Abstract
Bacterial spores are encased in a multilayered proteinaceous shell, called the coat. In many Bacillus spp., the coat protects against environmental assault and facilitates germination. In Bacillus anthracis, the spore is the etiological agent of anthrax, and the functions of the coat likely contribute to virulence. Here, we characterize a B. anthracis spore protein, called Cotβ, which is encoded only in the genomes of the Bacillus cereus group. We found that Cotβ is synthesized specifically during sporulation and is assembled onto the spore coat surface. Our analysis of a cotβ null mutant in the Sterne strain reveals that Cotβ has a role in determining coat-surface morphology but does not detectably affect germination. In the fully virulent Ames strain, a cotβ null mutation has no effect on virulence in a murine model of B. anthracis infection.
Keywords: Bacillus anthracis, coat, germination, spore assembly, anthrax
Introduction
Bacterial spores can resist extremes of temperature, pH, toxic chemicals and predation by other microorganisms (Klobutcher et al., 2006; Setlow, 2006). Studies in Bacillus subtilis demonstrate that spore resistance properties are derived from concentrically organized shells that encase the spore (Aronson & Fitz-James, 1976; Driks, 1999; Henriques et al., 2004). A critical protective structure is the coat, a protein shell that encases bacterial spores of all species. In some species, such as B. subtilis, the coat is the external surface of the spore. Unlike B. subtilis, Bacillus anthracis and Bacillus cereus possess an additional balloon-like structure, called the exosporium, that encases the entire spore (Holt & Leadbetter, 1969; Aronson & Fitz-James, 1976). The B. subtilis coat is composed of 60–80 protein species which are organized into distinctly staining inner and outer layers as visualized using thin-section electron microscopy (TEM) (Warth et al., 1963; Kim et al., 2006). The B. anthracis coat also has multiple layers, but these are more difficult to visualize because the coat is relatively thin (Holt & Leadbetter, 1969; Aronson & Fitz-James, 1976). Analysis of the composition and assembly of spore structures are critical steps towards understanding the molecular basis of spore resistance.
In addition to its protective roles, the B. subtilis coat also facilitates germination (Aronson & Fitz-James, 1976; Setlow, 2006). In B. anthracis, the etiological agent of anthrax, the spore is the infectious form of the bacterium, and the protective and germination-regulating functions of the coat (Kim et al., 2004; Giorno et al., 2007) are very likely to be important to virulence. During germination, the spore rehydrates and swells. The coat accommodates these changes in volume by relaxing a series of folds on the dormant spore coat surface (Chada et al., 2003; Plomp et al., 2005, 2007). Soon after rehydration begins, the coat is shed, permitting further expansion of cell volume and completion of the conversion to the vegetative state (Santo & Doi, 1974).
The spore is formed during a multistage developmental process called sporulation. An early step in sporulation is the division of the cell into two unequally sized compartments by an asymmetrically positioned septum. Next, the smaller forespore compartment, which will become the spore, is engulfed by the larger mother cell. Ultimately, the mature spore is released into the milieu after mother cell lysis. In B. subtilis, spore coat assembly begins with synthesis of the spore coat proteins in the mother cell, just after the appearance of the sporulation septum (Zheng & Losick, 1990).
In this study, we characterize the coat protein Cotβ, which is present in the genomes of B. anthracis, B. cereus, Bacillus thuringiensis, Bacillus mycoides, Bacillus pseudomycoides and Bacillus weihenstephanensis (members of the so-called B. cereus group) but not in any other as yet sequenced species, and which we identified in a search for novel B. anthracis spore proteins. We show that, in the Sterne strain of B. anthracis, Cotβ is at the spore coat surface, and has a major effect on the topography of the coat surface. We further demonstrate that, in the fully virulent Ames strain, cotβ is not necessary for infection in an animal model.
Materials and methods
General methods
Escherichia coli strains were cultured in Luria–Bertani medium and antibiotics were used at standard concentrations (Sambrook et al., 1989). All B. anthracis strains are congenic with either the Ames or Sterne 34F2 strains. For the Ames and Sterne derivatives of B. anthracis, we used erythromycin at 5 μg mL−1 and kanamycin at 20 μg mL−1, and for Sterne derivates, we used tetracycline at 5 μg mL−1. Routine molecular biological techniques were performed using standard methodologies (Sambrook et al., 1989). For atomic force-, fluorescence-, and immunofluorescence microscopy (IFM) experiments, Sterne strain spores were prepared by exhaustion sporulation in liquid Difco sporulation medium (DSM) (Harwood & Cutting, 1990). Spores judged to be dormant using phase contrast microscopy were collected and washed 5–10 times in sterile deionized water and analyzed immediately, as described below. For sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), spores of the Sterne and Ames strains were generated by culturing in DSM medium at 37 °C as described (Giorno et al., 2007). For immunoblot analysis, reverse transcriptase (RT)-PCR analysis and animal challenge assays, spores of the Sterne and Ames strains (and derivatives) were prepared in Leighton–Doi broth (Leighton & Doi, 1971) either at room temperature (for immunoblot) or at 37 °C (for RT-PCR and challenge assays), and then purified by centrifugation through a Hypaque-76 gradient (Nycomed Inc.). MS was performed as described previously (Lai et al., 2003). To monitor cotβ transcription in the Ames strain, mRNA was purified between 2 and 4 h after the onset of sporulation and RT-PCR analysis was performed as described previously (Bozue et al., 2005). The plasmids used in this study to generate recombinant B. anthracis strains are listed in Table 1 and their construction is described in Supporting Information. Recombinant B. anthracis Sterne strains were generated by electroporation using unmethylated plasmid DNA (Koehler et al., 1994). Recombinant Ames strains were generated using a protocol described previously (Mendelson et al., 2004). A strain in which cotβ was inactivated, Ames-JAB-7, was obtained by screening for kanamycin resistance and erythromycin sensitivity (encoded by plasmid pEO-3). The disruption of the cotβ gene in the chromosome was confirmed using PCR. Spore proteins were extracted as described previously (Little & Driks, 2001), and were fractionated on 12% bis-Tris Gels (Invitrogen) and stained with either the Gel Code Blue system (Pierce) or Coomassie Brilliant Blue, or were electrotransferred to a polyvinylidene fluoride (PVDF) membrane in a 20% methanol, 3.7% SDS, Nupage Transfer Buffer (Invitrogen), in an Owl Panther transfer unit (Owl Separation Systems), and then stained with India Ink in a phosphate-buffered saline (PBS) solution (0.4% Tween-20, 0.1% India Ink) or for immunoblotting, were blocked in a 5% nonfat milk solution and then incubated with rabbit anti-green fluorescent protein (GFP) antibodies (Molecular Probes) at a dilution of 1 : 2000. After washing, membranes were incubated with anti-rabbit secondary antibodies conjugated to horseradish peroxidase (BioRad) at a dilution of 1 : 4000. Bands were visualized using the 1-Step 4CN reagent (Pierce).
Table 1.
Strains and plasmids
| Strain | Genotype/description | Sources |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | Cloning host | Lab stock |
| GM2163 | dam dcm; for transformation of B. anthracis | Lab stock |
| GM1684 | dam | T. Koehler |
| Sterne strains | ||
| 34F2 | pXO1+ pXO2−, wild type | P. Jackson |
| RG61 | pXO1+ pXO2−, cotβ∷km | This study |
| RG134 | pXO1+ pXO2−, cotβΩpAS5 | This study |
| MGM68 | pXO1+ pXO2−, cotβ∷km | This study |
| RG56 | pXO1+ pXO2−, cotE∷km | Giorno et al. (2007) |
| MGM37 | pXO1+ pXO2−, cotE∷km, cotβΩpAS5 | This study |
| Ames strains | ||
| Ames | pXO1+ pXO2+, wild type | Little & Knudson (1986) |
| Ames-JAB-7 | pXO1+ pXO2+, cotβ∷km | This study |
| Ames-JAB-10 | pXO1+ pXO2+, cotβΩpEO3-cotβ-gfp | This study |
| Name | Description | Sources |
| Plasmids | ||
| pMR1 | Used to introduce kanr into the chromosome by marker replacement | Giorno et al. (2007) |
| pMR2 | Used to replace cotβ with kanr | This study |
| pAS1 | Used to introduce gfp-fusion genes into the chromosome | Lab stock |
| pAS5 | pAS1 bearing 3′ fragment of cotβ | This study |
| pEO3 | E. coli/Bacillus shuttle vector | Mendelson et al. (2004) |
| pJRS182 | Origin of the Ωkm-2 cassette | Perez-Casal et al. (1991) |
| pJRS100.2 | Source of the Ωkm-2 cassette | M. Caparon |
| pEO3-cotβ | pEO3+1.3 kb fragment containing cotβ | This study |
| pEO3-cotβ-Ωkm-2 | pEO3-cotβ bearing Ωkm-2 | This study |
| pEO3-cotβ-gfp | pEO3 containing cotβ-gfp | This study |
Results and discussion
Roles of Cotβ in structure and resistance
We have calculated, that c. 10–20 B. anthracis-specific spore proteins remain to be identified (Kim et al., 2006; Giorno et al., 2007). Because these proteins are likely to play roles in species-specific spore properties, we seek to identify these proteins using SDS-PAGE fractionation of spore extracts followed using MS analysis of excised bands (Lai et al., 2003). Using this approach, we identified a peptide from a B. anthracis spore protein with a predicted mass of 8.4 kDa that migrates as a c. 10 kDa species (currently annotated as BAS1956 in the Sterne strain and BA2104 in the Ames strain) which we designate Cotβ (Fig. 1) [see Kim et al. (2004) for precedent for the use of Greek letters to name B. anthracis coat proteins]. This peptide does not match any sequences other than Cotβ and its orthologues in publicly available databases.
Fig. 1.

Identification of a novel spore protein in Bacillus anthracis. Bacillus anthracis coat proteins from the wild-type Sterne strain were extracted from spores, subjected to 16% SDS-PAGE and stained with Coomassie Blue G-250. The band indicated by an arrowhead was cut out and analyzed using matrix-assisted laser desorption/ionization time-of-flight MS. The peptide sequence is aligned with the best-fit ORF (BAS1956) from the annotated genome of the Sterne strain (inset). Molecular weight markers are indicated in kDa.
To analyze the role of cotβ in spore function and assembly, we inactivated it by replacing the gene with a kanamycin resistance cassette in the Sterne and Ames strains of B. anthracis, resulting in strains MGM68 and Ames-JAB-7, respectively. These mutations are unlikely to affect the transcription of the surrounding genes for three reasons: (1) the kanamycin resistance cassette is flanked by transcriptional termination sequences (Perez-Casal et al., 1991), (2) RT-PCR analysis shows that cotβ is encoded by a separate mRNA transcript than that of the immediately upstream ORF (data not shown), and (3) the downstream ORF is oriented in the opposite direction from cotβ.
Both mutant strains sporulated normally and produced spores that were indistinguishable from wild type, based on phase-contrast light microscopy and TEM (data not shown). To assess general spore resistance properties, we subjected the Sterne strain cotβ mutant (MGM68) to bleach, lysozyme, and heat challenges (using the methods described in Harwood & Cutting, 1990; Young & Setlow, 2003). We detected no significant differences between the mutant and wild-type strains (data not shown). Additionally, there were no readily detectable differences in the protein compositions of the extractible portion of the spore coat proteins from wild-type and mutant spores, as judged using one-dimensional SDS-PAGE (data not shown). We visualized bands in these gels both by staining with Coomassie Brilliant Blue, and by India ink staining after electrotransfer to a PVDF membrane, which allows detection of bands not stained by Coomassie (data not shown). In B. subtilis, the presence of multiple spore proteins in a single band is well documented (Kuwana et al., 2002). We infer, therefore, that a protein other than Cotβ is also present at this location in the gel (Fig. 1).
To determine whether Cotβ has a role in directing coat-surface topography, we examined the Sterne strain cotβ mutant spore surface using atomic force microscopy (AFM), which produces topographical relief maps. We have found that AFM can detect morphological defects not revealed using TEM (Chada et al., 2003; McPherson et al., 2005; Giorno et al., 2007). Previously, we showed that the B. anthracis spore coat surface is spanned by ridges that extend, for the most part, from pole-to-pole along the long axis of the spore (Chada et al., 2003; Wang et al., 2007). In c. 85% of B. anthracis spores, the ridges appear in pairs, in close apposition (Fig. 2a), as opposed to well-separated single ridges for the case of B. subtilis (Chada et al., 2003; Plomp et al., 2005; Wang et al., 2007). In contrast to wild-type B. anthracis spores, < 7% of cotβ mutant Sterne strain spores (of 150 spores examined) possessed clear ridges. Instead, the coat surface has a smooth appearance, but with a cleft extending across the length of the spore (Fig. 2b and c). We note that this phenotype is very unlikely to be due to a mutation in an unlinked gene as the cotβ null mutation (in strain MGM68) was introduced by transduction from a strain (RG61) in which it was originally created (see Supporting Information). The B. subtilis coat-surface protein CotB also controls coat topography (Donovan et al., 1987; Isticato et al., 2001; Chada et al., 2003). Possibly, a role in coat-surface morphology is typical of coat-surface proteins in all species.
Fig. 2.
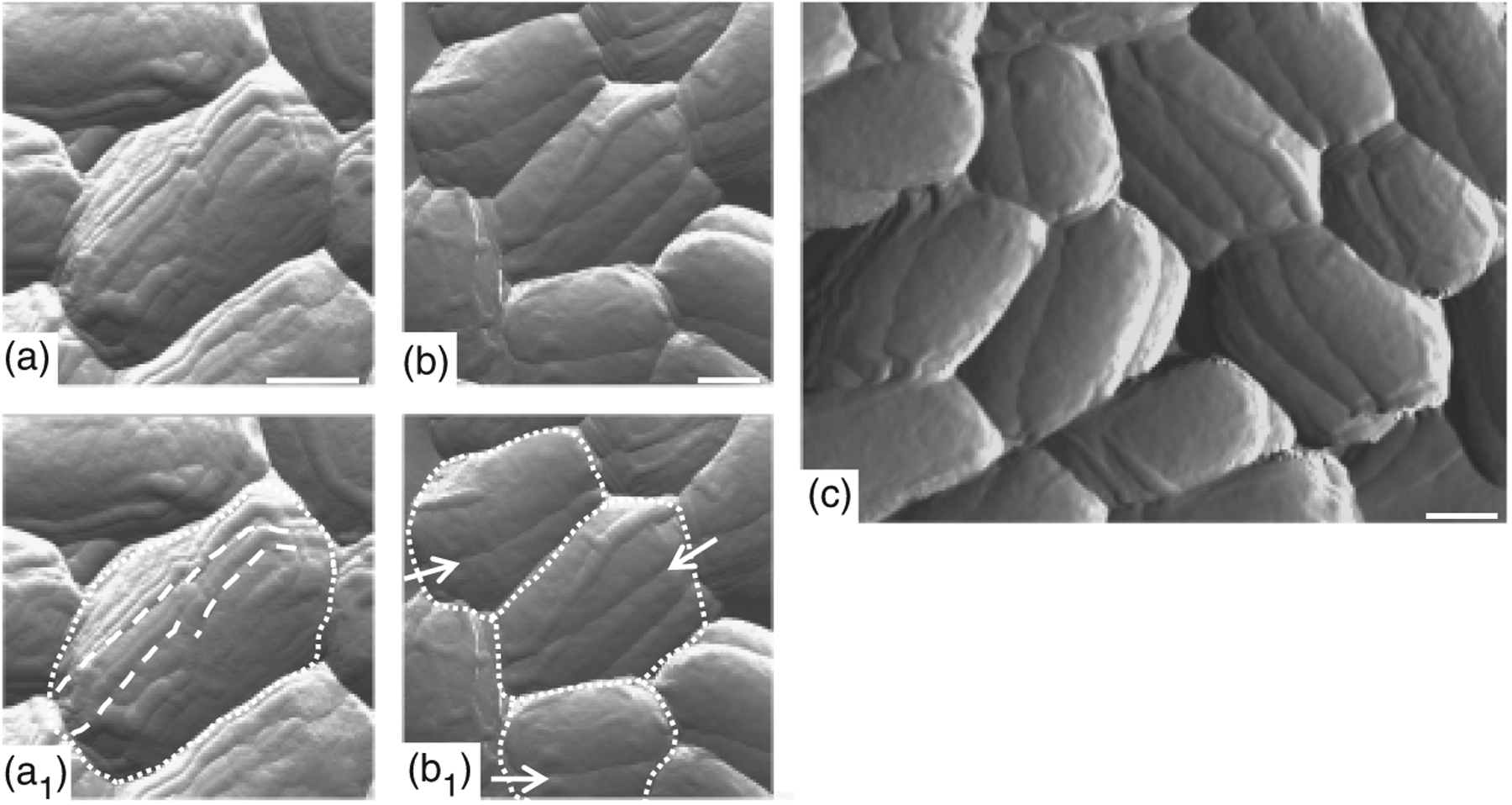
Effect of cotβ on spore coat-surface topography. Wild-type Sterne (a and a1) and cotβ mutant (strain MGM68) spores (b, b1, and c) were analyzed by AFM in the tapping mode. Spore outlines and spore coat ridges imaged in (a) and (b) are indicated by white-dotted lines and arrows in (a1) and (b1), respectively. Scale bars represent 0.5 μm in (a), (b) and (c).
Physiological roles of Cotβ
Because defects in the coat can affect germination (Henriques & Moran, 2006), we analyzed the ability of cotβ mutant spores to germinate using a variety of assays (see Supporting Information) that measure distinct steps in germination (Harwood & Cutting, 1990). However, we found that cotβ mutant spores germinated identically to the wild-type strains (data not shown), suggesting that Cotβ does not have a role in germination.
To detect a role for Cotβ in pathogenesis, we infected female BALB/c mice intranasally (Lyons et al., 2004) with either c.1 × 106 wild-type Ames or cotβ mutant (Ames-JAB-7) spores. Infection with wild-type spores resulted in a typical survival curve, with 93.4% of animals (out of 15 animals) having succumbed by day 6 postinfection. The survival curves of animals challenged with cotβ spores or wild-type spores were indistinguishable (data not shown).
Timing of Cotβ synthesis and subcellular localization
The observation that Cotβ affects coat-surface morphology focused our attention on its subcellular location. To study this, we introduced cotβ-gfp at the cotβ locus (generating strain RG134) via a Campbell-type single reciprocal recombination, resulting in a chromosomal copy of the fusion gene at the endogenous locus. The endogenous, wild-type, copy of cotβ is very likely to be inactive in RG134 because its promoter is separated from the coding sequence by the inserted plasmid backbone. We then used fluorescence microscopy to localize Cotβ-GFP in sporulating cells and spores. To estimate the stage of development, we imaged the sporangial cell envelopes with the lipophilic counter stain FM4–64 (Pogliano et al., 1999) as well as using phase-contrast microscopy (data not shown). Because sporulation had to be carried out at room temperature to visualize GFP-localization (see Supporting Information), the progression through sporulation was much slower than the c. 8 h required at 37 °C (see below).
We first detected Cotβ-GFP when the forespore was engulfed (stage III) (Losick et al., 1986) (at about 24 h of culturing, Fig. 3a), consistent with σE-control of cotβ transcription (Hilbert & Piggot, 2004). Sequences resembling the − 10 and − 35 RNA polymerase-binding sites of the B. subtilis σE consensus sequence (Eichenberger et al., 2003) are present upstream of cotβ (at nucleotide positions − 16 and − 39, relative to the beginning of the ORF). At stage III, Cotβ-GFP was dispersed throughout the mother cell cytoplasm. When about 90% of the cells in the culture had reached stage IV, when forespores dehydrate and become refractile, we detected caps or rings of fluorescence around the forespores, as well as dispersed fluorescence in the mother cell cytoplasm (Fig. 3a). Released spores were surrounded by rings or caps of fluorescence. These rings were more pronounced after deconvolution (Fig. 3b). We argue that this fluorescence pattern is indicative of a coat localization for Cotβ, as we have shown previously that GFP alone does not target any particular location in sporulating cells of B. subtilis (Little & Driks, 2001), and a cap or ring-like localization pattern is consistent with that of a coat or exosporium protein as demonstrated by several laboratories (Pogliano et al., 1995; Bauer et al., 1999; Asai et al., 2001; Ozin et al., 2001; Eichenberger et al., 2003; van Ooij et al., 2004; Kim et al., 2006; R. Giorno, J. Bozue, M. Mallozzi et al., unpublished data). However, when viewed in the context of the temporal progression of sporulation, this localization pattern differs in an interesting way from previous studies, in that there is a distinct stage during which Cotβ is present but not yet deposited around the forespore. It would appear, therefore, that an as yet unidentified signal acts prior to stage IV to trigger Cotβ deposition. This dynamic localization pattern is consistent with observations of other B. anthracis spore proteins (R. Giorno, J. Bozue, M. Mallozzi et al., unpublished data).
Fig. 3.
Fluorescence microscopic and Western blot analysis of Cotβ-GFP in Ames and Sterne strain spores and sporulating cells. (a) Fluorescence microscopic localization of Cotβ-GFP in strain RG134. Cells were grown at room temperature and analyzed 24 h (corresponding to stage III), 28 h (corresponding to stage IV), and 48 h (after spore release) after inoculation. Membrane staining (MEM) is shown in the upper panels, GFP fluorescence (GFP) is shown in the middle panels, and the merged (MERGE) image is shown in the lower panels. Forespore membranes are not stained due to the exclusion of the dye by the coat. The sporangia pictured in the merged images are cartooned below for clarity; spores and forespores are indicated by dark grey ovals, and mother cells are indicated by white rounded rectangles. Released spores in (a) were prepared by water washing. Fluorescence of Hypaque-purified released spores was indistinguishable from that of water-washed spores (data not shown). (b) Deconvolved micrograph from a spore from strain MGM37. (c) Spore coat extracts of cotβ-gfp fusion bearing spores, from strains Ames-JAB-10 and RG134 (lanes 1 and 2, respectively), or from the wild-type Ames strain spores (lane 3) were fractionated using SDS-PAGE and transferred to a PVDF membrane, and then probed with anti-GFP antibodies. The asterisk (*) indicates a cross-reacting species, and arrowheads indicate the Cotβ-GFP-specific doublet that is present in both fusion-bearing strains. (d) After sporulation for 2 days at room temperature, spores bearing cotβ-gfp (from strain RG134, panels 1 and 2), or bearing cotβ-gfp and a cotE mutation (from strain MGM37, panels 3 and 4) were collected and imaged by fluorescence (β-GFP) or IFM (α-GFP).
Unexpectedly, we did not detect fluorescent rings in a version of the Ames strain bearing cotβ-gfp (Ames-JAB-10) (data not shown). We confirmed that the cotβ gene was transcribed in the Ames strain using RT-PCR analysis of mRNA collected from sporangia (data not shown). To confirm that Cotβ-GFP was synthesized and present on spores, we extracted spore proteins from Ames-JAB-10 spores (as well as from RG134 spores), fractionated them using SDS-PAGE and analyzed them by Western blot analysis using anti-GFP antibodies. We detected Cotβ-GFP-specific bands in both fusion-bearing strains (Fig. 3c, lanes 1 and 2). These bands migrate as a doublet of about 37 kDa, consistent with the expected size of the fusion protein (37.5 kDa). However, the intensity of the doublet bands from strain Ames-JAB-10 was substantially less than strain RG134, and three additional bands were present in the RG134 extracts (migrating between 45 and 50 kDa) which were not detected in Ames-JAB-10. These data suggest that, in the Ames strain, Cotβ-GFP is made (albeit at a level below the limit of detection using fluorescence microscopy). The reason for the lower abundance of Cotβ-GFP in cells from the Ames strain background is unknown. We note, however, that the adenine at position −13 (relative to the beginning of the ORF) in the Sterne strain is missing in the Ames strain, altering a putative σE consensus sequence. Possibly, this alteration reduces cotβ transcription (and presumably Cotβ abundance). The identities of the 45–50 kDa RG134-specific bands are unknown, although they apparently contain at least part of the fusion protein. A reasonable speculation is that they represent posttranslationally modified Cotβ-GFP.
To determine the location of Cotβ within the spore more precisely, we first performed IFM on Cotβ-GFP-bearing spores (from strain RG134) using anti-GFP antibodies. While we could detect GFP fluorescence (Fig. 3d, panel 1), we did not detect a signal by IFM (Fig. 3d, panel 2) presumably because antibodies are prevented from binding by the presence of the exosporium. We then carried out IFM on cells bearing cotβ-gfp, but where the exosporium is absent due to a mutation in cotE (in strain MGM37) (Giorno et al., 2007). IFM analysis of strain MGM37 revealed rings of fluorescence. These rings, as well as those due to GFP-fluorescence, were interrupted at several positions around the spore (Fig. 3d, panels 3 and 4). We infer that Cotβ-GFP is present beneath the exosporium surface (although it could also be present elsewhere in the exosporium) and, most likely, on the coat surface. Interestingly, GFP-fluorescence in MGM37 spores was reduced relative to wild type (Fig. 3d, compare panels 1 and 3). Therefore, although some Cotβ-GFP is assembled in the absence of CotE, a significant amount of Cotβ-GFP likely assembles in a CotE-dependent manner.
Antigens specific to B. cereus-group spores (such as Cotβ) could be used in future technologies to discriminate these spores from nonharmful spores, to improve medical diagnostics, food safety, and possibly biodefense. However, because Cotβ is not required for disease in the model we analyzed, it is not an optimal marker for detecting a biological weapon, as it could be removed by a technically capable enemy. Nonetheless, because Cotβ is conserved in food-borne disease causing strains of B. cereus it could serve as a marker for pathogens in the food supply.
In summary, we have characterized Cotβ, a B. cereus group-specific protein in B. anthracis present on the spore coat surface. The abundance of Cotβ in Sterne strain spores is significantly greater than in Ames strain spores, providing evidence that spore composition varies among B. anthracis strains. While Cotβ does not detectably contribute to spore resistance, germination, or virulence (as measured by a murine intranasal challenge assay), it has a significant role in coat-surface architecture, similar to the B. subtilis coat-surface protein CotB (Chada et al., 2003).
Supplementary Material
Acknowledgements
This work was supported by grants GM53989 and AI53365 from the National Institutes of Health (NIH) (A.D.), Department of the Army Award Number W81XWH-04-01-0307 (P.E.), and the United States Army Medical Research and Materiel Command under Projects 02-4-5C-018 (J.A.B.), 02-4-5C-023 (S.W.) and 04-0-IL-002 (S.W.). Research was conducted in compliance with the Animal Welfare Act, and other federal statutes and regulations relating to animals and experiments involving animals, and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals, National Research Council, 1996. The facility where this research was conducted is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the United States Army. We thank the Michigan Proteome Consortium for the identification of Cotβ.
References
- Aronson AI & Fitz-James P (1976) Structure and morphogenesis of the bacterial spore coat. Bacteriol Rev 40: 360–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai K, Takamatsu H, Iwano M, Kodama T, Watabe K & Ogasawara N (2001) The Bacillus subtilis yabQ gene is essential for formation of the spore cortex. Microbiology 147: 919–927. [DOI] [PubMed] [Google Scholar]
- Bauer T, Little S, Stover AG & Driks A (1999) Functional regions of the Bacillus subtilis spore coat morphogenetic protein CotE. J Bacteriol 181: 7043–7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozue JA, Parthasarathy N, Phillips LR, Cote CK, Fellows PF, Mendelson I, Shafferman A & Friedlander AM (2005) Construction of a rhamnose mutation in Bacillus anthracis affects adherence to macrophages but not virulence in guinea pigs. Microb Pathogenesis 38: 1–12. [DOI] [PubMed] [Google Scholar]
- Chada VG, Sanstad EA, Wang R & Driks A (2003) Morphogenesis of Bacillus spore surfaces. J Bacteriol 185: 6255–6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan W, Zheng LB, Sandman K & Losick R (1987) Genes encoding spore coat polypeptides from Bacillus subtilis. J Mol Biol 196: 1–10. [DOI] [PubMed] [Google Scholar]
- Driks A (1999) Bacillus subtilis spore coat. Microbiol Mol Biol R 63: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenberger P, Jensen ST, Conlon EM et al. (2003) The sigmaE regulon and the identification of additional sporulation genes in Bacillus subtilis. J Mol Biol 327: 945–972. [DOI] [PubMed] [Google Scholar]
- Giorno R, Bozue J, Cote C et al. (2007) Morphogenesis of the Bacillus anthracis spore. J Bacteriol 189: 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood CR & Cutting S (1990) Molecular Biological Methods for Bacillus. John Wiley and Sons, Sussex, UK. [Google Scholar]
- Henriques AO & Moran CP Jr (2006) Structure, assembly, and function of the spore surface layers. Annu Rev Microbiol 61: 555–558. [DOI] [PubMed] [Google Scholar]
- Henriques AO, Costa TV, Martins LO & Zilhao R (2004) The functional architecture and assembly of the coat. Bacterial Spore Formers: Probiotics and Emerging Applications (Ricca E, Henriques AO & Cutting SM, eds), pp. 65–86. Horizon Biosciences, Norfolk. [Google Scholar]
- Hilbert DW & Piggot PJ (2004) Compartmentalization of gene expression during Bacillus subtilis spore formation. Microbiol Mol Biol R 68: 234–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt SC & Leadbetter ER (1969) Comparative ultrastructure of selected aerobic spore-forming bacteria: a freeze-etching study. Bacteriol Rev 33: 346–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isticato R, Cangiano G, Tran HT, Ciabattini A, Medaglini D, Oggioni MR, De Felice M, Pozzi G & Ricca E (2001) Surface display of recombinant proteins on Bacillus subtilis spores. J Bacteriol 183: 6294–6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Hahn M, Grabowski P, McPherson DC, Otte MM, Wang R, Ferguson CC, Eichenberger P & Driks A (2006) The Bacillus subtilis spore coat protein interaction network. Mol Microbiol 59: 487–502. [DOI] [PubMed] [Google Scholar]
- Kim HS, Sherman D, Johnson F & Aronson AI (2004) Characterization of a major Bacillus anthracis spore coat protein and its role in spore inactivation. J Bacteriol 186: 2413–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klobutcher LA, Ragkousi K & Setlow P (2006) The Bacillus subtilis spore coat provides “eat resistance” during phagocytic predation by the protozoan Tetrahymena thermophila. Proc Natl Acad Sci USA 103: 165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler TM, Dai Z & Kaufman-Yarbray M (1994) Regulation of the Bacillus anthracis protective antigen gene: CO2 and a trans-acting element activate transcription from one of two promoters. J Bacteriol 176: 586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwana R, Kasahara Y, Fujibayashi M, Takamatsu H, Ogasawara N & Watabe K (2002) Proteomics characterization of novel spore proteins of Bacillus subtilis. Microbiology 148: 3971–3982. [DOI] [PubMed] [Google Scholar]
- Lai EM, Phadke ND, Kachman MT, Giorno R, Vazquez S, Vazquez JA, Maddock JR & Driks A (2003) Proteomic analysis of the spore coats of Bacillus subtilis and Bacillus anthracis. J Bacteriol 185: 1443–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton TJ & Doi RH (1971) The stability of messenger ribonucleic acid during sporulation in Bacillus subtilis. J Biol Chem 246: 3189–3195. [PubMed] [Google Scholar]
- Little S & Driks A (2001) Functional analysis of the Bacillus subtilis morphogenetic spore coat protein CotE. Mol Microbiol 42: 1107–1120. [DOI] [PubMed] [Google Scholar]
- Little SF & Knudson GB (1986) Comparative efficacy of Bacillus anthracis live spore vaccine and protective antigen vaccine against anthrax in the guinea pig. Infect Immun 52: 509–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick R, Youngman P & Piggot PJ (1986) Genetics of endospore formation in Bacillus subtilis. Annu Rev Genet 20: 625–669. [DOI] [PubMed] [Google Scholar]
- Lyons CR, Lovchik J, Hutt J, Lipscomb MF, Wang E, Heninger S, Berliba L & Garrison K (2004) Murine model of pulmonary anthrax: kinetics of dissemination, histopathology, and mouse strain susceptibility. Infect Immun 72: 4801–4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson DC, Kim H, Hahn M, Wang R, Grabowski P, Eichenberger P & Driks A (2005) Characterization of the Bacillus subtilis spore morphogenetic coat protein CotO. J Bacteriol 187: 8278–8290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson I, Tobery S, Scorpio A, Bozue J, Shafferman A & Friedlander AM (2004) The NheA component of the non-hemolytic enterotoxin of Bacillus cereus is produced by Bacillus anthracis but is not required for virulence. Microb Pathogenesis 37: 149–154. [DOI] [PubMed] [Google Scholar]
- Ozin AJ, Samford CS, Henriques AO & Moran CP Jr (2001) SpoVID guides SafA to the spore coat in Bacillus subtilis. J Bacteriol 183: 3041–3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Casal J, Caparon MG & Scott JR (1991) Mry, a trans-acting positive regulator of the M protein gene of Streptococcus pyogenes with similarity to the receptor proteins of two-component regulatory systems. J Bacteriol 173: 2617–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomp M, Leighton TJ, Wheeler KE & Malkin AJ (2005) The high-resolution architecture and structural dynamics of Bacillus spores. Biophys J 88: 603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomp M, Leighton TJ, Wheeler KE, Hill HD & Malkin AJ (2007) In vitro high-resolution structural dynamics of single germinating bacterial spores. Proc Natl Acad Sci USA 104: 9644–9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogliano J, Osborne N, Sharp MD, Abanes-De Mello A, Perez A, Sun YL & Pogliano K (1999) A vital stain for studying membrane dynamics in bacteria: a novel mechanism controlling septation during Bacillus subtilis sporulation. Mol Microbiol 31: 1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogliano K, Harry E & Losick R (1995) Visualization of the subcellular location of sporulation proteins in Bacillus subtilis using immunofluorescence microscopy. Mol Microbiol 18: 459–470. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF & Maniatis T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- Santo LY & Doi RH (1974) Ultrastructural analysis during germination and outgrowth of Bacillus subtilis spores. J Bacteriol 120: 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P (2006) Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J Appl Microbiol 101: 514–525. [DOI] [PubMed] [Google Scholar]
- van Ooij C, Eichenberger P & Losick R (2004) Dynamic patterns of subcellular protein localization during spore coat morphogenesis in Bacillus subtilis. J Bacteriol 186: 4441–4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Krishnamurthy SN, Jeong J-S, Driks A, Mehta M & Gingras BA (2007) Finger-printing species and strains of Bacilli spores by distinctive coat surface morphology. Langmuir 23: 10230–10234. [DOI] [PubMed] [Google Scholar]
- Warth AD, Ohye DF & Murrell WG (1963) The composition and structure of bacterial spores. J Cell Biol 16: 579–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SB & Setlow P (2003) Mechanisms of killing of Bacillus subtilis spores by hypochlorite and chlorine dioxide. J Appl Microbiol 95: 54–67. [DOI] [PubMed] [Google Scholar]
- Zheng LB & Losick R (1990) Cascade regulation of spore coat gene expression in Bacillus subtilis. J Mol Biol 212: 645–660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


