Remdesivir inhibits the viral RNA–dependent RNA polymerase, thereby interrupting the viral replication process. It decreases the time to recovery in hospitalized coronavirus disease 2019 patients with lower respiratory tract infections; however, older adult patients with underlying cardiovascular disease and those taking beta-blockers, when treated with remdesivir, have bradycardia as an adverse effect. This article provides a brief overview of remdesivir-induced bradycardia, diagnosis, and treatment.
Key Words: bradycardia, cardiotoxicity, COVID-19, remdesivir
Abstract
Remdesivir, a viral RNA–dependent RNA polymerase inhibitor, found extensive use in coronavirus disease 2019–infected patients because it curbs the viral load expansion. Among patients hospitalized as a result of lower respiratory tract infection, remdesivir proved to improve recovery time; however, remdesivir also can induce significant cytotoxic effects on cardiac myocytes. In this narrative review, we discuss the pathophysiological mechanism of remdesivir-induced bradycardia and diagnostic and management strategies for these patients. We conclude that further research is necessary to understand better the mechanism of bradycardia in coronavirus disease 2019 patients with or without cardiovascular disorder treated with remdesivir.
Key Points
Remdesivir decreases the time to recovery in hospitalized coronavirus disease 2019 patients with lower respiratory tract infections.
Remdesivir causes various cardiovascular effects, including hypotension, bradycardia, atrial fibrillation, QRS widening, prolonged QT interval, torsades de pointes, and cardiac arrest.
Bradycardia is the most common cardiac adverse effect caused by remdesivir.
Management criteria include discontinuation of remdesivir and adding atropine or dopamine to refractory cases.
Remdesivir is a viral inhibitor that interrupts replication by inhibiting the viral RNA–dependent RNA polymerase. Presently, remdesivir is effective against nonsegmented negative-sense RNA viruses such as filoviridae, paramyxoviridae, pneumoviridae, and coronaviridae families.1 In the current scenario of coronavirus disease 2019 (COVID-19), remdesivir is used in patients with oxygen saturation of less than 94%. Furthermore, it has proved to be a drug of significance because it decreases the time to recovery in hospitalized COVID-19 patients with lower respiratory tract infections.2
Bradycardia is defined as an arrhythmia with a heart rate of fewer than 60 beats per minute. It could be a normal finding in young athletes, or it may be caused by damage to the conduction system from intrinsic or extrinsic factors. Intrinsic factors include idiopathic degeneration (aging), infarction or ischemia, infiltrative diseases, collagen vascular disease, myotonic muscular dystrophy, surgical trauma, and familial and infectious diseases. Extrinsic factors include autonomically mediated syndromes, drugs (eg, beta-blockers, calcium-channel blockers, digoxin, clonidine), hypothyroidism, hypothermia, neurologic disorders, and electrolyte imbalances (hypokalemia and hyperkalemia).3 The usual causes of the pathology reside in the sinus node, atrioventricular nodal tissue, and the specialized His-Purkinje conduction system. The common symptoms of bradycardia are syncope, presyncope, transient dizziness, fatigue, dyspnea on exertion, and symptoms of heart failure or cerebral hypoperfusion leading to confusion.4
Remdesivir causes various cardiovascular effects, including hypotension, bradycardia, atrial fibrillation rhythm, QRS widening, prolonged QT interval, torsades de pointes, and cardiac arrest.5 In addition, because of complications in older adult patients with underlying cardiovascular disease and use of beta-blockers, a discussion of remdesivir-induced bradycardia is essential.6 The section below discusses the pathophysiology of remdesivir-induced bradycardia, diagnosis, and treatment in detail.
Pathophysiology
Remdesivir is a nucleotide analog prodrug commonly used in hospitalized COVID-19 patients. Inside the cell, it is converted to an active nucleotide triphosphate form, which is responsible for its antiviral properties. This active triphosphate form is similar to adenosine triphosphate (ATP), acts as the substitute in place of ATP for viral RNA–dependent RNA polymerases, inhibits enzyme activity, and decreases viral production.1 Remdesivir is primarily metabolized by the action of plasma hydrolases carboxylesterase 1 (80% of metabolism) and cathepsin A (10%), and the remainder by the action of cytochrome P450 enzymes.7 Remdesivir levels should be monitored carefully when coadministered with other medications that can act on cytochrome enzymes. Remdesivir is predominantly eliminated from the body in urine (74%) and feces (14%).8
Remdesivir has been shown to cause many adverse effects such as anemia, hepatotoxicity (increased liver enzymes), cutaneous rash, renal toxicity, and hypotension, and a spectrum of cardiac adverse effects such as bradycardia atrial fibrillation, hypotension, and QT prolongation.9 According to a WHO Individual Case Safety Reports database comprising 2603 patients, bradycardia was the most common cardiac adverse effect caused by remdesivir. In the study conducted among 2603 COVID-19 patients undergoing remdesivir treatment, 302 patients showed cardiac results, with bradycardia affecting 94 patients (31%). Affected individuals, more commonly men (53, 56%), were aged 6 to 90 years with a mean age of 61.2 years. The median onset of bradycardia was within 3 days. From the study of Touafchia et al, Figures 1 and 2 depict remdesivir-induced bradycardia distribution over sex and age, respectively.10 Bradycardia associated with remdesivir use is caused by two probable mechanisms: mitochondrial dysfunction and sinus node dysfunction.6
Fig. 1.
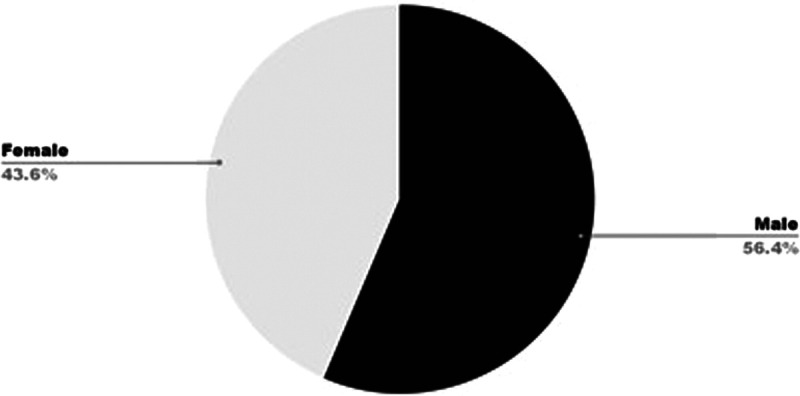
Remdesivir-induced bradycardia: case distribution according to sex.
Fig. 2.
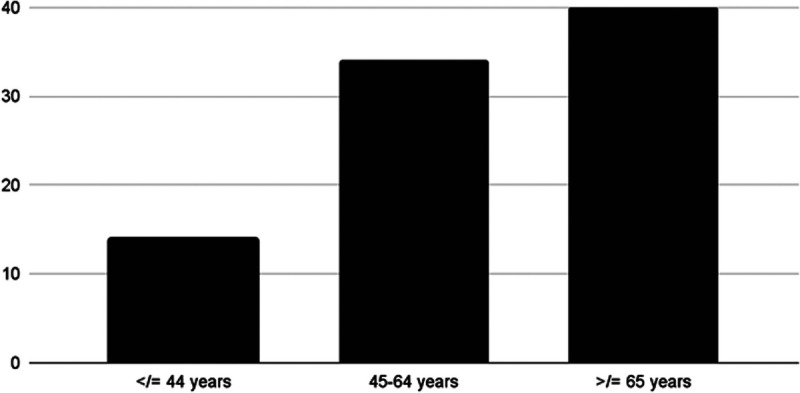
Remdesivir-induced bradycardia: case distribution according to age.
As discussed earlier, remdesivir acts as an antiviral agent; however, it also crosses and reacts with human mitochondrial RNA polymerase, causing its inhibition and thus leading to mitochondrial dysfunction. This is a common mechanism for drug-induced cardiotoxicity and is one of the probable mechanisms behind remdesivir-induced bradycardia.11
Another mechanism by which remdesivir can cause bradycardia is through its suppression of the sinoatrial (SA) node. As discussed earlier, remdesivir in its triphosphate form has structural similarity to ATP, which is metabolized to adenosine that is known to cause electrophysiological changes in the heart.1 Adenosine suppresses the automaticity at the sinus node in the heart, exerting a negative chronotropic effect, and generates a negative dromotropic impact by inhibiting atrioventricular nodal conduction.1 In the heart, adenosine acts on the SA node through the A1 (adenosine) receptor, activates outward potassium channels, and inhibits inward calcium channels. This results in the hyperpolarization of the SA node cell membrane, causing shortening of the action potential duration and leading to decreased heart rate, as shown in Figure 3.12
Fig. 3.
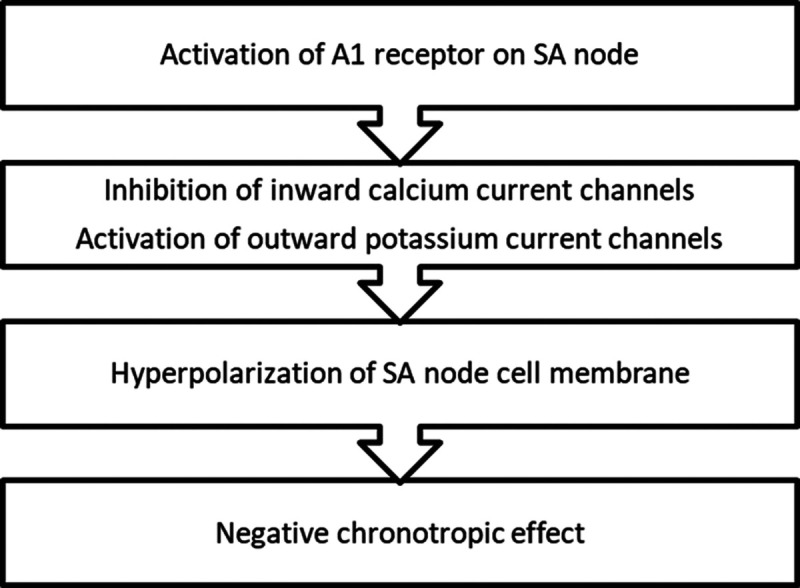
Adenosine-mediated negative chronotropic effect. A1, adenosine; SA, sinoatrial.
Diagnosis/Treatment
Bradycardia is recognized as an adverse reaction because remdesivir use has been growing. It can be diagnosed by documenting a pulse rate of less than 60 beats per minute and confirmed with the help of a 12-point electrocardiogram (ECG). It also is essential to rule out other etiologies of bradycardia by doing a thorough workup. This includes a physical examination, complete metabolic panel, thyroid function tests, urine toxicology, and drug screening. Remdesivir can be identified as the main culprit in an ECG showing marked sinus bradycardia in patients who initially had normal telemetry monitor and ECG findings before the dose and did not receive any other medications that could cause bradycardia.13 The University of Illinois Chicago Drug Information Group recommends a baseline ECG and heart rate for all patients before receiving remdesivir and continuous cardiac monitoring during treatment, especially among those with underlying cardiovascular disease, older adults, and those taking a beta-blocker.14
Bradycardia should be managed with continued monitoring, symptomatic treatment, and discontinuation of remdesivir. Reports have shown the resolution of bradycardia after discontinuation of the drug. One study included 166 COVID-19 patients; 100 patients received remdesivir and the other 66 patients did not receive remdesivir. After the 5-day course of remdesivir, sinus bradycardia developed in 21 patients from the remdesivir group and three patients in the control group who did not receive remdesivir. Later, bradycardia was resolved in all of the patients from the remdesivir group after discontinuation of the drug.15 If bradycardia persists, then additional management should include atropine 0.5 mg every 3 to 5 minutes and/or a dopamine infusion of 3 or 5 μg/kg/min.14 If the symptoms and heart rate of the patient do not improve, the patient is a candidate for a temporary pacemaker.16
Conclusions
Remdesivir has proven to be beneficial in hospitalized COVID-19 patients; however, besides its undisputed use in the pandemic, it has led to many adverse effects. As such, it is essential to be vigilant during treatment with remdesivir because it has the potential to cause serious adverse effects. Recently, bradycardia has proven to be a common complication of remdesivir use. The probable mechanisms of remdesivir-induced bradycardia are either through mitochondrial dysfunction or inhibition of the SA node. Patients with preexisting cardiac abnormalities should receive a baseline ECG before the drug is initiated. Management generally involves discontinuation of the drug or atropine or dopamine in refractory cases. Further research is needed on this topic to gain more insight into the mechanism behind the association of bradycardia and remdesivir in both patients with or without an underlying cardiovascular disorder.
Footnotes
The authors did not report any financial relationships or conflicts of interest.
Contributor Information
Sai Gautham Kanagala, Email: gauthamkanagala@gmail.com.
Hardeep Dholiya, Email: hardeepdholiya@gmail.com.
Poonam Jhajj, Email: poonamkjhajj@gmail.com.
Vasu Gupta, Email: drgupta.vasu@gmail.com.
Sachin Gupta, Email: docsachin2111@gmail.com.
Shiau-ing Wu, Email: shiauingwu@gmail.com.
Rohit Jain, Email: drrohitjain2010@gmail.com.
References
- 1.Gubitosa JC Kakar P Gerula C, et al. Marked sinus bradycardia associated with remdesivir in COVID-19: a case and literature review. JACC Case Rep 2020;2(14):2260–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beigel JH Tomashek KM Dodd LE, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med 2020;383:1813–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mangrum JM, DiMarco JP. The evaluation and management of bradycardia. N Engl J Med 2000;342:703–709. [DOI] [PubMed] [Google Scholar]
- 4.Sidhu S, Marine JE. Evaluating and managing bradycardia. Trends Cardiovasc Med 2020;30:265–272. [DOI] [PubMed] [Google Scholar]
- 5.Nabati M, Parsaee H. Potential cardiotoxic effects of remdesivir on cardiovascular system: a literature review. Cardiovasc Toxicol 2022;22:268–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ching PR, Lee C. Remdesivir-associated bradycardia. BMJ Case Rep 2021;14:e245289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Humeniuk R Mathias A Kirby BJ, et al. Pharmacokinetic, pharmacodynamic, and drug-interaction profile of remdesivir, a SARS-CoV-2 replication inhibitor. Clin Pharmacokinet 2021;60:569–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Center for Biotechnology Information—PubChem . Compound summary: remdesivir. https://pubchem.ncbi.nlm.nih.gov/compound/Remdesivir. Accessed June 20, 2022.
- 9.Gupta AK Parker BM Priyadarshi V, et al. Cardiac adverse events with remdesivir in COVID-19 infection. Cureus 2020;12:e11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Touafchia A Bagheri H Carrié D, et al. Serious bradycardia and remdesivir for coronavirus 2019 (COVID-19): a new safety concerns. Clin Microbiol Infect 2021;27:791.e5–791.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Day LB, Abdel-Qadir H, Fralick M. Bradycardia associated with remdesivir therapy for COVID-19 in a 59-year-old man. CMAJ 2021;193:E612–E615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mustafa SJ Morrison RR Teng B, et al. Adenosine receptors and the heart: role in regulation of coronary blood flow and cardiac electrophysiology. Handb Exp Pharmacol 2009(193):161–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacinto JP Patel M Goh J, et al. Remdesivir-induced symptomatic bradycardia in the treatment of COVID-19 disease. HeartRhythm Case Rep 2021;7:514–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soni R; Drug Information Group . What literature is available describing the occurrence of bradycardia with remdesivir? https://dig.pharmacy.uic.edu/faqs/2021-2/october-2021-faqs/what-literature-is-available-describing-the-occurrence-of-bradycardia-with-remdesivir. Accessed June 20, 2022.
- 15.Attena E Albani S Maraolo AE, et al. Remdesivir-induced bradycardia in COVID-19: a single center prospective study. Circ Arrhythm Electrophysiol 2021;14:e009811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hafeez Y, Grossman SA. Sinus Bradycardia. Treasure Island, FL: StatPearls Publishing; 2022. [PubMed] [Google Scholar]


