Abstract
The development of transgenic invertebrate preparations in which the activity of specifiable sets of neurons can be recorded and manipulated with light represents a revolutionary advance for studies of the neural basis of behavior. However, a downside of this development is its tendency to focus investigators on a very small number of “designer” organisms (e.g., C. elegans and Drosophila), potentially negatively impacting the pursuit of comparative studies across many species, which is needed for identifying general principles of network function. The present article illustrates how optical recording with voltage-sensitive dyes in the brains of non-transgenic gastropod species can be used to rapidly (i.e., within the time course of single experiments) reveal features of the functional organization of their neural networks with single-cell resolution. We outline in detail the dissection, staining, and recording methods used by our laboratory to obtain action potential traces from dozens to ~150 neurons during behaviorally relevant motor programs in the CNS of multiple gastropod species, including one new to neuroscience – the nudibranch Berghia stephanieae. Imaging is performed with absorbance voltage-sensitive dyes and a 464-element photodiode array that samples at 1,600 frames/second, fast enough to capture all action potentials generated by the recorded neurons. Multiple several-minute recordings can be obtained per preparation with little to no signal bleaching or phototoxicity. The raw optical data collected through the methods described can subsequently be analyzed through a variety of illustrated methods. Our optical recording approach can be readily used to probe network activity in a variety of non-transgenic species, making it well suited for comparative studies of how brains generate behavior.
Introduction
The development of transgenic lines of invertebrates such as Drosophila and C. elegans has provided powerful systems in which the neural bases of behavior may be optically interrogated and manipulated. However, these special preparations may have the downside of reducing enthusiasm for neural circuit studies of non-transgenic species, particularly with respect to the introduction of new species into neuroscience research. Focusing exclusively on one or two model systems is detrimental to the search for general principles of network function, because comparative studies represent an essential route by which such principles are discovered1 , 2 , 3 , 4 . Our aim here is to demonstrate a large-scale imaging approach for obtaining rapid insight into the functional structure of gastropod neural networks, in an effort to facilitate comparative studies of neural network function.
Gastropod mollusks such as Aplysia, Lymnaea, Tritonia, Pleurobranchaea and others have long been used to investigate principles of neural network function, in large part because their behaviors are mediated by large, often individually identifiable neurons located on the surface of ganglia, making them readily accessible to recording techniques5 . In the 1970s, voltage-sensitive dyes (VSDs) that can integrate into the plasma membrane were developed that soon enabled the first electrode-free recordings of the action potentials generated by multiple neurons6 . Here, we demonstrate our use of VSDs to examine network activity in several species of gastropods, including one new to neuroscience, Berghia stephanieae. The imaging device is a commercially available 464-element photodiode array (PDA) that samples at 1,600 frames/second (Figure 1), which, when used with fast absorbance VSDs, reveals the action potentials of all recorded neurons7 . The signals recorded by all diodes are displayed immediately after acquisition and superimposed on an image of the ganglion in the PDA acquisition software, making it possible to investigate neurons of interest with sharp electrodes in the same preparation8 , 9 .
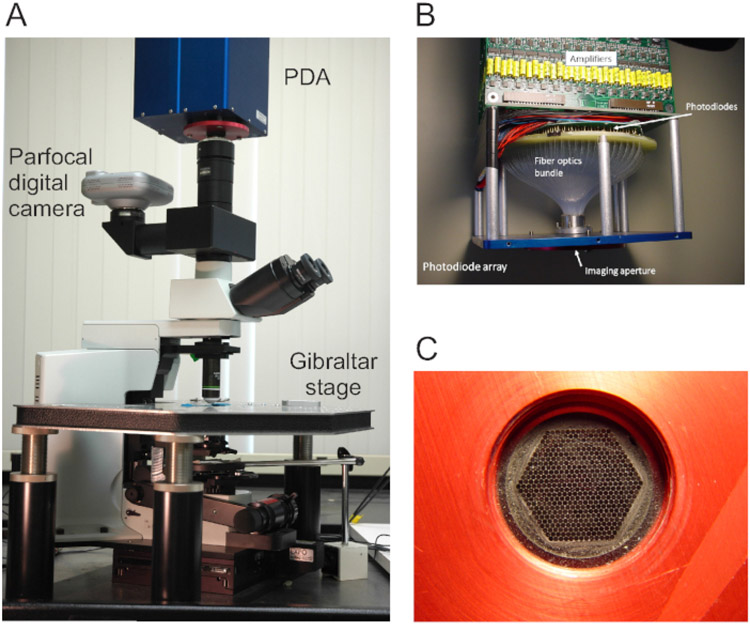
Figure 1: Views of the optical imaging rig and photodiode array (PDA).
(A) The optical imaging rig, featuring the PDA, digital camera, microscope, and stage. (B) The internal design of the PDA, in which fiber optics connect the imaging aperture to 464 photodiodes. A row of amplifiers is located above the photodiodes. (C) The hexagonal face of the imaging aperture, onto which the area being imaged is focused.
In the raw PDA data, many diodes redundantly record the larger neurons, and many also contain mixed signals from multiple neurons. A turning point was the development of an automated spike-sorting method using independent component analysis to rapidly process each raw 464-channel PDA data set into a new set of traces, in which every recorded neuron appears in a separate trace containing just its action potentials10 , 11 .
In this article we outline the essential steps involved in obtaining large-scale action potential recordings from gastropod nervous systems with a photodiode array and fast absorbance VSDs. We moreover illustrate analytical methods that can be employed for clustering and mapping the optically recorded neurons with respect to their functional ensembles, and for characterizing population-level features that often are not apparent through simple inspection of the firing traces12 , 13 .
Protocol
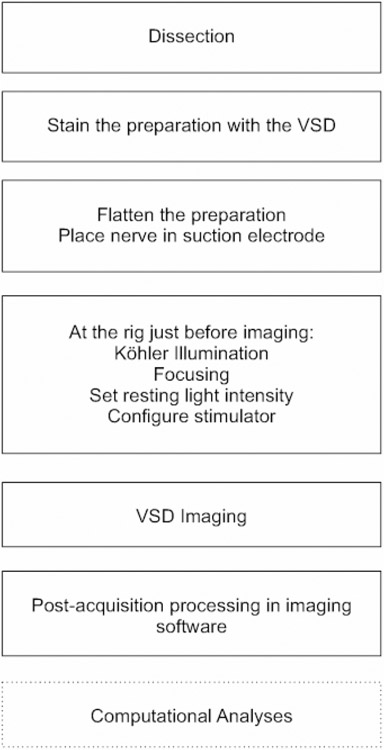
NOTE: The workflow outlined below is summarized in Figure 2.
Figure 2: A flowchart illustrating the essential workflow in obtaining optical recordings.
The essential steps in the VSD imaging protocol, from dissection and staining through the details of imaging, are depicted in this flowchart.
1. Minimize vibration
If possible, ensure the rig is on the ground floor, and use a spring-based isolation table, which dampens a wider range of vibration frequencies than air tables.
If using a spring-based table, make sure it is floating (it needs to be adjusted every time one adds or takes something off the table).
-
Reduce vibration-based noise in the imaging room as much as possible, even to the extent of shutting off airflow during imaging if necessary. Minimize any vibration stemming from fluid turbulence in perfusion systems.
NOTE: The neural preparation must not move during acquisition. Movements of any kind produce shifts of contrast edges across the diodes, leading to artifactual signals. If the procedure involves a stimulus during acquisition, it must not induce preparation movement.
2. Run a pinhole test to enable proper alignment of ganglion photos with the PDA data
Place a piece of aluminum foil with 3 small holes poked in it on a microscope slide. Take an image of the three holes with the digital camera mounted on its parfocal photoport.
Using the imaging software that comes with the PDA, acquire a short file (e.g., 5 s). Partway through the acquisition, tap the table to induce vibration artifacts that will be very visible around the edges of the pinholes, allowing for the image of the pinholes to be precisely aligned with the optical data.
- Use the Superimpose function in the imaging software, found in menu item “Display ∣ Page Superimpose ∣ Superimpose Traces w/External Image ∣ Superimpose Image,” to overlay the diode data onto the photo of the pinholes, and then iteratively adjust the x, y, and magnification settings for the photo until the pinholes sit directly atop the pinhole artifacts in the diode data.
-
Save these numbers to align preparation images taken with the camera with the diode data in future experiments.NOTE: Pinhole alignment of the PDA only needs to be performed once after the PDA is mounted on the microscope, until it is rotated or removed, at which time it must be done again.
-
3. Dissections for three marine gastropod species
For species that grow to large size, such as Tritonia and Aplysia, start with smaller individuals, which have thinner, less opaque ganglia, easing obtaining sufficient light for optimal signal-to-noise.
-
Have ready filtered artificial seawater to be used as saline for the dissections and imaging experiments.
NOTE: In all subsequent steps of the protocol, “saline” denotes artificial seawater.
- Dissection of Tritonia diomedea
- Place an animal in the refrigerator for about 20 min to anesthetize it.
- For larger animals, expose the brain by holding the animal in one hand, letting the head end drape over the forefinger to expose the “neck.” For smaller animals, pin them dorsal side up in a wax-lined dissection dish before exposing the brain.
-
Using dissection scissors, make a 3-4 cm midline incision on the dorsal side of the animal, above the buccal mass (which can be felt through the body wall).NOTE: The needed portion of the CNS, consisting of fused, bilateral cerebropleural and pedal ganglia, is orange and distinct in appearance from the surrounding tissue; it lies immediately posterior to the rhinophores and atop the buccal mass.
- Excise the CNS by severing those nerves innervating the animal’s body using forceps and microdissection scissors, keeping intact all those nerves connecting the central ganglia. Leave a long length of pedal nerve 3 (PdN3), or whichever nerve will be stimulated.
- Use minutien pins to affix the CNS to the bottom of a saline-filled elastomer-lined dish for further dissection. Maintain the preparation temperature at 11 °C by perfusing the dish with saline delivered with a feedback-controlled, in-line Peltier cooling system using a peristaltic pump.
- Using forceps and microdissection scissors, carefully remove the loosely adhering layer of the connective tissue from around the CNS. Leave the fine sheath closely adhering to the ganglia.
-
Briefly (~10 s) dip the ganglia in a solution of 0.5% glutaraldehyde in saline. Place the ganglia back in the saline-perfused elastomer-lined dish, allowing saline to wash away the glutaraldehyde before beginning VSD staining.NOTE: This light fix of the connective tissue and its intrinsic muscles will help prevent movement during imaging.
- Dissection of Aplysia californica
- Anesthetize an approximately 40 g animal by injecting ~20 mL of 350 mM MgCl2 into the body through the ventral surface (foot).
- Use pins to position the animal ventral side up in a wax-lined dissection dish.
-
Using dissection scissors, make a 2-3 cm midline incision along the most anterior extent of the foot. Pin down the flaps of the foot on either side of the incision to reveal part of the CNS and buccal mass.NOTE: The needed portion of the CNS, consisting of fused cerebral ganglia, and closely apposed, bilateral pleural and pedal ganglia, is yellow-orange and distinct in appearance from the surrounding tissue; it sits dorsal and posterolateral to the bulbous muscular buccal mass.
- Use forceps and dissection scissors to carefully dissect away the buccal mass, revealing the cerebral ganglia.
- Excise the CNS by severing those nerves innervating the animal’s body using forceps and microdissection scissors, keeping intact all those nerves connecting the central ganglia. Leave a long length of pedal nerve 9 (PdN9), or whichever nerve will be stimulated.
- Use minutien pins to position the CNS in a saline-filled elastomer-lined dish. Maintain the preparation temperature at 15-16 °C by perfusing the dish with saline passing through a Peltier cooling device.
- Using forceps and microdissection scissors, remove excessive connective tissue from the CNS, and dissect away a superficial portion of the sheath on the ganglion or ganglia to be imaged. Be careful during this process to not make a hole in the sheath, which would result in neurons spilling out from within.
- Briefly (~20 s) dip the ganglia in a solution of 0.5% glutaraldehyde in saline. Place the ganglia back in the saline-perfused elastomer-lined dish, allowing saline to wash away the glutaraldehyde before beginning VSD staining.
- Dissection of Berghia stephanieae
- Place an animal in the refrigerator for about 20 min to anesthetize it.
- Using an elastomer-lined dish filled with room-temperature saline, place minutien pins in both the head and tail.
-
Using microdissection scissors, make a 5-7 mm dorsal incision superficial to the CNS.NOTE: The eyes, dark spots that reside within the animal beside the CNS, conveniently mark the position of the needed portion CNS, which consists of bilaterally fused cerebropleural and pedal ganglia and sits atop the buccal mass.
- Excise the CNS by severing those nerves innervating the animal’s body using forceps and microdissection scissors. Leave any nerve being stimulated sufficiently long for a suction electrode.
4. Stain the preparation with a voltage-sensitive dye
- Prepare stock solutions of either RH155 (also known as NK3041) or RH482 (also known as NK3630 or JPW1132).
- RH155: Dissolve 5.4 mg of solid dye in 1 mL of 100% EtOH, pipetting 29 μL into each of 34 microcentrifuge tubes. With the contents of each tube exposed to the air, let them dry overnight in the dark. Cap and place the resulting solid aliquots of RH155, each containing 0.15 mg, in a −20 °C freezer.
-
RH482: Dissolve 2 mg of solid dye in 100 μL of DMSO, divide the solution into 20 aliquots of 5 μL, each containing 0.1 mg of RH482, and store in a −20 °C freezer.NOTE: For Tritonia and Aplysia, either bath perfusion or pressure application may be used to load the VSD RH155 into the membranes of the neurons in the preparation. Pressure application has the advantage of exposing just the ganglion being imaged to the VSD.
- For bath perfusion, add 5 mL of saline to each of two of the above aliquots of solid RH155 and vortex into solution, producing a combined solution of 10 mL containing 0.03 mg/mL RH155.
- Perfuse in the dark (to avoid photobleaching) for 1 to 1.5 h at 11 °C for Tritonia and at 16 °C for Aplysia. Maintain the temperature by passing the perfusion solution through a Peltier cooling system.
- For pressure application, add 500 μL saline to one aliquot of RH155, and vortex to produce a dye concentration of 0.3 mg/mL.
- Draw about 200 μL of the solution into polyethylene tubing using a handheld microdispenser, ensuring that there is a good match between the tube diameter and the diameter of the ganglion to be stained.
- Use a micromanipulator to carefully place the end of the tube over the target ganglion, lowering it until it forms a snug seal on the ganglion. Use the type of cooling system described above to keep the ganglia at the desired temperature.
- Dim the room lights to avoid photobleaching and turn the microdispenser applicator knob every 5 min to force more dye onto the ganglion.
- Check at 30 min to confirm that good staining is occurring, and then continue for a total staining time of about 1 h.
- For staining in Berghia, add 1 mL of saline to a frozen aliquot of RH482, and vortex to dissolve.
- Transfer 200 μL of this solution into a microcentrifuge tube containing 800 μL of saline and vortex into solution, producing a final staining solution of 0.02 mg/ml RH482 in saline with 0.1% DMSO.
- Place the entire CNS into the microcentrifuge tube, wrapping the tube in aluminum foil to avoid photobleaching, and shake by hand every 5-6 min for about 1 h. Store the remaining 800 μL of the first solution in the refrigerator, and use for up to 3 days to stain subsequent preparations.
5. Flatten the preparation and set up for nerve stimulation
NOTE: The steps in this section should be performed under minimal illumination or with green light to minimize photobleaching.
After staining, immerse the CNS in saline within the imaging chamber and place under a dissecting microscope.
Place pieces of silicone (for Tritonia or Aplysia) or blobs of petroleum jelly (for Berghia) to the left and right of the ganglion/ganglia to be imaged.
-
Press an appropriately sized piece of a glass or plastic coverslip down onto the preparation to flatten it. Press firmly but not so hard as to damage the neurons.
NOTE: Flattening the convex surface of the preparation in this manner will render a greater number of neurons parfocal, thereby increasing the number of neurons recorded, and will furthermore help to immobilize the preparation during imaging.
- If stimulating a nerve to elicit a fictive motor program, prepare a suction electrode whose front tip is approximately as wide as the nerve diameter. Accomplish this by carefully melting a segment of PE-100 polyethylene tubing over a flame while gently pulling both ends of the tubing segment and then cutting the resulting taper at the desired point.
- Draw a small volume of saline through the tapered end of a polyethylene suction electrode, followed by the end of the nerve to be stimulated, by attaching a length of thick-walled, flexible polymer tubing to the back end of the electrode and using mouth suction to apply negative pressure.
- Confirm that the saline in the electrode lacks bubbles that could interrupt electrical conduction.
6. Preparation and optimization for imaging
Move the chamber to the imaging rig. Start the saline perfusion through the recording chamber and place the temperature probe near the preparation. Set the temperature controller for the temperature desired for the species being imaged (for Tritonia, 11 °C, for Aplysia, 15-16 °C, or for Berghia, 26-27 °C).
Place one chlorided silver wire down the suction electrode, making sure that it contacts the saline in the electrode, and put the other Ag-AgCl wire (the return path) into the bath saline, near the suction electrode.
- Lower the water immersion lens into the saline. Close the base diaphragm, and then raise or lower the substage condenser and adjust the focus until the edges of the diaphragm are in sharp focus, creating Kohler illumination.
- Focus on the region of the preparation to be imaged. Bias focusing on smaller neurons over larger ones, as optical signals originating in larger neurons are more likely than smaller ones to be registered even if they are slightly out of focus.
- Take a picture of the ganglion to be imaged with the parfocal digital camera.
-
With the control panel gain switch set to 1x, inspect the resting light intensity (RLI) in the imaging software by clicking on the “RLI” button and checking the average RLI of the diodes. Adjust the voltage level sent from a stimulator to the LED lamp power supply and continue checking the average RLI level until it is in the desired range (usually around 3-4 V).NOTE: High RLIs, corresponding to approximately 3-4 V on the PDA, are desirable. The higher the light, the better the signal-to-noise ratio of the optical signals, however, this must be balanced against a faster rate of photobleaching at higher RLIs. This risk is minimized by using high-NA objective lenses. The water immersion objective lenses used are 10x/0.6 NA, 20x/0.95 NA, 40x/0.8 NA, and 40x/1.15 NA.
- Set the control panel gain switch to 100x for the recording.
-
If stimulating a nerve, set the desired voltage, frequency, and duration on a separate stimulator from that used to set the light level. Confirm that TTL triggering between the control panel and stimulator is properly configured.NOTE: Sample nerve stimulus parameters in each species are as follows: Tritonia PdN3, 2 s, 10 Hz pulse train of 5 ms, 10 V pulses; Aplysia PdN9, 2.5 s, 20 Hz pulse train of 5 ms, 8 V pulses; a Berghia pedal nerve, 2 s, 10 Hz pulse train of 5 ms, 5 V pulses.
- Double-check that the spring or air table is floating.
7. Optical recording
Turn off or dim the room lights, including any overhead fluorescent lighting.
-
Set the desired file duration, the pathway, and file name, and then click on the “Take Data” button in the imaging software to acquire files up to the capacity of the computer’s available RAM. Remain still during the optical recording, as small vibrations can introduce large artifacts into the optical recording data.
NOTE: For acquisitions that would exceed the computer’s available RAM, a custom-made C++ acquisition program is available through Dr. Jian-young Wu of Georgetown University.
- To view data immediately after acquisition, use the Superimpose function in the imaging software to superimpose the data collected by all 464 diodes over the image of the ganglion taken earlier of the preparation7 . Click on any of the diodes represented in the software to expand what they recorded on a separate trace screen.
- Achieve exact alignment of the diodes with respect to the preparation by entering the x, y, and magnification factors as previously determined by the pinhole test.
- To maximize action potential visibility and improve the neuron yield for subsequent spike-sorting14 , impose a bandpass Butterworth filter with 5 Hz and 100 Hz cutoffs (available in imaging software) to remove both low- and high-frequency noise.
- To save filtered optical data as a text file for further analysis in a scientific computing platform, first select the “TP filter” box just under the “Page” screen in the imaging software. Then, select “Save Page as ASCII” from the “Output” tab and enter the desired filename into the dialog box that appears.
Representative Results
Tritonia
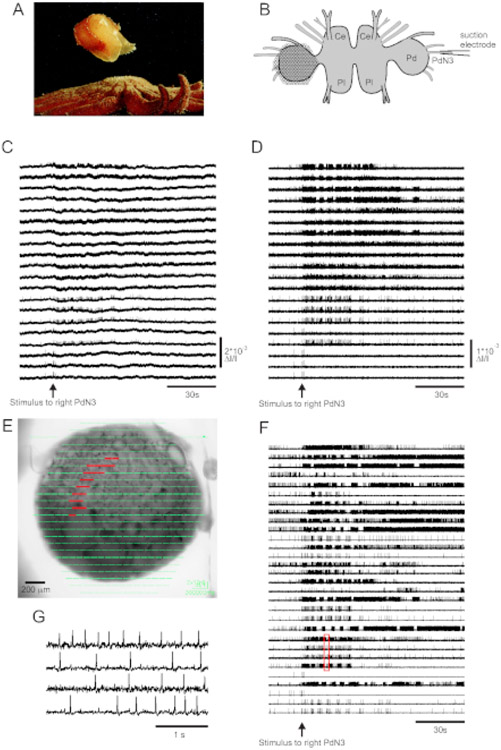
Skin contact with its seastar predator triggers Tritonia diomedea’s escape swim, consisting of a rhythmic series of whole-body flexions that propel it away to safety (Figure 3A). In isolated brain preparations, a brief stimulus to pedal nerve 3 (PdN3) elicits the rhythmic swim motor program (SMP) for this behavior, which is readily recognizable in optical recordings from the pedal ganglia. Figure 3B depicts the layout of a VSD imaging experiment designed to record the firing activity of neurons on the dorsal surface of the left Tritonia pedal ganglion, over which the PDA was positioned, as a stimulus to the contralateral (right) PdN3 elicits the SMP. Raw and filtered data (bandpass Butterworth filter, 5 and 100 Hz cutoffs) from 20 diodes recording activity before, during and after stimulation of PdN3 are shown in Figures 3C,D respectively. The nerve stimulus was delivered 20 s into the 2 min file. Immediately after acquisition, the signals measured by all 464 diodes of the recording array can be topographically displayed over an image of the preparation in the imaging software (Figure 3E). At this point, many traces contain spikes recorded redundantly from the same neurons, and some traces contain spikes from more than one neuron. Spike-sorting the filtered diode traces with ICA yielded 53 unique neuronal traces, 30 of which are shown in Figure 3F. The kinetics of individual spikes can be appreciated in Figure 3G, which expands an excerpt of four traces from Figure 3F (red box); the accuracy of the ICA spike-sorting algorithm was previously verified using simultaneous sharp electrode recordings, which showed that all spikes in the sorted traces correspond to intracellularly recorded spikes from individual Neurons11 , 14 .
Figure 3: Results from Tritonia diomedea, illustrating raw, filtered, and spike-sorted data.
(A) Tritonia escaping from the predatory sea star Pycnopodia helianthoides through its swim, which consists of alternating dorsal and ventral flexions of the body. (B) Schematic of the imaging setup. Ce=cerebral lobe of the cerebropleural ganglion; Pl = pleural lobe of the cerebropleural ganglion; Pd = pedal ganglion. (C) Raw data from 20 photodiodes, displaying activity in the left pedal ganglion to stimulation of the contralateral PdN3 (stimulus indicated by the arrow). (D) Filtered data from the same diodes as in C (5 and 100 Hz bandpass Butterworth filter). (E) Imaging software output in which compressed traces collected by all 464 diodes are superimposed topographically over an image of the preparation. The positions of the 20 diodes whose traces are shown in C and D are highlighted in red. (F) Thirty selected single-neuron traces generated by spike-sorting via ICA. (G) An expanded view of four single-neuron traces, corresponding to the red box in F, displays their action potentials at higher temporal resolution.
Aplysia
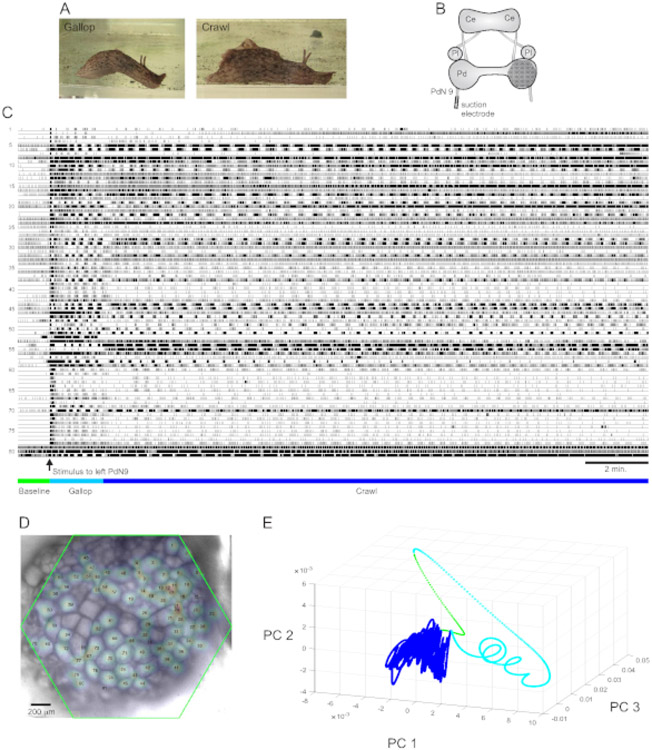
A strongly aversive tail stimulus to Aplysia californica elicits a stereotyped two-part rhythmic escape response15 . The first phase of the response is a gallop of several cycles of head lunges and tail pulls that move the animal quickly forward. This is typically followed by a period of crawling, involving repeated waves of head-to-tail muscular contractions that drive the animal forward at a slower speed for several minutes (Figure 4A). To capture these escape motor programs in optical recordings, the PDA was focused on the dorsal surface of the right pedal ganglion in an isolated brain preparation, and a suction electrode was placed on the contralateral (left) pedal nerve 9 (PdN9; Figure 4B). One minute into a continuous 20 min optical recording (Figure 4C), PdN9 was stimulated to elicit the gallop-crawl motor program sequence. The probabilistic Gaussian spatial distributions of the signals from all 81 recorded neurons were mapped onto the ganglion (Figure 4D). Dimensionality reduction applied to the full recording revealed that the gallop (cyan) and crawl (dark blue) phases of the escape program occupied distinct areas and formed different trajectories, spiral- and loop-like, respectively, in principal component space (Figure 4E).
Figure 4: Results from Aplysia californica, illustrating long-duration recording, signal mapping, and dimensionality reduction.
(A) The two phases of Aplysia’s sequential escape motor program, the gallop and the crawl. (B) Schematic of the imaging setup. Ce = cerebral ganglion; Pl = pleural ganglion; Pd = pedal ganglion. (C) A 20 min recording of 81 neurons in the right pedal ganglion responding to a stimulus to the contralateral PdN9 (indicated by the arrow). Green, cyan, and dark blue bars below the traces indicate the pre-stimulus period, the gallop, and the crawl phases of the escape motor program, respectively. (D) An image of the preparation with mapped probabilistic Gaussian distributions of the locations of all 81 neuronal signal sources identified by ICA. The green outline represents the position of the hexagonal face of the PDA with respect to the ganglion. The numbers on each Gaussian correspond to the trace numbers in C. (E) Dimensionality reduction using principal component analysis plotting the first three principal components against each other over the course of the 20 min file. The pre-stimulatory baseline, gallop, and crawl epochs are shown in green, cyan, and dark blue, respectively. See Videos 1-3 for animations of neuronal firing corresponding to this recording.
Three videos based on the Aplysia recording depicted in Figure 4 demonstrate further types of analyses that can be performed on such datasets. Video 1 animates the firing of all recorded neurons over the full duration of the recording. The initial post-stimulatory period of the escape motor program was characterized by a gallop, in which activity in the ganglion was marked by alternating bursting of different functional clusters (Video 2). The gallop subsequently transitioned to a crawl, in which the activity across neuronal clusters remained broadly phasic but assumed a counterclockwise rotational trajectory in the ganglion (Video 3). The latter two videos also incorporate consensus clustering, which reveals the firing and locations of the different functional ensembles for the gallop and crawl phases of the escape response separately. Note that many neurons assigned to the same cluster in both the gallop and crawl phases exhibited physical proximity to one another in the ganglion, consistent with prior findings12 .
Berghia
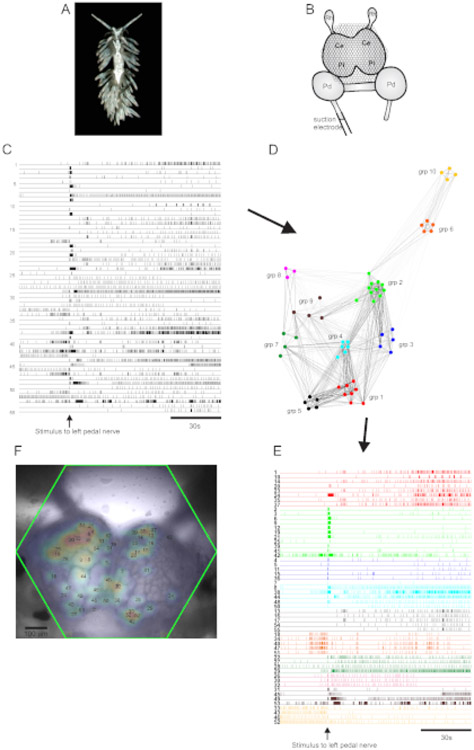
The aeolid nudibranch Berghia stephanieae (Figure 5A) represents a new model system for neuroscience. The imaging setup for a typical Berghia experiment is shown in Figure 5B. To elicit widescale neuronal activity, a suction electrode was placed on the most prominent left pedal nerve, and a nerve stimulus was delivered 30 s into a 2 min recording. ICA-processed traces revealed both spontaneous and stimulus-evoked activity in 55 neurons (Figure 5C). Community detection via consensus clustering identified ten distinct functional ensembles, which are depicted in Figure 5D in a network graph and in Figure 5E, which reorganizes the traces shown in Figure 5C based on their clustering assignments. Gaussian distributions of the signals from all recorded neurons are superimposed on an image of the preparation in Figure 5F to indicate the positions of all 55 recorded neurons.
Figure 5: Results from Berghia stephanieae, a new species for neuroscience, illustrating network graphing, functional clustering, and bilateral signal mapping.
(A) A Berghia specimen. (B) Schematic of the imaging setup. Ce = cerebral lobe of the cerebropleural ganglion; Pl = pleural lobe of the cerebropleural ganglion; Pd = pedal ganglion; Rh = rhinophore ganglion. (C) Traces displaying the spontaneous and stimulus-evoked activity of 55 bilateral neurons in the cerebropleural ganglia (delivery of the stimulus is indicated by the arrow). (D) A network graph displaying the ten functional ensembles, each assigned a unique color, identified through consensus clustering. Nodes in this plot represent neurons, where distance in network space represents the degree of firing correlation within and between ensembles. (E) The traces in C are rearranged and color-coded (following the color scheme of D) into functional ensembles. (F) An image of the preparation showing the mapped locations of the signals from every recorded neuron, and the trace numbers in C and E to which they correspond.
Discussion
One of the most important details in implementing our large-scale VSD imaging approach is to minimize vibration, which produces movements of contrast edges across the diodes, resulting in large artifactual signals. Because absorbance VSDs produce very small-percentage changes in light intensity with action potentials, vibration artifacts, if not prevented, can obscure the neuronal signals of interest. We employ several methods to minimize vibration artifacts. First, our imaging room is situated on the ground floor, which isolates the preparation from vibrations related to building air-handling equipment and many other sources. Second, a spring-based isolation table was used, which other PDA users have confirmed provides better vibration dampening than the more common air table16 . Third, water immersion objectives were used, which eliminate image fluctuations arising from surface ripples. Fourth, the preparation being imaged was lightly pressed between the chamber coverslip bottom and a coverslip fragment pressed down from above that is held in place by silicone plugs or petroleum jelly, further stabilizing the preparation. This also flattens the convex surface of the ganglion or ganglia being imaged, resulting in more neurons in the plane of focus of the objective, which increases the number of neurons recorded.
To maximize the signal-to-noise ratio for the very small changes in the degree of VSD light absorbance resulting from an action potential, it is essential to achieve near-saturating light through the preparation to the PDA, while at the same time minimizing photobleaching of the dye. To this end, we typically work at 3-4 V of resting light intensity, as measured with the PDA control panel gain switch in the 1x position (the PDA’s 464 amplifiers saturate at 10 V of light). During data acquisition this gain factor is changed to 100x. Getting sufficient light to reach 3-4 V as measured by the PDA can be accomplished in several ways. First, use an ultrabright LED light source that delivers a wavelength appropriate to the absorption properties of the absorbance dye in use. Accordingly, a 735-nm LED collimated lamp was used, which overlaps with the optimal absorption wavelengths of RH155 and RH482. Second, if necessary, use a flip-top substage condenser that concentrates the light from the LED light source to a smaller area. Third, adjust the condenser height to achieve Kohler illumination, which ensures high, even brightness and maximal image quality. Fourth, ensure there are no heat filters in the optical pathway, which can attenuate the LED lamp’s 735-nm wavelength. Fifth, remove diffusers, if more light is needed, from the optical pathway. Sixth, use high-NA objectives, which provide high spatial resolution, and allow sufficient levels of light to reach the PDA at lower lamp intensities. This has allowed us to minimize photobleaching to the extent that we can obtain several acquisition files of 10-20 min duration per preparation using the same light intensity across all files and without a significant loss of signal amplitude or the need for re-staining. Crucially, if the experimenter wishes to track neurons across these longer files, ensure that the focal plane does not change, and that the preparation does not move. Finally, an additional way to route sufficient light to the PDA is to use younger animals, which have thinner, and thus less opaque ganglia.
From time to time we find that the signal-to-noise ratio of the optical signals deteriorates and/or the motor program rhythms are suboptimal (e.g., slow or abnormal). When this begins to occur consistently, we mix fresh solutions of VSD. Aliquots of VSD typically remain viable for about 6 months in a −20 °C freezer. Relatedly, it is worth noting that for Berghia, the best results have so far been obtained with the absorbance VSD RH482. As RH482 is more lipophilic than RH155, it may better stain Berghia’s comparatively smaller neurons or remain in the neuronal membranes more effectively at the higher recording saline temperature used for this tropical species.
One limitation of PDA-based imaging of neural activity relates to the AC coupling of the voltage signals in hardware before the 100x preamplification step: although this represents a necessary feature to remove the large DC offset produced by the high resting light level required by this technique, the AC coupling intrinsic to the PDA precludes the measurement of slow changes in membrane potential, such as those associated with synaptic inputs. If recording slow or steady-state potential changes is desired, a DC-coupled CMOS camera imaging system can be utilized to capture subthreshold activity. Byrne and colleagues recently used such a setup with RH155 to image the activity of neurons in the buccal ganglion of Aplysia17 , 18 . We have used both systems and found that the CMOS camera, due to its much higher density of detectors (128 x 128), generates 50x larger data files for the same imaging time7 . The PDA’s smaller files facilitate faster processing and analysis. This also enables extended single-trial recordings (Figure 4) and studies of learning, in which data from multiple trials are concatenated into one large file before spike-sorting, allowing network organization to be tracked as learning develops19 .
In other camera-based investigations, fluorescent VSDs have been used by Kristan and colleagues to examine network function in the segmental ganglia of the leech. In one influential study this led to the identification of a neuron involved in the animal’s decision to swim or to crawl20 . In another study, Kristan et al. examined the extent to which the leech’s swimming and crawling behaviors are driven by multifunctional vs. dedicated circuits21 . More recently, Wagenaar and colleagues used a two-sided microscope for voltage imaging that allows them to record from almost all neurons in a leech segmental ganglion22 . In contrast to many camera-based imaging methods, an advantage of our PDA-based imaging method is rapid and unbiased spike-sorting by ICA, a form of blind source separation that involves no decisions about neuronal boundaries for results processing.
With respect to the choice of VSDs, one advantage of the absorbance dyes RH155 and RH482 is the little-to-no phototoxicity associated with them23 , 24 , enabling longer recording times than is typical for fluorescent VSDs. Moreover, the fast absorbance VSDs we use are well suited for recording the overshooting somatic action potentials in gastropod preparations, which are typically 80 mV in amplitude. As shown in Figure 3G, our optical method can record action potential undershoots (none of our recordings are trace-averaged): this suggests that the VSDs we use should be able to discern action potentials in other model systems that attenuate to some degree and thus are not overshooting by the time they reach the soma. Nevertheless, our optical approach may not be ideal for species that are known to exhibit highly attenuated action potentials when recorded in the soma.
Much current research on neural networks is being focused on a small number of designer transgenic species. However, neuroscience benefits from the study of a wide variety of phylogenetically distinct species. Studying many different species provides insights into how circuits evolve25 , 26 , and illuminates principles of network function that may be common across phyla1 , 2 , 3 , 4 , 27 . We have so far applied our imaging method to a number of gastropod species, including Aplysia californica8 , 11 , 12 , 13 , 14 , 28 , Tritonia diomedea8 , 9 , 11 , 14 , 19 , 28 , Tritonia festiva28 , Pleurobranchaea californica (unpublished data), and most recently Berghia stephanieae (Figure 5). An appeal of this approach is that it can be readily applied to many species, with no need for transgenic animals. We wish to acknowledge that our use of VSD imaging with fast absorbance dyes and a PDA follows in the footsteps of pioneering work that accomplished this in semi-intact, behaving Navanax29 and Aplysia30 preparations. Our emphasis on the rapidity of our approach is in part an answer to concerns that many investigators may be increasingly reluctant to initiate network studies in new species due to fears that years of study will be necessary to characterize basic network organization before being able to explore scientific questions of broad interest to neuroscience31 . Accordingly, our goal here is to demonstrate a technique that greatly speeds the process – to the point that significant same-day insights into network organization may be obtained from single preparations.
Video 1: Animation of the full, 20-minute Aplysia escape locomotor program.
The opacity of the white shapes overlying 81 individual neurons in the right pedal ganglion (left panel) was driven by the corresponding neuronal traces (right panel) and varied linearly as a function of average spike rate (binned per every 0.61 s of real time in the recording). For each neuron, full opacity was normalized to its maximum firing rate over the duration of the recording. One second of elapsed time in the video represents 12.2 s of real time. The scale bar corresponds to real time, with the green, cyan, and dark blue lines below the traces indicating the pre-stimulus baseline, gallop, and crawl phases of the escape locomotor program, respectively. The yellow boxes around the gallop phase and a portion of the crawl phase indicate the recording excerpts used to generate the animations in Videos 2 and 3.
Video 2: Animation of the gallop phase of the Aplysia escape locomotor program.
Consensus clustering was performed on all 81 recorded neurons in just the gallop phase of the motor program to derive the functional ensembles, using the approach and software described and made available in ref.12 . Neuronal ensembles exhibiting largely tonic or irregular firing patterns during this phase of the escape program were omitted from this video. The action potentials of the neurons belonging to the black and olive-green ensembles can be heard in the audio track of the video, with the corresponding neurons and traces highlighted. Average spike rates were normalized as in Video 1 and with equivalent time binning; 1 s of elapsed time in the video corresponds to 6.1 s of real time.
Video 3: Animation of the crawl phase of the Aplysia escape locomotor program.
Consensus clustering was performed on all 81 recorded neurons in just the crawl phase of the motor program to derive the functional ensembles. Ensembles exhibiting largely tonic or irregular firing patterns during this phase of the motor program were omitted from this video. Average spike rates were normalized as in Videos 1 and 2 and with equivalent time binning; 1 s of elapsed time in the video corresponds to approximately 12.2 s of real time.
Acknowledgments
This work was supported by NSF 1257923 and NIH 1U01NS10837. The authors wish to acknowledge Jean Wang’s assistance in the laboratory.
Footnotes
A complete version of this article that includes the video component is available at http://dx.doi.org/10.3791/61623.
Disclosures
The authors have nothing to disclose.
References
- 1.Miller CT Hale ME Okano H Okabe S Mitra P Comparative Principles for Next-Generation Neuroscience. Frontiers in Behavioral Neuroscience. 13 (12) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenowitz EA, Zakon HH Emerging from the bottleneck: benefits of the comparative approach to modern neuroscience. Trends in Neuroscience. 38 (5), 273–278 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolker J Model organisms: There's more to life than rats and flies. Nature. 491 (7422), 31–33 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Carlson BA Diversity matters: the importance of comparative studies and the potential for synergy between neuroscience and evolutionary biology. JAMA Neurology. 69 (8), 987–993 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Chase R Behavior and its neural control in gastropod molluscs. Oxford University Press. Oxford, UK: (2002). [Google Scholar]
- 6.Salzberg BM, Grinvald A, Cohen LB, Davila HV, Ross WN Optical recording of neuronal activity in an invertebrate central nervous system: simultaneous monitoring of several neurons. Journal of Neurophysiology. 40 (6), 1281–1291 (1977). [DOI] [PubMed] [Google Scholar]
- 7.Frost WN et al. Monitoring Spiking Activity of Many Individual Neurons in Invertebrate Ganglia. Advances in Experimental Medicine and Biology. 859, 127–145 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frost WN, Wang J, Brandon CJ A stereo-compound hybrid microscope for combined intracellular and optical recording of invertebrate neural network activity. Journal of Neuroscience Methods. 162 (1-2), 148–154 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frost WN, Wu J-Y Voltage-sensitive dye imaging. In Basic Electrophysiological Methods. Edited by Covey E, Carter M, 169–195, Oxford University Press. Oxford, UK: (2015). [Google Scholar]
- 10.Brown GD, Yamada S, Sejnowski TJ Independent component analysis at the neural cocktail party. Trends in Neuroscience. 24 (1), 54–63 (2001). [DOI] [PubMed] [Google Scholar]
- 11.Hill ES, Moore-Kochlacs C, Vasireddi SK, Sejnowski TJ, Frost WN Validation of independent component analysis for rapid spike sorting of optical recording data. Journal of Neurophysiology. 104 (6), 3721–3731 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruno AM, Frost WN, Humphries MD Modular deconstruction reveals the dynamical and physical building blocks of a locomotion motor program. Neuron. 86 (1), 304–318 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruno AM, Frost WN, Humphries MD A spiral attractor network drives rhythmic locomotion. ELife. 6, e27342 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill ES, Bruno AM Vasireddi SK, Frost WN ICA applied to VSD imaging of invertebrate neuronal networks. In Independent Component Analysis for Audio and Biosignal Applications. Edited by Naik GR, 235–246. InTech. London: (2012). [Google Scholar]
- 15.Jahan-Parwar B, Fredman SM Neural control of locomotion in Aplysia: role of the central ganglia. Behavioral and Neural Biology. 27 (1), 39–58 (1979). [DOI] [PubMed] [Google Scholar]
- 16.Jin W, Zhang RJ, Wu JY Voltage-sensitive dye imaging of population neuronal activity in cortical tissue. Journal of Neuroscience Methods. 115 (1), 13–27 (2002). [DOI] [PubMed] [Google Scholar]
- 17.Neveu CL et al. Unique Configurations of Compression and Truncation of Neuronal Activity Underlie l-DOPA-Induced Selection of Motor Patterns in Aplysia. eNeuro. 4 (5), ENEURO.0206-17.2017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai Z, Neveu CL, Baxter DA, Byrne JH, Aazhang B Inferring neuronal network functional connectivity with directed information. Journal of Neurophysiology. 118 (2), 1055–1069 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill ES, Vasireddi SK, Wang J, Bruno AM, Frost WN Memory Formation in Tritonia via Recruitment of Variably Committed Neurons. Current Biology. 25 (22), 2879–2888 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Briggman KL, Abarbanel HD, Kristan WB Jr. Optical imaging of neuronal populations during decision-making. Science. 307 (5711), 896–901 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Briggman KL, Kristan WB Jr. Imaging dedicated and multifunctional neural circuits generating distinct behaviors. Journal of Neuroscience. 26 (42), 10925–10933 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomina Y, Wagenaar DA A double-sided microscope to realize whole-ganglion imaging of membrane potential in the medicinal leech. ELife. 6, e29839 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang PY, Jackson MB Interpretation and optimization of absorbance and fluorescence signals from voltage-sensitive dyes. Journal of Membrane Biology. 196 (2), 105–116 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Parsons TD, Salzberg BM, Obaid AL, Raccuia-Behling F, Kleinfeld D Long-term optical recording of patterns of electrical activity in ensembles of cultured Aplysia neurons. Journal of Neurophysiology. 66, 316–333 (1991). [DOI] [PubMed] [Google Scholar]
- 25.Katz PS Evolution of central pattern generators and rhythmic behaviours. Philosophical Transactions of the Royal Society of London, Series B. 371 (1685), 20150057 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moroz LL Biodiversity Meets Neuroscience: From the Sequencing Ship (Ship-Seq) to Deciphering Parallel Evolution of Neural Systems in Omic's Era. Integrative and Comparative Biology. 55 (6), 1005–1017 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frost WN, Tian L-M, Hoppe TA, Mongeluzi DL, Wang J A cellular mechanism for prepulse inhibition. Neuron. 40, 991–1001 (2003). [DOI] [PubMed] [Google Scholar]
- 28.Hill ES, Vasireddi SK, Bruno AM, Wang J, Frost WN Variable neuronal participation in stereotypic motor programs. PLoS One. 7 (7), e40579 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.London JA, Zecevic D, Cohen LB Simultaneous optical recording of activity from many neurons during feeding in Navanax. Journal of Neuroscience. 7 (3), 649–661 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu J, Cohen LB, Falk CX Neuronal activity during different behaviors in Aplysia: A distributed organization. Science. 263 (5148), 820–823 (1994). [DOI] [PubMed] [Google Scholar]
- 31.Marder E Searching for insight. In Invertebrate Neurobiology. Edited by North G, Greenspan RJ, 1–18, Cold Spring Harbor Laboratory Press. Cold Spring Harbor, NY: (2007). [Google Scholar]