Abstract
The application potential of spirulina meal in aquaculture feeds has been well summarized in several descriptive reviews. Nevertheless, they converged on compiling results from all possible relevant studies. Little available quantitative analysis regarding the pertinent topics has been reported. This quantitative meta-analysis was performed to investigate the influences of dietary spirulina meal (SPM) addition on responsive variables in aquaculture animals, including final body weight (FBW), specific growth rate (SGR), feed conversion ratio (FCR), protein efficiency ratio (PER), condition factor (CF), and hepatosomatic index (HSI). The pooled standardized mean difference (Hedges' g) and 95% confidence limit were computed to quantify the primary outcomes based on random-effects model. The sensitivity and subgroup analyses were carried out to evaluate the validity of the pooled effect size. The meta-regression analysis was conducted to investigate the optimal inclusion of SPM as a feed supplement and the upper threshold of SPM usage for substituting fishmeal in aquaculture animals. The results indicated that on the whole, dietary SPM addition significantly improved FBW, SGR, and PER; statistically decreased FCR of animals; had no significant influence on CF and HSI. The growth-enhancing effect of SPM inclusion in the form of feed additive was significant; however, the effect was indistinctive in the form of feedstuff. Furthermore, the meta-regression analysis displayed that the optimal levels of SPM as a feed supplement in fish and shrimp diets were 1.46%-2.26% and 1.67%, respectively. Additionally, up to 22.03%-24.53% and 14.95%-24.85% of SPM as fishmeal substitute did not have a negative effect on growth and feed utilization in fish and shrimp, respectively. Therefore, SPM is a promising fishmeal substitute and a growth-promoting feed additive for sustainable aquaculture of fish and shrimp.
1. Introduction
The global animal protein demand is projected to double by 2050 due to ever increasing human population and growing protein consumption per capita [1]. The amount of protein supplied by global aquaculture accounted for 8% of total animal source protein for human consumption [2], and per capita, consumption is increasing faster than meat and dairy consumption [3]. The aquafeed industry is still dependent on marine ingredients sourced from wild-captured forage fish [4]. Therefore, searching suitable and sustainable alternatives for fishmeal and fish oil is an important approach to achieve continuable growth of aquaculture in production. Spirulina, as one of the most extensively used microalgae, has a highly nutritional profile that contains high crude protein content (59%-63% of dry weight), enough n-3 polyunsaturated fatty acids (γ-linoleic acid), and vitamins and minerals [5, 6]. Some bioactive substances are also detected in this cyanobacterium, such as phenols, β-carotene, chlorophylls, and phycobiliprotein, and they exhibited various biological properties including antioxidant and anti-inflammatory activities [7, 8]. Additionally, recent studies have shown that Spirulina could also be cultivated in aquaculture effluent [9, 10], and this is regarded as win-win pattern to treat aquaculture wastewater and spirulina meal production.
In recent years, increasingly studies are accumulating about the application of spirulina meal in fish feed. The spirulina meal was regarded as a functional additive in the diet formulation in some studies, and the supplementation level was comparatively lower. In these studies, they mainly concentrated on growth performance, antioxidative properties [11], immunoprotective effect [12], and pigmentation capability [13, 14] of dietary spirulina meal supplementation for its bioactive compounds. In other studies, spirulina meal was treated as a dietary protein source to substitute fishmeal or other protein sources due to its high protein content and the inclusion level was higher. Considering the nutritional properties of Spirulina, practicability analysis of spirulina meal in aquaculture feeds has been well documented in some descriptive reviews [5, 15–20], but they converged on compiling different results from all possible relevant studies. No quantitative reviews regarding the pertinent topics have been reported.
Meta-analysis is a statistically analytical technique to quantitatively combine the results of independent experiments on the same topic, to overall comprehend a problem, determine variation sources, or construct a meta-regression model describing the relationship between variables [21]. Although this analytical technique has been applied in aquaculture nutrition in recent years [22–24], this analytic method has not been widely accepted by the experts due to the short time of application in aquaculture nutrition and the possible difference existed in various cultured species. It is well known that the requirement for dietary nutrients, replacement level of fishmeal by other protein sources or optimal addition level of a dietary additive/ingredient in certain aquaculture species is a range value not a point value under the various aquaculture environment and dietary formulation background. Meta-regression analysis based on the existing data across fish species in the literatures may yield an optimal range of nutrient requirements or substitution levels of fishmeal for fish or shrimps, which could be used for reference to the unstudied cultured species. Given that Spirulina is one of the most common microalgae in industrial production, it is necessary to extract data from relevant studies and synthesize the results through meta-analysis. However, there is no systematic review and meta-analysis about the application of spirulina meal in aquafeed across various cultured species. Therefore, the objective of the present study was to conduct meta-analysis to systematically assess the effects of dietary spirulina meal as a functional additive or fishmeal alternative on growth and feed utilization in fish across different studies and species.
2. Methods and Materials
2.1. Search Strategy
Two researchers established and finalized the literature search strategy. To assess the influence of dietary inclusion of spirulina meal on growth performance (final body weight (FBW), specific growth rate (SGR), feed utilization (feed conversion ratio (FCR), protein efficiency ratio (PER), morphological parameters (condition factor (CF), and hepatosomatic index (HSI) were determined in fish and shrimps. Electronic databases including Web of Science, China National Knowledge Infrastructure (CNKI), and Google Scholar were searched from the inception date until 3rd of March 2022, for the discovery of relevant studies according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [25]. The key words and index terms were applied: Topic = (Spirulina or Arthrospira or Arthrospira maxima or Spirulina maxima), Topic = (fish or fishes or shrimp∗), and Topic = (diet or diets or dietary or feed or aquafeed∗ or aquafeed∗). The search was limited to published literatures in English and Chinese and to full-text articles.
2.2. Study Selection
2.2.1. Selection of Studies
Citations and abstracts of all retrieved literatures were downloaded to Endnote X9 (for Window, Thomson Reuters, Philadelphia, PA, USA), and duplicates were excluded. Firstly, the titles and abstracts of the searched papers were evaluated by two separate researchers, and off-topic literatures were removed. Secondly, full-text articles of potential studies were downloaded and scrutinized by the same researchers to filter the eligible studies. Thirdly, the third researcher double-checked and finalized the included studies. Lastly, two independent researchers extracted the data from the included studies for meta-analysis. Inconsistencies were resolved by acceptance of a third researcher.
2.2.2. Eligibility Criteria
Articles could be incorporated in this systematic review if they meet the following criteria: (1) the randomized controlled design and procedure were abided by; (2) the experimental animals were fish or shrimps; (3) spirulina meal was supplemented solely in the diet as an additive without large differences in diet nutrient composition or as a fishmeal substitute; (4) at least one of the following parameters was determined in both control and experimental treatments: FBW, SGR, FCR (or feed efficiency), PER, CF, or HSI; (5) mean value, experimental replication, and error estimation (e.g., standard deviation (SD) and standard error (SE)) of each parameter were exhibited either numerically or graphically to compute the effect size and confidence intervals. The studies were excluded when one of the following scenarios existed: (1) the supplemental level of spirulina meal was not clearly indicated; (2) the FM content was not manifested when it was replaced by spirulina meal; (3) animals grew super slowly (SGR was 0.33%/d for 90-day feeding period); (4) the FM was substituted by spirulina meal and other meals; (5) the feeding period was less than 28 days; (6) the experimental replication was one; (7) the extracts of spirulina were used, such as β-carotene and phycocyanin.
2.3. Data Extraction
A sum of 58 articles were adopted for the current meta-analysis and the following information were gleaned from the result tables in each eligible article: first author's surname; journal; year published; study design including cultured species, initial body weight, number of replicates, number of animals per replicate, and feeding period of the trial; culture condition containing salinity and water temperature; contents of spirulina meal (SPM) and fishmeal (FM) in control and experimental diets; outcome measurements (FBW, SGR, FCR, PER, CF, and HSI), and homologous SD, SE, or pooled SE. When the results of the target variables were presented in the form of a graph, Origin 8.5 software was implemented to extract data. Moreover, the trophic levels of fish and shrimp were acquired from FishBase (http://www.fishbase.se/) and earlier studies [26–28]. One author extracted data from each study and another author proofread all data entries for accuracy. In several studies, feed efficiency (FE), but not the FCR, was used to evaluate the feed utilization of animals. The mean and error of FE were transformed to the counterparts of FCR in accordance with our previous study [29], and the equations were as follows: SDFCR = SDFE/(MeanFE × MeanFE); MeanFCR = 1/MeanFE.
The equations of growth parameters in the included studies were shown as follows:
Specific growth rate (SGR, %/d) = 100 × [ln (FBW) − ln (IBW)]/days
Feed conversion ratio (FCR) = feed intake/(final body weight − initial body weight)
Protein efficiency ratio (PER, %) = 100 × body weight gain/protein intake
Condition factor (CF, %) = 100 × final body weight of each fish or shrimp (g)/(final body length of each fish or shrimp)3
Hepatosomatic index (HSI, %) = 100 × final liver weight of each fish (g) or final hepatopancreas weight of each shrimp/final weight of each fish or shrimp (g)
2.4. Data Synthesis and Analysis
All statistical analyses and graphical approaches were performed in the R version 4.0.2 platform. The metafor package was used to calculate effect size, sampling variation, and 95% confidence interval (95% CI) and to analyze heterogeneity and sensitivity. The segmented package was implemented to judge the breakpoint in the broken-line regression. The ggplot2 package was applied to construct the forest plots and regression plots.
2.4.1. Effect Size Computation and Heterogeneity Analysis
Standardized mean difference (SMD) effect size was expressed by Hedges' g statistic and the computational formula referred to our previous study [29]. In some studies, standard error (SE) or pooled standard error (PSE) were used to assess within-group variation. The SE or PSE was transformed to standard deviation (SD) through the following equation: SD = SE × sqrt (n), where n is the number of study replicates. The Hedges' g effect size and corresponding 95% CI were computed by a random-effects model for each outcome indicator (FBW, SGR, FCR, PER, CF, and HSI) considering that the outcome indicators were continuous variables. A positive Hedges' g value (lower 95% CI>0) indicates that the outcome indicator of fish or shrimp was significantly higher in SPM supplemented group compared with SPM free group. A negative Hedges' g value (upper 95% CI<0) represents that the outcome indicator was significantly lower in spirulina meal supplemented treatment compared with spirulina free treatment. The 95% CI of Hedges' g estimate encompassing zero denotes that no significant variations of the indicators were observed between control group and experimental group. The Hedges' g values could also be explained as follows: small effect (g = 0.20 − 0.49), medium effect (g = 0.50 − 0.79), and large effect (g = 0.80 and above). Cochran's Q statistic and I2 statistic were implemented to quantitatively categorize heterogeneity across studies as follows: no heterogeneity: 0 < I2 ≤ 25%; low heterogeneity: 25% < I2 ≤ 50%; moderate heterogeneity: 50% < I2 ≤ 75%; high heterogeneity ≥75%.
2.4.2. Sensitivity Analysis and Publication Bias
Sensitivity analysis was performed to identify outliers and influential data points by using the Cook's D distance and R-Student value. Once being identified as influential points, these data were removed from the dataset, and the overall Hedge's g, CI, and I2 were reanalyzed. The publication bias was evaluated by constructing funnel plots. Moreover, the trim and fill method was conducted to assess missing studies and modify the overall effect size. Begg's and Egger's tests were also used, and the statistical level was set at p < 0.1 [30]. If the results of Begg's and Egger's tests were incompatible, the Egger's test was adopted as a reference.
2.4.3. Subgroup Analysis and Meta-regression Analysis
Effects of experimental species, SPM as a feed additive or feedstuff, on trophic levels of aquatic animals were tested by subgroup analysis. We classified the subgroups in three groups: (1) the experimental animals were categorized as fish and shrimp; (2) the fish was further subdivided into freshwater fish and marine fish according to culture environment; (3) SPM was defined as a feed additive when the inclusion level in the diet was less than 4% (≤4%) and as a feed ingredient when the inclusion level was more than 4% (>4%); (4) the rank of trophic level of animals was defined as follows according to earlier study: low trophic level: 2 ≤ trophic level<3, medium trophic level: 3 ≤ trophic level<4, and high trophic level: trophic level ≥4 [29].
In view of the inclusion level of SPM being a continuous variable, the random-effects meta-regression analysis was conducted to probe into the regression relationship between the inclusion content of SPM and the effect size of each outcome indicator. The datasets of FBW, SGR, FCR, CF, and HSI were divided into two subsets according to use of spirulina meal as a feed additive or ingredient, respectively. These subsets were then subdivided into two smaller subsets based on the experimental animals being fish or shrimp. When the SPM was viewed as additive, the optimal levels of SPM were estimated through the fitted regression equations. In addition, the superior limits of SPM usage as a protein ingredient were assessed by the intersection point of the fittest regression curve and Hedge's g at zero. The datasets of PER, CF, and HSI were not metaregressed due to relatively smaller sample capacity. Furthermore, local polynomial regression was conducted to determine the tendency between spirulina meal contents and Hedge's g values using the ‘loess' method of geom_smooth function in the ggplot2 package when exploring the best fitted curve. Subsequently, linear, quadratic, cubic, and broken-line regressions were chosen to fit the data, and the best fitted curve/equation was selected. In addition, we used the segmented function in the segmented package to determine the breakpoint and piecewise equations in the broken-line regression.
3. Results
3.1. Study Selection Process
The study selection process was detailed in Figure 1. The initial literature search yielded 410 publications, of which three repeated articles were omitted and 100 were excluded after reviewing the titles and 225 based on abstract assessment. Full texts of 82 probably eligible publications were retrieved for further evaluation, of which 24 articles were removed for the following reasons. Twelve articles lacked of diet formulas or big differences existed in the nutrient composition of experimental diets; six studies did not indicate the error values (standard deviation, standard error, or pooled standard error) of the parameters; one article only had one replicate; four articles had super slow growth rate. After filtration, 58 articles were finally included in this review.
Figure 1.
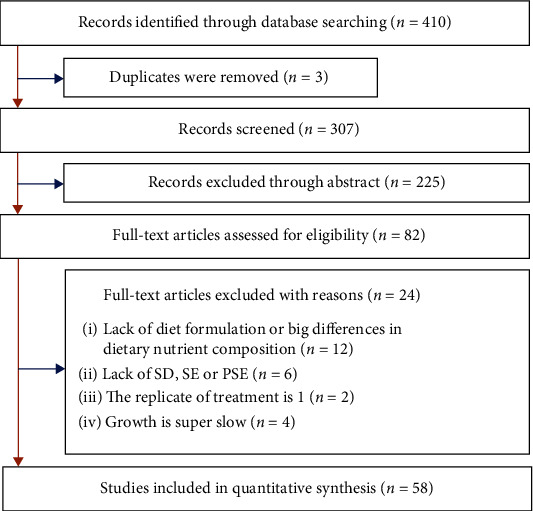
Flow diagram of eligible study selection according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The SD, SE, and PSE represented standard deviation, standard error, and pooled standard error, respectively.
3.2. Characteristics of Included Studies
Information of the included articles were recapitulated in Table S1 of Supplemental Materials. The publication year of the incorporated studies ranged from 2005 to 2022. Twenty-eight species were encompassed in the study. Six shrimp species were studied, including Fenneropenaeus chinensis [31], Litopenaeus vannamei ([32–34], and [35]), Macrobrachium rosenbergii [36], Marsupenaeus japonicus [37], Neocaridina davidi [38], Penaeus monodon ([39, 40], and [41]), and 22 fish species included Amphilophus citrinellus × Cichlasoma trimaculatum [42], Astronotus ocellatus [12], Barilius bendelisis [43], Carassius auratus [44], Carassius auratus gibelio [45–47], Clarias gariepinus [48, 49], Clarias macrocephalus [50], Cyprinus carpio ([51–53], and [13, 14]), Cyrtocara moorii [54, 55], Dicentrarchus labrax [56], Megalobrama amblycephala [57], Mugil liza [17–20], Oncorhynchus mykiss ([58–61], and [62]), Oplegnathus fasciatus [63], Oreochromis niloticus ([64–77], and [78]), Pagrus pagrus [79], Pangasinodon gigas [80], Pelteobagrus fulvidraco ([81–83], and [11]), Piaractus mesopotamicus [84], Salmo trutta caspius [85, 86], Solea solea [87, 88], and Trichopodus trichopterus [89]. Among the 28 species, 11 belonged to low trophic species, 16 were medium trophic species, and 1 was high trophic species (O. mykiss). The dietary content of SPM as feed additives in the experimental treatment scoped from 0.025% to 4%, and the extent of SPM as feed ingredients was from 4.5% to 59.7%. A sum of 620 comparisons (n = 148 for FBW, n = 149 for SGR, n = 147 for FCR, n = 77 for PER, n = 65 for CF, and n = 34 for HSI) between the control group and spirulina meal supplemented group were carried out in this study (Tables 1 and 2 and Table S2). Considering that dietary spirulina meal supplementation generated no impact on morphological indices (CF and HSI), the meta-analyzed results were placed in Tables S2, S7, and S8 and Figures S5, S6, and S7 in Supplemental Materials.
Table 1.
Effect size calculation for final body weight (FBW) and specific growth rate (SGR) comparison between control treatment and spirulina meal supplemented treatment across aquaculture species through random-effects model.
| Effect size (random-effects model) for FBW | Effect size (random-effects model) for SGR | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| k | I 2 | Hedges' g value | SE | CL | P value | k | I 2 | Hedges' g value | SE | CL | P value | |
| All species | 148 | 99.09 | 1.141 | 0.290 | 0.572 to 1.709 | <0.0001 | 149 | 98.69 | 0.788 | 0.227 | 0.343 to 1.234 | 0.001 |
| Subgroups | ||||||||||||
| Species category | ||||||||||||
| Fish species | 124 | 98.66 | 0.871 | 0.279 | 0.323 to 1.419 | 0.002 | 123 | 98.59 | 0.829 | 0.257 | 0.326 to 1.332 | 0.001 |
| Shrimps | 24 | 99.75 | 2.731 | 1.100 | 0.580 to 4.882 | 0.013 | 26 | 98.84 | 0.614 | 0.483 | -0.334 to 1.561 | 0.204 |
| Fish + habitat | ||||||||||||
| Freshwater fish | 108 | 98.54 | 0.754 | 0.305 | 0.157 to 1.350 | 0.013 | 108 | 98.26 | 0.694 | 0.274 | 0.156 to 1.232 | 0.011 |
| Marine fish | 16 | 98.42 | 1.645 | 0.679 | 0.314 to 2.976 | 0.015 | 15 | 98.64 | 1.795 | 0.702 | 0.420 to 3.170 | 0.011 |
| Supplemental types | ||||||||||||
| Additives | 78 | 98.86 | 1.645 | 0.396 | 0.870 to 2.420 | <0.0001 | 68 | 97.16 | 1.016 | 0.265 | 0.496 to 1.536 | 0.0001 |
| Ingredients | 70 | 99.20 | 0.572 | 0.418 | -0.247 to 1.391 | 0.171 | 81 | 99.17 | 0.580 | 0.358 | -0.122 to 1.281 | 0.105 |
| Trophic level | ||||||||||||
| Low trophic level | 80 | 99.12 | 1.831 | 0.465 | 0.920 to 2.742 | <0.0001 | 79 | 98.55 | 0.845 | 0.357 | 0.146 to 1.544 | 0.018 |
| Medium trophic level | 54 | 98.28 | 0.671 | 0.307 | 0.069 to 1.272 | 0.029 | 58 | 98.52 | 1.018 | 0.300 | 0.430 to 1.605 | 0.001 |
| High trophic level (O. mykiss) | 14 | 98.73 | -1.019 | 0.852 | -2.688 to 0.650 | 0.231 | 12 | 97.90 | -0.737 | 0.671 | -2.053 to 0.579 | 0.273 |
k: sample size (no. of comparison); I2: percentage variation across studies due to heterogeneity; SE: standard error; CL: confidence limits (lower and upper).
Table 2.
Effect size calculation for feed conversion ratio (FCR) and protein efficiency ratio (PER) comparison between control treatment and spirulina meal supplemented treatment across aquaculture species through random-effects model.
| Effect size (random-effects model) for FCR | Effect size (random-effects model) for PER | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| k | I 2 | Hedges' g value | SE | CL | P value | k | I 2 | Hedges' g value | SE | CL | P value | |
| All species | 147 | 97.71 | -0.700 | 0.190 | -1.072 to -0.328 | 0.0002 | 77 | 96.69 | 0.796 | 0.207 | 0.390 to 1.202 | 0.0001 |
| Subgroups | ||||||||||||
| Species category | ||||||||||||
| Fish | 121 | 97.29 | -0.615 | 0.202 | -1.011 to -0.218 | 0.002 | 63 | 95.94 | 1.003 | 0.224 | 0.565 to 1.442 | <0.0001 |
| Shrimp | 26 | 98.73 | -1.089 | 0.508 | -2.085 to -0.093 | 0.032 | 14 | 97.82 | -0.109 | 0.471 | -1.032 to 0.815 | 0.818 |
| Fish + habitat | ||||||||||||
| Freshwater fish | 107 | 97.31 | -0.575 | 0.214 | -0.995 to -0.155 | 0.007 | 49 | 95.90 | 1.076 | 0.248 | 0.591 to 1.561 | <0.0001 |
| Marine fish | 14 | 97.09 | -0.910 | 0.635 | -2.155 to 0.334 | 0.152 | 14 | 96.06 | 0.728 | 0.531 | -0.313 to 1.768 | 0.170 |
| Supplemental types | ||||||||||||
| Additives | 70 | 96.06 | -0.810 | 0.203 | -1.207 to -0.413 | <0.0001 | 36 | 91.52 | 1.100 | 0.181 | 0.745 to 1.454 | <0.0001 |
| Ingredients | 77 | 98.33 | -0.596 | 0.321 | -1.224 to 0.032 | 0.063 | 41 | 97.62 | 0.513 | 0.358 | -0.190 to 1.215 | 0.153 |
| Trophic level | ||||||||||||
| Low trophic level | 81 | 98.06 | -0.691 | 0.289 | -1.258 to -0.124 | 0.017 | 45 | 95.70 | 0.801 | 0.243 | 0.325 to 1.277 | 0.001 |
| Medium trophic level | 57 | 97.33 | -0.606 | 0.261 | -1.118 to -0.094 | 0.020 | 32 | 97.59 | 0.813 | 0.372 | 0.083 to 1.542 | 0.029 |
| High trophic level (O. mykiss) | 9 | 91.10 | -1.261 | 0.557 | -2.352 to -0.170 | 0.024 | — | — | — | — | — | — |
k: sample size (no. of comparison); I2: percentage variation across studies due to heterogeneity; SE: standard error; CL: confidence limits (lower and upper).
3.3. Effect of Dietary SPM Addition on FBW
A total of 148 comparisons were executed to investigate the overall effect of dietary SPM inclusion on FBW of aquatic animals (Table 1), and the results of publication bias for FBW were shown in Table S3 and Figure S1. The results suggested that dietary SPM addition significantly improved FBW compared to control group (Hedges' g = 1.14, 95%CI = 0.57 to 1.71, P < 0.0001) with big heterogeneity (I2 = 99.09%, Pheterogeneity < 0.0001). Subgroup analysis was carried out in this study to explore the sources of such high heterogeneity. According to possible factors, the FBW dataset was decomposed based on experimental animals, habitat of cultured fish, usage of spirulina meal, and trophic level (Table 1 and Figure 2). The subgroup analysis displayed that dietary supplementation of spirulina meal significantly improved FBW in fish (Hedge's g = 0.87, P = 0.002), freshwater fish (g = 0.75, P = 0.013), marine fish (g = 1.65, P = 0.015), shrimp (g = 2.73, P = 0.013), additives subgroup (g = 1.65, P < 0.0001), low trophic level species (g = 1.83, P < 0.0001), and medium trophic level species (g = 0.67, P = 0.029). Whereas, species with high trophic levels (g = −1.02, P = 0.23) and a subgroup of ingredients (g = 0.57, P = 0.17) were not significantly affected by dietary addition of SPM.
Figure 2.
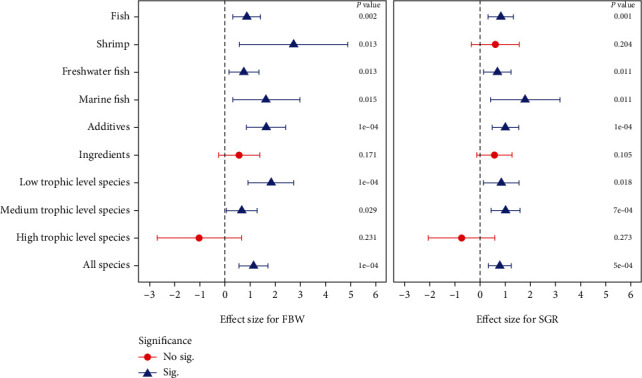
Hedges' g comparisons for FBW and SGR (mean ± 95% confidence interval) subgroup analysis (random-effects model). The confidence interval intersecting with the dashed line indicated no significant differences between the control group and treatment group, and vice versa.
Moreover, meta-regression was also performed to explore the possible relationship between the effect size of FBW (EFFBW) and SPM inclusion level as a feed additive or a feed ingredient in fish and shrimp. As a feed additive, a significantly quadratic relationship between EFFBW and SPM inclusion level in fish was observed (P = 0.02, R2 = 0.121), and optimal level of Spirulina was estimated to be 1.46% through the fitted equation (Figure 3(a)). As a feed ingredient, the best fittest curve was also a quadratic equation for fish (P < 0.0001, R2 = 0.437), and the upper limit of SPM level in fish diet was calculated to be 24.53%, exceeding which negative influence of SPM on FBW of fish would emerge (Figure 3(b)). As for shrimp, the broken-line regression was the better fitted one, and the biggest usage of spirulina meal in the diet was estimated as 14.95% (Figure 3(b)).
Figure 3.
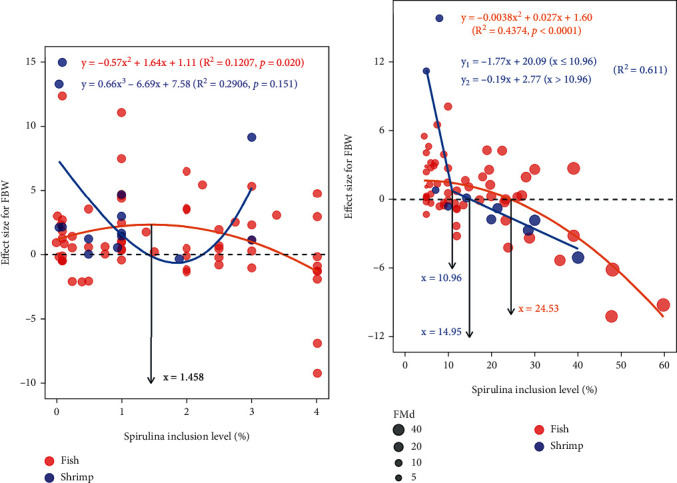
Random-effects model regression of spirulina meal inclusion level as a feed additive (a) and feed ingredient (b) on the effect size of FBW. The circle size represents the replaced level (%) of fishmeal by SPM, in other words, it means the difference of fishmeal content between the control group and SPM supplemented group.
3.4. Effect of Dietary SPM Addition on SGR
A sum of 149 comparisons for examining the influence of dietary SPM addition on SGR in aquaculture animals were shown in Table 1, and the results of publication bias for SGR were exhibited in Table S4 and Figure S2. The results indicated that dietary SPM supplementation had a significantly beneficial effect on SGR in all species (Hedges' g = 0.79, 95%CI = 0.34 to 1.23, P = 0.001) with high heterogeneity (I2 = 98.69%, Pheterogeneity < 0.0001). In the subgroup analysis, the overall trend of EFSGR in each subgroup was exactly alike as to those of EFFBW except for the shrimp subgroup. In this subgroup, the effect of SPM on SGR in shrimp showed no significant difference (g = 0.61, 95%CI = −0.33 to 1.56, P = 0.204).
As a feed additive, SPM inclusion level showed a significantly cubic relationship with EFSGR in fish (P = 0.04, R2 = 0.102), and optimum level of SPM based on cubic equation was recommended to be 2.26% (Figure 4(a)). As a feed ingredient, quadratic regression was the best fitted curve both in fish and shrimp, and the highest usage of SPM in fish and shrimp were estimated to be 23.94% and 24.85%, respectively (Figure 4(b)).
Figure 4.
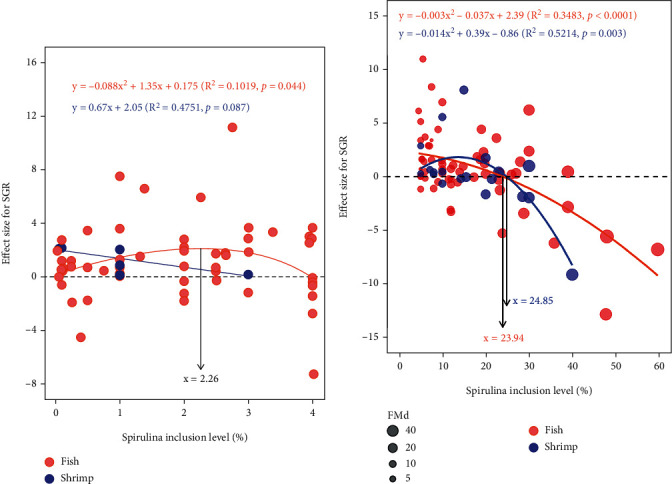
Random-effects model regression of spirulina meal inclusion level as a feed additive (a) and feed ingredient (b) on the effect size of SGR. The circle size represents the replaced level (%) of fishmeal by SPM, in other words, it means the difference of fishmeal content between the control group and SPM supplemented group.
3.5. Effect of Dietary SPM Addition on FCR
A sum of 147 comparisons were carried out to investigate the influence of dietary administration of SPM on FCR in aquaculture animals (Table 2 and Figure 5), and the results of publication bias for FCR were exhibited in Table S5 and Figure S3. We found that SPM included in diets clearly decreased FCR of fish and shrimp (Hedges' g = −0.70, 95%CI = −1.07 to − 0.33, P = 0.0002). The subgroup analysis manifested that dietary spirulina addition noteworthily reduced FCR in all subgroups excepting the subgroups of ingredients and marine fish, in which there were no obvious differences in FCR between the control group and SPM supplemented group (g = −0.60, P = 0.06 for ingredients group; g = −0.91, P = 0.15 for marine fish group) (Table 2 and Figure 5).
Figure 5.
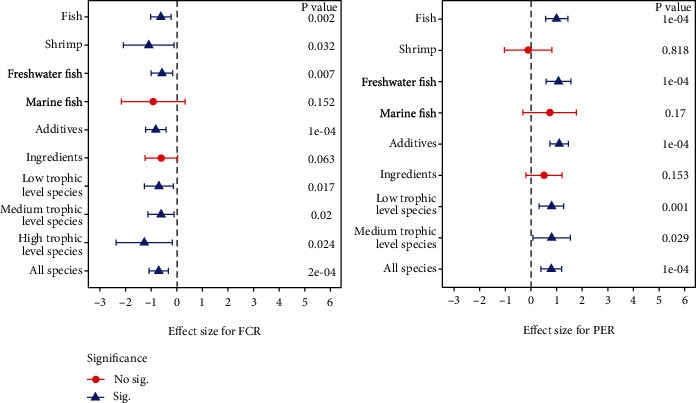
Hedges' g comparisons for FCR and PER (mean ± 95% CI) subgroup analysis (random-effects model). The confidence interval intersecting with the dashed line indicated no significant differences between the control group and treatment group, and vice versa.
In the meta-regression, there was a significantly negatively linear correlation between the effect size of FCR (EFFCR) and spirulina level in fish diet (P = 0.048, R2 = 0.062) (Figure 6(a)) when spirulina was treated as a feed additive. Optimum level of SPM in shrimp diet based on the quadratic equation was advised to be 1.67% (Figure 6(a)). As a feedstuff, linear regression was the best fitted curve both in fish and shrimp, and the upper limits of spirulina meal usage in fish and shrimp were calculated separately to be 22.03% and 20.00% (Figure 6(b)).
Figure 6.
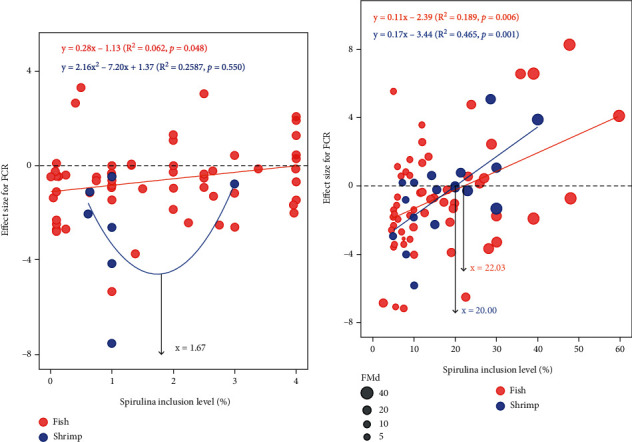
Random-effects model regression of spirulina meal inclusion level as a feed additive (a) and feed ingredient (b) on the effect size of FCR. The circle size represents the replaced level (%) of fishmeal by SPM, in other words, it means the difference of fishmeal content between the control group and SPM supplemented group.
3.6. Effect of Dietary SPM Addition on PER
A sum of 77 comparisons were conducted to examine the effect of dietary addition of SPM on PER in aquaculture animals (Table 2 and Figure 5), and the results of publication bias for PER were exhibited in Table S6 and Figure S4. We discovered that dietary addition of SPM significantly improved PER in fish and shrimp (Hedges' g = 0.80, 95%CI = 0.39 to 1.20, P = 0.0001) with high heterogeneity (I2 = 96.69%, Pheterogeneity < 0.0001). The subgroup analysis manifested that dietary SPM addition clearly increased PER in all subgroups excepting shrimp, marine fish, and ingredient subgroups. There were no obvious variations in PER for shrimp subgroup (g = −0.11, P = 0.82), marine fish group (g = 0.73, P = 0.17), and ingredient subgroup (g = 0.51, P = 0.15) between control group and SPM supplemented group (Table 2 and Figure 5). Due to the small sample size, meta-regression analysis between effect size of PER and SPM inclusion levels in fish and shrimp was not performed.
4. Discussion
Spirulina meal (SPM) has garnered increasing attention in the past few years and has been extensively studied as a novel protein source and a functional feed additive in aquafeeds. There existed big differences in the application efficacy of SPM in different aquaculture animals when it was regarded as a protein source in the diet formula. Fish meal could be 100% substituted by SPM with higher growth rate and better feed utilization in tilapia [69], while in C. moorii and A. citrinellus × C. trimaculatum, a small quantity of replacement for FM resulted in inferior growth and feed utilization [42, 54, 55]. Likewise, SPM, as a functional additive, exerted a negative influence on growth performance in some studies [82]. Whereas, high potency has been observed in rainbow trout when the supplemental dose of SPM was optimal [61]. The efficacy instability of SPM was also well reflected in the current study, in which large confidence intervals of overall effect size were noticed in FBW (0.51 to 1.71), SGR (0.34 to 1.23), FCR (-1.07 to -0.33), and PER (0.39 to 1.20). In spite of the high heterogeneity, the immutable fact was that the mean effect sizes of FBW, SGR, FCR, and PER was favorable for animals and were 1.14, 0.79, -0.70, and 0.80, respectively. Meanwhile, the average effect sizes of CF and HSI were not significantly affected by SPM administration. It is indicative that on average dietary, SPM inclusion significantly improved growth performance and feed utilization of aquaculture animals.
Although the pooled effect sizes of outcome parameters on the whole dataset validated the benefits of SPM on growth performance, the degree of a cross-study heterogeneity was large and this meta-analysis clearly averaged out some key influential factors, including cultured species (fish and shrimp), use of SPM (ingredient and additive), trophic levels of animals (low, medium, and high), and inclusion level of SPM. The primary target of a subgroup analysis is to recognize either consistency or big variances in the magnitude of the treatment effect among different groupings [90]. Therefore, the subgroup analysis was employed to characterize the effects of some of the aforementioned main covariates. Meta-regression aims at distinguishing whether a linear relationship exists between an outcome measure and a continuous variable [91]. Thus, the meta-regression analysis was conducted to explore the most probable relationship between the pooled effect size of each parameter and SPM inclusion level. We could determine the optimal concentration of SPM in the diet as a feed additive by means of meta-regression analysis and identify the upper threshold of SPM substituting fishmeal in diet beyond which the negative effects on growth might occur.
The subgroup analysis revealed that dietary SPM addition significantly improved growth and feed utilization in fish with large sample sizes (k = 124 for FBW, 123 for SGR, 121 for FCR, and 63 for PER). From the mathematical point of view, the majority of Hedge's g from the fish subsets of FBW, SGR, and PER are distributed at more than zero (75 in FBW, 91 in SGR, and 53 in PER). Meanwhile, most of data in the FCR subset distributed at less than zero (91 in FCR). Therefore, the overall synthesized results of the growth of fish fed SPM were beneficial for fish. The SPM was applied in fish diet in the forms of a feed supplement and a FM substitute. When the SPM was included in the fish diet as a supplement, the growth-promoting effects might be attributed to its high nutrient intensity (high protein content, γ-linolenic acid, etc.) and bioactive compounds (polysaccharides, carotenoids, chlorophyll, etc.) [17–20]. This could also be mirrored in the regression charts, in which most of the red dots dispersed above the dashed line (g = 0) for FBW and SGR subgrouping (Figures 3(a) and 4(a)). For FCR subsets, the red dots scattered mainly below the reference line (Figure 6(a)). When the SPM was used in the form of a feed ingredient, low and medium substitution levels of fishmeal (not exceeding a certain replacement levels) benefiting the growth of fish compared to the control diets were recorded in many studies [17–20, 36, 42, 46, 47, 60, 74]. Apart from the active components in SPM, this growth-promoting effect may be also correlated with the modified composition of gut microbiota by SPM inclusion in fish diets. It has been reported that adding 15% SPM in zebrafish diet significantly increased the abundance of beneficial bacteria in the gut such as Cetobacterium and decreased the proportion of pernicious bacteria such as Vibrio [92]. Cetobacterium is one of the most common bacteria in the intestine of freshwater fish and could produce vitamin B12, which is essential nutrient for fish [93], and Vibrio is one of the most prevalent pathogens and leads to vibriosis in fish [94]. Similarly, SPM has also been found to alter the intestinal microbiota structure in Yellow River carp and exert a positive influence on health [13, 14]. Regarding the shrimp subgroup, SPM supplementation significantly altered FBW and FCR, but had no significant differences on SGR and PER. This may be due to the small sample size in this dataset (k = 24 for FBW, 26 for SGR, 26 for FCR, and 14 for PER).
In terms of additive subgroups, SPM addition noteworthily enhanced growth performance in fish and shrimp, which could be ascribed to its easy digestibility [95], and abundant antioxidants, including phycocyanin, polysaccharide, polyunsaturated fatty acids, vitamins, carotenoids, and other bioactive compounds [96]. It was because of the strong antioxidation properties of the Spirulina, some energy for metabolic processes could be saved for animal growth. Whereas in the ingredient subgroup, SPM inclusion had no significant effect on animal growth. This may be attributed to the negative impact of high substitution levels of fishmeal by SPM in some studies [74, 83]. We also conducted the subgroup analysis based on the trophic level of an animal. It has been found that SPM supplementation significantly affected FBW, SGR, FCR, and PER in low and medium trophic species. In the high trophic species (O. mykiss) subgroup, there were no significant variances in FBW and SGR between the control group and SPM group. It could be correlated with the feeding habit of the animal, low and medium trophic animals belonging to herbivores or omnivore, they could digest some microalgae well in the gut in the natural waters from an evolutionary point of view. However, O. mykiss appertains to carnivores, the effect of dietary SPM as a supplement or a feed ingredient on growth was poor in this fish [58, 61, 62].
To investigate the optimal inclusion of SPM as a feed supplement and the superior limit of SPM inclusion level for substituting fishmeal in fish and shrimp, the meta-regression between the effect sizes of outcome measures (FBW, SGR, and PER) and SPM inclusion level was carried out. It indicated that the optimal addition level of SPM in fish diet was estimated to be 1.46%-2.26% based on the effect sizes of FBW and SGR with SPM inclusion level. In addition, the lowest value of FCR effect size was assessed at 1.67% of SPM inclusion level in shrimp. The upper limit of SPM inclusion level replacing fishmeal ranged from 22.03% to 24.53% in fish. This scope was agreed with previous studies, in which the maximal usage of SPM meal was estimated at 23.03% in P. fulvidraco [83] and 22.50% in M. liza [17–20] from the viewpoint of growth. Whereas this extent of SPM was broader from 14.95% to 24.85% in shrimp, this may be related to the small sample size in shrimp subsets. This also may be due to different shrimp species in the FBW subset and SGR subset. There were three shrimp species in the FBW subset, including L. vannamei [32], N. davidi [38], and M. rosenbergii [36], whereas the SGR subclass had another species (P. monodon). This shrimp species, with an omnivorous feeding habit, was demonstrated to have the capability to utilize at least 20.00% SPM in the diet without jeopardizing growth performance [40]. Therefore, the maximum usage of SPM in shrimp diet reached 24.85%.
5. Conclusion
In a nutshell, the present quantitative meta-analysis indicated that dietary SPM addition significantly improved final body weight, specific growth rate, and protein efficiency ratio, and simultaneously significantly decreased the feed conversion ratio of aquaculture animals. In addition, the growth-enhancing effect of SPM inclusion was significant when it was applied in the form of feed additive (the inclusion level was less than 4%); however, the effect was indistinctive when it was utilized as a feedstuff. Furthermore, the meta-regression analysis displayed that the optimal addition levels of SPM as a growth-promoting feed supplement in fish and shrimp diets were 1.46%-2.26% and 1.67%, respectively. Furthermore, application of SPM in diet could efficiently regulate the body color in fish according previous studies. Therefore, despite high price of SPM, it could be also applied as a diet additive currently for aquatic animals having higher demand for body color in the market, such as P. fulvidraco and some ornamental fish. Additionally, up to 22.03%-24.53% and 14.95%-24.85% of SPM as fishmeal substitute in fish and shrimp diets did not have a negative effect on growth and feed utilization, respectively. Due to high price of SPM, it could be put into practice in aquafeeds as main protein sources in the future when the market price declines sharply.
Acknowledgments
This work was supported by S&T Program of Hebei (21326703D), Scientific Research Fund of Hebei Normal University (L2020B23), Hebei MATRT (HBCT2018180205, HBCT2018170205), Science and Technology Project of Hebei Education Department (QN2022125), and Natural Science Foundation of Hebei Province (C2022205034).
Data Availability
Data are available on request from the authors.
Conflicts of Interest
The authors declare that they have no conflict of interest in this manuscript.
Authors' Contributions
Ling Li was responsible for investigation, methodology, formal analysis, and writing the original draft. Haiyan Liu was responsible for data curation and validation. Peiyu Zhang was responsible for conceptualization, funding acquisition, project administration, and writing the review and editing.
Supplementary Materials
The detailed information of included studies in this meta-analysis was listed in Table S1 of Supplemental Materials. The meta-analysis for CF and HSI were presented in Table S2 and Figure S7. In addition, publication bias for each outcome indicator (FBW, SGR, FCR, PER, CF, and HSI) in this study evaluated by methods of Egger's regression test, Begg's rank correlation test, and funnel plot were shown in Tables S3-S8 and Figures S1-S6, respectively. Furthermore, R code in this meta-analysis was also shared in the Supplemental Materials.
References
- 1.Henchion M., Hayes M., Mullen A. M., Fenelon M., Tiwari B. Future protein supply and demand: strategies and factors influencing a sustainable equilibrium. Food . 2017;6(7):p. 53. doi: 10.3390/foods6070053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyd C. E., McNevin A. A., Davis R. P. The contribution of fisheries and aquaculture to the global protein supply. Food security . 2022;14(3):805–827. doi: 10.1007/s12571-021-01246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schar D., Klein E. Y., Laxminarayan R., Gilbert M., Van Boeckel T. P. Global trends in antimicrobial use in aquaculture. Scientific Reports . 2020;10(1):p. 21878. doi: 10.1038/s41598-020-78849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naylor R. L., Hardy R. W., Buschmann A. H., et al. A 20-year retrospective review of global aquaculture. Nature . 2021;591(7851):551–563. doi: 10.1038/s41586-021-03308-6. [DOI] [PubMed] [Google Scholar]
- 5.Alagawany M., Taha A. E., Noreldin A., El-Tarabily K. A., Abd El-Hack M. E. Nutritional applications of species of Spirulina and Chlorella in farmed fish: a review. Aquaculture . 2021;542:p. 736841. doi: 10.1016/j.aquaculture.2021.736841. [DOI] [Google Scholar]
- 6.Grosshagauer S., Kraemer K., Somoza V. The true value of Spirulina. Journal of Agricultural and Food Chemistry . 2020;68(14):4109–4115. doi: 10.1021/acs.jafc.9b08251. [DOI] [PubMed] [Google Scholar]
- 7.Carcea M., Sorto M., Batello C., et al. Nutritional characterization of traditional and improved dihe, alimentary blue-green algae from the lake Chad region in Africa. LWT- Food Science and Technology . 2015;62(1):753–763. doi: 10.1016/j.lwt.2014.10.039. [DOI] [Google Scholar]
- 8.Chentir I., Hamdi M., Li S., Doumandji A., Markou G., Nasri M. Stability, bio-functionality and bio-activity of crude phycocyanin from a two- phase cultured Saharian Arthrospira sp. strain. Algal Research . 2018;35:395–406. doi: 10.1016/j.algal.2018.09.013. [DOI] [Google Scholar]
- 9.Cardoso L. G., Duarte J. H., Andrade B. B., et al. Spirulina sp. LEB 18 cultivation in outdoor pilot scale using aquaculture wastewater: high biomass, carotenoid, lipid and carbohydrate production. Aquaculture . 2020;525:p. 735272. doi: 10.1016/j.aquaculture.2020.735272. [DOI] [Google Scholar]
- 10.Cardoso L. G., Lombardi A. T., de Jesus Silva J. S., et al. Scaling-up production of Spirulina sp. LEB18 grown in aquaculture wastewater. Aquaculture . 2021;544:p. 737045. doi: 10.1016/j.aquaculture.2021.737045. [DOI] [Google Scholar]
- 11.Xia Y., Liu C., Fei S., et al. Arthrospira platensis additive enhances the growth performance and antioxidant response in hybrid yellow catfish (Pelteobagrus fulvidraco ♀ × Pelteobagrus vachelli ♂) Aquaculture Reports . 2021;20:p. 100721. doi: 10.1016/j.aqrep.2021.100721. [DOI] [Google Scholar]
- 12.Mohammadiazarm H., Maniat M., Ghorbanijezeh K., Ghotbeddin N. Effects of spirulina powder (Spirulina platensis) as a dietary additive on Oscar fish, Astronotus ocellatus: assessing growth performance, body composition, digestive enzyme activity, immune-biochemical parameters, blood indices and total pigmentation. Aquaculture Nutrition . 2021;27(1):252–260. doi: 10.1111/anu.13182. [DOI] [Google Scholar]
- 13.Ren H. T., Du M. X., Zhou J., An H. Y. Effect of Spirulina and Ferrous Fumarate on Intestinal Morphology and the Diversity of Gut Microbiota of Yellow River Carp. Biological Trace Element Research . 2022;200(9):4142–4149. doi: 10.1007/s12011-021-02993-8. [DOI] [PubMed] [Google Scholar]
- 14.Ren H. T., Zhao X. J., Huang Y., Xiong J. L. Combined effect of Spirulina and ferrous fumarate on growth parameters, pigmentation, digestive enzyme activity, antioxidant enzyme activity and fatty acids composition of Yellow River carp (Cyprinus carpio) Aquaculture Reports . 2021;21:p. 100776. doi: 10.1016/j.aqrep.2021.100776. [DOI] [Google Scholar]
- 15.Ragaza J. A., Hossain M. S., Meiler K. A., Velasquez S. F., Kumar V. A review on Spirulina: alternative media for cultivation and nutritive value as an aquafeed. Reviews in Aquaculture . 2020;12(4):2371–2395. doi: 10.1111/raq.12439. [DOI] [Google Scholar]
- 16.Zhang F., Man Y. B., Mo W. Y., Wong M. H. Application of Spirulina in aquaculture: a review on wastewater treatment and fish growth. Reviews in Aquaculture . 2020;12(2):582–599. doi: 10.1111/raq.12341. [DOI] [Google Scholar]
- 17.Rosas V. T., Bessonart M., Romano L. A., Tesser T. B. Fishmeal substitution for Arthrospira platensis in juvenile mullet (Mugil liza) and its effects on growth and non-specific immune parameters. Revista Colombiana de Ciencias Pecuarias . 2019;32(1):3–13. doi: 10.17533/udea.rccp.v32n1a01. [DOI] [Google Scholar]
- 18.Rosas V. T., Monserrat J. M., Bessonart M., Magnone L., Romano L. A., Tesser M. B. Fish oil and meal replacement in mullet (Mugil liza) diet with Spirulina (Arthrospira platensis) and linseed oil. Comparative Biochemistry and Physiology, Part C: Toxicology & Pharmacology . 2019;218:46–54. doi: 10.1016/j.cbpc.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Rosas V. T., Monserrat J. M., Bessonart M., Magnone L., Romano L. A., Tesser M. B. Comparison of β-carotene and Spirulina (Arthrospira platensis) in mullet (Mugil liza) diets and effects on antioxidant performance and fillet colouration. Journal of Applied Phycology . 2019;31(4):2391–2399. doi: 10.1007/s10811-019-01773-1. [DOI] [Google Scholar]
- 20.Rosas V. T., Poersch L. H., Romano L. A., Tesser M. B. Feasibility of the use of Spirulina in aquaculture diets. Reviews in Aquaculture . 2019;11(4):1367–1378. doi: 10.1111/raq.12297. [DOI] [Google Scholar]
- 21.Gurevitch J., Koricheva J., Nakagawa S., Stewart G. Meta-analysis and the science of research synthesis. Nature . 2018;555(7695):175–182. doi: 10.1038/nature25753. [DOI] [PubMed] [Google Scholar]
- 22.Konnert G. D. P., Gerrits W. J. J., Gussekloo S. W. S., Schrama J. W. Balancing protein and energy in Nile tilapia feeds: a meta-analysis. Reviews in Aquaculture . 2022;14(4):1757–1778. doi: 10.1111/raq.12671. [DOI] [Google Scholar]
- 23.Liland N. S., Araujo P., Xu X. X., et al. A meta-analysis on the nutritional value of insects in aquafeeds. Journal of Insects as Food and Feed . 2021;7(5):743–759. doi: 10.3920/JIFF2020.0147. [DOI] [Google Scholar]
- 24.Maas R. M., Verdegem M. C. J., Wiegertjes G. F., Schrama J. W. Carbohydrate utilisation by tilapia: a meta-analytical approach. Reviews in Aquaculture . 2020;12:1851–1866. doi: 10.1111/raq.12413. [DOI] [Google Scholar]
- 25.Knobloch K., Yoon U., Vogt P. M. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. Journal of Cranio-Maxillofacial Surgery . 2011;39(2):91–92. doi: 10.1016/j.jcms.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Fan S., Wang B., Liu H., et al. Trophodynamics of organic pollutants in pelagic and benthic food webs of lake Dianchi: importance of ingested sediment as uptake route. Environmental Science & Technology . 2017;51(24):14135–14143. doi: 10.1021/acs.est.7b03681. [DOI] [PubMed] [Google Scholar]
- 27.Guo K., Zhao W., Wang S., Liu B., Zhang P. Food web structure and trophic levels in a saltwater pond sea cucumber and prawn polyculture system. Acta Oceanologica Sinica . 2016;35(4):58–62. doi: 10.1007/s13131-016-0834-9. [DOI] [Google Scholar]
- 28.Mirzajani A., Ghane A., Bagheri S., et al. Diet survey and trophic position of Macrobrachium nipponense in the food web of Anzali wetland. Wetlands . 2020;40(5):1229–1239. doi: 10.1007/s13157-020-01278-5. [DOI] [Google Scholar]
- 29.Li L., Liu H. Y., Xie S. Q., Zhang P. Y., Yang Z. C. Effects of taurine supplementation on growth performance and feed utilization in aquatic animals: a meta-analysis. Aquaculture . 2022;551:p. 737896. doi: 10.1016/j.aquaculture.2022.737896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu B., Li Z., Wang C., et al. Effects of fermented feed supplementation on pig growth performance: A meta- analysis. Animal Feed Science and Technology . 2020;259:p. 114315. doi: 10.1016/j.anifeedsci.2019.114315. [DOI] [Google Scholar]
- 31.Kim C. J., Yoon S. K., Kim H. I., Park Y. H., Oh H. M. Effect of Spirulina platensis and probiotics as feed additives on growth of shrimp Fenneropenaeus chinensis. Journal of Microbiology and Biotechnology . 2006;16:1248–1254. [Google Scholar]
- 32.Macias-Sancho J., Poersch L. H., Bauer W., Romano L. A., Wasielesky W., Tesser M. B. Fishmeal substitution with Arthrospira (Spirulina platensis) in a practical diet for Litopenaeus vannamei: Effects on growth and immunological parameters. Aquaculture . 2014;426-427:120–125. doi: 10.1016/j.aquaculture.2014.01.028. [DOI] [Google Scholar]
- 33.Pakravan S., Akbarzadeh A., Sajjadi M. M., Hajimoradloo A., Noori F. Partial and total replacement of fish meal by marine microalga Spirulina platensis in the diet of Pacific white shrimp Litopenaeus vannamei: growth, digestive enzyme activities, fatty acid composition and responses to ammonia and hypoxia stress. Aquaculture Research . 2017;48(11):5576–5586. doi: 10.1111/are.13379. [DOI] [Google Scholar]
- 34.Silva-Neto J. F., Nunes A. J. P., Sabry-Neto H., Sá M. V. C. Spirulina meal has acted as a strong feeding attractant for Litopenaeus vannamei at a very low dietary inclusion level. Aquaculture Research . 2012;43(3):430–437. doi: 10.1111/j.1365-2109.2011.02846.x. [DOI] [Google Scholar]
- 35.Liu L., Cai X., Ai Y., et al. Effects of Lactobacillus pentosus combined with Arthrospira platensis on the growth performance, immune response, and intestinal microbiota of Litopenaeus vannamei. Fish & Shellfish Immunology . 2022;120:345–352. doi: 10.1016/j.fsi.2021.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Qu Y. H., Li X., Wang H. L., et al. Effects of partial replacement of fish meal by Spirulina on growth performance, nutrient apparent digestibility coefficients, whole-body composition and serum biochemical indices of Macrobrachium rosenbergii. Chinese Journal of Animal Nutrition . 2021;33(4):2187–2198. [Google Scholar]
- 37.Chien Y.-H., Shiau W. C. The effects of dietary supplementation of algae and synthetic astaxanthin on body astaxanthin, survival, growth, and low dissolved oxygen stress resistance of kuruma prawn, Marsupenaeus japonicus Bate. Journal of Experimental Marine Biology and Ecology . 2005;318(2):201–211. doi: 10.1016/j.jembe.2004.12.016. [DOI] [Google Scholar]
- 38.Namaei Kohal M., Esmaeili Fereidouni A., Firouzbakhsh F., Hayati I. Effects of dietary incorporation of Arthrospira (Spirulina) platensis meal on growth, survival, body composition, and reproductive performance of red cherry shrimp Neocaridina davidi (Crustacea, Atyidae) over successive spawnings. Journal of Applied Phycology . 2018;30(1):431–443. doi: 10.1007/s10811-017-1220-5. [DOI] [Google Scholar]
- 39.Abdel-Warith A.-W. A., Fath El-Bab A. F., Younis E.-S. M. I., et al. Using of chitosan nanoparticles (CsNPs), Spirulina as a feed additives under intensive culture system for black tiger shrimp (Penaeus monodon) Journal of King Saud University - Science . 2020;32(8):3359–3363. doi: 10.1016/j.jksus.2020.09.022. [DOI] [Google Scholar]
- 40.Sivakumar N., Sundararaman M., Selvakumar G. J. I., Jo A. R. Evaluation of growth performance of Penaeus monodon (Fabricius) fed diet with partial replacement of fishmeal by Spirulina platensis (Sp) meal. Indian Journal of Animal Research . 2018;52:1721–1726. doi: 10.18805/ijar.B-3438. [DOI] [Google Scholar]
- 41.Kolanchinathan P., Kumari P. R., Raja K., John G., Balasundaram A. Analysis of feed composition and growth parameters of Penaeus monodon supplemented with two probiotic species and formulated diet. Aquaculture . 2022;549:p. 737740. doi: 10.1016/j.aquaculture.2021.737740. [DOI] [Google Scholar]
- 42.Sornsupharp B., Lomthaisong K., Dahms H. U., Sanoamuang L. O. Effects of dried fairy shrimp Streptocephalus sirindhornae meal on pigmentation and carotenoid deposition in flowerhorn cichlid; Amphilophus citrinellus (Günther, 1864) × Cichlasoma trimaculatum (Günther, 1867) Aquaculture Research . 2015;46(1):173–184. doi: 10.1111/are.12172. [DOI] [Google Scholar]
- 43.Jha G. N., Sarma D., Qureshi T. A. Effect of Spirulina (Spirulina platensis) and marigold (Tagetes erecta) fortified diets on growth, body composition and total carotenoid content of Barilius bendelisis. The Indian Journal of Animal Sciences . 2012;82:336–340. [Google Scholar]
- 44.James R., Vasudhevan I., Sampath K. Interaction of Spirulina with different levels of vitamin E on growth, reproduction, and coloration in goldfish (Carassius auratus) Israeli Journal of Aquaculture . 2009;61:330–338. doi: 10.46989/001c.20567. [DOI] [Google Scholar]
- 45.Cao S., Han D., Xie S., et al. Effects of dietary fishmeal replacement with Spirulina platensis powder on the growth performance, feed utilization and protein deposition in juvenile gibel carp (Carassis auratus gibelio var. CAS) Acta Hydrobiologica Sinica . 2016;40(4):647–654. [Google Scholar]
- 46.Cao S., Zhang P., Zou T., et al. Replacement of fishmeal by spirulina Arthrospira platensis affects growth, immune related-gene expression in gibel carp (Carassius auratus gibelio var. CAS III), and its challenge against Aeromonas hydrophila infection. Fish & Shellfish Immunology . 2018;79:265–273. doi: 10.1016/j.fsi.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 47.Cao S. P., Zou T., Zhang P. Y., et al. Effects of dietary fishmeal replacement with Spirulina platensis on the growth, feed utilization, digestion and physiological parameters in juvenile gibel carp (Carassis auratus gibelio var. CAS III) Aquaculture Research . 2018;49(3):1320–1328. doi: 10.1111/are.13590. [DOI] [Google Scholar]
- 48.Raji A. A., Alaba P. A., Yusuf H., et al. Fishmeal replacement with Spirulina platensis and Chlorella vulgaris in African catfish (Clarias gariepinus) diet: effect on antioxidant enzyme activities and haematological parameters. Research in Veterinary Science . 2018;119:67–75. doi: 10.1016/j.rvsc.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 49.Raji A. A., Jimoh W. A., Abu Bakar N. H., et al. Dietary use of Spirulina (Arthrospira) and Chlorella instead of fish meal on growth and digestibility of nutrients, amino acids and fatty acids by African catfish. Journal of Applied Phycology . 2020;32(3):1763–1770. doi: 10.1007/s10811-020-02070-y. [DOI] [Google Scholar]
- 50.Chainapong T., Traichaiyaporn S., Deming R. L. Effect of dietary Spirulina platensis on the fatty acid composition in flesh and ovary of walking catfish (Clarias macrocephalus) Chiang Mai Journal of Science . 2018;45:129–135. [Google Scholar]
- 51.Ansarifard F., Rajabi Islami H., Shamsaie Mehrjan M., Soltani M. Effects of Arthrospira platensis on growth, skin color and digestive enzymes of koi, Cyprinus carpio. Iranian Journal of Fisheries Sciences . 2018;17:381–393. [Google Scholar]
- 52.Ramakrishnan C. M., Haniffa M., Manohar M., Dhanaraj M., Arockiaraj A. J., Arunsingh S. Effects of probiotics and spirulina on survival and growth of juvenile common carp (Cyprinus carpio) Israeli Journal of Aquaculture . 2008;60:128–133. doi: 10.46989/001c.20484. [DOI] [Google Scholar]
- 53.Sun X., Chang Y., Ye Y., et al. The effect of dietary pigments on the coloration of Japanese ornamental carp (koi, Cyprinus carpio L.) Aquaculture . 2012;342-343:62–68. doi: 10.1016/j.aquaculture.2012.02.019. [DOI] [Google Scholar]
- 54.Erdogan F. Effects of Spirulina platensis as a feed additive on growth and coloration of blue dolphin cichlids (Cyrtocara moorii Boulunger, 1902) Aquaculture Research . 2019;50(9):2326–2332. doi: 10.1111/are.14112. [DOI] [Google Scholar]
- 55.Erdogan F. Effects ofSpirulina platensisas a feed additive on growth and coloration of blue dolphin cichlids (Cyrtocara mooriiBoulunger, 1902) Aquaculture Research . 2019;50(9):2326–2332. doi: 10.1111/are.14112. [DOI] [Google Scholar]
- 56.Guroy B., Guroy D., Bilen S., et al. Effect of dietary Spirulina (Arthrospira platensis) on the growth performance, immune-related gene expression and resistance to Vibrio anguillarum in European seabass (Dicentrarchus labrax) Aquaculture Research . 2022;53(6):2263–2274. doi: 10.1111/are.15745. [DOI] [Google Scholar]
- 57.Jiang W., Miao L., Lin Y., Ci L., Liu B., Ge X. Spirulina (Arthrospira) platensis as a protein source could improve growth, feed utilisation and digestion and physiological status in juvenile blunt snout bream (Megalobrama amblycephala) Aquaculture Reports . 2022;22:p. 100932. doi: 10.1016/j.aqrep.2021.100932. [DOI] [Google Scholar]
- 58.Güroy B., Güroy D., Mantoğlu S., et al. Dietary Spirulina (Arthrospira platensis, Gomont, 1892) improved the flesh quality and shelf life of rainbow trout (Oncorhynchus mykiss, Walbaum, 1792) fed fish meal or plant-based diet. Aquaculture Research . 2019;50(9):2519–2527. doi: 10.1111/are.14206. [DOI] [Google Scholar]
- 59.Sheikhzadeh N., Mousavi S., Khani Oushani A., Firouzamandi M., Mardani K. Spirulina platensis in rainbow trout (Oncorhynchus mykiss) feed: effects on growth, fillet composition, and tissue antioxidant mechanisms. Aquaculture International . 2019;27(6):1613–1623. doi: 10.1007/s10499-019-00412-3. [DOI] [Google Scholar]
- 60.Teimouri M., Amirkolaie A. K., Yeganeh S. The effects of Spirulina platensis meal as a feed supplement on growth performance and pigmentation of rainbow trout (Oncorhynchus mykiss) Aquaculture . 2013;396-399:14–19. doi: 10.1016/j.aquaculture.2013.02.009. [DOI] [Google Scholar]
- 61.Kermani P., Babaei S., Abedian-Kenari A., Hedayati M. Growth performance, plasma parameters and liver antioxidant enzymes activities of rainbow trout (Oncorhynchus mykiss) juvenile fed on Spirulina platensis extract. Iranian Journal of Fisheries Sciences . 2020;19:1463–1478. [Google Scholar]
- 62.Twibell R., Johnson R., Hyde N., Gannam A. Evaluation of Spirulina and plant oil in diets for juvenile steelhead (Oncorhynchus mykiss) Aquaculture . 2020;528:p. 735598. doi: 10.1016/j.aquaculture.2020.735598. [DOI] [Google Scholar]
- 63.Kim S. S., Rahimnejad S., Kim K. W., Lee K. J. Partial replacement of fish meal with spirulina pacifica in diets for parrot fish (Oplegnathus fasciatus) Turkish Journal of Fisheries and Aquatic Sciences . 2013;13:197–204. [Google Scholar]
- 64.Abdel-Tawwab M., Ahmad M. H. Live Spirulina (Arthrospira platensis) as a growth and immunity promoter for Nile tilapia, Oreochromis niloticus (L.), challenged with pathogenic Aeromonas hydrophila. Aquaculture Research . 2009;40(9):1037–1046. doi: 10.1111/j.1365-2109.2009.02195.x. [DOI] [Google Scholar]
- 65.Abdel-Warith A., Elsayed E. Use of Arthrospira platensis as a feed additive to improve growth performance, feed utilization, body composition, and immune response of Nile tilapia, Oreochromis niloticus. Journal of Scientific and Industrial Research . 2019;78:681–686. [Google Scholar]
- 66.Al-Deriny S. H., Dawood M. A. O., Elbialy Z. I., El-Tras W. F., Mohamed R. A. Selenium nanoparticles and Spirulina alleviate growth performance, hemato-biochemical, immune-related genes, and heat shock protein in Nile tilapia (Oreochromis niloticus) Biological Trace Element Research . 2020;198(2):661–668. doi: 10.1007/s12011-020-02096-w. [DOI] [PubMed] [Google Scholar]
- 67.Al-Deriny S. H., Dawood M. A. O., Zaid A. A. A., et al. The synergistic effects of Spirulina platensis and Bacillus amyloliquefaciens on the growth performance, intestinal histomorphology, and immune response of Nile tilapia (Oreochromis niloticus) Aquaculture Reports . 2020;17:p. 100390. doi: 10.1016/j.aqrep.2020.100390. [DOI] [Google Scholar]
- 68.Belal E. B., Khalafalla M. M. E., El-Hais A. M. A. Use of spirulina (Arthrospira fusiformis) for promoting growth of Nile tilapia fingerlings. African Journal of Microbiology Research . 2012;6(35):6423–6431. doi: 10.5897/AJMR12.288. [DOI] [Google Scholar]
- 69.El-Sheekh M., El-Shourbagy I., Shalaby S., Hosny S. Effect of feeding Arthrospira platensis (Spirulina) on growth and carcass composition of hybrid red tilapia (Oreochromis niloticus × Oreochromis mossambicus) Turkish Journal of Fisheries and Aquatic Sciences . 2014;14(2):471–478. doi: 10.4194/1303-2712-v14_2_18. [DOI] [Google Scholar]
- 70.Grassi T. L. M., Oliveira D. L., Paiva N. M., et al. Microbial biomass as an antioxidant for tilapia feed. Aquaculture Research . 2018;49(8):2881–2890. doi: 10.1111/are.13753. [DOI] [Google Scholar]
- 71.Mahmoud M. M. A., El-Lamie M. M. M., Kilany O. E., Dessouki A. A. _Spirulina_ (Arthrospira platensis) supplementation improves growth performance, feed utilization, immune response, and relieves oxidative stress in Nile tilapia (Oreochromis niloticus) challenged with Pseudomonas fluorescens. Fish & Shellfish Immunology . 2018;72:291–300. doi: 10.1016/j.fsi.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 72.Plaza I., García J. L., Galán B., de la Fuente J., Bermejo-Poza R., Villarroel M. Effect of Arthrospira supplementation on Oreochromis niloticus gut microbiota and flesh quality. Aquaculture Research . 2019;50(5):1448–1458. doi: 10.1111/are.14020. [DOI] [Google Scholar]
- 73.Plaza I., García J. L., Villarroel M. Effect of spirulina (Arthrospira platensis) supplementation on tilapia (Oreochromis niloticus) growth and stress responsiveness under hypoxia. Spanish Journal of Agricultural Research . 2018;16(1, article e0606) doi: 10.5424/sjar/2018161-11698. [DOI] [Google Scholar]
- 74.Velasquez S. F., Chan M. A., Abisado R. G., et al. Dietary Spirulina (Arthrospira platensis) replacement enhances performance of juvenile Nile tilapia (Oreochromis niloticus) Journal of Applied Phycology . 2016;28(2):1023–1030. doi: 10.1007/s10811-015-0661-y. [DOI] [Google Scholar]
- 75.Abdel-Tawwab M., El-Saadawy H. A., El-Belbasi H. I., Abd El-Hameed S. A. A., Attia A. A. Dietary spirulina (Arthrospira platenesis) mitigated the adverse effects of imidacloprid insecticide on the growth performance, haemato-biochemical, antioxidant, and immune responses of Nile tilapia. Comparative Biochemistry and Physiology. C . 2021;247:p. 109067. doi: 10.1016/j.cbpc.2021.109067. [DOI] [PubMed] [Google Scholar]
- 76.Shalata H. A., Bahattab O., Zayed M. M., et al. Synergistic effects of dietary sodium butyrate and Spirulina platensis on growth performance, carcass composition, blood health, and intestinal histomorphology of Nile tilapia (Oreochromis niloticus) Aquaculture Reports . 2021;19:p. 100637. doi: 10.1016/j.aqrep.2021.100637. [DOI] [Google Scholar]
- 77.Siringi J. O., Turoop L., Njonge F. Growth and biochemical response of Nile tilapia (Oreochromis niloticus) to Spirulina (Arthrospira platensis) enhanced aquaponic system. Aquaculture . 2021;544:p. 737134. doi: 10.1016/j.aquaculture.2021.737134. [DOI] [Google Scholar]
- 78.Mabrouk M. M., Ashour M., Labena A., et al. Nanoparticles of Arthrospira platensis improves growth, antioxidative and immunological responses of Nile tilapia (Oreochromis niloticus) and its resistance to Aeromonas hydrophila. Aquaculture Research . 2022;53(1):125–135. doi: 10.1111/are.15558. [DOI] [Google Scholar]
- 79.Chatzifotis S., Vaz Juan I., Kyriazi P., Divanach P., Pavlidis M. Dietary carotenoids and skin melanin content influence the coloration of farmed red porgy (Pagrus pagrus) Aquaculture Nutrition . 2011;17(2):e90–e100. doi: 10.1111/j.1365-2095.2009.00738.x. [DOI] [Google Scholar]
- 80.Meng-umphan K. Production of generation-2 Mekong giant catfish (Pangasinodon gigas) cultured with Spirulina sp. Maejo International Journal of Science and Technology . 2008;2:559–567. [Google Scholar]
- 81.Liu C., Li Y., Chen Z., et al. Effects of dietary whole and defatted Arthrospira platensis (cyanobacterium) on growth, body composition and pigmentation of the yellow catfish Pelteobagrus fulvidraco. Journal of Applied Phycology . 2021;33(4):2251–2259. doi: 10.1007/s10811-021-02445-9. [DOI] [Google Scholar]
- 82.Liu C., Liu H., Han D., et al. Effects of dietary Arthrospira platensis supplementation on the growth performance, antioxidation and immune related-gene expression in yellow catfish (Pelteobagrus fulvidraco) Aquaculture Reports . 2020;17:p. 100297. doi: 10.1016/j.aqrep.2020.100297. [DOI] [Google Scholar]
- 83.Liu C., Liu H., Xu W., et al. Effects of dietary Arthrospira platensis supplementation on the growth, pigmentation, and antioxidation in yellow catfish (Pelteobagrus fulvidraco) Aquaculture . 2019;510:267–275. doi: 10.1016/j.aquaculture.2019.05.067. [DOI] [Google Scholar]
- 84.Carneiro W. F., Castro T. F. D., Reichel T., de Castro Uzeda P. L., Martínez-Palacios C. A., Murgas L. D. S. Diets containing Arthrospira platensis increase growth, modulate lipid metabolism, and reduce oxidative stress in pacu (Piaractus mesopotamicus) exposed to ammonia. Aquaculture . 2022;547:p. 737402. doi: 10.1016/j.aquaculture.2021.737402. [DOI] [Google Scholar]
- 85.Roohani A. M., Abedian Kenari A., Fallahi Kapoorchali M., et al. Effect of spirulina Spirulina platensis as a complementary ingredient to reduce dietary fish meal on the growth performance, whole-body composition, fatty acid and amino acid profiles, and pigmentation of Caspian brown trout (Salmo trutta caspius) juveniles. Aquaculture Nutrition . 2019;25(3):633–645. doi: 10.1111/anu.12885. [DOI] [Google Scholar]
- 86.Roohani A. M., Kapoorchali M. F., Kenari A. A., Borani M. S., Zorriezahra M. J. Hematite- biochemical and immune response of Caspian brown trout (Salmo troutta caspius, Kessler, 1877) juveniles fed different levels of spirulina (Spirulina platensis) Iranian Journal of Fisheries Sciences . 2020;19:1153–1174. [Google Scholar]
- 87.Galafat A., Vizcaino A. J., Saez M. I., et al. Evaluation of Arthrospira sp. enzyme hydrolysate as dietary additive in gilthead seabream (Sparus aurata) juveniles. Journal of Applied Phycology . 2020;32(5):3089–3100. doi: 10.1007/s10811-020-02141-0. [DOI] [Google Scholar]
- 88.Shawky W. A., El-Sayed H. S., Saleh N. E., Ismael A. A., AFM E.-S. Evaluation of microalgae-supplemented diets and enriched decapsulated artemia cyst powder as novel diets for post-weaned common sole (Solea solea) larvae. Aquaculture Nutrition . 2021;27(4):1042–1051. doi: 10.1111/anu.13245. [DOI] [Google Scholar]
- 89.Khanzadeh M., Fereidouni A. E., Berenjestanaki S. S. Effects of partial replacement of fish meal with Spirulina platensis meal in practical diets on growth, survival, body composition, and reproductive performance of three-spot gourami (Trichopodus trichopterus) (Pallas, 1770) Aquaculture International . 2016;24(1):69–84. doi: 10.1007/s10499-015-9909-4. [DOI] [Google Scholar]
- 90.Dijkman B., Kooistra B., Bhandari M. How to work with a subgroup analysis. Canadian Journal of Surgery . 2009;52:515–522. [PMC free article] [PubMed] [Google Scholar]
- 91.Baker W. L., Michael White C., Cappelleri J. C., Kluger J., Coleman C. I., From the Health Outcomes, Policy, and Economics (HOPE) Collaborative Group Understanding heterogeneity in meta-analysis: the role of meta-regression. International Journal of Clinical Practice . 2009;63(10):1426–1434. doi: 10.1111/j.1742-1241.2009.02168.x. [DOI] [PubMed] [Google Scholar]
- 92.Ma K., Chen S. W., Wu Y., et al. Dietary supplementation with microalgae enhances the zebrafish growth performance by modulating immune status and gut microbiota. Applied Microbiology and Biotechnology . 2022;106(2):773–788. doi: 10.1007/s00253-021-11751-8. [DOI] [PubMed] [Google Scholar]
- 93.Ramírez C., Coronado J., Silva A., Romero J. Cetobacterium is a major component of the microbiome of giant amazonian fish (Arapaima gigas) in Ecuador. Animals . 2018;8(11):p. 189. doi: 10.3390/ani8110189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ina-Salwany M. Y., Al-saari N., Mohamad A., et al. Vibriosis in fish: a review on disease development and prevention. Journal of Aquatic Animal Health . 2019;31(1):3–22. doi: 10.1002/aah.10045. [DOI] [PubMed] [Google Scholar]
- 95.Vonshak A. Spirulina Platensis Arthrospira: Physiology, Cell-Biology and Biotechnology . CRC press; 1997. [Google Scholar]
- 96.Han P., Li J., Zhong H., et al. Anti-oxidation properties and therapeutic potentials of Spirulina. Algal Research . 2021;55:p. 102240. doi: 10.1016/j.algal.2021.102240. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The detailed information of included studies in this meta-analysis was listed in Table S1 of Supplemental Materials. The meta-analysis for CF and HSI were presented in Table S2 and Figure S7. In addition, publication bias for each outcome indicator (FBW, SGR, FCR, PER, CF, and HSI) in this study evaluated by methods of Egger's regression test, Begg's rank correlation test, and funnel plot were shown in Tables S3-S8 and Figures S1-S6, respectively. Furthermore, R code in this meta-analysis was also shared in the Supplemental Materials.
Data Availability Statement
Data are available on request from the authors.


