Abstract
Introduction
Ventilator-associated events (VAEs) are one of the main sources of concern in critically ill patients due to the high frequency and mortality. We conducted this analysis to compare the effects of open endotracheal suctioning system with closed one on the incidences of VAEs in adult patients receiving mechanical ventilation (MV).
Materials and methods
A comprehensive literature search was performed in PubMed, Scopus, Cochrane Library, and hand searching bibliographies of retrieved articles. The search was confined to randomized controlled trials with human adults comparing closed tracheal suction systems (CTSS) vs open tracheal suction systems (OTSS) in prevention of ventilator-associated pneumonia (VAP). Full-text articles were used in order to extract the data. Data extraction was only started after completing the quality assessment.
Results
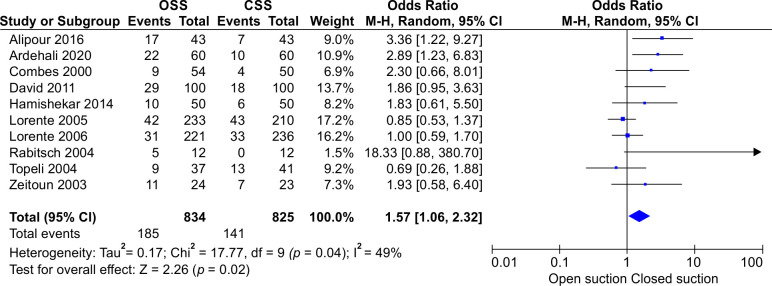
The search resulted in 59 publications. Among them, 10 were identified as eligible for meta-analysis. There was a significant increase in incidence of VAP when using OTSS compared to CTSS, so that OCSS increased the incidence of VAP by 57% (OR 1.57, 95% CI 1.063–2.32, p = 0.02).
Discussion
Our results showed that using CTSS can significantly decrease VAP development compared to OTSS. This conclusion does not yet mean the routine use of CTSS as a standard VAP prevention measure for all patients since individual patient’s disease and cost are other factors that should be in mind when determining the choice of the suctioning system. High-quality trials with a larger sample size are highly recommended.
How to cite this article
Sanaie S, Rahnemayan S, Javan S, Shadvar K, Saghaleini SH, Mahmoodpoor A. Comparison of Closed vs Open Suction in Prevention of Ventilator-associated Pneumonia: A Systematic Review and Meta-analysis. Indian J Crit Care Med 2022;26(7):839–845.
Keywords: Closed, Prevention, Suction, Ventilator-associated pneumonia
Highlights
Ten clinical trials were identified in this systematic review and meta-analysis regarding comparison of CTSS vs OTSS. All the included studies had high or unknown risk of bias regarding blinding of participants and outcomes. This meta-analysis has shown that using CTSS can significantly decrease VAP development compared to OTSS.
Introduction
Ventilator-associated pneumonia is one of the most serious and common infections in critically ill patients under MV. Its incidence differs between almost 5 and 50% in mechanically ventilated patients.1–4 As VAP increases the morbidity [intensive care unit (ICU) length of stay, duration of MV, and hospital stay] and mortality, its prevention seems an essential step in the management of mechanically ventilated patients.5–7 Based on VAP prevention bundle, suctioning has an important role in the pathogenesis of VAP.8 Endotracheal suctioning is routinely performed in mechanically ventilated patients to clear secretions. Currently, there are two different types of suctioning systems. Closed tracheal suction systems which employ multiuse suctioning catheters and let endotracheal suctioning without disconnecting of patient from the ventilator resulting in decreased contamination, maintenance of positive pressure ventilation, and oxygenation.9–11 It seems that during OTSS, the ventilator is disconnected which together with the negative vacuum pressure and fewer physiologic disturbances during suctioning like increased heart rate and mean arterial pressure, and decreased arterial deoxygenation lead to intense loss of lung volume and subsequent hypoxemia. Previous systematic reviews could not reach such a strong evidence regarding the priority of each intervention on VAP because of underreporting, low quality of the included trials (low sample size of trials and a potential for publication bias), and incomplete data sets of included studies.12–17 Based on the lack of data in this field, we performed a systematic review and meta-analysis to reevaluate the efficacy of CTSS use compared to OTSS use in prevention of VAP in mechanically ventilated adult patients.
Materials and Methods
A comprehensive literature search was performed in PubMed, Scopus, Cochrane Library, and hand searching bibliographies of retrieved articles. ProQuest was searched to achieve additional grey literature. For the Cochrane Library, the Database of Systematic Reviews, the database of abstracts of reviews of effects (DARE), and the Cochrane central register of controlled trials (CENTRAL) were searched. The following keywords were used: VAP, “ventilator associated pneumonia”, “ventilator associated events”, “open suction”, and “closed suction”. The search was confined to randomized controlled trials (RCTs) with human adults that were published after 2000 and in English language. The latest search was performed in June 2020.
Our primary research question was to make a comparison of CTSS vs OTSS in prevention of VAP. For this reason, we searched for clinical trials investigating the onset of VAP in OTSS and CTSS. We used a broad inclusion strategy to achieve the maximum sensitivity. The inclusion criteria were as the following: (1) Clinical studies published up to June 2020; (2) Articles with available data about onset of VAP in CTSS and/or OTSS. Final inclusion of the studies was determined by two reviewers who were independently making the final decision on inclusion of the studies. For studies reported in more than one publication, data abstraction was performed using all publications; however, we included just one report in our study. The studies meeting the following criteria were excluded: (1) Review articles, case reports, and conference abstracts; (2) The animal studies; (3) Original articles not containing data on onset of VAP in CTSS and/or OTSS; (4) Articles that were inaccessible after two times of mailing for paper request from the corresponding author; (5) Articles with unclear data description; (6) Duplicate reports; (7) Not randomized articles; (8) Articles before year 2000. Furthermore, the randomization procedure was critically appraised. To prevent manipulation of the allocation process, the method of assigning the patients to either CTSS or OTSS should be adequately concealed for both patient and clinician (healthcare worker). This method was judged by two reviewers without masking of author or source, using four ratings for quality of allocation concealment: (A) Adequate concealment of the allocation; (B) Uncertainty about adequate concealment of allocation; (C) Allocation definitely not adequately concealed; (D) Allocation concealment not used. Discrepancies in ratings were resolved through discussion between reviewers. No additional information was sought from the original authors. Reporting quality assessment of included articles was performed according to the consolidated standards of reporting trials (CONSORT). The CONSORT statement is used worldwide to improve the reporting of RCTs.18 We considered studies with 10 points or more as studies with moderate to good study quality. However, all the selected studies were included in the systematic review, regardless of their score. Additionally, Cochrane Risk of Bias 2 (RoB2) tool in RevMan 5.3 was used to assess the final included studies. Three authors assessed the quality of each article independently. In the times of disagreement, the final decision was made consulting with two other authors. Data extraction was only started after completing the reporting quality and risk of bias assessment. Full text articles were used in order to extract the data. Two authors independently performed the data extraction from text and tables. The authors of the original studies were contacted in case of lacking essential data in the text.
Data Analysis
From each study, data were extracted on the outcomes measured. The synthesis of data was performed using random effect models. To assess heterogeneity of treatment effects across the studies, I2 statistic was computed in Review Manager (version 5.3). I2 is derived from Cochrane’s Q statistic. It measures the extent of inconsistency among the studies’ results, and the outcome is interpreted as the percentage of total variation across studies that is due to heterogeneity rather than chance.19 A value of 0% indicates that all variability in effect estimates is due to chance and that none is due to heterogeneity. Larger values show that most of the variability is due to heterogeneity rather than chance.
Results
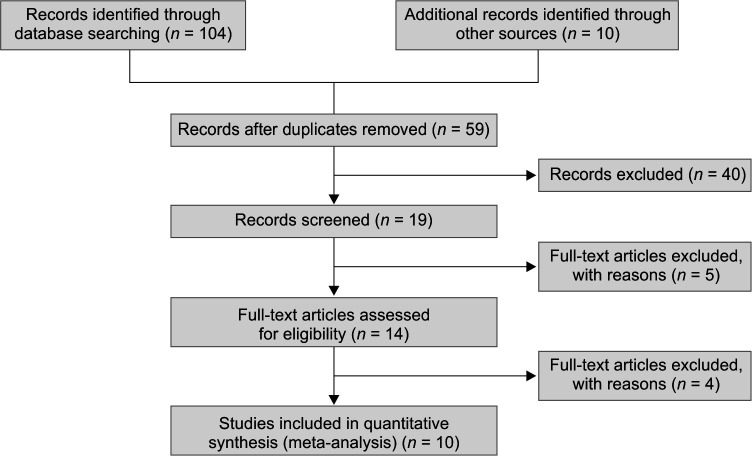
The search resulted in 59 publications. Forty articles were excluded at first screening based on their titles and abstracts because they met the exclusion criteria such as irrelevant purposes, unclear indicators, review articles, case reports, conference abstracts, and animal models. Full texts of the remaining 19 potentially related studies were obtained. Five studies were excluded because of not meeting the inclusion criteria and the remaining 14 studies, after removing four articles due to not being randomized, 10 were included for this systematic review and meta-analysis. The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flowchart of the study is shown in Flowchart 1. Study characteristics and results of the nine studies that are included in the systematic review are summarized in Table 1. Risk of bias assessment of included studies based on the Cochrane RoB2 tool in RevMan 5.3 for RCTs are summarized in Figure 1. All the included articles had risk of bias regarding not being blind both for participants and outcomes.
Flowchart 1.
Flow diagram of the study
Table 1.
Study characteristics and results of the included studies in the systematic review
| Author | Year | Study type | Sample size | CSS | OSS | p-value | Conclusion | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CSS | OSS | VAP | No VAP | VAP | No VAP | ||||||
| 1 | Alipour et al.23 | 2016 | Clinical trial (prospective randomized) | 43 | 43 | 7 | 36 | 17 | 26 | 0.016 | The incidence of VAP was significantly lower in closed method compared to the open method |
| 2 | Ardehali et al.24 | 2020 | Clinical trial (prospective randomized) | 60 | 60 | 10 | 50 | 22 | 38 | 0.637 | There was not any significant difference between groups regarding incidence of VAP |
| 3 | Hamishekar et al.25 | 2014 | Clinical trial (prospective randomized) | 50 | 50 | 6 | 44 | 10 | 40 | 0.27 | There was not any significant difference between groups regarding incidence of VAP |
| 4 | Combes et al.26 | 2000 | Prospective randomized study | 50 | 54 | 4 | 46 | 9 | 45 | 0.07 | Closed suction nonsignificantly reduced incidence of VAP without any adverse effect |
| 5 | Topeli et al.27 | 2004 | Prospective, randomized, controlled trial | 41 | 37 | 13 | 28 | 9 | 28 | 0.47 | There was no difference between the groups in terms of the frequency of development of VAP, mortality in the MICU, length of MICU stay and duration of MV |
| 6 | Rabitsch et al.20 | 2004 | Prospective randomized study | 12 | 12 | 0 | 12 | 5 | 7 | 0.037 | CSS reduced the incidence of VAP when compared with OSS |
| 7 | David et al.28 | 2011 | Open-labelled randomized controlled trial | 100 | 100 | 18 | 82 | 29 | 71 | 0.07 | Using the clinical criteria, the incidence of VAP was higher with OES than with CES. When tested for superiority, CES was associated with a trend to a reduced incidence of VAP (odds ratio, 1.86; 95% CI, 0.91–3.83; p = 0.067). The number needed to treat with CES to prevent 1 VAP was 9 (95% CI, −0.7 to 22.7) |
| 8 | Lorente et al.29 | 2005 | Clinical trial (prospective randomized) | 210 | 233 | 43 | 167 | 42 | 191 | 0.62 | There was not any significant difference between groups regarding incidence of VAP |
| 9 | Lorente et al.30 | 2006 | Clinical trial (prospective randomized) | 236 | 221 | 33 | 203 | 31 | 190 | 0.99 | There was not any significant difference between groups regarding incidence of VAP |
| 10 | Zeitoun et al.31 | 2003 | Prospective randomized study | 23 | 24 | 7 | 16 | 11 | 13 | 0.278 | Use of a closed suction system did not decrease the incidence of nosocomial pneumonia when compared with the open system |
Fig. 1.
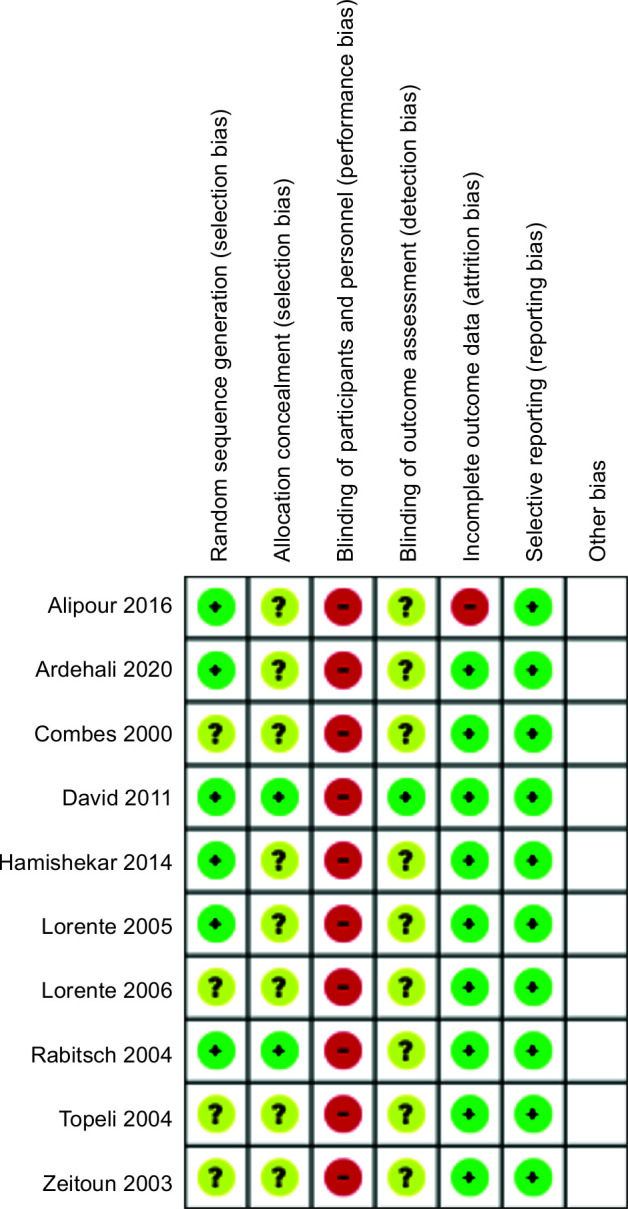
Risk of bias in the included studies
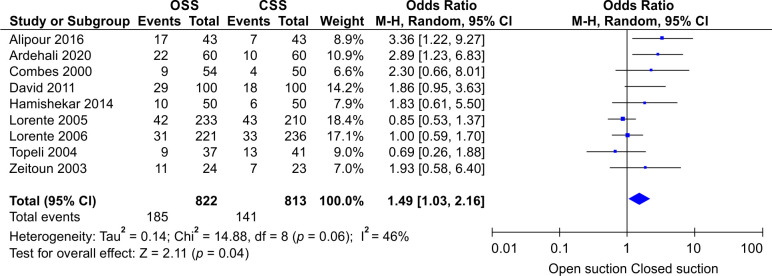
Heterogeneity was tested by the heterogeneity statistic Q and quantified using I2. Results showed almost a high heterogeneity among the studies (I2 = 49%, p = 0.04). Therefore, the use of random effect model in this study was correct and appropriate. That is, the studies used have been extracted from different communities. This can be observed and inferred due to the different OR values for studies in the first part of the output. The Forest plot of the study for incidence of VAP is presented in Figure 2. It shows that the incidence of VAP in CSS is significantly lower than OSS (OR: 1.57, CI 95%; 1.06–2.32, p = 0.02). The funnel plot is presented in Figure 3. The funnel plot can be used to check for the presence or absence of publication bias. Asymmetry will indicate the existence of diffusion biases. The symmetry of this graph indicates no diffusion bias. However, it seems that the study of Rabitsch et al. behaves relatively differently from other studies.20 Sensitivity analysis showed that, after excluding the study by Rabitsch et al., the result from meta-analysis changed from (OR: 1.57, CI 95%; 1.06–2.32, p = 0.02) to (OR: 1.49, CI 95%; 1.03–2.16, p = 0.04), which is presented in Figure 4.
Fig. 2.
Forest plot showing the results of the studies
Fig. 3.
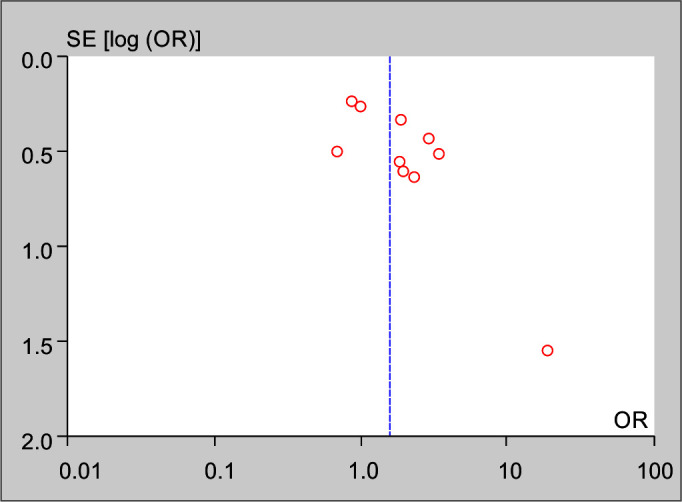
Funnel plot of the study
Fig. 4.
Forest plot showing the results of the sensitivity analysis
Discussion
The results of this meta-analysis showed that OTSS was associated with a significant increase (57%) in VAP frequency compared with CTSS. Previous studies have shown that CTSS tends to decrease the development of VAP, but the insignificant results of some studies can be due to less statistical power, the different method for VAP definition, heterogeneity of samples, small trial sizes, and wide confidential interval.
In this study, all the included studies had high or unknown risk of bias regarding blinding of participants and outcomes. However, it was accepted, while the nature of these studies was not compatible with blinding since the setting of the studies was technically impossible to be blinded while the difference between CTSS and OTSS was completely obvious on the first sight.
Bacterial transfer from naso-oropharynx and gut to the lower respiratory tract in mechanically ventilated patients via endotracheal tube cuff or by direct cross contamination during nursing intervention results in VAP development.21 It seems that CTSS as compared with OTSS prevents cross transmission of bacteria in intubated patients on MV, but previous systematic review and meta-analysis could not show a significant reduction of VAP by using CTSS.13–17,22 The reason for these insignificant results in the previous meta-analysis can be due to different included studies. First of all, the previously published meta-analyses were mostly conducted before 2010 and therefore have included articles before 2000, which have not been included in present meta-analysis.11,14,16,17 There was only one meta-analysis published after 2010, which had included some articles in languages other than English that are not included in the present meta-analysis. On the other hand, our meta-analysis includes the newest published articles regarding the onset of VAP in CTSS vs OTSS, which were not included in previous meta-analyses.12 In addition, heterogeneity of the patients in included studies can contribute to the different results of the previous meta-analysis in comparison to the present study as the patients from included studies were from various kinds of ICUs such as surgical, medical, neurosurgical, and trauma and the included studies in previous meta-analyses and the current meta-analysis are different. On the other hand, there was a high possibility for false-positive respiratory tract infections among patients with CTSS in some of the trials since in these trials, the specimens for microbiological testing were not taken by a new and sterile system which increases the risk of tracheal secrete contamination from reused catheters and bias for subsequent diagnosis of pneumonia. Regarding the trials included in our systematic review and meta-analysis, they have shown different results on reduction of VAP; three of them showed a significant reduction and the other seven did not show a significant reduction of VAP.23–31 In addition, duration of hospital or ICU stay, duration of MV, and mortality rate were other outcomes assessed in these trials which had no difference between CTSS and OTSS. In the previous guidelines, the priority for the use of either CTSS or OTSS for VAP prevention was considered as an unresolved issue. Some guidelines have favored CTSS over OTSS use for cost and safety considerations, without of a favorable evidence supporting CTSS use for the prevention of VAP.32–35 One of the problems in performed trials was the inconsistency regarding the optimal time of CTSS exchange. As it differ from 24 to 168 hours in different studies, meta-regression analysis showed no relation between VAP development and CTSS exchange. Although most companies have recommended routine exchange of closed suction, most studies were underpowered, so there is no strong evidence to support this recommendation.36,37
This study has some limitations. First, patients’ demographic data, study protocols including prophylactic antibiotics, oral care, time of CTSS exchange, and the risk of VAP were not uniform across the trials. Second, we did not include non-English language trials in this meta-analysis, which can reduce the generalizability of our results. Third, nearly half of our trials lacked information on sequence generation, allocation concealment, and blinding of outcome assessors which to some extent was due to the special design nature of the studies. Finally, most of the trials had small sample size which could lead to lower power of the included studies. Thus, although the results from the current study are reliable based on their exact methodology and high overall power, future trials with larger sample size and possible solutions for blinding of participants and outcomes are needed for reaching to nonbiased results.
Conclusion
Our results showed that using CTSS can significantly decrease VAP development compared to OTSS. This conclusion does not yet mean the routine use of CTSS as a standard VAP prevention measure for all patients since individual patient’s disease and cost are other factors which should be in mind when determining the choice of the suctioning system. High-quality trials with a larger sample size is highly recommended.
Authors’ Contribution
AM had full access to all data in the study and took responsibility for the integrity of data and the accuracy of the data analysis. SS designed the study and analyzed the results. SR, SHS, and SJ interpreted the data and wrote the paper. AM, KS, SS, and SR performed the systematic search and checked papers methodology and bias. SJ and SHS advised on the conception and design of the study. All authors vouched for the respective data and analysis, approved the final version, and agreed to publish the manuscript.
Availability of Data and Materials: The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Orcid
Sarvin Sanaie https://orcid.org/0000-0003-2325-5631
Sama Rahnemayan https://orcid.org/0000-0003-0641-4178
Sahar Javan https://orcid.org/0000-0002-5167-6639
Kamran Shadvar https://orcid.org/0000-0003-4433-9949
Seied-Hadi Saghaleini https://orcid.org/0000-0003-4996-4372
Ata Mahmoodpoor https://orcid.org/0000-0002-4361-6230
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Chastre J, Fagon J-Y. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165(7):867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 2.Rello J, Ollendorf DA, Oster G, Vera-Llonch M, Bellm L, Redman R, et al. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122(6):2115–2121. doi: 10.1378/chest.122.6.2115. [DOI] [PubMed] [Google Scholar]
- 3.Vincent JL, Bihari DJ, Suter PM, Bruining HA, White J, Nicolas-Chanoin MH, et al. The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. EPIC International Advisory Committee. JAMA. 1995;274(8):639–644. 7637145 [PubMed] [Google Scholar]
- 4.Hunter JD. Ventilator associated pneumonia. BMJ. 2012;344:e3325. doi: 10.1136/bmj.e3325. [DOI] [PubMed] [Google Scholar]
- 5.Melsen WG, Rovers MM, Bonten MJM. Ventilator-associated pneumonia and mortality: a systematic review of observational studies. Crit Care Med. 2009;37(10):2709–2718. doi: 10.1097/ccm.0b013e3181ab8655. [DOI] [PubMed] [Google Scholar]
- 6.Safdar N, Dezfulian C, Collard HR, Saint S. Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med. 2005;33(10):2184–2193. doi: 10.1097/01.ccm.0000181731.53912.d9. [DOI] [PubMed] [Google Scholar]
- 7.Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039–2046. doi: 10.1001/jamainternmed.2013.9763. [DOI] [PubMed] [Google Scholar]
- 8.Morris AC, Hay AW, Swann DG, Everingham K, McCulloch C, McNulty J, et al. Reducing ventilator-associated pneumonia in intensive care: impact of implementing a care bundle. Crit Care Med. 2011;39(10):2218–2224. doi: 10.1097/CCM.0b013e3182227d52. [DOI] [PubMed] [Google Scholar]
- 9.Cereda M, Villa F, Colombo E, Greco G, Nacoti M, Pesenti A. Closed system endotracheal suctioning maintains lung volume during volume-controlled mechanical ventilation. Intensive Care Med. 2001;27(4):648–654. doi: 10.1007/s001340100897. [DOI] [PubMed] [Google Scholar]
- 10.Maggiore SM, Lellouche F, Pigeot J, Taille S, Deye N, Durrmeyer X, et al. Prevention of endotracheal suctioning-induced alveolar derecruitment in acute lung injury. Am J Respir Crit Care Med. 2003;167(9):1215–1224. doi: 10.1164/rccm.200203-195OC. [DOI] [PubMed] [Google Scholar]
- 11.Jongerden IP, Rovers MM, Grypdonck MH, Bonten MJ. Open and closed endotracheal suction systems in mechanically ventilated intensive care patients: a meta-analysis. Crit Care Med. 2007;35(1):260–270. doi: 10.1097/01.CCM.0000251126.45980.E8. [DOI] [PubMed] [Google Scholar]
- 12.Kuriyama A, Umakoshi N, Fujinaga J, Takada T. Impact of closed versus open tracheal suctioning systems for mechanically ventilated adults: a systematic review and meta-analysis. Intensive Care Med. 2015;41(3):402–411. doi: 10.1007/s00134-014-3565-4. [DOI] [PubMed] [Google Scholar]
- 13.Overend TJ, Anderson CM, Brooks D, Cicutto L, Keim M, McAuslan D, et al. Updating the evidence-base for suctioning adult patients: a systematic review. Can Respir J. 2009;16(3):e6–e17. doi: 10.1155/2009/872921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peter JV, Chacko B, Moran JL. Comparison of closed endotracheal suction versus open endotracheal suction in the development of ventilator-associated pneumonia in intensive care patients: an evaluation using meta-analytic techniques. Indian J Med Sci. 2007;61(4):201–211. 17401257 [PubMed] [Google Scholar]
- 15.Siempos II, Vardakas KZ, Falagas ME. Closed tracheal suction systems for prevention of ventilator-associated pneumonia. Br J Anaesth. 2008;100(3):299–306. doi: 10.1093/bja/aem403. [DOI] [PubMed] [Google Scholar]
- 16.Subirana M, Solà I, Benito S. Closed tracheal suction systems versus open tracheal suction systems for mechanically ventilated adult patients. Cochrane database Syst Rev. 2007;2007(4):CD004581. doi: 10.1002/14651858.CD004581.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vonberg R-P, Eckmanns T, Welte T, Gastmeier P. Impact of the suctioning system (open vs. closed) on the incidence of ventilation-associated pneumonia: meta-analysis of randomized controlled trials. Intensive Care Med. 2006;32(9):1329–1335. doi: 10.1007/s00134-006-0241-3. [DOI] [PubMed] [Google Scholar]
- 18.Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabitsch W, Köstler WJ, Fiebiger W, Dielacher C, Losert H, Sherif C, et al. Closed suctioning system reduces cross-contamination between bronchial system and gastric juices. Anesth Analg. 2004;99(3):886. doi: 10.1213/01.ANE.0000143353.85428.39. [DOI] [PubMed] [Google Scholar]
- 21.Mahmoodpoor A, Peyrovi-far A, Hamishehkar H, Bakhtyiari Z, Mirinezhad MM, Hamidi M, et al. Comparison of prophylactic effects of polyurethane cylindrical or tapered cuff and polyvinyl chloride cuff endotracheal tubes on ventilator-associated pneumonia. Acta Med Iran. 2013;51(7):461–466. 23945890 [PubMed] [Google Scholar]
- 22.Niël-Weise BS, Snoeren RLMM, van den Broek PJ. Policies for endotracheal suctioning of patients receiving mechanical ventilation: a systematic review of randomized controlled trials. Infect Control Hosp Epidemiol. 2007;28(5):531–536. doi: 10.1086/513726. [DOI] [PubMed] [Google Scholar]
- 23.Alipour N, Manouchehrian N, Sanatkar M, Anvari HMP, Jahromi MSS. Evaluation of the effect of open and closed tracheal suction on the incidence of ventilator associated pneumonia in patients admitted in the intensive care unit. Arch Anesthesiol Crit Care. 2016;2(2):193–196. SE-Research Article(s). Available from: https://aacc.tums.ac.ir/index.php/aacc/article/view/79. [Google Scholar]
- 24.Ardehali SH, Fatemi A, Rezaei SF, Forouzanfar MM, Zolghadr Z. The effects of open and closed suction methods on occurrence of ventilator associated pneumonia; a comparative study. Arch Acad Emerg Med. 2020;8(1):e8. 32021989 [PMC free article] [PubMed] [Google Scholar]
- 25.Hamishekar H, Shadvar K, Taghizadeh M, Golzari SE, Mojtahedzadeh M, Soleimanpour H, et al. Ventilator-associated pneumonia in patients admitted to intensive care units, using open or closed endotracheal suctioning. Anesthesiol Pain Med. 2014;4(5):e21649. doi: 10.5812/aapm.21649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Combes P, Fauvage B, Oleyer C. Nosocomial pneumonia in mechanically ventilated patients, a prospective randomised evaluation of the Stericath closed suctioning system. Intensive Care Med. 2000;26(7):878–882. doi: 10.1007/s001340051276. [DOI] [PubMed] [Google Scholar]
- 27.Topeli A, Harmanci A, Cetinkaya Y, Akdeniz S, Unal S. Comparison of the effect of closed versus open endotracheal suction systems on the development of ventilator-associated pneumonia. J Hosp Infect. 2004;58(1):14–19. doi: 10.1016/j.jhin.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 28.David D, Samuel P, David T, Keshava SN, Irodi A, Peter JV. An open-labelled randomized controlled trial comparing costs and clinical outcomes of open endotracheal suctioning with closed endotracheal suctioning in mechanically ventilated medical intensive care patients. J Crit Care. 2011;26(5):482–488. doi: 10.1016/j.jcrc.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Lorente L, Lecuona M, Martín MM, García C, Mora ML, Sierra A. Ventilator-associated pneumonia using a closed versus an open tracheal suction system. Crit Care Med. 2005;33(1):115–119. doi: 10.1097/01.ccm.0000150267.40396.90. [DOI] [PubMed] [Google Scholar]
- 30.Lorente L, Lecuona M, Jiménez A, Mora ML, Sierra A. Tracheal suction by closed system without daily change versus open system. Intensive Care Med. 2006;32(4):538–544. doi: 10.1007/s00134-005-0057-6. [DOI] [PubMed] [Google Scholar]
- 31.Zeitoun SS, de Barros ALBL, Diccini S. A prospective, randomized study of ventilator-associated pneumonia in patients using a closed vs. open suction system. J Clin Nurs. 2003;12(4):484–489. doi: 10.1046/j.1365-2702.2003.00749.x. [DOI] [PubMed] [Google Scholar]
- 32.AARC Clinical Practice Guidelines. Endotracheal suctioning of mechanically ventilated patients with artificial airways 2010. Respir Care. 2010;55(6):758–764. 20507660 [PubMed] [Google Scholar]
- 33.Dodek P, Keenan S, Cook D, Heyland D, Jacka M, Hand L, et al. Evidence-based clinical practice guideline for the prevention of ventilator-associated pneumonia. Ann Intern Med. 2004;141(4):305–313. doi: 10.7326/0003-4819-141-4-200408170-00011. [DOI] [PubMed] [Google Scholar]
- 34.Hess DR, Kallstrom TJ, Mottram CD, Myers TR, Sorenson HM, Vines DL. Care of the ventilator circuit and its relation to ventilator-associated pneumonia. Respir Care. 2003;48(9):869–879. 14513820 [PubMed] [Google Scholar]
- 35.Muscedere J, Dodek P, Keenan S, Fowler R, Cook D, Heyland D. Comprehensive evidence-based clinical practice guidelines for ventilator-associated pneumonia: prevention. J Crit Care. 2008;23(1):126–137. doi: 10.1016/j.jcrc.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 36.Darvas JA, Hawkins LG. The closed tracheal suction catheter: 24 hour or 48 hour change? Aust Crit care Off J Confed Aust Crit Care Nurses. 2003;16(3):86–92. doi: 10.1016/s1036-7314(03)80005-x. [DOI] [PubMed] [Google Scholar]
- 37.Kollef MH, Prentice D, Shapiro SD, Fraser VJ, Silver P, Trovillion E, et al. Mechanical ventilation with or without daily changes of in-line suction catheters. Am J Respir Crit Care Med. 1997;156(2 Pt 1):466–472. doi: 10.1164/ajrccm.156.2.9612083. [DOI] [PubMed] [Google Scholar]