Abstract
Primary immune thrombocytopenia (ITP) is the most common acquired autoimmune bleeding disorder. Abnormally increased levels of High Mobility Group Box 1 (HMGB1) protein associate with thrombocytopenia and therapeutic outcome in ITP. Previous studies proposed that a natural inhibitor of HMGB1, 18β-glycyrrhetinic acid (18β-GA), could be used for its anti-inflammatory and immune-modulatory effects, although its ability to correct immune balance in ITP is unclear. In this study, we showed that plasma HMGB1 correlated negatively with platelet counts in ITP patients, and confirmed that 18β-GA stimulated the production of regulatory T cells (Treg), restored the balance of CD4+ T-cell subsets and enhanced the suppressive function of Treg through blocking the effect on HMGB1 in patients with ITP. HMGB1 short hairpin RNA interference masked the effect of 18β-GA in Treg of ITP patients. Furthermore, we found that 18β-GA alleviated thrombocytopenia in mice with ITP. Briefly, anti-CD61 immune-sensitized splenocytes were transferred into severe combined immunodeficient mice to induce a murine model of severe ITP. The proportion of circulating Treg increased significantly, while the level of plasma HMGB1 and serum antiplatelet antibodies decreased significantly in ITP mice along 18β-GA treatment. In addition, 18β-GA reduced phagocytic activity of macrophages towards platelets both in ITP patients and ITP mice. These results indicate that 18β-GA has the potential to restore immune balance in ITP via inhibition of HMGB1 signaling. In short, this study reveals the role of HMGB1 in ITP, which may serve as a potential target for thrombocytopenia therapy.
Introduction
Primary immune thrombocytopenia (ITP) is an acquired autoimmune disease characterized by immune-mediated platelet destruction or impaired platelet production or both. Related studies have shown that disturbed helper T-cell subsets are involved in ITP. Platelet autoreactive CD4+ effector T cells are excessively activated, exhibiting decreased apoptosis, whereas CD4+ CD25+ Foxp3+ regulatory T cells (Treg) are numerically and functionally impaired.1 In addition, autoantibody-sensitized platelets are targeted and destroyed by the mononuclear macrophage system in ITP.2
Treg, as immunosuppressive T cells, play a key role in self-tolerance in many autoimmune diseases. Many studies have shown that Treg are defective in patients with chronic ITP. Both clinical and basic mechanistic studies have shown that a variety of treatments, including glucocorticoids, rituximab, thrombopoietin-receptor agonists, intravenous immunogloblin, and histone deacetylase inhibitors, exert therapeutic effects and modulate the expansion and regulatory functions of Tregs,3-7 among which dexamethasone, thrombopoietin-receptor agonists, intravenous immunoglobulin, and chidamide were also shown to attenuate phagocytosis of the monocyte-macrophage system.2,7-9
High Mobility Group Box 1 (HMGB1) protein, a non-histone nuclear protein, plays a critical role not only inside the cell as a DNA chaperone, but also outside of the cell as a damage-associated molecular pattern (DAMP) molecule. Of note, HMGB1 was found to mediate the development and progression of autoimmune diseases, acting on cell surface receptors, and triggering intracellular signaling. The expression of HMGB1 is upregulated in hepatitis B virus infection, accompanied by an increase in the level of pro-inflammatory factors, such as interleukin (IL)-6 and IL-17, affecting the balance of Th17/Treg.10 Stimulation of HMGB1 results in markedly reduced expression of Foxp3 and secretion of IL-10 from Treg in mice and humans.11,12 Recently, it has been reported that glycyrrhizin prevents release of HMGB1 induced by severe acute respiratory syndrome coronavirus-2 and inhibits viral replication.13 Studies have also shown higher levels of HMGB1 in the spleen, serum and plasma of patients with ITP, compared with those in healthy controls.14,15 HMGB1 is associated with an imbalance of Treg/Th17 cells and is involved in the pathogenesis of ITP.16 As 18β-glycyrrhetinic acid (18β-GA) is known to bind to HMGB1 directly,17 it has been used as a novel pharmacological inhibitor of HMGB1 cytokine activities in several clinical and experimental studies.18,19 18β-GA, the main active metabolite of glycyrrhiza, can be obtained by glucuronidase hydrolysis of glycyrrhiza after oral administration. There is evidence showing that 18β-GA is associated with regulating the homeostasis of Th1/Th2/Th17/Treg,20 for example, by triggering a curative Th1 response in experimental visceral leishmaniasis, attenuating airway inflammation in allergic asthma by reducing Th2 cytokines through upregulation of Foxp3 and downregulation of STAT6/GATA-3/RORγt expression, suppressing the differentiation of Th17 cells in experimental autoimmune encephalomyelitis, and inhibiting the development of hepatocellular carcinoma by modulating Treg expression.21-24 18β-GA has already been marketed in a variety of dosages for the treatment of liver dysfunction18 and specific cutaneous inflammation.25 Luo et al. reported the effect of licorice, another precursor of 18β-GA, in raising platelet counts, which also proved to be well-tolerated in ITP patients.26 However, to our knowledge, no previous studies have focused on the roles and the mechanism of action of 18β-GA in patients with ITP.
Therefore, the current study was performed to evaluate whether the inhibitor of HMGB1 restored immune balance in patients and mice with ITP. Moreover, we investigated the effects of 18β-GA on the modulation of Treg and the monocyte-macrophage system in vivo and in vitro, as well as the underlying molecular mechanisms. Our aim was to provide new insights into the treatment of ITP.
Methods
Patients and controls
A total of 42 patients (25 females and 17 males; 18-66 years old; median age, 47.5 years) with newly diagnosed primary ITP and without prior treatment were enrolled in this study between April 2018 and November 2021 at the Department of Hematology, Qilu Hospital, Shandong University, China. The patients’ platelet counts ranged between 1×109/L and 38×109/L (median count, 13×109/L) and they were diagnosed based on previously recommended criteria.27 Twenty-five age-matched healthy volunteers were included (13 females and 12 males; 18-65 years old; median age, 35 years) with platelet counts ranging between 155×109/L and 311×109/L (median count, 256×109/L). The subjects’ demographics and key clinical information are summarized in Online Supplementary Table S1. All study subjects donated 20 mL of EDTA-anticoagulated venous peripheral blood. The study protocol was approved by the Medical Ethics Committee of Qilu Hospital, Shandong University. All patients provided written informed consent before enrollment into the study, in accordance with the Declaration of Helsinki.
Animal model
A murine model of active ITP was established as previously reported with modifications.28 The strains and backgrounds of the mice are detailed in the Online Supplementary Methods. Briefly, we immunized CD61-knock-out mice with CD61+ platelets. Splenocytes from these immunized mice were engrafted into CD61+ severe combined immunodeficient (SCID) mice to construct the active ITP murine model. ITP mice were then randomly separated into groups that received the same volume of either 18β-GA (30 mg/kg,29 intraperitoneal injection, CAS471-53-4, Sigma-Aldrich, USA), the HMGB1 inhibitor lycopene (5 mg/kg,30 intragastric administration, S3943, Selleck, USA), or 3% dimethylsulfoxide (DMSO, intraperitoneal injection, Sigma-Aldrich, USA) every 2 days from day 1 after radiation and splenocyte infusion. Platelets were counted weekly, and ITP mice were euthanized after 5 weeks. Peripheral blood, spleen, thymus, inguinal lymph nodes, and liver were removed to prepare single-cell suspensions for analyses. All animal experiments were performed with the approval of the Animal Care and Use Committee of Shandong University and undertaken in accordance with the Institutional Guidelines for the Care and Use of Laboratory Animals.
The methods for Treg depletion, the detection of serum anti-platelet CD61-specific antibodies, multiple cytokine analysis, and the analysis of platelet retention using living liver and spleen imaging of ITP mice; cell isolation from ITP patients and healthy controls, immunophenotyping of Treg and monocyte-derived macrophages, suppressive capacity of Treg on the proliferation of CD4+ CD25– effector T cells and CD8+ cytotoxic T lymphocyte-induced platelet apoptosis, lentiviral interference of HMGB1 in Treg and macrophages, determination of acetylation level of plasma HMGB1 and the redox state test of HMGB1, and western blotting in ITP patients; phagocytic activity of macrophages, quantitative real-time polymerase chain reaction (RT-PCR), enzyme-linked immunosorbent assay (ELISA) in ITP patients and mice; and statistical analysis are explained in the Online Supplementary Data.
Results
18β-GA increased the number of Treg without apparent induction of apoptosis in peripheral blood mononuclear cells from patients with immune thrombocytopenia
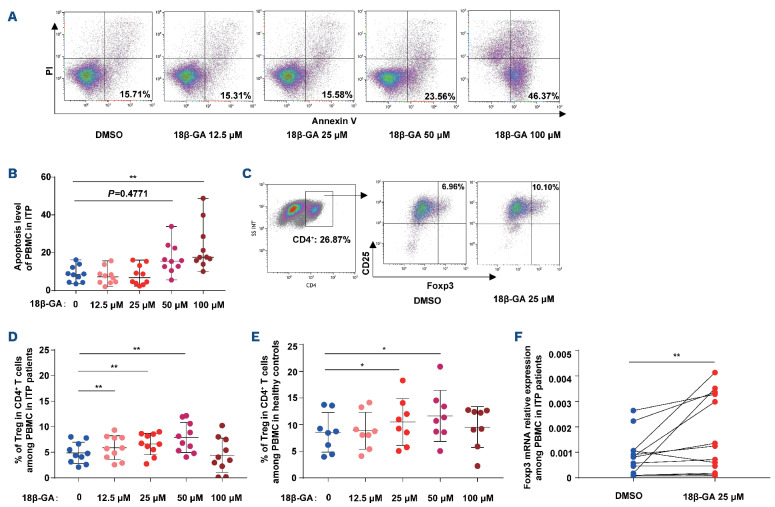
We found that 18β-GA only induced apoptosis of peripheral blood mononuclear cells (PBMC) from patients with ITP at the higher doses of 50 µM and 100 µM (Figure 1A, B).
Subsequently, we tested whether 18β-GA affects Treg, using PBMC isolated from ITP patients and healthy controls. We noted that 25 mM and 50 mM 18β-GA increased the number of Treg (Figure 1C-E). Moreover, we detected that Foxp3 mRNA expression in the group treated with 25 mM 18β-GA was higher than that in the DMSO-treated PBMC from ITP patients (Figure 1F). However, the percentages of CD4+ T cells among the PBMC remained unchanged after the respective treatments (Online Supplementary Figure S1). We therefore selected 25 mM 18β-GA as the optimal dose for cell experiments, as it induced an increase in Treg number without apparently causing apoptosis.
Figure 1.
18P-GA increased the number of Treg without apparent induction of apoptosis in peripheral blood mononuclear cells from patients with immune thrombocytopenia. (A) Representative density plots of apoptosis in cultures of peripheral blood mononuclear cells (PBMC) from patients with immune thrombocytopenia (ITP). The percentage of annexin V-positive and propidium iodide (PI)-negative cells among PBMC represents the rate of cell apoptosis. (B) 18β-GA induced apoptosis of PBMC from ITP patients after 3 days at the doses of 50 mM and 100 mM (n=10; Friedman test, ****P<0.0001). Multiple comparisons: **P=0.0069. (C) The gating strategy and representative density plots for identification of CD4+ CD25+ Foxp3+ Treg. (D) In the culture of PBMC from ITP patients, the percentages of Treg were significantly increased 3 days after 18β-GA treatment at the doses of 12.5 mM, 25 µM and 50 mM (n=10; one-way analysis of variance [ANOVA]; *P=0.0167). Multiple comparisons: dimethylsulfoxide (DMSO) vs. 18(3-GA 12.5 mM, **P=0.0051; DMSO vs. 18(3-GA 25 DM, **P=0.0031; DMSO vs. 18(3-GA 50 mM, **P=0.0076. (E) In the culture of PBMC from healthy controls, the percentages of Treg were significantly increased at 25 mM and 50 mM (n=8, one-way ANOVA, *P=0.0173). Multiple comparisons: DMSO vs. 18(3-GA 25 mM, *P=0.0403; DMSO vs. 18(3-GA 50 mM, *P=0.0386. (F) Foxp3 mRNA expression of the 18(3-GA group was higher than that of controls (n=13, Wilcoxon matched-pairs signed rank test, **P=0.0046). DMSO vs. 18(3-GA: 0.0008 (0.0001, 0.0026) vs. 0.0012 (0.0001, 0.0041).
Figure 2.
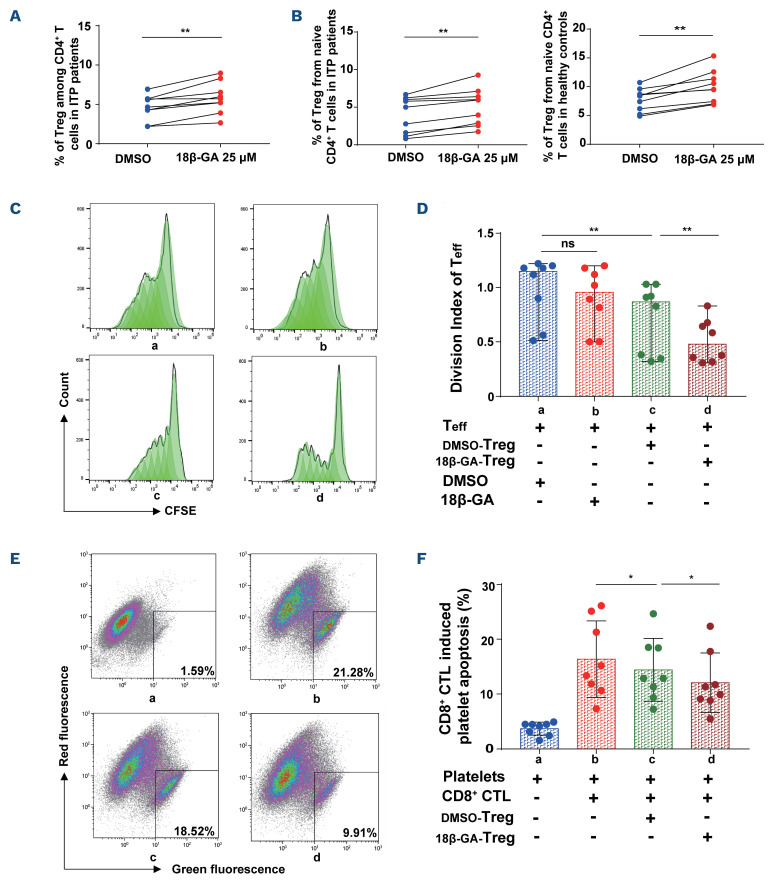
18β-GA increased the generation and enhanced the suppressive function of Treg from CD4+ T and naïve CD4+ T cells among patients with immune thrombocytopenia. (A) The percentage of Treg in the culture of CD4+ T cells from patients with immune throm-bocytopenia (ITP) was increased after 3 days by stimulation with 18β-GA at 25 mM. Paired t test, 4.6200±1.6010 vs. 5.8367±1.9671, n=9; **P=0.0024. (B) 18β-GA increased the generation of Treg from naïve CD4+ T cells from ITP patients after 3 days. Paired t test, 4.0067±2.3976 vs. 5.0967±2.4695, n=9, **P=0.0018. 18β-GA also increased Treg generation from isolated naïve CD4+ T cells from healthy controls. Paired t test, 7.7333±1.9871 vs. 10.0156±2.8094, n=9, **P=0.0012. (C) Representative histogram plots of CFSE of CD4+ CD25– effector T cells. a: DMSO-Te ff, b: 18β-GA-Te ff, c: Te ff+DMSO-Treg, d: Te ff+18β-GA-Treg. (D) Division index of effector T-cell proliferation after co-culture with Treg. a: DMSO-Te ff, b: 18β-GA-Te ff, c: Te ff+DMSO-Treg, d: Te ff+18β-GA-Treg. Wilcoxon matched-pairs signed rank test, n=8. Treg significantly suppressed proliferation of effector T cells (**Pac=0.0078). Compared with DMSO, 18β-GA significantly enhanced the inhibitory function of Treg (**Pcd=0.0078). (E) The gated dot-plots illustrate the apoptosis of platelets. a: Platelets, b: CD8+ CTL+platelets, c: DMSO-Treg+CD8+ CTL+platelets, d: 18β-GA-Treg+CD8+ CTL+platelets. (F) A significant reduction was observed in CD8+ CTL-induced platelet apoptosis from ITP patients cultured with 18β-GA-Treg in vitro. a: Platelets, b: CD8+ CTL+platelets, c: DMSO-Treg+CD8+ CTL+platelets, d: 18β-GA-Treg+CD8+ CTL+platelets. Paired t test, n=8. Treg markedly decreased the platelet apoptosis mediated by CD8+ CTL (*Pbc=0.0354). After treatment with 18β-GA, Treg significantly reduced CD8+ CTL induced platelet destruction, compared with Treg after treatment with DMSO (*Pcd=0.0497). CFSE: 5(6)-carboxyfluorescein diacetate N-succinimidyl ester (CFSE); Teff: T effector cells; DMSO: dimethylsulfoxide; CTL: cytotoxic T lymphocyte.
18β-GA increased Treg generation from isolated CD4+ T/naïve CD4+ T cells from patients with immune thrombocytopenia and enhanced the suppressive function of the Treg
We noted that the percentage of Treg increased after stimulating CD4+ T cells from patients with ITP with 25 mM 18β-GA (Figure 2A). Furthermore, peripheral Treg induced from naïve CD4+ T cells in patients with ITP and healthy controls were also significantly expanded after treatment with 18β-GA (Figure 2B).
We then explored whether 18β-GA enhances the suppressive function of Treg from patients with ITP. After drug elution, we co-cultured 5(6)-carboxyfluorescein diacetate N-succinimidyl ester (CFSE)-labeled CD4+ CD25- effector T cells with Treg. The division index of effector T cells was calculated using Flow Jo software. We confirmed that 18β-GA enhanced the suppressive function of Treg towards CD4+ CD25- effector T cells (Figure 2C, D). We observed that 18β-GA alone had no apparent effect on the proliferation of effector T cells; however, Treg markedly suppressed the proliferation of effector T cells after treatment with DMSO or 18β-GA. Furthermore, we found that, compared with DMSO, 18β-GA significantly enhanced Treg function in suppression of effector T-cell proliferation. In addition, we noted that Treg from ITP patients treated with either DMSO or 18β-GA reduced CD8+ cytotoxic T lymphocyte (CTL)-mediated platelet destruction, although the Treg treated with 18β-GA significantly reduced CD8+ CTL-induced platelet apoptosis, compared with that of Treg treated with DMSO (Figure 2E, F). These findings suggest that 18β-GA enhanced the immunosuppressive ability of Treg.
Figure 3.
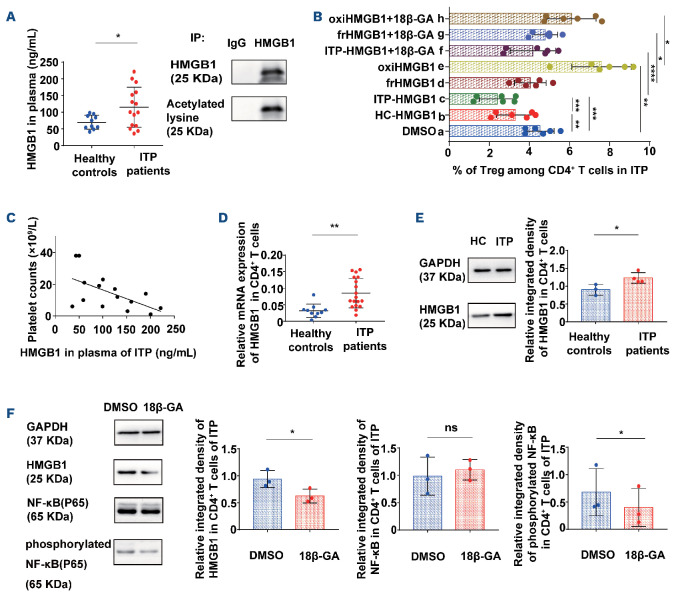
18β-GA inhibited the expression of HMGB1 in CD4+ T cells from patients with immune thrombocytopenia by suppressing NF-KB signaling. (A) The levels of HMGB1 in plasma of patients with immune thrombocytopenia (ITP) were higher than those in healthy controls (unpaired Student t test, *P=0.0335, 69.8793±20.7887 vs. 114.8579±60.2011, healthy controls n=10, ITP patients n=15). Immunoprecipitation of plasma HMGB1 with anti-HMGB1 antibodies in ITP patients. Western blotting with the anti-human HMGB1 antibody and acetylated-lysine antibody. Plasma HMGB1 in ITP patients was obviously acetylated. (B) The percentage of Treg stimulated with different redox states of HMGB1. 18β-GA inhibited the effect of HMGB1 with different redox states on Treg. a: DMSO; b: HC-HMGB1 (HMGB1 from healthy controls); c: ITP-HMGB1 (HMGB1 from ITP patients); d: ITP-frHMGB1 (ITP-fully reduced HMGB1); e: ITP-oxiHMGB1 (ITP-oxidized HMGB1); f: ITP-HMGB1+18β-GA; g: ITP-frHMGB1+18β-GA; h: ITP-oxiHMGB1+18β-GA. n=6. Paired t test, **Pab=0.0013; ***Pac=0.0001; ***Pbc=0.0006; **Pae=0.0023; ****Pcf<0.0001; *Pdg=0.0109; *Peh=0.0303. (C) The level of HMGB1 in plasma correlated negatively with the platelet count in routine blood tests in ITP patients, n=15, Pearson correlation, r=-0.5796, R2=0.3359, *P=0.0236. (D) HMGB1 mRNA expression in CD4+ T cells from ITP patients at the time of enrollment were higher than that in healthy controls (unpaired Student t test, **P=0.0014, 0.0314±0.0203 vs. 0.0852±0.0449, healthy controls n=10, ITP patients n=18). (E) Relative integrated density of HMGB1 of isolated CD4+ T cells from ITP patients and healthy controls after 3 days cultured by western blotting (unpaired t test, *P=0.0367, healthy controls n=3, ITP patients n=4). (F) 18β-GA reduced HMGB1 expression (*P=0.0182) and phosphorylated NF-KB expression (*P=0.0476) of CD4+ T cells from ITP patients after 3 days (paired t test, n=3). IP: immunoprecipitation; HC: healthy controls; DMSO: dimethylsulfoxide; ns: not significant.
18β-GA inhibited the expression of HMGB1 in CD4+ T cells from patients with immune thrombocytopenia by suppressing NF-κB signaling
Using ELISA, we found that the expression of HMGB1 in the plasma of active ITP patients was higher than that in healthy controls. We also found that the plasma HMGB1 in patients with ITP was acetylated, showing that it was released from the nuclei of cells31 (Figure 3A). Next, we compared the proliferation of HMGB1-treated Treg. HMGB1 was enriched from the plasma of healthy controls and ITP patients (HC-HMGB1 and ITP-HMGB1, respectively). The proportions of Treg were significantly decreased with HC-HMGB1 and ITP-HMGB1, and augmented with oxidized HMGB1 (ITP-oxiHMGB1), compared with the DMSO group. Moreover, Treg expansion was stimulated in the ITP-HMGB1 and fully reduced HMGB1 (ITP-frHMGB1) groups, while it was suppressed in the ITP-oxiHMGB1 group, after 18β-GA treatment (Figure 3B).
The level of HMGB1 in plasma correlated inversely with platelet counts of patients with ITP (Figure 3C). Moreover, the mRNA expression of HMGB1 in CD4+ T cells from ITP patients was higher than that of healthy controls (Figure 3D). We further investigated whether CD4+ T cells could secrete HMGB1 directly after cell culture. Our western blotting results showed an increase in HMGB1 expression in CD4+ T cells from patients with active ITP, compared with the expression in healthy controls (Figure 3E). The nuclear factor κB (NF-κB) signaling pathway is the major downstream pathway of HMGB1 intracellularly and extra-cellularly.32 We demonstrated that expression of phosphorylated NF-κB (P65) and the levels of HMGB1 decreased significantly in CD4+ T cells of patients with ITP after 18β-GA stimulation (Figure 3F). Moreover, the level of phosphorylation of heat shock factor 1 (HSF1) in CD4+ T cells from ITP patients was significantly increased after 18β-GA modulation. The mRNA expression of HMGB1 was significantly increased after addition of HSF1 inhibitor, and this effect could not be reversed by 18β-GA (Online Supplementary Figure S2).
18β-GA inhibited the expression of HMGB1, in turn increasing the number and restoring the immunosuppressive capacity of Treg from patients with immue thrombocytopenia
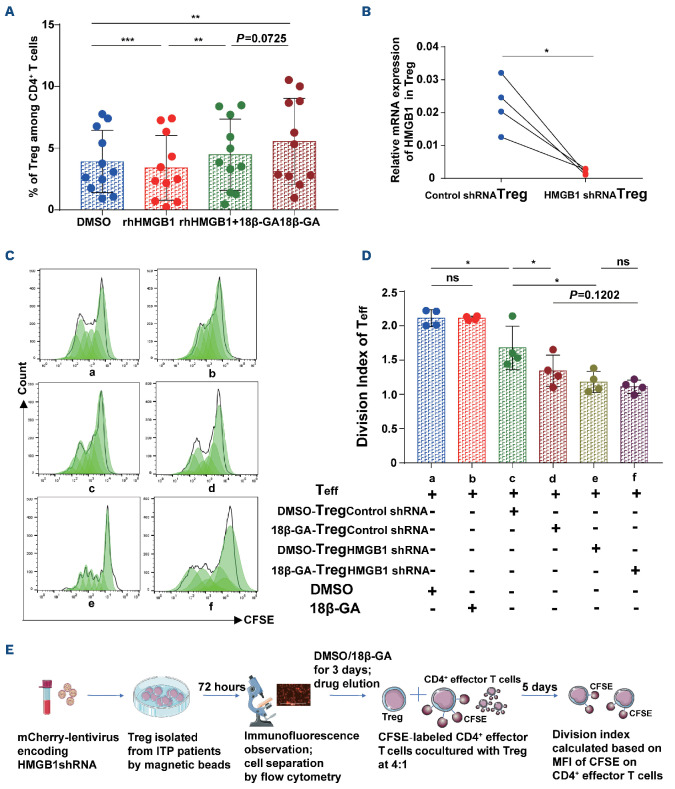
We used recombinant human HMGB1 protein (rhHMGB1) in cell experiments to simulate the high level of HMGB1 in the plasma of patients with ITP. We demonstrated that, compared with DMSO, 18β-GA increased Treg generation from isolated CD4+ T cells from ITP patients (18β-GA vs. DMSO; P=0.0069) (Figure 4A). However, we noticed that this change was apparently attenuated with additional treatment of rhHMGB1 at 100 ng/mL (18β-GA vs. rhHMGB1+18β-GA; P=0.0725). We also found that HMGB1 itself reduced the number of Treg directly (DMSO vs. rhHMGB1; P=0.0007), whereas this phenomenon was reversed when cells were also treated with 18β-GA (rhHMGB1 vs. rhHMGB1+18β-GA; P=0.0036) for 3 days. Next, to determine whether the mechanism of action of 18β-GA was mediated by HMGB1, we performed a lentiviral interference test (Figure 4B-E). We observed that the transfection with short hairpin (sh)RNA successfully silenced the HMGB1 in Treg with a knockdown efficiency of ≥78.7% as indicated by RT-PCR (Figure 4B). The cells were then divided into six groups (Figure 4C, D). We found that, compared to Treg transfected with the control lentivirus, Treg transfected with HMGB1 shRNA had a stronger immunosuppressive ability on effector T cells, similar to that produced by 18β-GA modulation, Moreover, HMGB1 shRNA interference masked the effect of 18β-GA in Treg of patients with ITP. We did not observe any significant statistical difference in the immunosuppressive ability of Treg transfected with HMGB1 shRNA after treatment with DMSO or 18β-GA.
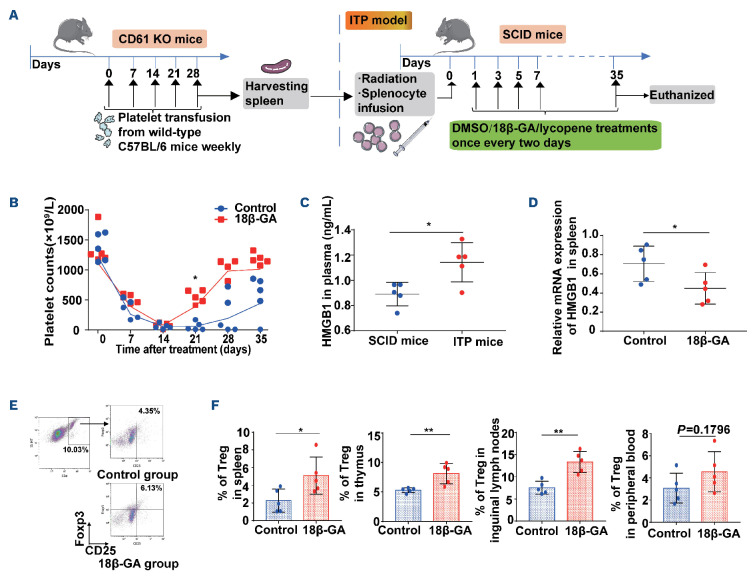
18β-GA ameliorated thrombocytopenia in an active murine model of immune thrombocytopenia via inhibition of HMGB1 signaling
We constructed a murine model of active ITP.28 We administered 18β-GA (30 mg/kg) or 3% DMSO via intraperitoneal injections every 2 days from day 1 after radiation and splenocyte transfusion (Figure 5A). Following the radiation and transfer of anti-CD61 immune-sensitized splenocytes into SCID mice, platelet counts dropped to a nadir on day 14. On day 21, significantly higher platelet counts were observed in the 18β-GA-treated group than in the control group (Figure 5B). The level of plasma HMGB1 in ITP mice was significantly higher than that in SCID mice without splenocyte transfusion (Figure 5C). Nevertheless, the mRNA expression of HMGB1 in mice spleen in the 18β-GA group was lower than that in the control group at day 35 (Figure 5D). It was also seen that 18β-GA increased Treg in peripheral blood, spleen, inguinal lymph nodes and thymus 35 days after splenic transfer (Figure 5E, F).
Figure 4.
18β-GA inhibited the expression of HMGB1 by increasing the number and restoring the immunosuppressive capacity of Treg from patients with immune thrombocytopenia. (A) Compared to dimethylsulfoxide (DMSO), 18β-GA increased the generation of Treg in CD4+ T cells from patients with immune thrombocytopenia (ITP), and this change was attenuated in the rhHMGB1 (100 ng/mL) group, n=11, paired t test, ***PDMSO vs. rhHMGB1=0.0007, **PrhHMGB1 vs. rhHMGB1+18β-GA=0.0036, **PDMSO vs. 18β-GA=0.0069. (B) HMGB1 shRNA transfection successfully silenced the HMGB1 gene in Treg of ITP patients after 72 hours; the knockdown efficiency of HMGB1 gene was ≥78.7% as determined by real-time quantitative polymerase chain reaction (paired t test, n=4, *P=0.0191). (C) Representative histogram of proliferation of CD4+ CFSE+ effector T cells. (D) After treatment with DMSO or 18β-GA, HMGB1 or control shRNA-transfected Treg were co-cultured with CD4+ CD25- effector T cells. The graph shows the division index of the proliferation of effector T cells. Effector T cells co-cultured with HMGB1 shRNA-Treg had a lower level of proliferation, compared with those co-cultured with control shRNA-Treg (*Pcd=0.0209, *Pce=0.0146). There was no statistically significant difference in the immuno-suppression of Treg transfected with HMGB1 shRNA after treatment with DMSO or 18β-GA (Pef=ns). Paired t test, n=4. (E) Flow chart of the lentiviral interference. Teff: T effector cells; CFSE: 5(6)-carboxyfluorescein diacetate N-succinimidyl ester; MFI: mean fluorescence intensity.
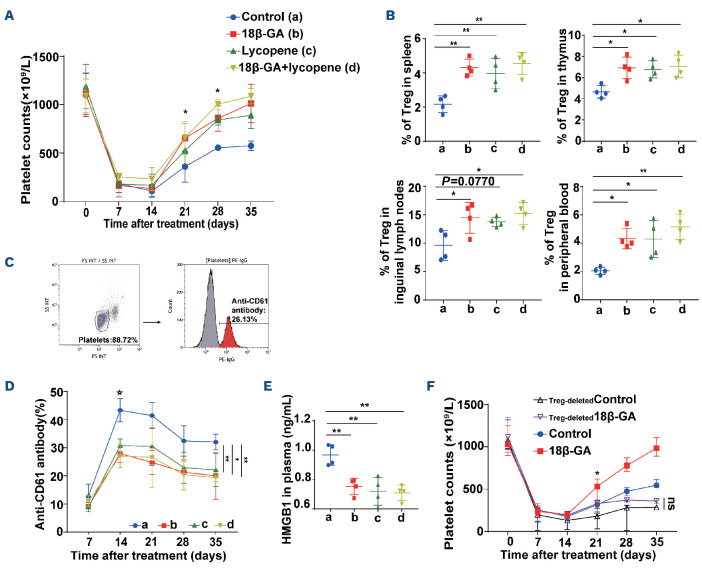
We observed that lycopene (5 mg/kg), another inhibitor of HMGB1,33 also alleviated thrombocytopenia in ITP mice, and induced similar effects on the elevation of platelet counts and upregulation of Treg in various organs as those induced by 18β-GA. Platelet counts dropped to their minimum levels on day 14 in each group, and platelet counts in mice treated with lycopene were significantly higher than those in the control group on day 28 (Figure 6A). The Treg population increased significantly in the spleen, thymus and peripheral blood by day 35 following treatment with lycopene (Figure 6B). We also found that the level of serum antiplatelet CD61-specific antibodies decreased significantly in the 18β-GA and lycopene groups, compared with that in the control group since day 14 (Figure 6C, D). Plasma HMGB1 expression was downregulated in the lycopene-modulated group, compared with that in the control group on day 35 (Figure 6E).
Figure 5.
18β-GA ameliorated thrombocytopenia in a murine model of active immune thrombocytopenia with increased Treg. (A) Immune thrombocytopenia (ITP) was established in radiated severe combined immunodeficient (SCID) mice with infusion of 5×104 splenocytes from CD61-knockout mice immunized against wildtype C57BL/6 mice platelets. Platelet counts were monitored weekly for 5 weeks. We defined the day of splenocyte infusion as day 0. The drug intervention was administered on day 1, and repeated every 2 days thereafter. (B-F) Treatment with 18β-GA (30 mg/kg) or control (3% dimethylsulfoxide [DMSO] in phosphate-buffered saline) was administered on day 1 and repeated every 2 days; n=5 for the control group and n=5 for the 18β-GA group. (B) On day 21, a significantly higher platelet count was observed in the 18β-GA-treated group than in the control group. The lines denote medians of platelet counts in each group as the data were not normally distributed. Significance among groups was determined by two-way analysis of variance, ****PTime<0.0001, ***Pplatelets=0.0002 (multiple comparisons: *P21=0.0158). (C) The level of plasma HMGB1 in mice with ITP was significantly higher than that in SCID mice. Unpaired t test, *P=0.0142. (D) Relative mRNA expression of HMGB1 in the spleen at day 35 was higher after 18β-GA treatment than after DMSO treatment. Unpaired t test, *P=0.0482. Peripheral blood, spleens, thymuses, inguinal lymph nodes and livers were harvested from ITP mice at day 35 after splenocyte transfer. (E) The gating strategy and representative density plots for identification of CD4+ CD25+ Foxp3+ Treg (F) 18β-GA-treated ITP mice had a higher percentage of Treg in spleen (*P=0.0340), thymus (**P=0.0075), inguinal lymph nodes (**P=0.0017), and peripheral blood (P=0.1796), compared with the control ITP mice. Differences between two groups were determined by an unpaired t test. KO: knockout.
In addition, we performed a Treg cell-depletion study and found that thrombocytopenia was exacerbated in ITP mice receiving Treg-depleted splenocytes, in which 18β-GA failed to raise platelet counts (Figure 6F). However, 18β-GA was still capable of raising platelet counts in ITP mice after CD19+ B-cell and CD8+ T-cell depletion (Online Supplementary Figure S3).
Moreover, we observed that, on day 35 after treatment with 18β-GA, the levels of proinflammatory cytokines such as interleukin (IL)-6, IL-12, tumor necrosis factor-alpha (TNF-a) and interferon-gamma (IFN-γ) were decreased, whereas the anti-inflammatory cytokine transforming growth factor-beta (TGF-β) increased in the serum of ITP mice (Online Supplementary Table S2).
18β-GA alleviated phagocytosis towards platelets by macrophages from mice and patients with immune thrombocytopenia
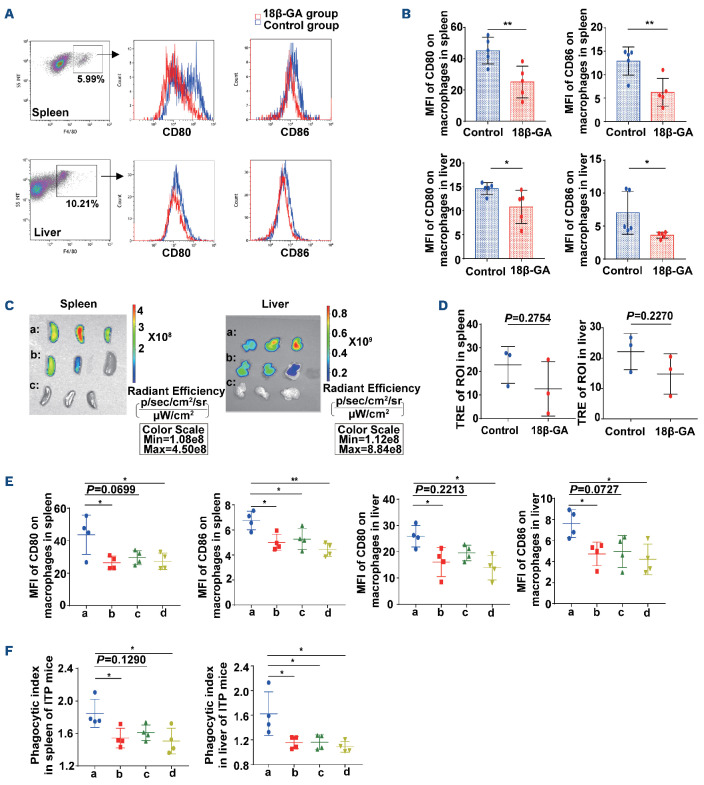
We quantified the mean fluorescence intensity of CD80/CD86 on F4/80+ macrophages on day 35 in ITP mice. It was observed that CD80 and CD86 expression on macrophages from spleen and liver decreased after 18β-GA treatment compared with the control group (Figure 7A, B). In addition, platelets residing within the spleen and liver of ITP mice on day 35 were assessed by total radiant efficiency in the region of interest with an in vivo live imaging system. The total radiant efficiency of the region of interest in the 18β-GA-treated group was lower than that in the DMSO-treated group, indicating weaker platelet phagocytosis in the spleen and liver after 18β-GA treatment (Figure 7C, D). Interestingly, we observed that lycopene also downregulated the expression of CD80/CD86 on F4/80+ macrophages (Figure 7E), and attenuated the phagocytic indices of macrophages towards platelets sensitized by anti-mouse CD41 antibody and labeled with 5-chloromethylfluorescein diacetate in spleen and liver of ITP mice on day 35 (Figure 7F).
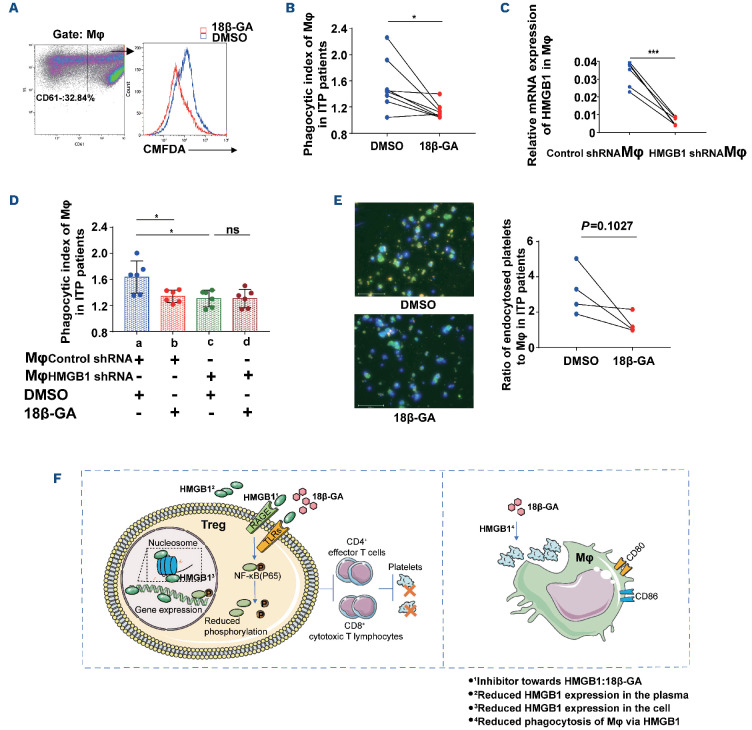
Furthermore, 18β-GA significantly reduced the phagocytic index of CD14+ monocyte-derived macrophages towards platelets in patients with ITP, consistent with results in ITP mice (Figure 8A, B). We blocked HMGB1 expression in monocyte-derived macrophages from patients with ITP (Figure 8C), and found that phagocytic indices were decreased (Figure 8D). Moreover, 18β-GA did not have a significant effect on phagocytic indices of macrophages after blockade of HMGB1. Microscopy images showed that the number of platelets phagocytosed by monocyte-derived macrophages from ITP patients in the 18β-GA-treated group was less than that in the DMSO group (Figure 8E).
In summary, the results of our in vitro and in vivo studies in ITP demonstrated that 18β-GA augmented the suppressive function of Treg via inhibition of HMGB1 signaling, with the ultimate effect of restoring the immune balance of the cell subset and improving thrombocytopenia. On the other hand, 18β-GA attenuated the phagocytotic activity of macrophages in ITP mice and patients by blocking HMGB1 (Figure 8F).
Discussion
The pathogenesis of ITP is complex and not fully elucidated, but includes antibody-mediated peripheral platelet destruction and CTL cytotoxicity towards platelets. Management of the condition remains a challenge.
18β-GA can play a protective role in psoriasis in mice through inhibition of inflammatory cytokines, and activation of Treg in both lymph nodes and the spleen.34 Carbenoxolone, as a derivative of GA, significantly reversed the severity and pathology in experimental autoimmune encephalomyelitis.35 IL-17-secreting and IFN-γ-secreting CD4+ T lymphocytes were remarkably lower in the spleen after carbenoxolone treatment in mice with experimental autoimmune encephalomyelitis. These data show that 18β-GA can regulate the homeostasis of CD4+ T helper cells, which is consistent with our research. Treg are important for maintaining self-tolerance and immune homeostasis in ITP. Insufficient production and impaired immunosuppressive activity of Treg contribute to loss of immune balance in patients with ITP. In our study, it was demonstrated that 18β-GA could upregulate the proportion and enhance the suppressive activity of Treg in cells of ITP patients. In the murine model of active ITP, 18β-GA significantly increased platelet counts, and enhanced the number and function of naturally occurring Treg in various organs in the ITP mice. Interestingly, a study by Aslam et al. showed that mice with active ITP had low numbers of peripheral splenic Treg, and that intravenous immunoglobulin therapy normalized this peripheral deficiency. Additionally, it was indicated that the peripheral Treg deficiency associated with thymic sequestration of functional Treg, and intravenous immunoglobulin therapy reduced the numbers of thymic Treg by allowing the cells’ release into the periphery, although the underlying mechanism of how intravenous immunoglobulin modulated Treg movements is undefined.5 Our in-vivo study, on the other hand, demonstrated that 18β-GA not only stimulated thymic expansion of Treg but also rescued the peripheral deficiency by increasing splenic, lymph node infiltrating and circulating Treg numbers. Whether this dual effect was a superimposed result of enhanced Treg induction and Treg release needs to be further investigated.
It is worth noting that ITP-specific therapies such as corticosteroids, intravenous immunoglobulin, rituximab, and thrombopoietin-receptor agonists restore Treg balance as an effect accompanying the rise in platelet counts in general, although via different mechanisms of action in ITP. In addition to well-established roles in hemostasis and thrombosis, platelets are increasingly considered as dynamic effector cells across immune and inflammatory continuums.36 Guo et al. found that T-cell-mediated thrombocytopenia was alleviated by allogeneic platelet transfusions in ITP.37 Thus, as versatile cells that orchestrate immune host responses, it is likely that a rising platelet mass will somehow affect Treg or vice versa. However, 18β-GA increased the number of Treg and enhanced their immunosuppressive function via HMGB1 blockade in the absence of platelets in our in vitro studies, indicating that the immunomodulatory effect of 18β-GA was platelet-independent. Moreover, the results of Treg-depletion in the ITP murine model also showed that the platelet-raising effect of 18β-GA required the involvement of Treg.
Figure 6.
18β-GA exerted a therapeutic effect by inhibiting HMGB1 in mice with immune thrombocytopenia. (A-E) Treatment with 18β-GA (30 mg/kg), lycopene (5 mg/kg), or control (3% dimethylsulfoxide in phosphate-buffered saline) was administered on day 1, and repeated every 2 days after murine models of active immune thrombocytopenia had been established, n=4 for each group. a: control, b: 18β-GA, c: lycopene, d: 18β-GA+lycopene. (A) On day 21, a significantly higher platelet count was observed in the 18β-GA-treated group than in the control group (*Pcontrol vs 18Β-GA=0.0128). On day 28, platelet counts increased significantly with treatment with 18β-GA (*Pcontrol vs 18Β-GA=0.0101) or lycopene (*Pcontrol vs lyco ene=0.0160). The lines denote the mean (± standard deviation [SD]) of platelet counts in each group as the data are normally distributed. Statistical significance among groups was determined by two-way analysis of variance (ANOVA), ****PTime<0.0001, **P latelets=0.0010. (B) The percentage of Treg in the spleen, thymus, inguinal lymph nodes, and peripheral blood on day 35. Statistical significance among groups was determined by one-way ANOVA, n=4 for each group. For spleens, **Pab=0.0028, **Pac=0.0098, **Pad=0.0012. For thymuses, *Pab=0.0168, *Pac=0.0243, *Pad=0.0109. For inguinal lymph nodes, *Pab=0.0346, *Pad=0.0144. For peripheral blood, *Pab=0.0155, *Pac=0.0167, **Pad=0.0016. (C) The gating strategy and representative plots for anti-CD61 antibody (%). (D) The level of serum antiplatelet CD61-specific antibodies decreased after day 14. The lines denote the mean (±SD) of % anti-CD61 antibody in each group as the data are normally distributed. * Significant differences among groups emerging on day 14. Two-way ANOVA. ***Pab=0.0003, **Pac=0.0037, ***Pad=0.0001 at day 14. At day 35, **Pab=0.0063, *Pac=0.0329, **Pad=0.0038. (E) Plasma HMGB1 levels in ITP mice at day 35. Statistical significance among groups was determined by one-way ANOVA **P b=0 0049 **P =0 0016 **P d=0 0012 (F) Thrombocytopenia was exacerbated in ITP mice receiving Treg-depleted splenocytes, where 18β-GA failed to raise platelet counts. The lines denote median (with range) of platelets in each group as the data are not normally distributed, n=4 for each group. Statistical significance among groups was determined by two-way ANOVA, and emerged on day 21: *PControl vs 18Β GA=0.0367. PTre deleted Control vs Tre deleted 18β GA=not significant (ns).
Figure 7.
18β-GA alleviated the phagocytosis of macrophages in mice with immune thrombocytopenia. (A) Representative histogram plots for mean fluorescence intensity (MFI) of CD80/CD86 on F4/80+ macrophages in spleen and liver of mice with immune thrombocytopenia (ITP). (B) 18β-GA-treated ITP mice had a lower MFI of CD80 and CD86 on F4/80+ macrophages in the spleen and liver, compared with the control ITP mice, n=5 for each group. Differences between two groups were determined by an unpaired t test. (**Pspleen-CD80=0.0095; **Pspleen-CD86=0.0079; *Pliver-CD80=0.0485; *Pliver-CD86=0.0489). (C, D) 18β-GA reduced the platelet retention in spleens and livers in the ITP mice model. Statistical results were calculated as the total radiant efficiency (TRE) in the region of interest (ROI) in the 18β-GA group or the group treated with dimethylsulfoxide (DMSO) divided by the TRE in the ROI in the negative control group. a: DMSO group; b: 18β-GA group; c: negative control group. The TRE of ROI in the 18β-GA group was lower than that in the DMSO group, n=3 for each group. Differences between two groups were determined by an unpaired t test. (E) Decreased CD80 and CD86 expression on day 35 on F4/80+ macrophages in the spleen and liver of ITP mice treated with 18β-GA or lycopene, n=4 for each group. a: control; b: 18β-GA; c: lycopene; d: 18β-GA+lycopene. Statistical significance among groups was determined by one-way analysis of variance (ANOVA). *P<0.05, **P<0.01. (F) 18β-GA and lycopene both attenuated the phagocytic indices on day 35 in spleen and liver of ITP mice, n=4 for each group. a: control; b: 18β-GA; c: lycopene; d: 18β-GA+lycopene. Statistical significance among groups was determined by one-way ANOVA. *P<0.05, **P<0.01.
Figure 8.
18β-GA alleviated the phagocytosis of macrophages in patients with immune thrombocytopenia. (A) Representative plots of intracellular fluorescence of monocyte-derived macrophages in patients with immune thrombocytopenia (ITP) after treatment with DMSO or 18β-GA. Intracellular 5-chloromethylfluorescein diacetate (CMFDA) fluorescence-positive scatters indicate platelets phagocytosed by monocyte-derived macrophages. CD61+ scatters indicate adhered but not-phagocytosed platelets. (B) 18β-GA (25 mM) inhibited phagocytosis of monocyte-derived macrophages from ITP patients towards anti-human CD41 antibody-coated platelets in vitro. Wilcoxon matched-pairs signed rank test, n=8, *P=0.0234. (C) HMGB1 shRNA transfection in monocyte-derived macrophages. HMGB1 shRNA transfection successfully silenced the HMGB1 gene in monocyte-derived macrophages from ITP patients after 72 hours; the knockdown efficiency of the HMGB1 gene was ≥81%, as determined by real-time quantitative polymerase chain reaction (paired t test, n=6, ***P=0.0003). (D) 18β-GA reduced phagocytic indices of monocyte-derived macrophages by blocking HMGB1 in ITP. Statistical significance among groups was determined by one-way analysis of variance, n=6, **P=0.0037. Multiple comparisons: *Pab=0.0457; *Pac=0.0107. (E) Microscopy images comparing phagocytosis towards CMFDA-labeled CD41-opsonized platelets by monocyte-derived macrophages of ITP patients in the presence of DMSO or 18β-GA. Bound but external platelets (CMFDA+APC-CD61: green and red) were distinguished from internalized platelets (CMFDA: green) using APC-anti human-CD61 antibody. Cell nuclei were labeled with DAPI (blue). Ratio of endocytosed platelets to macrophages: the numbers of endocytic platelets and macrophages were calculated by Imagepro Plus 6.0 software, under 200× magnification. Differences between two groups were determined by a paired t test, n=4. (F) Diagram to show the effect of 18β-GA acting on Treg and macrophages via HMGB1 signaling. Mϕ: macrophages; ns: not significant.
In addition, 18β-GA reduced the phagocytosis of macrophages towards autologous platelets in ITP. 18β-GA down-regulated CD80/CD86 expression and attenuated phagocytosis of macrophages in ITP. Zhao et al. demonstrated that TNF-a blockade corrected the number and function of monocytes and macrophages, leading to remarkable attenuation of antibody-mediated platelet destruction in ITP.38 Our in vivo studies indicated that the level of TNF-a also decreased after 18β-GA treatment. Liu et al. showed that a shift in the balance of FcγR toward inhibitory FcγRIIb on monocytes was accompanied by a considerable decrease in monocyte/macrophage phagocytic capacity in ITP.2,8 Zhao et al. found that low-dose chidamide, a type of histone deacetylase inhibitor, restores immune balance in ITP by attenuating macrophage phagocytosis of antibody-coated platelets.7 Miao et al. reported that nuclear HMGB1 promotes the phagocytic ability of macrophages.39,40 Our study also indicated that 18β-GA reduced phagocytosis of macrophages by blocking HMGB1 in ITP.
The active ITP murine model is currently the optimal model for mimicking human, chronic ITP as it encompasses both antibody-mediated and cell-mediated platelet and megakaryocyte destruction.28 We performed cell-depletion studies and found that 18β-GA was still capable of raising platelet counts after CD19+ B-cell and CD8+ T-cell depletion in ITP mice, indicating that 18β-GA has therapeutic potential in patients with either cell-mediated or antibody-mediated thrombocytopenia. However, the use of licorice and its derivatives in ITP might have potential adverse risks, such as headache, and edema.18 The immune modulating potency of 18β-GA in ITP is related to downregulation of HMGB1 expression, as shown by lentivirus interference tests and pharmacological use of rhHMGB1 in vitro, as well as lycopene treatment in ITP mice. GA is directly combined with HMGB1, quenching the physiological activity of HMGB1 for therapeutic purposes.41 HMGB1, a recently discovered cytokine of interest that mediates the response to infection, injury, inflammation and immunoregulation, has become a prospective therapeutic target in several pathological conditions. HMGB1 could exacerbate the imbalance of peripheral immune cell subsets. Li et al. showed that enriched HMGB1 in patients with chronic hepatitis B shifted the Treg/Th17 balance to Th17 dominance via the TLR4-IL-6 pathway.10 Administration of recombinant mouse HMGB1 aggravated airway inflammation and induced Th2, Th17 polarization in asthmatic mice, and HMGB1 could directly induce differentiation of Th2, Th17 cells in vitro through activating the Toll-like receptor (TLR)2, TLR4, receptor for advanced glycation end products (RAGE)-NF-κB signal pathway in CD4 naïve T cells as Li et al. reported.42 Importantly, HMGB1 stimulation can result in marked downregulated expression of CTLA-4 as well as Foxp3 expression and secretion of IL-10 from splenic Treg in mice.11 It has been suggested that effectively inhibiting HMGB1 expression could be a feasible way to treat liver failure, by enhancing Treg activity in patients with chronic hepatitis B.43 We found that, compared with controls, there was increased expression of HMGB1 in plasma and CD4+ T cells of ITP patients, as well as in plasma of ITP mice. The immune regulatory function of 18β-GA could be apparently attenuated by the addition of rhHMGB1 in vitro. The lentivirus interference test showed that HMGB1 is key to the effect of 18β-GA in Treg of ITP. Inhibition of lycopene in a murine model of active ITP had similar effects as those of 18β-GA. A negative correlation between plasma level of HMGB1 and baseline platelet count was documented in ITP patients, and upregulated Treg and downregulated HMGB1 levels were observed together with elevation of platelet count in treated ITP mice. Therefore, HMGB1 has potential values in the diagnosis and management of ITP. Its levels may be indicative of disease severity and prognosis of ITP to a certain extent.
Treg expansion was weakened with ITP-HMGB1 in comparison to HC-HMGB1, suggesting that the redox state of HMGB1 is responsible for immune balance in ITP. Furthermore, wildtype and fully reduced HMGB1 inhibited the proliferation of Treg, whereas both signals were turned off when HMGB1 was oxidized. Extracellular HMGB1 can act both as a chemokine and as a pro-inflammatory mediator, the latter effect depending on the redox state of three cysteines: C23 and C45 must form a disulfide bond within the first HMG-box domain of HMGB1 Box A, whereas the unpaired C106 within Box B must be in the thiol state.44 The cytokine-stimulating and chemotactic activities of HMGB1 are mutually exclusive. In contrast, HMGB1 terminally oxidized to sulfonates potentially induces immune tolerance and upregulates FoxP3 and CD206.45 However, whether ITP-frHMGB1 is partially oxidized and converted into disulfide HMGB1, which plays a pro-inflammatory role, needs further study. It has been reported that glycyrrhizin attenuated disulfide-HMGB1-induced depressive behaviour.46 Consistently, our results showed that the combination of ITP-HMGB1 and 18β-GA expanded the proportion of Treg, compared with HMGB1 alone. Previous literature described that the binding site of glycyrrhizin to HMGB1 covers specific region on the Cys-23 and Cys-45 side chains, blocking oxidant accessibility to the thiol groups, which protects them from oxidants.47
In addition, as a late inflammatory mediator, HMGB1 responds to the early inflammatory mediator TNF-a, prolonging inflammatory responses during acute liver failure and acute kidney injury; however, glycyrrhizin inhibited pyroptosis in TNF-induced M1 macrophages by inhibition of HMGB1. 48 Glycyrrhizin significantly reduced the degree of ferroptosis during acute liver failure by suppressing oxidative stress via HMGB1 inhibition.49 As 18β-GA has comprehensive effects involving inflammation, oxidation, etc. in other disease settings, as mentioned above,48,49 it is worth exploring whether these mechanisms are involved in immune modulation targeting the upstream pathogenesis of ITP in future studies.
Our results have shown that the phosphorylation of NFKB decreased in CD4+ T cells after 18β-GA treatment. In previous studies, HMGB1 was found to bind to cell surface receptors once released from cells. Classic HMGB1 receptors include the RAGE, TLR2 and TLR4, and NF-KB is a very important downstream factor of these signaling pathways.32,50 Signaling through RAGE leads to activation of NFKB, as well as to signal transduction through ERK and p38, which promotes the production of cytokines (TNF, IL-6 and IFN-y). HMGB1-dependent activation of TLR2 and TLR4 leads to NF-KB activation through a MyD88-dependent mechanism. Treg selectively expressed several members of the TLR family. TLR ligands can directly modulate the suppressive capacity of Treg.51 Our results provide evidence that 18β-GA inhibits the expression of HMGB1 and phosphorylation of NF-KB, and both restores the immunosuppressive function of Treg and induces their proliferation in ITP. Whether this is related to TLR ligands in ITP remains to be studied further. Treatment of HeLa cells with glycyrrhizin derivatives resulted in enhanced phosphorylation and acquisition of DNA-binding ability of HSF1.52 It has been reported that HSF1 binds directly with the HMGB1 promoter and negatively regulates HMGB1, involving the TLR4/MyD88/NF-KB signal pathway in asthma.53 HSF1 inhibits H2O2-induced cardiomyocyte death through suppression of HMGB1.54 Our study also proved that 18β-GA inhibited the mRNA expression of HMGB1 via phosphorylation of HSF1.
Abnormal cytokine profiles are closely associated with immune imbalance in ITP, and therapeutic options for ITP are associated with correction of cytokine abnormalities. Consistent with the imbalance of Th1/Th2 and Th17/Treg subsets, the levels of IFN-y and IL-17a were increased, whereas IL-4 and IL-10 levels were decreased in ITP. Other cytokines, including IL-6, TGF-β, and TNF-a, have also been described to be involved in ITP. In our study, TGF-β was significantly elevated by 18β-GA. However, the levels of TNF-a, IFN-y, IL-6 and IL-12 reduced after 18β-GA administration. These data are consistent with the restoration of immune homeostasis in ITP.
Loss of immune homeostasis is a major characteristic of ITP, and restoring the immune balance is the top priority. 18β-GA promoted immune balance in ITP by stimulating Treg proliferation and alleviating phagocytosis of monocytes and macrophages. Although the precise mechanism of the interaction between HMGB1 and Treg remains to be elucidated, HMGB1 is emerging as a novel and potential immunoregulatory checkpoint offering new strategies for immune-therapeutics of ITP. This provides a promising therapeutic option in newly diagnosed ITP patients. Large-scale prospective randomized clinical trials, including long-term follow-up and analysis of sustained response, are needed to validate our findings in the future.
Supplementary Material
Acknowledgments
We would like to thank Alexandra H. Marshall (Marshall Medical Communications) and Editage (www.editage.com) for English language editing.
Funding Statement
Funding: This work was supported by grants from the National Natural Science Foundation of China (n. 81900121, n. 81973994, n. 81900123); Major Research and Development Plan of Shandong Province (2021LCZX05); Young Taishan Scholar Foundation of Shandong Province (n. tsqn201909175); Natural Science Foundation for Distinguished Young Scholars of Shandong Province (ZR2021JQ28); Clinical Research Center of Shandong University (n. 2020SDUCRCC009); and the Graduate Education Reform Project of Shandong University (n. XYJG2020141).
References
- 1.McKenzie CG, Guo L, Freedman J, Semple JW. Cellular immune dysfunction in immune thrombocytopenia (ITP). Br J Haematol 2013;163(1):10-23. [DOI] [PubMed] [Google Scholar]
- 2.Liu XG, Ma SH, Sun JZ, et al. High-dose dexamethasone shifts the balance of stimulatory and inhibitory Fcgamma receptors on monocytes in patients with primary immune thrombocytopenia. Blood. 2011;117(6):2061-2069. [DOI] [PubMed] [Google Scholar]
- 3.Stasi R, Cooper N, Del Poeta G, et al. Analysis of regulatory T-cell changes in patients with idiopathic thrombocytopenic purpura receiving B cell-depleting therapy with rituximab. Blood. 2008;112(4):1147-1150. [DOI] [PubMed] [Google Scholar]
- 4.Bao W, Bussel JB, Heck S, et al. Improved regulatory T-cell activity in patients with chronic immune thrombocytopenia treated with thrombopoietic agents. Blood. 2010;116(22):4639-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aslam R, Hu Y, Gebremeskel S, et al. Thymic retention of CD4+CD25+FoxP3+ T regulatory cells is associated with their peripheral deficiency and thrombocytopenia in a murine model of immune thrombocytopenia. Blood. 2012;120(10):2127-2132. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Wang Z, Hu S, Zhao X, Cao L. Correction of abnormal T cell subsets by high-dose dexamethasone in patients with chronic idiopathic thrombocytopenic purpura. Immunol Lett. 2013;154(1-2):42-48. [DOI] [PubMed] [Google Scholar]
- 7.Zhao HY, Ma YH, Li DQ, et al. Low-dose chidamide restores immune tolerance in ITP in mice and humans. Blood. 2019;133(7):730-742. [DOI] [PubMed] [Google Scholar]
- 8.Liu XG, Liu S, Feng Q, et al. Thrombopoietin receptor agonists shift the balance of Fcγ receptors toward inhibitory receptor IIb on monocytes in ITP. Blood. 2016;128(6):852-861. [DOI] [PubMed] [Google Scholar]
- 9.Nagelkerke SQ, Dekkers G, Kustiawan I, et al. Inhibition of FcγR-mediated phagocytosis by IVIg is independent of IgG-Fc sialylation and FcγRIIb in human macrophages. Blood. 2014;124(25):3709-3718. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Wang FP, She WM, et al. Enhanced high-mobility group box 1 (HMGB1) modulates regulatory T cells (Treg)/T helper 17 (Th17) balance via toll-like receptor (TLR)-4-interleukin (IL)-6 pathway in patients with chronic hepatitis B. J Viral Hepat. 2014;21(2):129-140. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Yao YM, Huang LF, et al. The potential effect and mechanism of high-mobility group box 1 protein on regulatory T cell-mediated immunosuppression. J Interferon Cytokine Res. 2011;31(2):249-257. [DOI] [PubMed] [Google Scholar]
- 12.Luo C, Liu H, Wang H, Wang J. Toll-like receptor 4 signaling in high mobility group box-1 protein 1 mediated the suppression of regulatory T-cells. Med Sci Monit. 2017;23:300-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gowda P, Patrick S, Joshi SD, Kumawat RK, Sen E. Glycyrrhizin prevents SARS-CoV-2 S1 and Orf3a induced high mobility group box 1 (HMGB1) release and inhibits viral replication. Cytokine. 2021;142:155496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang PF, Zhang GY, Liu HY, et al. [Expression of HMGB1 in spleen of adult patients with chronic and refractory immune thrombocytopenia and its significance]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2018;26(2):516-521. [DOI] [PubMed] [Google Scholar]
- 15.Wang R, Cao Q, Bai ST, Wang L, Sheng GY. Potential role and mechanism for high mobility group box1 in childhood chronic immune thrombocytopenia. Eur Rev Med Pharmacol Sci. 2019;23(24):10931-10941. [DOI] [PubMed] [Google Scholar]
- 16.Zhang G, Yang P, Liu X, et al. HMGB1 is increased in patients with immune thrombocytopenia and negatively associates with Tregs. Thromb Res. 2022;213:128-136. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi H, Kidachi Y, Kamiie K, Noshita T, Umetsu H. Structural insight into the ligand-receptor interaction between glycyrrhetinic acid (GA) and the high-mobility group protein B1 (HMGB1)-DNA complex. Bioinformation. 2012;8(23):1147-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li JY, Cao HY, Liu P, Cheng GH, Sun MY. Glycyrrhizic acid in the treatment of liver diseases: literature review. Biomed Res Int. 2014;2014:872139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavone L, Muzzi M, Mencucci R, et al. 18β-glycyrrhetic acid inhibits immune activation triggered by HMGB1, a pro-inflammatory protein found in the tear fluid during conjunctivitis and blepharitis. Ocul Immunol Inflamm. 2011;19(3):180-185. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Zhao Y, An N, et al. 18β-glycyrrhetic acid modulates Th1/Th17/Th22/regulatory T cells homeostasis via HMGB1/NF-κB signaling pathway in immune thrombocytopenia. Blood. 2018;132(Suppl 1):1144. [Google Scholar]
- 21.Ukil A, Biswas A, Das T, Das PK. 18 Beta-glycyrrhetinic acid triggers curative Th1 response and nitric oxide up-regulation in experimental visceral leishmaniasis associated with the activation of NF-kappa B. J Immunol. 2005;175(2):1161-1169. [DOI] [PubMed] [Google Scholar]
- 22.Kim SH, Hong JH, Lee JE, Lee YC. 18beta-glycyrrhetinic acid, the major bioactive component of Glycyrrhizae radix, attenuates airway inflammation by modulating Th2 cytokines, GATA-3, STAT6, and Foxp3 transcription factors in an asthmatic mouse model. Environ Toxicol Pharmacol. 2017;52:99-113. [DOI] [PubMed] [Google Scholar]
- 23.Endong L, Shijie J, Sonobe Y, et al. The gap-junction inhibitor carbenoxolone suppresses the differentiation of Th17 cells through inhibition of IL-23 expression in antigen presenting cells. J Neuroimmunol. 2011;240-241:58-64. [DOI] [PubMed] [Google Scholar]
- 24.Kuang P, Zhao W, Su W, et al. 18beta-glycyrrhetinic acid inhibits hepatocellular carcinoma development by reversing hepatic stellate cell-mediated immunosuppression in mice. Int J Cancer 2013;132(8):1831-1841. [DOI] [PubMed] [Google Scholar]
- 25.Kowalska A, Kalinowska-Lis U. 18β-glycyrrhetinic acid: its core biological properties and dermatological applications. Int J Cosmet Sci. 2019;41(4):325-331. [DOI] [PubMed] [Google Scholar]
- 26.Luo YG, Liu YQ, Hu J. [Clinical study on effect of recombinant roasted licorice decoction combined with low-dose glucocorticoids in treating idiopathic thrombocytopenic purpura]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2001;21(7):501-503. [PubMed] [Google Scholar]
- 27.Neunert C, Lim W, Crowther M, et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117(16):4190-4207. [DOI] [PubMed] [Google Scholar]
- 28.Chow L, Aslam R, Speck ER, et al. A murine model of severe immune thrombocytopenia is induced by antibody- and CD8+ T cell-mediated responses that are differentially sensitive to therapy. Blood. 2010;115(6):1247-1253. [DOI] [PubMed] [Google Scholar]
- 29.Shen S, Zhou M, Huang K, et al. Blocking autophagy enhances the apoptotic effect of 18beta-glycyrrhetinic acid on human sarcoma cells via endoplasmic reticulum stress and JNK activation. Cell Death Dis. 2017;8(9):e3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu CB, Wang R, Yi YF, Gao Z, Chen YZ. Lycopene mitigates β-amyloid induced inflammatory response and inhibits NF-κB signaling at the choroid plexus in early stages of Alzheimer's disease rats. J Nutr Biochem. 2018;53:66-71. [DOI] [PubMed] [Google Scholar]
- 31.Evankovich J, Cho SW, Zhang R, et al. High mobility group box 1 release from hepatocytes during ischemia and reperfusion injury is mediated by decreased histone deacetylase activity. J Biol Chem. 2010;285(51):39888-39897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5(4):331-342. [DOI] [PubMed] [Google Scholar]
- 33.Musumeci D, Roviello GN, Montesarchio D. An overview on HMGB1 inhibitors as potential therapeutic agents in HMGB1-related pathologies. Pharmacol Ther. 2014;141(3):347-357. [DOI] [PubMed] [Google Scholar]
- 34.Chen H, Liu H, Tang B, et al. The protective effects of 18β-glycyrrhetinic acid on imiquimod-induced psoriasis in mice via suppression of mTOR/STAT3 signaling. J Immunol Res. 2020;2020:1980456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y, Hu Z, Li R, et al. The effects of carbenoxolone against experimental autoimmune encephalomyelitis in a mouse model. Neuroimmunomodulation. 2020;27(1):19-27. [DOI] [PubMed] [Google Scholar]
- 36.Guo L, Shen S, Rowley JW, et al. Platelet MHC class I mediates CD8+ T-cell suppression during sepsis. Blood. 2021;138(5):401-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo L, Yang L, Speck ER, et al. Allogeneic platelet transfusions prevent murine T-cell-mediated immune thrombocytopenia. Blood. 2014;123(3):422-427. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Y, Xu P, Guo L, et al. Tumor necrosis factor-a blockade corrects monocyte/macrophage imbalance in primary immune thrombocytopenia. Thromb Haemost. 2021;121(6):767-781. [DOI] [PubMed] [Google Scholar]
- 39.Miao J, Ye S, Lan J, et al. Nuclear HMGB1 promotes the phagocytic ability of macrophages. Exper Cell Res. 2020;393(1):112037. [DOI] [PubMed] [Google Scholar]
- 40.Miao J, Ye P, Lan J, et al. Paeonol promotes the phagocytic ability of macrophages through confining HMGB1 to the nucleus. Int Immunopharmacol. 2020;89(Pt B):107068. [DOI] [PubMed] [Google Scholar]
- 41.Bailly C, Vergoten G. Glycyrrhizin: an alternative drug for the treatment of COVID-19 infection and the associated respiratory syndrome? Pharmacol Ther 2020;214:107618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li R, Wang J, Zhu F, et al. HMGB1 regulates T helper 2 and T helper17 cell differentiation both directly and indirectly in asthmatic mice. Mol Immunol. 2018;97:45-55. [DOI] [PubMed] [Google Scholar]
- 43.Wang LW, Chen H, Gong ZJ. High mobility group box-1 protein inhibits regulatory T cell immune activity in liver failure in patients with chronic hepatitis B. Hepatobiliary Pancreat Dis Int 2010;9(5):499-507. [PubMed] [Google Scholar]
- 44.Yang H, Lundbäck P, Ottosson L, et al. Redox modifications of cysteine residues regulate the cytokine activity of HMGB1. Mol Med. 2021;27(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hubert P, Roncarati P, Demoulin S, et al. Extracellular HMGB1 blockade inhibits tumor growth through profoundly remodeling immune microenvironment and enhances checkpoint inhibitor-based immunotherapy. J Immunother Cancer. 2021;9(3):e001966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lian YJ, Gong H, Wu TY, et al. Ds-HMGB1 and fr-HMGB induce depressive behavior through neuroinflammation in contrast to nonoxid-HMGB1. Brain Behav Immun. 2017;59:322-332. [DOI] [PubMed] [Google Scholar]
- 47.Mollica L, De Marchis F, Spitaleri A, et al. Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem Biol. 2007;14(4):431-441. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y, Zhang H, Chen Q, et al. TNF-a/HMGB1 inflammation signalling pathway regulates pyroptosis during liver failure and acute kidney injury. Cell Prolif. 2020;53(6):e12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y, Chen Q, Shi C, Jiao F, Gong Z. Mechanism of glycyrrhizin on ferroptosis during acute liver failure by inhibiting oxidative stress. Mol Med Rep. 2019;20(5):4081-4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan S, Liu Z, Xu Z, Liu J, Zhang J. High mobility group box 1 (HMGB1): a pivotal regulator of hematopoietic malignancies. J Hematol Oncol. 2020;13(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lewkowicz P, Lewkowicz N, Sasiak A, Tchórzewski H. Lipopolysaccharide-activated CD4+CD25+ T regulatory cells inhibit neutrophil function and promote their apoptosis and death. J Immunol. 2006;177(10):7155-7163. [DOI] [PubMed] [Google Scholar]
- 52.Kawashima D, Asai M, Katagiri K, Takeuchi R, Ohtsuka K. Reinvestigation of the effect of carbenoxolone on the induction of heat shock proteins. Cell Stress Chaperones. 2009;14(5):535-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shang L, Wang L, Shi X, et al. HMGB1 was negatively regulated by HSF1 and mediated the TLR4/MyD88/NF-κB signal pathway in asthma. Life Sci. 2020;241:117120. [DOI] [PubMed] [Google Scholar]
- 54.Yu Y, Liu M, Zhang L, et al. Heat shock transcription factor 1 inhibits H2O2-induced cardiomyocyte death through suppression of high-mobility group box 1. Mol Cell Biochem. 2012;364(1-2):263-269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.