ABSTRACT
The coexistence of osteoporosis and chronic kidney disease (CKD) is an evolving healthcare challenge in the face of increasingly aging populations. Globally, accelerating fracture incidence causes disability, impaired quality of life and increased mortality. Consequently, several novel diagnostic and therapeutic tools have been introduced for treatment and prevention of fragility fractures. Despite an especially high fracture risk in CKD, these patients are commonly excluded from interventional trials and clinical guidelines. While management of fracture risk in CKD has been discussed in recent opinion-based reviews and consensus papers in the nephrology literature, many patients with CKD stages 3–5D and osteoporosis are still underdiagnosed and untreated. The current review addresses this potential treatment nihilism by discussing established and novel approaches to diagnosis and prevention of fracture risk in patients with CKD stages 3–5D. Skeletal disorders are common in CKD. A wide variety of underlying pathophysiological processes have been identified, including premature aging, chronic wasting, and disturbances in vitamin D and mineral metabolism, which may impact bone fragility beyond established osteoporosis. We discuss current and emerging concepts of CKD–mineral and bone disorders (CKD-MBD) and integrate management of osteoporosis in CKD with current recommendations for management of CKD-MBD. While many diagnostic and therapeutic approaches to osteoporosis can be applied to patients with CKD, some limitations and caveats need to be considered. Consequently, clinical trials are needed that specifically study fracture prevention strategies in patients with CKD stages 3–5D.
Keywords: biomarkers, bone mineral density, fracture risk, mineral metabolism, renal osteodystrophy
INTRODUCTION
Large epidemiologic studies demonstrate an increasing fracture risk with more advanced stages of chronic kidney disease (CKD), compared with the general population. The simultaneous presence of traditional risk factors for bone fragility and CKD-specific mineral and bone disorders (CKD-MBD) poses a challenge to clinical evaluation, treatment and prevention. The 2017 update of the Kidney Disease: Improving Global Outcomes Initiative CKD-MBD guidelines recommend determination of bone mineral density (BMD) for fracture risk assessment in all stages of CKD, including dialysis patients, if results will impact treatment decisions [1]. Recent developments in diagnostic methodology and pathophysiologic understanding, and an increasing therapeutic armamentarium for the treatment and prevention of fragility fractures, open new possibilities for the clinician. Several systematic reviews of osteoporosis medication in CKD have been published recently, demonstrating an efficacy of different classes of bone-specific drugs for improvement of BMD and for fracture risk reduction [2, 3]. Thus, it may be time for a re-evaluation of the diagnostic and therapeutic strategies for fracture prevention in CKD.
This review summarizes current knowledge in the field, based on a recent on-line seminar on management of fracture risk in CKD, organized by the CKD-MBD Working Group of the European Renal Association (ERA), with inclusion of relevant literature published after that event. New and established methods for fracture risk prediction and prevention are discussed with focus on their relevance for patients with advanced CKD stages 4–5D.
EVALUATION OF FRACTURE RISK
FRAX for fracture risk prediction in CKD
FRAX is a computer-based fracture risk assessment tool, which can be found online at https://www.sheffield.ac.uk/FRAX. It has now been calibrated in 78 countries or territories, and 6 million calculations are performed annually in over 170 countries [4]. It calculates the 10-year probability of a major osteoporotic fracture as well as the 10-year probability of a hip fracture. The tool was developed based on specific risk factors, with or without femoral neck BMD as measured by dual-energy x-ray absorptiometry (DXA). FRAX includes the competing risk of death in the fracture risk assessment. This means that the risk of death, associated with other factors such as age, which precludes the occurrence of fracture, has been taken into account. However, FRAX currently does not include falls, which is another important and independent risk factor for fracture. Although there is a high prevalence of sarcopenia in CKD (up to 70%) and an increasing incidence of falls with CKD severity, there is no direct evidence that sarcopenia in CKD is associated with increased risk of falls and fracture [5, 6].
Several studies have shown that the validity of risk prediction for people with CKD stages 3a and 3b is similar to those with estimated glomerular filtration rate (eGFR) >60 mL/min/1.73 m2 [7–9]. However, these studies found that FRAX either under- or overestimates the fracture risk in both CKD and non-CKD. Possible reasons for these discrepancies are differences in methods used to identify fractures and the comparison of fracture risk in the study populations to the nationwide population risk used in FRAX. Overall, provided that these shortcomings are taken into account, FRAX can be used as a reliable fracture risk assessment tool in CKD stages 1–3.
There are few data on the role of FRAX in fracture risk prediction for CKD stages 4–5D, including dialysis patients [7, 8, 10, 11]. The next FRAX update may include risk factors such as recurrent falls, but the inclusion of CKD as a risk factor remains elusive. Renal osteodystrophy (ROD) impairs bone quality and is highly prevalent in CKD stages 4–5D [12]. However, important information on fracture risk relating to the type of ROD is unavailable, and a sufficiently powered study is unlikely to be achievable. Therefore, the question remains whether FRAX underestimates fracture risk in CKD patients who have ROD. Additionally, the increased mortality risk of CKD patients may require a re-adjustment to the competing risk of death. Furthermore, assessing ‘imminent’ fracture risk (within 1–2 years from an incident fracture) may appear clinically appropriate and is a current matter of research. Thus, development of new models or adjustment of the FRAX score with factors such as ‘recent’ fracture (rather than just fracture) or fracture site are currently being tested [13]. Such concern appears highly relevant in the context of CKD and should be evaluated in this population. Nonetheless, patients with any stage of CKD, including dialysis patients, who are currently identified as having high fracture risk by FRAX should be considered for treatment. Country-specific intervention thresholds for the general population are recommended, awaiting further evidence for specific thresholds in CKD [14].
DXA in CKD
The central role of bone mass in the operational definition of osteoporosis was recognized by the World Health Organization in 1994 with the publication of the BMD-derived T-score as a working definition of osteoporosis [15]. This did not ignore the contribution of other skeletal factors to fracture risk (e.g. high bone turnover, bone geometry, microarchitectural disruption, etc.), but simply recognized that BMD, measured by DXA, could capture about 70%–85% of bone strength in ex vivo studies and, more importantly, showed strong predictive ability for incident osteoporotic fractures in many studies [16]. Within the setting of CKD, it was initially assumed that a number of additional ‘uraemic’ factors might also influence skeletal strength and fracture risk and that the role for BMD measurements might be somewhat more limited [17]. This has proven not to be the case, with studies showing a similar gradient of risk for future fracture in CKD as in non-CKD populations [1, 18]. Nonetheless, the role of DXA-measured BMD in individual patient management is something that requires close collaboration and understanding between nephrologists and their local providers of BMD measurements, as a number of caveats in interpretation of BMD need to be considered. First, a T-score of –2.5 or less may not equal osteoporosis; impaired mineralization, e.g. osteomalacia, will also give rise to a low T-score, a situation in which some osteoporosis therapy might be potentially harmful. Secondly, many patients will be at high fracture risk despite having a T-score greater than –2.5, especially those with prior fractures. This has given rise to the development of tools for assessing absolute fracture risk such as FRAX, as discussed above [8]. Thirdly, the T-score definition of osteoporosis is inappropriate in certain clinical situations, particularly in children and young adults where the Z-score is more appropriate [19]. Finally, the finding of a low T-score or Z-score does not identify the cause, so that a comprehensive medical evaluation should be considered {e.g. to exclude malabsorption, the nature of an underlying ROD [hyperparathyroidism (HPT), adynamic bone] and/or senile osteoporosis, etc.}. The management of these, often complex, patients is enhanced by the development of multidisciplinary teams involving kidney and bone health expertise.
DXA scan derived Trabecular Bone Score
Trabecular Bone Score (TBS) is a grey-level textural parameter derived from DXA spine scans (other skeletal sites are becoming available) which provides information related to bone microarchitecture, with lower values indicating heterogenous or disrupted microarchitecture [20]. There is compelling evidence that TBS provides BMD- and FRAX-independent input to future fracture risk in the non-CKD population [21], and a FRAX-adjustment for TBS has been made available [22, 23]. More limited data suggest that the role of TBS in fracture risk prediction in the setting of CKD stage 5D and after kidney transplantation may be similar to that in non-CKD populations [24]. However, in patients with CKD stages 3–4, two large cohort studies found diverging results concerning the ability of TBS to predict fracture incidence: while Naylor et al. [25] found an association with incident fractures in patients with mostly CKD stage 3A, Rampersad et al. [26] found no association of TBS with fracture incidence in patients with CKD stages 3–4. These diverging findings may be due to differences in study design, e.g. severity of CKD and fracture identification. Thus, additional evidence is needed to further elucidate the role of TBS in CKD.
Novel bone imaging
HR-pQCT
Assessment of bone microstructure has traditionally relied on histomorphometry of bone biopsy specimens, as imaging modalities available for routine clinical use such as conventional computed tomography (CT) or DXA lack adequate resolution to study cortical or trabecular structure in detail. With the advent of new methods such as peripheral quantitative computed tomography (pQCT), high-resolution pQCT (HR-pQCT) and micro magnetic resonance imaging (µMRI) it is now possible to analyse bone microstructure non-invasively and thus more easily and longitudinally as well. However, it is important to note that all three techniques discussed here take images from the distal radius or distal tibia, anatomical locations where fractures do not have as serious clinical consequences as fractures of the vertebrae or the femoral neck. This limitation also applies to histomorphometry of bone biopsy samples, which are usually collected from the iliac crest. Furthermore, radius and tibia have different mechanical as well as metabolic properties and functions compared with the iliac crest, so comparisons between micro-imaging modalities (QCT, HR-pQCT and µMRI) and bone histomorphometry are bound to yield only modest correlations for parameters of bone microstructure [27]. The majority of data on bone microstructure in CKD patients has been generated using HR-pQCT [27–35], which overcomes the shortcomings of pQCT and µMRI. While pQCT lacks the resolution to identify individual trabeculae and thus is more or less confined to structural studies of the cortex [36], the use of µMRI is currently limited by its availability at only a few specialized centres [37]. HR-pQCT has a resolution of approximately 80 µm, which allows for detailed examination of the trabecular compartment of bone (the typical trabecular width is 100–200 µm) as well as cortical porosity (Ct.Po). Generally speaking, increases in Ct.Po are expected to weaken mechanical bone strength. Interestingly, Ct.Po measured by HR-pQCT was found to increase over time in patients with CKD [38] and after kidney transplantation [34]. However, Ct.Po, measured by HR-pQCT, was not superior to BMD, determined by DXA, for identification of HD patients with prevalent fragility fractures [31]. Another cortical parameter associated with bone stability is the buckling ratio (BR), which is calculated by dividing the distance from the bone surface to the centre of the bone mass by the cortical thickness. Most studies describing BR are based on QCT data. A higher BR is associated with increased fracture risk [39, 40] and treatment with osteoporosis medication can improve the BR [41, 42]. Increased BRs compared with healthy controls have been described in dialysis patients [43] and after kidney transplantation [44]. HR-pQCT images can be reconstructed in 2D and 3D (Fig. 1) and can be used for virtual stress testing by estimating failure load using finite element analysis [45]. In a recent large international cohort, failure load outperformed individual parameters of microarchitecture, aBMD and FRAX as independent predictor of any incident fracture and incident major osteoporotic fractures in older men and women [46]. HR-pQCT studies have demonstrated that bone microarchitecture and failure load are significantly more compromised in patients with CKD [28, 35], including dialysis patients [29] with a history of bone fracture compared with patients without previous fracture, and that bone microstructure deteriorates rapidly in CKD patients [20]. However, in cross-sectional studies the power to discriminate patients according to fracture status was similar for HR-pQCT and conventional DXA [28, 29, 35].
Figure 1:
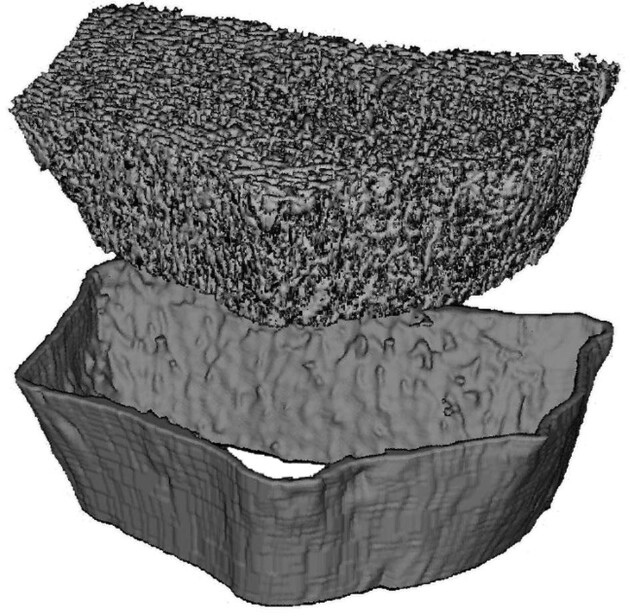
Three-dimensional (3D) reconstruction of the distal radius of a dialysis patient. Source: Department of Radiology, Medical University Vienna, Austria.
Future research using HR-pQCT and µMRI could yield highly interesting insights into fracture prediction, pathophysiology of bone dynamics (e.g. spontaneous development and refilling of cortical porosity), effects of different treatments on bone microarchitecture, and, possibly, with the use of MRI-spectroscopy, also non-invasive analysis of bone composition and quality.
18F-sodium fluoride positron emission tomography
Positron emission tomography (PET) is a non-invasive imaging method that enables measurement of the molecular function in tissues and organs by using short-lived radioactive isotopes. It monitors the movement of markers in vivo, without interfering with normal body functions. 18F-sodium fluoride (18F-NaF) PET allows assessment of regional bone turnover [47, 48]. The tracer 18F-NaF is a sensitive bone-seeking compound with high and rapid bone uptake and plasma clearance [49], rendering a half-life of 110 min. 18F-NaF reflects osteoblast activity and can therefore determine bone remodelling [50, 51].
18F-NaF PET correlates with both dynamic and static histomorphometric parameters in bone biopsies [50, 51]. In a study of dialysis patients [50], 18F-NaF PET had an area under the curve of 0.82 to discriminate histomorphometrically determined low turnover from non-low turnover bone disease, with a sensitivity of 76% and a specificity of 78%. 18F-NaF PET is also a feasible method to follow the effect of both antiresorptive and anabolic treatment of osteoporosis in patients with normal kidney function longitudinally [52, 53]. The advantages of the method are that it is non-invasive, quick and reproducible, and enables assessment of regional bone turnover. Disadvantages are radiation exposure and its limited availability.
In the field of cardiovascular disease, 18F-NaF PET has shown potential to broaden our understanding of the pathophysiological mechanisms underlying atherosclerosis and vascular calcification. The precise mechanism of vascular uptake of 18F-NaF is unclear, but it seems to adsorb with high affinity to calcified deposits within plaques [54]. Indications of higher tracer uptake in culprit lesions after myocardial infarction have been presented [55]. Moreover, 18F-NaF uptake in the arterial wall correlates with risk factors for atherosclerosis [56]. A recent study demonstrated independent positive associations between 18F-NaF uptake in bone, histomorphometric parameters of bone turnover in bone biopsies and arterial calcification in end-stage renal disease patients [57], in line with a previous study of bone biopsy-based histomorphometric parameters of bone turnover and coronary calcification in dialysis patients [58].
In summary, 18F-NaF PET seems to be a promising diagnostic tool when assessing bone turnover and treatment in patients with ROD. Future studies will determine 18F-NaF PET’s role in imaging of atherosclerosis and in exploring bone-vascular cross talk.
Bone biopsy
(Quantitative) histomorphometric analysis of bone is still the ‘gold standard’ for diagnosis and specific classification of the different types of ROD. Compared with other techniques for the evaluation of bone status, it also allows additional information on bone cell surface, number, and activity. Several (immuno-)histochemical stainings to evaluate cellular activity may be performed. Bone histomorphometry can be combined with other techniques such as µ-CT, for additional information on bone microarchitecture. However, a bone biopsy has several constraints. It is invasive, and as the histomorphometric analysis is laborious, time consuming, expensive (reimbursement is lacking in most countries), and requires specific expertise and complex diagnostic interpretation, it is only performed in a limited number of labs.
In the setting of CKD stages 4–5D, a bone biopsy is traditionally recommended in case of (i) suspected osteomalacia [persistent bone pain, multiple fractures, hypocalcemia, hypophosphatemia, elevated bone-specific alkaline phosphatase (BALP)], (ii) unexplained discordance between total ALP and/or BALP levels and parathyroid hormone (PTH), e.g. BALP >25–30 µg/L and PTH <100 pmol/L, in the presence of a normal liver function, (iii) unexplained persistent or severe hypo- or hypercalcemia/hypophosphatemia, and (iv) before prescription of anti-osteoporotic treatment (e.g. bisphosphonates, denosumab or teriparatide). However, a recent consensus statement by the ERA and the International Osteoporosis Foundation states that inability to perform a biopsy does not justify withholding treatment to CKD patients with high fracture risk [14]. Overall, the quantitative bone histomorphometric analysis, by providing information on bone turnover and mineralization, may help guide prevention and treatment of ROD. While the risk prediction of fractures can reasonably be based on BMD measurements in CKD stages 1–3, fracture risk evaluation is more complex in CKD stages 4–5D, as all subtypes of ROD may result in low BMD [59]. Additionally, subtypes of ROD can affect bone quality differently [12], thus, correct diagnosis of ROD may potentially improve fracture risk prediction in CKD stages 4–5D.
The iliac crest is the preferred site for a bone biopsy because it is easily accessible, only minimally affected by mechanical strain, and sampling from this site is associated with very low morbidity. Bone biopsies at the iliac crest can be obtained in a vertical or a horizontal direction. Whilst in the past, the great majority of bone biopsies were taken using a 7.5 mm Bordier–Meunier trephine, today up to 40% of biopsy procedures are performed with smaller, disposable, Jamshidi-type 4 mm trephines [60]. As demonstrated recently by Novel-Catin et al. [61], quantitative histomorphometric analysis of 3–3.5 mm hemi-biopsies fully matched with the whole-sample 7.5 mm biopsy (N = 68) in 91% of cases.
Quantitative bone histomorphometry encompasses the analysis of a series of both static (e.g. amount of osteoid, number of osteoblasts, bone area/volume, number of osteoclasts) and dynamic (e.g. bone formation rate, mineralization lag time, mineral apposition rate) parameters. In order to allow the analysis of dynamic parameters, double labelling with fluorochrome compounds such as demeclocycline or tetracycline is necessary prior to the bone biopsy procedure [61]. These compounds are incorporated into newly mineralized bone and emit a green-yellow colour under UV light (Fig. 2). Following sampling, the biopsy is directly fixed in 70% ethanol and may be stored at 4°C until shipping at environmental temperature (no refrigeration is necessary).
Figure 2:
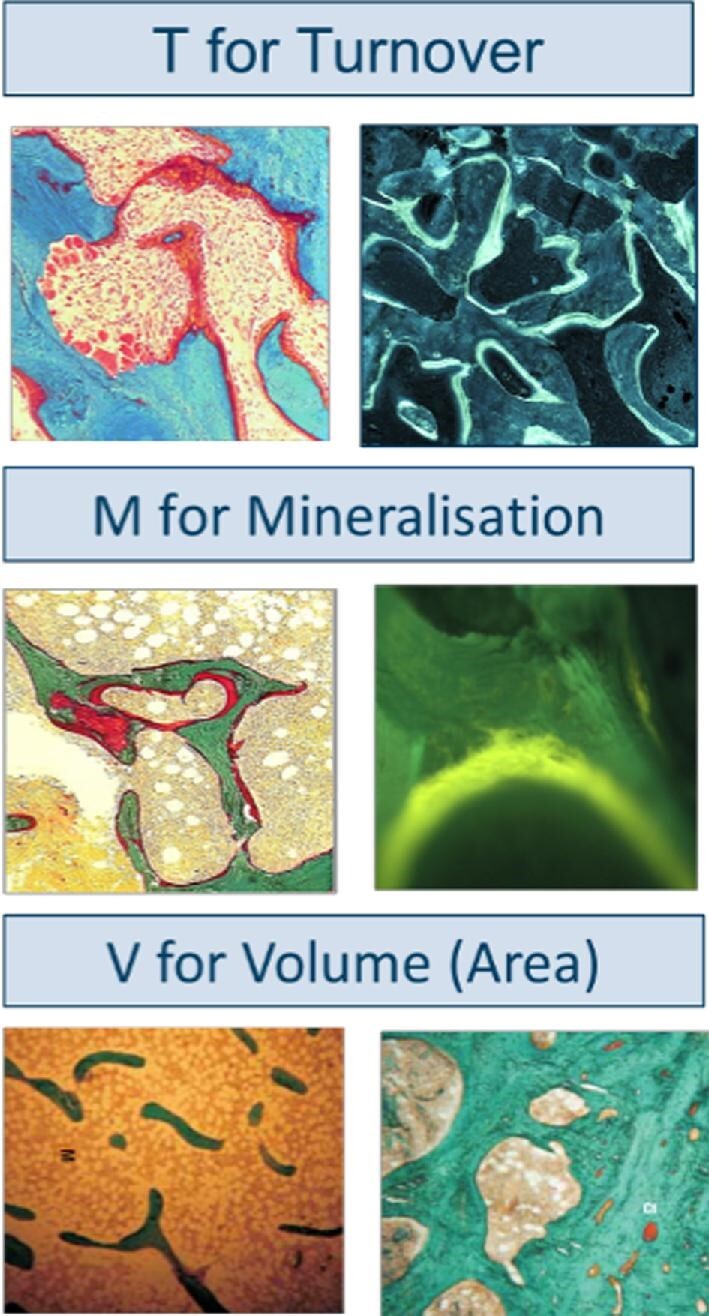
Representative Goldner stained and fluorescent tetracycline labelled sections (two upper right photographs) as examined for evaluation of turnover (T), mineralization (M) and volume (V). Source: Laboratory of Pathophysiology, Department of Biomedical Sciences, University of Antwerp, Belgium.
The classic description of the histologic abnormalities of ROD includes hyperparathyroid bone disease (osteitis fibrosa), adynamic bone, osteomalacia and mixed uraemic osteodystrophy. In 2006, the KDIGO consensus conference agreed on a new classification of ROD [62] that addresses the most important bone abnormalities, which include changes in bone turnover (T), mineralization (M) and volume (V) (Fig. 2). The TMV classification offers more precise information than the previously used classification system [63].
Clinical evaluation of bone mechanical properties
Bone fragility is related to the mechanical properties of bone tissue, i.e. the bone's capacity to resist elastic deformation, plastic deformation and crack propagation. Determinants of bone material mechanical properties include matrix composition, microarchitecture, geometry, shape, remodelling dynamics, water content and occurrence of micro-damages. Disturbances in these parameters may contribute to increased bone fragility in the presence of normal bone mass [64, 65]. Standardized methods for the direct determination of mechanical bone properties comprise bending tests, and micro- and nanoindentation. Until recently, these methods were restricted to the use in ex vivo experimental studies or bone biopsies. The OsteoProbe® is a novel hand-held device for the in vivo determination of impact microindentation (IMI) of cortical bone, which in clinical studies was able to identify patients with prior fractures—both with and without CKD [66–69]. This device measures indentation depth in bone by IMI and calculates a bone material strength index (BMSi) by comparison with indentation depth in a standardized material [64]. Experimental studies have identified cortical material composition (e.g. the mineral-to-matrix ratio and the content of water or advanced glycation end-products), rather than microarchitecture or porosity as determinants of BMSi [70, 71]. Although BMSi is associated with BMD in postmenopausal osteoporosis [72] and in CKD (Johansson M, Qureshi AR, Jankowska M, Lindholm B, Haarhaus M. presentation at ERA-EDTA annual congress 2021), the presence of a previous fracture is associated with lower BMSi, independent of BMD [67, 69]. Thus, IMI may evolve as a clinical tool to complement DXA in fracture risk evaluation. However, although the OsteoProbe® is approved for clinical use, evidence from large, prospective studies is still lacking.
Bone turnover markers in CKD
While imaging modalities to assess bone health perform reasonably well in assessing bone volume or mineral content, circulating markers derived from the formation or degradation of bone reflect bone turnover, and as such are complementary to imaging techniques currently used in clinical practice. Besides reflecting this different aspect of bone, these biomarkers have the advantage of being easily obtainable by a simple blood sample, rendering them suitable as screening tools for the diagnosis of bone turnover, and they can be followed longitudinally, to monitor effect of treatments that modulate bone turnover. General disadvantages of bone biomarkers are that many are not firmly associated with future fracture risk in general populations, unless at extreme values, and that several biomarkers have unpredictable kinetics in the setting of CKD. As disorders of bone turnover commonly found in CKD are more severe than in non-CKD populations and may impact bone fragility, the question remains whether bone biomarkers better reflect fracture risk in CKD. In fact, in a study on haemodialysis patients, BALP was a better predictor of fracture incidence than BMD at different sites or PTH [73].
PTH is the most frequently used biomarker for bone turnover in CKD. It is, however, important to realise, that, while PTH is a regulator of bone turnover, it does not reflect turnover per se. PTH may also reflect parathyroid disease, PTH molecules may be biologically inert, and PTH resistance on bone level can exist, all conditions in which PTH value fails as indicator of bone turnover. In contrast, circulating BALP is mainly derived from active osteoblasts and reflects turnover more directly. In the absence of liver disease, BALP comprises approximately 50% of total circulating ALP activity; thus, total ALP is commonly used as biomarker for routine control of bone formation in CKD. Clinically used target ranges for PTH [74] are derived from associations with mortality, not with bone histology or incidence rates of fractures. With these pitfalls in mind, a large study found that intact PTH had an area under the receiver operator characteristics curve (AUROC) of 0.701, with an optimal cut-off of 104 pg/mL for distinguishing low from non-low bone turnover, as assessed by bone histomorphometry in dialysis patients [75]. For the distinction between high and non-high turnover, an AUROC of 0.724 was found with an optimal intact PTH cut-off of 323 pg/mL [75]. The AUROCs for BALP were 0.757 and 0.711, with cut-off values of 33.1 U/L and 42.1 U/L for low and high turnover, respectively [75]. In that study, combining these two markers did not increase diagnostic performance. The most promising novel biomarkers in the CKD setting are procollagen type I N-terminal propeptide (PINP) and tartrate resistant acid phosphatase 5b (TRaP5b), reflecting bone formation and resorption, respectively [76]. PINP is cleaved from the N-terminal site of newly formed bone collagen as a triple helix polypeptide, the concentration of which is independent of kidney function. However, PINP also circulates as monomers, which do accumulate in CKD [77]. It is therefore of importance that the assay used detects intact PINP. TRaP5b is an osteoclast-derived enzyme, and its serum levels associate with the number and size of these cells [78]. Circulating levels are not influenced by kidney function or by dialysis [79]. Two recent studies indicate that osteoblast and osteoclast markers may outperform PTH as a biomarker of bone turnover in CKD [80, 81], especially when two markers are combined [81]. However, while the ability to discriminate high from non-high turnover was similar in CKD 4–5D and after kidney transplantation, the accuracy of biomarkers to distinguish between low and non-low turnover was lower in kidney transplant recipients [81].
TREATMENT OPTIONS
Non-pharmacological measures
Lifestyle and diet
Lifestyle factors influence fracture risk in osteoporosis, and patients with CKD are not exempt from these risks. Excessive alcohol intake and current smoking increase the risk of hip fractures in CKD [82], and a range of dietary factors have been linked to either low BMD or fractures, including a low protein intake [83], low fish intake [84] and lack of vitamin K [85]. A sufficient intake of calcium and vitamin D are crucial for maintenance of bone health (discussed in depth in a separate paragraph). More important than any single nutrient, however, is the overall nutritional status. There is a well-known direct relationship between body mass index and bone mass [86], and low body weight or weight loss are consistent risk factors for fracture in CKD patients, as well as in the general population [82, 87, 88].
Exercise
Loss of muscle mass and function, sarcopenia contributes to falls, fractures and overall mortality [89]. Evaluation of muscle function is simple and easy to perform in clinic by tests such as the short physical performance battery [90]. In CKD, both self-reported physical function [91] and low scores on tests such as the 6-minute walking test [92] associate with falls and fractures. Though no interventional trial has evaluated the effect of exercise on fracture risk in CKD, resistance training is capable of eliciting a normal, anabolic muscle-response even in CKD stage 5D [93], resulting in increased muscle strength and improved physical performance [93, 94].
Mechanical loading is also a key component in maintaining bone mass, and weight-bearing exercise has positive effects on the skeleton throughout life [95, 96]. A recent meta-analysis concluded that resistance training may also increase BMD in patients with CKD stages 3–5, mainly at the peripheral skeleton [97]. Progressive resistance training resulted in a small increase in whole-body bone mineral content after 12 weeks in patients receiving haemodialysis, when compared with sham (lightweight) exercise [93].
Falls
The propensity to fall is a strong, independent risk factor for fracture [98]. The fall risk is high in CKD, particularly in CKD stage 5D, with a recent fall reported in as many as 50% of elderly patients receiving haemodialysis [5, 99]. Reduced physical performance, malnutrition, depression and central nervous system-affecting drugs increase the risk of falls specifically in CKD [87, 100]. Very little has been published on initiatives aimed at reducing risk of falls in late-stage CKD. One single-centre study demonstrated a reduction in falls in the haemodialysis clinic—among patients, staff or visitors—by a pragmatic approach targeting factors involved in falls, such as slippery floors and poor lighting [101].
Drugs
CKD patients are treated with a multitude of drugs, many of which affect bone health. Loop-diuretics increase renal calcium excretion and increase the risk of fractures in the background population, likely due to induction of secondary HPT (SHPT) [102]. A similar effect was not seen in CKD stage 5D [103]. Thiazide diuretics increase tubular reabsorption of calcium, with small, positive effects on bone mass and fracture risk [104, 105]. As thiazides are considered ineffective with eGFR <30 mL/min/1.73 m2 [106], there are currently no data available on the effect this class of diuretics may have on bone or mineral metabolism in CKD stages 3–5. This may change with the recent introduction of chlorthalidone as an efficient blood-pressure lowering thiazide in patients with CKD stage 4 [107].
The increased fracture risk seen with use of proton pump inhibitors (PPI) has received attention recently, although the precise mechanism is yet unknown [102, 108]. In a Danish registry-based study, PPI use increased the fracture risk for patients receiving haemodialysis to a similar level to that in the background population [103]. Treatment with PPI—but not with H2 receptor agonists—was also associated with increased risk of fractures in kidney transplant recipients [109].
Unfractionated heparin (UFH) use is associated with 5%–10% bone loss and increased risk of vertebral fractures in young, pregnant women [110]. Although these data are observational and have been criticized for the lack of appropriate controls [111], they are supported by experimental studies demonstrating inhibition of bone formation with rapid trabecular bone loss with the use of UFH [112]. A similar signal has not been found for low molecular weight heparin (LMWH) [113]. In a recent study, intradialytic UFH use was associated with greater bone loss at the spine over a 2-year period in patients receiving chronic intermittent haemodialysis, when compared with LMWH [114].
Bone loss and increased fracture risk are well-known side effects of systemic treatment with glucocorticoids. A daily dose of just 5 mg prednisolone increases the fracture risk by ∼20%, with a dose-dependent increase in risk up to 60% with daily doses of 20 mg [115]. Cumulative glucocorticoid dose is a determinant of bone loss after kidney transplantation [116, 117], and steroid-sparing regimens are associated with reduced risk of fractures [118]. Glucocorticoids are also the cornerstone therapy for multiple glomerular disorders. Efforts to reduce glucocorticoid exposure through steroid-minimization, and/or combined immunosuppressive protocols are ongoing [119].
Conclusions regarding non-pharmacological interventions to lower fracture risk in CKD are summarized in Table 1 and Fig. 3.
Table 1:
Non-pharmacological interventions to reduce fracture risk in CKD.
| Lifestyle factor | Intervention |
|---|---|
| Drugs | Avoid long-term use of central nervous system–affecting drugs and proton pump inhibitors if possible |
| The preference of low molecular weight heparin to unfractionated heparin may be advocated | |
| Glucocorticoid dose and duration should be reduced to a minimum, and fracture risk assessment must be considered mandatory for any patient initiating systemic steroid treatment | |
| Diet and nutrition | Ensure sufficient nutrition, paying special attention to the calcium balance |
| Promote cessation of smoking and moderation of alcohol intake | |
| Physical function | Encourage exercise, to maintain physical function and reduce the risk of falls |
| Resistance training may be particularly beneficial to skeletal health | |
| Falls risk | Risk of falls should be evaluated—and acted upon |
Figure 3:
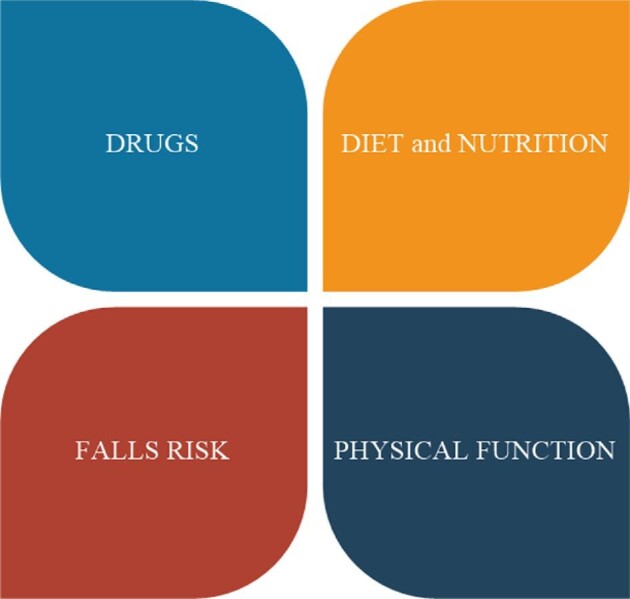
Targetable lifestyle factors associated with fracture risk in CKD.
Pharmacotherapy for fracture prevention in CKD
A range of pharmacological agents have been developed for fracture prevention in osteoporosis, which are generally safe and effective in CKD stages 1–3 [2, 3, 14]. Despite a sparsity of large clinical trials, specifically targeting fracture risk in CKD stages 4–5D, evidence from post hoc analyses of randomized and controlled trials (RCTs), moderately sized prospective clinical trials and observational studies supports pharmacological fracture risk reduction in all stages of CKD, although some caveats and limitations need to be considered, and we advise to obtain informed consent when considering off-label use of osteoporosis medication in CKD. Advantages and disadvantages of osteoporosis medications in CKD stages 4–5D are summarized in Table 2.
Table 2:
Advantages and disadvantages of osteoporosis medications in CKD 4–5D.
| Advantages | Disadvantages | |
|---|---|---|
| Calcium | Can reduce risk of sHPT and skeletal mineralization defects | Excessive use may increase cardiovascular risk and risk for kidney stones |
| Lowers phosphorus load | ||
| May improve BMD in combination with vitamin D | ||
| Vitamin D | Can reduce risk of sHPT and skeletal mineralization defects | Stimulates FGF23 |
| May improve BMD in combination with calciumActive vitamin D preferrable in CKD 5DNutritional vitamin D preferable in CKD 4–5 | Excessive use may increase cardiovascular riskActive vitamin D should be restricted to patients with SHPT and can in that case also be used as adjuvant therapy with bone-specific agents | |
| Bisphosphonates | Can improve BMD in all stages of CKD | Increased systemic retention |
| Persistent effect after cessationNo evidence for increased cardiovascular risk | May be associated with CKD progression in CKD 4–5 and reduced residual renal function in CKD 5D | |
| Occasional reports of AKI with intravenous use | ||
| Denosumab | Can improve BMD in all stages of CKDNo evidence of increased cardiovascular or renal riskNo dose adaptation needed in any stages of CKD, including CKD 5D | Risk for hypocalcemia (especially in severe HPT)Rapid BMD deterioration and increased fracture risk after cessation |
| PTH analogues | Can improve BMD in all stages of CKD | Safety uncertain |
| May improve suppressed BFR | Optimal dosing uncertain | |
| May aggravate existing hyperparathyroidism | ||
| Romosozumab | May improve BMD in all stages of CKD | May induce hypocalcaemia |
| Anabolic and antiresorptive effect | Cardiovascular safety uncertain | |
| Optimal dosing uncertain | ||
| HRT | Can improve BMD in all stages of CKD | Safety uncertain |
| Limited to early menopause |
AKI, acute kidney injury.
Calcium and vitamin D
Calcium and vitamin D are commonly used in pharmacological treatment strategies for fracture prevention in osteoporosis, and in the treatment and prevention of SHPT in CKD [120]. Early SHPT diagnosis and treatment is crucial for the management of patients with CKD [120]. Elevated PTH and abnormal calcium and phosphate levels are frequently observed from stage 3 CKD onwards [121], and it is estimated that 40%–82% of stage 3b/4 CKD patients have SHPT. Recently, Geng et al. [122] evaluated the relationship between baseline PTH levels and long-term risk of fractures, vascular events and death in a large cohort of stage 3–4 CKD patients. The study found that among these patients, high PTH was an independent risk factor in multivariable adjusted models predicting fracture, vascular events and death.
Furthermore, dysregulation of calcium and phosphorous homeostasis in CKD leads to decreased renal phosphate excretion, increased serum phosphate, elevated levels of fibroblast growth factor 23 (FGF-23) and reduced synthesis of 1,25(OH)2 vitamin D. Elevated FGF-23 expression downregulates residual renal 1-alpha-hydroxylase (CYP27B1), which further exacerbates deficiency of 1,25(OH)2 vitamin D, acting as an additional driver to SHPT. Continuous stimulation of the parathyroid glands by a combination of elevated serum phosphate, decreased serum calcium and markedly reduced serum 1,25(OH)2 vitamin D levels leads to increased PTH synthesis and release [123].
Vitamin D deficiency is common in patients with CKD, particularly in patients with proteinuria, due to loss of 25-hydroxy (25-OH) vitamin D with vitamin D binding protein [124], and is associated with fractures [125]. The advantage of native vitamin D is maintenance of feedback mechanisms in the synthesis of of 1,25(OH)2 vitamin D and possible effects due to extrarenal hydroxylation, yielding extrarenal production of 1,25(OH)2 vitamin D. Vitamin D supplementation should be prescribed early in the course of renal disease. For treatment and prevention of vitamin D deficiency in CKD patients, recommendations for the general population could be indicative. However, uncertainty exists with respect to optimal levels of 25-OH vitamin D and the dietary reference intake (DRI) for native vitamin D to achieve these levels. While the US Institute of Medicine recommends a DRI of 600 IU/day for ages 1 to 70 years, and 800 IU/day for 71 years and older to achieve serum 25-OH vitamin D levels of 16-20 ng/mL (40–50 nmol/L) [126], alternative algorithms result in somewhat higher DRIs for non-CKD populations [127–129]. For patients with CKD, an intake of 800 IU/day has been recommended [130], which may need to be modified to achieve the desired target level of 25-OH vitamin D. In postmenopausal osteoporosis, single therapy with calcium or vitamin D has little effect on fractures, while the combination of both may prevent incidence of non-vertebral fractures [131]. However, a negative feedback mechanism of active vitamin D on additional vitamin D activation may reduce the effect of native vitamin D treatment beyond vitamin D repletion on fracture risk [132]. The efficacy of native and active vitamin D substitution for fracture risk reduction in CKD stages 4–5D is not sufficiently studied and different indications may apply for management of osteoporosis and secondary hyperparathyroidism in this setting.
Long-term calcium deficiency may predispose to osteoporosis, but BMD loss related to age or menopause cannot be prevented and/or treated with calcium supplementation only [133, 134]. Furthermore, there may be negative effects associated with high doses of calcium on enhanced risk of nephrolithiasis, arrhythmias, and cardiovascular risk, although these results remain inconclusive [135]. This consumption of excessive amounts of calcium in adults can be especially harmful in patients with CKD [136], particularly in the presence of hypercalcemia, low PTH levels, adynamic bone, concurrent warfarin treatment and/or existing cardiovascular calcifications [137]. The intake of moderate doses (up to 1000 mg/day) of oral calcium in combination with antiresorptive treatment for 1 year improved BMD but did not increase the risk of cardiovascular calcifications or arterial stiffness [138]. However, high doses may be of potential harm, at least theoretically [139]. Therefore, a reasonable approach could be to encourage an appropriate intake of calcium primarily through the diet [126], and to complete with moderate pharmacologic calcium supplementation only if the nutritional intake is insufficient. The routine use of pharmacological supplementation of calcium or calcium-based phosphate binders for all patients with CKD stages 4–5D cannot be recommended [140].
Antiresorptive treatment
Antiresorptive agents are currently the most prescribed bone-specific drugs for fracture prevention in non-CKD populations. They comprise bisphosphonates and denosumab, the latter being a monoclonal antibody against receptor activator of nuclear factor kappa-B ligand. Antiresorptives improve BMD by slowing bone turnover and allowing for an increased mineralization of resorption cavities [141]. Whereas the positive effect of bisphosphonates on BMD may diminish with time [142], denosumab has a continued effect on BMD for up to 10 years, possibly due to sustained bone modelling [141, 143]. A robust body of evidence indicates a continuous fracture risk reduction by bisphosphonates, which persists for several years after treatment cessation [142], and fracture prevention by denosumab has been demonstrated for at least 10 years in postmenopausal women with normal kidney function [144] and CKD stages 1–3 [145]. It can be speculated that the persistent fracture risk reduction by bisphosphonates may be related to long skeletal retention times and a persistent suppressive effect on bone turnover for years after discontinuation. In contrast, bone turnover increases rapidly after discontinuation of denosumab, paralleled by a deterioration of BMD and an increased fracture risk [146]. Sequential treatment with an anabolic agent outperforms bisphosphonates in the ability to prevent this rapid decline of BMD following discontinuation of denosumab [147]. Due to impaired renal clearance of bisphosphonates with risk for systemic accumulation in the setting of CKD and occasional reports of acute kidney failure associated with intravenous administration, bisphosphonates are relatively contraindicated in CKD stages 4–5D, and their use is off-label in most countries when eGFR is <30 mL/min/1.73 m2. Small studies in dialysis patients suggest a positive effect on BMD without increased risk for negative outcomes [148]. A recent observational study indicated similar effects of bisphosphonates on BMD gain in patients with CKD stages 1–3a and non-CKD patients, while the finding of a possible decreased effect in more advanced CKD was biased by very low patient numbers [149]. In addition, a moderate risk for CKD progression in patients with CKD stages 3b–5 was found in a large observational study, while bisphosphonate treatment was associated with improved survival, but only after propensity score matching [150]. Denosumab neither is cleared by the kidneys nor does it affect kidney function negatively, thus, it is not contraindicated in CKD stages 4–5D [14]. Several observational reports and some small RCTs indicate moderate to large effects on BMD without accelerated cardiovascular risk in end-stage renal disease [148]. A risk for hypocalcaemia exists following treatment with denosumab. This risk may be exaggerated by concomitant treatment with calcimimetics. Oral calcium and vitamin D can reduce the risk of severe or symptomatic hypocalcemia [151, 152]. Atypical femoral fractures and osteonecrosis of the jaw are rare complications of antiresorptive treatments, which do not occur more often in CKD than in other populations. Severe suppression of bone turnover has been discussed as an additional limitation, based on the concept that adynamic bone is a ‘disease’ and may contribute to negative bone and cardiovascular outcomes. The generalizability of this concept for any type of low bone turnover in CKD and, more specifically, to CKD patients on antiresorptive agents, has recently been challenged [148]. In summary, antiresorptive agents are safe and effective in CKD 1–3. Their use in CKD stages 4–5D can be beneficial, but should be based on individual evaluation, awaiting more direct evidence for fracture prevention in these patients.
Anabolic treatment
Currently, worldwide, two recombinant PTH analogues, teriparatide and abaloparatide, are available for the treatment of osteoporosis in postmenopausal women with high fracture risk. Post hoc analyses of pivotal trials demonstrated comparable efficacy on fracture risk reduction and BMD increases in patients with normal kidney function as compared with patients with CKD stage 1–3 and normal endogenous PTH levels [153, 154]. Regarding safety, in those with CKD, teriparatide more often induced hypercalcemia and hyperuricemia, but without accompanying increased incidence of clinical events such as nephrolithiasis or gout. Therefore, in CKD stage 1–3 patients with high fracture risk without elevated endogenous PTH, treatment with PTH analogues seems effective and safe, if adequately monitored.
For CKD stage 4–5 limited data are available. A small pilot study in haemodialysis patients with histomorphometrically proven adynamic bone demonstrated an increase in lumbar spine (but not femoral neck) BMD with daily-dosed teriparatide for 6 months [155]. Two small Japanese studies with weekly-dosed teriparatide demonstrated comparable effects in haemodialysis patients with biochemical signs of adynamic bone. In these studies, and in a Japanese post-marketing study in CKD stage 4–5 patients, teriparatide administration did not result in serious adverse events [156–158]. However, there are drawbacks to teriparatide administration in general. Consolidation therapy with antiresorptive treatment is needed after the use of PTH analogues and treatment duration is limited to 2 years because of an association of its long-term use with the development of osteosarcoma in rodent studies [159, 160]. In 2021, the United States Food and Drug Administration removed the time limit for treatment with teriparatide if a patient remains at or has returned to having a high risk for fracture [161].
To summarize, PTH analogues are effective and safe for fracture risk reduction in CKD stage 1–3 patients without metabolic derangements and with high fracture risk. In those with CKD stage 4–5 and signs of adynamic bone, PTH analogues can be considered for fracture risk reduction on an individual basis. Caution is needed because of a lack of data in this specific population, but identifying the CKD patient that may benefit from this form of anabolic treatment might be rewarding.
Romosozumab
Romosozumab is a fully human monoclonal antibody against sclerostin. Sclerostin, encoded by the gene SOST, is an osteocyte-secreted glycoprotein that has been identified as a pivotal regulator of bone formation. By inhibiting the Wnt and bone morphogenetic protein signalling pathways, sclerostin impedes osteoblast proliferation and function, thereby decreasing bone formation. In clinical trials, romosozumab resulted in an increase in BMD to a greater extent than alendronate and teriparatide with a decrease in risk of vertebral and nonvertebral fractures in postmenopausal women [162–164]. Romosozumab also increased the spine and hip BMD compared with placebo in men with osteoporosis [165]. Bone turnover marker data from these trials suggest an uncoupling of bone remodelling in favour of bone formation, which could be an asset in patients with CKD, acknowledging the high prevalence of low bone turnover in this patient population. Of equally great interest to CKD patients is the observation that BMD gains in postmenopausal women are accentuated in cortical bone [166], which is commonly affected in CKD. Unfortunately, clinical studies definitely proving the efficacy of romosozumab in the setting of CKD are neither available, nor currently scheduled. An observational report of 1 year of romosozumab treatment in haemodialysis patients demonstrated a positive effect on BMD without an increased incidence of cardiovascular events, compared with age- and gender-matched controls [167]. However, 61.5% of romosozumab-treated patients were pre-treated with bisphosphonates, which was stopped at initiation of romosozumab treatment. A recent post hoc analysis on data of two registration trials showed that the efficacy and safety of romosozumab versus alendronate or placebo among postmenopausal women with osteoporosis was similar at different levels of kidney function [168]. While these data on bone outcomes are reassuring, a numerically higher incidence of cardiovascular adverse events in the romosozumab group [164, 169] warrants caution and calls for additional safety data, especially in high-risk patients, which certainly includes patients with CKD. The putative higher cardiovascular risk may be explained by accentuated vascular calcification [170]. Furthermore, sclerostin may contribute to phosphate homeostasis by stimulating FGF23 expression in bone [171]. Of note, romosozumab therapy can induce profound hypocalcemia in patients with CKD stages 4–5D, which may be exaggerated by concomitant treatment with calcimimetics, necessitating close monitoring of serum calcium after initiation of romosozumab treatment in this population [172].
Menopausal hormone therapy and SERMs
The hypothalamic–pituitary–gonadal axis is disrupted in CKD [173]. Consequently, early menopause or hypogonadism are highly prevalent among CKD patients. Hormone replacement therapy (HRT) may be hypothesized to play a prominent role in the management of osteoporosis in CKD. Questions regarding the benefit–risk profile [174], lack of data from RCTs and availability of more potent alternatives has dampened the enthusiasm for post-menopausal HRT and selective oestrogen receptor modulators (SERMs) in the general population. However, current evidence reveals a benefit–risk profile that supports HRT treatment in women who have recently (<10 years) become menopausal, have menopausal symptoms and are <60 years old, with a low baseline risk for adverse events [175]. The limited data from RCTs precludes clear guidance with regard to HRT and SERMs in CKD patients [14, 176]. Acknowledging the increased cardiovascular risks (including a high risk for thromboembolic events) in patients with CKD, the benefit–risk profile may prove to be inferior.
Calcimimetics and parathyroidectomy
Hypo- and hypercalcaemia modulate PTH release and activation of its receptor (PTHR) in the kidneys, intestines, and bone, with the aim of restoring calcium equilibrium. Clinicians dealing with secondary hyperparathyroidism might be more attracted by the effects on the PTH/PTHR than on the calcium/Calcium sensing receptor (CaSR) system. However, the role of CaSR could be relevant since CaSR knockout animals die after birth, while the PTH-knockout and the double PTH-CaSR knockout animals survive. Therefore, distinct roles can be considered for the PTH/PTHR and Ca/CaSR systems when treating CKD patients.
Administration of high doses of PTH to PTH knockout and double PTH/CaSR knockout mice results in lower cortical and trabecular resorption in the latter, illustrating the complementary role of CaSR on PTH-induced bone effects [177]. In 5/6 nephrectomized rats developing ROD, PTH bone effects are mitigated by calcimimetics through PTH suppression, but direct bone effects are also possible as well, since bone cells express CaSR. Indeed, after parathyroidectomy and during PTH infusion to abolish treatment-related PTH declines, calcimimetics promoted bone formation rate and anabolic pathways in osteoblasts [178]. These results clearly demonstrate that, when treating ROD, direct effects of calcimimetics on bone may occur independently of PTH suppression and with potential effects on fracture rate [179]. In clinical practice, calcimimetics suppress PTH and can modify the bone phenotype [180]. Although there is a lack of high-quality evidence to support an effect of cinacalcet on fracture risk reduction in CKD stages 4–5D, post hoc analyses of placebo-controlled trials suggest that some effect may exist [181, 182]. Further subgroup analysis of the Evaluation of Cinacalcet Hydrochloride Therapy to Lower Cardiovascular Events (EVOLVE) trial indicates that it may be prudent to consider calcium balance when treating patients with high fracture risk with cinacalcet [181].
As for parathyroidectomy, in both primary [183] and secondary hyperparathyroidism [184], BMD increases after surgery in particular in patients with osteoporosis. In addition, a large study from US Renal Data System consistently shows that parathyroidectomy invariably diminishes fracture risk in haemodialysis patients [185]. However, as a trade-off, hospitalization rates may increase significantly early after and within 1 year of surgery [186].
As a whole, among the number of factors affecting bone strength [187], PTH reduction, either surgically or pharmacologically, is expected to reduce fracture rate in CKD patients with hyperparathyroidism, through different mechanisms and possibly according to the baseline bone status.
CONCLUSIONS
The increasing awareness of the high risk for fragility fractures among patients with CKD calls for improved guidance for evaluation, prevention, and treatment. The current review summarizes the evolving evidence in support of fracture risk evaluation and treatment in patients with all stages of CKD. We demonstrate that established diagnostic and therapeutic strategies may be safely and effectively applied also in patients with more advanced CKD and that risks, including suppression or stimulation of bone turnover, can be managed, based on individual patient evaluation. New diagnostic and therapeutic tools have the potential to improve tailoring of treatment strategies to individual patient needs. In spite of the current scarcity of evidence from randomized controlled trials for fracture prevention in CKD stages 4–5D, we advocate an active approach, based on the accumulating evidence, to close the treatment gap and meet the need of patients who are at risk for or suffer from fragility fractures. This approach has the potential to improve quality of life and reduce the increased mortality risk associated with fragility fractures.
ACKNOWLEDGEMENTS
The CKD-MBD Working Group is an official body of the ERA.
Contributor Information
Mathias Haarhaus, Division of Renal Medicine, Department of Clinical Science, Intervention and Technology, Karolinska University Hospital, Karolinska Institutet, Stockholm, Sweden; Diaverum Sweden, Malmö, Sweden.
Louise Aaltonen, Turku University Hospital, Kidney Center, Department of Medicine, Turku, Finland.
Daniel Cejka, Department of Medicine III – Nephrology, Hypertension, Transplantation, Rheumatology, Geriatrics, Ordensklinikum Linz - Elisabethinen Hospital, Linz, Austria.
Mario Cozzolino, Department of Health Sciences, Renal Division, University of Milan, ASST Santi Paolo e Carlo, Milan, Italy.
Renate T de Jong, Amsterdam UMC location Vrije Universiteit Amsterdam, Department of Internal Medicine and Endocrinology, Amsterdam, The Netherlands; Amsterdam Gastroenterology Endocrinology Metabolism Institute, Amsterdam, The Netherlands.
Patrick D'Haese, Laboratory of Pathophysiology, Department of Biomedical Sciences, University of Antwerp, Antwerp, Belgium.
Pieter Evenepoel, Department of Nephrology, University Hospitals Leuven, Leuven, Belgium; Department of Microbiology Immunology and Transplantation, Nephrology and Renal Transplantation Research Group, KU Leuven-University of Leuven, Leuven, Belgium.
Marie-Hélène Lafage-Proust, INSERM U1059, CHU, Université de Lyon, Saint-Etienne, France.
Sandro Mazzaferro, Nephrology Unit at Policlinico Umberto I Hospital and Department of Translation and Precision Medicine, Sapienza University of Rome, Rome, Italy.
Eugene McCloskey, Academic Unit of Bone Metabolism, Centre for Integrated research in Musculoskeletal Ageing, Mellanby Centre for Musculoskeletal Research, Department of Oncology & Metabolism, University of Sheffield, Sheffield, UK.
Syazrah Salam, Department of Oncology and Metabolism, University of Sheffield, Sheffield, UK and Sheffield Kidney Institute, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, UK.
Hanne Skou Jørgensen, Department of Microbiology Immunology and Transplantation, Nephrology and Renal Transplantation Research Group, KU Leuven-University of Leuven, Leuven, Belgium; Aarhus University Hospital, Department of Kidney Diseases, Aarhus, Denmark; Aarhus University, Department of Clinical Medicine, Aarhus, Denmark.
Marc Vervloet, Department of Nephrology, Amsterdam University Medical Center, VU University Amsterdam, Amsterdam, The Netherlands; Amsterdam Cardiovascular Sciences, Amsterdam University Medical Center, Amsterdam, The Netherlands.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
CONFLICT OF INTEREST STATEMENT
M.H. is a full-time employee of Diaverum Sverige AB and advisory board member at Resverlogix, all unrelated to the submitted work. L.A. has nothing to disclose. D.C. reports grants from Vifor and Amgen, consulting fees from Vifor, UCB, Stada and Gedeon Richter, and honoraria from Vifor, UCB and Takeda. M.C. reports honoraria and travel support from Vifor and honoraria from Amgen, all unrelated to the submitted work. He is chair of the European Best Practice Committee, Council Member of the European Renal Association and Member of the CKJ Editorial Board. R.T.d.J. has received a grant from Takeda, unrelated to the submitted work. P.D'H. has nothing to disclose. P.E. reports grants from Amgen and grants, consulting fees, travel support and honoraria from Vifor FMC, all unrelated to the submitted work. M.-H.L.-P. reports grants and non-financial support from Amgen, unrelated to the submitted work. S.M. reports honoraria and travel support from Vifor Pharma, unrelated to the submitted work. He is board member of the Società Italiana Nefrologia. E.M. has receied grants from the Royal Osteoporosis Society and ObsEva, consulting fees and honoraria from Amgen and UCB, consulting fees from Fresenius Kabi, and is chair of Osteoporosis 2000 and member of NOGG Advisory Group. H.S.J. and S.S. have nothing to disclose. M.V. reports grants from the Dutch Kidney Foundation and from the Dutch Ministry of Economic Affairs, grants and honoraria from Vifor Pharma, Amgen and Fresenius Medical Care, grants from AstraZeneca, Otsuka, Kyowa Kirin, GSK and Baxter, all unrelated to the submitted work. He is member of the DIPAC trial advisory board chair of the European Renal Association CKD-MBD Working Group.
REFERENCES
- 1. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group . KDIGO 2017 Clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl 2017;7:1–59. 10.1016/j.kisu.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hara T, Hijikata Y, Matsubara Yet al. Pharmacological interventions versus placebo, no treatment or usual care for osteoporosis in people with chronic kidney disease stages 3-5D. Cochrane Database Syst Rev 2021;7:CD013424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen CH, Lo WC, Hu PJet al. Efficacy of osteoporosis medications for patients with chronic kidney disease: an updated systematic review and network meta-analysis. Front Pharmacol 2022;13:822178. 10.3389/fphar.2022.822178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kanis JA, Johansson H, Harvey NCet al. A brief history of FRAX. Arch Osteoporos 2018;13:118. 10.1007/s11657-018-0510-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bowling CB, Bromfield SG, Colantonio LDet al. Association of reduced eGFR and albuminuria with serious fall injuries among older adults. Clin J Am Soc Nephrol 2016;11:1236–43. 10.2215/CJN.11111015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rashid A, Chaudhary Hauge S, Suetta Cet al. “Sarcopenia and risk of osteoporosis, falls and bone fractures in patients with chronic kidney disease: a systematic review”. PLoS One 2022;17:e0262572. 10.1371/journal.pone.0262572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Naylor KL, Garg AX, Zou Get al. Comparison of fracture risk prediction among individuals with reduced and normal kidney function. Clin J Am Soc Nephrol 2015;10:646–53. 10.2215/CJN.06040614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Whitlock RH, Leslie WD, Shaw Jet al. The fracture risk assessment tool (FRAX®) predicts fracture risk in patients with chronic kidney disease. Kidney Int 2019;95:447–54. 10.1016/j.kint.2018.09.022 [DOI] [PubMed] [Google Scholar]
- 9. Desbiens LC, Sidibé A, Beaudoin Cet al. Comparison of fracture prediction tools in individuals without and with early chronic kidney disease: a population-based analysis of CARTaGENE. J Bone Miner Res 2020;35:1048–57. 10.1002/jbmr.3977 [DOI] [PubMed] [Google Scholar]
- 10. Przedlacki J, Buczyńska-Chyl J, Koźmiński Pet al. The utility of FRAX® in predicting bone fractures in patients with chronic kidney disease on hemodialysis: a two-year prospective multicenter cohort study. Osteoporos Int 2018;29:1105–15. 10.1007/s00198-018-4406-z [DOI] [PubMed] [Google Scholar]
- 11. Przedlacki J, Buczyńska-Chyl J, Koźmiński Pet al. FRAX prognostic and intervention thresholds in the management of major bone fractures in hemodialysis patients: a two-year prospective multicenter cohort study. Bone 2020;133:115188. 10.1016/j.bone.2019.115188 [DOI] [PubMed] [Google Scholar]
- 12. Malluche HH, Porter DS, Monier-Faugere MCet al. Differences in bone quality in low- and high-turnover renal osteodystrophy. J Am Soc Nephrol 2012;23:525–32. 10.1681/ASN.2010121253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iconaru L, Charles A, Baleanu Fet al. Prediction of an imminent fracture after an index fracture - models derived from the Frisbee Cohort. J Bone Miner Res 2022;37:59–67. 10.1002/jbmr.4432 [DOI] [PubMed] [Google Scholar]
- 14. Evenepoel P, Cunningham J, Ferrari Set al. European consensus statement on the diagnosis and management of osteoporosis in chronic kidney disease stages G4-G5D. Nephrol Dial Transplantat 2021;36:42–59. 10.1093/ndt/gfaa192 [DOI] [PubMed] [Google Scholar]
- 15. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser 1994;843:1–129 [PubMed] [Google Scholar]
- 16. Johnell O, Kanis JA, Oden Aet al. Predictive value of BMD for hip and other fractures. J Bone Miner Res 2005;20:1185–94. 10.1359/JBMR.050304 [DOI] [PubMed] [Google Scholar]
- 17. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group . KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int 2009;76(Suppl 113):S1–130. [DOI] [PubMed] [Google Scholar]
- 18. Gómez-Islas VE, García-Fong KR, Aguilar-Fuentes REet al. Evaluation of bone densitometry by dual-energy x-ray absorptiometry as a fracture prediction tool in women with chronic kidney disease. Bone Rep 2020;13:100298. 10.1016/j.bonr.2020.100298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. International Society for Clinical Densitometry. ISCD Official Position Adults. 2019. https://iscd.org/learn/official-positions/adult-positions/ [Google Scholar]
- 20. Silva BC, Leslie WD, Resch Het al. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res 2014;29:518–30. 10.1002/jbmr.2176 [DOI] [PubMed] [Google Scholar]
- 21. McCloskey EV, Odén A, Harvey NCet al. A meta-analysis of trabecular bone score in fracture risk prediction and its relationship to FRAX. J Bone Miner Res 2016;31:940–8. 10.1002/jbmr.2734 [DOI] [PubMed] [Google Scholar]
- 22. McCloskey EV, Odén A, Harvey NCet al. Adjusting fracture probability by trabecular bone score. Calcif Tissue Int 2015;96:500–9. 10.1007/s00223-015-9980-x [DOI] [PubMed] [Google Scholar]
- 23. McCloskey E, Harvey NC, Lorentzon Met al. Trabecular bone score adjustment for the fracture risk assessment tool (FRAX®). Calcif Tissue Int 2022;111:226–7. 10.1007/s00223-022-00994-w [DOI] [PubMed] [Google Scholar]
- 24. Shevroja E, Lamy O, Hans D. Review on the utility of trabecular bone score, a surrogate of bone micro-architecture, in the chronic kidney disease spectrum and in kidney transplant recipients. Front Endocrinol 2018;9:561. 10.3389/fendo.2018.00561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Naylor KL, Prior J, Garg AXet al. Trabecular bone score and incident fragility fracture risk in adults with reduced kidney function. Clin J Am Soc Nephrol 2016;11:2032–40. 10.2215/CJN.00720116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rampersad C, Whitlock RH, Leslie WDet al. Trabecular bone score in patients with chronic kidney disease. Osteoporos Int 2020;31:1905–12. 10.1007/s00198-020-05458-1 [DOI] [PubMed] [Google Scholar]
- 27. Marques ID, Araújo MJ, Graciolli FGet al. Biopsy vs. peripheral computed tomography to assess bone disease in CKD patients on dialysis: differences and similarities. Osteoporos Int 2017;28:1675–83. 10.1007/s00198-017-3956-9 [DOI] [PubMed] [Google Scholar]
- 28. Nickolas TL, Stein E, Cohen Aet al. Bone mass and microarchitecture in CKD patients with fracture. J Am Soc Nephrol 2010;21:1371–80. 10.1681/ASN.2009121208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cejka D, Patsch JM, Weber Met al. Bone microarchitecture in hemodialysis patients assessed by HR-pQCT. Clin J Am Soc Nephrol 2011;6:2264–71. 10.2215/CJN.09711010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nickolas TL, Stein EM, Dworakowski Eet al. Rapid cortical bone loss in patients with chronic kidney disease. J Bone Miner Res 2013;28:1811–20. 10.1002/jbmr.1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bielesz B, Patsch JM, Fischer Let al. Cortical porosity not superior to conventional densitometry in identifying hemodialysis patients with fragility fracture. PLoS One 2017;12:e0171873. 10.1371/journal.pone.0171873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Negri AL, Del Valle EE, Zanchetta MBet al. Evaluation of bone microarchitecture by high-resolution peripheral quantitative computed tomography (HR-pQCT) in hemodialysis patients. Osteoporos Int 2012;23:2543–50. 10.1007/s00198-011-1890-9 [DOI] [PubMed] [Google Scholar]
- 33. Trombetti A, Stoermann C, Chevalley Tet al. Alterations of bone microstructure and strength in end-stage renal failure. Osteoporos Int 2013;24:1721–32. 10.1007/s00198-012-2133-4 [DOI] [PubMed] [Google Scholar]
- 34. Nishiyama KK, Pauchard Y, Nikkel LEet al. Longitudinal HR-pQCT and image registration detects endocortical bone loss in kidney transplantation patients. J Bone Miner Res 2015;30:554–61. 10.1002/jbmr.2358 [DOI] [PubMed] [Google Scholar]
- 35. Ghasem-Zadeh A, Bui M, Seeman Eet al. Bone microarchitecture and estimated failure load are deteriorated whether patients with chronic kidney disease have normal bone mineral density, osteopenia or osteoporosis. Bone 2022;154:116260. 10.1016/j.bone.2021.116260 [DOI] [PubMed] [Google Scholar]
- 36. Russo CR, Taccetti G, Caneva Pet al. Volumetric bone density and geometry assessed by peripheral quantitative computed tomography in uremic patients on maintenance hemodialysis. Osteoporos Int 1998;8:443–8. 10.1007/s001980050089 [DOI] [PubMed] [Google Scholar]
- 37. Sharma AK, Toussaint ND, Elder GJet al. Magnetic resonance imaging based assessment of bone microstructure as a non-invasive alternative to histomorphometry in patients with chronic kidney disease. Bone 2018;114:14–21. 10.1016/j.bone.2018.05.029 [DOI] [PubMed] [Google Scholar]
- 38. Nickolas TL, Stein EM, Dworakowski Eet al. Rapid cortical bone loss in patients with chronic kidney disease. J Bone Miner Res 2013;28:1811–20. 10.1002/jbmr.1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Borggrefe J, de Buhr T, Shrestha Set al. Association of 3D geometric measures derived from quantitative computed tomography with hip fracture risk in older men. J Bone Miner Res 2016;31:1550–8. 10.1002/jbmr.2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ito M, Wakao N, Hida Tet al. Analysis of hip geometry by clinical CT for the assessment of hip fracture risk in elderly Japanese women. Bone 2010;46:453–7. 10.1016/j.bone.2009.08.059 [DOI] [PubMed] [Google Scholar]
- 41. Chiba K, Yamada S, Yoda Iet al. Effects of monthly intravenous ibandronate on bone mineral density and microstructure in patients with primary osteoporosis after teriparatide treatment: the MONUMENT study. Bone 2021;144:115770. 10.1016/j.bone.2020.115770 [DOI] [PubMed] [Google Scholar]
- 42. Borggrefe J, Graeff C, Nickelsen TNet al. Quantitative computed tomographic assessment of the effects of 24 months of teriparatide treatment on 3D femoral neck bone distribution, geometry, and bone strength: results from the EUROFORS study. J Bone Miner Res 2010;25:472–81. 10.1359/jbmr.090820 [DOI] [PubMed] [Google Scholar]
- 43. Negri AL, Barone R, Lombas Cet al. Evaluation of cortical bone by peripheral quantitative computed tomography in continuous ambulatory peritoneal dialysis patients. Hemodial Int 2006;10:351–5. 10.1111/j.1542-4758.2006.00128.x [DOI] [PubMed] [Google Scholar]
- 44. Negri AL, Lombas C, Cuevas Cet al. Evaluation of cortical bone by peripheral quantitative computed tomography in renal transplant recipients. Transplant Proc 2005;37:1020–2. 10.1016/j.transproceed.2004.12.056 [DOI] [PubMed] [Google Scholar]
- 45. Boutroy S, Van Rietbergen B, Sornay-Rendu Eet al. Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res 2008;23:392–9. 10.1359/jbmr.071108 [DOI] [PubMed] [Google Scholar]
- 46. Samelson EJ, Broe KE, Xu Het al. Cortical and trabecular bone microarchitecture as an independent predictor of incident fracture risk in older women and men in the bone microarchitecture international consortium (BoMIC): a prospective study. Lancet Diabetes Endocrinol 2019;7:34–43. 10.1016/S2213-8587(18)30308-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Al-Beyatti Y, Siddique M, Frost MLet al. Precision of ¹⁸F-fluoride PET skeletal kinetic studies in the assessment of bone metabolism. Osteoporos Int 2012;23:2535–41. 10.1007/s00198-011-1889-2 [DOI] [PubMed] [Google Scholar]
- 48. Even-Sapir E, Mishani E, Flusser Get al. 18F-Fluoride positron emission tomography and positron emission tomography/computed tomography. Semin Nucl Med 2007;37:462–9. 10.1053/j.semnuclmed.2007.07.002 [DOI] [PubMed] [Google Scholar]
- 49. Czernin J, Satyamurthy N, Schiepers C. Molecular mechanisms of bone 18F-NaF deposition. J Nucl Med 2010;51:1826–9. 10.2967/jnumed.110.077933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Aaltonen L, Koivuviita N, Seppänen Met al. Correlation between (18)F-sodium fluoride positron emission tomography and bone histomorphometry in dialysis patients. Bone 2020;134:115267. 10.1016/j.bone.2020.115267 [DOI] [PubMed] [Google Scholar]
- 51. Piert M, Zittel TT, Becker GAet al. Assessment of porcine bone metabolism by dynamic [18F]fluoride ion PET: correlation with bone histomorphometry. J Nucl Med 2001;42:1091–100. [PubMed] [Google Scholar]
- 52. Frost ML, Cook GJ, Blake GMet al. A prospective study of risedronate on regional bone metabolism and blood flow at the lumbar spine measured by 18F-fluoride positron emission tomography. J Bone Miner Res 2003;18:2215–22. 10.1359/jbmr.2003.18.12.2215 [DOI] [PubMed] [Google Scholar]
- 53. Frost ML, Moore AE, Siddique Met al. ¹⁸F-fluoride PET as a noninvasive imaging biomarker for determining treatment efficacy of bone active agents at the hip: a prospective, randomized, controlled clinical study. J Bone Miner Res 2013;28:1337–47. 10.1002/jbmr.1862 [DOI] [PubMed] [Google Scholar]
- 54. Irkle A, Vesey AT, Lewis DYet al. Identifying active vascular microcalcification by (18)F-sodium fluoride positron emission tomography. Nat Commun 2015;6:7495. 10.1038/ncomms8495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Joshi NV, Vesey AT, Williams MCet al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet North Am Ed 2014;383:705–13. 10.1016/S0140-6736(13)61754-7 [DOI] [PubMed] [Google Scholar]
- 56. Arani LS, Gharavi MH, Zadeh MZet al. Association between age, uptake of 18 F-fluorodeoxyglucose and of 18 F-sodium fluoride, as cardiovascular risk factors in the abdominal aorta. Hell J Nucl Med 2019;22:14–19. [DOI] [PubMed] [Google Scholar]
- 57. Aaltonen L, Koivuviita N, Seppänen Met al. Association between bone mineral metabolism and vascular calcification in end-stage renal disease. BMC Nephrol 2022;23:12. 10.1186/s12882-021-02652-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Asci G, Ok E, Savas Ret al. The link between bone and coronary calcifications in CKD-5 patients on haemodialysis. Nephrol Dial Transplant 2011;26:1010–5. 10.1093/ndt/gfq491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Miller P. The role of bone biopsy in patients with chronic renal failure. Clin J Am Soc Nephrol 2008;3Suppl 3:S140–50. 10.2215/CJN.02430508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Evenepoel P, D'Haese P, Bacchetta Jet al. Bone biopsy practice patterns across Europe: the European renal osteodystrophy initiative-a position paper. Nephrol Dial Transplant 2017;32:1608–13. [DOI] [PubMed] [Google Scholar]
- 61. Novel-Catin E, Pelletier S, Fouque Det al. Quantitative histomorphometric analysis of halved iliac crest bone biopsies yield comparable ROD diagnosis as full 7.5 mm wide samples. Bone 2020;138:115460. 10.1016/j.bone.2020.115460 [DOI] [PubMed] [Google Scholar]
- 62. Moe S, Drueke T, Cunningham Jet al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from kidney disease: improving global outcomes (KDIGO). Kidney Int 2006;69:1945–53. 10.1038/sj.ki.5000414 [DOI] [PubMed] [Google Scholar]
- 63. Evenepoel P, Behets GJS, Laurent MRet al. Update on the role of bone biopsy in the management of patients with CKD-MBD. J Nephrol 2017;30:645–52. 10.1007/s40620-017-0424-8 [DOI] [PubMed] [Google Scholar]
- 64. Schoeb M, Hamdy NAT, Malgo Fet al. Added value of impact microindentation in the evaluation of bone fragility: a systematic review of the literature. Front Endocrinol 2020;11:15. 10.3389/fendo.2020.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Malluche HH, Porter DS, Mawad Het al. Low-energy fractures without low T-scores characteristic of osteoporosis: a possible bone matrix disorder. J Bone Joint Surg 2013;95:e1391–6. 10.2106/JBJS.L.01281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Holloway-Kew KL, Rufus-Membere P, Anderson KBet al. Bone material strength index is associated with prior fracture in men with and without moderate chronic kidney disease. Bone 2020;133:115241. 10.1016/j.bone.2020.115241 [DOI] [PubMed] [Google Scholar]
- 67. Rozental TD, Walley KC, Demissie Set al. Bone material strength index as measured by impact microindentation in postmenopausal women with distal radius and hip fractures. J Bone Miner Res 2018;33:621–6. 10.1002/jbmr.3338 [DOI] [PubMed] [Google Scholar]
- 68. Malgo F, Hamdy NAT, Papapoulos SEet al. Bone material strength index as measured by impact microindentation is low in patients with fractures irrespective of fracture site. Osteoporos Int 2017;28:2433–7. 10.1007/s00198-017-4054-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Malgo F, Hamdy NA, Papapoulos SEet al. Bone material strength as measured by microindentation in vivo is decreased in patients with fragility fractures independently of bone mineral density. J Clin Endocrinol Metab 2015;100:2039–45. 10.1210/jc.2014-4346 [DOI] [PubMed] [Google Scholar]
- 70. Rokidi S, Bravenboer N, Gamsjaeger Set al. Impact microindentation assesses subperiosteal bone material properties in humans. Bone 2020;131:115110. 10.1016/j.bone.2019.115110 [DOI] [PubMed] [Google Scholar]
- 71. Samakkarnthai P, Sfeir JG, Atkinson EJet al. Determinants of bone material strength and cortical porosity in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 2020;105:e3718–29. 10.1210/clinem/dgaa388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rudang R, Zoulakis M, Sundh Det al. Bone material strength is associated with areal BMD but not with prevalent fractures in older women. Osteoporos Int 2016;27:1585–92. 10.1007/s00198-015-3419-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Iimori S, Mori Y, Akita Wet al. Diagnostic usefulness of bone mineral density and biochemical markers of bone turnover in predicting fracture in CKD stage 5D patients—a single-center cohort study. Nephrol Dial Transplant 2012;27:345–51. 10.1093/ndt/gfr317 [DOI] [PubMed] [Google Scholar]
- 74. Ketteler M, Block GA, Evenepoel Pet al. Executive summary of the 2017 KDIGO chronic kidney disease-mineral and bone disorder (CKD-MBD) guideline update: what's changed and why it matters. Kidney Int 2017;92:26–36. 10.1016/j.kint.2017.04.006 [DOI] [PubMed] [Google Scholar]
- 75. Sprague SM, Bellorin-Font E, Jorgetti Vet al. Diagnostic accuracy of bone turnover markers and bone histology in patients with CKD treated by dialysis. Am J Kidney Dis 2016;67:559–66. 10.1053/j.ajkd.2015.06.023 [DOI] [PubMed] [Google Scholar]
- 76. Vervloet MG, Brandenburg VM, CKD-MBD working group of ERA-EDTA. Circulating markers of bone turnover. J Nephrol 2017;30:663–70. 10.1007/s40620-017-0408-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Koivula MK, Ruotsalainen V, Bjorkman Met al. Difference between total and intact assays for N-terminal propeptide of type I procollagen reflects degradation of pN-collagen rather than denaturation of intact propeptide. Ann Clin Biochem 2010;47:67–71. 10.1258/acb.2009.009110 [DOI] [PubMed] [Google Scholar]
- 78. Lv Y, Wang G, Xu Wet al. Tartrate-resistant acid phosphatase 5b is a marker of osteoclast number and volume in RAW 264.7 cells treated with receptor-activated nuclear κB ligand. Exp Ther Med 2015;9:143–6. 10.3892/etm.2014.2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yamada S, Inaba M, Kurajoh Met al. Utility of serum tartrate-resistant acid phosphatase (TRACP5b) as a bone resorption marker in patients with chronic kidney disease: independence from renal dysfunction. Clin Endocrinol (Oxf) 2008;69:189–96. 10.1111/j.1365-2265.2008.03187.x [DOI] [PubMed] [Google Scholar]
- 80. Salam S, Gallagher O, Gossiel Fet al. Diagnostic accuracy of biomarkers and imaging for bone turnover in renal osteodystrophy. J Am Soc Nephrol 2018;29:1557–65. 10.1681/ASN.2017050584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jørgensen HS, Behets G, Viaene Let al. Diagnostic accuracy of noninvasive bone turnover markers in renal osteodystrophy. Am J Kidney Dis 2022;79:667–76.e1. 10.1053/j.ajkd.2021.07.027 [DOI] [PubMed] [Google Scholar]
- 82. Stehman-Breen CO, Sherrard DJ, Alem AMet al. Risk factors for hip fracture among patients with end-stage renal disease. Kidney Int 2000;58:2200–5. 10.1111/j.1523-1755.2000.00394.x [DOI] [PubMed] [Google Scholar]
- 83. Chauveau P, Vendrely B, El Haggan Wet al. Body composition of patients on a very low-protein diet: a two-year survey with DEXA. J Ren Nutr 2003;13:282–7. 10.1016/S1051-2276(03)00117-1 [DOI] [PubMed] [Google Scholar]
- 84. Jørgensen HS, Eide IA, Hartmann Aet al. Plasma n-3 polyunsaturated fatty acids and bone mineral density in renal transplant recipients. J Ren Nutr 2016;26:196–203. 10.1053/j.jrn.2015.11.007 [DOI] [PubMed] [Google Scholar]
- 85. Evenepoel P, Claes K, Meijers Bet al. Poor vitamin K status is associated with low bone mineral density and increased fracture risk in end-stage renal disease. J Bone Miner Res 2019;34:262–9. 10.1002/jbmr.3608 [DOI] [PubMed] [Google Scholar]
- 86. Johansson H, Kanis JA, Odén Aet al. A meta-analysis of the association of fracture risk and body mass index in women. J Bone Miner Res 2014;29:223–33. 10.1002/jbmr.2017 [DOI] [PubMed] [Google Scholar]
- 87. Jadoul M, Albert JM, Akiba Tet al. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int 2006;70:1358–66. 10.1038/sj.ki.5001754 [DOI] [PubMed] [Google Scholar]
- 88. Evenepoel P, Claes K, Meijers Bet al. Bone mineral density, bone turnover markers, and incident fractures in de novo kidney transplant recipients. Kidney Int 2019;95:1461–70. 10.1016/j.kint.2018.12.024 [DOI] [PubMed] [Google Scholar]
- 89. Yeung SSY, Reijnierse EM, Pham VKet al. Sarcopenia and its association with falls and fractures in older adults: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 2019;10:485–500. 10.1002/jcsm.12411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Reese PP, Cappola AR, Shults Jet al. Physical performance and frailty in chronic kidney disease. Am J Nephrol 2013;38:307–15. 10.1159/000355568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Delgado C, Shieh S, Grimes Bet al. Association of self-reported frailty with falls and fractures among patients new to dialysis. Am J Nephrol 2015;42:134–40. 10.1159/000439000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. West SL, Jamal SA, Lok CE. Tests of neuromuscular function are associated with fractures in patients with chronic kidney disease. Nephrol Dial Transplant 2012;27:2384–8. 10.1093/ndt/gfr620 [DOI] [PubMed] [Google Scholar]
- 93. Rosa CSDC, Nishimoto DY, Souza GDEet al. Effect of continuous progressive resistance training during hemodialysis on body composition, physical function and quality of life in end-stage renal disease patients: a randomized controlled trial. Clin Rehabil 2018;32:899–908. 10.1177/0269215518760696 [DOI] [PubMed] [Google Scholar]
- 94. Kirkman DL, Mullins P, Junglee NAet al. Anabolic exercise in haemodialysis patients: a randomised controlled pilot study. J Cachexia Sarcopenia Muscle 2014;5:199–207. 10.1007/s13539-014-0140-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bolam KA, van Uffelen JG, Taaffe DR. The effect of physical exercise on bone density in middle-aged and older men: a systematic review. Osteoporos Int 2013;24:2749–62. 10.1007/s00198-013-2346-1 [DOI] [PubMed] [Google Scholar]
- 96. Zehnacker CH, Bemis-Dougherty A. Effect of weighted exercises on bone mineral density in post menopausal women. A systematic review. J Geriatr Phys Ther 2007;30:79–88. 10.1519/00139143-200708000-00007 [DOI] [PubMed] [Google Scholar]
- 97. Cardoso DF, Marques EA, Leal DVet al. Impact of physical activity and exercise on bone health in patients with chronic kidney disease: a systematic review of observational and experimental studies. BMC Nephrol 2020;21:334. 10.1186/s12882-020-01999-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yusuf AA, Hu Y, Chandler Det al. Predictors of imminent risk of fracture in Medicare-enrolled men and women. Arch Osteoporos 2020;15:120. 10.1007/s11657-020-00784-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Rossier A, Pruijm M, Hannane Det al. Incidence, complications and risk factors for severe falls in patients on maintenance haemodialysis. Nephrol Dial Transplant 2012;27:352–7. 10.1093/ndt/gfr326 [DOI] [PubMed] [Google Scholar]
- 100. Wang AY, Sherrington C, Toyama Tet al. Muscle strength, mobility, quality of life and falls in patients on maintenance haemodialysis: a prospective study. Nephrology 2017;22:220–7. 10.1111/nep.12749 [DOI] [PubMed] [Google Scholar]
- 101. Heung M, Adamowski T, Segal JHet al. A successful approach to fall prevention in an outpatient hemodialysis center. Clin J Am Soc Nephrol 2010;5:1775–9. 10.2215/CJN.01610210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Mortensen SJ, Mohamadi A, Wright CLet al. Medications as a risk factor for fragility hip fractures: a systematic review and meta-analysis. Calcif Tissue Int 2020;107:1–9. 10.1007/s00223-020-00688-1 [DOI] [PubMed] [Google Scholar]
- 103. Hansen D, Olesen JB, Gislason GHet al. Risk of fracture in adults on renal replacement therapy: a Danish national cohort study. Nephrol Dial Transplant 2016;31:1654–62. 10.1093/ndt/gfw073 [DOI] [PubMed] [Google Scholar]
- 104. van der Burgh AC, Oliai Araghi S, Zillikens MCet al. The impact of thiazide diuretics on bone mineral density and the trabecular bone score: the Rotterdam Study. Bone 2020;138:115475. 10.1016/j.bone.2020.115475 [DOI] [PubMed] [Google Scholar]
- 105. Bokrantz T, Schiöler L, Boström KBet al. Antihypertensive drug classes and the risk of hip fracture: results from the swedish primary care cardiovascular database. J Hypertens 2020;38:167–75. 10.1097/HJH.0000000000002245 [DOI] [PubMed] [Google Scholar]
- 106. Ali S, Navaneethan SD, Virani SSet al. Revisiting diuretic choice in chronic kidney disease. Curr Opin Nephrol Hypertens 2022;31:406–13. 10.1097/MNH.0000000000000814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Agarwal R, Sinha AD, Cramer AEet al. Chlorthalidone for hypertension in advanced chronic kidney disease. N Engl J Med 2021;385:2507–19. 10.1056/NEJMoa2110730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ghebre YT. Proton pump inhibitors and osteoporosis: is collagen a direct target? Front Endocrinol 2020;11:473. 10.3389/fendo.2020.00473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Lyu B, Jorgenson MR, Hansen KEet al. Proton pump inhibitors, but not H2-receptor antagonists, are associated with incident fractures among kidney transplant recipients. Transplantation 2020;104:2609–15. 10.1097/TP.0000000000003178 [DOI] [PubMed] [Google Scholar]
- 110. Barbour LA, Kick SD, Steiner JFet al. A prospective study of heparin-induced osteoporosis in pregnancy using bone densitometry. Am J Obstet Gynecol 1994;170:862–9. 10.1016/S0002-9378(94)70299-3 [DOI] [PubMed] [Google Scholar]
- 111. Backos M, Rai R, Thomas Eet al. Bone density changes in pregnant women treated with heparin: a prospective, longitudinal study. Hum Reprod 1999;14:2876–80. 10.1093/humrep/14.11.2876 [DOI] [PubMed] [Google Scholar]
- 112. Muir JM, Andrew M, Hirsh Jet al. Histomorphometric analysis of the effects of standard heparin on trabecular bone in vivo. Blood 1996;88:1314–20. 10.1182/blood.V88.4.1314.bloodjournal8841314 [DOI] [PubMed] [Google Scholar]
- 113. Gajic-Veljanoski O, Phua CW, Shah PSet al. Effects of long-term low-molecular-weight heparin on fractures and bone density in non-pregnant adults: a systematic review with meta-analysis. J Gen Intern Med 2016;31:947–57. 10.1007/s11606-016-3603-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Yang S, Niu Q, Gan Let al. Effect of long-term use of unfractionated or low-molecular-weight heparin on bone mineral density in maintenance hemodialysis patients. Hemodial Int 2020;24:374–82. 10.1111/hdi.12854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 2002;13:777–87. 10.1007/s001980200108 [DOI] [PubMed] [Google Scholar]
- 116. Evenepoel P, Behets GJ, Viaene Let al. Bone histomorphometry in de novo renal transplant recipients indicates a further decline in bone resorption 1 year posttransplantation. Kidney Int 2017;91:469–76. 10.1016/j.kint.2016.10.008 [DOI] [PubMed] [Google Scholar]
- 117. Julian BA, Laskow DA, Dubovsky Jet al. Rapid loss of vertebral mineral density after renal transplantation. N Engl J Med 1991;325:544–50. 10.1056/NEJM199108223250804 [DOI] [PubMed] [Google Scholar]
- 118. Nikkel LE, Mohan S, Zhang Aet al. Reduced fracture risk with early corticosteroid withdrawal after kidney transplant. Am J Transplant 2012;12:649–59. 10.1111/j.1600-6143.2011.03872.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Hendra H, Salama AD. Steroids as treatment for glomerulonephritis: time for a rethink. Nephrol Dial Transplant 2022;37:1212–7. 10.1093/ndt/gfaa267 [DOI] [PubMed] [Google Scholar]
- 120. Bover J, Bailone L, López-Báez Vet al. Osteoporosis, bone mineral density and CKD-MBD: treatment considerations. J Nephrol 2017;30:677–87. 10.1007/s40620-017-0404-z [DOI] [PubMed] [Google Scholar]
- 121. Levin A, Bakris GL, Molitch Met al. Prevalence of abnormal serum vitamin d, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 2007;71:31–38. 10.1038/sj.ki.5002009 [DOI] [PubMed] [Google Scholar]
- 122. Geng S, Kuang Z, Peissig PLet al. Parathyroid hormone independently predicts fracture, vascular events, and death in patients with stage 3 and 4 chronic kidney disease. Osteoporos Int 2019;30:2019–25. 10.1007/s00198-019-05033-3 [DOI] [PubMed] [Google Scholar]
- 123. Cunningham J, Locatelli F, Rodriguez M. Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol 2011;6:913–21. 10.2215/CJN.06040710 [DOI] [PubMed] [Google Scholar]
- 124. Capelli I, Cianciolo G, Gasperoni Let al. Nutritional vitamin D in CKD: should we measure? Should we treat? Clin Chim Acta 2020;501:186–97. 10.1016/j.cca.2019.11.010 [DOI] [PubMed] [Google Scholar]
- 125. Ambrus C, Almasi C, Berta Ket al. Vitamin D insufficiency and bone fractures in patients on maintenance hemodialysis. Int Urol Nephrol 2011;43:475–82. 10.1007/s11255-010-9723-x [DOI] [PubMed] [Google Scholar]
- 126. Ross AC, Manson JE, Abrams SAet al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011;96:53–8. 10.1210/jc.2010-2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Cashman KD, Fitzgerald AP, Kiely Met al. A systematic review and meta-regression analysis of the vitamin D intake-serum 25-hydroxyvitamin D relationship to inform European recommendations. Br J Nutr 2011;106:1638–48. 10.1017/S0007114511005058 [DOI] [PubMed] [Google Scholar]
- 128. Cashman KD, Ritz C, Kiely Met al. Improved dietary guidelines for vitamin D: application of individual participant data (IPD)-level meta-regression analyses. Nutrients 2017;9:469. 10.3390/nu9050469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Mo M, Wang S, Chen Zet al. A systematic review and meta-analysis of the response of serum 25-hydroxyvitamin D concentration to vitamin D supplementation from RCTs from around the globe. Eur J Clin Nutr 2019;73:816–34. 10.1038/s41430-019-0417-x [DOI] [PubMed] [Google Scholar]
- 130. Giannini S, Mazzaferro S, Minisola Set al. Raising awareness on the therapeutic role of cholecalciferol in CKD: a multidisciplinary-based opinion. Endocrine 2018;59:242–59. 10.1007/s12020-017-1369-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Avenell A, Mak JC, O'Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev 2014;CD000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ringe JD. Plain vitamin D or active vitamin D in the treatment of osteoporosis: where do we stand today? Arch Osteoporos 2020;15:182. 10.1007/s11657-020-00842-0 [DOI] [PubMed] [Google Scholar]
- 133. Bauer DC. Clinical practice. Calcium supplements and fracture prevention. N Engl J Med 2013;369:1537–43. 10.1056/NEJMcp1210380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Bolland MJ, Leung W, Tai Vet al. Calcium intake and risk of fracture: systematic review. BMJ 2015;351:h4580. 10.1136/bmj.h4580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Bolland MJ, Grey A, Avenell Aet al. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women's Health Initiative limited access dataset and meta-analysis. BMJ 2011;342:d2040. 10.1136/bmj.d2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Spiegel DM, Brady K. Calcium balance in normal individuals and in patients with chronic kidney disease on low- and high-calcium diets. Kidney Int 2012;81:1116–22. 10.1038/ki.2011.490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Torregrosa JV, Bover J, Cannata Andía Jet al. Spanish Society of Nephrology recommendations for controlling mineral and bone disorder in chronic kidney disease patients (S.E.N.-M.B.D.). Nefrologia 2011;31Suppl 1:3–32. [DOI] [PubMed] [Google Scholar]
- 138. Iseri K, Watanabe M, Yoshikawa Het al. Effects of denosumab and alendronate on bone health and vascular function in hemodialysis patients: a randomized, controlled trial. J Bone Miner Res 2019;34:1014–24. 10.1002/jbmr.3676 [DOI] [PubMed] [Google Scholar]
- 139. Rudnicki M, Hyldstrup L, Petersen LJet al. Effect of oral calcium on noninvasive indices of bone formation and bone mass in hemodialysis patients: a randomized double-blind placebo-controlled study. Miner Electrolyte Metab 1994;20:130–4. [PubMed] [Google Scholar]
- 140. Hill KM, Martin BR, Wastney MEet al. Oral calcium carbonate affects calcium but not phosphorus balance in stage 3-4 chronic kidney disease. Kidney Int 2013;83:959–66. 10.1038/ki.2012.403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Seeman E, Martin TJ. Antiresorptive and anabolic agents in the prevention and reversal of bone fragility. Nat Rev Rheumatol 2019;15:225–36. 10.1038/s41584-019-0172-3 [DOI] [PubMed] [Google Scholar]
- 142. Eriksen EF, Díez-Pérez A, Boonen S. Update on long-term treatment with bisphosphonates for postmenopausal osteoporosis: a systematic review. Bone 2014;58:126–35. 10.1016/j.bone.2013.09.023 [DOI] [PubMed] [Google Scholar]
- 143. Dempster DW, Chines A, Bostrom MPet al. Modeling-based bone formation in the human femoral neck in subjects treated with denosumab. J Bone Miner Res 2020;35:1282–8. 10.1002/jbmr.4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Bone HG, Wagman RB, Brandi MLet al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 2017;5:513–23. 10.1016/S2213-8587(17)30138-9 [DOI] [PubMed] [Google Scholar]
- 145. Broadwell A, Chines A, Ebeling PRet al. Denosumab safety and efficacy among participants in the FREEDOM extension study with mild to moderate chronic kidney disease. J Clin Endocrinol Metab 2021;106:397–409. 10.1210/clinem/dgaa851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Dennison EM, Cooper C, Kanis JAet al. Fracture risk following intermission of osteoporosis therapy. Osteoporos Int 2019;30:1733–43. 10.1007/s00198-019-05002-w [DOI] [PubMed] [Google Scholar]
- 147. Zhang C, Song C. Combination therapy of PTH and antiresorptive drugs on osteoporosis: a review of treatment alternatives. Front Pharmacol 2020;11:607017. 10.3389/fphar.2020.607017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Haarhaus M, Evenepoel P, European Renal Osteodystrophy (EUROD) workgroup. et al.Differentiating the causes of adynamic bone in advanced chronic kidney disease informs osteoporosis treatment. Kidney Int 2021;100:546–58. 10.1016/j.kint.2021.04.043 [DOI] [PubMed] [Google Scholar]
- 149. Abrahamsen B, Ernst MT, Smith CDet al. The association between renal function and BMD response to bisphosphonate treatment: real-world cohort study using linked national registers. Bone 2020;137:115371. 10.1016/j.bone.2020.115371 [DOI] [PubMed] [Google Scholar]
- 150. Robinson DE, Ali MS, Pallares Net al. Safety of oral bisphosphonates in moderate-to-severe chronic kidney disease: a binational cohort analysis. J Bone Miner Res 2021;36:820–32. 10.1002/jbmr.4235 [DOI] [PubMed] [Google Scholar]
- 151. Chen CL, Chen NC, Liang HLet al. Effects of denosumab and calcitriol on severe secondary hyperparathyroidism in dialysis patients with low bone mass. J Clin Endocrinol Metab 2015;100:2784–92. 10.1210/jc.2015-1259 [DOI] [PubMed] [Google Scholar]
- 152. Block GA, Bone HG, Fang Let al. A single-dose study of denosumab in patients with various degrees of renal impairment. J Bone Miner Res 2012;27:1471–9. 10.1002/jbmr.1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Bilezikian JP, Hattersley G, Mitlak BHet al. Abaloparatide in patients with mild or moderate renal impairment: results from the ACTIVE phase 3 trial. Curr Med Res Opin 2019;35:2097–102. 10.1080/03007995.2019.1656955 [DOI] [PubMed] [Google Scholar]
- 154. Miller PD, Schwartz EN, Chen Pet al. Teriparatide in postmenopausal women with osteoporosis and mild or moderate renal impairment. Osteoporos Int 2007;18:59–68. 10.1007/s00198-006-0189-8 [DOI] [PubMed] [Google Scholar]
- 155. Cejka D, Kodras K, Bader Tet al. Treatment of hemodialysis-associated adynamic bone disease with teriparatide (PTH1-34): a pilot study. Kidney Blood Press Res 2010;33:221–6. 10.1159/000316708 [DOI] [PubMed] [Google Scholar]
- 156. Nishikawa A, Yoshiki F, Taketsuna Met al. Safety and effectiveness of daily teriparatide for osteoporosis in patients with severe stages of chronic kidney disease: post hoc analysis of a postmarketing observational study. Clin Interv Aging 2016;Volume 11:1653–9. 10.2147/CIA.S120175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Sumida K, Ubara Y, Hoshino Jet al. Once-weekly teriparatide in hemodialysis patients with hypoparathyroidism and low bone mass: a prospective study. Osteoporos Int 2016;27:1441–50. 10.1007/s00198-015-3377-6 [DOI] [PubMed] [Google Scholar]
- 158. Yamamoto J, Nakazawa D, Nishio Set al. Impact of weekly teriparatide on the bone and mineral metabolism in hemodialysis patients with relatively low serum parathyroid hormone: a pilot study. Ther Apher Dial 2020;24:146–53. 10.1111/1744-9987.12867 [DOI] [PubMed] [Google Scholar]
- 159. Leder BZ, Tsai JN, Jiang LAet al. Importance of prompt antiresorptive therapy in postmenopausal women discontinuing teriparatide or denosumab: the Denosumab and Teriparatide Follow-up study (DATA-Follow-up). Bone 2017;98:54–58. 10.1016/j.bone.2017.03.006 [DOI] [PubMed] [Google Scholar]
- 160. Watanabe A, Yoneyama S, Nakajima Met al. Osteosarcoma in Sprague-Dawley rats after long-term treatment with teriparatide (human parathyroid hormone (1-34)). J Toxicol Sci 2012;37:617–29. 10.2131/jts.37.617 [DOI] [PubMed] [Google Scholar]
- 161. Miller PD, Lewiecki EM, Krohn Ket al. Teriparatide: label changes and identifying patients for long-term use. Cleve Clin J Med 2021;88:489–93. 10.3949/ccjm.88a.21011 [DOI] [PubMed] [Google Scholar]
- 162. Cosman F, Crittenden DB, Adachi JDet al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med 2016;375:1532–43. 10.1056/NEJMoa1607948 [DOI] [PubMed] [Google Scholar]
- 163. McClung MR, Grauer A, Boonen Set al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med 2014;370:412–20. 10.1056/NEJMoa1305224 [DOI] [PubMed] [Google Scholar]
- 164. Saag KG, Petersen J, Brandi MLet al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med 2017;377:1417–27. 10.1056/NEJMoa1708322 [DOI] [PubMed] [Google Scholar]
- 165. Lewiecki EM, Blicharski T, Goemaere Set al. A phase III randomized placebo-controlled trial to evaluate efficacy and safety of romosozumab in men with osteoporosis. J Clin Endocrinol Metab 2018;103:3183–93. 10.1210/jc.2017-02163 [DOI] [PubMed] [Google Scholar]
- 166. Langdahl BL, Libanati C, Crittenden DBet al. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open-label, phase 3 trial. Lancet North Am Ed 2017;390:1585–94. 10.1016/S0140-6736(17)31613-6 [DOI] [PubMed] [Google Scholar]
- 167. Sato M, Inaba M, Yamada Set al. Efficacy of romosozumab in patients with osteoporosis on maintenance hemodialysis in Japan; an observational study. J Bone Miner Metab 2021;39:1082–90. 10.1007/s00774-021-01253-y [DOI] [PubMed] [Google Scholar]
- 168. Miller PD, Adachi JD, Albergaria BHet al. Efficacy and safety of romosozumab among postmenopausal women with osteoporosis and mild-to-moderate chronic kidney disease. J Bone Miner Res 2022;37:1437–45. 10.1002/jbmr.4563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Lewiecki EM, Dinavahi RV, Lazaretti-Castro Met al. One year of romosozumab followed by two years of denosumab maintains fracture risk reductions: results of the FRAME extension study. J Bone Miner Res 2019;34:419–28. 10.1002/jbmr.3622 [DOI] [PubMed] [Google Scholar]
- 170. Evenepoel P, D'Haese P, Brandenburg V. Romosozumab in postmenopausal women with osteopenia. N Engl J Med 2014;370:1664. [DOI] [PubMed] [Google Scholar]
- 171. Ito N, Prideaux M, Wijenayaka ARet al. Sclerostin directly stimulates osteocyte synthesis of fibroblast growth factor-23. Calcif Tissue Int 2021;109:66–76. 10.1007/s00223-021-00823-6 [DOI] [PubMed] [Google Scholar]
- 172. Hsu CP, Maddox J, Block Get al. Influence of renal function on pharmacokinetics, pharmacodynamics, and safety of a single dose of romosozumab. J Clin Pharmacol 2022;62:1132–41. 10.1002/jcph.2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Vellanki K, Hou S. Menopause in CKD. Am J Kidney Dis 2018;71:710–9. 10.1053/j.ajkd.2017.12.019 [DOI] [PubMed] [Google Scholar]
- 174. Rossouw JE, Anderson GL, Prentice RLet al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA 2002;288:321–33. [DOI] [PubMed] [Google Scholar]
- 175. Rozenberg S, Al-Daghri N, Aubertin-Leheudre Met al. Is there a role for menopausal hormone therapy in the management of postmenopausal osteoporosis? Osteoporos Int 2020;31:2271–86. 10.1007/s00198-020-05497-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Ishani A, Blackwell T, Jamal SAet al. The effect of raloxifene treatment in postmenopausal women with CKD. J Am Soc Nephrol 2008;19:1430–8. 10.1681/ASN.2007050555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Xue Y, Xiao Y, Liu Jet al. The calcium-sensing receptor complements parathyroid hormone-induced bone turnover in discrete skeletal compartments in mice. Am J Physiol Endocrinol Metab 2012;302:E841–51. 10.1152/ajpendo.00599.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Díaz-Tocados JM, Rodríguez-Ortiz ME, Almadén Yet al. Calcimimetics maintain bone turnover in uremic rats despite the concomitant decrease in parathyroid hormone concentration. Kidney Int 2019;95:1064–78. 10.1016/j.kint.2018.12.015 [DOI] [PubMed] [Google Scholar]
- 179. Mazzaferro S, Pasquali M. Direct bone effects of calcimimetics in chronic kidney disease? Kidney Int 2019;95:1012–4. 10.1016/j.kint.2019.01.045 [DOI] [PubMed] [Google Scholar]
- 180. Behets GJ, Spasovski G, Sterling LRet al. Bone histomorphometry before and after long-term treatment with cinacalcet in dialysis patients with secondary hyperparathyroidism. Kidney Int 2015;87:846–56. 10.1038/ki.2014.349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Moe SM, Abdalla S, Chertow GMet al. Effects of cinacalcet on fracture events in patients receiving hemodialysis: the EVOLVE trial. J Am Soc Nephrol 2015;26:1466–75. 10.1681/ASN.2014040414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Cunningham J, Danese M, Olson Ket al. Effects of the calcimimetic cinacalcet HCl on cardiovascular disease, fracture, and health-related quality of life in secondary hyperparathyroidism. Kidney Int 2005;68:1793–800. 10.1111/j.1523-1755.2005.00596.x [DOI] [PubMed] [Google Scholar]
- 183. VanderWalde LH, Liu IL, Haigh PI. Effect of bone mineral density and parathyroidectomy on fracture risk in primary hyperparathyroidism. World J Surg 2009;33:406–11. 10.1007/s00268-008-9720-8 [DOI] [PubMed] [Google Scholar]
- 184. Chou FF, Chen JB, Lee CHet al. Parathyroidectomy can improve bone mineral density in patients with symptomatic secondary hyperparathyroidism. Arch Surg 2001;136:1064–8. 10.1001/archsurg.136.9.1064 [DOI] [PubMed] [Google Scholar]
- 185. Rudser KD, de Boer IH, Dooley Aet al. Fracture risk after parathyroidectomy among chronic hemodialysis patients. J Am Soc Nephrol 2007;18:2401–7. 10.1681/ASN.2007010022 [DOI] [PubMed] [Google Scholar]
- 186. Ishani A, Liu J, Wetmore JBet al. Clinical outcomes after parathyroidectomy in a nationwide cohort of patients on hemodialysis. Clin J Am Soc Nephrol 2015;10:90–97. 10.2215/CJN.03520414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Pimentel A, Ureña-Torres P, Zillikens MCet al. Fractures in patients with CKD-diagnosis, treatment, and prevention: a review by members of the European Calcified Tissue Society and the European Renal Association of Nephrology Dialysis and Transplantation. Kidney Int 2017;92:1343–55. 10.1016/j.kint.2017.07.021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.


