Abstract
Aim
To assess and compare the effectiveness of midazolam vs midazolam and ketamine combination in the management of young uncooperative pediatric patients.
Materials and methods
The research question was developed by using population, intervention, comparison, outcome, and study design framework. The literature search was performed using three electronic databases: PubMed, Scopus, and EBSCOhost. The risk of bias of the studies was independently appraised using the Cochrane Handbook for Systematic Reviews of Interventions.
Results
Out of 98 preliminary records, five studies were selected for analysis. Three hundred forty-six uncooperative children were randomized through the five randomized controlled trials (RCTs), with a mean age of 5.8 years. Midazolam with ketamine was the most successful combination for delivering rapid and sufficient analgosedation in uncooperative children. The clinical efficiency of midazolam and ketamine combination had an overall success rate of 84% when compared to ketamine and midazolam alone. 50% of children in the midazolam and ketamine group demonstrated calm behavior, compared to 37% in the midazolam group. 44% of the children experienced modest intra and/or postoperative adverse effects that did not necessitate any special treatment.
Conclusion
Midazolam and ketamine combination is more efficient than midazolam alone with respect to ease of treatment and clinical efficiency.
How to cite this article
Rathi GV, Padawe D, Takate V, et al. Comparative Evaluation of Ease of Dental Treatment and Clinical Efficiency of Midazolam vs Midazolam and Ketamine Combination for Sedation in Young Uncooperative Pediatric Patients: A Systematic Review. Int J Clin Pediatr Dent 2022;15(6):680-686.
Keywords: Clinical efficiency, Ease of dental treatment in uncooperative children, Midazolam, Midazolam and ketamine combination
Introduction
Worldwide, millions of children under the age of six are affected with early childhood caries (ECC).1–3 ECC is a variety of decay that alters children's teeth and is one of the most frequent dental illnesses today, causing discomfort, infection, a higher chance of developing dental cavities in primary and permanent teeth, and, finally, detrimental implications for permanent tooth eruption. These symptoms can range from demineralization to tooth structural loss or whole crown disintegration, indicating a dynamic and active decay process with alternating stages of destruction and repair.4–6
Half of all children worldwide have one or more carious primary teeth by the end of toddlerhood, but the importance of these teeth should not be overlooked because, as previously stated, they play an important role in the eruption of healthy permanent teeth, good nutrition, and one's esthetic appearance.5,6 In research published in 2018, Ganesh et al. found that the average prevalence of ECC in India was 49.6%. With a frequency of 63%, Andhra Pradesh has the greatest prevalence of ECC, while Sikkim has the lowest (41.92%).7
Dental treatment using rotary burs under local anesthesia (non-pharmacological treatment approach)8 and/or general anesthesia (GA) (pharmacological treatment approach)9 are used to mitigate this negative impact. For a variety of causes, dental care has been seen as an anxiety-inducing stigma by many children. Preschoolers, dental personnel, and dental care may all suffer as a result of dental fear. Many clinical studies have observed that kids with more levels of dental anxiety had more carious, fracture, and restored tooth surfaces than children with lower levels.10–12 As a result, various behavior management techniques are used to control this anxiety.
Many parents/guardians, refuse to comply with treatment under local anesthesia because of their children's fear of injection and associated behavior management issues. On the contrary, GA is often known to be a very expensive procedure and it necessitates the use of skilled personnel such as anesthetists, specialist nurses, and a variety of other services.13 In these circumstances, sedation can be seen as a means of mitigating discomfort and making dental care more convenient.
Sedatives include nitrous oxide, midazolam and other benzodiazepines, various sedative-hypnotics, and psychosedative drugs taken orally or systemically.14 According to Ashley et al.'s meta-analysis, the use of oral midazolam in doses ranging from 0.25 to 1 mg/kg is correlated with more cooperative behavior than placebo.13 Midazolam, on the contrary, is a quick-acting anxiolytic with a short half-life, making it only appropriate for minor dental treatment procedures. As a result, it is advantageous to locate a second sedative agent that may be taken in conjunction with midazolam to apply its own favorable effects (sedation and analgesia) to the clinical situation.15–17 Ketamine is one such sedative that is frequently used in conjunction with midazolam.
Oral sedation using the midazolam and ketamine relationship is safe and effective in several dental studies.18 According to Roelofse et al., the sedative effect of midazolam (1 mg) alone was much greater than the midazolam (0.35 mg) and ketamine combination.19 The pharmaceutical mixture's most notable benefit is that it minimizes the need for greater drug dosages. The primary goal of premedication is to produce drowsiness and anxiolysis to facilitate clinical and diagnostic procedures. Because midazolam is not often the sole sedative used for procedural sedation, it is worth looking into literature, if any clinical studies that show how midazolam compares to a combination of midazolam and ketamine in lowering dental anxiety in children. To our knowledge, no complete sedation evaluation has been undertaken prior to pediatric dental procedures.
Hence, the present review aimed at assessing and comparing the effectiveness of midazolam vs midazolam and ketamine combination in the management of young uncooperative pediatric patients in terms of ease of treatment and clinical efficiency.
Materials and Methods
Protocol Registration
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)20 protocols were referred to conduct this review and the same was registered at National Institute for Health Research PROSPERO International Prospective Register for Systematic Reviews (CRD42021236700).
Review Question
The population (P), intervention (I), comparison (C), and outcome (0) framework were used to generate the research question. In the management of uncooperative children (P), is midazolam (I) more effective than midazolam and ketamine combination (C) with respect to ease of treatment and clinical efficiency?
Search Strategy
Electronic databases like EBSCOhost, Cochrane Library, and PubMed were searched. Methodological Medical Subject Heading (MeSH) words were constructed using the PICO-formatted question. For each database, these techniques were tweaked as needed. The Cochrane Highly Sensitive Search Technique (CHSSS) was used to locate randomized trials, and the search strategy included controlled vocabulary and free text words (RCTs).
Inclusion Criteria
The inclusion criteria for the present review were—peer-reviewed scientific journals from 2005 to 2020, full articles in English, RCTs, studies that compared midazolam and ketamine combination vs midazolam and any other group, schoolchildren of any age-group, having the physical status of American Society of Anesthesiologists (ASA) type I, having at least two carious teeth without any pulp involvement, and parents willing to give consent.
Exclusion Criteria
The exclusion criteria for the present review were—reviews, manuscripts with incomplete data, cross-sectional studies, case reports, case series, animal studies or in vitro studies, abstracts, parents who did not wish to participate, articles in any other language except English, and definite positive behavior toward dental treatment.
Study Selection and Data Extraction Process
Based on the defined inclusion and exclusion norms, two reviewers (RG and PD) independently reviewed the title and abstract of the selected papers. The reviewers read the papers separately and extracted the data using a data extraction form created specifically for this study. The following information was included on this form—author's name, year of publication, place, sample size, age of children, intervention, comparator, ease of treatment completion, sedation score, onset of sedation, recovery time, adverse effects, and overall success. Any discrepancies between the two reviewers were addressed by consulting a third reviewer (TV).
Quality Assessment of Included Studies
This study's bias risk was assessed following the methods specified in the Cochrane Handbook for Systematic Reviews of Interventions.21 Random sequence creation, allocation concealment, blinding tested in three groups: participant, operator, and outcome assessor, inadequate outcome data, selective reporting, and other biases were all considered.
Results
Study Selection Process
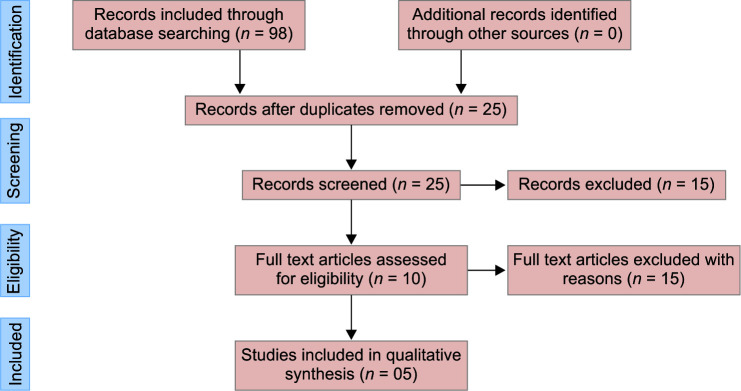
Figure 1 depicts the search results and study selection procedure. There were 98 preliminary records found in the electronic database searches: 48 from EBSCHOhost, 31 from The Cochrane Central Register of Controlled Trials, and 19 from Medline. When the references of the resulting papers were screened, no more acceptable research was located. The reference management program was used to manually remove duplicates, resulting in 73 records. After reading the title and abstract, 68 were eliminated. The full-text screening was used to analyze the resultant five publications, and they were all eligible for qualitative synthesis. Table 1 outlines the technique followed by the included studies to evaluate and compare the efficacy of midazolam with midazolam and ketamine combination in the management of young uncooperative pediatric patients. The chosen studies were carried out in the following three countries: Brazil (n = 3, 60%), India (1), and Nepal (1).
Fig 1.
PRISMA flow diagram
Table 1.
Characteristics of included studies
| Author/Year/Place | Sample size (M/F) | Age | Intervention/Dose (M) | Comparator/Dose (MK) | Ease of treatment completion/behavior of child during treatment | Sedation score | Onset of sedation | Recovery time | Adverse effects | Overall success |
|---|---|---|---|---|---|---|---|---|---|---|
| Koirala/200622/Nepal | 120 | 2–9 years | Midazolam: 0.5 mg/kg orally | Combination of midazolam 0.4 mg/kg and ketamine 3 mg/kg orally | MK: most convenient | MK: best sedation | MK: shortest time required | M: shortest | None | – |
| Bahetwar/201123/India | 45 (M: 22, F: 23) | 2–6 years | Midazolam: 0.3 mg/kg intranasally | MK (0.2 and 4 mg/kg, respectively) | M: 72% MK: 84% |
M: 84% MK: 89% |
M: 5–10 minutes MK: 4–10 minutes |
M: 26–40 minutes MK: 35–49 minutes |
Vomiting MK: 1 |
M: 69% MK: 84% |
| Moreira/201318/Brazil | 41 | Under 36 months | Oral midazolam (MS) at a dose of 1.0 mg⁄kg | MK [a combination of oral midazolam (0.5 mg⁄ kg) and ketamine (3 mg⁄ kg)] | OSUBRS score During dental exam PS: 12.5 ± 5.5 MS: 13.6 ± 4.5 MK: 12.6 ± 4.4 During treatment PS: 12.5 ± 5.2 MS: 14.0 ± 3.8 MK: 8.6 ± 4.1 |
MK: longer and effective session (p = 0.04) | – | – | Agitation MK: 1 Vomiting MK: 4 |
– |
| Antunes/201624/Brazil | 56 | Under 4 years | Moderate sedation with midazolam (dose 1 mg/kg, maximum 20 mg) administered orally | Moderate sedation with the association of midazolam (0.5 mg/kg, maximum 20 mg) and ketamine (3 mg/kg dose, maximum 50 mg) | OSUBRS score Quiet behavior M: 2.9 times MK: 4.3 times |
– | – | – | No adverse events | |
| Sado-Filho/201925/Brazil | 84 (M: 43, F: 41) |
Under 7 years | Oral midazolam (MO) (1.0 mg/kg, maximum 20.0 mg) | KMO: combination of ketamine (4.0 mg/kg, maximum 100.0 mg) and midazolam (0.5 mg/kg, maximum 5.0 mg) by oral route KMIN: combination of intranasal ketamine (4.0 mg/kg, maximum 100.0 mg) and midazolam (0.2 mg/kg, maximum 5.0 mg) |
OSUBRS score Quiet behavior MO: 10.7% KMO: 42.9% KMIN: 21.4% |
– | – | – | MO: 39% KMO: 53.6% KMIN: 39.3% |
MO: 32.1% KMO: 46.4% KMIN: 50.0% |
Characteristics of Included Studies (Table 1)
Children in the trials varied in age from 1 to 10 years. All the RCTs had a mean age of 5.8 years (approximately). In total, 346 uncooperative children were randomized through the five RCTs. Children in all the RCTs were found to be uncooperative at the start of the study. Three of the included RCTs documented the use of restraint devices like papoose boards or pedi wrap to protect or restrain children during dental procedures.18,22–25
Drugs used were midazolam alone vs midazolam and ketamine combination, which was administered orally or intranasally. However, just one study compared midazolam and ketamine to a placebo.19 Intranasal administration was done using an insulin injection syringe without a needle.24 During intraoral administration, to mask the bitter taste of midazolam, sweetening agents were added.23,25
In 2006, Koirala et al.22 reported that the therapy was more easily administered in the midazolam and ketamine population when compared to midazolam alone. In addition, the time required for sedation to begin was shortest in the midazolam and ketamine group. Midazolam when used alone, had the quickest recovery time. The midazolam and ketamine combination had the highest sedation score. The present RCT concluded that midazolam with ketamine was the most successful combination for delivering rapid and sufficient analgosedation in uncooperative children.
Bahetwar et al.,23 in 2011, reported that the time needed for the onset of sedation differed significantly between the three groups: midazolam, ketamine, and combination. Children recovered quickly when sedated with midazolam alone and slowest when sedated with midazolam and ketamine combination. The midazolam and ketamine combination had an overall success rate of 84% when compared to ketamine and midazolam alone.
When sedatives were used in groups of midazolam and ketamine combination and midazolam alone during therapy sessions, Moreira et al.18 found that the children in the combination group had the least Ohio State University Behavioral Rating Scale (OSUBRS) ratings, indicating that they behaved better than the children in the midazolam alone group (p = 0.003) or no sedation group (p = 0.03). The mean OSUBRS scores in the midazolam and ketamine combination group did not differ significantly from the behavior seen in the same children during the treatment session (repeated measurements) (p = 0.06).
Antunes et al.,24 in 2016, concluded that the children who received sedation with midazolam displayed totally quiet behavior during follow-up (2.9 times more). The trial also observed that children who received midazolam and ketamine combination were 4.3 times more likely to display cooperative behavior. The authors also revealed that 10 months after the baseline consultation, 50% of children in the midazolam and ketamine group demonstrated calm behavior, compared to 37% in the midazolam group, 25% in the general anesthetic group, and 17% in the no-drug group.
Sado-Filho et al.,25 in 2019 conducted a study to evaluate the rate of success by the dichotomous variable “quiet behavior for at least 60% of the session length.” Based on this parameter, the highest success was reported in intranasal midazolam and ketamine combination (50%) followed by oral midazolam and ketamine combination (46.4%). The lowest success rate was reported when midazolam was used alone (32.1%).
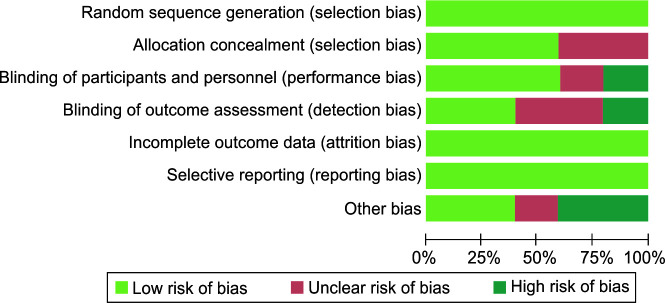
Quality Assessment
None of the papers included in this study were rated as low risk. At some of the factors that were evaluated, all five trials exhibited an unknown risk of bias. In this systematic review, only two studies had a high risk of bias (Table 2 and Fig. 2).
Table 2.
Quality assessment of included studies
| Author name, year | Selection bias | Reporting bias | Others | Performance bias | Attrition bias | ||
|---|---|---|---|---|---|---|---|
| Random sequence generation | Allocation concealment | Blinding participants and personnel | Blinding outcome | ||||
| Koirala, 200622 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Bahetwar, 201123 | Low risk | Unclear | Low risk | Low risk | Low risk | Low risk | Low risk |
| Moreira, 201318 | Low risk | Unclear | Low risk | Low risk | High risk | High risk | Low risk |
| Antunes, 201624 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Sado- Filho, 201925 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
Fig 2.
Quality assessment of included studies
Discussion
Early childhood caries is a worldwide oral health issue that continues to concern toddlers and preschool children all over the world. It is a multifactorial disease that develops over time as a function of the dynamic combination of three causes: vulnerable tooth surfaces, plaque bacteria, and carbohydrate diet.26–29 This disease's trend is mainly related to feeding habits.30,31 There may be other risk factors that are specific to the age-group. These include early mutans streptococci colonization and on-demand breastfeeding and snacking, which establishes an atmosphere that promotes the growth and development of mutans streptococci in dental plaque. Other risk factors for the growth of ECC include low socioeconomic status, as well as social determinants such as mother's educational level and cultural attitudes.32–34
Providing effective dental services to children with ECC can be a difficult task. Most children accept dental treatment with ease. But anxious, frightened, and uncooperative toddlers and preschoolers, as well as those who exhibit disruptive behavior, need behavior modification prior to dental procedures.35 Because of lack of psychological and emotional maturity, children under the age of three are not likely to cooperate with invasive procedures. From childhood through age 2 or 3 years, these children have underdeveloped cognitive abilities, a severely restricted repertoire of coping capacities, short to insignificant attention spans, and essentially no experience coping with anxiety.36
Traditional restorative techniques involve the use of rotary handpieces, burs, and local anesthesia for symptomatic management.37 These procedures are most commonly associated with dental anxiety, which is “a sensation of concern regarding dental treatment that is not necessarily tied to a specific external stimuli.”38 Protective stabilization, sedation, and GA are specialized behavior guidance methods that are widely used and taught in pediatric dental educational programs.39 Protective stabilization, according to the American Academy of Pediatric Dentistry (AAPD) recommendations, can lead to substantial effects such as bodily or psychological injury, lack of dignity, and any infringement of the patient's rights.40 Pediatric dentists frequently worry about how to deliver the most beneficial treatment, while limiting the danger of a traumatic dental experience for the kid. Hence, moderate sedation is considered to be more safe behavior management method for such children.
A number of sedative drugs, doses, and administration techniques have been researched in pediatric dentistry in an attempt to find the best and safest alternative. Midazolam is the sedative agent, routinely preferred in such situations. It is a benzodiazepine that has a quick onset of action. It has anxiolytic, muscle relaxant, and anticonvulsant properties, and is now most often used in children to induce anterograde amnesia and avoid prolonged seizures. However, it has a short duration of action and lacks an analgesic effect.41–45
Premedication protocol that combined midazolam's anxiolytic action with ketamine's analgesic function resulted in improved pediatric behavior than both medications alone, according to research in general pediatrics.46–49 Various studies reported that oral sedation with midazolam and ketamine combination is the safest and most efficient in pediatric dentistry in the management of young uncooperative children.
A double-blind trial was performed by Koirala et al.22 to assess the safety and effectiveness of orally administered newer sedatives and analgesics for conscious sedation in 120 children. No complications arose in any patient subjected to midazolam and ketamine combination. The author further concluded that if ketamine cannot be used, midazolam and tramadol combination can be used, and if only a single agent is permissible then midazolam is the ideal choice of sedative.
Bahetwar et al.23 conducted a study involving 45 uncooperative children (2–6 years) to assess and compare the safety and efficiency of intranasal administration of midazolam, ketamine, and combination to produce moderate sedation. A statistically significant difference was observed between midazolam and ketamine groups, with respect to the onset of sedation (p < 0.01). Midazolam (69%), ketamine (89%), and midazolam and ketamine combination (84%) displayed a statistically significant difference in success rates. Hence, the study concluded that intranasal ketamine displayed the highest success rate in modifying the behavior of uncooperative children to accept treatment.
Moreira et al.18 conducted a randomized controlled experiment to see if children's behavior (36 months) improves following treatment with oral midazolam and ketamine combination vs midazolam alone vs no sedation. The authors were able to perform more restorations in a single appointment, as midazolam and ketamine combination allowed the operators to extend the appointments easily. According to the authors, the most probable reason for this finding is that ketamine possesses an analgesic effect, which may have improved the value of midazolam sedation without compromising the upper airway reflex.
Antunes et al.24 assessed the children's behavior in consecutive dental appointments using different behavior management techniques (no sedation, oral sedation with midazolam, oral sedation with midazolam and ketamine combination, or GA) for behavior control. The phenomenon of “implicit memory” was also used to explain the long-term effects of ketamine as a sedative in this study. Midazolam has an effect on the patient's memory in terms of perceptual recognition and facilitation. Children who have received a modest dosage of midazolam remain alert to verbal and tactile stimulation but fail to recognize photographs presented after the sedative has been delivered (“explicit memory”).
Sado-Filho et al.25 carried out a randomized, controlled, parallel-design trial to assess the efficiency of intranasal midazolam and ketamine combination, oral midazolam and ketamine combination, and oral midazolam as the key factor of the behavioral guiding technique during dental treatment for preschoolers. According to the experiment, 44% of the children experienced modest intra and/or postoperative adverse effects that did not necessitate any special treatment.
Conclusion
The present systematic review concludes that the midazolam and ketamine combination is more efficient than midazolam alone with respect to ease of treatment and clinical efficiency.
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Kassebaum NJ, Smith AGC, Bernabé E, et al. Global, regional, and national prevalence, incidence, and disability-adjusted life years for oral conditions for 195 countries, 1990–2015: a systematic analysis for the global burden of diseases, injuries, and risk factors. J Dent Res. 2017;96(4):380–387. doi: 10.1177/0022034517693566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tinanoff N, Baez RJ, Diaz Guillory C, et al. Early childhood caries epidemiology, aetiology, risk assessment, societal burden, management, education, and policy: global perspective. Int J Paediatr Dent. 2019;29(3):238–248. doi: 10.1111/ipd.12484. [DOI] [PubMed] [Google Scholar]
- 3.Pitts N, Baez R, Diaz-Guallory C, et al. Early childhood caries: IAPD Bangkok declaration. Int J Paediatr Dent. 2019;29:384–386. doi: 10.1111/ipd.12490. [DOI] [PubMed] [Google Scholar]
- 4.Nematollahi H, Mehrabkhani M, Sheykhani M. Assessing the relationship between diet and prevalence of early childhood caries in Birjand preschool children. J Dent. 2007;8(1):70–85. [Google Scholar]
- 5.Wagle M, D'Antonio F, Reierth E, et al. Dental caries and preterm birth: a systematic review and meta-analysis. BMJ Open. 2018;8(3):e018556. doi: 10.1136/bmjopen-2017-018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broumand S, Sharififar S, Alikhani S. The study of caries free indicator of milk teeth in children age 3–6 at dare care center affiliated to health centers of army. 2006 [Google Scholar]
- 7.Ganesh A, Muthu MS, Mohan A, et al. Prevalence of early childhood caries in India—a systematic review. Indian J Pediatr. 2019;86(3):276–286. doi: 10.1007/s12098-018-2793-y. [DOI] [PubMed] [Google Scholar]
- 8.Dorri M, Martinez, -, Zapata MJ, Walsh T, et al. Atraumatic restorative treatment versus conventional restorative treatment for managing dental caries. Cochrane Database Syst Rev. 2017;12(12):CD008072. doi: 10.1002/14651858.cd008072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knapp R, Gilchrist F, Rodd HD, et al. Change in children's oral health-related quality of life following dental treatment under general anesthesia for the management of dental caries: a systematic review. Int J Paediatr Dent. 2017;27(4):302–312. doi: 10.1111/ipd.12259. [DOI] [PubMed] [Google Scholar]
- 10.Rantavuori K, Lahti S, Hausen H, et al. Dental fear and oral health and family characteristics of Finnish children. Acta Odontol Scand. 2004;62(4):207–213. doi: 10.1080/00016350410001586. [DOI] [PubMed] [Google Scholar]
- 11.Nicolas E, Bessadet M, Collado V, et al. Factors affecting dental fear in french children aged 5–12 years. Int J Pediatr Dent. 2010;20(5):366–373. doi: 10.1111/j.1365-263X.2010.01054.x. [DOI] [PubMed] [Google Scholar]
- 12.Townend E, Dimigen G, Fung D. A clinical study of child dental anxiety. Behav Res Ther. 2000;38(1):31–46. doi: 10.1016/s0005-7967(98)00205-8. [DOI] [PubMed] [Google Scholar]
- 13.Ashley PF, Chaudhary M, Lourenço-Matharu L. Sedation of children undergoing dental treatment. Cochrane Database Syst Rev. 2018;12(12):CD003877. doi: 10.1002/14651858.cd003877.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conway A, Rolley J, Sutherland JR. Midazolam for sedation before procedures. Cochrane Database of Syst Rev. 2016;2016(5):CD009491. doi: 10.1002/14651858.cd009491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies FC, Waters M. Oral midazolam for conscious sedation of children during minor procedures. J Accid Emerg Med. 1998;15(4):244–248. doi: 10.1136/emj.15.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dionne R. Oral midazolam syrup: a safer alternative for pediatric sedation. Compend Contin Educ Dent. 1999;20(3):221–222. [PubMed] [Google Scholar]
- 17.Nathan JE, Vargas KG. Oral midazolam with and without meperidine for management of the difficult young pediatric dental patient. A retrospective study. Pediatr Dent. 2002;24(2):129–138. [PubMed] [Google Scholar]
- 18.Moreira TA, Costa PS, Costa LR, et al. Combined oral midazolam–ketamine better than midazolam alone for sedation of young children: a randomized controlled trial. Int J Pediatr Dent. 2013;23(3):207–215. doi: 10.1111/j.1365-263X.2012.01246.x. [DOI] [PubMed] [Google Scholar]
- 19.Roelofse JA, Shipton EA, de la Harpe CJ, et al. Intranasal Sufentanil/Midazolam versus Ketamine/Midazolam for analgesia/sedation in the pediatric population prior to undergoing multiple dental extractions under general anesthesia: a prospective, double-blind, randomized comparison. Anesth Prog. 2004;51(4):114–121. [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzla J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Chichester, UK: John Wiley & Sons; 2019. [Google Scholar]
- 22.Koirala B, Pandey RK, Saksen AK, et al. A comparative evaluation of newer sedatives in conscious sedation. J Clin Pediatr Dent. 2006;30(4):273–276. doi: 10.17796/jcpd.30.4.540025283p827511. [DOI] [PubMed] [Google Scholar]
- 23.Bahetwar SK, Pandey RK, Saksena AK, et al. A comparative evaluation of intranasal midazolam, ketamine and their combination for sedation of young uncooperative pediatric dental patients: a triple blind randomized crossover trial. J Clin Pediatr Dent. 2011;35(4):415–420. doi: 10.17796/jcpd.35.4.l43h3354705u2574. [DOI] [PubMed] [Google Scholar]
- 24.Antunes DE, Viana KA, Costa PS, et al. Moderate sedation helps improve future behavior in pediatric dental patients—a prospective study. Braz Oral Res. 2016;30(1):e107. doi: 10.1590/1807-3107BOR-2016.vol30.0107. [DOI] [PubMed] [Google Scholar]
- 25.Sado-Filho J, Viana KA, Corrêa-Faria P, et al. Randomized clinical trial on the efficacy of intranasal or oral ketamine-midazolam combinations compared to oral midazolam for outpatient pediatric sedation. PLoS One. 2019;14(3):e0213074. doi: 10.13039/501100003593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramos-Gomez F, Kinsler J, Askaryar H. Understanding oral health disparities in children as a global public health issue: how dental health professionals can make a difference. Journal of public health policy. 2020;41(2):114–124. doi: 10.1057/s41271-020-00222-5. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. WHO Expert Consultation on Public Health Intervention against Early Childhood Caries: Report of a Meeting, Bangkok, Thailand, 26–28 January 2016. World Health Organization; 2017. [Google Scholar]
- 28.Kawashita Y, Kitamura M, Saito T. Monitoring time-related trends in dental caries in permanent teeth in Japanese national surveys. Int Dent J. 2012;62(2):100–105. doi: 10.1111/j.1875-595X.2011.00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Grauwe A, Aps JK, Martens LC. Early childhood caries (ECC): what's in a name? Eur J Paediatr Dent. 2004;5(2):62–70. [PubMed] [Google Scholar]
- 30.Feldens CA, Giugliani ER, Duncan BB, et al. Long-term effectiveness of a nutritional program in reducing early childhood caries: a randomized trial. Community Dent Oral Epidemiol. 2010;38(4):324–332. doi: 10.1111/j.1600-0528.2010.00540.x. [DOI] [PubMed] [Google Scholar]
- 31.Ribeiro NM, Ribeiro MA. Breastfeeding and early childhood caries: a critical review. J Pediatr (Rio J) 2004;80(5 Suppl):S199–S210. doi: 10.2223/1241. [DOI] [PubMed] [Google Scholar]
- 32.Cui L, Li X, Tian Y, et al. Breastfeeding and early childhood caries: a meta-analysis of observational studies. Asia Pac J Clin Nutr. 2017;26(5):867–880. doi: 10.6133/apjcn.082016.09. [DOI] [PubMed] [Google Scholar]
- 33.Seow WK. Biological mechanisms of early childhood caries. Community Dent Oral Epidemiol. 1998;26(1 Suppl):8–27. doi: 10.1111/j.1600-0528.1998.tb02090.x. [DOI] [PubMed] [Google Scholar]
- 34.Martens L, Vanobbergen J, Willems S, et al. Determinants of early childhood caries in a group of inner-city children. Quintessence Int. 2006;37(7):527–536. [PubMed] [Google Scholar]
- 35.Law CSL, Blain S. Approaching the pediatric dental patient: a review of nonpharmacologic behavior management strategies. J Calif Dent Assoc. 2003;31(9):703–713. [PubMed] [Google Scholar]
- 36.al-Rakaf H, Bello LL, Turkustani A, et al. Intra-nasal midazolam in conscious sedation of young pediatric dental patients. Int J Pediatr Dent. 2001;11(1):33–40. doi: 10.1046/j.1365-263x.2001.00237.x. [DOI] [PubMed] [Google Scholar]
- 37.Folayan MO, Idehen EE, Ojo OO. The modulating effect of culture on the expression of dental anxiety in children: a literature review. Int J Paediatr Dent. 2004;14(4):241–245. doi: 10.1111/j.1365-263X.2004.00563.x. [DOI] [PubMed] [Google Scholar]
- 38.Skaret E, Soevdsnes EK. Behavioural science in dentistry. The role of the dental hygienist in prevention and treatment of the fearful dental patient. Int J Dent Hyg. 2005;3(1):2–6. doi: 10.1111/j.1601-5037.2004.00109.x. [DOI] [PubMed] [Google Scholar]
- 39.Adair SM, Rockman RA, Schafer TE, et al. Survey of behavior management teaching in pediatric dentistry advanced education programs. Pediatr Dent. 2004;26(2):151–158. [PubMed] [Google Scholar]
- 40.American Academy of Pediatric Dentistry. Guideline on behavior guidance for the pediatric dental patient. Pediatr Dent. 2011;33:161–173. [Google Scholar]
- 41.Lima ARA, Costa LRRS, Costa PSS. A randomized, controlled, crossover trial of oral midazolam and hydroxyzine for pediatric dental sedation. Pesqui Odontol Bras. 2003;17(3):206–211. doi: 10.1590/s1517-74912003000300002. [DOI] [PubMed] [Google Scholar]
- 42.Heard C, Smith J, Creighton P, et al. A composition of four sedation techniques for pediatric dental surgery. Pediatr Anaesth. 2010;20(10):924–930. doi: 10.1111/j.1460-9592.2010.03402.x. [DOI] [PubMed] [Google Scholar]
- 43.Shapira J, Kupietzky A, Kadari A, et al. Comparison of oral midazolam with and without hydroxyzine in the sedation of pediatric dental patients. Pediatr Dent. 2004;26(6):492–496. [PubMed] [Google Scholar]
- 44.Day PF, Power AM, Hibbert SA, et al. Effectiveness of oral midazolam for pediatric dental care: a retrospective study in two specialist centers. Eur Arch Pediatr Dent. 2006;7(4):228–235. doi: 10.1007/BF03262557. [DOI] [PubMed] [Google Scholar]
- 45.Wan K, Jing Q, Zhao JZ. Evaluation of oral midazolam as conscious sedation for pediatric patients in oral restoration. Chin Med Sci J. 2006;21(3):163–166. [PubMed] [Google Scholar]
- 46.Astuto M, Disma N, Crimi E. Two doses of oral ketamine, given with midazolam, for premedication in children. Minerva Anestesiol. 2002;68(7-8):593–598. [PubMed] [Google Scholar]
- 47.Darlong V, Shende D, Subramanyam MS, et al. Oral ketamine or midazolam or low dose combination for premedication in children. Anaesth Intensive Care. 2004;32(2):246–249. doi: 10.1177/0310057x0403200214. [DOI] [PubMed] [Google Scholar]
- 48.Ghai B, Grandhe RP, Kumar A, et al. Comparative evaluation of midazolam and ketamine with midazolam alone as oral premedication. Pediatr Anaesth. 2005;15(7):554–559. doi: 10.1111/j.1460-9592.2004.01523.x. [DOI] [PubMed] [Google Scholar]
- 49.Darlong V, Shende D, Singh M, et al. Low- versus high-dose combination of midazolam–ketamine for oral premedication in children for ophthalmologic surgeries. Singapore Med J. 2011;52(7):12–16. [PubMed] [Google Scholar]