Abstract
It has been reported that the growth of Ralstonia solanacearum is suppressed at the rhizoplane of tomato plants and that tomato bacterial wilt is suppressed in plants grown in a soil (Mutsumi) in Japan. To evaluate the biological factors contributing to the suppressiveness of the soil in three treated Mutsumi soils (chloroform fumigated soil; autoclaved soil mixed with intact Mutsumi soil; and autoclaved soil mixed with intact, wilt-conducive Yamadai soil) infested with R. solanacearum, we bioassayed soil samples for tomato bacterial wilt. Chloroform fumigation increased the extent of wilt disease. More of the tomato plant samples wilted when mixed with Yamadai soil than when mixed with Mutsumi soil. Consequently, the results indicate that the naturally existing population of microorganisms in Mutsumi soil was significantly able to reduce the severity of bacterial wilt of tomato plants. To characterize the types of bacteria present at the rhizoplane, we isolated rhizoplane bacteria and classified them into 22 groups by comparing their 16S restriction fragment length polymorphism patterns. In Yamadai soil a single group of bacteria was extremely predominant (73.1%), whereas in Mutsumi soil the distribution of the bacterial groups was much more even. The 16S rDNA sequence analysis of strains of dominant groups suggested that gram-negative bacteria close to the β-proteobacteria were most common at the rhizoplane of the tomato plants. During in vitro assays, rhizoplane bacteria in Mutsumi soil grew more vigorously on pectin, one of the main root exudates of tomato, compared with those in Yamadai soil. Our results imply that it is difficult for the pathogen to dominate in a diversified rhizobacterial community that thrives on pectin.
Although many studies have been devoted to controlling the disease, bacterial wilt of tomato plants caused by Ralstonia solanacearum still commonly occurs in tropical and warm temperate areas of the world (5). However, some soils are known to have a low incidence of the disease (7). In an area of Yamaguchi Prefecture, Japan, tomato plants have been cultivated with little occurrence of bacterial wilt despite more than a decade of continuous monoculture, so we began research to determine how the occurrence of bacterial wilt is suppressed in this area. In a previous report (14), we compared the population dynamics of R. solanacearum in nonrhizosphere soil, rhizoplane, roots, and stems of tomato plants in a wilt-conducive soil and in a suppressive soil that were both newly infested with the pathogen. Survival of the pathogen in nonrhizosphere soil was better in the suppressive soil than in the conducive soil, while multiplication of the pathogen at the tomato rhizoplane was better in the conducive soil than in the suppressive soil. Therefore, we deduced that the suppression of the multiplication of the pathogen at the rhizoplane was one of the reasons for the lower incidence of bacterial wilt of tomato in this suppressive soil.
In this study, to evaluate the role of biotic factors in the suppressiveness of tomato bacterial wilt, we bioassayed tomato plants in three treated soils. Then, the types of rhizoplane bacteria were characterized by 16S ribosomal DNA (rDNA)-restriction fragment length polymorphism (RFLP) analysis. In addition, the growth on pectin, which is thought to be a main root exudate of tomato (15), and the polygalacturonase activity (8, 16, 18) of rhizoplane microorganisms from the tested soils were investigated.
MATERIALS AND METHODS
Soil samples.
Two soil samples, Mutsumi and Yamadai (14), were used in this study. Mutsumi soil was obtained from a commercial greenhouse field in Yamaguchi Prefecture in May 1996. The sampled field has received annual dressings of cow manure, and tomato plants have been cultivated there for 8 consecutive years with little occurrence of bacterial wilt. Yamadai soil was taken from a field of ryegrass at Yamaguchi University. Both soil samples were sieved (4 mm) and stored at room temperature with a water potential of −25 kPa until further experiments. Soil pH, texture, and organic carbon and total nitrogen contents are shown in Table 1. Before each experiment, soil samples were preincubated for 2 weeks at 30°C with a water potential of −6.2 kPa.
TABLE 1.
Chemical and physical properties of soils (before experiment)a
| Soil type | pH (H2O) | Organic C (%) | Total N (%) | CEC (cmol[+]/kg) | Soil components (%)
|
Soil texture | ||
|---|---|---|---|---|---|---|---|---|
| Sand | Silt | Clay | ||||||
| Mutsumi | 6.5 | 4.28 | 0.42 | 27.5 | 48 | 29 | 23 | CL |
| Yamadai | 4.4 | 1.62 | 0.15 | 13.8 | 55 | 25 | 20 | CL |
CEC, cation exchange capacity; CL, clay loam.
Assessment of suppressiveness of tomato bacterial wilt in treated Mutsumi soil.
Mutsumi soil was separated into three subsamples and treated as follows to modify its microbial characteristics. Two of the subsamples were autoclaved for 20 min at 121°C on 3 consecutive days to sterilize them completely and then mixed with either intact Mutsumi soil or intact Yamadai soil (ratio of 9:1 by dry weight). The other subsample was fumigated with chloroform vapor in a vacuum chamber for 24 h because this treatment is assumed to have little influence on the physicochemical properties of the soil in contrast to autoclaving, although it does not completely sterilize the microorganisms in soil.
Each of these treated soils was preincubated for 30 days at 30°C with a water potential of −6.2 kPa in a chamber sealed to prevent contamination from outside microorganisms. Tomato seeds (cultivar Momotarou) were immersed in sterile water for a day, sown into pots containing either type of soil sample, and cultivated in a growth chamber (30°C; light, 12 h; dark, 12 h). Ten days after germination, the seedlings were transplanted to pots containing the same type of soil sample that had been infested with R. solanacearum strain SL8 (14). These infested soils were prepared just before transplanting by spraying SL8 inoculum suspension (105 cells/ml) on the surface of the treated soil and then mixing them into the soil to achieve a final population of about 103 cells per g of dry soil (14). Pots containing the seedlings were kept in a growth chamber (30°C; light, 12 h; dark, 12 h) with the soil water potential maintained at about −6.2 kPa by the addition of sterile water twice a day. The number of wilted plants was recorded for 60 days. Bioassays were obtained by using 16 plants each in triplicate determinations (total, 48 plants) per soil sample. Some of the wilted seedlings were tested for the causative agent for tomato wilting by cutting the stem to find the white viscous suspension characteristic of the pathogen (12) and then that suspension was streaked onto Hara and Ono’s selective medium (4).
Isolation of root surface bacteria.
To isolate the rhizoplane bacteria, tomato plants were cultivated in Mutsumi and Yamadai soils as described above except that the pathogen was not inoculated into the soil. At about 2 weeks after the transplanting, tomato seedlings were carefully removed from two pots. Five seedlings grown in each pot were combined, and soil particles adhering to the roots were removed in sterile water by using sterilized forceps. Each set of roots was then weighed and washed by shaking four times in a 100-ml flask containing sterile water (20 times the weight of the fresh roots) to collect rhizoplane bacteria, as was reported previously (14). All of the washing solution from each set of root samples was collected in a 100-ml flask, serially diluted, and spread on the surface of YG agar plates (yeast extract, 1.0 g; K2HPO4, 0.3 g; KH2PO4, 0.2 g; MgSO4 · 7H2O, 0.2 g; agar, 15 g; water, 1 liter [pH 6.8]). Then, 250 colonies on the YG agar plate at a dilution level of 10−5 were isolated from the rhizoplane of tomato plants grown in each soil type. The isolated bacteria were suspended in 50% glycerol and kept at −80°C until further experiments.
DNA extraction from rhizoplane isolates.
Bacterial cells were lysed by different methods depending on whether they were gram negative or gram positive. Gram-negative bacteria were grown overnight in 10% solution of nutrient broth (Difco), after which 1.5-ml samples of culture were pelleted by centrifugation and washed twice in 1 ml of 1 M NaCl. Bacterial pellets were resuspended in 0.3 ml of lysis buffer (0.1 M Tris, pH 9.0; 0.1 M NaCl; 1% sodium dodecyl sulfate) and vortexed. After centrifugation, the supernatants were transferred to clean 1.5-ml tubes. Gram-positive bacteria were cultured overnight on YG agar plates. Each colony was suspended in 0.3 ml of lysis buffer containing proteinase K (1 mg/ml) (Boehringer Mannheim), incubated overnight at 37°C, and then incubated at 100°C for 5 min to inactivate the proteinase K. After centrifugation, the supernatants were transferred to clean 1.5-ml tubes and purified in a 1-ml mixture containing phenol, chloroform, and isoamyl alcohol (25:24:1). The DNA extracts were diluted 1:10 and used for PCR.
16S rDNA amplification.
PCR was carried out on samples of individual extracts of DNA by using the following primers: 5′-AGA GTT TGA TCM TGG CTC AG-3′ (positions 8 to 27 in Escherichia coli numbering) as the forward primer and 5′-TAC CTT GTT ACG ACT T-3′ (positions 1507 to 1492 in E. coli numbering) as the reverse primer. Each 50-μl sample of PCR reaction mixture consisted of 5 μl of 10× PCR buffer (TaKaRa), 4 μl of 0.2 mM deoxynucleoside triphosphate mixture (TaKaRa), 1 μl of each primer (500 nM), 1 U of Ex-Taq DNA polymerase (TaKaRa), 1 μl of template DNA, and sterile distilled water to up to 50 μl of reaction volume. PCR amplifications were performed in an automated thermal cycler with an initial denaturing (94°C for 1 min), followed by 35 cycles of denaturation (94°C for 1 min), annealing (50°C for 1 min), and extension (72°C for 1.5 min) and concluded by a single final extension (65°C for 1 min). After PCR, the amplified DNA was purified by polyethylene glycol precipitation (6), vacuum dried, and dissolved in 10 μl of sterile distilled water.
Restriction endonuclease digestion and gel electrophoresis.
To generate restriction fragments, these PCR products were digested for 10 h at 37°C with the restriction endonuclease HinfI (10 U) and MboI (10 U) in the reaction buffer supplied by the manufacturer. For each digestion reaction, 3 to 10 μl of amplified DNA was used, depending on the amount of amplified DNA. Digests were separated on 2% agarose gel in 1× TBE with a molecular marker, 100-bp DNA Ladder (GIBCO-BRL), and characterized by gel image analysis (Gel Doc 100; Bio-Rad).
Dendrogram and cluster analysis.
To compare the types and diversity of bacteria present at the rhizoplanes of the tomato plants grown in Mutsumi and Yamadai soil samples, band patterns of 16S rDNA digested with HinfI and MboI were analyzed for genetic similarity (cluster analysis) by Ward methods (17). Dendrograms were constructed according to the genetic similarity.
Sequence analysis of 16S rDNA.
After the results of the cluster analysis were considered, strains for partial 16S rDNA sequencing were randomly selected in the dominant clusters in Mutsumi and Yamadai rhizoplane bacteria to ascertain their phylogenetic positions. DNA sequences corresponding to positions 8 through 531 in E. coli 16S rRNA numbering were determined with an automated DNA sequencer (ABI 377; Perkin-Elmer). Homology searching of the sequence data was carried out in comparison with sequences in the nucleotide database at DDBJ/EMBL/GenBank by using BLAST (1).
Polygalacturonase activity of rhizoplane microorganisms.
Tomato rhizoplane microorganisms were extracted by four washes of the roots 2 weeks after the transplantation as described above. The number of bacteria in the washing solution was measured by direct microscopic counting after ethidium bromide staining (19). Then, 107 bacterial cells were inoculated into pectin medium (final volume, 10 ml; 10 g of pectin, 0.2 g of MgSO4 · 7H2O, 0.5 g of NH4NO3, 0.5 g of KH2PO4, 0.002 g of ZnSO4 · 7H2O, 0.001 g of CuSO4, 0.001 g of MnCl2, and 0.001 g of Na2MoO4 in either 1 liter of 0.05 M [pH 4.0] sodium acetate buffer or 1 liter of 0.05 M [pH 6.0] sodium citrate buffer; final concentration of bacteria, 106 cells/ml). Some of the pectin media were supplemented with cycloheximide (final concentration, 100 mg/liter) to suppress the fungal activities. Inoculated samples were incubated at 30°C shaken at 150 rpm for 12 h; the number of bacterial cells in the samples was then determined by microscopic counting (19). Subsequently, to provide clear supernatants to use in measuring polygalacturonase activity (3), the samples were centrifuged at 10,000 rpm for 15 min. Reaction mixtures containing 0.5 ml of the supernatant, 0.5 ml of 0.5% polygalacturonic acid, and 0.005 M EDTA were incubated at 37°C for 60 min in either 0.1 M sodium acetate buffer (pH 4.0) or 0.1 M sodium citrate buffer (pH 6.0). The reaction was terminated by the addition of Somogy’s reagent (20); the amount of reducing sugar was then measured by using the method of Nelson (13). One unit of polygalacturonase activity was defined as the amount of enzyme that produced 1 μmol of oligogalacturonate per min under the assay conditions.
RESULTS
Severity of tomato bacterial wilt in variously treated Mutsumi soils.
To demonstrate that the suppression of tomato bacterial wilt in Mutsumi soil was due to the organisms residing in it, we used chloroform-fumigated soil since the fumigation is assumed to have little influence on the physicochemical properties of the soil compared to the autoclaving process. After 30 days of preincubation, soil microbial diversity, based on the appearance of the colonies in the fumigated soil, appeared to be reduced compared with that in the nonfumigated soil, as was reported previously (22), although the number of microorganisms was higher in the fumigated soil (data not shown).
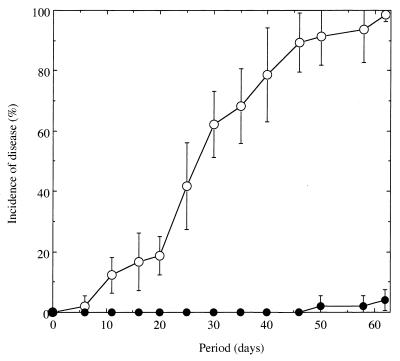
No symptoms of wilt were observed in tomato plants grown in the nonfumigated Mutsumi soil during 50 days of cultivation after transplantation (Fig. 1). In the chloroform-fumigated soil, plants began to wilt 6 days after being transplanted, and almost all of the tomato plants had succumbed by day 62 (Fig. 1). Before the wilting, no apparent differences in plant growth were observed between the two soil samples (data not shown).
FIG. 1.
Occurrence of tomato bacterial wilt grown in chloroform-fumigated (○) and nonfumigated (●) Mutsumi soil infested with R. solanacearum SL8 at an initial density of 103 CFU/g of soil. Standard errors are indicated by bars or are within each symbol.
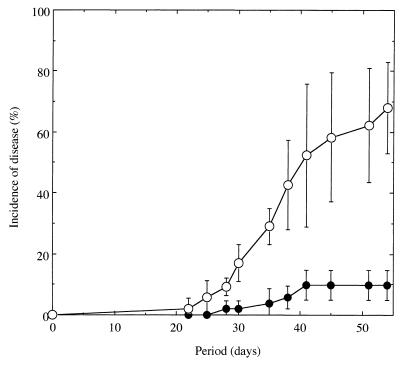
To compare how well microorganisms indigenous to Mutsumi and Yamadai soils suppress tomato bacterial wilt, autoclaved Mutsumi soil was mixed with either intact Mutsumi or intact Yamadai soil (ratio of 9:1 by dry weight) and then inoculated with the pathogen. In the mixture of autoclaved Mutsumi and intact Mutsumi soil, 10% of the plants wilted by 41 to 54 days after transplantation (Fig. 2). In the mixture of autoclaved Mutsumi and intact Yamadai soils, plants began to wilt at 22 days after transplantation, and 66% of the plants had wilted by day 54 (Fig. 2).
FIG. 2.
Occurrence of tomato bacterial wilt grown in nine parts autoclaved Mutsumi soil mixed with either one part intact Mutsumi soil (●) or 1 part intact Yamadai soil (○) and infested with R. solanacearum SL8 at an initial density of 103 CFU/g of dry soil. Standard errors are indicated by bars or are within each symbol.
The white viscous suspension characteristic of the pathogen was found in all of the stems of the wilted seedlings tested for the causative agent of wilting. The colonies grown from the white viscous suspension on Hara and Ono’s selective medium were identified as pathogenic R. solanacearum based on the colony appearance, indicating that the wilted seedlings were infected with the pathogen.
RFLP diversity of rhizoplane isolates.
We carried out our initial assessment of bacterial diversity by using the plate count method. The number of culturable bacteria was slightly higher in samples from the rhizoplanes of tomato plants grown in Mutsumi soil than from those grown in Yamadai soil (Table 2). Mutsumi samples also had greater numbers of culturable actinomycetes and fluorescent pseudomonads than in the Yamadai samples. The Mutsumi samples had 18 times more culturable fluorescent pseudomonads than the Yamadai samples. The morphology of the colonies that formed on YG agar from Mutsumi samples showed more diversity than those from the Yamadai samples (data not shown).
TABLE 2.
Numbers of microorganisms at the rhizoplanes of tomato plants (before inoculation of pathogen)
| Sample | Mean CFU/g (fresh weight)a
|
|||
|---|---|---|---|---|
| Bacteria (107) | Fungi (103) | Actinomycetes (106) | Fluorescent pseudomonads (103) | |
| Mutsumi | ||||
| Soil | 5.7 (1.3) | 46 (2.0) | 17 (0.2) | 2.9 (0.8) |
| Rhizoplane | 43 (11) | 61 (14) | 13 (5.0) | 220 (10) |
| Yamadai | ||||
| Soil | 4.1 (1.4) | 130 (30) | 5.0 (0.9) | 0.2 (0.2) |
| Rhizoplane | 22 (2.0) | 170 (40) | 2.5 (0.2) | 12 (1.2) |
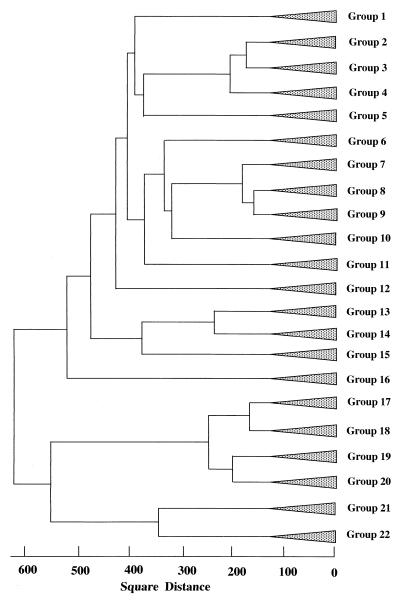
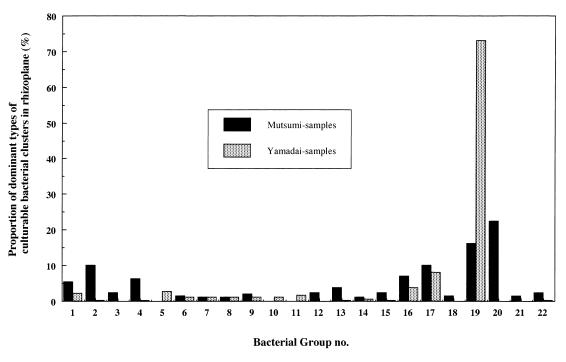
In 16S rDNA-RFLP analysis, 130 bacterial strains from the rhizoplanes in Mutsumi soils and 186 strains from the rhizoplanes in Yamadai soils were successfully analyzed of the 250 total strains isolated from each rhizoplane. As for the rest of the isolated strains (i.e., 184 strains), DNA was not successfully extracted or amplified during PCR. Analysis of the strains by 16S rDNA-RFLP analysis generated 96 different fragment patterns. Rhizoplane isolates from Mutsumi yielded 56 fragment patterns and Yamadai samples yielded 45. Figure 3 shows the results of a cluster analysis of restriction fragment patterns. Strains within a square distance of 130 were combined into a group, resulting in 22 groups formed from 316 isolates. In Mutsumi soil, group 20 (22.3%), group 19 (16.1%), and group 17 (10%) were predominant at tomato rhizoplane (Fig. 4). In Yamadai soil, group 19 (73.1%) was extremely dominant, followed by group 17 (8.1%) (Fig. 4). The culturable bacterial community at the Yamadai rhizoplane was greatly biased compared with that at Mutsumi rhizoplane.
FIG. 3.
Relationships among 22 groups composed of 316 isolates of culturable bacteria from the rhizoplanes of tomato plants. The square distance (genetic similarity) was determined from 16S rDNA-RFLP patterns obtained by digestion with HinfI and MboI by the Ward method. Isolates within 130 of the square distance were combined into a group.
FIG. 4.
Percentage of 16S rDNA-RFLP pattern groups in the rhizoplanes of tomato plants in Yamadai (gray bar) or Mutsumi (black bar) soils.
Ascertainment of the phylogenetic position of the dominant groups of culturable isolates at the tomato rhizoplane.
The phylogenetic positions of several strains from the dominant bacterial groups of the Yamadai and Mutsumi tomato rhizoplane samples were estimated through an analysis of 16S rDNA sequences. Table 3 shows the results of homology searching with BLAST. Thirteen strains examined in group 19 were close to Burkholderia pickettii (homology, 97.6%), which belongs to the β-subdivision of proteobacteria. The strains of group 17, predominant in both tomato rhizoplanes, showed high homology to Xanthomonas sacchari (γ-proteobacteria; homology, 93.5%), Burkholderia sp. (β-proteobacteria; homology, 92.4%), and Hydrogenophaga palleronii (β-proteobacteria; homology, 94.5%). All examined strains from group 20 matched well with Pseudomonas sp. strain P51 (β-proteobacteria; homology, 97.6%). The results indicate that the major part of the bacterial isolates in the tomato rhizoplane from both soils are β-proteobacteria.
TABLE 3.
Presumption of phylogenetic position determined by using the 16S rDNA sequence of dominant culturable bacteria at the rhizoplanes of tomato plants
| Bacterial cluster | Dominant ratio at rhizoplane (%)
|
Closest sequences in DNA database
|
|||||
|---|---|---|---|---|---|---|---|
| Mutsumi | Yamadai | Accession no. | Species | Phylogenetic position | Homologya (%) | No. of sequenced strain | |
| Group 1 | 5.4 | 2.2 | D83363 | Staphylococcus epidermidis | Gram-positive bacteria | 98.0 | 1 |
| Group 2 | 10 | 0.1 | D16273 | Bacillus megaterium | Gram-positive bacteria | 83.6 | 2 |
| Group 16 | 6.9 | 3.8 | X87261 | Sphingomonas chlorophenolica | Proteobacteria α subdivision | 95.1 | 2 |
| Group 17 | 10 | 8.1 | U37344 | Burkholderia sp. | Proteobacteria β subdivision | 92.4 | 1 |
| AF019073 | Hydrogenophaga palleronii | Proteobacteria β subdivision | 94.5 | 2 | |||
| Y10776 | Xanthomonas sacchari | Proteobacteria γ subdivision | 93.5 | 1 | |||
| Group 19 | 16.1 | 73.1 | X67042 | Pseudomonas pickettii | Proteobacteria β subdivision | 97.6 | 13 |
| Group 20 | 22.3 | 0 | AF015487 | Pseudomonas sp. strain P51 | Proteobacteria β subdivision | 97.6 | 4 |
The highest percentage among tested strains in each group is shown.
Group 1 and group 2 were relatively abundant groups in the rhizoplane from Mutsumi soil: strains from group 1 were phylogenetically close to Staphylococcus epidermidis (homology, 98.0%), and strains from group 2 were phylogenetically close to Bacillus megaterium (homology, 83.6%). Both species are low-content G+C gram-positive bacteria. Consequently, the bacterial community at the tomato rhizoplane of the Mutsumi soil was phylogenetically diverse compared with that at the tomato rhizoplane of the Yamadai soil.
Polygalacturonase activity and growth rate of microbial communities at the tomato rhizoplane.
Rhizoplane microorganisms from Mutsumi soil showed a higher polygalacturonase activity both at pH 6.0 and 4.0 than those from Yamadai soil (Table 4). The number of bacteria after 12 h of incubation was also higher in samples from Mutsumi soil than in those from Yamadai soil. These results suggest that the rhizoplane microbial population in the Mutsumi soil samples had a greater ability to assimilate pectin than did the Yamadai microbial population.
TABLE 4.
Polygalacturonase activity and growth rate of rhizoplane microbial population
| Samplea | Activity of polygalacturonase (U)
|
Bacterial cell no. after 12 h of incubation in pectin medium (cells/ml)b
|
||
|---|---|---|---|---|
| pH 6.0 | pH 4.0 | pH 6.0 | pH 4.0 | |
| Mutsumi | 1.2 | 0.78 | 2.7 (0.4) × 109 | 6.9 (2.2) × 106 |
| Mutsumi with cycloheximide | NDc | 0.41 | 1.9 (0.3) × 109 | 7.3 (2.1) × 106 |
| Yamadai | 0 | 0.32 | 3.1 (0.5) × 108 | 2.8 (0.9) × 106 |
| Yamadai with cycloheximide | 0.41 | 0 | 2.3 (0.6) × 108 | 2.3 (0.8) × 106 |
Alone or supplemented with cycloheximide (100 ppm) to suppress fungal activity.
As determined by microscopic counting (19). The standard errors are given in parentheses. The initial number was 1.0 × 106 cells/ml.
ND, not determined.
In samples that received cycloheximide, greater polygalacturonase activity and higher numbers of bacteria were found in the Mutsumi samples than in the Yamadai samples. This result suggests that bacteria were mainly responsible for the difference in polygalacturonase activities between the two soils.
DISCUSSION
In our previous study, we found that under laboratory conditions tomato plants grown in Mutsumi soil were not as susceptible to bacterial wilt as those grown in Yamadai soil and that the growth of R. solanacearum was suppressed at the rhizoplane of tomato plants in Mutsumi soil (14). In order to evaluate the biotic factors that suppress tomato bacterial wilt in Mutsumi soil, we compared the incidence of bacterial wilt in soils that were treated in three different ways. In chloroform-fumigated soil, where the physicochemical properties of the soil are assumed to be quite similar to those of nonfumigated soil, the incidence of bacterial wilt was greater than in nonfumigated Mutsumi soil (Fig. 1), indicating that the organisms in natural Mutsumi soil are involved in the suppressiveness of bacterial wilt. In the Mutsumi soil that was autoclaved and mixed with intact Yamadai soil, the incidence of bacterial wilt was higher than when autoclaved Mutsumi soil was mixed with intact Mutsumi soil (Fig. 2). Owing to the low ratio (9:1) of the mixtures, the physicochemical properties of the soils were likely similar, so the native microorganisms in the intact Mutsumi soil must be responsible for the suppressive ability of Mutsumi soil. One hypothesis is that the suppression of colonization and multiplication of R. solanacearum at the rhizoplane in Mutsumi soil (14) is due to the competitive ability of the indigenous microbial community present at the rhizoplane.
To compare the make-up of the populations of bacteria present at the rhizoplane in wilt-suppressive and -conducive soils, we characterized culturable bacteria at the rhizoplane by 16S rDNA-RFLP analysis. At the Yamadai rhizoplane, a soil conducive to tomato bacterial wilt, a single type (group 19) predominates (73.1%). In contrast, the distribution of 16S rDNA-RFLP clusters showed a much more even distribution of diverse bacteria at the Mutsumi rhizoplane (Fig. 3).
In the rhizosphere, where the plant meets the soil, there can be steep gradients in water content, pH, and other environmental factors (2). A rhizobacterial community with greater diversity would present more candidates suitable for the plant-influenced environment and would provide more competition to the extreme dominance of a single species at the rhizoplane when roots alter environmental conditions. Thus, R. solanacearum might fail to colonize and multiply at the tomato rhizoplane in Mutsumi soil. In contrast, a single species, R. solanacearum, might easily dominate at the tomato rhizoplane in Yamadai soil owing to the simple composition of the indigenous bacterial population.
In vitro enzyme assay revealed that the rhizoplane of tomato plants in Mutsumi soil has a bacterial population that vigorously utilizes pectin (Table 4). This result, together with previous reports (9, 10), implies that, in Mutsumi soil, the presence of bacteria with a strong competitive ability to utilize pectin at rhizoplane retards the multiplication of R. solanacearum.
Further work is needed to clarify the mechanism of tomato bacterial wilt suppression in Mutsumi soil.
ACKNOWLEDGMENTS
We thank T. Tokunaga, Yamaguchi Agricultural Experiment Station, T. Fujimoto, Hagi Agricultural Improvement Center, and T. Tokunaga, Mutsumi Town, for their assistance in soil sampling. We also thank S. Takaki, Fumakilla, Ltd., for his helpful discussions.
This research was partially supported by a grant-in-aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan to M.N. (09760059).
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Drew M C. Root function, development, growth and mineral nutrition. In: Lynch J M, editor. The rhizosphere. New York, N.Y: Wiley Interscience; 1989. pp. 35–57. [Google Scholar]
- 3.Guevara M A, Gonzalez-Jaen M T, Estevez P. Multiple forms of pectic lyase and polygalacturonase from Fusarium oxysporum f.sp. radicislycopersici: regulation of their synthesis by galacturonic acid. Can J Microbiol. 1997;43:245–253. [Google Scholar]
- 4.Hara H, Ono K. Ecological study on the bacterial wilt of tobacco, caused by Pseudomonas solanacearum. Bull Okayama Tob Exp Stn. 1983;42:127–138. [Google Scholar]
- 5.Hartman G L, Elphinstone J G. Advance in the control of Pseudomonas solanacearum race 1 in a major food crop. In: Hayward A C, Hartman G L, editors. Bacterial wilt, the disease and its causative agent, Pseudomonas solanacearum. Wallingford, United Kingdom: CAB International; 1994. pp. 157–177. [Google Scholar]
- 6.Hiraishi A. Analysis and phylogenetic study of 16S rRNA gene by using PCR (1) Bull Jpn Soc Microb Ecol. 1995;10:31–42. [Google Scholar]
- 7.Ho W C, Chern L L, Ko W H. Pseudomonas solanacearum-suppressive soils in Taiwan. Soil Biol Biochem. 1988;120:489–492. [Google Scholar]
- 8.Husain A, Kelman A. The role of pectic and cellulolytic enzymes in pathogenesis by Pseudomonas solanacearum. Phytopathology. 1958;48:377–386. [Google Scholar]
- 9.Ikeda K, Toyota K, Kimura M. Role of extracellular pectinase in the rhizoplane competence of a rhizobacterium Burkholderia picketti MSP3RIF. Soil Biol Biochem. 1998;30:323–329. [Google Scholar]
- 10.Tans-Kersten J, Guan Y, Allen C. Ralstonia solanacearum pectin methylesterase is required for growth on methylated pectin but not for bacterial wilt virulence. Appl Environ Microbiol. 1998;64:4918–4923. doi: 10.1128/aem.64.12.4918-4923.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin J P. Use of acid, rose bengal and streptomycin in the plate method for estimating soil fungi. Soil Sci. 1950;69:215–232. [Google Scholar]
- 12.Misaghi I J, Olsen M W, Billotte J M, Sonoda R M. The importance of rhizobacterial mobility in biocontrol of bacterial wilt of tomato. Soil Biol Biochem. 1992;24:287–293. [Google Scholar]
- 13.Nelson N. A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem. 1944;153:375–380. [Google Scholar]
- 14.Nishiyama M, Shiomi Y, Suzuki S, Marumoto T. Suppression of growth of Ralstonia solanacearum, tomato bacterial wilt agent, on/in tomato seedlings in a suppressive soil in Japan. Soil Sci Plant Nutr. 1999;45:79–87. [Google Scholar]
- 15.Oades J M. Mucilages at the root surface. J Soil Sci. 1978;29:1–16. [Google Scholar]
- 16.Ofuya C O. Physical properties of pectic polysaccharidase of Pseudomonas solanacearum from Nigeria. Curr Microbiol. 1984;10:141–146. [Google Scholar]
- 17.Orloci L. An agglomerative method for classification of plant communities. J Ecol. 1967;55:193–206. [Google Scholar]
- 18.Sand D C, Rovira A D. Isolation of fluorescent pseudomonads with a selective medium. Appl Microbiol. 1970;20:513–514. doi: 10.1128/am.20.3.513-514.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Someya T. Three-dimensional observation of soil bacteria in organic debris with a confocal laser scanning microscope. Soil Microorg (Tokyo) 1995;46:61–69. [Google Scholar]
- 20.Somogyi M M. Notes on sugar determination. J Biol Chem. 1952;195:19–23. [PubMed] [Google Scholar]
- 21.Takaki S, Kitamura A, Marumoto T. Control of Fusarium disease using antagonistic actinomycetes 1. Screening of antagonistic actinomycetes to Fusarium oxysporum. Soil Microorg (Tokyo) 1992;39:35–40. [Google Scholar]
- 22.Toyota K, Kimura M. Growth of the bacterial wilt pathogen Pseudomonas solanacearum introduced into soil colonized by individual soil bacteria. Soil Biol Biochem. 1996;28:1489–1494. [Google Scholar]