Abstract
This study was designed to extend research on motor skill development in autism spectrum disorder using a dual-task skill. Nine autistic and 18 non-autistic youths walked without grasping or while reaching to grasp a small or large object. Step extremity ratio, percent time in double support, and normalized speed were quantified. We hypothesized that gait would differ between autistic and non-autistic youth and that differences would be moderated by the phase (approach and grasp) and the complexity of the task (walking and grasping versus walking alone). Although gait parameters were similar during the walking-only trials, the combined task resulted in slower speed and shorter steps in autistic youth, particularly during the grasp phase. These findings, while in a small sample, offer preliminary evidence that autistic youth who show typical gait during simple assessments of motor ability may have difficulties in more complex tasks that require the coordination of movements.
Keywords: dual-task, task complexity, kinematics, motor development, gait
Introduction
During the first few years of life, most children experience tremendous changes in their capacity to perform both gross and fine motor skills. While newborn infants are fully dependent on others, by around six months of age, many infants have developed sufficient neck and core strength to be able to sit upright unaided1 and can reach for objects with one hand2. At approximately nine months of age, most children can navigate their environment by scooting or crawling3,4 and can use visual and proprioceptive feedback to bang two cubes together2. While independent upright locomotion usually begins to emerge around the child’s first birthday, immature control of posture and gait, and large stride-to-stride variability lead to frequent falls in emerging walkers5,6. Around that same time, precision grasping also appears, and children can hold a crayon or stack two blocks2. While it’s important to consider that gait and motor control in general can be examined through numerous kinematic and kinetic variables, with many maturing at different timescales, stable walking and more adult-like gait patterns become evident around three years of age7 along with the ability to copy a circle and awkwardly use scissors2. However, despite the motor milestones of the first 3–5 years, subtle changes in neuromuscular control8–10 and proprioception11 as well as continued refinement of locomotor12–14 and upper limb kinematic patterns15 persist throughout the first decade of life for most children.
Motor skill development does not always follow a similar trajectory in autistic children. Note that identity-first language (“autistic” and “non-autistic”) will be used for this paper16 in consideration of the expressed identity-first preference of autistic individuals17. A growing body of literature indicates that autistic individuals exhibit delays in motor skill development and coordination18–21 and sensorimotor processing22. These sensory and motor skill delays are evident in early childhood23,24 and persist through adulthood25–28.
Gait abnormalities in autistic children were noted as early as 1943 when Kanner29 wrote that autistic children demonstrated “clumsy” gait and gross motor patterns. The first study to use objective kinematic and kinetic measures to quantify gait characteristics in autistic youth reported reduced stride lengths and increased stance times in the autistic youth when compared to typically developing controls30. Several studies have been conducted in more recent years to further quantify differences in gait between autistic individuals and aged-matched, non-autistic individuals across a variety of developmental ages. In a pair of recent literature reviews, Kindregan and colleagues31 and Lum and collagues32 summarized the results of several studies that used kinematic and kinetic measures to assess gait in autistic individuals. Spatiotemporal measures such as step/stride length, walking speed, and percent of the gait cycle spent in double support (i.e. time during the gait cycle when both feet are in contact with the ground) were found to differ between autistic and non-autistic individuals in some studies33–35, whereas no differences were found in other studies36–39.
The variations in participants and methodology noted in the Kindegran et al.31 and Lum et al. 32 reviews can explain some of the differences in results. For example, participants ages varied between five and 19 years of age across studies. Recently Manicolo et al.40 suggested that development of mature gait may be slower in autism due to delays in the development of key brain regions, resulting in greater differences in spatiotemporal gait patterns in childhood than during adolescence. Methodological differences may also have played a role in findings across studies. Specifically, participants were free to walk at self-selected speeds across these studies. Bennett et al.41 recently showed that autistic youth exhibit similar sagittal plane biomechanics to non-autistic youth when both groups are asked to walk at standardized speeds despite differences in the frontal plane. Finally, it is also possible that simple walking tasks, which are well practiced and performed countless times per day, are not challenging enough to consistently elicit subtle kinematic differences between autistic and non-autistic individuals. Instead, gait abnormalities may only become evident as the complexity of the motor task is increased42–43. This would be consistent with the complex information processing model of autism44 and may also explain why “real world” tasks performed by autistic individuals in everyday life may result in observations of poorer performance, while no differences are found in lab and clinically based testing paradigms.
We hypothesized that more complex tasks that required participants to coordinate movements of the upper and lower limbs could provide a useful paradigm for investigating differences in motor control in autistic individuals compared to age-matched, non-autistic individuals. Specifically, in the current study, we employed a paradigm where participants were asked to reach for, grasp, and lift an object as they walked by. This paradigm is of interest because it requires the coordination of a continuous, repetitive skill (walking) with a discrete movement (reach-to-grasp). This poses a significant challenge to the motor planning and control system. Research has suggested that discrete and cyclical movements involve different motor planning and control mechanisms, an idea referred to as the two primitives theory45–47. Performing a gait and grasping task simultaneously, therefore, requires the planning of a discrete movement during the performance of a cyclic movement as the object is approached and then the integration of these two primitives to achieve a coordinated and efficient grasp.
Decreased performance on executive function and divided attention tasks has been reported in autistic children, youths, and adults as compared to age-matched, non-autistic peers48–53 suggesting that a dual-task paradigm combining upper and lower limb motor tasks could be particularly challenging for autistic individuals. Successfully grasping a target while walking requires on-line processing of visual information about the size, shape, and orientation of the target to execute the grasp while also maintaining the postural control necessary to safely navigate toward the target. In the current study, we further taxed the motor control system by adding reach-to-grasp to the movement goal. In non-autistic children (4–6 years-old), Cherng et al.54 found that dual-task costs to gait were greater when children performed a difficult upper-limb motor task (i.e. carrying a tray with marbles) compared to a simple motor task (carrying an empty tray). Similarly, Memari et al.55 reported that visual secondary tasks significantly influenced postural sway in autistic youths when compared to age-matched, non-autistic youths. Therefore, in this study, we sought to extend the research on complex motor skill development in autism spectrum disorder (ASD) by taxing the motor control system using a real world, dual-task skill to quantify spatiotemporal gait and grasp performance. As a first step in using this dual-task paradigm, we focused on youths ages 10–14 years-old to minimize the effect of developmental changes evident in gait and grasping patterns during the first decade of life. We hypothesized that spatiotemporal gait parameters would differ significantly between autistic and non-autistic youth and that these differences would be moderated by both the phase of the movement (approach and grasp) and the complexity of the task (walking and grasping versus walking alone).
Materials and Methods
Participants
This study received approval from the University of Wisconsin-Madison’s Institutional Review Board. Parent/guardian written informed consent and participant assent were obtained consistent with the declaration of Helsinki as revised in 2000. Participants were recruited through the Waisman Center’s participant registry and community flyers. Autistic participants were included if they had a previous clinical diagnosis of autistic disorder, Asperger’s syndrome, or pervasive developmental disorder not otherwise specified, which was confirmed by a parent or caregiver via a checkbox on a demographic questionnaire. An ASD diagnosis was additionally confirmed by author BGT using the Autism Diagnostic Observation Scale-2nd edition56. Autistic participants were excluded if the family reported a known medical cause of ASD (i.e. fragile-X testing, tuberous sclerosis), hypoxia-ischemia, seizure disorder, or other neurological disorders. The Wechsler Abbreviated Scale of Intelligence, 2nd edition (WASI-II)57 was performed to confirm that participants did not have co-occurring intellectual disorder (full-scale IQ < 70).
Non-autistic participants were required to screen low for ASD (i.e., a score of less than eight on the Social Communication Questionnaire58). Further, non-autistic participants could not have a first-degree family member with an ASD diagnosis, as motor difficulties may be present within the broader autism phenotype59.
Eighteen non-autistic participants between the ages of 10 and 14 years were recruited to participate in the study. Due to a combination of shut-downs related to the COVID-19 pandemic and planned demolition of the building that housed our lab space, we were only able to recruit 10 autistic participants. Further, one autistic participant was excluded because he/she did not complete several conditions within the experimental protocol. Participant groups were similar in age, height, and performance IQ, but differed in full-scale and verbal IQ (Table 1). There was also a higher percentage of female participants in the non-autistic group than the autistic group.
Table 1.
Descriptive statistics for the demographic information in each group
| Autistic (n=9) | Non-autistic (n=18) | p-Value | |
|---|---|---|---|
| % Males | 88.9 | 55.6 | |
| Age, Mean(SD) | 11.66 (0.70) | 11.75 (1.12) | 0.835 |
| Age, Range | 10.94 – 13.03 | 10.28 – 13.83 | |
| Height, Mean (SD) | 150.67 (12.32) | 154.78 (12.14) | 0.417 |
| Height, Range | 135 – 168 | 136 – 178 | |
| FSIQ, Mean(SD) | 111.44 (7.55) | 124.56 (11.93) | 0.006** |
| FSIQ, Range | 100 – 122 | 100 – 143 | |
| VIQ, Mean(SD) | 108.67 (11.87) | 125.11 (13.43) | 0.005** |
| VIQ, Range | 90 – 128 | 95 – 144 | |
| PIQ, Mean(SD) | 112.22 (14.29) | 118.78 (12.25) | 0.226 |
| PIQ, Range | 93 – 137 | 93 – 141 |
FSIQ, Full scale IQ; VIQ, Verbal IQ; PIQ, Performance IQ
Procedures
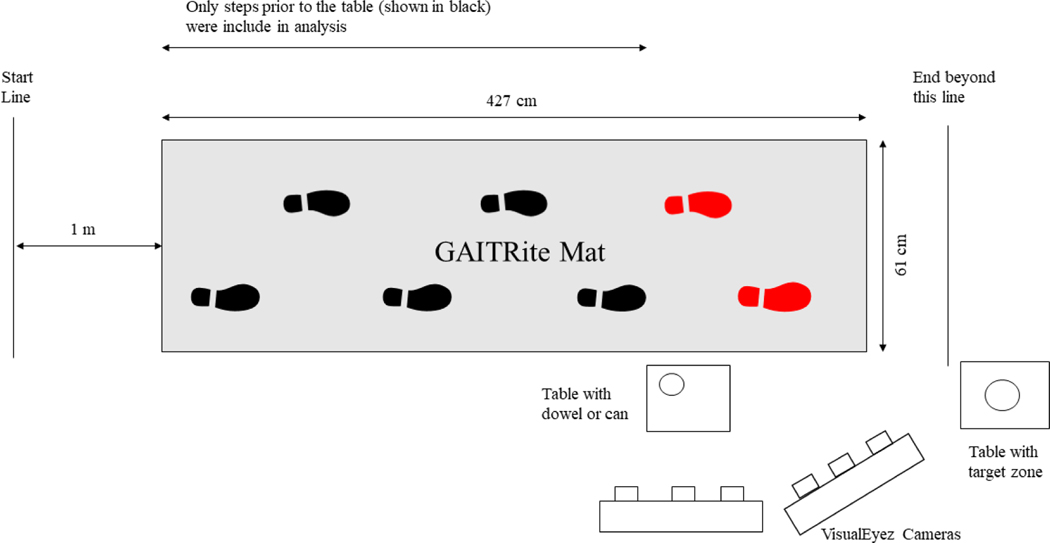
Spatiotemporal gait variables were collected using the GAITRite system (CIR Systems, Franklin, New Jersey, USA). The GAITRite walkway is a 427 cm long X 61 cm wide pressure sensitive mat consisting of 48 × 336 sensors. To collect upper body kinematics, two VisualEyez (Phoenix Technologies Incorporated) cameras were daisy chained and synchronized to increase the capture space. The cameras were mounted on separate tripods positioned laterally to the GAITRite walkway (Figure 1). The GAITRite and motion capture systems collected data at a sampling rate of 120 Hz and were time synchronized using an external pulse.
Figure 1.
Diagram of experimental setup with dimensions. The participant walked along the gait pad to pick up the target object on the table.
At the beginning of the experimental session, parents/guardians and the participants filled out a demographic questionnaire. We then measured the participant’s height (cm), by having them stand with their back against a wall on which a tape measure was affixed. Leg length was measured bilaterally by palpating for the greater trochanter of the femur and measuring to the lateral malleolus. For the right leg only, we also measured the length from greater trochanter to the floor (with shoes removed). This final measurement was used to set the height of the table on which the target object was positioned for each participant.
Three light-emitting diodes (LEDs) were attached to an ACE™ brand adjustable brace (8” at the widest point) that the participant wore across their chest. The brace was adjusted to a comfortable fit using the Velcro closure. Two shoulder straps, attached to the brace and crossed in back, prevented the brace from falling during the walking trials. The center LED was in line with the sternum and the remaining two LEDs were placed below and to the right and left of the center LED. This arrangement created a plane which was used as a reference for whole body movement. Five LEDs were attached to the participant’s right arm at the head of the humerus, lateral epicondyle, medial epicondyle, ulnar styloid process, and radial styloid process. Two additional LEDs were placed on wedges of putty taped to the nail beds of the right thumb and index finger using Transpore (3M) medical tape. Placing the LEDs on putty ensured that the LEDs remained visible to the VisualEyez cameras during object contact.
All participants were asked to remove their shoes for the length of the experiment to avoid the influence of shoe type on gait characteristics. Participants were instructed to begin by standing behind a start line which was located 1m from the start of the mat. This minimized the effect of acceleration on the first few steps recorded by the GaitRite mat. Once given a “go” cue, participants were asked to walk at a comfortable pace towards a table located 427 cm from the start position. Once they reached the table they were asked to grasp and lift a small (1.5 cm x 1.5 cm x 10 cm) or large (7 cm x 7 cm x 10 cm) cylindrical object with a precision grasp while continuing to walk forward. They were asked to perform the task without stopping at the table, and instead to continue walking toward a second table on which they were asked to place the object within a designated target zone. Participants were instructed to approach and grasp the object from the side while walking and were also encouraged to grasp the object as close to the top as comfortable to prevent obstruction of finger LEDs.
The experimental session started with three practice trials (walk small, walk large, walk forward) that were not recorded. These practice trials were used to ensure that participants understood the directions and allowed for the repositioning of any LEDs that were not visible throughout the trial. Once the practice trials were completed, the collection of experimental data began. Participants performed 40 trials separated into two blocks. Block 1 included 10 trials in which the participant walked and grasped the small object with the index finger and thumb, 10 trials where they walked and grasped the large object with the index finger and thumb, and 10 trials where they walked the length of the mat without grasping an object. Trials for the three walking conditions were presented in a randomized order. Block 2 was comprised of 10 trials in which the participant was asked to stand stationary next to the table on which the object was placed and grasp either the small object (5 trials) or the large object (5 trials). For the stationary trials, participants were asked to grasp the object with the index finger and thumb, vertically lift the object a few inches above the table surface, hold that position for three seconds, and place the object back onto the table. Block 2 trials were presented in a random order but were always presented after Block 1.
Data Processing
The GAITRite system collects numerous spatiotemporal gait parameters. However, due to the smaller sample size of the study, we chose to focus on three main variables of interest in the current study: step extremity ratio (SER), percent of gait cycle spent in double support (DS), and normalized speed (NSpeed). For all measures, only the steps up to the location of the object/table were considered in the analyses. Therefore, total trial time was defined as the time from the first footfall on the mat to the time when the marker on the index finger reached the location of the target in the forward direction (see Figure 1). All gait measures were normalized to leg-length to account for variation in participant height. Step-extremity ratio was calculated by dividing step length by leg length for both the right and left sides and then finding the average of the left and right values. The percent of the gait cycle spent in double support was calculated by dividing the total amount of time spent with both feet in contact with the mat by the total time needed to complete the trial and then multiplying that value by 100. Speed (cm/s) was calculated by dividing the distance between the initial and final footfall by the time needed to cover this distance. Speed was normalized by dividing the individual’s speed by their average leg length.
Each trial was divided into the approach phase (all steps leading up to the table on which the target rested) and the grasp phase (the single step during which the object was contacted for the small and large grasping conditions and the step during which the index finger marker reached the location where the target would normally be placed in the forward condition). Averages for SER, DS, and NSpeed were calculated for the 10 trials in each walking condition for each participant for both the approach and grasp phases.
To quantify the grasp portion of the task, we used the LEDs located on the participant’s index finger and thumb to calculate peak grasp aperture. The LED position data were rotated into a meaningful coordinate system and smoothed with a low-pass Butterworth filter with a cutoff frequency of 10 Hz. A customized computer program (KinSys, eh?soft, Madison, WI) was used to compute the grasp aperture. Aperture was defined as the resultant distance between the index finger and thumb markers, and a single peak value was determined for each trial. Trials in which the index finger or thumb were blocked for more than five consecutive frames during the reach-to-grasp movement were excluded from the analysis. When data were missing for four consecutive frames or less, the missing frames were linearly interpolated. Averages were then calculated for the valid trials within each condition and participant.
Statistical Analysis
Prior to performing comparisons, the assumptions of normality, homogeneity of the regression slopes and linearity of the relationship between the covariate (IQ) and average SER, DS and NSpeed variables were assessed. Homogeneity of the regression slopes and the linear relationships between the covariate and all dependent variables were found. Violation of the normality assumption was found for SER and was corrected using a square root transformation. SER, DS and NSpeed were then compared across condition, phase and age using a 3 Condition (walk small, walk large, forward) X 2 Phase (approach, grasp) X 2 Group (non-autistic, autistic) repeated measures Analyses of Covariance (ANCOVA), with full-scale IQ as a covariate. Because we hypothesized that spatiotemporal gait parameters would differ significantly between autistic and non-autistic youth and that these differences would be moderated by both the phase of the movement (approach and grasp) and the complexity of the task (walking and grasping versus walking alone) only 3-way interactions were interpreted.
Aperture measures were compared across conditions and between groups using a 2 Condition (walk, stand) X 2 Size (small, large) X 2 Group (non-autistic, autistic) repeated measures ANCOVA, with full-scale IQ as a covariate.
For all statistical analyses, an a priori alpha level of p < 0.05 was used, and significant main effects were further compared using the Bonferroni post-hoc test. When the sphericity assumption was violated, degrees of freedom using the Greenhouse-Geisser correction were reported.
Results
Table 2 shows the results of the 3 Condition X 2 Phase X 2 Group repeated measures ANCOVA on the spatiotemporal gait measures (SER, DS, and NSpeed).
Table 2.
Results of the ANCOVAs comparing spatiotemporal gait parameters across Condition, Phase and Group.
| Measure | Condition X Phase X Group | Condition X Group | Phase X Group | Condition X Phase | Condition (small, large, forward) | Phase (approach, grasp) | Group (non-autistic, autistic) |
|---|---|---|---|---|---|---|---|
| Step Extremity Ratio (SER) | F2,48=4.893 p = 0.012 ηp2=0.169 |
F2,48=3.77 p = 0.030 ηp2=0.136 |
F1,24=5.052 p = 0.034 ηp2=0.174 |
F2,50=53.28 p < 0.001 ηp2=0.681 |
F2,50=48.32 p < 0.001 ηp2=0.659 |
F1,25=119.18 p < 0.001 ηp2=0.83 |
F1,24=0.14 p = 0.712 ηp2=0.006 |
|
| |||||||
| Double Support (DS) | F1.35.23=1.646 p = 0.203 ηp2=0.064 |
F1.54,38.58=3.949 p = 0.038 ηp2=0.141 |
F1,25=1.415 p = 0.246 ηp2=0.056 |
F1.41,35.23=7.48 p = 0.005 ηp2=0.23 |
F1.54,38.58=30.77 p < 0.001 ηp2=0.55 |
F1,25=53.63 p < 0.001 ηp2=0.68 |
F1,25=1.146 p = 0.295 ηp2=0.046 |
|
| |||||||
| Normalized Speed (NVel) | F1.36,34.1=4.157 p = 0.039 ηp2=0.148 |
F2,50=3.279 p = 0.046 ηp2=0.12 |
F1,25=2.613 p = 0.119 ηp2=0.098 |
F1.36,34.1=39.5 p < 0.001 ηp2=0.612 |
F2,50=50.11, p < 0.001 ηp2=0.67 |
F1,25=128.76 p < 0.001 ηp2=0.84 |
F1,25=0.23 p = 0.636 ηp2=0.009 |
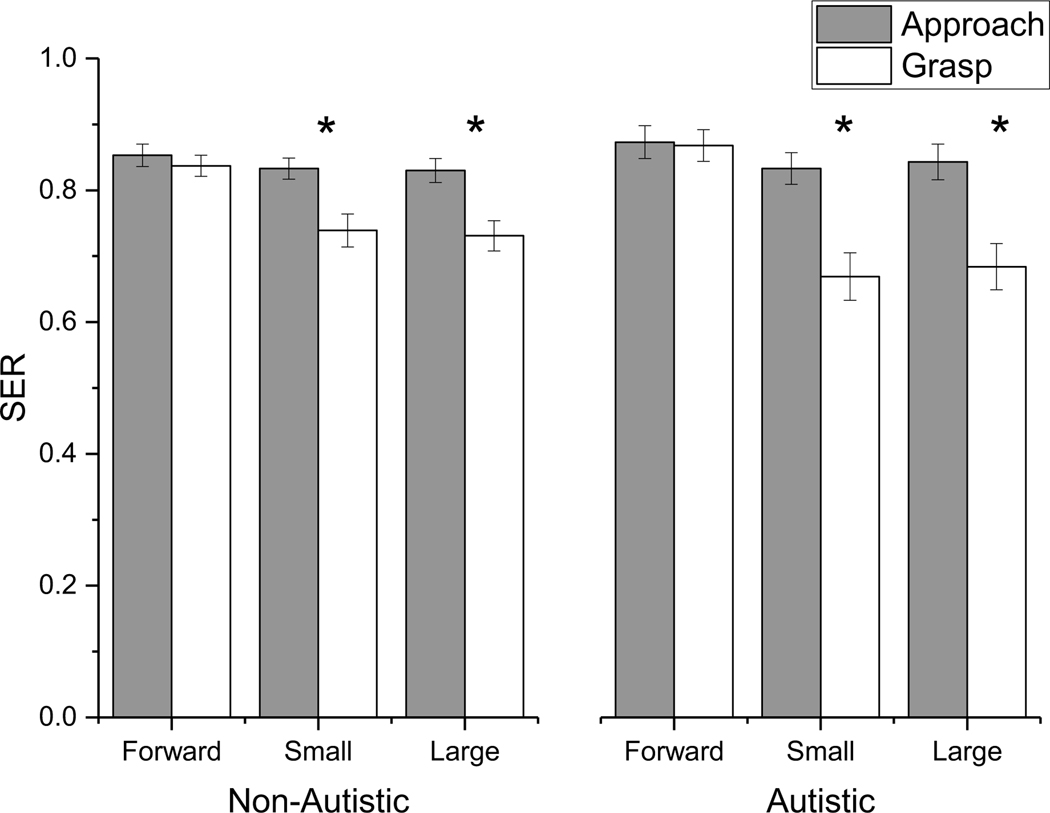
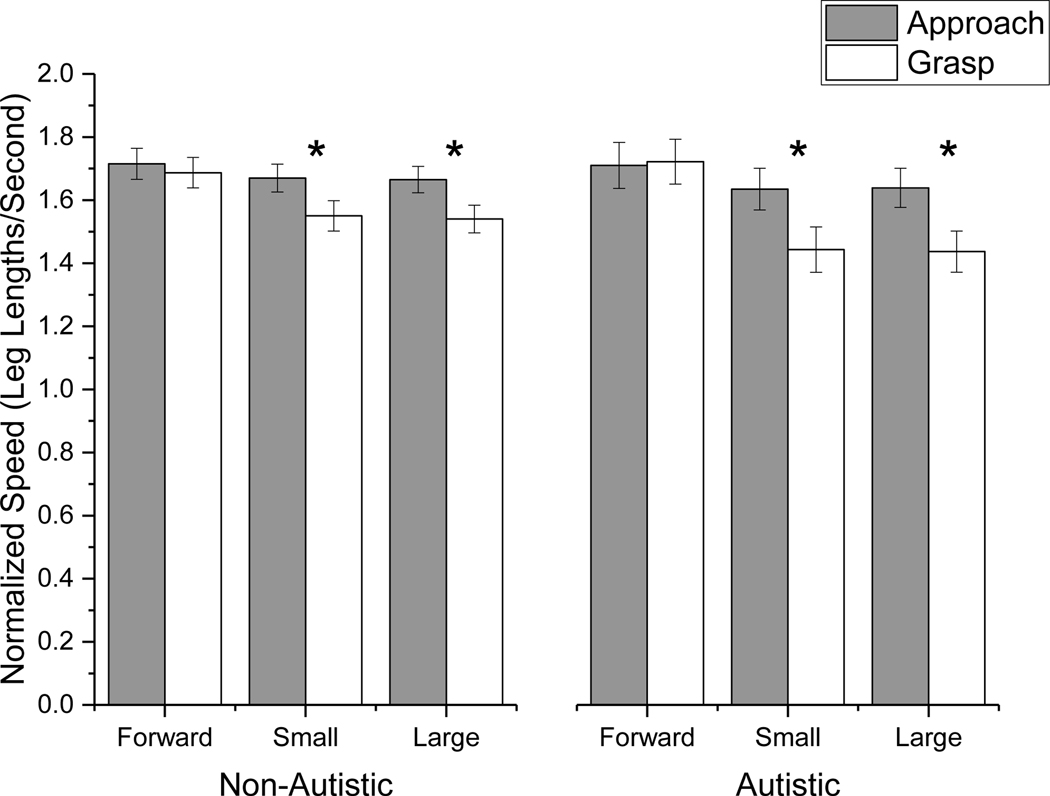
A significant three-way interaction was found for SER. Simple main effects analysis with Bonferroni correction indicated that both groups decreased their SER when reaching for the small and large targets in the grasp phase when compared to the approach phase (p < 0.05), however, the decrease was larger for the autistic group than the non-autistic group (Figure 2). There was also a significant Condition X Group X Phase interaction for NSpeed. Simple main effects analysis with Bonferroni correction indicated that both groups decreased their speed in the grasp phase compared to the approach phase when reaching for small and large targets (p < 0.05). This decrease was larger for the autistic than the non-autistic group. In contrast to SER and NSpeed, the three-way interaction was not significant for time spend in double support.
Figure 2.
Significant 3-way interaction between Group, Phase and Condition for step extremity ratio (SER). * represent significant main effects of phase at the Bonferroni corrected p value. Both groups decreased their SER when reaching for the small and large targets in the grasp phase when compared to the approach phase, however, the decrease was larger for the autistic group than the non-autistic group.
For the grasp, results indicated no differences in peak aperture or peak aperture variability regardless of whether participants were walking or standing (p > 0.05). Furthermore, there were no peak aperture or variability differences between the groups. The only main effects for this measure were related to the size of the target (F1,25=153.9, p<0.001, ηp2=0.86). As expected, participants used a larger peak aperture when grasping the large target (104.6 ± 2.3 mm) than when grasping the small target (67.0 ± 2.23 mm)
Follow-up Analysis
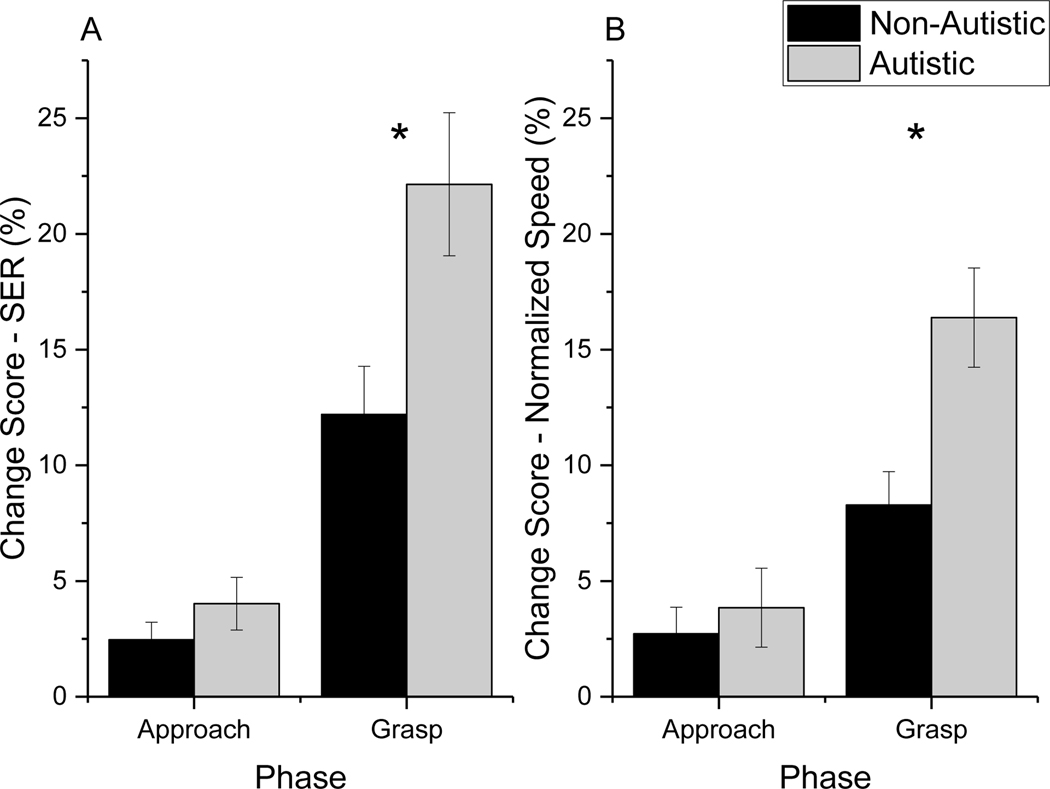
To further understand whether SER and NSpeed were altered to a greater extent in the grasp conditions compared to the walk forward condition depending on Group, we conducted follow-up analyses on the change scores for these two measures. We calculated the change in performance relative to the forward condition using the following equations: Change Score Small = (forward – small)/small*100; Change Score Large = (forward – large)/large*100. Change scores were compared using 2 Condition (small, large) X 2 Phase (approach, grasp) X 2 Group (non-autistic, autistic) repeated measures ANCOVAs, with full scale - IQ as a covariate. For this analysis, we were particularly interested in the Phase X Group interaction. As shown in Table 3 and Figures 4A and B, there were significant Phase X Group interactions for SER and NSpeed. Change scores for both SER and NSpeed were similar for the non-autistic and autistic groups for the approach phase, however, during the grasp phase, the autistic youths changed their step extremity ratio and speed to a much larger extent than their non-autistic peers.
Table 3.
Follow-up analysis comparing change scores in the spatiotemporal gait parameters across Condition, Phase and Group.
| Measure | Condition X Phase X Group | Condition X Group | Phase X Group | Condition X Phase | Condition (small, large) | Phase (approach, grasp) | Group (non-autistic, autistic) |
|---|---|---|---|---|---|---|---|
| Change Score - SER | F2,50=0.169 p = 0.685 ηp2<0.007 |
F1,25=0.576 p = 0.455 ηp2<0.085 |
F1,25=6.657 p = 0.016* ηp2=0.217 |
F1,25=0.017 p = 0.897 ηp2=0.001 |
F1,25=0.006 p = 0.941 ηp2<0.001 |
F1,25=88.51 p < 0.001* ηp2=0.78 |
F1,25=5.256 p = 0.031* ηp2=0.18 |
|
| |||||||
| Change Score - NSpeed | F2,50=0.013 p = 0.910 ηp2=0.001 |
F1,25=0.077 p = 0.784 ηp2=0.003 |
F1,25=4.661 p = 0.041* ηp2=0.163 |
F1,25=0.297 p = 0.679 ηp2=0.007 |
F1,25=0.015 p = 0.702 ηp2=0.006 |
F1,25=42.75 p < 0.001* ηp2=0.63 |
F1,25=5.932 p = 0.023* ηp2=0.198 |
Figure 4.
Interaction between Group and Phase for the ANCOVAs comparing the percent change in A) Step Exremity Ratio and B) Normalized Speed between the grasping and walk forward conditions. Larger change score values indicated shorter (SER) or slower (NSpeed) performance on the grasping tasks as compared to the forward walking tasks. Results indicated that autistic youths decreased their SER and NSpeed in the grasp phase to a greater extent than non-autistic youth. The * represent significant simple main effects with Bonferroni correction.
Discussion
Prior work examining motor function in autistic individuals has commonly focused on either upper or lower limb tasks in isolation and has varied in the extent to which clinically meaningful movement has been quantified (see Moseley and Pulvermuller21 for review). In line with the two primitives theory45–47 and the complex information processing model of ASD44, we hypothesized that the complexity of a motor task that combined discrete and cyclical movement would reveal motor differences in autism to a greater extent than a simple gait or reaching task alone, thereby revealing the conditions under which motor challenges may be most apparent in autistic youths. We investigated this dual-task motor performance at a time in development during which gait and grasping performance should be near maturity14,15. The study found that both groups altered their gait patterns during the more complex part of the task, but this change was even more pronounced in the autistic youths, such that autistic youths walked more slowly, and with shorter steps during the more challenging walk with grasp conditions as compared to the non-autistic youths. In contrast, grasp aperture did not differ by group or task, suggesting that autistic and non-autistic youths altered gait behavior but conserved grasp kinematics. Together, these findings suggest that autistic individuals may successfully complete the reaching task by significantly altering the walking task to maintain gait stability. This preliminary work provides proof of concept that systematically altering the complexity of combined tasks using the upper and lower limbs can provide an important window into the trade-offs that occur during complex dual-task information processing in autistic individuals.
While clinical and observational reports have described “clumsiness” in autistic individuals60, prior studies examining gait characteristics of autistic individuals have failed to provide overwhelming evidence for the presence or absence of atypical gait patterns (as reviewed in Kindregan et al.31 and Lum et al.32). Our preliminary results may shed some light on these disparate findings. Specifically, we found that during typical-speed, straight-line-gait testing procedures, several spatiotemporal gait measures did not differ between autistic and non-autistic individuals. However, at the point in the task when individuals were required to combine a discrete upper limb task with a cyclical lower limb task, autistic youths spent more time in double support, had a slower gait speed, and took shorter steps. Thus, considering the current findings, it is possible that the walking paradigms selected for the previous studies may have played a significant role in the extent to which autistic and non-autistic individuals differed.
These results align with the complex information processing model of ASD44 that suggests that task performance in the face of increasing task complexity may be impacted more in autistic individuals than in non-autistic individuals61,62, even in motor tasks. These results are also similar to Memari et al.55, who reported that visual secondary tasks significantly influenced postural sway in autistic youths. While task complexity can be modulated in various ways (i.e., dual-tasking, task familiarity, distractions, etc.), an advantage of the present study is that we systematically increased complexity by combining two familiar motor functions (i.e., continuous walking and discrete grasping) that individually should be at or near maturity in this age range but that, according to the two primitives theory, should be more challenging in combination. Indeed, the present results revealed similar motor patterns between the groups in the gait-only task, but during the combined gait and reach-to-grasp task, autistic individuals experienced a significantly greater impact on motor function. Therefore, when the task is more complex (potentially more similar to what is observed in natural environments) poorer performance in autistic individuals may become apparent. In terms of clinical applicability, these findings suggest that autistic youth who show typical gait patterns during more simple assessments of motor ability may yet have difficulties in more real-life tasks that require a combination of movements (i.e., dressing, cooking, cleaning/organization, etc.).
In the current study, both groups of participants altered gait while keeping the grasp aperture unchanged when compared to grasp alone. Carnahan et al.63 suggested that a hierarchical trade-off between the grasp and gait movements can occur, depending on movement priorities and safety. Therefore, based on the task requirements and environmental conditions, participants may have chosen to prioritize safe gait over safe grasp by slowing down and taking shorter steps. In the current study, objects were rarely knocked or dropped (only 6 times across 540 trials), and no participants fell. Therefore, this strategy of maintaining gait stability while leaving grasp unaltered was successful. Future studies are needed to determine whether this “gait stability” strategy is consistent in both groups of youths across different scenarios (e.g. grasping a fragile object, stepping over an obstacle), or whether both groups adapt grasp kinematics to environmental requirements as needed. Prior work from our lab using a bimanual grasping task across a larger age range revealed that autistic youth did not alter motor plans when faced with more challenging grasp scenarios34. In fact, we showed that they used smaller grasp apertures in the more complex conditions. Therefore, it is of interest to determine how the parallel planning of gait and grasp is modified as task complexity is further increased. A third phase of the movement, namely carrying the object after the grasp, could also provide an interesting window into combined upper and lower limb performance. However, this was beyond the scope of the current study.
While the present study focused on this dual-task paradigm within a relatively narrow age range (10–14 years-old), these findings raise interesting questions regarding motor development in autistic individuals. In non-autistic children and youth, the ability to perform secondary tasks while walking has been found to improve with age throughout development, with key gait measures such as step length, cadence, and stride velocity remaining undisturbed in non-autistic youths (7–10 years) and young adults for simple walking and carrying tasks64,65. However, with a more complex upper limb task, gait can still be influenced even in non-autistic adults. Specifically, Abbruzzese et al.65 found that when the complexity of the dual-task was increased (carrying a tray with a cup of water placed on it), gait measures were influenced in both non-autistic youths and young adults. Further, the dual-task costs were greater in non-autistic youths than young adults, suggesting that dual-task gait in school-aged children requires increased cognitive processes such as executive and attentional functions and has not yet reached adult capacity54. As expected, all individuals in the current study walked more slowly and took shorter steps during the more complex combined reaching and walking tasks. However, for autistic individuals the cost of grasping an object while walking was particularly evident during the grasp phase of the combined task. Intriguingly, the gait characteristics observed in the autistic 10–14 year-olds are similar to what have been reported previously in older, non-autistic adults at increased risk of falls (for review see Muir-Hunter and Wittwer66). While it is unclear whether the same mechanisms underlie these dual-task gait changes in autistic youths compared to older adults, the similarities in gait changes during dual-task paradigms are striking, and future research should investigate how dual-task gait may develop with age in autistic individuals, may correspond with individual differences in executive function, and may align (or differ) between autistic youths and non-autistic older adults.
Limitations and Future Directions
One limitation of the current study relates to the smaller number of participants, particularly in the group of autistic participants. Unfortunately, due to the COVID-19 pandemic and a change in research facilities at our institution, it is no longer possible to continue recruiting participants for this study. However, our preliminary results demonstrate that our novel paradigm provides an interesting and intriguing window into complex movement coordination in autistic youth. This paradigm may provide a tool capable of distinguishing subtle differences in autistic and non-autistic youth that are difficult to observe in conventional lab tests. With the small age range chosen for this study, during which motor skill has begun to stabilize, we hoped to increase the homogeneity of the group while providing proof-of-concept data for the efficacy of this experimental paradigm. The patterns of significant differences between groups across several spatiotemporal variables suggests that our experimental paradigm could be a valuable tool for systematically investigating motor challenges in autistic individuals. A second limitation relates to the IQ differences between the autistic and non-autistic groups. We statistically controlled for differences in full-scale IQ within our analyses, however, since our task requires significant executive function resources, these differences may play a role in our results. Follow-up studies should be conducted with a larger sample size, matched on IQ, to replicate and extend these findings. A third limitation is not knowing if participants walked as they typically would on the gait mat with the LEDs attached, which may limit the external validity of these findings. Finally, future work should consider gait during the third phase of the task: carrying the object after the grasp.
Figure 3.
Significant 3-way interaction between Group, Phase and Condition for normalized speed. * represent significant main effects of phase at the Bonferroni corrected p value. Both groups decreased their speed when reaching for the small and large targets in the grasp phase when compared to the approach phase, however, the decrease was larger for the autistic group than the non-autistic group.
Footnotes
Disclosure of interest
The authors report no conflict of interest
References
- 1.Sheridan M. The developmental progress of infants and young children: Ministry of Health reports on public health and medical subjects, (Vol. 102). London (England). Her Majesties Stationary Office; 1960. [Google Scholar]
- 2.Gerber RJ, Wilks T, Erdie-Lalena C. Developmental milestones: Motor development. Pediatr Rev; 2010; 31(7): 267–277: Scopus. 10.1542/pir.31-7-267. [DOI] [PubMed] [Google Scholar]
- 3.Bottos M, Barba BD, Stefani D, Pettenà G, Tonin C, D’Este A. Locomotor Strategies Preceding Independent Walking: Prospective Study Of Neurological And Language Development In 424 Cases. Dev Med Child Neurol; 1989; 31(1): 25–34: 10.1111/j.1469-8749.1989.tb08408.x. [DOI] [PubMed] [Google Scholar]
- 4.Robson P. Prewalking locomotor movements and their use in predicting standing and walking. Child Care Health Dev; 1984; 10(5): 317–330: 10.1111/j.1365-2214.1984.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 5.Breniere Y, Bril B. Why do children walk when falling down while adults fall down in walking? C R Acad Sci III; 1988. : 307(11), 617. [PubMed] [Google Scholar]
- 6.Shumway-Cook A, Woollacott MH. Motor Control: Theory and practical applications. Philadelphia (USA). Lippincott Williams and Wilkins, 1995. [Google Scholar]
- 7.Sutherland D, Olshen R, Biden E. The Development of Mature Walking. London (England). Mac Keith Press. 1988 [Google Scholar]
- 8.Beck RJ, Andriacchi TP, Kuo KN, Fermier RW, Galante JO. Changes in the gait patterns of growing children.: J Bone and Joint Surg. 1981; 63(9): 1452–1457: 10.2106/00004623-198163090-00012. [DOI] [PubMed] [Google Scholar]
- 9.Preis S, Klemms A, Müller K. Gait analysis by measuring ground reaction forces in children: Changes to an adaptive gait pattern between the ages of one and five years. Dev Med Child Neurol. 1997; 39(4): 228–233: 10.1111/j.1469-8749.1997.tb07416.x. [DOI] [PubMed] [Google Scholar]
- 10.Sutherland DH, Olshen R, Cooper L, Woo S. The development of mature gait. J Bone and Joint Surg. 1980; 62: 336–353. [PubMed] [Google Scholar]
- 11.Pickett K, Konczak J. Measuring kinaesthetic sensitivity in typically developing children. Dev Med Child Neurol. 2009; 51(9): 711–716. 10.1111/j.1469-8749.2008.03229.x. [DOI] [PubMed] [Google Scholar]
- 12.Rose-Jacobs R. Development of Gait at Slow, Free, and Fast Speeds in 3- and 5-Year-Old Children. Phys Ther. 1983; 63(8): 1251–1259. 10.1093/ptj/63.8.1251 [DOI] [PubMed] [Google Scholar]
- 13.Slaton DS. Gait Cycle Duration in 3-Year-Old Children. Phys Ther. 1985; 65(1): 17–21: 10.1093/ptj/65.1.17. [DOI] [PubMed] [Google Scholar]
- 14.Hausdorff JM., Zemany L, Peng CK, Goldberger AL. Maturation of gait dynamics: Stride-to-stride variability and its temporal organization in children. J Appl Physiol. 1999; 86(3): 1040–1047: 10.1152/jappl.1999.86.3.1040. [DOI] [PubMed] [Google Scholar]
- 15.Kuhtz-Buschbeck JP, Stolze H, Jöhnk K, Boczek-Funcke A, & Illert M. Development of prehension movements in children: A kinematic study. Exp Brain Res. 1998;122(4): 424–432: 10.1007/s002210050530. [DOI] [PubMed] [Google Scholar]
- 16.Bottema-Beutel K, Kapp SK, Lester JN, Sasson NJ, & Hand BN. Avoiding Ableist Language: Suggestions for Autism Researchers. Autism in Adulthood. 2021; 3(1): 18–29: 10.1089/aut.2020.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kenny L, Hattersley C, Molins B, Buckley C, Povey C, & Pellicano E. Which terms should be used to describe autism? Perspectives from the UK autism community. Autism. 2016; 20(4): 442–462: 10.1177/1362361315588200. [DOI] [PubMed] [Google Scholar]
- 18.Bhat AN. Motor Impairment Increases in Children With Autism Spectrum Disorder as a Function of Social Communication, Cognitive and Functional Impairment, Repetitive Behavior Severity, and Comorbid Diagnoses: A SPARK Study Report. Autism Res. 2020; 14(1): 202–219: 10.1002/aur.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhat AN, Landa RJ, & Galloway JC. Current perspectives on motor functioning in infants, children, and adults with autism spectrum disorders. Phys Ther. 2011; 91(7): 1116–1129. [DOI] [PubMed] [Google Scholar]
- 20.Fournier KA, Hass CJ, Naik SK, Lodha N, Cauraugh JH. Motor Coordination in Autism Spectrum Disorders: A Synthesis and Meta-Analysis. J Autism Dev Disord. 2010; 40(10): 1227–1240. 10.1007/s10803-010-0981-3. [DOI] [PubMed] [Google Scholar]
- 21.Moseley RL, Pulvermüller F. What can autism teach us about the role of sensorimotor systems in higher cognition? New clues from studies on language, action semantics, and abstract emotional concept processing. Cortex. 2018; 100: 149–190: 10.1016/j.cortex.2017.11.019 [DOI] [PubMed] [Google Scholar]
- 22.Wozniak RH, Leezenbaum NB, Northrup JB, West KL, Iverson JM. The development of autism spectrum disorders: Variability and causal complexity. Wiley Interdisciplinary Reviews: Cogn Sci. 2017; 8(1–2): e1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ketcheson L, Hauck JL, Ulrich D. The levels of physical activity and motor skills in young children with and without autism spectrum disorder, aged 2–5 years. Autism. 2018; 22(4): 414–423: 10.1177/1362361316683889. [DOI] [PubMed] [Google Scholar]
- 24.Lloyd M, MacDonald M, Lord C. Motor skills of toddlers with autism spectrum disorders. Autism. 2013; 17(2): 133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosconi MW, Sweeney JA. Sensorimotor dysfunctions as primary features of autism spectrum disorders. Sci China Life Sci. 2015; 58(10): 1016–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linke AC, Kinnear MK, Kohli JS, Fong CH, Lincoln AJ, Carper RA, Müller RA. Impaired motor skills and atypical functional connectivity of the sensorimotor system in 40- to 65-year-old adults with autism spectrum disorders. Neurobiol Aging. 2020; 85: 104–112: 10.1016/j.neurobiolaging.2019.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim YH, Lee HC, Falkmer T, Allison GT, Tan T, Lee WL, Morris SL. Effect of Visual Information on Postural Control in Adults with Autism Spectrum Disorder. J Autism Dev Disord. 2019; 49(12): 4731–4739: 10.1007/s10803-018-3634-6. [DOI] [PubMed] [Google Scholar]
- 28.Travers BG, Bigler ED, Duffield TC, Prigge MDB, Froehlich AL, Lange N, Alexander AL, & Lainhart JE. Longitudinal development of manual motor ability in autism spectrum disorder from childhood to mid-adulthood relates to adaptive daily living skills. Dev Sci. 2017; 20(4): e12401: 10.1111/desc.12401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943; 2(3): 217–250. [PubMed] [Google Scholar]
- 30.Vilensky J, Damasio AR, Maurer RG. Gait distrubances in patients with autistic behavior: A preliminary study. Arch Neurol. 10981; 38:646–649. [DOI] [PubMed] [Google Scholar]
- 31.Kindregan D, Gallagher L, & Gormley J. Gait deviations in children with autism spectrum disorders: A review. Autism Res Treat. 2015: doi: 10.1155/2015/741480. Epub 2015 Apr 2. PMID: 25922766; PMCID: PMC4398922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lum JAG, Shandley K, Albein-Urios N, Kirkovski M, Papdopoulos N, Wilson RB, Enticott PG, Rinehart NJ. Meta-Analysis reveals gait anomalies in autism. Autism Res, 2021: 14: 733–747. [DOI] [PubMed] [Google Scholar]
- 33.Nobile M, Perego P, Piccinini L, Mani E, Rossi A, Bellina M, & Molteni M. Further evidence of complex motor dysfunction in drug naïve children with autism using automatic motion analysis of gait. Autism, 2011: 15(3): 263–283. 10.1177/1362361309356929 [DOI] [PubMed] [Google Scholar]
- 34.Weiss MJ, Moran MF, Parker ME, & Foley JT. Gait analysis of teenagers and young adults diagnosed with autism and severe verbal communication disorders. Frontiers in Integrative Neuroscience, (2013): 7, 33: 10.3389/fnint.2013.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pauk J, Zawadzka N, Wasilewska A, & Godlewski P. Gait deviations in children with classic high-functioning autism and low-functioning Autism. Journal of Mechanics in Medicine and Biology, (2017): 17(3): 10.1142/S0219519417500427 [DOI] [Google Scholar]
- 36.Morrison S, Armitano CN, Raffaele CT, Deutsch SI,Neumann SA, Caracci H, & Urbano MR (2018).Neuromotor and cognitive responses of adults with autism spectrum disorder compared to neurotypical adults. Exp Brain Res, 2018: 236, 2321–2332: 10.1007/s00221-018-5300-9. [DOI] [PubMed] [Google Scholar]
- 37.Rinehart NJ, Tonge BJ, Bradshaw JL, Iansek R, Enticott PG, McGinley J, Gait function in high-functioning autism and Asperger’s disorder: evidence for basal-gaglia and cerebellar involvement? Eur Cild Adolesc Psychiatry, 2006: 15(5), 256–264: doi: 10.1007/s00787-006-0530-y. [DOI] [PubMed] [Google Scholar]
- 38.Calhoun M, Longworth M, Chester VL, Gait patterns in children with autism, Clin Biomech, 2011, 26(2): 200–206, 10.1016/j.clinbiomech.2010.09.013 [DOI] [PubMed] [Google Scholar]
- 39.Nayate A, Tonge BJ, Bradshaw JL, McGinley JL, Iansek R, Rinehart NJ, Differentiation of high-functioning autism and Asperger’s disorder based on neuromotor behavior, J Autism Dev Disord, 2012, 42(5): 707–717: doi: 10.1007/s10803-011-1299-5. [DOI] [PubMed] [Google Scholar]
- 40.Manicolo O, Brotzmann M, Hagmann-von Arx P, Grob A, & Weber P. (2019). Gait in children with infantile/atypical autism: Age-dependent decrease in gait variability and associations with motor skills. Europ J Paed Neurol, 2019, 23(1): 117–125: 10.1016/j.ejpn.2018.09.011 [DOI] [PubMed] [Google Scholar]
- 41.Bennett HJ, Ringleb SI, Bobzien J, Haegele JA, Walking lower extremity biomechanics of adolescents with autism spectrum disorder. J Biomech. 2021, 119: doi: 10.1016/j.jbiomech.2021.110332. [DOI] [PubMed] [Google Scholar]
- 42.Rinehart NJ, Bellgrove MA, Tonge BJ, Brereton AV, Howells-Rankin D, Bradshaw JL. An Examination of Movement Kinematics in Young People with High-functioning Autism and Asperger’s Disorder: Further Evidence for a Motor Planning Deficit. J Autism Dev Disord. 2006; 36(6): 757–767: 10.1007/s10803-006-0118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodgers RA, Travers BG, Mason AH. Bimanual Reach to Grasp Movements in Youth With and Without Autism Spectrum Disorder. Front Psychol. 2019; 9: 2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Minshew NJ, & Goldstein G. Autism as a disorder of complex information processing. Ment Retard Dev Disabil Res Rev. 1998; 4(2): 129–136: 10.1002/(SICI)1098-2779(1998)4:2. [DOI] [Google Scholar]
- 45.Sternad D, Marino H, Charles S, Duarte M, DiPietro L, & Hogan N. Transitions between discrete and rhythmic primitives in a unimanual task. Front Comput Neurosci. 2013; 7: 10.3389/fncom.2013.00090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hogan N, Sternad D. Dynamic primitives in the control of locomotion. Front Comput Neurosci. 2013; 7: 10.3389/fncom.2013.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Mourik AM, Beek PJ. Discrete and cyclical movements: Unified dynamics or separate control? Acta Psychol. 2004; 117(2): 121–138: 10.1016/j.actpsy.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 48.Hughes C, Russell J, Robbins TW. Evidence for executive dysfunction in autism. Neuropsychologia. 1994; 32(4): 477–492: 10.1016/0028-3932(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 49.Ozonoff S, Rogers SJ, Pennington BF. Asperger’s Syndrome: Evidence of an Empirical Distinction from High-Functioning Autism. J Child Psychol Psychiatry. 1991; 32(7): 1107–1122: 10.1111/j.1469-7610.1991.tb00352.x [DOI] [PubMed] [Google Scholar]
- 50.Prior M, Hoffmann W. Brief report: Neuropsychological testing of autistic children through an exploration with frontal lobe tests. J Autism Dev Disord. 1990; 20(4): 581–590: 10.1007/BF02216063 [DOI] [PubMed] [Google Scholar]
- 51.Russell J. Autism as an executive disorder. Oxford(England): Oxford University Press; 1998. [Google Scholar]
- 52.Russell J, Jarrold C, Henry L. Working Memory in Children with Autism and with Moderate Learning Difficulties. J Child Psychol Psychiatry. 1996; 37(6): 673–686; 10.1111/j.1469-7610.1996.tb01459.x. [DOI] [PubMed] [Google Scholar]
- 53.Kenworthy L, Black DO, Harrison B, Rosa A. della, Wallace GL. Are Executive Control Functions Related to Autism Symptoms in High-Functioning Children? Child Neuropsychol. 2009; 15(5): 425–440: 10.1080/09297040802646983. [DOI] [PubMed] [Google Scholar]
- 54.Cherng RJ, Liang LY, Hwang IS, Chen JY. The effect of a concurrent task on the walking performance of preschool children. Gait Posture. 2007; 26(2): 231–237: 10.1016/j.gaitpost.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 55.Memari AH, Ghanouni P, Shayestehfar M, Ziaee V, Moshayedi P. Effects of visual search vs. Auditory tasks on postural control in children with autism spectrum disorder. Gait Posture. 2014; 39(1): 229–234: 10.1016/j.gaitpost.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 56.Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop S. Autism diagnostic observation schedule–2nd edition (ADOS-2). Los Angeles(CA): Western Psychological Corporation; 2012 [Google Scholar]
- 57.Wechsler D. (2011). WASI-II: Wechsler abbreviated scale of intelligence. PsychCorp. [Google Scholar]
- 58.Rutter M, Bailey A, Lord C. SCQ: Social Communication Questionnaire. Los Angeles (CA) : Western Psychological Services; ; 2003. [Google Scholar]
- 59.Mosconi MW, Kay M, D’Cruz AM, Guter S, Kapur K, Macmillan C, Stanford LD, Sweeney JA. Neurobehavioral Abnormalities in First-Degree Relatives of Individuals With Autism. Arch Gen Psychiatry. 2010; 67(8): 830–840: 10.1001/archgenpsychiatry.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hallett M, Lebiedowska MK, Thomas SL, Stanhope SJ, Denckla MB, & Rumsey J. Locomotion of autistic adults. Arch Neurol. 1993; 50(12): 1304–1308. [DOI] [PubMed] [Google Scholar]
- 61.Williams DL, Goldstein G, Minshew NJ. Neuropsychologic functioning in children with autism: Further evidence for disordered complex information-processing. Child Neuropsychology: Child Neuropsychol. 2006; 12(4–5): 279–298: 10.1080/09297040600681190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Williams DL, Minshew NJ, Goldstein G. Further understanding of complex information processing in verbal adolescents and adults with autism spectrum disorders. Autism. 2015; 19(7): 859–867: 10.1177/1362361315586171 [DOI] [PubMed] [Google Scholar]
- 63.Carnahan H, Mcfadyen BJ, Cockell DL, Halverson AH. The combined control of locomotion and prehension. Neurosci Res Commun. 1996; 19(2); 91–100: 10.1002/(SICI)1520-6769(199609)19:2. [DOI] [Google Scholar]
- 64.Boonyong S, Siu KC, van Donkelaar P, Chou LS, Woollacott MH. Development of postural control during gait in typically developing children: The effects of dual-task conditions. Gait Posture. 2012; 35(3): 428–434: 10.1016/j.gaitpost.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abbruzzese LD, Rao AK, Bellows R, Figueroa K, Levy J, Lim E, Puccio L. Effects of manual task complexity on gait parameters in school-aged children and adults. Gait Posture. 2014; 40(4) : 658–663 : 10.1016/j.gaitpost.2014.07.017 [DOI] [PubMed] [Google Scholar]
- 66.Muir-Hunter SW, Wittwer JE. Dual-task testing to predict falls in community-dwelling older adults: A systematic review. Physiotherapy. 2016; 102(1): 29–40: 10.1016/j.physio.2015.04.011. [DOI] [PubMed] [Google Scholar]