Abstract
In vitro ammonia-oxidizing bacteria are capable of oxidizing hydrocarbons incompletely. This transformation is accompanied by competitive inhibition of ammonia monooxygenase, the first key enzyme in nitrification. The effect of hydrocarbon pollution on soil nitrification was examined in situ. In a microcosm study, adding diesel fuel hydrocarbon to an uncontaminated soil (agricultural unfertilized soil) treated with ammonium sulfate dramatically reduced the amount of KCl-extractable nitrate but stimulated ammonium consumption. In a soil with long history of pollution that was treated with ammonium sulfate, 90% of the ammonium was transformed into nitrate after 3 weeks of incubation. Nitrate production was twofold higher in the contaminated soil than in the agricultural soil to which hydrocarbon was not added. To assess if ammonia-oxidizing bacteria acquired resistance to inhibition by hydrocarbon, the contaminated soil was reexposed to diesel fuel. Ammonium consumption was not affected, but nitrate production was 30% lower than nitrate production in the absence of hydrocarbon. The apparent reduction in nitrification resulted from immobilization of ammonium by hydrocarbon-stimulated microbial activity. These results indicated that the hydrocarbon inhibited nitrification in the noncontaminated soil (agricultural soil) and that ammonia-oxidizing bacteria in the polluted soil acquired resistance to inhibition by the hydrocarbon, possibly by increasing the affinity of nitrifying bacteria for ammonium in the soil.
Bioremediation is the most recent technology used for cleaning areas contaminated with hydrocarbon derivatives. The approach that has been exploited most consists of stimulation of the soil endogenous microflora by adding an electron acceptor and/or nutriments, in particular nitrogen in the form of ammonium salts (2, 43, 44). This nitrogen source is exploited mainly by microbial biomass for growth and production of degradative enzymes. Some of the ammonium may be transformed into nitrite and nitrate by the nitrification pathway (26). A number of in vitro studies have shown that pure cultures of Nitrosomonas europaea oxidize a wide variety of hydrocarbon substrates through the action of ammonia monooxygenase, the first key enzyme in the autotrophic nitrification process. In contrat, in situ studies of the effects of hydrocarbon soil pollution on nitrification apparently have not been performed previously. The common hydrocarbon substrates include alkanes, alkenes, and aromatic and chlorinated aliphatic compounds (13, 15, 27, 40). Transformation of hydrocarbons via the ammonia monooxygenase pathway may be considered competitive cooxidation which reduces the rate and extent of ammonia oxidation. The oxidation products obtained from alternative hydrocarbon substrates are not assimilated by N. europaea and accumulate in the culture medium. It is thought that in this case the nitrifying bacteria present in a polluted environment initiate a syntrophic pathway that provides intermediates for heterotrophic bacteria. Thus, nitrifying bacteria appear to be excellent candidates for hydrocarbon remediation because it may be possible to enhance the biodegradative capacity of these ubiquitous soil bacteria by adding ammonia and oxygen in order to support hydrocarbon cometabolism.
However, if bacteria are to be used effectively in bioremediation schemes, it is important to obtain information concerning the nitrification process that occurs in the presence of hydrocarbon in a natural soil medium; studies performed with pure cultures ignore interactions of bacteria and environmental components and bacterial diversity (36, 37).
The objective of the present study was to determine the effect of adding a hydrocarbon fuel on nitrification and nitrifying bacteria in an uncontaminated agricultural soil and in a soil with a long history of pollution. We present evidence that nitrifying bacteria in polluted soil were characterized by a lower affinity for hydrocarbons and that the apparent inhibition of nitrification observed in the presence of hydrocarbons resulted not from a competitive effect but from immobilization of nitrogen in the microbial biomass.
MATERIALS AND METHODS
Soil samples.
Two loamy soils were used in this study. A soil that was contaminated with diesel fuel was obtained from an abandoned area of a petroleum refinery. This soil was characterized by absence of vegetation and a management program. The operation at the refinery ceased 12 years ago. The other soil was obtained from a unfertilized agricultural plot that had been planted with ryegrass for at least 3 years. All of the inorganic nitrogen in this soil was derived from mineralization of organic nitrogen. Soil samples were collected aseptically from the upper 20 cm and were stored at 4°C. Before use, they were sieved (mesh size, 2 mm) and kept at room temperature for 24 h. Characteristics of the soils are summarized in Table 1.
TABLE 1.
Properties of the soils used in this investigation
| Soil | Soil texture | Particle size distribution (%)
|
Moisture content (%) | pH (H2O) | % C | % N | N-NO3− concn (μg/g)a | N-NH4+ concn (μg/g)a | TPH concn (μg/g)b | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sand | Silt | Clay | |||||||||
| Agricultural | Loam | 8 | 80 | 12 | 11 | 7.4 | 1.2 | 0.14 | 6.5 | <1.0 | NDc |
| Polluted | Loam | 15 | 63 | 22 | 9 | 8.2 | 1.28 | 0.05 | <0.5 | <1.0 | 2,500–4,000 |
Determined on a dry weight basis.
The total petroleum hydrocarbon (TPH) content was determined on a dry weight basis by using infrared spectroscopy.
ND, not detected.
Incubation experiment.
Laboratory microcosms were used for the incubation experiment. Each sample was separated into 500-g (dry weight) portions, and each portion was transferred to a sterile 2,000-ml Mason jar capped with a lid fitted with a rubber septum. Triplicate jars were prepared for each treatment. The microcosms were incubated for 4 weeks at 28°C in the dark and were aerated weekly to maintain aerobic conditions. The mineral N content was determined each week. Total mineralized carbon contents were determined by using sterile 500-ml Mason jars. Triplicate jars (50 g [dry weight] of soil/jar) were used for each soil treatment. Total mineralized carbon contents were measured weekly by titrating the CO2 trapped during incubation in NaOH (25). All water-soluble substrates were dissolved in a volume of water sufficient to adjust the water content of the soil to 20% on the basis of wet weight. Substrates were neutralized with NaOH, if necessary, before they were added. All substrates were added to samples by spraying them with a 2-ml syringe. Ammonium was added in the form of (NH4)2SO4, and 150 μg of P/g (dry weight) of soil was added as KH2PO4 to prevent any limitation of activity by nutriment imbalance. Diesel fuel (density at 15°C, 0.83 kg/liter) was purchased from Petro-FINA Belgium. Nitrapyrin (90% pure) was obtained from Sigma Chemical, was dissolved in 100 mM dimethyl sulfoxide at a concentration of 0.5% (wt/wt), and was added at a concentration of 10 μg/g of soil. To obtain maximal inhibition of nitrification, the nitrapyrin solution was mixed with the ammonium salt solution before it was added to the soil.
Analytical procedures.
Exchangeable ammonium, nitrite, and nitrate contents were determined after soil samples were extracted with 1 M KCl (1:5, wt/vol) for 2 h by using a Tecator Aquatec model 5400 autoanalyzer with a detection level of 0.1 ppm of N for all three compounds. Mineral nitrogen was quantified colorimetrically by the indophenol (ammonium) and cadmium reduction (nitrate) methods (16).
Petroleum hydrocarbon in the polluted soil was extracted with carbon tetrachloride and was analyzed quantitatively by infrared spectrophotometry and qualitatively with a gas chromatograph (GC) equipped with a flame ionization detector (FID) (29).
Enumeration of nitrifying bacteria.
Ammonia- and nitrite-oxidizing bacteria were enumerated by a most-probable-number (MPN) procedure (30). Suspensions of 5.0 g of moist soil and 45 ml of sterile phosphate buffer containing 139 mg of K2HPO4 per liter and 27 mg of KH2PO4 per liter (pH 7.0) were shaken at 100 rpm for 2 h. Subsamples of the suspensions were diluted in sterile microtiter plates containing the appropriate medium for the ammonium- and nitrite-oxidizing bacteria (42). Twelve replicates were made per dilution. Samples were incubated for a maximum of 3 months at 28°C in the dark. The number of nitrifying bacteria was determined with Cochran’s tables (10) after detection of NO2− with the Griess reagent (33).
DNA extraction.
DNA was extracted from 0.5-g portions of soil in 2-ml microcentrifuge tubes containing 0.5 ml of 100 mM phosphate buffer (pH 8), 0.5 ml of a 10% sodium dodecyl sulfate solution (100 mM NaCl, 500 mM Tris [pH 8], 10% sodium dodecyl sulfate), and 2.5 g of 0.1-mm-diameter zirconia beads. The tubes were shaken at 2,000 rpm for 20 min in a bead mill homogenizer. The supernatant was recovered by centrifugation for 5 min at 12,000 × g and was extracted with a phenol-chloroform mixture (3). After ethanol precipitation, the DNA was resuspended in 100 μl of TE (10 mM Tris, 0.1 mM EDTA).
PCR amplification.
Crude DNA was purified by gel filtration on Sephadex G-200 (39). PCR amplification of 16S ribosomal DNA fragments was carried out by using the CTO primers specific for ammonia oxidizers belonging to the β subgroup of the class Proteobacteria (19) and primer FGPS specific for the genus Nitrobacter (12). Each reaction mixture (total volume, 50 μl) was prepared as recommended by the manufacturer by using 2.5 U of Expand High Fidelity polymerase (Boehringer Mannheim). To minimize amplification inhibition in the PCR, 400 ng of bovine serum albumin per μl was added to the PCR mixture (20). The thermal cycle included an initial denaturation step consisting of 94°C for 120 s, followed by 35 cycles consisting of denaturation at 94°C for 30 s, annealing at 57°C with the CTO primers and at 50°C with FGPS for 30 s, and elongation at 68°C for 60 s. The cycle was completed by a final elongation step consisting of 72°C for 5 min.
RESULTS AND DISCUSSION
Hydrocarbon in the polluted soil.
The concentrations of total petroleum hydrocarbons, as estimated by infrared spectroscopy analysis, ranged from 2,500 to 4,000 μg/g of soil (Table 1). The large differences in the total petroleum hydrocarbon concentrations in samples resulted from the heterogeneous distribution of petroleum hydrocarbons in the soil. A GC-FID analysis revealed that polyaromatic hydrocarbons and aliphatic hydrocarbons (C11 to C19) were the dominant pollutants (data not shown).
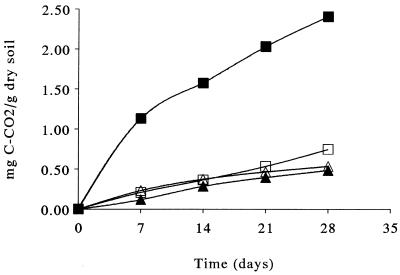
Several reports have documented that mineralization (conversion to CO2) of the hydrocarbons in polluted soils is enhanced by adding mineral N (2, 43, 44). With our sample of polluted soil this effect was observed in the presence of an extra dose of diesel fuel but not in the initial soil sample (Fig. 1). The lack of enhancement of mineralization of the indigenous hydrocarbons could have been due to restricted access of microorganisms to hydrocarbons that were adsorbed or trapped in microaggregates and/or were not easy to mineralize (18).
FIG. 1.
Cumulative CO2 evolution during incubation of polluted soil amended with 300 μg of NH4+ N/g (dry weight) of soil (▵), 4,000 μg of diesel fuel/g (dry weight) of soil (□), or 300 μg of NH4+ N/g (dry weight) of soil plus 4,000 μg of diesel fuel/g (dry weight) of soil (■) and unamended polluted soil (▴). Data are averages based on three replicates; the error bars are smaller than the symbols.
Nitrogen in polluted soil.
The levels of NH4+ N and NO3− N extracted with KCl were below the limit of detection (0.5 μg/g) in the polluted soil (Table 1). This situation did not evolve during the 4-week incubation period (data not shown). The total-N content of this soil was also very low (0.05%) (Table 1). The low N content was probably due to the absence of any N management of the abandoned area which was the source of the soil.
In the agricultural soil, the concentration of nitrate increased from 6.5 to 15 μg of NO3− N/g (dry weight) of soil after 4 weeks of incubation. However, the concentration of ammonium did not change. This showed that nitrification in this soil depended on mineralization of organic nitrogen. Generally, the populations and in situ activities of nitrifiers may be limited by the rate of production of ammonium during mineralization of organic nitrogen. This can easily be demonstrated in many soils by adding ammonium and observing stimulation of growth of the nitrifier population (5). In contrast, the contaminated soil was characterized by low total-N contents and no accumulation of any form of inorganic nitrogen. This probably resulted from the absence of organic nitrogen available for mineralization.
Nitrifiers in polluted soil.
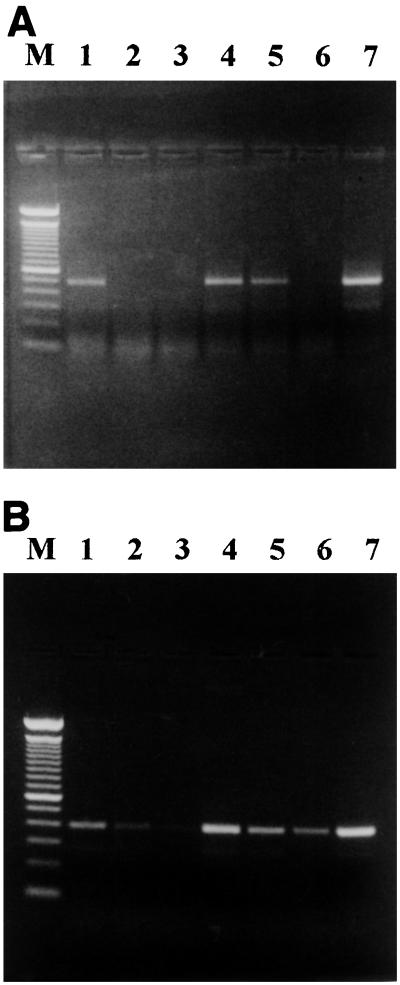
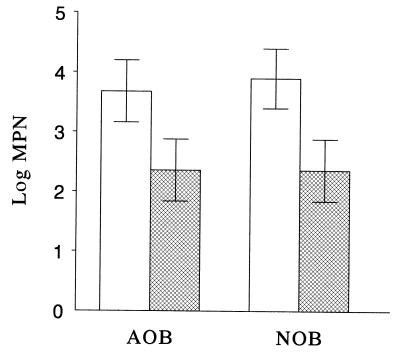
Ammonia-oxidizing bacteria belonging to the β subgroup of the Proteobacteria and nitrite-oxidizing bacteria belonging to the genus Nitrobacter are the principal autotrophic nitrifiers that have been found in soils (7, 36). Use of PCR amplification primers CTO and FGPS, which are specific for the 16S rRNA genes of ammonia-oxidizing bacteria (19) and nitrite-oxidizing bacteria (12), respectively, with DNA extracted from the polluted and agricultural soils produced the expected amplification products (465-bp product for ammonia-oxidizing bacteria and 397-bp product for nitrite-oxidizing bacteria) (Fig. 2, lanes 1 and 3). A PCR analysis of serial dilutions of DNA extracted from both soils revealed that the lower detection limit for ammonia-oxidizing bacteria and nitrite-oxidizing bacteria was approximately 10 times higher in the polluted soil than in the agricultural soil (Fig. 2). This result supports the finding that there was a clear quantitative distinction between the MPN values for nitrifying bacteria in polluted and agricultural soils (Fig. 3). The lower value obtained by the MPN procedure showed that the PCR product obtained with DNA extracted from polluted soil was derived from living nitrifiers and not from dead cells or DNA adsorbed to soil particles (1, 21). Moreover, it demonstrated that this group of bacteria persisted in hydrocarbon-polluted soil for several years in the absence of an energy supply (ammonium and nitrite) and in the presence of the polluting hydrocarbons.
FIG. 2.
Agarose gel analysis of 16S ribosomal DNA from agricultural and polluted soils amplified with primers CTO (A) and FGPS (B). Lanes 1 through 3, DNA from agricultural soil; lanes 4 through 6, DNA from polluted soil; lane 7, positive control (N. europaea [A] or Nitrobacter winogradskyi [B]). Lanes 1 and 4 contained undiluted soil DNA; lanes 2 and 5 contained soil DNA diluted 1:10; lanes 3 and 6 contained soil DNA diluted 1:100; and lane M contained a 1-kb DNA ladder size standard (Gibco BRL).
FIG. 3.
MPN of ammonia-oxidizing bacteria (AOB) and nitrite-oxidizing bacteria (NOB) in agricultural soil (open bars) and polluted soil (cross-hatched bars). The error bars indicate the 95% confidence limits.
Effect of ammonium addition.
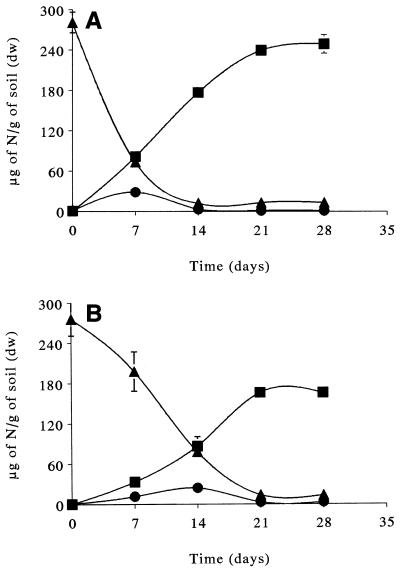
Addition of 300 μg of NH4+ N/g (dry weight) of soil to the polluted soil was followed by a large and rapid increase in the mineral nitrate content of the soil, which was about 240 μg of NO3− N/g (dry weight) of soil after 3 weeks of incubation (Fig. 4A). The concomitant decrease in the ammonium concentration attained 95% of the initial value. In contrast, the rate of nitrification of 300 μg of NH4+ N/g (dry weight) of soil added to an agricultural soil was apparently slower (Fig. 5). Previous laboratory studies have shown that the rate of nitrification in agricultural soils after ammonium sulfate is added depends on the soil properties and that the inhibitory effect of ammonium on nitrification occurs at concentrations of >300 μg of N/g (23, 24, 38). The dominant factors that control the rates of nitrification in many soils are (i) the supply of NH4+ substrate, (ii) the acidity, (iii) the water content, and (iv) the temperature (8, 28). These factors were equivalent or optimal for nitrification in the two soils used in this investigation. This suggests that the observed difference in the rates of nitrification for the two soils resulted from a qualitative difference in the populations of nitrifying bacteria.
FIG. 4.
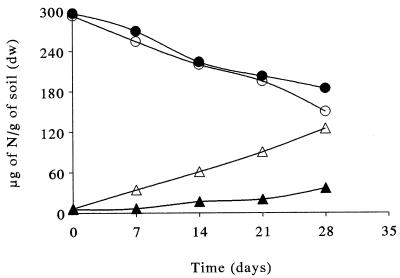
Changes in the concentrations of ammonium (▴), nitrate (■), and nitrite (●) during incubation of polluted soil amended with 300 μg of NH4+ N/g (dry weight) of soil (A) or 4,000 μg of diesel fuel/g (dry weight) of soil plus 300 μg of NH4+ N/g (dry weight) of soil (B). dw, dry weight.
FIG. 5.
Changes in nitrate (▵ and ▴) and ammonium (○ and ●) concentrations in agricultural soil amended with 300 μg of NH4+ N/g (dry weight) of soil in presence (▵ and ○) and in the absence (▴ and ●) of 4,000 μg of diesel fuel/g (dry weight) of soil. The error bars are smaller than the symbols. dw, dry weight.
In the agricultural soil, the disappearance of ammonium reflected the appearance of nitrate (Fig. 5). In the polluted soil, the ammonium concentration was reduced to 30% of its initial value during the first week, but the nitrate concentration represented only 30% of the observed value after 3 weeks of incubation (Fig. 4A). During the first week, most of the ammonium consumed was apparently in the form of nitrification intermediates other than nitrite, and the transitory amount of nitrite which accumulated was very small (Fig. 4A). The high rate of ammonium consumption in the first week suggested that the first key enzyme in nitrification, ammonia monooxygenase, is not a limiting factor. Thus, the ammonia-oxidizing bacteria in polluted soil seem to be dominated by a small number of bacteria with a high affinity for ammonium.
Effect of a nitrification inhibitor.
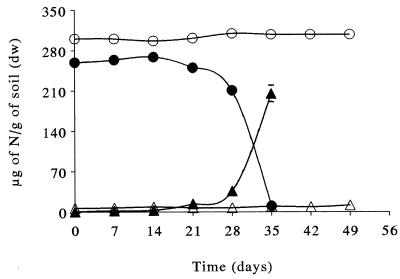
Previous laboratory studies showed that nitrapyrin was an effective nitrification inhibitor and that the degrees of inhibition were different in different soils (4, 9) and varied with the genus and strain of ammonia-oxidizing bacteria (6). In the agricultural soil, production of nitrate and disappearance of ammonium were not observed even after 50 days of incubation (Fig. 6) in the presence of the nitrification inhibitor nitrapyrin (10 μg/g [dry weight]) (9). In contrast, in the polluted soil, nitrification and ammonium consumption started after a lag period of about 3 weeks. The nitrapyrin was completely consumed after 3 weeks in the polluted soil, as shown by the GC-FID analysis (data not shown). Degradation of nitrapyrin and degradation of the intermediates (also inhibitors of nitrification [4]) in polluted soil could have been due to the action of the nitrifying bacteria, as observed in vitro (41). The rapid degradation of nitrapyrin in the polluted soil but not in the agricultural soil could have been due to the high specific activity of nitrifying bacteria in the polluted soil.
FIG. 6.
Effects of nitrapyrin (10 μg/g [dry weight] of soil) on the concentrations of nitrate (▵ and ▴) and ammonium (○ and ●) in agricultural soil (▵ and ○) and polluted soil (▴ and ●) amended with 300 μg of NH4+ N/g (dry weight) of soil. dw, dry weight.
The complete inhibition of nitrification by nitrapyrin in the two soils examined during the first few weeks of incubation suggested that the contribution of heterotrophic nitrification to nitrate production was insignificant. In fact, nitrapyrin is a specific inhibitor of chemoautotrophic ammonia oxidation but not heterotrophic ammonia oxidation (11, 34) and has been used as a differential inhibitor in environmental studies (32). Moreover, the soils which we used had neutral pH values, and contributions to nitrate production by organotrophic microorganisms have been observed only in acid soils that are rich in organic matter (17, 31, 32).
Effect of ammonium and diesel fuel.
In order to compare the effects of a hydrocarbon on nitrification in the contaminated and uncontaminated soils, both soils were amended with 4,000 μg of diesel fuel per g and 300 μg of NH4+ N/g (dry weight) of soil. This was done because the hydrocarbons present in the contaminated soil were apparently not accessible to microorganisms and had no effect on nitrification.
Addition of a hydrocarbon to the agricultural soil resulted in a lower increase in nitrate production compared to the same soil when diesel fuel was not added; the evolution of ammonium was apparently not affected by the diesel fuel addition (Fig. 5). The decrease in the ammonium concentration in the presence of the hydrocarbon resulted in large part from immobilization by incorporation into organic components (i.e., microbially synthesized amino acids and proteins) (14). Consequently, nitrification was directly affected by the hydrocarbon addition, so that decreases in denitrification were not expected during this incubation period.
When the previously polluted soil was supplemented with an extra dose of diesel fuel (Fig. 4B), the ammonium concentration was apparently not affected. Nevertheless, the plateau concentration of nitrate was 30% lower than the concentration observed in the absence of extra added hydrocarbon (Fig. 4A). The total amount of ammonium consumed after 4 weeks suggested that the low level of nitrate production in the polluted soil and in the presence of an extra dose of diesel fuel was mainly due to immobilization of nitrogen by heterotrophic bacteria rather than to inhibition of nitrifying bacteria by hydrocarbons. Under the conditions which we used, the availability of ammonium to nitrifying bacteria appeared to be a limiting factor for nitrification.
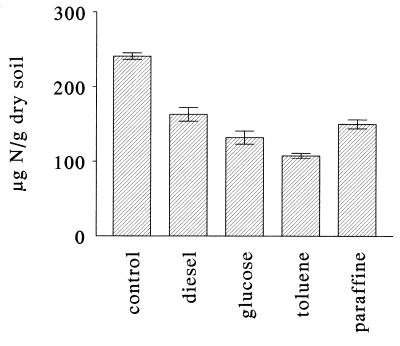
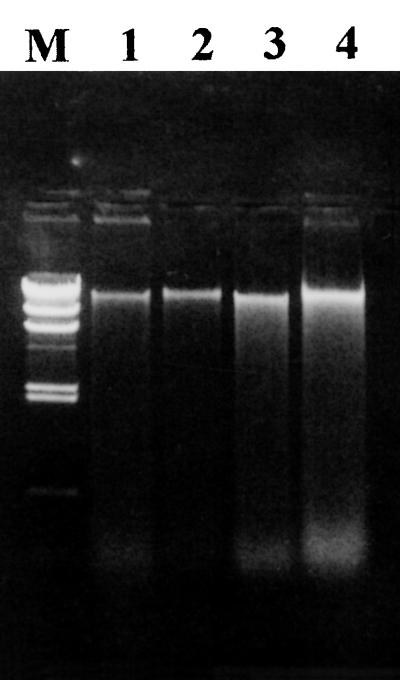
In order to check that the apparent inhibition of nitrification observed when polluted soil was exposed to diesel fuel was the result of immobilization of NH4+ N by hydrocarbon-stimulated microbial activity, we compared the effects of hydrocarbons and glucose on the amounts of ammonium and nitrate detected after incubation of soil treated with 300 μg of NH4+ N/g (Fig. 7). After incubation, the KCl-extracted ammonium values for all treatments were comparable to the value observed in the presence of ammonium alone. This result showed that hydrocarbons were as effective as glucose for inducing immobilization of NH4+ N. The conclusion that nitrogen was immobilized by heterotrophic bacteria stimulated by addition of a carbon source was confirmed by the amounts of DNA extracted from the polluted soil amended with both ammonium and diesel fuel, which were higher than the amounts extracted from the polluted soil amended with diesel fuel alone or with ammonium alone (Fig. 8). In fact, several studies have shown that changes in the DNA contents of environmental samples corresponded to changes in the population densities determined by using direct count epifluorescence microscopy (22, 35).
FIG. 7.
Effects of different hydrocarbons and glucose on the amounts of NO3− N after 4 weeks of incubation of polluted soil treated with 300 μg of NH4+ N/g (dry weight) of soil.
FIG. 8.
Agarose gel electrophoresis of DNA extracted from polluted soil after 4 weeks of incubation. Lane 1, untreated soil; lane 2, soil treated with 300 μg of NH4+ N/g (dry weight) of soil; lane 3, soil treated with 4,000 μg of diesel fuel/g (dry weight) of soil; lane 4, soil treated with 300 μg of NH4+ N/g (dry weight) of soil and 4,000 μg of diesel fuel/g (dry weight) of soil; lane M, DNA molecular weight marker II (Boehringer Mannheim).
The observation that a hydrocarbon did not have a direct effect on the activity of nitrifying bacteria could be explained by the high affinity of ammonia-oxidizing bacteria for ammonium in the polluted soil. This apparently did not occur in the agricultural soil, in which a significant amount of ammonium was always present (Fig. 5).
Conclusions.
Addition of hydrocarbon to an uncontaminated soil stimulated immobilization of nitrogen and reduced nitrification. In the absence of a bioremediation program (nutriment addition), the N immobilized in situ was derived from mineralization of the available organic nitrogen. In the case of long-term pollution and in the absence of a bioremediation program, the soil was excessively poor in nitrogen. Perhaps the same situation occurred in the contaminated soil used in this study, which had a long history of pollution, because initially this soil had a low total-N content and a small population of nitrifying bacteria. Apparently, nitrifying bacteria persisted in contaminated soil containing a limited amount of ammonium for several years. These conditions reduced the number of nitrifying bacteria but probably selected an ammonia-oxidizing community with a higher ammonium affinity and a higher activity than the community in the agricultural soil. The ammonia-oxidizing bacterial community in contaminated soil was not directly affected by a hydrocarbon addition, possibly because of its high affinity for ammonium.
A contaminated soil is a nitrogen-limited environment, and the adaptations which we observed may be very important for nitrifier survival in a ammonium-limited soil. Whether these adaptations are due to physiological plasticity or to the presence of strains specialized for living in an ammonium-limited and hydrocarbon-polluted soil will be examined by using the new molecular techniques for studying the diversity of these organisms (19).
ACKNOWLEDGMENTS
J.D. thanks the David & Alice Van Buuren Foundation and M.J.P. thanks the Federal Ministry of Agriculture of Belgium for financial support.
REFERENCES
- 1.Aardema B W, Lorenz M G, Krumbein W E. Protection of sediment-adsorbed transforming DNA against enzymatic inactivation. Appl Environ Microbiol. 1983;46:417–420. doi: 10.1128/aem.46.2.417-420.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen-King R M, Barker J F, Gillham R W, Jensen B K. Substrate- and nutrient-limited toluene biotransformation in sandy soil. Environ Toxicol Chem. 1994;13:693–705. [Google Scholar]
- 3.Assubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Green Publishing Association and Wiley-Interscience; 1989. [Google Scholar]
- 4.Bedard C, Knowles R. Physiology, biochemistry, and specific inhibitors of CH4, NH4+, and CO oxidation by methanotrophs and nitrifiers. Microbiol Rev. 1989;53:68–84. doi: 10.1128/mr.53.1.68-84.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belser L W. Population ecology of nitrifying bacteria. Annu Rev Microbiol. 1979;33:309–333. doi: 10.1146/annurev.mi.33.100179.001521. [DOI] [PubMed] [Google Scholar]
- 6.Belser L W, Schmidt E L. Inhibitory effect of nitrapyrin on three genera of ammonia-oxidizing nitrifiers. Appl Environ Microbiol. 1981;41:819–821. doi: 10.1128/aem.41.3.819-821.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boks E, Koops H-P. The genus Nitrobacter and related genera. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. 2nd ed. New York, N.Y: Springer-Verlag; 1992. pp. 414–430. [Google Scholar]
- 8.Bremner J M, McCarty G W. Inhibition of nitrification in soil by allelochemicals derived from plants and plants residues. In: Bollag J M, Stotzky G, editors. Soil biochemistry. New York, N.Y: Maecel Dekker, Inc.; 1993. pp. 181–218. [Google Scholar]
- 9.Bundy L G, Bremner J M. Inhibition of nitrification in soils. Soil Sci Soc Am Proc. 1973;37:396–398. [Google Scholar]
- 10.Cochran W G. Estimation of bacterial densities by means of the “most probable number.”. Biometrics. 1950;6:105–106. [PubMed] [Google Scholar]
- 11.Crawford D M, Chalk P M. Mineralization and immobilization of soil and fertilizer nitrogen with nitrification inhibitors and solvents. Soil Biol Biochem. 1992;24:559–568. [Google Scholar]
- 12.Degrange V, Bardin R. Detection and counting of Nitrobacter populations in soil by PCR. Appl Environ Microbiol. 1995;61:2093–2098. doi: 10.1128/aem.61.6.2093-2098.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hyman M R, Murton I B, Arp J D. Interaction of ammonia monooxygenase from Nitrosomonas europaea with alkanes, alkenes, and alkynes. Appl Environ Microbiol. 1998;64:3187–3190. doi: 10.1128/aem.54.12.3187-3190.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joergensen R G, Schmaedeke F, Windhorst K, Meyer B. Biomass and activity of microorganisms in a fuel oil contaminated soil. Soil Biol Biochem. 1995;27:1137–1143. [Google Scholar]
- 15.Keener W K, Arp D J. Transformation of aromatic compounds by Nitrosomonas europaea. Appl Environ Microbiol. 1994;60:1914–1920. doi: 10.1128/aem.60.6.1914-1920.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keeney D R, Nelson D W. Nitrogen—Inorganic Forms. In: Page A, editor. Methods of soil analysis, part 2. Chemical and microbiological properties. Madison, Wis: American Society of Agronomy, Inc., Crop Science Society of America, Inc., and Soil Science Society of America, Inc.; 1982. pp. 643–693. [Google Scholar]
- 17.Killham K. Nitrification in coniferous forest soils. Plant Soil. 1990;128:31–44. [Google Scholar]
- 18.Knaebel D B, Federle T W, McAvoy D C, Vestal J R. Effect of mineral and organic soil constituents on microbial mineralization of organic compounds in a natural soil. Appl Environ Microbiol. 1994;60:4500–4508. doi: 10.1128/aem.60.12.4500-4508.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kowalchuk G A, Stephen J R, De Boer W, Prosser J I, Embley T M, Woldendorp J W. Analysis of ammonia-oxidizing bacteria of the β-subdivision of the class Proteobacteria in coastal sand dunes by denaturing gradient gel electrophoresis and sequencing of PCR-amplified 16S ribosomal DNA fragments. Appl Environ Microbiol. 1997;63:1489–1497. doi: 10.1128/aem.63.4.1489-1497.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kreader C A. Relief of amplification inhibition in PCR with bovine serum albumin or T4 gene 32 protein. Appl Environ Microbiol. 1996;62:1102–1106. doi: 10.1128/aem.62.3.1102-1106.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorenz M G, Wackernagel W. Adsorption of DNA to sand and variable degradation rates of adsorbed DNA. Appl Environ Microbiol. 1987;53:2948–2952. doi: 10.1128/aem.53.12.2948-2952.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lund V, Goksoyr J. Effects of water fluctuations on microbial mass and activity in soil. Microb Ecol. 1980;6:115–123. doi: 10.1007/BF02010550. [DOI] [PubMed] [Google Scholar]
- 23.Malhi S S, McGill W B. Nitrification in three Alberta soils: effect of temperature, moisture and substrate concentration. Soil Biol Biochem. 1982;14:393–399. [Google Scholar]
- 24.Nishio T, Fujimoto T. Kinetics of nitrification of various amounts of ammonium added to soils. Soil Biol Biochem. 1990;22:51–55. [Google Scholar]
- 25.Öhlinger R. Soil respiration by titration. In: Schinner F, editor. Methods in soil biology. Berlin, Germany: Springer-Verlag; 1995. pp. 95–98. [Google Scholar]
- 26.Prosser J I. Autotophic nitrification in bacteria. Adv Microb Physiol. 1989;30:125–181. doi: 10.1016/s0065-2911(08)60112-5. [DOI] [PubMed] [Google Scholar]
- 27.Rasche M E, Hicks R E, Hyman M R, Arp D J. Oxidation of monohalogenated ethanes and n-chlorinated alkanes by whole cells of Nitrosomonas europaea. J Bacteriol. 1990;172:5368–5373. doi: 10.1128/jb.172.9.5368-5373.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robertson G P. Nitrification and denitrification in humid tropical ecosystems: potential controls on nitrogen retention. In: Proctor J, editor. Mineral nutriments in tropical forest and savannah ecosystems. British Ecological Society Special Publication Number 9. Oxford, United Kingdom: Blackwell Scientific; 1989. pp. 55–69. [Google Scholar]
- 29.Rodier J. L’analyse de l’eau. 7th ed. Paris, France: Bordas; 1984. Micropollutants organiques; pp. 391–466. [Google Scholar]
- 30.Rowe R, Todd R, Waide J. Microtechnique for most-probable-number analysis. Appl Environ Microbiol. 1977;33:675–680. doi: 10.1128/aem.33.3.675-680.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schimel J P, Firestone M K, Killham K S. Identification of heterotrophic nitrification in a Sierran forest soil. Appl Environ Microbiol. 1984;48:802–806. doi: 10.1128/aem.48.4.802-806.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt E L. Nitrification in soil. In: Stevenson F J, editor. Nitrogen in agricultural soils. Madison, Wis: American Society of Agronomy; 1982. pp. 253–288. [Google Scholar]
- 33.Schmidt E L, Belser L W. Nitrifying bacteria. In: Page A, editor. Methods of soil analysis, part 2. Chemical and microbiological properties. Madison, Wis: American Society of Agronomy, Inc., Crop Science Society of America, Inc., and Soil Science Society of America, Inc.; 1982. pp. 1027–1042. [Google Scholar]
- 34.Shattuck G E, Alexander M. A differential inhibitor of microorganisms. Soil Sci Soc Am Proc. 1963;27:600–601. [Google Scholar]
- 35.Shi Y, Zwolinski M D, Schreiber M E, Bahr J M, Sewell G W, Hickey W J. Molecular analysis of microbial community structures in pristine and contaminated aquifiers: field and laboratory microcosm experiments. Appl Environ Microbiol. 1999;65:2143–2150. doi: 10.1128/aem.65.5.2143-2150.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stephen J R, McCaig A E, Smith Z, Prosser J I, Embley T M. Molecular diversity of soil and marine 16S rDNA sequence related to β-subgroup ammonia-oxidizing bacteria. Appl Environ Microbiol. 1996;62:4147–4154. doi: 10.1128/aem.62.11.4147-4154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stephen J R, Kowalchuk G A, Bruns M V, McCaig A E, Phillips C G, Embley T M, Prosser J I. Analysis of β-subgroup proteobacterial ammonia oxidizer population in soil by denaturing gradient gel electrophoresis analytical and hierarchical phylogenetic probing. Appl Environ Microbiol. 1998;64:2958–2965. doi: 10.1128/aem.64.8.2958-2965.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stojanovic B J, Alexander M. Effect of inorganic nitrogen on nitrification. Soil Sci. 1958;86:208–215. [Google Scholar]
- 39.Tsai Y L, Olson B H. Rapid method for separation of bacterial DNA from humic substances in sediments for polymerase chain reaction. Appl Environ Microbiol. 1992;58:2292–2295. doi: 10.1128/aem.58.7.2292-2295.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vannelli T, Logan M, Arciero D M, Hooper A B. Degradation of halogenated hydrocarbon aliphatic compounds by the ammonia oxidizing bacterium Nitrosomonas europaea. Appl Environ Microbiol. 1990;56:1169–1171. doi: 10.1128/aem.56.4.1169-1171.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vannilli T, Hooper A B. Oxidation of nitrapyrin to 6-chloropicolinic acid by the ammonia-oxidizing bacterium Nitrosomonas europaea. Appl Environ Microbiol. 1992;58:2321–2325. doi: 10.1128/aem.58.7.2321-2325.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verhagen F J M, Laandbroek H J. Competition for ammonium between nitrifying and heterotrophic bacteria in dual energy-limited chemostats. Appl Environ Microbiol. 1991;57:3255–3263. doi: 10.1128/aem.57.11.3255-3263.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walworth J L, Reynolds C M. Bioremediation of a petroleum-contaminated cryic soil: effects of phosphorus, nitrogen, and temperature. J Soil Contam. 1995;4:299–310. [Google Scholar]
- 44.Walworth J L, Woolard C R, Braddock F, Reynolds C M. Enhancement and inhibition of soil petroleum bioremediation through the use of fertilizer nitrogen: an approach to determining optimum levels. J Soil Contam. 1997;6:465–480. [Google Scholar]