ABSTRACT
Campylobacter jejuni is a leading cause of foodborne bacterial gastroenteritis worldwide, and raw or undercooked chicken meat is considered the major source of human campylobacteriosis. In this study, we identified 36 compounds that showed inhibitory effects on C. jejuni growth at low concentrations by screening a chemical compound library. Three of the 36 compounds were herbal compounds, including tryptanthrin (TRP), an indoloquinazoline alkaloid. TRP has been reported to have a variety of biological properties, such as antimicrobial, anti-inflammatory, and antitumor activities, but there was previously no information about its anti-C. jejuni activity. We further conducted in vitro and in vivo experiments to evaluate the potential of TRP for the control of C. jejuni in chicken farms. The MIC of TRP for C. jejuni was much lower than that of 13 other herbal compounds that were previously reported to have anti-C. jejuni activities. Time-kill assays under growing and nongrowing conditions demonstrated that TRP has bactericidal activity against C. jejuni. In addition, TRP showed a narrow-spectrum antimicrobial effect against C. jejuni, and there was little potential for the development of TRP-resistant C. jejuni during serially passaged culture. In chick infection experiments, the administration of TRP in drinking water significantly reduced the cecal colonization of C. jejuni when TRP was used either before or after C. jejuni infection. These data suggest that TRP is effective for the control of C. jejuni in chicken farms.
IMPORTANCE Campylobacter is a widespread pathogen in the food chain of chickens. Once chickens become infected, large numbers of Campylobacter cells are excreted in their feces. The development of an effective material for reducing the amount of Campylobacter in the chicken intestinal tract will make it possible to reduce the contamination of the food chain with Campylobacter and to produce safe and secure chicken meat. In the present study, in vivo experiments revealed that the use of an herbal compound, tryptanthrin, significantly reduced the number of Campylobacter cells in the chicken gut by a bactericidal mechanism. Furthermore, our in vitro experiments demonstrated that, compared with the other herbal compounds, tryptanthrin achieved antimicrobial activity against C. jejuni at the lowest concentration. The use of tryptanthrin may lead to the development of a novel control measure for reducing the colonization of C. jejuni in the food chain.
KEYWORDS: Campylobacter jejuni, chemical compound library, chicken, herbal compound, tryptanthrin
INTRODUCTION
Campylobacter is the most common cause of bacterial foodborne disease in humans worldwide. The World Health Organization (WHO) estimated that more than 95 million cases of foodborne illness caused by Campylobacter species occurred worldwide in 2010 (1). In the European Union, the annual cost associated with human campylobacteriosis was estimated to be €2.4 billion (2). Contamination by Campylobacter jejuni and Campylobacter coli throughout chicken food supply chains, from farm to commercial meats, is responsible for the frequent occurrence of this infection in humans (3–5). Of the two Campylobacter species, C. jejuni is the predominant cause of campylobacteriosis in many countries, including those in the European Union and the United States (6, 7).
Because a large amount of C. jejuni (up to 108 or 109 cells per gram of feces) is present in the feces of chickens infected with this bacterium (8), chicken farm environments are highly contaminated with C. jejuni: the corresponding infection is rapidly disseminated throughout chicken flocks within farms and is therefore easily transmitted to other chicken farms through wildlife, insects, and vendors (5, 9). It is difficult to control the C. jejuni contamination of carcasses from chicken intestinal contents by current treatment techniques because the chicken body surface is also highly contaminated by C. jejuni via feces (10, 11). Therefore, the widespread contamination of the food supply chains is attributed to the large amounts of Campylobacter present in the intestinal tracts of infected chickens.
Previous studies showed that an approximately 10-fold or thousandfold reduction in C. jejuni cells on chicken carcasses significantly reduced the incidence of campylobacteriosis in humans (12, 13). Therefore, a reduction in the number of C. jejuni in the chicken gut is an essential strategy for controlling campylobacteriosis in humans. To date, many studies have been conducted to develop preventive measures against Campylobacter for use on chicken farms, such as feed and water additives, vaccination, prebiotics and probiotics, bacteriocins, and bacteriophages (5, 12, 14). In recent years, there has been increasing interest in the use of natural substances to control Campylobacter on chicken farms and processing plants because of consumer demands for antibiotic-free products. Many researchers have proposed controlling Campylobacter in the chicken gut by using feed or water additives, including natural substances, such as plant-derived essential oils and organic acids, that are known to show antimicrobial activities in vitro (14, 15). However, there are few reports of in vivo studies demonstrating the anti-Campylobacter effects of natural substances in the chicken gut. Certain herbal essential oils and compounds, such as carvacrol and thymol, which are present in several herbal plants, have achieved a statistically significant reduction in C. jejuni in the chicken gut, but the effects were limited (16, 17).
In the present study, we screened a chemical compound library to identify novel and effective natural substances for controlling Campylobacter colonization in the chicken gut. We demonstrated that tryptanthrin (TRP), a known herbal compound, showed strong antimicrobial activity against C. jejuni at low concentrations. We further performed in vitro and in vivo experiments to evaluate the anti-C. jejuni activity of TRP, especially whether TRP was able to reduce the amount of C. jejuni in the chicken gut.
RESULTS
Potent anti-C. jejuni activity of TRP.
We identified 36 compounds that inhibited the growth of the two C. jejuni strains (NCTC 11168 and 81-176) at a rate of more than 99.0% at 10 μM through the high-throughput screening of 1,926 compounds. Of the 36 compounds, 23 were not commercially available or were used as antibiotics. The remaining 13 compounds consisted of 3 herbal compounds and 10 other compounds. TRP, one of the three herbal compounds, is an indoloquinazoline alkaloid that was first isolated from natural indigo and later identified in various natural sources (18). Certain herbal compounds were reported to show antimicrobial effects against C. jejuni, and therefore, we compared the antimicrobial activities of TRP against two C. jejuni strains (NCTC 11168 and 81-176) with those of 13 herbal compounds that are known to have antimicrobial activities against C. jejuni (19–24). As shown in Table 1, the MICs of TRP for the C. jejuni NCTC 11168 and 81-176 strains were 6.25 and 3.12 μM, respectively. Among the previously reported 13 herbal compounds, cinnamaldehyde showed the lowest MIC for both NCTC 11168 and 81-176 strains (200 and 100 μM, respectively). However, the MIC of TRP was 32-fold lower than that of cinnamaldehyde.
TABLE 1.
MICs of herbal compounds against C. jejuni
| Classa | Compound | MIC (μM) of: |
|
|---|---|---|---|
| NCTC 11168 | 81-176 | ||
| Organic heterocyclic compound | Tryptanthrin | 6.25 | 3.12 |
| Organochalcogen compounds | Cinnamaldehyde | 200 | 100 |
| Benzyl isothiocyanate | 800 | 800 | |
| Allyl isothiocyanate | >800 | >800 | |
| Perillaldehyde | >800 | >800 | |
| Phenols | Carvacrol | 400 | 200 |
| Eugenol | 800 | 400 | |
| Rosmarinic acid | >800 | >800 | |
| Thymol | >800 | >800 | |
| Terpenoids | Citral | 800 | 800 |
| Cineole | >800 | >800 | |
| Alcohol | Linalool | >800 | >800 |
| Hydrocarbons | p-Cymene | >800 | >800 |
| α-Pinene | >800 | >800 | |
Herbal compounds are classified according to the ChEBI database.
Antimicrobial spectra of TRP.
We investigated the antimicrobial spectra of TRP against three species of Campylobacter that are known to be pathogenetic in humans and animals (C. jejuni, C. coli, and C. fetus) and seven species of gut bacteria (Escherichia coli, Enterobacter cloacae, Klebsiella pneumoniae, Proteus mirabilis, Salmonella enterica, Enterococcus faecalis, and Clostridium perfringens), as shown in Table 2. The MICs of TRP for 20 strains of C. jejuni belonging to different sequence types ranged from 0.78 to 6.25 μM. In addition, the MICs of TRP for four strains of C. coli and one strain of C. fetus (CFF018) were 6.25 or 12.5 μM, but those for the other three strains of C. fetus and all seven strains of gut bacteria were greater than 100 μM.
TABLE 2.
Antibacterial spectrum of TRP
| Bacterial species | Strain | Source | Sequence type (clonal complex)a | MIC (μM) |
|---|---|---|---|---|
| Campylobacter jejuni | NCTC 11168 | Human | 43 (21) | 3.12 |
| 81-176 | Human | 604 (42) | 3.12 | |
| ATCC 33560 | Bovine | 403 (403) | 3.12 | |
| 11-164 | Chicken | 6849 (354) | 3.12 | |
| CJ069 | Chicken | 50 (21) | 3.12 | |
| CJ049 | Chicken | 4526 (21) | 1.56 | |
| CJ060 | Chicken | 22 (22) | 3.12 | |
| CJ046 | Chicken | 45 (45) | 3.12 | |
| CJ012 | Chicken | 3503 (48) | 3.12 | |
| CJ061 | Chicken | 61 (61) | 3.12 | |
| CJ067 | Chicken | 257 (257) | 1.56 | |
| CJ070 | Chicken | 4063 (283) | 6.25 | |
| CJ038 | Chicken | 3911 (353) | 1.56 | |
| CJ007 | Chicken | 4052 (353) | 3.12 | |
| CJ021 | Chicken | 354 (354) | 0.78 | |
| CJ019 | Chicken | 5402 (354) | 6.25 | |
| CJ001 | Chicken | 443 (443) | 3.12 | |
| CJ011 | Chicken | 460 (460) | 3.12 | |
| CJ004 | Chicken | 5262 (464) | 6.25 | |
| CJ037 | Chicken | 607 (607) | 3.12 | |
| Campylobacter coli | ATCC BAA-1061 | Chicken | 1063 (828) | 12.5 |
| CC003 | Chicken | 854 (828) | 12.5 | |
| CC001 | Chicken | 1767 (828) | 6.25 | |
| CC002 | Chicken | 4172 (828) | 12.5 | |
| Campylobacter fetus | CFF009 | Bovine | 2 (not provided) | >100 |
| CFF045 | Bovine | 3 (not provided) | >100 | |
| CFF018 | Bovine | 5 (not provided) | 6.25 | |
| CFF028 | Bovine | 6 (not provided) | >100 | |
| Escherichia coli | ATCC 23736 | Unknown | NT | >100 |
| Enterobacter cloacae | ATCC 13047 | Human | NT | >100 |
| Klebsiella pneumoniae | ATCC 9997 | Unknown | NT | >100 |
| Proteus mirabilis | ATCC 29906 | Unknown | NT | >100 |
| Salmonella enterica | LT2 | Unknown | NT | >100 |
| Enterococcus faecalis | ATCC 19433 | Unknown | NT | >100 |
| Clostridium perfringens | ATCC 13124 | Unknown | NT | >100 |
Not provided, clonal complex of C. fetus are not provided by PubMLST database; NT, not tested.
Time-kill kinetics of TRP for C. jejuni under growing conditions.
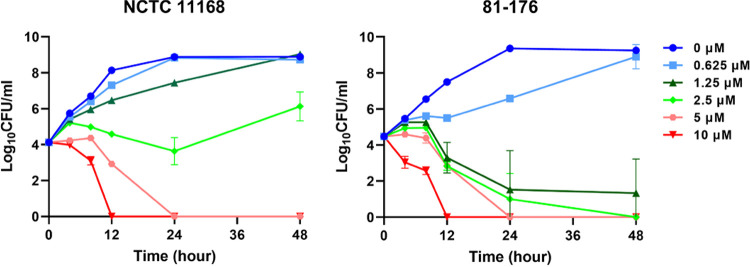
We cultured C. jejuni NCTC 11168 and 81-176 strains in Mueller-Hinton (MH) broth with various TRP concentrations (0, 0.625, 1.25, 2.5, 5, and 10 μM) to evaluate in depth the antimicrobial effect of TRP on C. jejuni under growing conditions. As shown in Fig. 1, viable cells of both the NCTC 11168 and 81-176 strains became undetectable by 24 h after inoculation with more than 5 μM TRP. In the presence of 1.25 and 2.5 μM TRP, the growth of the NCTC 11168 strain was observed, but viable cells of the 81-176 strain were hardly observed by 48 h after inoculation. TRP concentrations under 0.625 μM did not show strong effects on the growth of either strain. These experiments clearly showed that TRP at low concentrations inhibited C. jejuni growth in a dose-dependent manner.
FIG 1.
Time-kill kinetics of TRP for C. jejuni under growing conditions. Time-kill curves of the C. jejuni NCTC 11168 and 81-176 strains in MH broth with different TRP concentrations (0, 0.625, 1.25. 2.5, 5, and 10 μM) under microaerophilic conditions. The data are shown as the mean values and SDs of the results from three independent measurements.
Time-kill kinetics of TRP for C. jejuni under nongrowing conditions.
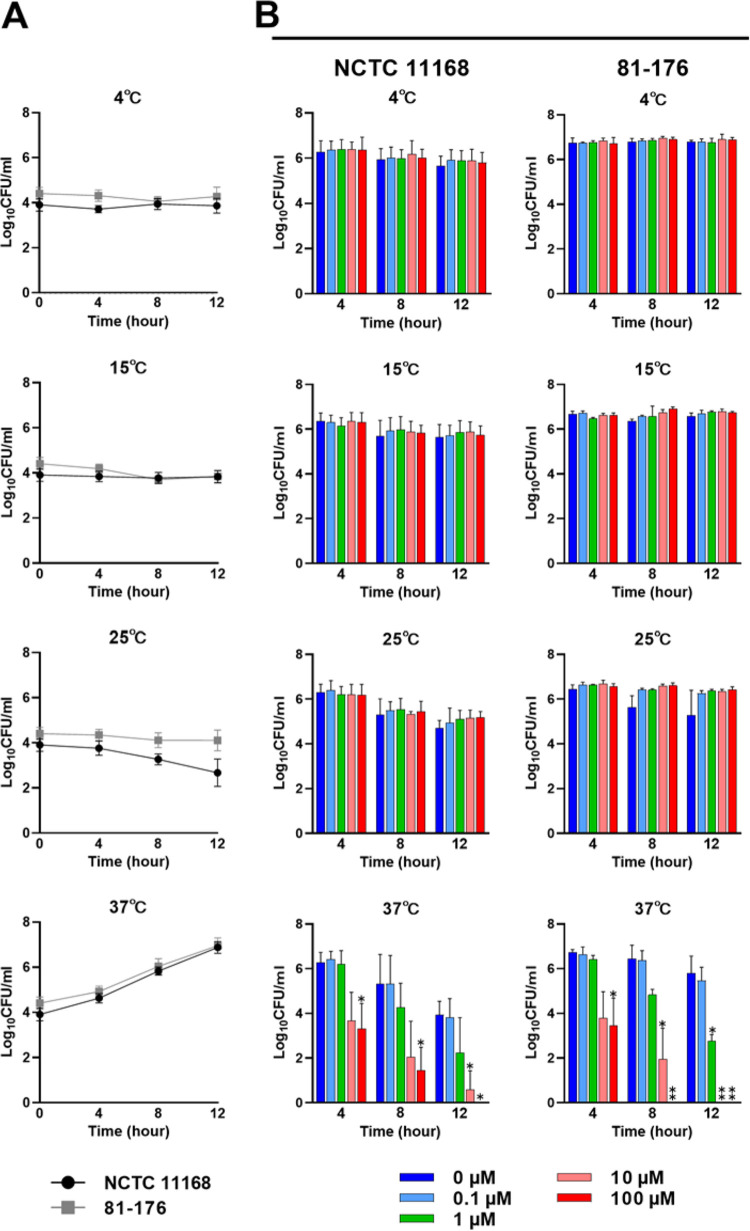
To assess the effect of TRP on C. jejuni under nongrowing conditions, the number of viable cells in phosphate-buffered saline (PBS) with various TRP concentrations was measured at different temperatures. First, we determined the growth of the C. jejuni NCTC 11168 and 81-176 strains in MH broth at different temperatures (4, 15, 25, and 37°C) under microaerobic conditions, as shown in Fig. 2A. The bacterial growth of both strains was observed at 37°C but not at 25°C (Fig. 2A). In the viability tests, the bacterial suspensions in PBS with various TRP concentrations (0, 0.1, 1, 10, and 100 μM) were incubated at different temperatures under aerobic conditions. There were no significant differences in cell viability among different TRP concentrations at 4, 15, and 25°C at any of the three time points for either the NCTC 11168 or 81-176 strains (Fig. 2B). In contrast, when the incubation temperature was 37°C, the cell numbers of C. jejuni treated with 1, 10, and 100 μM TRP were significantly lower than those without TRP treatment at several time points for both strains (P < 0.05 and P < 0.01).
FIG 2.
Time-kill kinetics of TRP for C. jejuni under nongrowing conditions. (A) Growth curves of the C. jejuni NCTC 11168 and 81-176 strains in MH broth at different temperatures (4, 15, 25, and 37°C) under microaerophilic conditions. (B) Viable cell counts of the C. jejuni NCTC 11168 and 81-176 strains in PBS at different temperatures (4, 15, 25, and 37°C) under aerobic conditions. The TRP concentrations were set at 0, 0.1, 1, 10, and 100 μM. The data are shown as the means and SDs of the results from three independent experiments. Asterisks indicate statistically significant differences from the control (PBS without TRP) by Student’s t test (*, P < 0.05; **, P < 0.01) at the same time point.
Potential for the development of TRP resistance in C. jejuni.
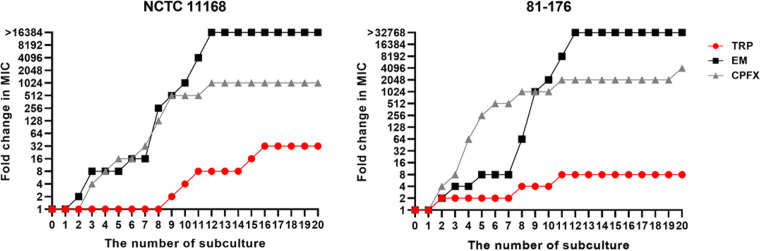
The potential for the development of TRP resistance in the C. jejuni NCTC 11168 and 81-176 strains was evaluated by comparing the MICs of TRP over 20 subcultures to those of two antimicrobials, erythromycin (EM) and ciprofloxacin (CPFX). The initial MICs of TRP, EM, and CPFX for the NCTC 11168 strain were 1.56, 0.5, and 0.125 μg/mL, respectively, and those for the 81-176 strain were 0.78, 0.25, and 0.0625 μg/mL, respectively. In addition, the final MICs of TRP, EM, and CPFX for the NCTC 11168 strain after 20 subcultures were 50, >8,192, and 128 μg/mL, respectively, and those for the 81-176 strain were 6.25, >8,192, and 256 μg/mL, respectively. The C. jejuni strains exposed to EM and CPFX rapidly acquired resistance to antimicrobials within 12 subcultures. During this period, the MICs of EM for the NCTC 11168 and 81-176 strains were increased more than 16,384- and 32,768-fold, respectively, and those of CPFX were increased 1,024- and 2,048-fold, respectively (Fig. 3). Notably, the maximum EM concentration in the determination of MICs was 8,192 μg/mL due to its solubility, but the growth of both NCTC 11168 and 81-176 strains was still observed at this concentration after 12 subcultures. In sharp contrast to those of EM and CPFX, the MICs of TRP for the NCTC 11168 and 81-176 strains were increased only 32- and 8-fold, respectively, even after 20 subcultures (Fig. 3).
FIG 3.
The development of resistance to TRP, EM, and CPFX of C. jejuni by serially passaged culture. The fold changes in the MICs of the NCTC 11168 and 81-176 strains were monitored up to 20 passages with sub-MICs of TRP, EM, and CPFX.
Effect of TRP on C. jejuni colonization in the chick gut.
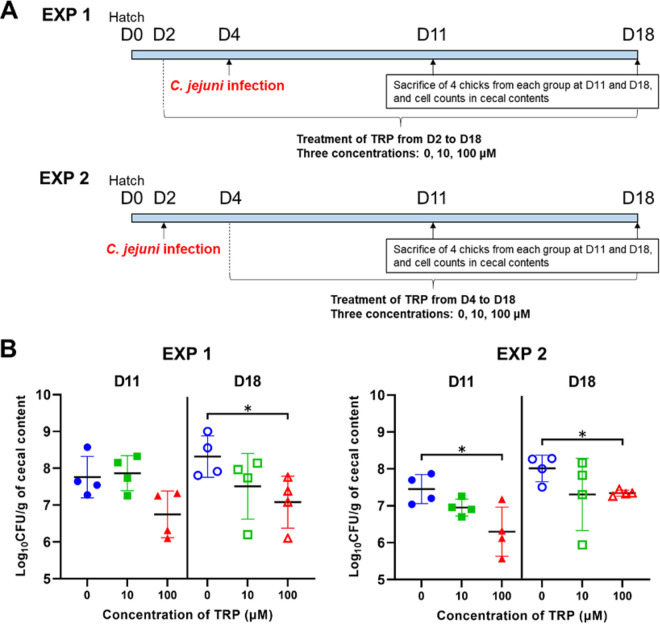
Figure 4A illustrates two types of infection experiments that were carried out to determine the effect of TRP on the colonization of C. jejuni in the chicken gut. In both experiments, 24 newly hatched 1-day-old chicks were assigned to three groups (8 chicks/group). Two of the three groups were treated with TRP, which was added to drinking water at final concentrations of 10 and 100 μM, and the control group was not treated with TRP. In experiment 1, TRP treatment was started 2 days before infection with the C. jejuni 11-164 strain. In experiment 2, TRP treatment was started 2 days after infection with the C. jejuni 11-164 strain. In both experiments, four chicks from each group were sacrificed at 11 and 18 days old, and the number of C. jejuni in their cecal contents was measured. The results of experiment 1 indicated that the numbers of C. jejuni in the 11- and 18-day-old chicks treated with 100 μM TRP (6.7 and 7.1 log10 CFU/g) were lower than those in the control group (7.8 and 8.3 log10 CFU/g), respectively, and a significant difference (P < 0.05) was observed for the 18-day-old chicks (Fig. 4B). In addition, the results of experiment 2 also showed that the numbers of C. jejuni in the 11- and 18-day-old chicks treated with 100 μM TRP (6.3 and 7.3 log10 CFU/g) were significantly lower (P < 0.05) than those in the control group (7.5 and 8.0 log10 CFU/g), respectively (Fig. 4B). When the chicks were treated with 10 μM TRP, the viable cell numbers of C. jejuni in the 18-day-old chicks in experiment 1 and the 11- and 18-day-old chicks in experiment 2 (7.5, 7.0, and 7.3 log10 CFU/g, respectively) were lower than those in the control group, but the difference was not significant (P > 0.05).
FIG 4.
The effect of TRP on the cecal colonization of C. jejuni in chicks. (A) Schedule for the animal experiments. Two types of experiments were conducted to evaluate the antibacterial effect against the C. jejuni 11-164 strain in the chick cecum. TRP treatments (10 and 100 μM added to drinking water) were started 2 days before infection in experiment 1 (EXP 1) and 2 days after infection in experiment 2 (EXP 2). The control groups were not treated with TRP. Four chicks from each group were sacrificed at 11 and 18 days old, and the numbers of C. jejuni in their cecal contents were quantified. (B) Each dot represents the amount of C. jejuni expressed as the log10 CFU per gram in the cecal content of each individual infected chick in experiments 1 and 2. Error bars represent the SDs derived from four chicks per group. All the data were analyzed by unpaired Student’s t test (*, P < 0.05).
DISCUSSION
Natural herbal compounds and essential oils have been previously proposed to control Campylobacter in chicken farms, but their effectiveness has not been clearly defined. As shown by the method of identification of TRP in the present study, compound libraries are useful tools for discovering novel antimicrobial compounds. There have been a few reports of using chemical library screening to identify novel anti-C. jejuni agents: Johnson et al. identified compounds that inhibited the flagellar expression and growth of C. jejuni (25), and Kumar et al. identified compounds that had anti-C. jejuni effects in Caco-2 cells (26). Both of these screening experiments identified synthetic compounds that showed anti-Campylobacter effects in vitro, but it remained unclear whether these compounds have the same effects in the chicken gut. In addition, consumers are generally concerned with the use of synthetic additives in animal feed and their possible inclusion in the meat that we consume (27). Therefore, we used a chemical compound library, which included natural substances, to find novel anti-Campylobacter agents that were expected to reduce the numbers of this pathogen in the chicken intestinal tract. TRP, which was identified as an anti-Campylobacter agent in the present study, is an herbal compound belonging to a unique chemical class with an indoloquinazoline structure that showed the lowest MIC values against C. jejuni compared to 13 plant molecules previously reported to exhibit anti-C. jejuni activities (Table 1). Furthermore, there have been no previous reports of the anti-C. jejuni and anti-C. coli activities of TRP, although this compound was shown to have antimicrobial activities against several bacteria, e.g., Escherichia coli (28), Bacillus subtilis (29), Staphylococcus aureus (30, 31), Mycobacterium tuberculosis (32, 33), and Helicobacter pylori (34). Among Campylobacter species, C. rectus, which is known as a periodontal pathogen, was the only species whose susceptibility to TRP was previously investigated (the MIC of TRP was 25 μg/mL, approximately 100 μM) (35). To our knowledge, this is the first report demonstrating the antimicrobial effect of TRP against both C. jejuni and C. coli.
TRP showed narrow-spectrum antimicrobial effects against C. jejuni and C. coli, i.e., the MICs of TRP for all strains of the two Campylobacter species were low (ranging from 0.78 to 12.5 μM), but those for most strains of C. fetus and all seven species of gut bacteria were greater than 100 μM (Table 2). Bandekar et al. investigated the effects of TRP and its nine derivatives on the growth and survival of the E. coli AS19 strain and found that 10 μg/mL (approximately 40 μM) TRP slowed growth and reduced the optical density at 650 nm (OD650) endpoint of E. coli by approximately 2-fold (28). However, we observed growth of the E. coli ATCC 23736 strain in the presence of 100 μM TRP (Table 2). In addition, the growth inhibition assay based on OD600 showed no significant difference in the growth of the E. coli ATCC 23736 strain with and without treatment with 100 μM TRP (data not shown). The difference in the results between these two studies may be due to the use of different strains in each experiment. Although further experiments are needed to determine the susceptibilities of various E. coli strains, TRP concentrations ranging from several dozens to hundreds of micromolar are considered to be required for the complete inhibition of E. coli growth. In contrast, TRP completely inhibited the growth of C. jejuni at concentrations lower than 5 or 6.25 μM in the present study (Table 1 and Fig. 1). These results suggested that C. jejuni is more susceptible to TRP than conventional gut bacteria. Johnson et al. reviewed the need for anti-Campylobacter compounds to be safe for both humans and livestock and to have narrow-spectrum effects to reduce the impact of these compounds on normal gut microbes (27). However, there have been no synthetic or natural compounds that satisfy these requirements. Although further studies on the effects of TRP on the microbiome in the chicken gut are needed, our results suggested that TRP has bacterial species-specific antimicrobial activity against C. jejuni and C. coli, with little effect on normal gut bacteria.
Our in vivo experiments demonstrated a remarkable antimicrobial effect of TRP that significantly reduced the numbers of cecal C. jejuni cells in infected chicks. Interestingly, a significant reduction was observed not only in experiment 1 (TRP treatment before C. jejuni infection) but also in experiment 2 (TRP treatment after C. jejuni infection), as shown in Fig. 4B. Our results suggested that TRP shows a bactericidal effect against C. jejuni in the chicken gut, and our in vitro experiments confirmed that relatively high TRP concentrations reduce the number of C. jejuni at its growth temperature (Fig. 1; Fig. 2A and B). Bactericidal effects of TRP and indigo plant extract on several bacteria other than Campylobacter, such as E. coli, Streptococcus mutans, Porphyromonas gingivaris, and Prevotella intermedia, have also been reported (28, 35). Therefore, TRP is suggested to be a promising agent for reducing the colonization of C. jejuni in the chicken gut.
Many studies have shown that plant-derived compounds and essential oils have antimicrobial properties against C. jejuni, but only a few of these compounds can significantly reduce the number of bacterial cells in the chicken gut (15). Arsi et al. investigated the application of carvacrol, thymol, and their combination as feed additives to prevent the cecal colonization of C. jejuni in chickens (16). Significant reductions (0.6 to 2.0 log10 CFU/mL) in C. jejuni were observed with the use of 1% carvacrol, 0.25% and 2% thymol, and 0.5% carvacrol and thymol combined. However, the body weight gains of the treated chickens were significantly reduced at higher concentrations (>0.5%) of both thymol and carvacrol. In contrast, our data demonstrated that cecal C. jejuni was significantly reduced, with reductions ranging from 0.7 to 1.2 log CFU/g cecal content, in chicks treated with 100 μM TRP (approximately 0.0025%). Importantly, there were no significant changes in body weight (P > 0.05) between the TRP-treated and control groups (see Fig. S1 in the supplemental material). These results indicated that TRP is a promising candidate for the control of C. jejuni without health hazards in chicken farms; however, the animal experiments in this study were limited to small sizes and short feeding periods. Further studies are necessary to evaluate the impacts of slaughter age and field conditions on the antimicrobial effect of TRP.
The antimicrobial mechanism of TRP remains unclear: Tripathi et al. reported that TRP showed a high affinity for an enoyl-acyl carrier protein reductase (InhA) in M. tuberculosis (33), while Bandekar et al. suggested that TRP and its derivatives bound DNA by intercalation in E. coli (28). In the present study, we evaluated the potential of C. jejuni to develop resistance to TRP by monitoring the changes in the MIC of TRP through continuous-passage cultures in the presence of TRP at sub-MICs. Because it was previously reported that C. jejuni developed resistance to EM and CPFX through in vitro plating experiments (36, 37), we compared the potential for developing resistance to TRP with that of developing resistance to EM and CPFX in the same manner. The results showed that TRP produced only small changes in the MIC values for both the C. jejuni NCTC 11168 and 81-176 strains, whereas EM and CPFX greatly increased the MIC values by >16,384- and 1,024-fold for the NCTC 11168 strain and by >32,768- and 4,096-fold for the 81-176 strain, respectively, after a 60-day experimental period (Fig. 3). Interestingly, a two- or three-step increase in the MICs of TRP was observed for both C. jejuni strains, suggesting that multiple low-level resistance-causing mutations occurred in the different responsible genes. Whole-genome sequencing analyses of TRP-resistant C. jejuni strains are being conducted to identify the antimicrobial resistance mechanism of TRP caused by these chromosomal mutations.
Recently, herbal plants and their extracts have been commonly used as feed and water additives in chicken farms worldwide. To date, TRP has been isolated from various plants, such as Persicaria tinctoria, Strobilanthes cusia, and Isatis tinctoria (18). The leaves of P. tinctoria and I. tinctoria were found to contain high TRP concentrations (38, 39); therefore, their extracts also exhibited antimicrobial effects (35, 40). These plants contain several antimicrobial compounds other than TRP, such as kaempferol, 6-methoxykaempferol, and 3,5,4′-trihydroxy-6,7-methylenedioxy flavone (41). Kataoka et al. reported that the combined oral administration of TRP and kaempferol tended to cause a further decrease in H. pylori cells in the stomach of Mongolian gerbils compared with the single administration of each compound, although no significant differences were observed (34). TRP shows an antimicrobial effect against C. jejuni at a low concentration and with a single administration to chickens, as shown in the present study, and it may be possible to achieve synergistic effects by combining TRP and other herbal compounds. Future research is expected to improve TRP-containing anti-C. jejuni agents and to develop feed or drinking water additives that are effective for the control of Campylobacter in chicken farms.
MATERIALS AND METHODS
Screening of anti-C. jejuni agents using a chemical compound library.
The validated compound library (consisting of 1,926 chemical compounds) obtained from the Drug Discovery Initiative (The University of Tokyo, Tokyo, Japan) was used for the screening of anti-C. jejuni agents. The chemical compounds were dissolved in 0.5 μL of dimethyl sulfoxide (DMSO) at a concentration of 2 mM in each well of a flat-bottom 96-well plate. Inhibition of the growth of C. jejuni was determined by a broth microdilution assay. Briefly, the C. jejuni 81-176 and 11-164 strains were grown in 3 mL of MH broth (Becton, Dickinson and Company, Sparks, MD, USA) overnight at 42°C under microaerophilic conditions. The bacterial culture was diluted with MH broth to a concentration of 106 CFU/well, which was set based on our previous study (42), and 99.5 μL of the dilution was added to each well of the 96-well plate, i.e., the final concentration of each compound was 10 μM. DMSO alone was used as a negative control, and 20 μg/mL chloramphenicol was used as a positive control. OD600 values were measured after incubating the 96-well plates for 72 h at 42°C under microaerobic conditions. The hit compounds were selected according to the screening methods of Kumar et al. (26). The growth inhibition rates (%) of C. jejuni strains were calculated as follows: 100 × (OD600 of the negative control − OD600 of the test compound)/(OD600 of the negative control − OD600 of the positive control). The compounds that inhibited the growth of the two C. jejuni strains at a rate of more than 99.0% were selected as hit compounds.
Susceptibility testing with a liquid dilution method.
In the present study, TRP, cinnamaldehyde, perillaldehyde, carvacrol, eugenol, rosmarinic acid, thymol, citral, cineole, linalool, p-cymene, and α-pinene were purchased from Fujifilm Wako Pure Chemical Co. (Osaka, Japan), and benzyl isothiocyanate and allyl isothiocyanate were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). These 14 herbal compounds were classified into six categories according to the ChEBI database (Table 1). The stock solution of each compound was prepared at 10 mM with DMSO (Fujifilm Wako Pure Chemical Co.). The stock solutions were diluted to a concentration of 800 μM in MH broth and serially diluted to concentrations ranging from 0.78 to 800 μM in 96-well plates. C. jejuni NCTC 11168 and 81-176 strains were inoculated into the diluted solutions at 106 CFU/well, and bacterial growth was evaluated after incubation in 96-well plates for 72 h at 42°C under microaerobic conditions.
To investigate the antibacterial spectrum of TRP, we used 28 Campylobacter and 7 non-Campylobacter strains (Table 2). The Campylobacter strains consisted of 20 C. jejuni, 4 C. coli, and 4 C. fetus strains. The non-Campylobacter strains consisted of gut bacteria, namely, E. coli, E. cloacae, K. pneumoniae, P. mirabilis, S. enterica, E. faecalis, and C. perfringens. The MIC values of TRP were determined by the standard microtiter broth dilution method. The stock solution of TRP was diluted to a concentration of 100 μM in MH broth and serially diluted to concentrations ranging from 0.1 to 100 μM in 96-well plates. Only the test for C. perfringens was conducted with dilutions of Brucella broth (Becton, Dickinson and Company) supplemented with 5 μg/mL hemin (Fujifilm Wako Pure Chemical Co.). The inoculum concentration of bacterial strains was 106 CFU/well. The inoculated plates were incubated at 42°C (for C. jejuni and C. coli) or 37°C (for C. fetus) for 72 h under microaerophilic conditions, 37°C for 24 h under aerobic conditions (for aerobic gut bacteria), or 37°C for 48 h under anaerobic conditions (for C. perfringens). The experiments were repeated three times, and the results of one representative experiment for each bacterial strain are shown in Table 2.
Time-kill assay under growing conditions.
To determine the time-kill kinetics of TRP against C. jejuni under growing conditions, 2-fold serial dilutions were prepared in MH broth ranging from 0.625 to 10 μM. The C. jejuni NCTC 11168 and 81-176 strains were inoculated into 3 mL of the diluted solutions and MH broth (0 μM TRP) at a concentration of 104 CFU/mL in sterile borosilicate tubes and incubated at 42°C under microaerophilic conditions. To quantify the number of viable bacteria, samples were obtained 4, 8, 12, 24, and 48 h after inoculation followed by serial dilutions, and 100 μL of each solution was spread onto MH agar (Becton, Dickinson and Company).
Time-kill assay under nongrowing conditions.
First, the growth of C. jejuni strains in MH broth at different temperatures was determined by the following procedure. The C. jejuni NCTC 11168 and 81-176 strains were inoculated as described in 3 mL of MH broth at a concentration of 104 CFU/mL and incubated at 4, 15, 25, and 37°C under microaerobic conditions. The number of bacteria was measured at 4, 8, and 12 h after inoculation as described above.
To determine the time-kill kinetics of TRP against C. jejuni under nongrowing conditions, the viable cells of the C. jejuni NCTC 11168 and 81-176 strains in the presence of TRP were measured at different temperatures. Tenfold serial dilutions of TRP from 0.1 to 100 μM were prepared in PBS. C. jejuni cells at a concentration of 106 CFU/mL were inoculated into 3 mL of the diluted solutions in sterile borosilicate tubes and incubated at 4, 15, 25, and 37°C under aerobic conditions. The number of viable cells was counted 4, 8, and 12 h after inoculation as described above.
Verification experiment for the development of drug resistance.
The development of the drug resistance of C. jejuni was verified by a previously described method (43) with some modifications. TRP, EM, and CPFX solutions were prepared in MH broth at concentrations ranging from 0.25 to 32 times the MICs of the C. jejuni NCTC 11168 and 81-176 strains. The ranges of the concentrations were modified depending on the results of each passage. EM and CPFX were obtained from Fujifilm Wako Pure Chemical Corporation. C. jejuni strains were inoculated into 500 μL of each of the dilutions of TRP, EM, or CPFX in 48-well plates and incubated at 42°C under microaerobic conditions. After 72 h of incubation, the bacterial cells at the second-highest concentration that showed growth were used for the subsequent culture. This subculture procedure was repeated 20 times.
Chick infection experiment.
Two types of chick infection experiments were conducted to determine the effect of TRP on the numbers of C. jejuni in the chicken intestinal tract. The experimental schedules are illustrated in Fig. 4A. Newly hatched 1-day-old chicks (L-M-6 strain) were obtained from Nisseiken Co., Ltd. (Tokyo, Japan). In experiment 1, TRP treatment was started 2 days before infection with the C. jejuni 11–164 strain. On the other hand, in experiment 2, TRP treatment was started 2 days after infection with the C. jejuni 11-164 strain. The treatment was performed by adding TRP to the drinking water at final concentrations of 10 and 100 μM, and the drinking water was changed every day. The chicks were allowed to freely drink water from a bell drinker. In both experiments, chicks were orally challenged with 106 CFU of the wild-type C. jejuni 11-164 strain through stomach tubes. Four chicks from each group were sacrificed at 11 and 18 days old, and the numbers of C. jejuni in their cecal contents were quantified by spreading serial dilutions on MH agar plates containing modified Preston Campylobacter selective supplement (catalog no. SR0204E; Oxoid Ltd., Basingstoke, UK) and 10 μg/mL nalidixic acid (Fujifilm Wako Pure Chemical Co.). These experiments were conducted in strict accordance with the guidelines of animal experimentation defined by the National Institute of Animal Health (NIAH), Japan. The protocol was approved by the committee on the Ethics of Animal Experiments of the NIAH (permit numbers 20-060 and 21-039).
Statistical analysis.
Differences in the results were tested using two-tailed unpaired Student's t tests. A P value of <0.05 was considered to indicate statistical significance (see the figure legends for specific values).
ACKNOWLEDGMENT
This research was partially supported by the Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research [BINDS]) from AMED under grant number JP21am0101086.
Footnotes
Supplemental material is available online only.
Contributor Information
Masahiro Kusumoto, Email: kusu555@affrc.go.jp.
Edward G. Dudley, The Pennsylvania State University
REFERENCES
- 1.Kirk MD, Pires SM, Black RE, Caipo M, Crump JA, Devleesschauwer B, Dopfer D, Fazil A, Fischer-Walker CL, Hald T, Hall AJ, Keddy KH, Lake RJ, Lanata CF, Torgerson PR, Havelaar AH, Angulo FJ. 2015. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLoS Med 12:e1001921. 10.1371/journal.pmed.1001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Food Safety Authority. 2011. Scientific opinion on Campylobacter in broiler meat production: control options and performance objectives and/or targets at different stages of the food chain. EFSA J 9:2105. 10.2903/j.efsa.2011.2105. [DOI] [Google Scholar]
- 3.Kaakoush NO, Castano-Rodriguez N, Mitchell HM, Man SM. 2015. Global epidemiology of Campylobacter infection. Clin Microbiol Rev 28:687–720. 10.1128/CMR.00006-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skarp CPA, Hanninen ML, Rautelin HIK. 2016. Campylobacteriosis: the role of poultry meat. Clin Microbiol Infect 22:103–109. 10.1016/j.cmi.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Wagenaar JA, French NP, Havelaar AH. 2013. Preventing Campylobacter at the source: why is it so difficult? Clin Infect Dis 57:1600–1606. 10.1093/cid/cit555. [DOI] [PubMed] [Google Scholar]
- 6.European Food Safety Authority. 2022. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2019–2020. EFSA J 20:e07209. 10.2903/j.efsa.2022.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention National Antimicrobial Resistance Monitoring System (NARMS). 2018. NARMS 2015 human isolates surveillance report. https://www.cdc.gov/narms/pdf/2015-NARMS-Annual-Report-cleared_508.pdf.
- 8.Bahrndorff S, Garcia AB, Vigre H, Nauta M, Heegaard PM, Madsen M, Hoorfar J, Hald B. 2015. Intestinal colonization of broiler chickens by Campylobacter spp. in an experimental infection study. Epidemiol Infect 143:2381–2389. 10.1017/S0950268814003239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sibanda N, McKenna A, Richmond A, Ricke SC, Callaway T, Stratakos AC, Gundogdu O, Corcionivoschi N. 2018. A review of the effect of management practices on Campylobacter prevalence in poultry farms. Front Microbiol 9:2002. 10.3389/fmicb.2018.02002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herman L, Heyndrickx M, Grijspeerdt K, Vandekerchove D, Rollier I, De Zutter L. 2003. Routes for Campylobacter contamination of poultry meat: epidemiological study from hatchery to slaughterhouse. Epidemiol Infect 131:1169–1180. 10.1017/s0950268803001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nauta MJ, Jacobs-Reitsma WF, Havelaar AH. 2007. A risk assessment model for Campylobacter in broiler meat. Risk Anal 27:845–861. 10.1111/j.1539-6924.2006.00834.x. [DOI] [PubMed] [Google Scholar]
- 12.Hermans D, Van Deun K, Messens W, Martel A, Van Immerseel F, Haesebrouck F, Rasschaert G, Heyndrickx M, Pasmans F. 2011. Campylobacter control in poultry by current intervention measures ineffective: urgent need for intensified fundamental research. Vet Microbiol 152:219–228. 10.1016/j.vetmic.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Rosenquist H, Nielsen NL, Sommer HM, Nørrung B, Christensen BB. 2003. Quantitative risk assessment of human campylobacteriosis associated with thermophilic Campylobacter species in chickens. Int J Food Microbiol 83:87–103. 10.1016/s0168-1605(02)00317-3. [DOI] [PubMed] [Google Scholar]
- 14.Dai L, Sahin O, Grover M, Zhang Q. 2020. New and alternative strategies for the prevention, control, and treatment of antibiotic-resistant Campylobacter. Transl Res 223:76–88. 10.1016/j.trsl.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Micciche A, Rothrock MJ, Jr, Yang Y, Ricke SC. 2019. Essential oils as an intervention strategy to reduce Campylobacter in poultry production: a review. Front Microbiol 10:1058. 10.3389/fmicb.2019.01058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arsi K, Donoghue AM, Venkitanarayanan K, Kollanoor-Johny A, Fanatico AC, Blore PJ, Donoghue DJ. 2014. The efficacy of the natural plant extracts, thymol and carvacrol against Campylobacter colonization in broiler chickens. J Food Saf 34:321–325. 10.1111/jfs.12129. [DOI] [Google Scholar]
- 17.Szott V, Reichelt B, Alter T, Friese A, Roesler U. 2020. In vivo efficacy of carvacrol on Campylobacter jejuni prevalence in broiler chickens during an entire fattening period. Eur J Microbiol Immunol (Bp) 10:131–138. 10.1556/1886.2020.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jahng Y. 2013. Progress in the studies on tryptanthrin, an alkaloid of history. Arch Pharm Res 36:517–535. 10.1007/s12272-013-0091-9. [DOI] [PubMed] [Google Scholar]
- 19.Dufour V, Alazzam B, Ermel G, Thepaut M, Rossero A, Tresse O, Baysse C. 2012. Antimicrobial activities of isothiocyanates against Campylobacter jejuni isolates. Front Cell Infect Microbiol 2:53. 10.3389/fcimb.2012.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedman M, Henika PR, Mandrell RE. 2002. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J Food Prot 65:1545–1560. 10.4315/0362-028x-65.10.1545. [DOI] [PubMed] [Google Scholar]
- 21.Kurekci C, Padmanabha J, Bishop-Hurley SL, Hassan E, Al Jassim RA, McSweeney CS. 2013. Antimicrobial activity of essential oils and five terpenoid compounds against Campylobacter jejuni in pure and mixed culture experiments. Int J Food Microbiol 166:450–457. 10.1016/j.ijfoodmicro.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Duarte A, Luís Â, Oleastro M, Domingues FC. 2016. Antioxidant properties of coriander essential oil and linalool and their potential to control Campylobacter spp. Food Control 61:115–122. 10.1016/j.foodcont.2015.09.033. [DOI] [Google Scholar]
- 23.Kovač J, Šimunović K, Wu Z, Klančnik A, Bucar F, Zhang Q, Možina SS. 2015. Antibiotic resistance modulation and modes of action of (-)-α-pinene in Campylobacter jejuni. PLoS One 10:e0122871. 10.1371/journal.pone.0122871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Šimunović K, Bucar F, Klančnik A, Pompei F, Paparella A, Smole Možina S. 2020. In vitro effect of the common culinary herb winter savory (Satureja montana) against the infamous food pathogen Campylobacter jejuni. Foods 9:537. 10.3390/foods9040537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson JG, Yuhas C, McQuade TJ, Larsen MJ, DiRita VJ. 2015. Narrow-spectrum inhibitors of Campylobacter jejuni flagellar expression and growth. Antimicrob Agents Chemother 59:3880–3886. 10.1128/AAC.04926-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar A, Drozd M, Pina-Mimbela R, Xu X, Helmy YA, Antwi J, Fuchs JR, Nislow C, Templeton J, Blackall PJ, Rajashekara G. 2016. Novel anti-Campylobacter compounds identified using high throughput screening of a pre-selected enriched small molecules library. Front Microbiol 7:405. 10.3389/fmicb.2016.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson TJ, Shank JM, Johnson JG. 2017. Current and potential treatments for reducing Campylobacter colonization in animal hosts and disease in humans. Front Microbiol 8:487. 10.3389/fmicb.2017.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bandekar PP, Roopnarine KA, Parekh VJ, Mitchell TR, Novak MJ, Sinden RR. 2010. Antimicrobial activity of tryptanthrins in Escherichia coli. J Med Chem 53:3558–3565. 10.1021/jm901847f. [DOI] [PubMed] [Google Scholar]
- 29.Honda G, Tabata M, Tsuda M. 1979. The antimicrobial specificity of tryptanthrin. Planta Med 37:172–174. 10.1055/s-0028-1097320. [DOI] [PubMed] [Google Scholar]
- 30.Kawakami J, Kakinami H, Matsushima N, Nakane A, Kitahara H, Nagaki M, Ito S. 2013. Structure–activity relationship analysis for antimicrobial activities of tryptanthrin derivatives using quantum chemical calculations. J Comput Chem Jpn 12:109–112. 10.2477/jccj.2012-0026. [DOI] [Google Scholar]
- 31.Kawakami J, Matsushima N, Ogawa Y, Kakinami H, Nakane A, Kitahara H, Nagaki M, Ito S. 2011. Antibacterial and antifungal activities of tryptanthrin derivatives. Trans Mat Res Soc Japan 36:603–606. 10.14723/tmrsj.36.603. [DOI] [Google Scholar]
- 32.Mitscher LA, Baker W. 1998. Tuberculosis: a search for novel therapy starting with natural products. Med Res Rev 18:363–374. . [DOI] [PubMed] [Google Scholar]
- 33.Tripathi A, Wadia N, Bindal D, Jana T. 2012. Docking studies on novel alkaloid tryptanthrin and its analogues against enoyl-acyl carrier protein reductase (InhA) of Mycobacterium tuberculosis. Indian J Biochem Biophys 49:435–441. [PubMed] [Google Scholar]
- 34.Kataoka M, Hirata K, Kunikata T, Ushio S, Iwaki K, Ohashi K, Ikeda M, Kurimoto M. 2001. Antibacterial action of tryptanthrin and kaempferol, isolated from the indigo plant (Polygonum tinctorium Lour.), against Helicobacter pylori-infected Mongolian gerbils. J Gastroenterol 36:5–9. 10.1007/s005350170147. [DOI] [PubMed] [Google Scholar]
- 35.Iwaki K, Koya-Miyata S, Kohno K, Ushio S, Fukuda S. 2006. Antimicrobial activity of Polygonum tinctorium Lour: extract against oral pathogenic bacteria. J Nat Med 60:121–125. 10.1007/s11418-005-0025-z. [DOI] [Google Scholar]
- 36.Hanninen ML, Hannula M. 2007. Spontaneous mutation frequency and emergence of ciprofloxacin resistance in Campylobacter jejuni and Campylobacter coli. J Antimicrob Chemother 60:1251–1257. 10.1093/jac/dkm345. [DOI] [PubMed] [Google Scholar]
- 37.Lin J, Yan M, Sahin O, Pereira S, Chang YJ, Zhang Q. 2007. Effect of macrolide usage on emergence of erythromycin-resistant Campylobacter isolates in chickens. Antimicrob Agents Chemother 51:1678–1686. 10.1128/AAC.01411-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iwaki K, Fujii M, Tatefuji T, Shibuya T, Ohta T, Fukuda S. 2005. Tryptanthrin content in Polygonum tinctorium leaves. ITE Lett Batteries New Technol Med 6:560–565. [Google Scholar]
- 39.Oberthür C, Hamburger M. 2004. Tryptanthrin content in Isatis tinctoria leaves-a comparative study of selected strains and post-harvest treatments. Planta Med 70:642–645. 10.1055/s-2004-827188. [DOI] [PubMed] [Google Scholar]
- 40.Honda G, Tosirisuk V, Tabata M. 1980. Isolation of an antidermatophytic, tryptanthrin, from indigo plants, Polygonum tinctorium and Isatis tinctoria. Planta Med 38:275–276. 10.1055/s-2008-1074877. [DOI] [PubMed] [Google Scholar]
- 41.Hashimoto T, Aga H, Chaen H, Fukuda S, Kurimoto M. 1999. Isolation and identification of anti-Helicobacter pylori compounds from Polygonum tinctorium Lour. Nat Med 53:27–31. [Google Scholar]
- 42.Iwata T, Chiku K, Amano K, Kusumoto M, Ohnishi-Kameyama M, Ono H, Akiba M. 2013. Effects of lipooligosaccharide inner core truncation on bile resistance and chick colonization by Campylobacter jejuni. PLoS One 8:e56900. 10.1371/journal.pone.0056900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang Y, Hang X, Jiang X, Zeng L, Jia J, Xie Y, Li F, Bi H. 2019. In vitro and in vivo activities of zinc linolenate, a selective antibacterial agent against Helicobacter pylori. Antimicrob Agents Chemother 63:e00004-19. 10.1128/AAC.00004-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Download aem.01701-22-s0001.pdf, PDF file, 0.1 MB (88.4KB, pdf)