Abstract
Weeping forsythia is a wide-spread shrub in China with important ornamental, medicinal and ecological values. It is widely distributed in China’s warm temperate zone. In plants, WRKY transcription factors play important regulatory roles in seed germination, flower development, fruit ripening and coloring, and biotic and abiotic stress response. To date, WRKY transcription factors have not been systematically studied in weeping forsythia. In this study, we identified 79 WRKY genes in weeping forsythia and classified them according to their naming rules in Arabidopsis thaliana. Phylogenetic tree analysis showed that, except for IIe subfamily, whose clustering was inconsistent with A. thaliana clustering, other subfamily clustering groups were consistent. Cis-element analysis showed that WRKY genes related to pathogen resistance in weeping forsythia might be related to methyl jasmonate and salicylic acid-mediated signaling pathways. Combining cis-element and expression pattern analyses of WRKY genes showed that more than half of WRKY genes were involved in light-dependent development and morphogenesis in different tissues. The gene expression results showed that 13 WRKY genes were involved in drought response, most of which might be related to the abscisic acid signaling pathway, and a few of which might be regulated by MYB transcription factors. The gene expression results under cold stress showed that 17 WRKY genes were involved in low temperature response, and 9 of them had low temperature responsiveness cis-elements. Our study of WRKY family in weeping forsythia provided useful resources for molecular breeding and important clues for their functional verification.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10709-023-00184-y.
Keywords: Cold stress, Drought stress, WRKY transcription factor, Gene expression
Introduction
Ornamental plants play an important role in urban landscape design and ecological construction, contributing to the improvement of the environment and landscape (Wang et al. 2014). Weeping forsythia (Forsythia suspensa (Thunb.) Vahl) is an Oleaceae plant, and is native to in Henan, Hebei, Shandong, Shanxi, Hubei, Shaanxi and other provinces in China (Yang et al. 2017). Weeping forsythia is a widely used garden ornamental plant, and is known for its excellent ornamental characteristics of early spring flowering, with flowers emerging spectacularly before spring leaves, and a large number of flowers.
Weeping forsythia also has important medicinal values. Its fruit contains forsythin, forsythoside A, α-pinene, β-pinene, terpinen-4-ol and other volatile oil components, which are widely used in traditional Chinese medicines for treating colds, such as Shuanghuanglian Oral Liquid and Lianhua Qingwen Oral Liquid (Xiang et al. 2021). Recent studies showed that weeping forsythia could relieve the symptoms of COVID-19 (Hu et al., 2021; Zhao et al., 2021). In recent years, weeping forsythia has been extensively planted artificially due to the dramatic reduction of natural resources. Therefore, studies of the horticulture, genetics and medicinal use of weeping forsythia are increasing. High-quality genome and whole genome sequencing data of weeping forsythia were published (Li et al. 2022a). Large-scale of transcriptome data of weeping forsythia provide new opportunities to analyze gene expression patterns in different tissues and different environmental stresses.
In horticultural plants whose genome data have been published recently, many gene families have been identified and analyzed, such as bZip family in Prunus mume (Li et al. 2022b), GDSL family in Pyrus spp. (Zhang et al. 2022a), HD-Zip and MdCLE peptide families in Malus × domestica (Zhang et al. 2022b, c). The studies on the distribution, gene structure, expression patterns and functional differentiation of these gene families in the genome provide useful resources for analyzing the evolutionary mechanisms of the gene families and for utilizing this information to guide for molecular breeding.
To date, no study on any gene family of weeping forsythia at the genome level has been described. WRKY gene family is one of the largest transcription factor families in higher plants. In 1994, the first WRKY protein was cloned from sweet potato (Ishiguro and Nakamura 1994). In recent years, with the publication of more plant genomes, a large number of WRKY members from different species have been cloned and identified (Wani et al. 2021). WRKY gene is named because of its N- terminal containing 7 highly conserved amino acid sequences composed of WRKYGQK (Song et al. 2018). WRKY gene is more likely to bind to the upstream W-box region of some gene promoters, which contains a core DNA sequence (T)(T)TGAC(C/T) sequence (Cheng et al. 2019).
WRKY gene is not constitutively expressed in plants. The expression of WRKY gene is induced by various environments, such as biotic and abiotic stress and different development stages of plants, and its expression is tissue-specific and participates in various physiological processes in plants (Rinerson et al. 2015). In cold stress, WRKY genes improve cold tolerance by regulating CBF signaling pathway (Huang et al. 2022). Recent studies showed that WRKY genes were involved into increasing the tolerance to adversity stress (Jiang et al. 2017), improving the immunity against pathogens (Pandey and Somssich 2009), participating in somatic embryogenesis (Yang et al., 2020), promoting the synthesis of secondary metabolites (Chen et al. 2017), regulating the flowering of plants, accelerating fruit ripening (Wang et al. 2017), and promoting fruit coloring (Cheng et al. 2017).
The number of WRKY gene family members varies greatly in different species, while its family members are large in most higher plants (Chen et al. 2019). The sequence homology of other amino acids in the WRKY gene family is not high except the conservative region. The diversity of gene structures of the WRKY family implies the diversity of their functions. To date, the WRKY family has been functionally and physiologically characterized in several ornamental plants, such as Dendrobium officinale (Wang et al. 2018), Acer truncatum (Li et al. 2021a), Prunus mume (Bao et al. 2019). However, WRKY family has not been described in weeping forsythia. To better understand the characteristics, evolution and functions of the WRKY genes in weeping forsythia, we provide a detailed overview of the number and classification, gene structure and expression of WRKY family at the genome level. Thus, our study improves the understanding of the WRKY family and provides key candidate genes related to cold and drought stresses in weeping forsythia.
Materials and methods
Data sources and sequence searches
The weeping forsythia genome was obtained from National Center for Biotechnology Information (accession no. JAHHPY000000000; Li et al., 2022). The keyword “WRKY” was used to search WRKY genes in the annotation file, and blasted the candidate genes in NCBI (https://blast.ncbi.nlm.nih.gov/; Altschul et al. 1990) to identify the WRKY domain. The genes with conserved WRKY domain were considered the true WRKY genes. ExPASy online tool (https://web.expasy.org/cgi-bin/protparam/protparam; Artimo et al., 2012) was used to predict and analyze the physicochemical properties of WRKY protein in weeping forsythia, including molecular weight, isoelectric point, amino acid number, fat index, instability index, and hydrophobicity. The subcellular localization of weeping forsythia WRKY genes was predicted by Plant-mPLoc online software (http://www.csbio.sjtu.edu.cn/bioinf/plant-multi/, Chou and Shen, 2010), and the online software SOPMA (https://npsa-prabi.ibcp.fr/cgi-bin/secpred_sopma.pl; Geourjon and Deleage, 1995) was used to predict the secondary structure of WRKY protein.
According to the identified WRKY gene ID of weeping forsythia and the genome sequence of weeping forsythia, the chromosome position information of WRKY gene family members was obtained. The position of WRKY gene of weeping forsythia on chromosome was visualized by TBtools software (Chen et al. 2020a). The genome and protein sequence data of Arabidopsis thaliana is from the Arabidopsis information resource (https://www.arabidopsis.org/; Eulgem et al., 2000).
Phylogenetic relationship and gene structure
A phylogenetic tree using the neighbor-joining (NJ) method (Jones et al. 1992) was constructed using the obtained amino acid sequences. The NJ tree was constructed using MEGA 7.0 (Kumar et al., 2016) with the Poisson model, the pairwise deletion option, and 1,000 bootstrap resampling times. Finally, the phylogenetic tree was visualized using FigTree v1.4.4 (Rambaut 2009). TBtools (Chen et al. 2020a) was used to visualize the introns and exons of all WRKY genes of weeping forsythia. The online database of MEME (https://meme-suite.org/meme/tools/meme, Bailey et al., 2009) was used to analyze the protein domains and conserved motifs of all WRKY genes in weeping forsythia. The analysis value of conserved motifs was set to 10, and the protein domains of WRKY family in weeping forsythia were visualized by TBtools (Chen et al. 2020a). TBtools (Chen et al. 2020a) was further used to extract the upstream 2 Kb sequence information of WRKY genes in weeping forsythia, and the online software PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/; Thijs et al., 2002) was predicted the possible cis-acting elements of WRKY genes, and the predicted results were visualized by TBtools (Chen et al. 2020a).
Expression patterns of WRKY gene in different tissues and under cold and drought stresses
The RNA-seq data of 79 weeping forsythia WRKY genes were downloaded from NCBI, including data from fruit, stem, leaf (accession no. SRR17386487-SRR17386495), and flower tissues (accession no., SRX11342985, SRX11342993 and SRX11342994). RNA-seq data from fruit, stem, leaf was obtained at harvest time (July) of the weeping forsythia fruit (Li et al., 2022). RNA-seq data of flower were obtained at the flower bud period (March). The gene expression data of weeping forsythia under drought stress were from Wuzhishan populations under 80% and 20% soil water content (accession no. SRX7503009, SRX7503010, SRX7503012-SRX7503015; Li et al. 2021b). The gene expression data of weeping forsythia under cold stress were from Wuzhishan populations at 25 ℃ and 4 ℃ (accession no. SRX7440183-SRX7440188; Li et al. 2021c).
Low-quality reads with more than 10% anonymous nucleotides (N) and more than 50% of bases possessing a value Q ≤ 10 were removed from raw sequencing data. The gene expression level of all genes in these samples was estimated by fragments per kilobase of transcript per million fragments mapped (FPKM) using StringTie (Pertea et al. 2015). The expression patterns in different tissues and in response to drought and cold stresses were analyzed using the R package Heatmap. Log2FPKM ≥ 1 was used as the threshold to screen the WRKY genes that were effectively expressed in different tissues of weeping forsythia. Under drought and cold stresses, FC ≥ 2 and FDR ≤ 0.05 were used as the thresholds to screen WRKY genes involved in stress response.
Results
Gene identification and sequence characteristics of the WRKY gene family
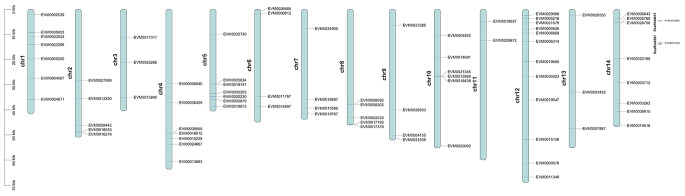
A total of 80 possible WRKY genes were identified from the weeping forsythia genome through genome annotation files. After further domain identification, 79 of them contained the WRKYGQK conserved domain, and these genes were identified as real WRKY genes. WRKY proteins varied greatly in weeping forsythia, amino acid length ranged from 146 (FsWRKY21) to 728 aa (FsWRKY27 and FsWRKY63), protein molecular weight ranged from 16.33 (FsWRKY21) to 80.32 Da (FsWRKY63), and isoelectric point ranged from 4.56 (FsWRKY65) to 9.84 (FsWRKY63). All these proteins were located in the nucleus and were hydrophilic proteins (Table S1). Among 79 WRKY proteins, 5 proteins (FsWRKY33, FsWRKY50, FsWRKY55, FsWRKY61, FsWRKY74) were stable proteins, and the rest were unstable proteins. The results of subcellular localization prediction showed that all 79 proteins were located in the nucleus (Table S1). The WRKY genes were randomly distributed on the chromosomes of weeping forsythia. Among them, the WRKY genes in Chr1 (8.9%), Chr4(8.9%), Chr5(8.9%), Chr12(15.2%) and Chr14(10.1%) were the most, and one gene was distributed in the unmounted fragments Scaffold397 and Scaffold413 respectively (Fig. 1).
Fig. 1.
Distribution map of WRKY gene family in the weeping forsythia genome
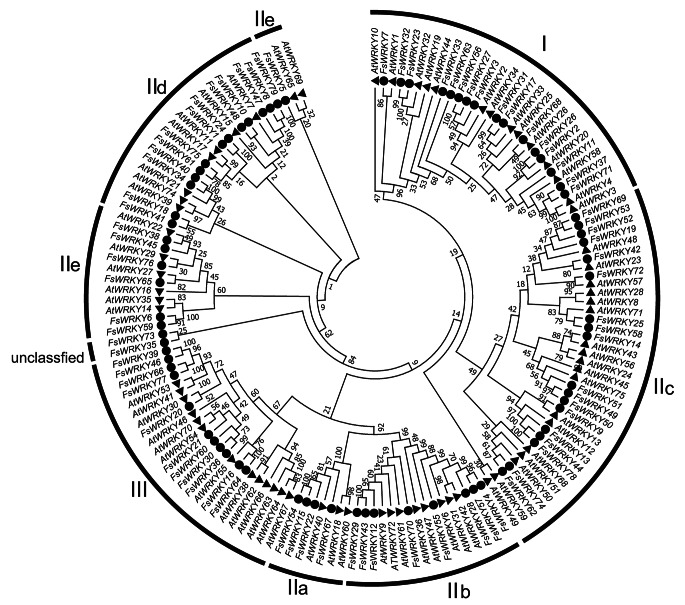
Phylogenetic relationship of WRKY protein in weeping forsythia
To better understand the phylogenetic relationship of WRKY protein of weeping forsythia, the phylogenetic tree of 79 WRKY proteins of weeping forsythia and 74 WRKY proteins of A. thaliana was constructed using the NJ method. The phylogenetic tree showed that the members of WRKY protein I, IIa, IIb, IIc, IId and III of weeping forsythia were grouped together well with the members of this subfamily of A. thaliana, but the members of IIe subfamily did not group together in one clade (Fig. 2). FsWRKY5, AtWRKY65 and FsWRKY69 grouped together separately, which was inconsistent with the classification results of WRKY protein in A. thaliana. Our results showed that members of IId were clustered into two clades, which indicated that IId subfamily had obvious genetic differentiation in weeping forsythia. Our clustering results were basically consistent with those of A. thaliana, except for the IIe subfamily. According to the classification of seven WRKY subfamilies of A. thaliana, we counted the number of members of WRKY gene of weeping forsythia in seven subfamilies, including 16 members in I subfamily, 4 members in IIa subfamily, 8 members in IIb subfamily, 18 members in IIc subfamily, 13 members in IId subfamily, 7 members in IIe subfamily, 11 members in type III, and two unclassified members.
Fig. 2.
Phylogenetic tree construction and analysis of WRKY proteins in weeping forsythia and Arabidopsis thaliana
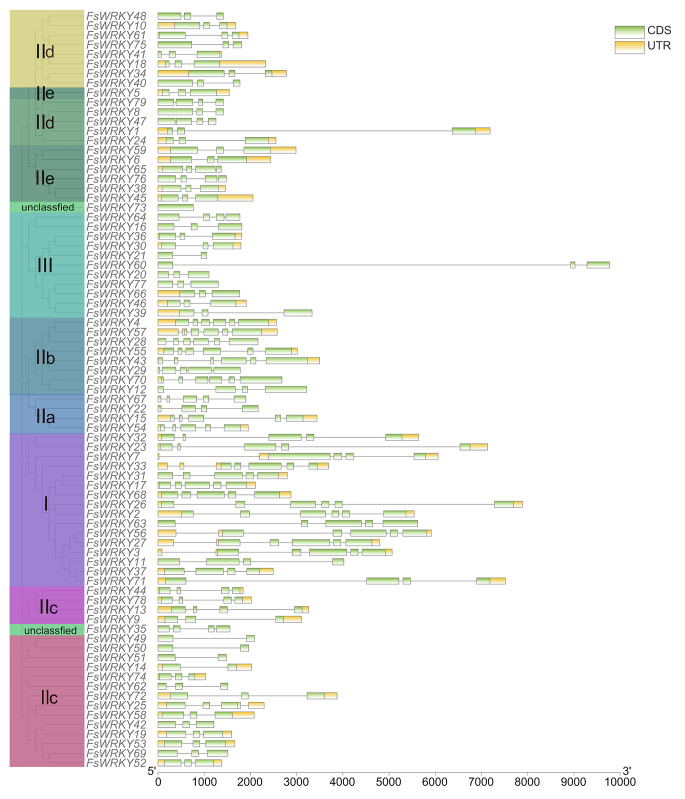
Gene structure and protein structure of WRKY family
In a gene family, the gene intron and exon structure can reflect the evolutionary relationship of the members of the gene family. We analyzed the WRKY gene sequences of all weeping forsythia and visualized the WRKY gene structure by using TBtools (Fig. 3). I subfamily has 4 to 6 exons and 3 to 6 introns, and the average gene length is larger than other subfamilies. IIa subfamily had 3 to 4 introns and 5 exons except FsWKRY22, which had 4. IIb subfamily had 4 to 6 exons and 4 to 5 introns. IIc subfamily has 2 to 4 exons and 1 to 3 introns. IId subfamily had 3 to 4 exons and 2 to 3 introns. IIe subfamily has 3 to 4 exons and 2 to 3 introns. III subfamily had 2 to 4 exons and 1 to 3 introns. The unclassified FsWKRY73 had no intron.
Fig. 3.
Gene structure analysis of WRKY genes of weeping forsythia
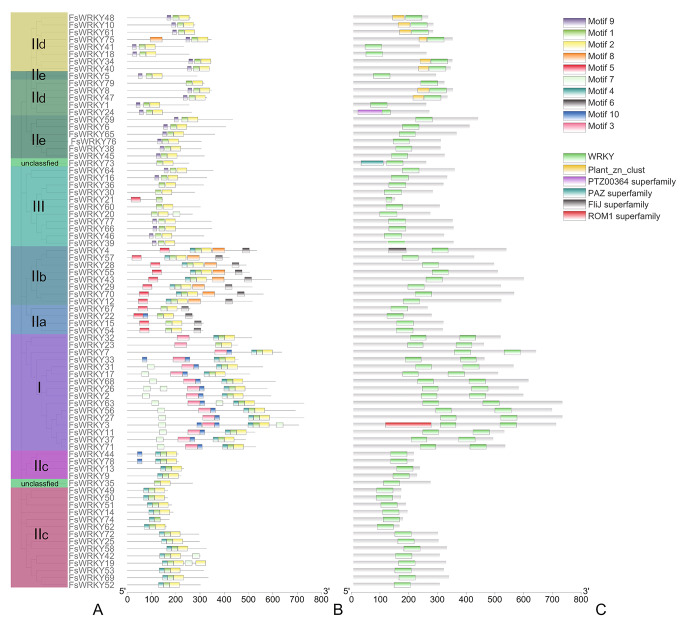
To further analyze the structure and function of WRKY protein in weeping forsythia, the conserved domain and conserved motif of WRKY family of weeping forsythia were analyzed (Fig. 4). Conservative domain analysis showed that 16 members of the I subfamily of weeping forsythia contained two WRKY domains, while the other subfamily members contained only one WRKY domain. In addition, among the 13 members of IId subfamily, 8 members had Plant_zn_clust conserved domain, which might indicate that they were zinc-dependent transcription factors. Conservative motif analysis showed that the WRKY domains were mostly composed of three combinations of conservative motif4, motif1, and motif2, motif9, motif1, and motif2, or motif 3 and motif10, among which the WRKY domain composed of motif 3 and motif10 was unique to I subfamily. The prediction of protein secondary structure of WRKY family showed that the proportion of alpha helix range from 7.47% (FsWRKY26) to 35.52% (FsWRKY67), beta turn range from 1.13% (FsWRKY64) to 10.78% (FsWRKY49), extended strand range from 5.35% (FsWRKY45) to 21.98% (FsWRKY13), and random coil range from 44.91% (FsWRKY49) to 77.97% (FsWRKY72) (Table S2). The results showed that the secondary structure of WRKY protein in weeping forsythia was mainly composed of alpha helix and random coil.
Fig. 4.
The protein motifs and protein domain analysis of WRKY genes of weeping forsythia. A, Phylogenetic tree of WRKY genes; B, Protein motif of WRKY genes; C, Protein domain of of WRKY genes
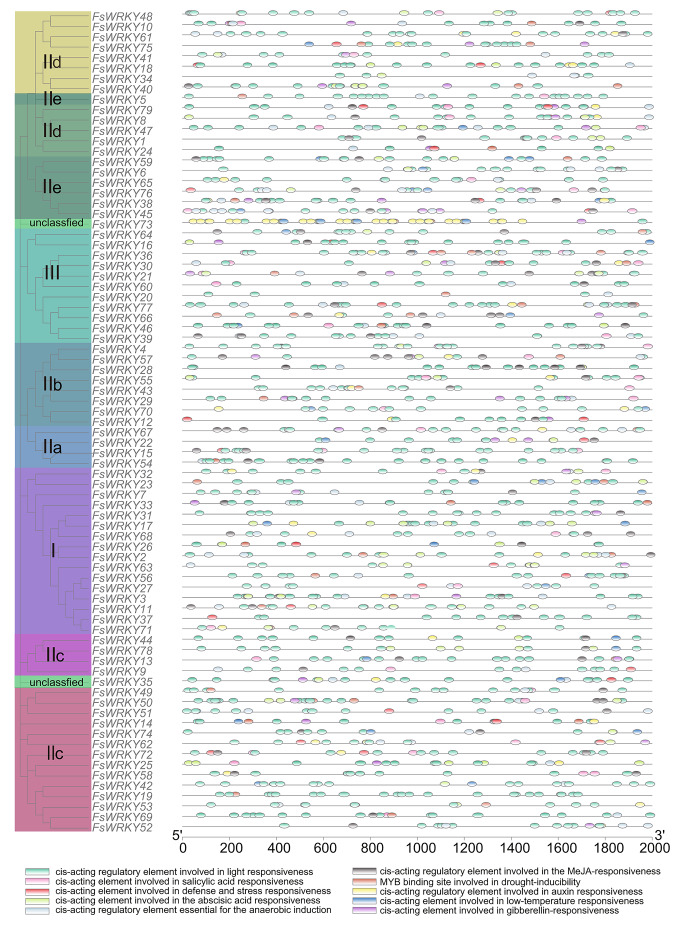
Cis-acting elements of WRKY gene family
A total of 1,910 possible cis-acting regulatory elements were identified in the upstream 2 Kb range of 79 WRKY genes (Table S3; Fig. 5). The results showed that there were many cis-acting elements in the promoter region of WRKY gene of weeping forsythia. In addition to a large number of light-responsive elements, cis-acting elements related to plant hormones, such as methyl jasmonate (MeJA), abscisic acid (ABA), gibberellin (GA), auxin, salicylic acid (SA) and cis-acting elements related to low temperature, such as drought, anaerobic, and defense and stress, were also found in WRKY genes of weeping forsythia. Among them, 42 cis-elements related to low temperature stress were distributed among 28 WRKY genes, and 52 cis-elements related to drought involving MYB transcription factors were distributed among 38 genes.
Fig. 5.
Cis-acting elements analysis of the promoters of WRKY genes of weeping forsythia
Expression patterns of WRKY gene in different tissues, cold and drought stresses
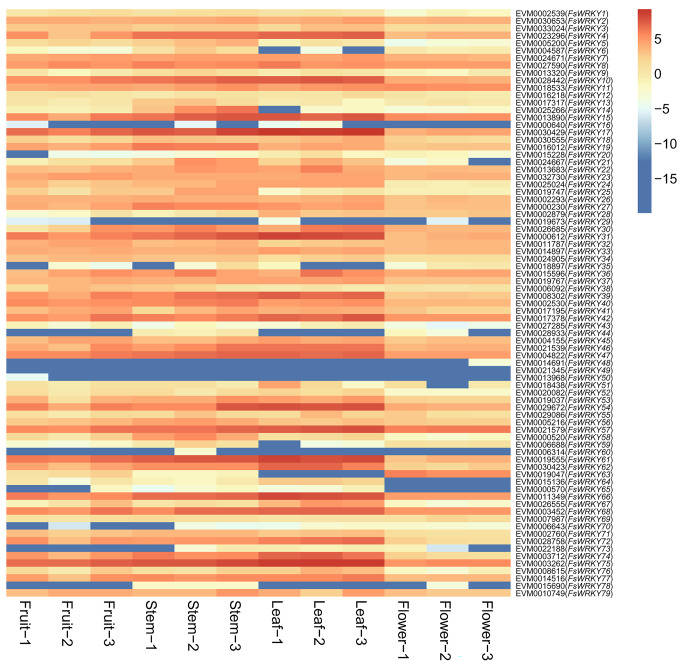
The expression of WRKY genes in fruits, stems, leaves and flowers of weeping forsythia were investigated. The results showed that 52 WRKY genes were effectively expressed in fruits and stems, 53 in leaves and 46 in flowers (Table S4; Fig. 6). Our results indicated that a large number of WRKY genes might be involved in the development and morphogenesis of fruit, stem, leaf and flower tissues in weeping forsythia.
Fig. 6.
Heat map of WRKY gene expression (Log2FPKM) in different tissues of weeping forsythia
The expression patterns of WRKY genes were further investigated under cold and drought stresses. We found that 17 WRKY genes responded significantly to cold stress (Table S5; Fig. 7), of which 9 (FsWRKY22, FsWRKY36, FsWRKY38, FsWRKY42, FsWRKY45, FsWRKY47, FsWRKY56, FsWRKY70, and FsWRKY75) had cis-elements related to low temperature stress, and 13 WRKY genes were significantly differentially expressed under drought stress (Table S6; Fig. 8), in which 4 genes had drought-related cis-elements involved in MYB transcription factors (FsWRKY19, FsWRKY54, FsWRKY56, and FsWRKY67), and 10 cis-elements with ABA responsiveness (FsWRKY4, FsWRKY8, FsWRKY17, FsWRKY19, FsWRKY27, FsWRKY28, FsWRKY34, FsWRKY54, FsWRKY74, and FsWRKY76), and two of them (FsWRKY19 and FsWRKY54) both had two kind of cis-elements.
Fig. 7.
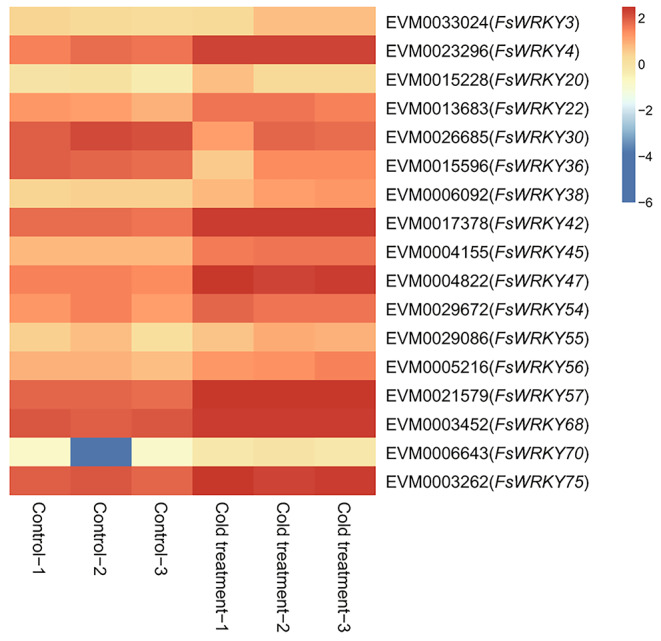
Heat map of WRKY gene expression (Log10FPKM) in response to cold stress of weeping forsythia
Fig. 8.
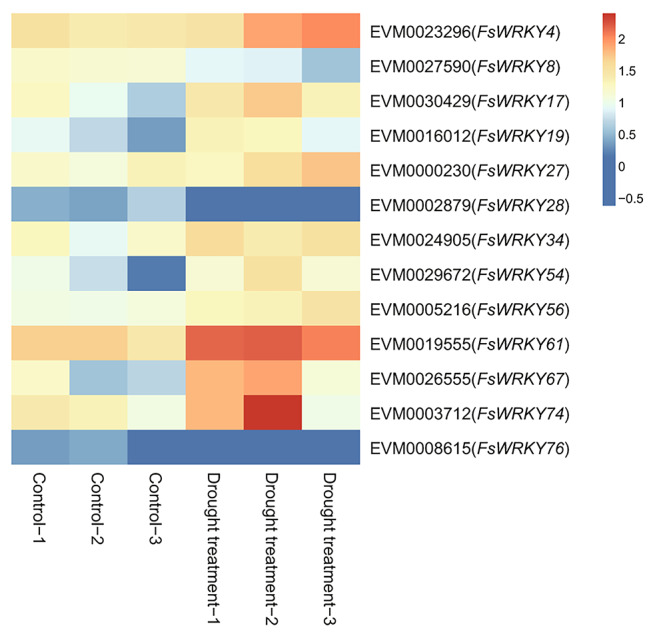
Heat map of WRKY gene expression (Log10FPKM) in response to drought stress of weeping forsythia
Discussion
We systematically analyzed WRKY genes in weeping forsythia at the genome level. We grouped WRKY genes in weeping forsythia according to the naming principle of the A. thaliana WRKY gene family (Eulgem et al. 2000). A total of 79 bZIP genes were identified in weeping forsythia, which is more than the number of WRKY genes (74 bZIP genes) identified in A. thaliana (Eulgem et al. 2000), Hippophae rhamnoides (48) (Wang et al. 2019a), Cucumis sativus (61) (Chen et al. 2020b), but less than the 109 members identified in Oryza sativa (Hwang et al. 2016), or the 126 members in Raphanus sativus (Karanja et al. 2017), 171 members in Triticum aestivum (Ning et al. 2017), and 81 members in Lycopersicon esculentum (Huang et al. 2012).
All WRKY genes of weeping forsythia were consistently clustered with the reference geneome of A. thaliana, except for the IIe subfamily which was differently clustered from A. thaliana. These results suggest that the IId subfamily of weeping forsythia has undergone genetic differentiation, and it was divided into two genetic clades.
In the two unclassified members, FsWRKY73 was special, being the only member without introns and having the shortest gene length. FsWRKY73 did not effective express in our investigated tissues of weeping forsythia, and it was not differentially expressed under drought and cold stresses. A special PAZ domain was found in FsWRKY73. At present, it is usually considered that PAZ domain is related to the binding of single-stranded RNA, which was likely to lead to gene silencing (Yan et al. 2003).
In the WRKY gene family, I subfamily contains two WRKY domains, which is considered as the oldest clade (Zhang and Wang, 2005). We identified 16 members in this subfamily in weeping forsythia, the same number as A. thaliana in this subfamily. The other six subfamilies are differentiated from I subfamily. In these newly differentiated subfamilies, except IIb subfamily, which had the same number of members as A. thaliana, the number of other subfamilies was slightly different. Not all II subfamilies of WRKY had zinc finger structure, only some members of IId subfamily had the conserved domain of Plant_zn_clust. This result indicated that the function of these members of IId subfamily might require the participation of zinc ions.
Except for the conserved WRKY domain, the WRKY gene varied greatly in length and structure. According to the number of members and clustering results, the members of each subfamily of WRKY gene in weeping forsythia did not have significant family expansion and contraction compared with that of WRKY gene in A. thaliana, and they might have more similar functions with that of A. thaliana.
At present, many studies had been conducted on the function of the WRKY genes. According to the existing research, WRKY gene functions mainly fall into the following three categories. First, it could increase the resistance to biotic stress, such as the resistance to fungi (Fan et al. 2017), bacteria (Qiu et al. 2007), and viruses (Zou et al. 2019). Second, it could improve the abiotic stress resistance. For example, WRKY genes have been shown to participate in response regulation in drought (He et al. 2016), temperature (Wang et al. 2019b), salt (Fang et al. 2021) and nutrition (Wang et al. 2019c) stresses. Third, WRKY genes are likely involved into plant development regulation. Specifically, WRKY genes were suggested to participate in somatic embryo genesis (Yang et al., 2020), promoted the synthesis of secondary metabolites in plants (Chen et al. 2017), regulated the flowering of plants (Lei et al., 2020), accelerated fruit ripening (Wang et al. 2017), and promoted fruit coloring (Cheng et al. 2017).
In WRKY genes of weeping forsythia, many cis-responsive elements (Fig. 5) of MeJA and SA were found in the upstream of the WRKY family members. The signal pathways mediated by MeJA and SA were usually associated with plant pathogen resistance (Halim et al. 2006; Findling et al. 2014). The expression patterns of WRKY gene in leaves, stems, fruits, flowers of weeping forsythia showed that more than half of WRKY genes were effectively expressed in these tissues. The WRKY genes might be involved in the development process and morphogenesis of leaf, stem, fruit, and flower tissues. In addition, the results of cis-element analysis showed that a large number of light responsive cis-regulatory elements in the upstream of these WRKY genes (Fig. 5). The development process and morphogenesis of leaves, stems, fruits, and flowers were usually high light-dependent (Franklin 2009). The effective expression of the WRKY genes in these tissues might be activated by light through light responsive cis-regulatory elements.
Two abiotic stresses, drought and cold stresses, are often encountered in the production and cultivation of weeping forsythia. Here, we investigated the expression patterns of genes in weeping forsythia leaves under drought and cold stresses. Under drought stress, 13 WRKY genes in weeping forsythia showed significant response expression, among which 4 genes had drought-related cis-elements with MYB binding sites, indicating that the responses of these 4 genes might be regulated by MYB transcription factors. Except for the drought-related cis-elements with MYB binding sites, 10 of these 13 genes had the ABA responsiveness cis-elements, indicating that most of the responding genes might be involved in ABA regulation pathways. In wheat, WRKY genes have been shown to participate in ABA-dependent way in response to abiotic stress (Budak et al. 2013). ABA signaling pathways is an important way to improve drought tolerance in plants, and WRKY genes play important roles in ABA signaling pathways (Wei et al. 2019). Under cold stress, 17 WRKY genes showed significant response expression, and 9 of the 17 genes had low temperature responsiveness cis-elements, which further confirmed that these genes were indeed related to cold stress. Previous studies have also confirmed that WRKY genes play important roles in improving cold tolerance in plants (Kim et al. 2016; Fei et al. 2022). Other genes which did not have low temperature responsiveness cis-elements might be activated by other signal pathways. On the whole, the genes involved in drought and cold stresses response in the WRKY gene family were significantly fewer than those involved in light-dependent development and morphogenesis of different tissues.
Conclusion
In this study, we identified 79 WRKY genes in weeping forsythia and classified them according to their naming rules in A. thaliana. A phylogenetic tree showed that, except for the IIe subfamily, clustering was inconsistent with A. thaliana clustering. Cis-element analysis showed that WRKY genes related to pathogen resistance in weeping forsythia might be related to Me-JA and SA-mediated signaling pathways. Combining cis-element and expression pattern analyses of WRKY genes showed that more than half of WRKY genes were involved in light-dependent development and morphogenesis in different tissues. The gene expression results showed that 13 WRKY genes were involved in drought response, most of which might be related to the ABA signaling pathway, and a few of which might be regulated by MYB transcription factors. The gene expression results under cold stress showed that 17 WRKY genes were involved in low temperature response, and 9 of them had low temperature responsiveness cis-elements. Our study of the WRKY family in weeping forsythia provided useful resources for molecular breeding and important clues for their functional verification.
Supplementary Information The online version contains supplementary material available at genetica.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Xin Sun for providing the seeds of weeping forsythia in the field.
Authors’ information
Ya-Lin Yang, Shu-Chen Wang, Fan Wang, Qian Li, Hong-Li Liu: Innovation Platform of Molecular Biology, College of Landscape and Art, Henan Agricultural University, Zhengzhou, China.
Samuel A. Cushman: Northern Arizona University, School of Forestry, Flagstaff, AZ, USA.
Yong Li: College of Life Science and Technology, Inner Mongolia Normal University, Huhehaote, China; State Key Laboratory of Tree Genetics and Breeding, Chinese Academy of Forestry, Beijing, China.
Funding
The authors acknowledge the funding from the Open Fund of State Key Laboratory of Tree Genetics and Breeding (Chinese Academy of Forestry) (Grant No. TGB2021004).
Data Availability
The datasets generated and/or analysed during the current study are available in the NCBI repository, https://www.ncbi.nlm.nih.gov/ and accession no. RNA-seq of fruits, stems, and leaves; SRR17386487-SRR17386495; RNA-seq of flowers, SRX11342985, SRX11342993 and SRX11342994; RNA-seq of drought stress, SRX7503009, SRX7503010, SRX7503012-SRX7503015; RNA-seq of cold stress, SRX7440183-SRX7440188; Genome data, JAHHPY000000000. Material samples are available from authors.
Declarations
Ethical approval and Consent to participate
Not applicable.
Human and Animal Ethics
Not applicable.
Consent for publication
Not applicable.
Competing Interest
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Artimo P, Jonnalagedda M, Arnold K, Baratin D, Csardi G, de Castro E, Duvaud S, Flegel V, Fortier A, Gasteiger E, Grosdidier A, Hernandez C, Ioannidis V, Kuznetsov D, Liechti R, Moretti S, Mostaguir K, Redaschi N, Rossier G, Xenarios I, Stockinger H. Expasy: Sib bioinformatics resource portal. Nucleic Acids Res. 2012;40:W597–W603. doi: 10.1093/nar/gks400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey T, Bodén M, Buske F, Frith M, Grant C, Clementi L, Ren J, Li W, Noble W. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37:W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao F, Ding AQ, Cheng TR, Wang J, Zhang QX. Genome-wide analysis of members of the WRKY gene family and their cold stress response in Prunus mume. Genes. 2019;10:911. doi: 10.3390/genes10110911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budak H, Kantar M, Kurtoglu KY (2013) Drought Tolerance in Modern and Wild Wheat. Sci World J 2013: 548246 [DOI] [PMC free article] [PubMed]
- Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, Xia R. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020;13:1194–1202. doi: 10.1016/j.molp.2020.06.009. [DOI] [PubMed] [Google Scholar]
- Chen C, Chen X, Han J, Lu W, Ren Z. Genome-wide analysis of the WRKY gene family in the cucumber genome and transcriptome-wide identification of WRKY transcription factors that respond to biotic and abiotic stresses. BMC Plant Biol. 2020;20:443. doi: 10.1186/s12870-020-02625-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MH, Yan TX, Shen Q, Lu X, Pan QF, Huang YR, Tang YL, Fu XQ, Liu M, Jiang WM, Lv ZY, Shi P, Ma YN, Hao XL, Zhang LD, Li L, Tang KX. Glandular trichome-specific WRKY 1 promotes artemisinin biosynthesis in Artemisia annua. New Phytol. 2017;214:304–316. doi: 10.1111/nph.14373. [DOI] [PubMed] [Google Scholar]
- Chen XJ, Li C, Wang H, Guo ZJ. WRKY transcription factors: evolution, binding, and action. Phytopathol Res. 2019;1:13. doi: 10.1186/s42483-019-0022-x. [DOI] [Google Scholar]
- Cheng MN, Huang ZJ, Hua QZ, Shan W, Kuang JF, Lu WJ, Qin YH, Chen JY. The WRKY transcription factor HpWRKY44 regulates CytP450-like1 expression in red pitaya fruit (Hylocereus polyrhizus) Hortic Res. 2017;4:17039. doi: 10.1038/hortres.2017.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng XK, Zhao YX, Jiang QS, Yang J, Zhao WS, Taylor IA, Peng YL, Wang DL, Liu JF. Structural basis of dimerization and dual w-box DNA recognition by rice WRKY domain. Nucleic Acids Res. 2019;47:4308–4318. doi: 10.1093/nar/gkz113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou KC, Shen HB. Plant-mPploc: a top-down strategy to augment the power for predicting plant protein subcellular localization. PLoS ONE. 2010;5:e11335. doi: 10.1371/journal.pone.0011335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulgem T, Rushton PJ, Robatzek S, Somssich IE. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000;5:199–206. doi: 10.1016/S1360-1385(00)01600-9. [DOI] [PubMed] [Google Scholar]
- Fan SJ, Dong LD, Han D, Zhang F, Wu JJ, Jiang LY, Cheng Q, Li RP, Lu WC, Meng FS, Zhang SZ, Xu PF (2017) GmWRKY31 and GmHDL56 enhances resistance to Phytophthora sojae by regulating defense-related gene expression in soybean. Front Plant Sci 8:781 [DOI] [PMC free article] [PubMed]
- Fang X, Li W, Yuan H, Chen H, Bo C, Ma Q, Cai R. Mutation of ZmWRKY86 confers enhanced salt stress tolerance in maize. Plant Physiol Biochem. 2021;167:840–850. doi: 10.1016/j.plaphy.2021.09.010. [DOI] [PubMed] [Google Scholar]
- Fei J, Wang YS, Cheng H, Su YB, Zhong YJ, Zheng L. The Kandelia obovata transcription factor KoWRKY40 enhances cold tolerance in transgenic Arabidopsis. BMC Plant Biol. 2022;22:274. doi: 10.1186/s12870-022-03661-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findling S, Fekete A, Warzecha H, Krischke M, Brandt H, Blume E, Mueller MJ, Berger S. Manipulation of methyl jasmonate esterase activity renders tomato more susceptible to Sclerotinia sclerotiorum. Funct Plant Biol. 2014;41:133–143. doi: 10.1071/FP13103. [DOI] [PubMed] [Google Scholar]
- Franklin KA. Light and temperature signal crosstalk in plant development. Curr Opin Plant Biol. 2009;12:63–68. doi: 10.1016/j.pbi.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Geourjon C, Deleage G. SOPMA: significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Comput Appl Biosci. 1995;11:681–684. doi: 10.1093/bioinformatics/11.6.681. [DOI] [PubMed] [Google Scholar]
- Halim VA, Vess A, Scheel D, Rosahl S. The role of salicylic acid and jasmonic acid in pathogen defence. Plant Biol. 2006;8:307–313. doi: 10.1055/s-2006-924025. [DOI] [PubMed] [Google Scholar]
- He GH, Xu JY, Wang YX, Liu JM, Li PS, Chen M, Ma YZ, Xu ZS. Drought-responsive WRKY transcription factor genes TaWRKY1 and TaWRKY33 from wheat confer drought and/or heat resistance in Arabidopsis. BMC Plant Biol. 2016;16:116. doi: 10.1186/s12870-016-0806-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Guan WJ, Bi Y, Zhang W, Li LJ, Zhang BL, Liu QQ, Song YL, Li XW, Duan ZP, Zheng QS, Yang ZF, Liang JY, Han MF, Ruan LG, Wu CM, Zhang YT, Jia ZH, Zhong NS. Efficacy and safety of lianhuaqingwen capsules, a repurposed chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine. 2021;85:153242. doi: 10.1016/j.phymed.2020.153242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Gao Y, Liu J, Peng X, Niu X, Fei Z, Cao S, Liu Y. Genome-wide analysis of WRKY transcription factors in Solanum lycopersicum. Mol Genet Genomics. 2012;287:495–513. doi: 10.1007/s00438-012-0696-6. [DOI] [PubMed] [Google Scholar]
- Huang XB, Cao LW, Fan JB, Ma GJ, Chen L. CdWRKY2-mediated sucrose biosynthesis and CBF-signalling pathways coordinately contribute to cold tolerance in bermudagrass. Plant Biotechnol J. 2022;20:660–675. doi: 10.1111/pbi.13745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SH, Kwon SI, Jang JY, Fang IL, Lee H, Choi C, Park S, Ahn I, Bae SC, Hwang DJ. OsWRKY51, a rice transcription factor, functions as a positive regulator in defense response against Xanthomonas oryzae pv. Oryzae. Plant Cell Rep. 2016;35:1975–1985. doi: 10.1007/s00299-016-2012-0. [DOI] [PubMed] [Google Scholar]
- Ishiguro S, Nakamura K. Characterization of a cDNA encoding a novel DNA-binding protein, SPF1, that recognizes SP8 sequences in the 5′ upstream regions of genes coding for sporamin and beta-amylase from sweet potato. Mol Gen Genet. 1994;244:563–571. doi: 10.1007/BF00282746. [DOI] [PubMed] [Google Scholar]
- Jiang JJ, Ma SH, Ye NH, Jiang M, Cao JS, Zhang JH. WRKY transcription factors in plant responses to stresses. J Integr Plant Biol. 2017;59:86–101. doi: 10.1111/jipb.12513. [DOI] [PubMed] [Google Scholar]
- Jones D, Taylor W, Thornton J. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci. 1992;8:275–282. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- Karanja BK, Fan LX, Xu L, Wang Y, Zhu XW, Tang MJ, Wang RH, Zhang F, Muleke EM, Liu LW. Genome-wide characterization of the WRKY gene family in radish (Raphanus sativus L.) reveals its critical functions under different abiotic stresses. Plant Cell Rep. 2017;36:1757–1773. doi: 10.1007/s00299-017-2190-4. [DOI] [PubMed] [Google Scholar]
- Kim CY, Vo KTX, Nguyen CD, Jeong DH, Lee SK, Kumar M, Kim SR, Park SH, Kim JK, Jeon JS. Functional analysis of a cold-responsive rice WRKY gene, OsWRKY71. Plant Biotechnol Rep. 2016;10:13–23. doi: 10.1007/s11816-015-0383-2. [DOI] [Google Scholar]
- Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Zheng TC, Li LL, Wang J, Cheng TR, Zhang QX. Genome-wide investigation of the bZip transcription factor gene family in Prunus mume: classification, evolution, expression profile and low-temperature stress responses. Hortic Plant J. 2022;8:230–242. doi: 10.1016/j.hpj.2021.01.009. [DOI] [Google Scholar]
- Li Y, Li X, Wei JT, Cai KW, Zhang HZ, Ge LL, Ren ZJ, Zhao CL, Zhao XY. Genome-wide identification and analysis of the WRKY gene family and cold stress response in Acer truncatum. Genes. 2021;12:1867. doi: 10.3390/genes12121867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Shi LC, Cushman SA. Transcriptomic responses and physiological changes to cold stress among natural populations provide insights into local adaptation of weeping forsythia. Plant Physiol Biochem. 2021;165:94–103. doi: 10.1016/j.plaphy.2021.05.020. [DOI] [PubMed] [Google Scholar]
- Li Y, Shi LC, Pei NC, Cushman SA, Si YT. Transcriptomic responses to drought stress among natural populations provide insights into local adaptation of weeping forsythia. BMC Plant Biol. 2021;21:273. doi: 10.1186/s12870-021-03075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang F, Pei NC, Li Q, Liu HL, Yuan WJ, Zhang HC. The updated weeping forsythia genome reveals the genomic basis for the evolution and the forsythin and forsythoside a biosynthesis. Hortic Plant J. 2022 doi: 10.1016/j.hpj.2022. [DOI] [Google Scholar]
- Ning P, Liu CC, Kang JQ, Lv JY. Genome-wide analysis of WRKY transcription factors in wheat (Triticum aestivum L.) and differential expression under water deficit condition. PeerJ. 2017;55:e3232. doi: 10.7717/peerj.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SP, Somssich IE. The role of WRKY transcription factors in plant immunity. Plant Physiol. 2009;150:1648–1655. doi: 10.1104/pp.109.138990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertea M, Pertea GM, Antonescu CM, Chang TC, Mendell JT, Salzberg SL. Stringtie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol. 2015;33:290–295. doi: 10.1038/nbt.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu DY, Xiao J, Ding XH, Xiong M, Cai M, Cao CL, Li XH, Xu CG, Wang SP. OsWRKY13 mediates rice disease resistance by regulating defense-related genes in salicylate- and jasmonate-dependent signaling. Mol Plant Microbe In. 2007;20:492–499. doi: 10.1094/MPMI-20-5-0492. [DOI] [PubMed] [Google Scholar]
- Rambaut A (2009) FigTree, a graphical viewer of phylogenetic trees. Institute of Evolutionary Biology University of Edinburgh
- Rinerson CI, Rabara RC, Tripathi P, Shen QXJ, Rushton PJ. The evolution of WRKY transcription factors. BMC Plant Biol. 2015;15:66. doi: 10.1186/s12870-015-0456-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Sun WH, Yang GF, Sun J (2018) WRKY transcription factors in legumes. BMC Plant Biol 18:243 [DOI] [PMC free article] [PubMed]
- Thijs G, Marchal K, Lescot M, Rombauts S, De Moor B, Rouze P, Moreau Y. A gibbs sampling method to detect overrepresented motifs in the upstream regions of coexpressed genes. J Comput Biol. 2002;9:447–464. doi: 10.1089/10665270252935566. [DOI] [PubMed] [Google Scholar]
- Wang GM, Zuo JC, Li XR, Liu YH, Yu JB, Shao HB, Li YZ. Low plant diversity and floristic homogenization in fast-urbanizing towns in Shandong peninsular, China: Effects of urban greening at regional scale for ecological engineering. Ecol Eng. 2014;64:179–185. doi: 10.1016/j.ecoleng.2013.12.054. [DOI] [Google Scholar]
- Wang L, Zhang XL, Wang L, Tian YA, Jia N, Chen SZ, Shi NB, Huang XM, Zhou C, Yu Y, Zhang ZQ, Pang XQ. Regulation of ethylene-responsive slWRKYs involved in color change during tomato fruit ripening. Sci Rep. 2017;7:16674. doi: 10.1038/s41598-017-16851-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MQ, Huang QX, Lin P, Zeng QH, Li Y, Liu QL, Zhang L, Pan YZ, Jiang BB, Zhang F. The overexpression of a transcription factor gene VbWRKY32 enhances the cold tolerance in Verbena bonariensis. Front Plant Sci. 2019;10:1746. doi: 10.3389/fpls.2019.01746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QZ, Shang XW, Tang WW, Li HP, Wen XP, Fan FH. Cloning and low phosphorus tolerance function analysis of PmWRKY164 from Pinus massoniana. J Agr Biotech. 2019;27:1016–1024. [Google Scholar]
- Wang T, Song Z, Wei L, Li LB. Molecular characterization and expression analysis of WRKY family genes in Dendrobium officinale. Genes Genom. 2018;40:265–279. doi: 10.1007/s13258-017-0602-z. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Feng RX, Zhang X, Su Z, Wei JR, Liu JF. Characterization of the Hippophae rhamnoides WRKY gene family and functional analysis of the role of the HrWRKY21 gene in resistance to abiotic stresses. Genome. 2019;62:689–703. doi: 10.1139/gen-2019-0024. [DOI] [PubMed] [Google Scholar]
- Wani SH, Anand S, Singh B, Bohra A, Joshi R. WRKY transcription factors and plant defense responses: latest discoveries and future prospects. Plant Cell Rep. 2021;40:1071–1085. doi: 10.1007/s00299-021-02691-8. [DOI] [PubMed] [Google Scholar]
- Wei W, Liang DW, Bian XH, Shen M, Xiao JH, Zhang WK, Ma B, Lin Q, Lv J, Chen X, Chen SY, Zhang JS. GmWRKY54 improves drought tolerance through activating genes in abscisic acid and Ca2+ signaling pathways in transgenic soybean. Plant J. 2019;100:384–398. doi: 10.1111/tpj.14449. [DOI] [PubMed] [Google Scholar]
- Xiang MF, Jin CT, Sun LH, Zhang ZH, Yao JJ, Li LC. Efficacy and potential mechanisms of chinese herbal compounds in coronavirus disease 2019: advances of laboratory and clinical studies. Chin Med. 2021;16:130. doi: 10.1186/s13020-021-00542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan KS, Yan S, Farooq A, Han A, Zeng L, Zhou MM. Structure and conserved RNA binding of the PAZ domain. Nature. 2003;426:469–474. doi: 10.1038/nature02129. [DOI] [PubMed] [Google Scholar]
- Yang J, Miao CY, Mao RL, Li Y. Landscape population genomics of forsythia (Forsythia suspensa) reveal that ecological habitats determine the adaptive evolution of species. Front Plant Sci. 2017;8:481. doi: 10.3389/fpls.2017.00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang PJ, Zhang H, Du JK, Qiao YS. Genome-wide identification and co-expression analysis of GDSL genes related to suberin formation during fruit russeting in pear. Hortic Plant J. 2022;8:153–170. doi: 10.1016/j.hpj.2021.11.010. [DOI] [Google Scholar]
- Zhang Q, Chen T, Wang X, Wang J, Gu K, Yu J, Hu D, Hao Y. Genome-wide identification and expression analyses of homeodomain-leucine zipper family genes reveal their involvement in stress response in apple (Malus × domestica) Hortic Plant J. 2022;8:261–278. doi: 10.1016/j.hpj.2021.04.003. [DOI] [Google Scholar]
- Zhang T, Li X, Zhao Q, Shi Y, Hao Y, You C. Genome-wide identification and functional characterization of the MdCLE peptide family in apple (Malus × domestica) Hortic Plant J. 2022;8:279–288. doi: 10.1016/j.hpj.2021.12.003. [DOI] [Google Scholar]
- Zhang YJ, Wang LJ. The WRKY transcription factor superfamily: its origin in eukaryotes and expansion in plants. BMC Evol Biol. 2005;5:1. doi: 10.1186/1471-2148-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Li W, Dai SJ, Liu RX, Xie ZP, Zhang SM, Yue XD. Alkaloids bearing rare skeletons from Forsythia suspensa with anti-inflammatory and anti-viral activities in vitro. Phytochemistry. 2021;186:112739. doi: 10.1016/j.phytochem.2021.112739. [DOI] [PubMed] [Google Scholar]
- Zou LJ, Yang F, Ma YH, Wu QG, Yi KX, Zhang DW. Transcription factor WRKY30 mediates resistance to Cucumber mosaic virus in Arabidopsis. Biochem Bioph Res Co. 2019;517:118–124. doi: 10.1016/j.bbrc.2019.07.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are available in the NCBI repository, https://www.ncbi.nlm.nih.gov/ and accession no. RNA-seq of fruits, stems, and leaves; SRR17386487-SRR17386495; RNA-seq of flowers, SRX11342985, SRX11342993 and SRX11342994; RNA-seq of drought stress, SRX7503009, SRX7503010, SRX7503012-SRX7503015; RNA-seq of cold stress, SRX7440183-SRX7440188; Genome data, JAHHPY000000000. Material samples are available from authors.