ABSTRACT
Vascular calcification, characterized by calcium deposition in the intimal and medial layers of the arterial wall, is frequently encountered in patients with chronic kidney disease (CKD) and leads to an enhanced risk of adverse cardiovascular (CV) outcomes. However, the underlying complex pathophysiology remains incompletely understood. Recently, Vitamin K supplementation aimed at correcting Vitamin K deficiency highly prevalent in CKD holds great promise to mitigate the progression of vascular calcification. This article discusses the functional Vitamin K status in CKD, the pathophysiology linking Vitamin K deficiency and vascular calcification, and reviews current literature from animal models, observational studies, and clinical trials across the different spectrum of CKD. While favorable effects of Vitamin K on vascular calcification and CV outcomes are suggested in animal and observational studies, most recently published clinical trials investigating the effects of Vitamin K on vascular health failed to support the beneficial role of Vitamin K supplementation, despite improving the functional status of Vitamin K. We address the potential reasons for these discrepancies and provide further perspective on Vitamin K research in CKD.
KEYWORDS: Arterial stiffness, Chronic kidney disease, Matrix Gla-protein, Vascular calcification, Vitamin K
INTRODUCTION
Cardiovascular (CV) disease is the leading cause of morbidity and mortality in patients with chronic kidney disease (CKD) and end-stage renal disease (ESRD) [1]. Several cardiac and vascular abnormalities are demonstrated to account for this increased risk of CV events and mortality. Identifying modifiable risk factors that contribute to the development of CV disease in CKD is particularly important. Beyond the well-established traditional risk factors, such as aging, diabetic mellitus, hypertension, hyperlipidemia, smoking, and obesity, several nontraditional risk factors, including dysregulation of fibroblast growth factors-23 and klotho, deposition of calcium and phosphorus, endothelial dysfunction, chronic inflammation, oxidative stress, gut-derived uremic toxins, and malnutrition, were also implicated in the pathogenesis of CV disease [2,3].
Vascular calcification, characterized by calcium deposition in the intimal and medial layers of the arterial wall, is one of the most special and relevant contributors responsible for the high CV burden in patients with CKD and ESRD [4]. However, the pathophysiology of this accelerated and extensive vascular calcification in CKD remains not completely understood, and a large body of research is ongoing to elucidate the complex pathogenesis.
Notably, growing evidence suggests a close association between Vitamin K deficiency and accelerated vascular calcification, which has drawn significant attention from researchers in recent years [5-9]. And Vitamin K supplementation is regarded as a promising approach to mitigate the progression of vascular calcification in CKD patients.
This review addressed and updated current evidence regarding the role of Vitamin K on vascular calcification in CKD from experimental animal models, observational, and clinical trials.
SOURCES, BIOABSORPTION, AND FUNCTION OF VITAMIN K
The Vitamin K family members, which share a naphthoquinone double ring in structure, are comprised of Vitamin K1 (phylloquinone) and Vitamin K2 (menaquinones, MKs), the latter of which includes MK-4 through to MK-13, named depending on the length of lipophilic isoprenoid side chain [10]. Among menaquinones, two isoforms, MK-4 and MK-7, are available in supplement form. As an essential micronutrient that needs to intake through food, Vitamin K1 is abundant in leafy green vegetables (such as spinach, broccoli, Brussels sprouts, cabbage, and kale), MK-4 is rich in Butter, eggs yolks, and animal-based foods, and MK-7 is derived from fermented food, such as Natto and cheeses [11,12]. In general, Vitamin K1 is more abundant in foods and accounts for the majority of Vitamin K intake in the human diet. Following oral intake, Vitamin K1 and K2 are absorbed via the small intestine, packed into the chylomicrons, and then transported to tissue by lipoproteins. Vitamin K1 is mainly accumulated in the liver and is responsible for coagulation function, whereas Vitamin K2 exerts a more extrahepatic distribution pattern and is believed to have diverse impacts on human health [13]. Notably, an adequate intake of Vitamin K2, but not Vitamin K1, is associated with a reduced risk of coronary heart disease [14].
Vitamin K is a crucial cofactor for various Vitamin K-dependent proteins in the body. In humans, at least 17 different Vitamin K-dependent proteins have been identified [10]. Apart from hemostasis function as the most well-known biological function of Vitamin K, emerging evidence suggests that this micronutrient is also implicated in diverse biological functions, such as CV and renal protection, bone health, glycemic control, cognitive function, and cancer prevention [12,15].
VITAMIN K STATUS IN CHRONIC KIDNEY DISEASE
Insufficient Vitamin K intake, as well as hypofunction of Vitamin K status, is commonly observed in CKD. Based on the Third National Health and Nutrition Examination Survey (NHANES III), up to 72% of CKD patients had inadequate daily Vitamin K intake [16]. Among patients undergoing hemodialysis (HD), a 70%–90% prevalence of inadequate Vitamin K intake was reported, which was two-fold higher than those with normal renal function [17]. The low intake of Vitamin K could be attributed to dietary potassium restriction in CKD. Accordingly, increased daily intake of leafy green vegetables with low to moderate potassium (such as broccoli, cabbage, and kale) and potassium-reduced cooking strategies may be helpful in improving Vitamin K status.
Apart from dietary factors, the expression of Vitamin K epoxide reductase in the thoracic aorta was reduced in CKD status, evidenced by a rat model of adenine-induced CKD [18]. Moreover, UbiA prenyltransferase domain-containing protein-1 (UBIAD1), a Menaquinone-4 bioconversion enzyme, is decreasingly expressed in the kidney [18,19]. Recently, lipoproteins, which account for uptake and transportation of the fat-soluble Vitamin K, have been shown to be altered profoundly in experimental and human CKD [20].
THE MECHANISMS LINK VITAMIN K STATUS AND VASCULAR CALCIFICATION IN CHRONIC KIDNEY DISEASE
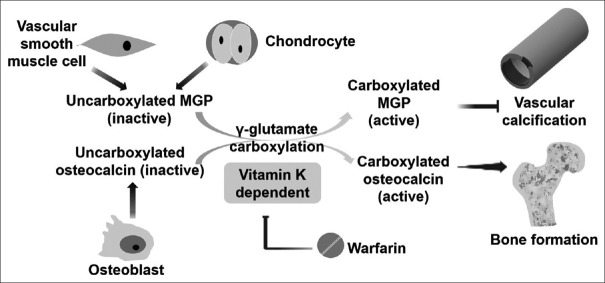
Among Vitamin K-dependent proteins, matrix Gla-protein (MGP) and osteocalcin mediate the close link between Vitamin K status and vascular calcification [5,6]. MGP is secreted from vascular smooth muscle cells and chondrocytes, whereas osteocalcin is secreted from osteoblast [5]. Uncarboxylated forms (inactive forms) of MGP and osteocalcin are carboxylated and activated through γ-glutamyl carboxylase, which converts specific glutamic acid residues into γ-carboxyglutamate. This carboxylation process is Vitamin K-dependent, in which reduced Vitamin K is oxidized to Vitamin K epoxide. The active forms of carboxylated MGP and osteocalcin then exert their effects by inhibiting vascular calcification and promoting bone formation, respectively [5,6]. Although the exact mechanisms remain unknown, the carboxylated MGP may inhibit vascular calcification by several potential mechanisms: (1) binding calcium ions or calcium crystals; (2) binding of bone morphogenetic protein-2, a growth factor transforming vascular smooth muscle cells into osteoblast-like cells; (3) anti-apoptosis of vascular smooth muscle cells; (4) binding to elastin, a potential substrate for calcification initiation [7,8]. Warfarin, a traditional anticoagulant, augments vascular calcification and retards bone formation by inhibiting Vitamin K epoxide reductase [Figure 1].
Figure 1.
The role of Vitamin K status in the pathogenesis of vascular calcification. CKD, chronic kidney disease; ucMGP: Uncarboxylated MGP, RCT: Randomized control trial, ESRD: End-stage renal disease, HD: Hemodialysis; HR: Hazard ratio; CV: Cardiovascular
Accordingly, both uncarboxylated forms of MGP and osteocalcin are regarded as functional biomarkers for Vitamin K status, as well as surrogate markers for vascular calcification [21] and bone health [22].
VITAMIN K AND VASCULAR HEALTH IN CHRONIC KIDNEY DISEASE: EVIDENCE FROM OBSERVATION STUDIES
The close association between Vitamin K intake amount, as well as its functional biomarkers, and clinical outcomes was consistently reported in observational studies. Among 3401 nondialysis CKD participants retrieved from the NHANES III with extensive follow-up for a median of 13.3 years, high Vitamin K intake had lower hazard ratios (HRs) for overall mortality (HR = 0.85; 95% confidence interval [CI]: 0.72–1.00) and CV mortality (HR = 0.78; 95% CI 0.64-0.95), compared with low Vitamin K intake [16]. In the Chronic Renal Insufficiency Cohort, one of the largest CKD cohorts in the United States, low serum uncarboxylated MGP levels and high phylloquinone levels were associated with 21%–29% and 16%–19% decreased risk of all-cause mortality among 3066 nondialysis CKD patients, after a median 12.8 follow-up years [23]. Consistently, high serum uncarboxylated MGP levels were also reported to increase the mortality risk in patients with CKD stage 5 [24], diabetic CKD [25], ESRD undergoing peritoneal dialysis [26], and kidney transplant recipients [27].
A cohort study in 387 chronic HD patients disclosed the relationship between warfarin use, the severity of vascular calcification, and mortality. Compared to those without warfarin use, patients on warfarin had a 2.6 and 2.9-folds increased odds ratios (ORs) for aortic calcification and iliac artery calcification, respectively and had a two-fold increased hazard risk of all-cause mortality (HR = 1.97, 95% CI: 1.02–3.84, P = 0.046) [28].
THE POTENTIAL BENEFIT OF VITAMIN K SUPPLEMENTATION: EVIDENCE FROM EXPERIMENTAL ANIMAL STUDIES
In a murine model, Scheiber et al. disclosed that 12-week high-dose Vitamin K2 supplementation inhibited CV calcification in CKD rats fed with a high phosphate diet [29]. McCabe et al. showed that warfarin-treated CKD rats increased pulse pressure and pulse wave velocity and enhanced calcium content in the thoracic and abdominal aorta, renal artery, and carotid artery after a 7-week intervention; conversely, those treated with high-dose Vitamin K1 inhibited the development of vascular calcification [30]. In another CKD rats model, warfarin treatment increased venous neo-intimal hyperplasia and calcification of arterialized veins, while Vitamin K2 supplementation mitigated the detrimental effects of warfarin [31]. Similarly, Kaesler et al. reported that supplementation of 4-week Vitamin K1 or K2 in uremic rats reduced the tissue expression of uncarboxylated MGP in the aorta, which was associated with a decline in aortic and renal calcium content [32].
CLINICAL TRIALS INVESTIGATING THE EFFECTS OF VITAMIN K ON VASCULAR HEALTH IN CHRONIC KIDNEY DISEASE
Emerging clinical trials investigated the effects of Vitamin K supplementation on serum uncarboxylated MGP, vascular calcification, and clinical outcomes across the CKD spectrum. These results are described in the following section and summarized in Table 1.
Table 1.
Summary of clinical trials of Vitamin K supplementation on vascular health in chronic kidney disease
| Author, year | Study design | Participants | Treatment | Study duration | Effects of Vitamin K | |
|---|---|---|---|---|---|---|
|
| ||||||
| Serum ucMGP | Vascular outcomes | |||||
|
| ||||||
| Nondialysis CKD | ||||||
| Kurnatowska et al., 2015 [34] | Double-blind RCT | 42 patients with stage 3-5 | Cholecalciferol 10 µg + Vitamin K2 90 µg daily versus 10 µg cholecalciferol alone | 9 months | Reduced in Vitamin D + K2 group | The progression of common carotid intima-media thickness attenuated in cholecalciferol + Vitamin K2 group |
| Witham et al., 2020 [33] | Double-blind RCT | 159 patients with stage 3b or 4 | Vitamin K2 400 µg daily versus placebo | 12 months | Reduced | No significant differences in pulse wave velocity (primary outcome) and augmentation index, office blood pressure, N-terminal pro B-type natriuretic peptide, short physical performance battery, grip strength, and risks of falls (secondary outcomes) |
|
| ||||||
| ESRD | ||||||
|
| ||||||
| Westenfeld et al., 2012 [35] | RCT | 53 chronic HD patients | Vitamin K2 45, 135, or 360 µg daily | 6 weeks | A dose-dependent decline | — |
| Caluwé et al., 2014 [36] | RCT | 200 chronic HD patients | Vitamin K2 360, 720 or 1080 µg thrice weekly | 8 weeks | A dose-dependent decline | — |
| Oikonomaki et al., 2019 [38] | RCT | 102 HD patients | Vitamin K2 200 µg daily versus control | 12 months | Reduced | The changes in aortic calcification didn’t differ between groups |
| Haroon et al., 2020 (Trevasc-HDK study) [41] | Open-label RCT | 200 HD patients | Vitamin K2 360 µg thrice weekly versus control | 18 months | Ongoing | |
| Levy-Schousboe et al., 2021 (RenaKvit) [37] | Double-blind RCT | 48 dialysis patients | Vitamin K2 360 µg daily versus placebo | 24 months | Reduced | The changes in pulse wave velocity, calcification of coronary arteries, aortic and mitral valves, and abdominal aorta didn’t differ between groups |
| De Vriese et al., 2020 (Valkyrie) [39] | RCT | 132 HD patients with atrial fibrillation | Warfarin versus rivaroxaban 10 mg daily versus rivaroxaban 10 mg daily + Vitamin K2 2000 µg trice weekly | 18 months | Elevated in warfarin group, and reduced in rivaroxaban and rivaroxaban + Vitamin K2 group | There were no significant changes regarding pulse wave velocity, as well as calcification of coronary artery, thoracic aorta, and cardiac valve among the three treatment arms |
| De Vriese et al., 2021 (Valkyrie) [40] | RCT | 132 HD patients with atrial fibrillation | 36 months | — | The competing risk-adjusted HR for composite of fatal and nonfatal CV events was 0.41 (95% CI, 0.25–0.68) in rivaroxaban group and 0.34 (95% CI, 0.19-0.61) in rivaroxaban + Vitamin K2 group, compared with warfarin group | |
|
| ||||||
| Renal transplantation | ||||||
|
| ||||||
| Mansour et al., 2017 [42] | Single-arm | 60 renal transplant recipients | Vitamin K2 360 µg daily | 8 weeks | Reduced | A 14.2% reduction in mean central pulse wave velocity was observed |
| Lees et al., 2021 (ViKTORIES) [43] | Double-blind RCT | 90 renal transplant recipients | Menadiol diphosphate 5 mg trice weekly versus placebo | 12 months | Reduced | The changes of vascular stiffness and calcification didn’t differ between groups |
CKD: Chronic kidney disease, ucMGP: Uncarboxylated matrix Gla-protein, RCT: Randomized control trial, ESRD: End-stage renal disease, HD: Hemodialysis, HR: Hazard ratio, CV: Cardiovascular, CI: Confidence interval
A double-blind, randomized control trial to investigate the effect of 12-month daily 400 mg Vitamin K2 supplementation on vascular stiffness among 159 CKD patients with stage 3b or 4 showed that, albeit a significant reduction in serum uncarboxylated MGP and osteocalcin levels in Vitamin K treatment group, there were no significant differences in pulse wave velocity as the primary outcome, as well as secondary outcomes, which included augmentation index, office blood pressure, N-terminal pro B-type natriuretic peptide, short physical performance battery, grip strength, and risks of falls [33]. In another small randomized, double-blind study, the effects of vascular calcification and atherosclerosis were compared between daily 10 mg cholecalciferol with 90 mg Vitamin K2 and cholecalciferol alone among 42 nondialysis CKD patients with stage 3–5. After 270 days of supplementation, there is a significant decline in serum uncarboxylated MGP and osteocalcin levels in those with Vitamin K2 supplementation. And, in cholecalciferol plus Vitamin K2 group, the progression of common carotid intima-media thickness tended to be attenuated compared to those with cholecalciferol alone [34].
In 53 patients undergoing chronic HD, Westenfeld et al. observed a 4.5- and 8.4-folds increase in serum levels of uncarboxylated MGP and osteocalcin, compared with 50 normal healthy control subjects. The high levels of uncarboxylated MGP and osteocalcin in HD group declined after 6-week supplementation with daily 45, 135, or 360 μg Vitamin K2, in a dose-dependent manner [35]. Similarly, a randomized control trial conducted by Caluwé et al. demonstrated that, among 200 patients undergoing chronic HD, 360, 720 or 1080 μg Vitamin K2 supplementation thrice weekly lowered serum uncarboxylated MGP in a dose-dependent manner [36].
A 2-year randomized, double-blind trial (RenaKvit trial) compared the effect of pulse wave velocity and arterial calcification between daily 360 μg Vitamin K2 and placebo among 48 dialysis patients [37]. Despite a significant reduction of uncarboxylated MGP in Vitamin K2 group, the pulse wave velocity changes didn’t differ between groups over the 2-year period. In addition, calcification of coronary arteries, aortic and mitral valves, and abdominal aorta progressed in both groups. Similarly, a significant decline in uncarboxylated MGP levels but not reduced aortic calcification in patients supplemented with 200 mg Vitamin K2 daily among 102 HD patients was reported in another prospective randomized interventional study after 12-month follow-up period [38].
The Valkyrie study compared the effects of warfarin, 10 mg rivaroxaban daily, or 10 mg rivaroxaban daily plus 2000 mg Vitamin K2 thrice weekly on vascular calcification and clinical outcomes among 132 HD patients with atrial fibrillation [39]. Compared to a significant elevation of uncarboxylated MGP levels in warfarin group, those in rivaroxaban and rivaroxaban plus Vitamin K2 group displayed a decline in uncarboxylated MGP levels after 6 months. After an 18-month period of intervention, there were no significant changes regarding pulse wave velocity, as well as calcification of coronary artery, thoracic aorta, and cardiac valve among the three treatment arms. However, after extended follow-up for at least another 18 months, rivaroxaban and rivaroxaban plus Vitamin K2 groups showed reduced HRs of a composite outcome of fatal CV disease, stroke, cardiac, and other vascular events, in comparison to the warfarin group [40].
Trevasc-HDK study, another randomized and open-label trial investigating the effects of 18-month oral Vitamin K2 supplementation (360 mg thrice weekly) on vascular calcification in 200 HD patients, is ongoing [41].
In 60 renal transplant recipients, a single-arm clinical trial evaluating the effects of 8-week Vitamin K2 supplementation (360 μg daily) on arterial stiffness showed a 55.1% reduction in mean uncarboxylated MGP and a 14.2% reduction in mean central PWV [42]. However, the ViKTORIES trial, a randomized, double-blind, placebo-controlled trial in 90 renal transplant recipients, failed to show any impact of 1-year supplemented 5-mg menadiol diphosphate trice weekly, a synthetic analogue of Vitamin K, on vascular stiffness and calcification [43].
INTERPRETATION OF CURRENT EVIDENCE
In summary, despite a decline in serum ucMGP levels, there is insufficient evidence to support the beneficial effects of Vitamin K supplementation on vascular calcification and stiffness in CKD and ESRD, as well as on CV events and mortality. Several possible reasons may be responsible for these findings. First, in patients with CKD, the pathogenesis of vascular calcification was multifactorial and was much more intricate than that in the general population. In this regard, a single strategy with Vitamin K supplementation may not be sufficient to reduce or mitigate the progression of vascular calcification. The combination of Vitamin K with other strategies aimed to exert synergic effects might be more effective. For example, through combination with Vitamin D, which promotes the production of Vitamin K-dependent protein, the randomized control trial conducted by Kurnatowska et al. reported the beneficial effects of Vitamin K2 plus cholecalciferol on vascular stiffness in CKD stage 3–5 [34]. Second, considering the chronicity nature and high burden of vascular calcification among CKD patients, the study duration of most clinical trials may not be long enough to observe the clinical significance; further clinical trials with extended follow-up duration should be attempted to evaluate the long-term effects of Vitamin K. This chronicity pathophysiology of vascular calcification in CKD patients may also explain, at least in part, the discrepancies between clinical trials and experimental animal models, the latter of which showed promising benefit results of Vitamin K supplementation [18,29,32]. Notably, extended follow-up of Valkyrie study for additional 18 months disclosed a reduced risk of composited fatal and non-fatal CV events in rivaroxaban group and rivaroxaban plus Vitamin K2 group, compared with warfarin group [40]. However, whether these results are attributed to the differences in Vitamin K status among groups or less safety profile of warfarin couldn’t be clarified due to a lack of placebo group. Third, the optimal dose of Vitamin K to achieve the best efficacy for vascular health remains unknown. High-dose Vitamin K supplementation may enhance its biological effects theoretically but may also raise the concern of increased adverse effects. For example, MK-7 supplementation could influence anticoagulation sensitivity and attenuate drug effects in those on anticoagulants [44]. Otherwise, most aforementioned clinical trials with Vitamin K2 doses ranging from 45 mg daily to 2000 mg trice weekly reported tolerable side effects without increased risks of thrombosis events. Fourth, whether the serum MGP levels, which reflect functional Vitamin K status, play a causal role in the pathogenesis of vascular calcification or are only a marker should be clarified further. In 188 HD patients, 6-week Vitamin K2 supplementation resulted in a significant decline in serum ucMGP levels, but no increase in carboxylated MGP levels, the latter of which independently predicted vascular calcification, all-cause and CV mortality in that study [45]. Finally, the effects of Vitamin K supplementation may be hampered by uremic status, given the observation that the pharmacokinetics and biodistribution were prominently altered in advanced CKD and ESRD [20]. Since some clinical trials in non-CKD populations, including healthy elderly population [46], diabetic mellitus [47], and post-menopausal women [48], reported a benefit of Vitamin K supplementation on vascular health, whether Vitamin K supplementation in patients with earlier stages of CKD exhibited better efficacy should be determined in further studies.
CONCLUSION
Taken together, while the close relationships between Vitamin K deficiency and enhanced CV risks were reported in observational studies and the beneficial effects of Vitamin K supplementation on vascular health were suggested in animal studies, most clinical trials failed to show convincing CV benefits of Vitamin K. More clinical studies with large sample size and extensive follow-up periods are encouraged. Furthermore, clinical studies directed at identifying other forms of synthetic Vitamin K and their optimizing effective dose, determining suitable candidates who may benefit from Vitamin K supplementation, as well as combining multiple strategies are also urgently needed to expand our understanding regarding this important issue.
Financial support and sponsorship
This study was supported by a grant from Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation (TCMF-EP 110-03), and the Ministry of Science and Technology (MOST 110-2314-B-303-012-) in Taiwan.
Conflicts of interest
Dr. Bang-Gee Hsu, an editorial board member at Tzu Chi Medical Journal, had no role in the peer review process of or decision to publish this article. The other author declared no conflicts of interest in writing this paper.
REFERENCES
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Yao Q, Pecoits-Filho R, Lindholm B, Stenvinkel P. Traditional and non-traditional risk factors as contributors to atherosclerotic cardiovascular disease in end-stage renal disease. Scand J Urol Nephrol. 2004;38:405–16. doi: 10.1080/00365590410031715. [DOI] [PubMed] [Google Scholar]
- 3.Filipska I, Winiarska A, Knysak M, Stompór T. Contribution of gut microbiota-derived uremic toxins to the cardiovascular system mineralization. Toxins (Basel) 2021;13:274. doi: 10.3390/toxins13040274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlieper G, Schurgers L, Brandenburg V, Reutelingsperger C, Floege J. Vascular calcification in chronic kidney disease: An update. Nephrol Dial Transplant. 2016;31:31–9. doi: 10.1093/ndt/gfv111. [DOI] [PubMed] [Google Scholar]
- 5.Shioi A, Morioka T, Shoji T, Emoto M. The inhibitory roles of Vitamin K in progression of vascular calcification. Nutrients. 2020;12:E583. doi: 10.3390/nu12020583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roumeliotis S, Duni A, Vaios V, Kitsos A, Liakopoulos V, Dounousi E. Vitamin K supplementation for prevention of vascular calcification in chronic kidney disease patients: Are we there yet? Nutrients. 2022;14:925. doi: 10.3390/nu14050925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wuyts J, Dhondt A. The role of Vitamin K in vascular calcification of patients with chronic kidney disease. Acta Clin Belg. 2016;71:462–7. doi: 10.1080/17843286.2016.1180770. [DOI] [PubMed] [Google Scholar]
- 8.Hou YC, Lu CL, Zheng CM, Chen RM, Lin YF, Liu WC, et al. Emerging role of Vitamins D and K in modulating uremic vascular calcification: The aspect of passive calcification. Nutrients. 2019;11:152. doi: 10.3390/nu11010152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stępień A, Koziarska-Rościszewska M, Rysz J, Stępień M. Biological role of Vitamin K-with particular emphasis on cardiovascular and renal aspects. Nutrients. 2022;14:262. doi: 10.3390/nu14020262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simes DC, Viegas CS, Araújo N, Marreiros C. Vitamin K as a diet supplement with impact in human health: Current evidence in age-related diseases. Nutrients. 2020;12:E138. doi: 10.3390/nu12010138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schurgers LJ, Vermeer C. Determination of phylloquinone and menaquinones in food. Effect of food matrix on circulating Vitamin K concentrations. Haemostasis. 2000;30:298–307. doi: 10.1159/000054147. [DOI] [PubMed] [Google Scholar]
- 12.Schwalfenberg GK. Vitamins K1 and K2: The emerging group of vitamins required for human health. J Nutr Metab 2017. 2017 doi: 10.1155/2017/6254836. 6254836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schurgers LJ, Vermeer C. Differential lipoprotein transport pathways of K-vitamins in healthy subjects. Biochim Biophys Acta. 2002;1570:27–32. doi: 10.1016/s0304-4165(02)00147-2. [DOI] [PubMed] [Google Scholar]
- 14.Geleijnse JM, Vermeer C, Grobbee DE, Schurgers LJ, Knapen MH, van der Meer IM, et al. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: The Rotterdam Study. J Nutr. 2004;134:3100–5. doi: 10.1093/jn/134.11.3100. [DOI] [PubMed] [Google Scholar]
- 15.Simes DC, Viegas CS, Araújo N, Marreiros C. Vitamin K as a powerful micronutrient in aging and age-related diseases: Pros and cons from clinical studies. Int J Mol Sci. 2019;20:E4150. doi: 10.3390/ijms20174150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheung CL, Sahni S, Cheung BM, Sing CW, Wong IC. Vitamin K intake and mortality in people with chronic kidney disease from NHANES III. Clin Nutr. 2015;34:235–40. doi: 10.1016/j.clnu.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Fusaro M, D’Alessandro C, Noale M, Tripepi G, Plebani M, Veronese N, et al. Low Vitamin K1 intake in haemodialysis patients. Clin Nutr. 2017;36:601–7. doi: 10.1016/j.clnu.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 18.McCabe KM, Booth SL, Fu X, Ward E, Adams MA, Holden RM. Vitamin K metabolism in a rat model of chronic kidney disease. Am J Nephrol. 2017;45:4–13. doi: 10.1159/000451068. [DOI] [PubMed] [Google Scholar]
- 19.Nakagawa K, Hirota Y, Sawada N, Yuge N, Watanabe M, Uchino Y, et al. Identification of UBIAD1 as a novel human menaquinone-4 biosynthetic enzyme. Nature. 2010;468:117–21. doi: 10.1038/nature09464. [DOI] [PubMed] [Google Scholar]
- 20.Kaesler N, Schreibing F, Speer T, Puente-Secades S, Rapp N, Drechsler C, et al. Altered Vitamin K biodistribution and metabolism in experimental and human chronic kidney disease. Kidney Int. 2022;101:338–48. doi: 10.1016/j.kint.2021.10.029. [DOI] [PubMed] [Google Scholar]
- 21.Schurgers LJ, Barreto DV, Barreto FC, Liabeuf S, Renard C, Magdeleyns EJ, et al. The circulating inactive form of matrix gla protein is a surrogate marker for vascular calcification in chronic kidney disease: A preliminary report. Clin J Am Soc Nephrol. 2010;5:568–75. doi: 10.2215/CJN.07081009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evenepoel P, Claes K, Meijers B, Laurent M, Bammens B, Naesens M, et al. Poor Vitamin K status is associated with low bone mineral density and increased fracture risk in end-stage renal disease. J Bone Miner Res. 2019;34:262–9. doi: 10.1002/jbmr.3608. [DOI] [PubMed] [Google Scholar]
- 23.Shea MK, Barger K, Booth SL, Wang J, Feldman HI, Townsend RR, et al. Vitamin K status, all-cause mortality, and cardiovascular disease in adults with chronic kidney disease: The Chronic Renal Insufficiency Cohort. Am J Clin Nutr. 2022;115:941–8. doi: 10.1093/ajcn/nqab375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dai L, Li L, Erlandsson H, Jaminon AM, Qureshi AR, Ripsweden J, et al. Functional Vitamin K insufficiency, vascular calcification and mortality in advanced chronic kidney disease: A cohort study. PLoS One. 2021;16:e0247623. doi: 10.1371/journal.pone.0247623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roumeliotis S, Roumeliotis A, Stamou A, Leivaditis K, Kantartzi K, Panagoutsos S, et al. The association of dp-ucMGP with cardiovascular morbidity and decreased renal function in diabetic chronic kidney disease. Int J Mol Sci. 2020;21:E6035. doi: 10.3390/ijms21176035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Q, Guo H, Cao S, Zhou Q, Chen J, Su M, et al. Associations of Vitamin K status with mortality and cardiovascular events in peritoneal dialysis patients. Int Urol Nephrol. 2019;51:527–34. doi: 10.1007/s11255-019-02080-x. [DOI] [PubMed] [Google Scholar]
- 27.Keyzer CA, Vermeer C, Joosten MM, Knapen MH, Drummen NE, Navis G, et al. Vitamin K status and mortality after kidney transplantation: A cohort study. Am J Kidney Dis. 2015;65:474–83. doi: 10.1053/j.ajkd.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 28.Fusaro M, Tripepi G, Noale M, Plebani M, Zaninotto M, Piccoli A, et al. Prevalence of vertebral fractures, vascular calcifications, and mortality in warfarin treated hemodialysis patients. Curr Vasc Pharmacol. 2015;13:248–58. doi: 10.2174/15701611113119990146. [DOI] [PubMed] [Google Scholar]
- 29.Scheiber D, Veulemans V, Horn P, Chatrou ML, Potthoff SA, Kelm M, et al. High-dose menaquinone-7 supplementation reduces cardiovascular calcification in a murine model of extraosseous calcification. Nutrients. 2015;7:6991–7011. doi: 10.3390/nu7085318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCabe KM, Booth SL, Fu X, Shobeiri N, Pang JJ, Adams MA, et al. Dietary Vitamin K and therapeutic warfarin alter the susceptibility to vascular calcification in experimental chronic kidney disease. Kidney Int. 2013;83:835–44. doi: 10.1038/ki.2012.477. [DOI] [PubMed] [Google Scholar]
- 31.Zaragatski E, Grommes J, Schurgers LJ, Langer S, Kennes L, Tamm M, et al. Vitamin K antagonism aggravates chronic kidney disease-induced neointimal hyperplasia and calcification in arterialized veins: Role of Vitamin K treatment? Kidney Int. 2016;89:601–11. doi: 10.1038/ki.2015.298. [DOI] [PubMed] [Google Scholar]
- 32.Kaesler N, Magdeleyns E, Herfs M, Schettgen T, Brandenburg V, Fliser D, et al. Impaired Vitamin K recycling in uremia is rescued by Vitamin K supplementation. Kidney Int. 2014;86:286–93. doi: 10.1038/ki.2013.530. [DOI] [PubMed] [Google Scholar]
- 33.Witham MD, Lees JS, White M, Band M, Bell S, Chantler DJ, et al. Vitamin K supplementation to improve vascular stiffness in CKD: The K4Kidneys randomized controlled trial. J Am Soc Nephrol. 2020;31:2434–45. doi: 10.1681/ASN.2020020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurnatowska I, Grzelak P, Masajtis-Zagajewska A, Kaczmarska M, Stefańczyk L, Vermeer C, et al. Effect of Vitamin K2 on progression of atherosclerosis and vascular calcification in nondialyzed patients with chronic kidney disease stages 3-5. Pol Arch Med Wewn. 2015;125:631–40. doi: 10.20452/pamw.3041. [DOI] [PubMed] [Google Scholar]
- 35.Westenfeld R, Krueger T, Schlieper G, Cranenburg EC, Magdeleyns EJ, Heidenreich S, et al. Effect of Vitamin K2 supplementation on functional Vitamin K deficiency in hemodialysis patients: A randomized trial. Am J Kidney Dis. 2012;59:186–95. doi: 10.1053/j.ajkd.2011.10.041. [DOI] [PubMed] [Google Scholar]
- 36.Caluwé R, Vandecasteele S, Van Vlem B, Vermeer C, De Vriese AS. Vitamin K2 supplementation in haemodialysis patients: A randomized dose-finding study. Nephrol Dial Transplant. 2014;29:1385–90. doi: 10.1093/ndt/gft464. [DOI] [PubMed] [Google Scholar]
- 37.Levy-Schousboe K, Frimodt-Møller M, Hansen D, Peters CD, Kjærgaard KD, Jensen JD, et al. Vitamin K supplementation and arterial calcification in dialysis: Results of the double-blind randomized, placebo-controlled RenaKvit trial. Clin Kidney J. 2021;14:2114–23. doi: 10.1093/ckj/sfab017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oikonomaki T, Papasotiriou M, Ntrinias T, Kalogeropoulou C, Zabakis P, Kalavrizioti D, et al. The effect of Vitamin K2 supplementation on vascular calcification in haemodialysis patients: A 1-year follow-up randomized trial. Int Urol Nephrol. 2019;51:2037–44. doi: 10.1007/s11255-019-02275-2. [DOI] [PubMed] [Google Scholar]
- 39.De Vriese AS, Caluwé R, Pyfferoen L, De Bacquer D, De Boeck K, Delanote J, et al. Multicenter randomized controlled trial of Vitamin K antagonist replacement by rivaroxaban with or without Vitamin K2 in hemodialysis patients with atrial fibrillation: The Valkyrie study. J Am Soc Nephrol. 2020;31:186–96. doi: 10.1681/ASN.2019060579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Vriese AS, Caluwé R, Van Der Meersch H, De Boeck K, De Bacquer D. Safety and efficacy of Vitamin K antagonists versus rivaroxaban in hemodialysis patients with atrial fibrillation: A multicenter randomized controlled trial. J Am Soc Nephrol. 2021;32:1474–83. doi: 10.1681/ASN.2020111566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haroon SW, Tai BC, Ling LH, Teo L, Davenport A, Schurgers L, et al. Treatment to reduce vascular calcification in hemodialysis patients using Vitamin K (Trevasc-HDK): A study protocol for a randomized controlled trial. Medicine (Baltimore) 2020;99:e21906. doi: 10.1097/MD.0000000000021906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mansour AG, Hariri E, Daaboul Y, Korjisn S, Alam AE, Protogerou AD, et al. Vitamin K2 supplementation and arterial stiffness among renal transplant recipients –A single-arm, single-center clinical trial. J Am Soc Hypertens. 2017;11:589–97. doi: 10.1016/j.jash.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Lees JS, Rankin AJ, Gillis KA, Zhu LY, Mangion K, Rutherford E, et al. The VIKTORIES trial: A randomized, double-blind, placebo-controlled trial of Vitamin K supplementation to improve vascular health in kidney transplant recipients. Am J Transplant. 2021;21:3356–68. doi: 10.1111/ajt.16566. [DOI] [PubMed] [Google Scholar]
- 44.Theuwissen E, Teunissen KJ, Spronk HM, Hamulyák K, Ten Cate H, Shearer MJ, et al. Effect of low-dose supplements of menaquinone-7 (Vitamin K2) on the stability of oral anticoagulant treatment: Dose-response relationship in healthy volunteers. J Thromb Haemost. 2013;11:1085–92. doi: 10.1111/jth.12203. [DOI] [PubMed] [Google Scholar]
- 45.Schlieper G, Westenfeld R, Krüger T, Cranenburg EC, Magdeleyns EJ, Brandenburg VM, et al. Circulating nonphosphorylated carboxylated matrix gla protein predicts survival in ESRD. J Am Soc Nephrol. 2011;22:387–95. doi: 10.1681/ASN.2010040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shea MK, O’Donnell CJ, Hoffmann U, Dallal GE, Dawson-Hughes B, Ordovas JM, et al. Vitamin K supplementation and progression of coronary artery calcium in older men and women. Am J Clin Nutr. 2009;89:1799–807. doi: 10.3945/ajcn.2008.27338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bellinge JW, Francis RJ, Lee SC, Bondonno NP, Sim M, Lewis JR, et al. The effect of Vitamin K1 on arterial calcification activity in subjects with diabetes mellitus: A post hoc analysis of a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2022;115:45–52. doi: 10.1093/ajcn/nqab306. [DOI] [PubMed] [Google Scholar]
- 48.Knapen MH, Braam LA, Drummen NE, Bekers O, Hoeks AP, Vermeer C. Menaquinone-7 supplementation improves arterial stiffness in healthy postmenopausal women. A double-blind randomised clinical trial. Thromb Haemost. 2015;113:1135–44. doi: 10.1160/TH14-08-0675. [DOI] [PubMed] [Google Scholar]