is primarily essential for the metabolism of glutathione and glutathionylated xenobiotics. However, elevated GGT levels may contribute to prooxidant activity, which can eventually lead to damage to different cell types, tissues and DNA caused by oxidative and nitrosative stress and the generation of deleterious ROS or RNS.67,68 Elevation in the activity of GGT in the plasma of mancozeb-treated rats, added to the other biochemical changes in this study affirms the hepatotoxic potential of the fungicide. Aberkane et al.69 reported that GGT elevation can cause damage of red blood cell membranes and cause the release of transition metals like iron, which can trigger chain, prooxidant reactions. This finding supports the ROS generation capacity of mancozeb and its ability to cause tissue damage. In addition, the increased level of total bilirubin content and decrease of total protein in the plasma of mancozeb-treated rats confirms the potential of the fungicide to cause damage and functional impairment of the hepatocytes. Supplementation with curcumin effectively prevented oxidative damages induced by mancozeb as demonstrated by the lower AST, ALT, AST, LDH, GGT, and total bilirubin concentrations compared to those of only mancozeb-treated rats.
Conclusion
The data obtained from the present study suggested that curcumin can effectively protect against mancozeb mediated hepatic abnormalities in rats by exerting antioxidant activity and reducing oxidative stress. Curcumin reduced or normalized raised levels of hepatic plasma markers (ALT, AST, ALP, LDH, GGT, and total bilirubin), the lipid peroxidation marker (MDA), and protein oxidation marker (AOPP). Curcumin supplementation additionally improved the hepatic levels of GSH, and vitamin C, as well as the activities of SOD, CAT, and GPx. However, more sensitive tests like western blot or real time PCR analysis may be necessary in future studies to confirm these effects.
Acknowledgments
The authors are grateful to Dr Sampson A. Oyebadejo for his skilful assistance in the histological analysis.
Contributor Information
Jonah Sydney Aprioku, Department of Experimental Pharmacology and Toxicology, Faculty of Pharmaceutical Sciences, University of Port Harcourt, East-West Road, Choba, Rivers State, PMB 5323, Nigeria.
Ayanabia Monica Amamina, Department of Experimental Pharmacology and Toxicology, Faculty of Pharmaceutical Sciences, University of Port Harcourt, East-West Road, Choba, Rivers State, PMB 5323, Nigeria.
Perpetua Amarachi Nnabuenyi, Department of Experimental Pharmacology and Toxicology, Faculty of Pharmaceutical Sciences, University of Port Harcourt, East-West Road, Choba, Rivers State, PMB 5323, Nigeria.
Authors’ contributions
JSA conceived and designed the study. AMA and PAN managed the literature searches. All authors contributed in conduction of the experiments and collection of the data. JSA handled the analysis of the data, and wrote the first draft of the manuscript. All authors approved the final manuscript.
Funding
None declared.
Conflicts of interest. There are no conflicts of interest to declare.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1. Verma S, Singh S, Sharma S, Tewari SK, Roy RK, Goel AK, Rana TS. Assessment of genetic diversity in indigenous turmeric (Curcuma longa) germplasm from India using molecular markers. Physiol Mol Biol Plants. 2015:21(2):233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kocaadam B, Şanlier N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit Rev Food Sci Nutr. 2017:57(13):2889–2895. [DOI] [PubMed] [Google Scholar]
- 3. Soleimani V, Sahebkar A, Hosseinzadeh H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: review. Phytother Res. 2018:32(6):985–995. [DOI] [PubMed] [Google Scholar]
- 4. Araújo CC, Leon LL. Biological activities of Curcuma longa L. Mem Inst Oswaldo Cruz. 2001:96(5):723–728. [DOI] [PubMed] [Google Scholar]
- 5. Labban L. Medicinal and pharmacological properties of turmeric (Curcuma longa): a review. Int J Pharm Biomed Sci. 2014:5:17–23. [Google Scholar]
- 6. Boroumand N, Samarghandian S, Hashemy SI. Immunomodulatory, anti-inflammatory, and antioxidant effects of curcumin. J HerbMed Pharmacol. 2018:7(4):211–219. [Google Scholar]
- 7. MacBean C. The pesticide manual [OP]: a world compendium. 16th ed. England: CABI; 2013. [Google Scholar]
- 8. Yang C, Hamel C, Vujanovic V, Gan Y. Fungicide: modes of action and possible impact on nontarget microorganisms. Int Sch Res Notices. 2011:2011:8–8. [Google Scholar]
- 9. Aroonvilairat S, Kespichayawattana W, Sornprachum T, Chaisuriya P, Siwadune T, Ratanabanangkoon K. Effect of pesticide exposure on immunological, hematological and biochemical parameters in Thai orchid farmers- a cross-sectional study. Int J Environ Res Public Health. 2015:12(6):5846–5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Srivastava AK, Mishra S, Ali W, Shukla Y. Protective effects of lupeol against mancozeb-induced genotoxicity in cultured human lymphocytes. Phytomedicine. 2016:23(7):714–724. [DOI] [PubMed] [Google Scholar]
- 11. Morales-Ovalles Y, Miranda-Contreras L, Peña-Contreras Z, Dávila-Vera D, Balza-Quintero A, Sánchez-Gil B, Mendoza-Briceño RV. Developmental exposure to mancozeb induced neurochemical and morphological alterations in adult male mouse hypothalamus. Environ Toxicol Pharmacol. 2018:64:139–146. [DOI] [PubMed] [Google Scholar]
- 12. Kackar R, Srivastava MK, Raizada RB. Assessment of toxicological effects of mancozeb in male rats after chronic exposure. Indian J Exp Biol. 1999:37(6):553–559. [PubMed] [Google Scholar]
- 13. Yahia D, El-Amir YO, Rushdi M. Mancozeb fungicide-induced genotoxic effects, metabolic alterations, and histological changes in the colon and liver of Sprague Dawley rats. Toxicol Ind Health. 2019:35(4):265–276. [DOI] [PubMed] [Google Scholar]
- 14. Bailey DC, Todt CE, Orfield SE, Denney RD, Snapp IB, Negga R, Montgomery KM, Bailey AC, Pressley AS, Traynor WL, et al. Caenorhabditis elegans chronically exposed to a Mn/Zn ethylenebis-dithiocarbamate fungicide show mitochondrial complex I inhibition and increased reactive oxygen species. Neurotoxicology. 2016:56:170–179. [DOI] [PubMed] [Google Scholar]
- 15. Sefi M, Elwej A, Chaâbane M, Bejaoui S, Marrekchi R, Jamoussi K, Gouiaa N, Boudawara-Sellemi T, El Cafsi M, Zeghal Net al. Beneficial role of vanillin, a polyphenolic flavoring agent, on maneb-induced oxidative stress, DNA damage, and liver histological changes in Swiss albino mice. Hum Exp Toxicol. 2019:38(6):619–631. [DOI] [PubMed] [Google Scholar]
- 16. Morgan DP. Recognition and management of pesticide poisonings. 3rd ed. Washington DC, US: Environmental Protection Agency; 1982 [Google Scholar]
- 17. Domico LM, Zeevalk GD, Bernard LP, Cooper KR. Acute neurotoxic effects of mancozeb and maneb in mesencephalic neuronal cultures are associated with mitochondrial dysfunction. Neurotoxicology. 2006:27(5):816–825. [DOI] [PubMed] [Google Scholar]
- 18. Ghelani H, Razmovski-Naumovski V, Chang D, Nammi S. Chronic treatment of curcumin improves hepatic lipid metabolism and alleviates the renal damage in adenine-induced chronic kidney disease in Sprague-Dawley rats. BMC Nephrol. 2019:20(1):431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Borra SK, Gurumurthy P, Mahendra J, Jayamathi KM, Cherian CN, Chand R. Antioxidant and free radical scavenging activity of curcumin determined by using different in vitro and ex vivo models. J Med Plants Res. 2013:7(36):2680–2690. [Google Scholar]
- 20. Hewlings SJ, Kalman DS. Curcumin: a review of its effect on human health. Foods. 2017:22:92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. El-Demerdash FM, Yousef MI, Radwan FME. Ameliorating effect of curcumin on sodium arsenite-induced oxidative damage and lipid peroxidation in different rat organs. Food Chem Toxicol. 2009:47(1):249–254. [DOI] [PubMed] [Google Scholar]
- 22. CCAC- Canadian Council on Animal Care . CCAC guidelines on: the care and use of farm animals in research, teaching and testing. Ottawo: Canadian Council on Animal Care; 2009. [Google Scholar]
- 23. Gautam DC, Kapoor L. Genotoxic effects of dithane M-45 on the bone marrow cells of mice in vivo. Experientia. 1991:47(3):280–282. [DOI] [PubMed] [Google Scholar]
- 24. Jagetia GC, Rajanikant GK. Effect of curcumin on radiation-impaired healing of excisional wounds in mice. J Wound Care. 2013:13(3):107–109. [DOI] [PubMed] [Google Scholar]
- 25. Tung BT, Hai NT, Son PK. Hepatoprotective effect of Phytosome Curcumin against paracetamol-induced liver toxicity in mice. Braz J Pharm Sci. 2017:53(1):e16136. [Google Scholar]
- 26. Uzunhisarcikli M, Aslanturk A. Hepatoprotective effects of curcumin and taurine against bisphenol A-induced liver injury in rats. Environ Sci Pollut Res Int. 2019:26(36):37242–37253. [DOI] [PubMed] [Google Scholar]
- 27. Lowry OH, Rosebrugh NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951:193(1):265–275. [PubMed] [Google Scholar]
- 28. Reitman S, Frankel SA. Colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminase. Am J Clin Pathol. 1957:28(1):56–63. [DOI] [PubMed] [Google Scholar]
- 29. Burd JF, Usategui-Gomez MA. Colorimetric assay for serum lactate dehydrogenase. Clin Chim Acta. 1973:46(3):223–227. [DOI] [PubMed] [Google Scholar]
- 30. Tietz NW. Textbook of clinical chemistry. Philadelphia, PA: WB Saunders; 1986 [Google Scholar]
- 31. Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978:52:302–310. [DOI] [PubMed] [Google Scholar]
- 32. Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, Jungers P, Descamps-Latscha B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996:49(5):1304–1313. [DOI] [PubMed] [Google Scholar]
- 33. Brehe JE, Burch HB. Enzymatic assay of glutathione. Anal Biochem. 1976:74(1):189–197. [DOI] [PubMed] [Google Scholar]
- 34. Angirekula S, Atti L, Atti S. Estimation of serum ascorbic acid (vitamin C) in the age related (senile) cataract: a case control study. Ann Clin Lab Res. 2018:06(01):217. [Google Scholar]
- 35. Aebi H. Catalase in vitro. Methods Enzymol. 1984:105:121–126. [DOI] [PubMed] [Google Scholar]
- 36. Sun M, Zigman S. An improved spectrophotometric assay for superoxide dismutase based on epinephrine autoxidation. Anal Biochem. 1978:90(1):81–89. [DOI] [PubMed] [Google Scholar]
- 37. Flohe L, Gunzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984:105:114–121. [DOI] [PubMed] [Google Scholar]
- 38. Ahmad MM, Rezk NA, Fawzy A, Sabry M. Protective effects of curcumin and silymarin against paracetamol induced hepatotoxicity in adult male albino rats. Gene. 2019:712:143966. [DOI] [PubMed] [Google Scholar]
- 39. Saber TM, Abo-Elmaaty AMA, Abdel-Ghany HM. Curcumin mitigates mancozeb-induced hepatotoxicity and genotoxicity in rats. Ecotoxicol Environ Saf. 2019:183:109467. [DOI] [PubMed] [Google Scholar]
- 40. Alarcan J, Waizenegger J, Solano MDM, Lichtenstein D, Luckert C, Peijnenburg A, Stoopen G, Sharma RP, Kumar V, Marx-Stoelting Pet al. Hepatotoxicity of the pesticides imazalil, thiacloprid and clothianidin – individual and mixture effects in a 28-day study in female Wistar rats. Food Chem Toxicol. 2020:140:111306. [DOI] [PubMed] [Google Scholar]
- 41. Michael B, Yano B, Sellers RS, Perry R, Morton D, Roome N, Johnson JK, Schafer K, Pitsch S. Evaluation of organ weights for rodent and non-rodent toxicity studies: a review of regulatory guidelines and a survey of current practices. Toxicol Pathol. 2007:35(5):742–750. [DOI] [PubMed] [Google Scholar]
- 42. Yahia E, Aiche MA, Chouabia A, Boulakoud MS. Biochemical and hematological changes following long term exposure to mancozeb. Adv Biores. 2015:6:83–86. [Google Scholar]
- 43. Du Y, Wang T, Jiang N, Ren R, Li C, Li C, Fu F. Sodium aescinate ameliorates liver injury induced by methyl parathion in rats. Exp Ther Med. 2012:3(5):818–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Razia S, Siddiqui A. Study on hepatic histopathological observations and the ameliorative effects of O. sanctum on mancozeb induced toxicity in albino mice. Int J Glob Sci Res. 2015:2:152–158. [Google Scholar]
- 45. Domico LM, Cooper KR, Bernard LP, Zeevalk GD. Reactive oxygen species generation by the ethylene-bis-dithiocarbamate (EBDC) fungicide mancozeb and its contribution to neuronal toxicity in mesencephalic cells. Neurotoxicology. 2007:28(6):1079–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Messarah M, Amamra W, Boumendjel A, Barkat L, Bouasla I, Abdennour C, Boulakoud MS, El Feki A. Ameliorating effects of curcumin and vitamin E on diazinon-induced oxidative damage in rat liver and erythrocytes. Toxicol Ind Health. 2013:29(1):77–88. [DOI] [PubMed] [Google Scholar]
- 47. Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee J, Chen S, Corpe C, Dutta A, Dutta SKet al. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. 2003:22(1):18–35. [DOI] [PubMed] [Google Scholar]
- 48. Jones DP, Go YM. Redox compartmentation of cellular stress. Diabetes Obes Metab. 2010:12(Suppl 2):116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Grosicka-Maciag E, Kurpio-Piec D, Szumito M, Grzela T, Rahden-Staroń I. Protective effect of N-acetyl-L-cysteine against maneb induced oxidative and apoptotic injury in Chinese hamster V79 cells. Food Chem Toxicol. 2011:49(4):1020–1025. [DOI] [PubMed] [Google Scholar]
- 50. Aprioku JS. Pharmacology of free radicals and the impact of reactive oxygen species on the testis. J Reprod Infertil. 2013:14(4):158–172. [PMC free article] [PubMed] [Google Scholar]
- 51. Ighodaro OM, Akinloye OA. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx): their fundamental role in the entire antioxidant defence grid. Alexandria J Med. 2018:54(4):287–293. [Google Scholar]
- 52. Amara IB, Saad HB, Hamdaoui L, Karray A, Boudawara T, Ali YB, Zeghal N. Maneb disturbs expression of superoxide dismutase and glutathione peroxidase, increases reactive oxygen species production, and induces genotoxicity in liver of adult mice. Environ Sci Pollut Res Int. 2015:22(16):12309–12322. [DOI] [PubMed] [Google Scholar]
- 53. Priyadarsini KI, Maity DK, Naik GH, Kumar MS, Unnikrishnan MK, Satav JG, Mohan H. Role of phenolic O-H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free Radic Biol Med. 2003:35(5):475–484. [DOI] [PubMed] [Google Scholar]
- 54. Lin X, Bai D, Wei Z, Zhang Y, Huang Y, Deng H, Huang X. Curcumin attenuates oxidative stress in RAW264.7 cells by increasing the activity of antioxidant enzymes and activating the Nrf2-Keap1 pathway. PLoS One. 2019:14(5):e0216711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ashrafizadeh M, Ahmadi Z, Mohammadinejad R, Farkhondeh T, Samarghandian S. Curcumin activates the Nrf2 pathway and induces cellular protection against oxidative injury. Curr Mol Med. 2020:20(2):116–133. [DOI] [PubMed] [Google Scholar]
- 56. Abd-Elhakim YM, Moselhy AAA, Aldhahrani A, Beheiry RR, Mohamed WAM, Soliman MM, Saffaf BA, El Deib MM. Protective effect of curcumin against sodium salicylate-induced oxidative kidney damage, nuclear factor-kappa dysregulation, and apoptotic consequences in rats. Antioxidants (Basel). 2021:10(6):826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Huang J, Yi Q, You Y, Chen Y, Niu T, Li Y, Zhang J, Ji X, Xu G, Zou Wet al. Curcumin suppresses oxidative stress via regulation of ROS/NF-κB signaling pathway to protect retinal vascular endothelial cell in diabetic retinopathy. Mol Cell Toxicol. 2021:17(3):367–376. [Google Scholar]
- 58. Buhrmann C, Mobasheri A, Busch F, Aldinger C, Stahlmann R, Montaseri A, Shakibaei M. Curcumin modulates nuclear factor kappaB (NF-kappaB)-mediated inflammation in human tenocytes in vitro: role of the phosphatidylinositol 3-kinase/Akt pathway. J Biol Chem. 2011:286(32):28556–28566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ni H, Jin W, Zhu T, Wang J, Yuan B, Jiang J, Liang W, Ma Z. Curcumin modulates TLR4/NF-κB inflammatory signaling pathway following traumatic spinal cord injury in rats. J Spinal Cord Med. 2015:38(2):199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lu C, Zhang F, Xu W, Wu X, Lian N, Jin H, Chen Q, Chen L, Shao J, Wu Let al. Curcumin attenuates ethanol-induced hepatic steatosis through modulating Nrf2/FXR signaling in hepatocytes. IUBMB Life. 2015:67(8):645–658. [DOI] [PubMed] [Google Scholar]
- 61. Di Tu Q, Jin J, Hu X, Ren Y, Zhao L, He Q. Curcumin improves the renal autophagy in rat experimental membranous nephropathy via regulating the PI3K/AKT/mTOR and Nrf2/HO-1 signaling pathways. Biomed Res Int. 2020:2020:7069052–7069012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang Y, Tang Q, Duan P, Yang L. Curcumin as a therapeutic agent for blocking NF-κB activation in ulcerative colitis. Immunopharmacol Immunotoxicol. 2018:40(6):476–482. [DOI] [PubMed] [Google Scholar]
- 63. Tizabi Y, Hurley LL, Qualls Z, Akinfiresoye L. Relevance of the anti-inflammatory properties of curcumin in neurodegenerative diseases and depression. Molecules. 2014:19(12):20864–20879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tonomura Y, Kato Y, Hanafusa H, Morikawa Y, Matsuyama K, Uehara K, Ueno M, Torii M. Diagnostic and predictive performance and standardized threshold of traditional biomarkers for drug-induced liver injury in rats. J Appl Toxicol. 2015:35(2):165–172. [DOI] [PubMed] [Google Scholar]
- 65. Amacher DE. Serum transaminase elevations as indicators of hepatic injury following the administration of drugs. Regul Toxicol Pharmacol. 1998:27(2):119–130. [DOI] [PubMed] [Google Scholar]
- 66. Singh A, Bhat TK, Sharma OP. Clinical biochemistry of hepatotoxicity. J Clin Toxicol. 2011:S4:001. [Google Scholar]
- 67. Whitfield JB. Gamma glutamyl transferase- review. Crit Rev Clin Lab Sci. 2001:38(4):263–355. [DOI] [PubMed] [Google Scholar]
- 68. Koenig G, Seneff S. Gamma-glutamyltransferase: a predictive biomarker of cellular antioxidant inadequacy and disease risk. Dis Markers. 2015:2015:818570–818518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Aberkane H, Stoltz JF, Galteau MM, Wellman M. Erythrocytes as targets for gamma-glutamyltranspeptidase initiated pro-oxidant reaction. Eur J Haematol. 2002:68(5):262–271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.