ABSTRACT
Viruses have brought humanity many challenges: respiratory infection, cancer, neurological impairment and immunosuppression to name a few. Virology research over the last 60+ years has responded to reduce this disease burden with vaccines and antivirals. Despite this long history, the COVID-19 pandemic has brought unprecedented attention to the field of virology. Some of this attention is focused on concern about the safe conduct of research with human pathogens. A small but vocal group of individuals has seized upon these concerns – conflating legitimate questions about safely conducting virus-related research with uncertainties over the origins of SARS-CoV-2. The result has fueled public confusion and, in many instances, ill-informed condemnation of virology. With this article, we seek to promote a return to rational discourse. We explain the use of gain-of-function approaches in science, discuss the possible origins of SARS-CoV-2 and outline current regulatory structures that provide oversight for virological research in the United States. By offering our expertise, we – a broad group of working virologists – seek to aid policy makers in navigating these controversial issues. Balanced, evidence-based discourse is essential to addressing public concern while maintaining and expanding much-needed research in virology.
KEYWORDS: COVID-19, Coronavirus, DURC, Gain of function, SARS-CoV-2, biosafety, influenza, pandemic, vaccines, zoonosis
COMMENTARY
Just 30,000 nucleotides of single-stranded RNA, neatly packaged as a coronavirus, brought the world to its knees socially, economically, ethically, and morally during the COVID-19 pandemic. COVID-19 has cast a harsh light on the many cracks, fissures and disparities in our public health system, and the inability to broadly come together to face a colossal crisis and focus on the needs of the most vulnerable. However, scientists worked together and responded to the threat with impressive speed, drawing on critical previous research on coronaviruses and other viral systems. Virologists, immunologists and microbiologists from around the globe collaborated together with scientists from allied disciplines, such as infectious diseases and epidemiology. They confronted the virus through research to understand its pathogenesis and transmission, through surveillance to track the emergence of variants, and through the development of rapid tests, vaccines, antivirals and monoclonal antibodies. The SARS-CoV-2 pandemic would have claimed a substantially larger number of lives and caused more economic disruption were it not for this unprecedented collaborative scientific response. Nevertheless, the SARS-CoV-2 pandemic has also brought virology under the microscope with concerns about safety of virology research and the uncertainties around the origins of SARS-CoV-2. Here we provide an evidence- based discourse to address key issues.
Virology research under scrutiny.
Congress has a constitutional mandate to provide oversight to federally funded research. As a new Congress convenes in the United States, there is an opportunity for oversight hearings related to research in virology and the virology community stands ready to partner with Congress and lend our expertise. Our hope is that these hearings will highlight the enormous contributions of virology, including gain-of-function experiments, to human health (Table 1). However, we fear that some may use any such hearings to discredit virology and virologists and – whether intentional or not – add fuel to an anti-science, fear-based movement. Should such hearings lead to Congress legislating restrictions on scientific research, the outcome could impede our ability to predict, prepare, and respond to emerging viral threats. An equally devastating outcome would be to sow even more public distrust in science, which would limit our ability to confront viruses in general and increase the human burden from viral diseases.
TABLE 1.
Human viral diseases for which virology research has delivered vaccines and antiviral drugs
| Disease | Vaccine | Antiviral |
|---|---|---|
| Adenovirus | Yes | No |
| AIDS | No | Yes |
| Cervical and Head/neck Cancer | Yes | No |
| COVID-19 | Yes | Yes |
| Ebola virus | Yes | Yes |
| Japanese encephalitis | Yes | No |
| Hepatitis A | Yes | No |
| Hepatitis B | Yes | Yes |
| Hepatitis C | No | Yes |
| Herpes (HSV and CMV) | No | Yes |
| Influenza | Yes | Yes |
| Measles | Yes | No |
| Mpox | Yes | Yes |
| Mumps | Yes | No |
| Polio | Yes | No |
| Rabies | Yes | No |
| Respiratory syncytial virus | No | Yes |
| Rotavirus | Yes | No |
| Rubella | Yes | No |
| Smallpox | Yes | Yes |
| Tick borne encephalitis | Yes | No |
| Yellow fever | Yes | No |
| Varicella and zoster | Yes | Yes |
The origin of SARS-CoV-2.
A major point of contention in discussions of the COVID-19 pandemic has been the origin of SARS-CoV-2, with two major camps arguing that the virus either originated from animal-to-human transmission (zoonosis) or by a laboratory leak (1–3). Most virologists have been open-minded about the possible origins of SARS-CoV-2 and have formed opinions based on the best available evidence, as is done for all scientific questions (4). While each of these possibilities is plausible and has been investigated, currently the zoonosis hypothesis has the strongest supporting evidence (5–8). Zoonosis involves transmission of the virus as a consequence of close proximity between humans and wild animals, a scenario that has occurred repeatedly over time, leading to the emergence of many viruses, including Ebola virus, other coronaviruses, influenza A virus, mpox virus, and others (9–11). The lab-origin hypothesis suggests an accident at best or nefarious actors at the worst. At this time and based on the available data, there is no compelling evidence to support either of these lab-origin scenarios. It is important that scientists, the public, and public figures follow the evidence and limit speculation that can become fodder for misinformation and conspiracy theories. For example, on January 1, 2023, USA Today published an article discussing where the next pandemic could originate and disproportionately emphasized risk from manmade threats and lab accidents while minimizing the fact that most pandemics are zoonoses and never mentioning how virological research could mitigate risk (12). Unfounded accusations of a lab leak event or nefarious research in Chinese laboratories will hasten the deterioration of important partnerships between the US and China that are critical for early detection and preparedness for seasonal influenza and future pandemics.
Gain-of-function research.
Despite the paucity of evidence for a laboratory-origin of SARS-CoV-2, discussion of this possibility has driven a second controversy to the forefront of science policy discussion: the use of gain-of-function approaches in virology. Although the phrase ‘gain-of-function’ is very problematic and inexact, it is commonly used, and we will use it here cautioning all its limitations (13). The source of concern in this area is that changing a virus to add new functionality may yield a dangerous pathogen. It is important to understand, however, that gain-of-function approaches incorporate a large proportion of all research because they are a powerful genetic tool in the laboratory. These include the development of cancer therapeutics, bacterial strategies for bioremediation, and the engineering of drought- or pest-resistant crops (Table 2). For example, some oncolytic viruses used to treat cancer mediate their effects because, using gain-of-function approaches, they have been endowed with new properties that kill tumors. At least two FDA-approved products resulted from providing viruses with new functions (Table 2). Gain-of-function research with pathogens of pandemic potential established that avian influenza viruses have the capacity to acquire mammalian transmissibility and that bat-associated coronaviruses posed a danger to humans years before COVID-19 (Table 2).
TABLE 2.
Examples of useful gain-of-function experiments
| Goal/result | Microbe | Gain-of-Function | Reference |
|---|---|---|---|
| Insect control | Baculovirus | Scorpion neurotoxin | (18) |
| Solid tumor therapy | Vaccinia | GM-CSF Expression | (19)a |
| Melanoma therapy | Herpes Simplex | GM-CSF Expression | (20) |
| COVID-19 vaccine | Adenovirus type 26 | Expression of SARS-CoV-2 spike protein | (21)a |
| Repair Cardiac pacemaker | Adenovirus | Expression of sinoatrial node transcription factor | (22) |
| Treatment of bacterial infectious diseases | Bacteriophages | Expression of various payloads to enhance activity | (23) |
| Treating Citrus tree greening disease | Citrus tristeza virus | Spinach Defensin expression | (24) |
| Enhanced Lithium Batteries | E4 and M13 bacteriophage | Modified coat protein for carbon nanotube and cation binding | (25) |
| Rabbit control through immunocontraception | Myxoma virus | Expression of rabbit zona pellucida glycoproteins | (26)b |
| Mouse control through immunocontraception | Ectromelia | Expression of mouse zona pellucida glycoproteins | (27)b |
| Faster computers | M13 bacteriophage | Increased electrical conductance | (28) |
| Established that H5N1 has capacity for mammalian transmissibility | H5N1 | Mutations leading to mammalian transmission | (29, 30) |
| Established danger from bat SARS-like coronavirus to humans | SARS-CoV | Bat coronavirus spike protein | (31) |
| Drought and salt resistance in plants | Arabidopsis | Over expression of vacuolar H+-ATPase | (32) |
| Resistance to dengue virus to reduce transmission | Mosquitoes | Transgenic expression of antibody to dengue virus | (33) |
| Resistance to freezing | Many species from plant to animals | Expression of anti-freeze proteins | (34) |
| Increase nitrogen fixation to reduce fertilizer need | Klebsiella variicola | 122-fold increase in nitrogen fixation genes | (35) |
| Develop a new vaccine against cryptococcosis | Cryptococcus neoformans | Expression of gamma- interferon | (36) |
| Hormones for human therapy (e.g. insulin) | E. coli | Synthesis of human hormone (e.g. insulin) | (37) |
| Enzymes for food prepn such as pectinases for improved juice production | Yeast species | Enzyme expression for industrial use | (38) |
| CAR T cells | Lentivirus | Cancer immunotherapy | (39) |
| Dengue vaccine | Dengue/yellow fever virus | Recombinant DNA technology replaces genetic sequences in the yellow fever vaccine with dengue virus sequences | (40)b |
FDA approved product.
Experiments done in viruses that classify as pathogens with pandemic potential.
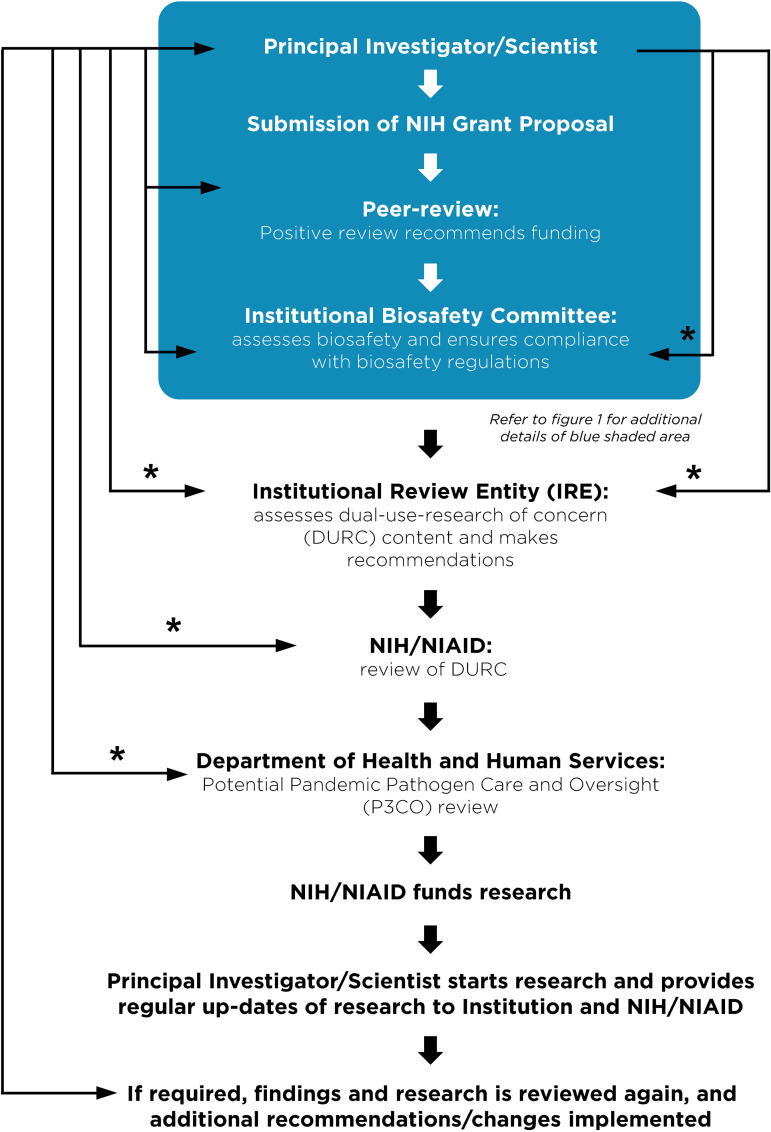
Despite these clear benefits, a narrative has developed suggesting that gain-of-function research is synonymous with high-risk or nefarious activity to engineer or enhance pandemic pathogens. In truth, gain-of-function research is a valuable experimental approach that virologists use to address essential questions. Virologists do not take their work lightly and thoughtfully propose experiments to address essential questions. Virologists do not operate in isolation to judge the risks of experiments: layers of regulation are in place such that risks are considered by individuals with diverse perspectives and expertise (Fig. 1). The vast majority of virology experiments could not enhance pandemic potential (referred to in the United States as gain-of-function research-of-concern). Those rare experiments that could are currently subject to stringent oversight through the U.S. Government under programs known as dual-use- research-of-concern (DURC) (14) and potential-pandemic-pathogens-care-and-oversight (P3CO) (15) (Fig. 2), and also by the vast majority of international publishers, including the American Society for Microbiology (ASM) and the Public Library of Science (PLOS) (Fig. 3). There are clearly experiments where the risks outweigh the benefits, and it is important that mechanisms exist to prevent such experiments. However, in many cases, gain-of-function research-of-concern can very clearly advance pandemic preparedness and the development of vaccines and antivirals. These tangible benefits often far outweigh the theoretical risks posed by modified viruses. Thus, it is important that oversight mechanisms faithfully consider both risks and benefits of these types of studies. It is equally important that the mechanisms used to provide oversight of gain-of-function research-of-concern be focused on research that is indeed of concern. Identifying research of concern is complex but it is critical that safeguards be both thoughtfully designed and implemented to avoid suppressing innovation in a field essential to mitigating infectious disease threats and with the potential to transform health.
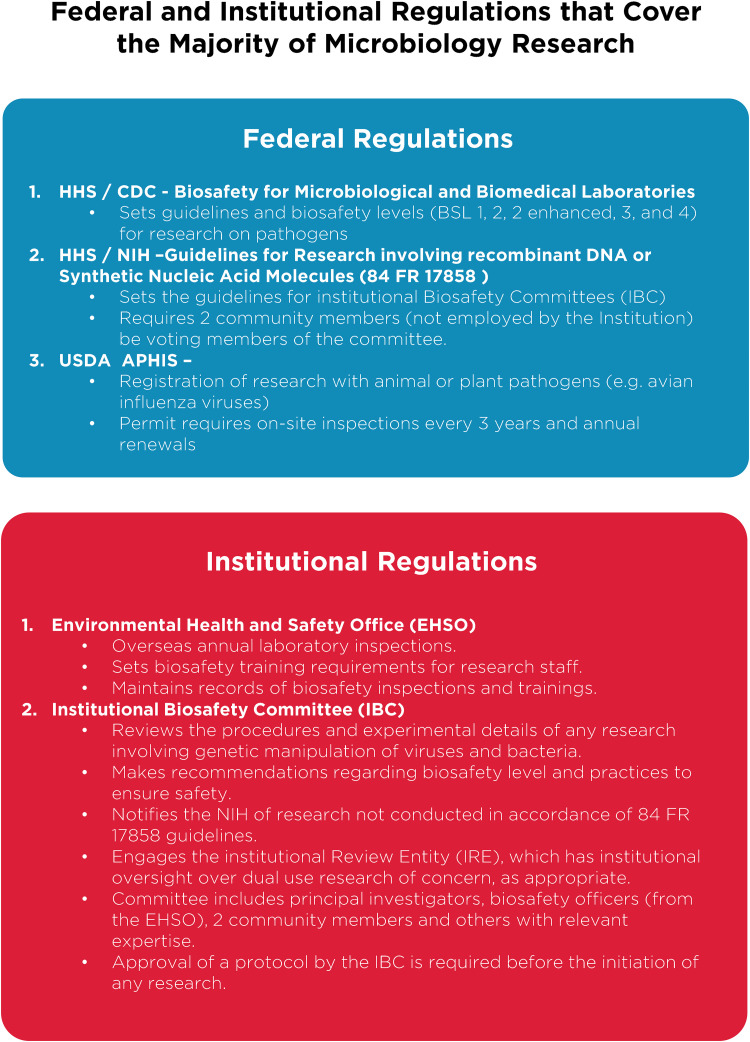
FIG 1.
Federal and institutional regulations. A brief breakdown of current U.S. Department of Health and Human Services regulations on microbiology research and the implementation of federal requirements by individual institutions, through various committees and processes. Important additional oversight that is not shown includes that provided by occupational health services to ensure safety of research personnel, and federal and institutional regulation of research involving vertebrate animals or human subjects, which are overseen by Institutional Animal Care and Use Committees (IACUC; animal research) and Institutional Review Boards (IRB; human subjects research).
FIG 2.
Current regulations surrounding funding, monitoring and approval of gain-of-function research of concern. A flow chart describing the steps a principal investigator must follow prior to receiving funding or initiating research considered dual-use-research-of-concern (DURC) on pathogens of pandemic potential (P3). The blue shaded box corresponds to the general practices that all investigators carry out at the institutional level described in Fig. 1. Research with the potential to enhance the properties of pathogen is referred for additional oversight. Asterisks (*) indicate an iterative process typically involving 1–3 reviews and revisions.
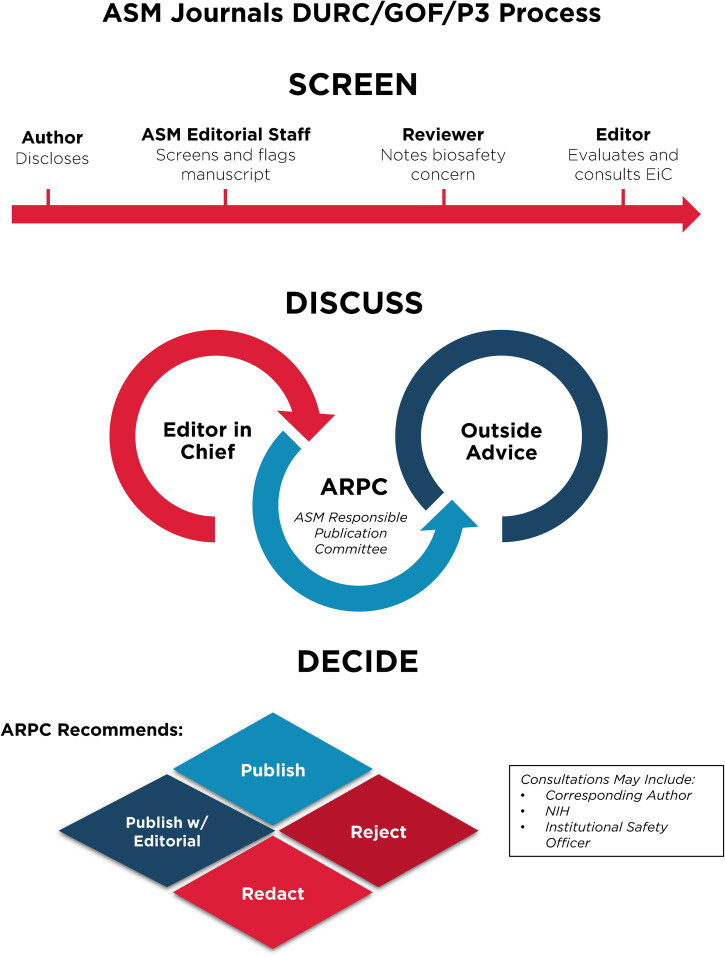
FIG 3.
Publishing process for articles involving gain-of-function research-of-concern or with pathogens of pandemic potential at ASM journals. Articles in the GOF/DURC/P3 category are flagged by multiple layers at the screening stage. This process will initiate discussion among the scientific editor in chief and other subject matter experts and lead to a publication decision after careful consideration of risks and benefits.
Existing regulation of virology research in the United States.
Without qualification, appropriate precautions should be taken to minimize laboratory accidents or the unjustified engineering of pathogens with enhanced pandemic potential. This is a shared goal for scientists and regulators. Virological research in the United States is subject to federal regulation through the Department of Health and Human Services and the U.S. Department of Agriculture for work involving human, animal, and plant pathogens. Federal policies are guided by the Biosafety in Microbiological and Biomedical Laboratories (BMBL) guidelines (16) published by the Centers for Disease Control and Prevention (CDC) and implemented by Institutional Biosafety Committees (IBCs) that manage the safety and practice of laboratory research at the local level (Fig. 1). The National Institutes of Health (NIH) established guidelines for the creation of IBCs in 1976 and updates these guidelines periodically. While each institution may have additional specific policies, the NIH requires that IBCs approve of research involving genetic manipulation of pathogens and that members of the local community are engaged through their inclusion on IBCs. As noted above, further oversight for research involving viruses with pandemic potential exists at multiple levels, including federal funding agencies (e.g., Department of Health and Human Services, Department of Defense), institutions (e.g., colleges, universities, and research institutes), and most accredited publishers of scientific research (Fig. 2 and 3) (17). It is important that we work to ensure appropriate and consistent implementation of these existing safeguards. We should also seek to develop international partnerships to ensure appropriate oversight is in place worldwide. While policy and infrastructure should frequently be reassessed with the goal of updating and improving biosafety and biosecurity, policy should not be changed rashly and without consideration of potential unintended consequences. Given the scientific complexities involved, such decisions should not be legislated. They should instead be made after full consideration by scientists with the relevant expertise – with appropriate oversight from Congress.
Conclusion.
As we enter the fourth year of the COVID-19 pandemic, it is clear that the scientific establishment has been the most effective shield protecting humanity from this calamity through the delivery of rapid tests, vaccines, antibody therapies, and small molecule antivirals. Millions are alive today that would otherwise be dead thanks to society’s investment in creating and maintaining a vibrant scientific enterprise. In the debate on further restrictions around gain-of-function research and new regulations on virology in general, it is important to appreciate the existing framework of regulations at the federal and institutional levels. It is also critical to recognize that our ability to respond rapidly to emerging viral threats is dependent on our ability to apply the tools of modern biology to viruses. Regulations that are redundant with current practice or overly cumbersome will lead to unwarranted constraints on pandemic preparation and response and could leave humanity more vulnerable to future disease outbreaks. Gain-of-function research has been an extremely valuable tool in the development of vaccines and antivirals (Table 2). It already has layers of regulations and checks in place to ensure that potentially unsafe research is immediately reported (Fig. 2). It is critical that policy makers, virologists, and biosafety experts work together to ensure that research is conducted safely, with the common goal of reducing the burden of disease caused by viruses.
ACKNOWLEDGMENTS
The opinions expressed reflect those of the authors, and not necessarily the opinions of ASM, the authors’ institutions, or funding agencies. We acknowledge Johnny Chang for his assistance with graphics.
No funding was used to support this commentary, but we wish to disclose funding from agencies received by authors. JCA None WJA NIH DCA NIH JDB NIH BWB CIHR, NSERC LB Associazione Italiana per la Ricerca sul Cancro, ICGEB SBM NIH DB NIH, Private Foundation DBM Private Foundation DCB NIH, Private Foundation ACB NIH SB None CRB NIH, NSF, Private Foundation AJB USDA, Biotechnology and Biological Sciences Research Council (BBSRC), UK CBB NIH, DOD CEC NIH SKC NIH PC NIH AC NIH GCC NIH ARC NIH, Private Foundation JMC NIH KC NIH BD NIH MDD NIH, Burroughs Wellcome Investigators in the Pathogenesis of Infectious Disease Program KD None JAD NIH, Private Foundation TSD NIH, Private Foundation, Heinz Endowments JDD Canadian Institutes of Health Research and Natural Sciences and Engineering Council of Canada DD NIH RRD NIH, USDA, Private Foundation WPD NIH, Zoetis LLC, Moderna, Coalition for Epidemics Preparedness Initiatives (CEPI) RED NIH NCE NIH, Howard Hughes Medical Institute ME NIH LWE None BAF NSF AFS NIH MLF NSF, USDA, Private Foundation LF Canadian Institutes of Health Research (CIHR) MBF NIH, Novavax, Astrazeneca, Pfizer, BARDA, Eli Lilly KF NIH, DOD, Bill and Melinda Gates Foundation MUG NIH MMG NIH, Private Foundation TG NIH DG NIH, Private Foundation AG-S NIH, DOD, Private Foundation APG NIH BAG NIH, Howard Hughes Medical Institute SPG NIH, Private Foundation FG NIH ALG None MHH NIH EH NIH NSH NIH, DOD, Private Foundation MTH NIH EEH NIH, Private Foundation BGH NIH, NSF SMH NIH ECH UK Medical Research Council, UK Medical Research Council JMH NIH MJI NIH, Via Nova Therapeutics WTJ NIH RFK NIH JPK NIH SMK NIH FK German research foundation (DFG), European research council (ERC) DMK NIH TK NIH, Moderna ML NIH LL NIH SSL NIH RAL NIH ASL NIH, Burroughs Wellcome Fund, Flu Lab, CDC BL NIH, NSF, Private Foundation DAL NIH SL NIH, Private donor's fund, Ohio State. startup fund RML NIH CBL NIH ACL NIH, Private Foundation MAL NIH JML NIH BM NIH GM NIH MTM NIH, DHS AMehle NIH, Private Foundation WAM NIH, Plant Sciences Institute, Iowa State University IM NIH CM NIH NJM NIH AMoscona NIH BCM NIH KM NIH JCM NIH EAM NIH MHN NIH JAN NIH CJN None JZN NIH CMO None AO NIH WAO NIH, Bill & Melinda Gates Foundation DAO NIH, Private Foundation JHJO NIH JSP NIH CRP NIH, Private Foundation AP NIH, Private Foundation, CDC PEP NIH JKP NIH, Private Foundation RKP NIH, DOD, Gilead Sciences, Enanta Pharmaceuticals SJP None JGP NIH DP NIH, Private Foundation MQM NIH, Private Foundation RR NIH CMR NIH, Private Foundation RJR NIH CJR NIH RS-G NIH MS NIH LMS NIH, Cornell University, Private donors, Fast Grants SS None JWS NIH SSC NIH, Private Foundation BLS NIH TS NIH, DOD GS NIH, Private Foundation VS NIH, Private Foundation GAS NIH, USDA JGS NIH KRS NIH MLS start-up funds from the University of Florida KS Australian government, NHRMC WIS NIH MSS NIH TCS NIH, USDA AWT NIH VLT NIH, Private Foundation BT NIH, DOD, Private Foundation SAT NIH SMT NIH ZT NIH KVD NIH, Private Foundation MV None NAW NIH DW NIH MPW Medical Research Council, UK (MR/W025647/1); UKRI mpox research consortium (BB/X011143/1) JBW NIH MDW NIH SKW NIH EAW NIH, Private Foundation SPJW NIH, Private Foundation, USAID, Vir Biotechnology BRGW Private Foundation CEW NIH, University of Michigan SWW NIH ADY NIH.
The views expressed in this article do not necessarily reflect the views of the journal or of ASM.
Funding Statement
No funding was used to support this commentary, but we wish to disclose funding from agencies received by authors. JCA None WJA NIH DCA NIH JDB NIH BWB CIHR, NSERC LB Associazione Italiana per la Ricerca sul Cancro, ICGEB SBM NIH DB NIH, Private Foundation DBM Private Foundation DCB NIH, Private Foundation ACB NIH SB None CRB NIH, NSF, Private Foundation AJB USDA, Biotechnology and Biological Sciences Research Council (BBSRC), UK CBB NIH, DOD CEC NIH SKC NIH PC NIH AC NIH GCC NIH ARC NIH, Private Foundation JMC NIH KC NIH BD NIH MDD NIH, Burroughs Wellcome Investigators in the Pathogenesis of Infectious Disease Program KD None JAD NIH, Private Foundation TSD NIH, Private Foundation, Heinz Endowments JDD Canadian Institutes of Health Research and Natural Sciences and Engineering Council of Canada DD NIH RRD NIH, USDA, Private Foundation WPD NIH, Zoetis LLC, Moderna, Coalition for Epidemics Preparedness Initiatives (CEPI) RED NIH NCE NIH, Howard Hughes Medical Institute ME NIH LWE None BAF NSF AFS NIH MLF NSF, USDA, Private Foundation LF Canadian Institutes of Health Research (CIHR) MBF NIH, Novavax, Astrazeneca, Pfizer, BARDA, Eli Lilly KF NIH, DOD, Bill and Melinda Gates Foundation MUG NIH MMG NIH, Private Foundation TG NIH DG NIH, Private Foundation AG-S NIH, DOD, Private Foundation APG NIH BAG NIH, Howard Hughes Medical Institute SPG NIH, Private Foundation FG NIH ALG None MHH NIH EH NIH NSH NIH, DOD, Private Foundation MTH NIH EEH NIH, Private Foundation BGH NIH, NSF SMH NIH ECH UK Medical Research Council, UK Medical Research Council JMH NIH MJI NIH, Via Nova Therapeutics WTJ NIH RFK NIH JPK NIH SMK NIH FK German research foundation (DFG), European research council (ERC) DMK NIH TK NIH, Moderna ML NIH LL NIH SSL NIH RAL NIH ASL NIH, Burroughs Wellcome Fund, Flu Lab, CDC BL NIH, NSF, Private Foundation DAL NIH SL NIH, Private donor's fund, Ohio State. startup fund RML NIH CBL NIH ACL NIH, Private Foundation MAL NIH JML NIH BM NIH GM NIH MTM NIH, DHS AMehle NIH, Private Foundation WAM NIH, Plant Sciences Institute, Iowa State University IM NIH CM NIH NJM NIH AMoscona NIH BCM NIH KM NIH JCM NIH EAM NIH MHN NIH JAN NIH CJN None JZN NIH CMO None AO NIH WAO NIH, Bill & Melinda Gates Foundation DAO NIH, Private Foundation JHJO NIH JSP NIH CRP NIH, Private Foundation AP NIH, Private Foundation, CDC PEP NIH JKP NIH, Private Foundation RKP NIH, DOD, Gilead Sciences, Enanta Pharmaceuticals SJP None JGP NIH DP NIH, Private Foundation MQM NIH, Private Foundation RR NIH CMR NIH, Private Foundation RJR NIH CJR NIH RS-G NIH MS NIH LMS NIH, Cornell University, Private donors, Fast Grants SS None JWS NIH SSC NIH, Private Foundation BLS NIH TS NIH, DOD GS NIH, Private Foundation VS NIH, Private Foundation GAS NIH, USDA JGS NIH KRS NIH MLS start-up funds from the University of Florida KS Australian government, NHRMC WIS NIH MSS NIH TCS NIH, USDA AWT NIH VLT NIH, Private Foundation BT NIH, DOD, Private Foundation SAT NIH SMT NIH ZT NIH KVD NIH, Private Foundation MV None NAW NIH DW NIH MPW Medical Research Council, UK (MR/W025647/1); UKRI mpox research consortium (BB/X011143/1) JBW NIH MDW NIH SKW NIH EAW NIH, Private Foundation SPJW NIH, Private Foundation, USAID, Vir Biotechnology BRGW Private Foundation CEW NIH, University of Michigan SWW NIH ADY NIH.
Footnotes
Ed. Note: This commentary is being published by the following ASM journals: Journal of Virology, mBio, and mSphere.
[This article was published on 26 January 2023 with the incorrect given name for Matthew Daugherty, omissions in the affiliations, and small errors in the text and footnotes. Corrections were made in the version posted on 7 February 2023. An additional footnote correction was made in the current version, posted 28 February 2023.]
REFERENCES
- 1.Cohen J. 2022. Studies bolster pandemic origin in Wuhan animal market. Science 375:946–947. 10.1126/science.adb1760. [DOI] [PubMed] [Google Scholar]
- 2.Maxmen A. 2022. Wuhan market was epicentre of pandemic's start, studies suggest. Nature 603:15–16. 10.1038/d41586-022-00584-8. [DOI] [PubMed] [Google Scholar]
- 3.Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. 2020. The proximal origin of SARS- CoV-2. Nat Med 26:450–452. 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casadevall A, Weiss SR, Imperiale MJ. 2021. Can Science Help Resolve the Controversy on the Origins of the SARS-CoV-2 Pandemic? mBio 12:e0194821. 10.1128/mBio.01948-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Worobey M, Levy JI, Malpica Serrano L, Crits-Christoph A, Pekar JE, Goldstein SA, Rasmussen AL, Kraemer MUG, Newman C, Koopmans MPG, Suchard MA, Wertheim JO, Lemey P, Robertson DL, Garry RF, Holmes EC, Rambaut A, Andersen KG. 2022. The Huanan Seafood Wholesale Market in Wuhan was the early epicenter of the COVID-19 pandemic. Science 377:951–959. 10.1126/science.abp8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Relman DA. 2020. Opinion: To stop the next pandemic, we need to unravel the origins of COVID-19. Proc Natl Acad Sci USA 117:29246–29248. 10.1073/pnas.2021133117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmes EC, Goldstein SA, Rasmussen AL, Robertson DL, Crits-Christoph A, Wertheim JO, Anthony SJ, Barclay WS, Boni MF, Doherty PC, Farrar J, Geoghegan JL, Jiang X, Leibowitz JL, Neil SJD, Skern T, Weiss SR, Worobey M, Andersen KG, Garry RF, Rambaut A. 2021. The origins of SARS-CoV-2: A critical review. Cell 184:4848–4856. 10.1016/j.cell.2021.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garry RF. 2022. The evidence remains clear: SARS-CoV-2 emerged via the wildlife trade. Proc Natl Acad Sci USA 119:e2214427119. 10.1073/pnas.2214427119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plowright RK, Parrish CR, McCallum H, Hudson PJ, Ko AI, Graham AL, Lloyd-Smith JO. 2017. Pathways to zoonotic spillover. Nat Rev Microbiol 15:502–510. 10.1038/nrmicro.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albery GF, Becker DJ, Brierley L, Brook CE, Christofferson RC, Cohen LE, Dallas TA, Eskew EA, Fagre A, Farrell MJ, Glennon E, Guth S, Joseph MB, Mollentze N, Neely BA, Poisot T, Rasmussen AL, Ryan SJ, Seifert S, Sjodin AR, Sorrell EM, Carlson CJ. 2021. The science of the host-virus network. Nat Microbiol 6:1483–1492. 10.1038/s41564-021-00999-5. [DOI] [PubMed] [Google Scholar]
- 11.Taubenberger JK, Kash JC. 2010. Influenza virus evolution, host adaptation, and pandemic formation. Cell Host Microbe 7:440–451. 10.1016/j.chom.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weintraub K. 2023. As COVID turns 3, experts worry where the next pandemic will come from — and if we will be ready, In USA Today. https://www.usatoday.com/story/news/health/2023/01/01/covid-anniversary-next-pandemic- expert-concern/10847848002/. [Google Scholar]
- 13.Dance A. 2021. The shifting sands of 'gain-of-function' research. Nature 598:554–557. 10.1038/d41586-021-02903-x. [DOI] [PubMed] [Google Scholar]
- 14.USDoHaH S. 2021. Dual Use Research of Concern 2021. https://www.phe.gov/s3/dualuse/Pages/default.aspx. Accessed.
- 15.USDoHaH S. 2021. Department of Health and Human Services (HHS) Framework for Guiding Funding Decisons about Proposed Research Involving Enhanced Potential Pandemic Pathogens https://www.phe.gov/s3/dualuse/Pages/p3co.aspx. Accessed
- 16.(ed). 2020. Biosafety in Microbiological and Biomedical Laboratories (BMBL). Centers for Disease Control and Prevention and U.S. National Institutes of Health., https://www.cdc.gov/labs/BMBL.html. Accessed [Google Scholar]
- 17.Casadevall A, Dermody TS, Imperiale MJ, Sandri-Goldin RM, Shenk T. 2015. Dual-Use Research of Concern (DURC) Review at American Society for Microbiology Journals. mBio 6:e01236. 10.1128/mBio.01236-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart LM, Hirst M, López Ferber M, Merryweather AT, Cayley PJ, Possee RD. 1991. Construction of an improved baculovirus insecticide containing an insect-specific toxin gene. Nature 352:85–88. 10.1038/352085a0. [DOI] [PubMed] [Google Scholar]
- 19.Kim JH, Oh JY, Park BH, Lee DE, Kim JS, Park HE, Roh MS, Je JE, Yoon JH, Thorne SH, Kirn D, Hwang TH. 2006. Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol Ther 14:361–370. 10.1016/j.ymthe.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Bommareddy PK, Patel A, Hossain S, Kaufman HL. 2017. Talimogene Laherparepvec (T-VEC) and Other Oncolytic Viruses for the Treatment of Melanoma. Am J Clin Dermatol 18:1–15. 10.1007/s40257-016-0238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gray JJ, Moughon S, Wang C, Schueler-Furman O, Kuhlman B, Rohl CA, Baker D. 2003. Protein- protein docking with simultaneous optimization of rigid-body displacement and side-chain conformations. J Mol Biol 331:281–299. 10.1016/s0022-2836(03)00670-3. [DOI] [PubMed] [Google Scholar]
- 22.Kapoor N, Liang W, Marbán E, Cho HC. 2013. Direct conversion of quiescent cardiomyocytes to pacemaker cells by expression of Tbx18. Nat Biotechnol 31:54–62. 10.1038/nbt.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meile S, Du J, Dunne M, Kilcher S, Loessner MJ. 2022. Engineering therapeutic phages for enhanced antibacterial efficacy. Curr Opin Virol 52:182–191. 10.1016/j.coviro.2021.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh D, Motghare M, Gowda S. 2018. Citrus greening: overview of the most severe disease of citrus. Adv Agric Res Technol J 2:83–100. [Google Scholar]
- 25.Lee YJ, Yi H, Kim WJ, Kang K, Yun DS, Strano MS, Ceder G, Belcher AM. 2009. Fabricating genetically engineered high-power lithium-ion batteries using multiple virus genes. Science 324:1051–1055. 10.1126/science.1171541. [DOI] [PubMed] [Google Scholar]
- 26.Mackenzie SM, McLaughlin EA, Perkins HD, French N, Sutherland T, Jackson RJ, Inglis B, Müller WJ, van Leeuwen BH, Robinson AJ, Kerr PJ. 2006. Immunocontraceptive effects on female rabbits infected with recombinant myxoma virus expressing rabbit ZP2 or ZP3. Biol Reprod 74:511–521. 10.1095/biolreprod.105.046268. [DOI] [PubMed] [Google Scholar]
- 27.Jackson RJ, Maguire DJ, Hinds LA, Ramshaw IA. 1998. Infertility in mice induced by a recombinant ectromelia virus expressing mouse zona pellucida glycoprotein 3. Biol Reprod 58:152–159. 10.1095/biolreprod58.1.152. [DOI] [PubMed] [Google Scholar]
- 28.Loke DK, Clausen GJ, Ohmura JF, Chong T-C, Belcher AM. 2018. Biological-templating of a segregating binary alloy for nanowire-like phase-change materials and memory. ACS Appl Nano Mater 1:6556–6562. 10.1021/acsanm.8b01508. [DOI] [Google Scholar]
- 29.Herfst S, Schrauwen EJ, Linster M, Chutinimitkul S, de WE, Munster VJ, Sorrell EM, Bestebroer TM, Burke DF, Smith DJ, Rimmelzwaan GF, Osterhaus AD, Fouchier RA. 2012. Airborne transmission of influenza A/H5N1 virus between ferrets. Science 336:1534–1541. 10.1126/science.1213362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imai M, Watanabe T, Hatta M, Das SC, Ozawa M, Shinya K, Zhong G, Hanson A, Katsura H, Watanabe S, Li C, Kawakami E, Yamada S, Kiso M, Suzuki Y, Maher EA, Neumann G, Kawaoka Y. 2012. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 486:420–428. 10.1038/nature10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menachery VD, Yount BL, Jr, Debbink K, Agnihothram S, Gralinski LE, Plante JA, Graham RL, Scobey T, Ge XY, Donaldson EF, Randell SH, Lanzavecchia A, Marasco WA, Shi ZL, Baric RS. 2015. A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence. Nat Med 21:1508–1513. 10.1038/nm.3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaxiola RA, Li J, Undurraga S, Dang LM, Allen GJ, Alper SL, Fink GR. 2001. Drought- and salt- tolerant plants result from overexpression of the AVP1 H+-pump. Proc Natl Acad Sci USA 98:11444–11449. 10.1073/pnas.191389398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buchman A, Gamez S, Li M, Antoshechkin I, Li HH, Wang HW, Chen CH, Klein MJ, Duchemin JB, Crowe JE, Jr, Paradkar PN, Akbari OS. 2020. Broad dengue neutralization in mosquitoes expressing an engineered antibody. PLoS Pathog 16:e1008103. 10.1371/journal.ppat.1008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naing AH, Kim CK. 2019. A brief review of applications of antifreeze proteins in cryopreservation and metabolic genetic engineering. 3 Biotech 9:329. 10.1007/s13205-019-1861-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wen A, Havens KL, Bloch SE, Shah N, Higgins DA, Davis-Richardson AG, Sharon J, Rezaei F, Mohiti-Asli M, Johnson A, Abud G, Ane JM, Maeda J, Infante V, Gottlieb SS, Lorigan JG, Williams L, Horton A, McKellar M, Soriano D, Caron Z, Elzinga H, Graham A, Clark R, Mak SM, Stupin L, Robinson A, Hubbard N, Broglie R, Tamsir A, Temme K. 2021. Enabling Biological Nitrogen Fixation for Cereal Crops in Fertilized Fields. ACS Synth Biol 10:3264–3277. 10.1021/acssynbio.1c00049. [DOI] [PubMed] [Google Scholar]
- 36.Wormley FL, Jr, Perfect JR, Steele C, Cox GM. 2007. Protection against cryptococcosis by using a murine gamma interferon-producing Cryptococcus neoformans strain. Infect Immun 75:1453–1462. 10.1128/IAI.00274-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riggs AD. 2021. Making, Cloning, and the Expression of Human Insulin Genes in Bacteria: The Path to Humulin. Endocr Rev 42:374–380. 10.1210/endrev/bnaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blanco P, Sieiro C, Villa TG. 1999. Production of pectic enzymes in yeasts. FEMS Microbiol Lett 175:1–9. 10.1111/j.1574-6968.1999.tb13595.x. [DOI] [PubMed] [Google Scholar]
- 39.June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. 2018. CAR T cell immunotherapy for human cancer. Science 359:1361–1365. 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 40.Juraska M, Magaret CA, Shao J, Carpp LN, Fiore-Gartland AJ, Benkeser D, Girerd-Chambaz Y, Langevin E, Frago C, Guy B, Jackson N, Duong Thi Hue K, Simmons CP, Edlefsen PT, Gilbert PB. 2018. Viral genetic diversity and protective efficacy of a tetravalent dengue vaccine in two phase 3 trials. Proc Natl Acad Sci USA 115:E8378–E8387. 10.1073/pnas.1714250115. [DOI] [PMC free article] [PubMed] [Google Scholar]