Abstract
The term “pulmonary renal syndrome” describes a clinical syndrome which is characterised by the presence of both diffuse alveolar haemorrhage and glomerulonephritis. It encompasses a group of diseases with distinctive clinical and radiological manifestations, as well as different pathophysiological processes. The most common diseases implicated are anti-neutrophil cytoplasm antibodies (ANCA)-positive small vessel vasculitis and anti-glomerular basement membrane (anti-GBM) disease. Prompt recognition is required as respiratory failure and end-stage renal failure can rapidly occur. Treatment includes a combination of glucocorticoids, immunosuppression, plasmapheresis and supportive measures. The use of targeted treatments has significantly reduced mortality. Thus, an understanding of pulmonary renal syndrome is essential for the respiratory physician.
Short abstract
Pulmonary renal syndrome is the combination of rapidly progressive glomerulonephritis (RPGN) and diffuse alveolar haemorrhage (DAH). Morbidity and mortality are high so prompt diagnosis and intervention are key for the respiratory physician. https://bit.ly/3UFAmR9
Educational aims
To gain an understanding of current knowledge on pulmonary renal syndrome.
To acquire an up-to-date method for approaching the workup of a patient with suspected pulmonary renal syndrome.
Introduction
Pulmonary renal syndrome is a potentially life-threatening condition defined as the combination of diffuse alveolar haemorrhage (DAH) and rapidly progressive glomerulonephritis (RPGN). It was first described by Goodpasture in 1919 [1]. The term Goodpasture's syndrome was adopted in 1958 to define a group of patients with similar characteristics of idiopathic pulmonary haemorrhage and glomerulonephritis [2]. The pathogenic role of the anti-glomerular basement membrane (anti-GBM) antibody in some of these cases was discovered 10 years later [3]. When it became clear that several different pathogenic mechanisms could lead to this clinical syndrome, the eponymous “Goodpasture's syndrome” was abandoned and pulmonary renal syndrome was introduced [4].
Pulmonary renal syndrome can be caused by many systemic autoimmune conditions with anti-neutrophil cytoplasm antibodies (ANCA)-associated vasculitis accounting for most cases. A significant number of patients present with rapidly progressive respiratory and/or renal failure and often need admission to the intensive care unit (ICU) for ongoing management. With recent advances in treatment, specifically the introduction of novel immunosuppression, mortality rates have improved but remain high with some reporting mortality rates of up to 50% [5].
Our aim is to provide an in-depth overview of pulmonary renal syndrome for the respiratory physician, focusing on treatment innovations.
Epidemiology and pathophysiology
Pulmonary renal syndrome is associated with several diseases. They can broadly be divided into ANCA-associated vasculitis (AAV) and immune complex-mediated vasculitis (table 1). AAV is the most common underlying cause, accounting for ∼70% of cases. Anti-GBM disease, an immune complex vasculitis, accounts for up to 20% of cases with the remaining 10% of cases attributable to less common conditions [6].
TABLE 1.
Differential diagnoses of pulmonary renal syndrome
| ANCA-associated vasculitis | Granulomatosis with polyangiitis (GPA) |
| Microscopic polyangiitis (MPA) | |
| Eosinophilic granulomatosis with polyangiitis (EGPA) | |
| Anti-GBM disease | Anti-GBM disease |
| ANCA-negative vasculitis | IgA disease |
| Cryoglobulinaemia | |
| Drug-induced vasculitis | Cocaine |
| d-penicillamine | |
| Autoimmune connective tissue disease | Systemic lupus erythematosus (SLE) |
| Polymyositis | |
| Diabetes mellitus | |
| Mixed connective tissue disease (MCTD) | |
| Systemic sclerosis |
Classifying the differential diagnoses of pulmonary renal syndromes into groups based on the underlying pathological process. ANCA: anti-neutrophil cytoplasm antibodies; GBM: glomerular basement membrane.
The specific pathological process depends on the underlying disease. In the majority of pulmonary renal syndromes, small vessel vasculitis affecting the alveoli and glomeruli is responsible [7, 8]. Inflammation arises through neutrophilic infiltration of the vascular endothelium, which affects the arterioles, venules and capillaries resulting in vessel wall destruction and necrosis. Necrosis can be fibrinoid or granulomatous in nature [9].
In the lung, in addition to the small vessel vasculitis and resultant necrosis, a distinct process has been identified within the alveolar wall/interstitium called necrotising pulmonary capillaritis. This can occur with other features of vasculitis as described but also in isolation. It can be distinguished by the marked influx of interstitial neutrophils which are undergoing leukocytoclasis or fragmentation. Pyknotic cells and nuclear dust accumulates within the lung parenchyma as these neutrophils are constantly undergoing apoptosis. The interstitium fills with these neutrophils, oedema and fibrin thrombi, and eventually undergoes fibrinoid necrosis. The integrity of interstitial capillaries is damaged during this process, allowing red blood cells to cross the now incompetent alveolar capillary basement membranes, entering the interstitial space and flooding the alveoli [10, 11].
In the kidney, fibrinoid deposition causes crescentic inflammation in the glomerulus, where inflammatory cells infiltrate Bowman's space with epithelial cell hyperplasia and fibrosis [12].
AAV
Most cases of pulmonary renal syndrome are caused by AAV and can be identified by ANCA antibody testing. AAVs include microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA) and eosinophilic GPA (EGPA). Serology is useful in differentiating between these, as the clinical and pathological differences are often subtle [8]. Up to 95% of patients with AAV have detectable ANCA on serum testing [13]. ANCA can be detected via indirect immunofluorescence and ELISA. A positive ANCA test can support a diagnosis of GPA, MPA or EGPA [14]. Antibodies can be directed against proteinase-3 (PR3) or myeloperoxidase (MPO) [15].
The incidence of GPA has been estimated globally between 0.5 and 20 cases per million [16–18]. The mean age at onset is 40–55 years [19]. It is more common in Caucasians [20], with a higher incidence in Europe and Australia [21]. Clinically, a triad of granulomatous inflammation of the respiratory tract, necrotising vasculitis and glomerulonephritis is present. Both the upper and lower respiratory tract can be affected. Upper respiratory tract manifestations include sinusitis, oral ulcerations, and subglottic or bronchial stenosis. Lower respiratory tract manifestations include cough, haemoptysis, chest pain and dyspnoea [22]. DAH is rare but when it does occur, it is typically subclinical and recurrent [23] and associated with renal involvement [24, 25].
The incidence of MPA is estimated at 2.7 to 24 cases per million [18, 26, 27]. The clinical features are similar to those seen in GPA, with the exception of granulomas. Skin lesions and arthralgias are common [10] and up to 80% of patients develop necrotic glomerulonephritis. DAH is seen in 30–50% of patients and it is frequently severe and life-threatening [23].
EGPA is a rare disorder with an incidence of less than 3 cases per million [8, 28]. Men are twice as likely to be affected as women with a typical age at onset of 40–60 years old [8]. Patients typically present with asthma and sinusitis initially with progression into an eosinophilia and vasculitis [29]. Eosinophilic infiltration can cause end-organ damage, such as diastolic cardiomyopathy, which is associated with a high mortality rate [30]. Renal involvement is usually mild in nature [10]. A positive ANCA is seen in 30–50% of cases and is associated with a more severe disease with renal disease, DAH and central nervous systemic involvement frequently reported. Overall, DAH is seen less frequently than in other AAVs [8].
Anti-GBM disease
Anti-GBM disease or Goodpasture's syndrome is rare. The incidence is estimated at 1 per million [9]. It is four times more common in men. Disease onset typically occurs between 20 and 30 years of age [8], and it is more common in Caucasian patients [9]. It is not a smoking-related disease, but it has been reported that smokers are more likely to develop DAH in the context of a diagnosis of anti-GBM disease with the leading hypothesis of increased alveolar permeability with smoking [23, 31].
Anti-GBM disease is caused by antibodies which are directed against type 4 collagen, a component of the glomerular basement membrane. The antibodies bind to the basement membrane activating a cascade which results in the disruption of Bowman's capsule with resultant proteinuria and crescent formation. There is an association with human leukocyte antigen (HLA) alleles, particularly HLA DRB1 and HLA DRW2 [32, 33]. However, most cases occur sporadically; suggesting that environmental factors may play a role [34].
Most patients present with symptoms and/or signs related to glomerulonephritis and DAH. Clinical onset has been associated with smoking and respiratory tract infections [31, 35]. The detected antibody level correlates with the severity of renal disease [3] and those with concurrent DAH tend to have more severe renal injury than those without DAH [36].
AAV and anti-GBM overlap
“Double positive disease” has been reported where both anti-GBM and ANCA antibodies are detected. The clinical significance is unclear, but it is hypothesised that ANCA-induced glomerular inflammation may expose disease epitopes that trigger an anti-GBM response [37, 38]. Up to 14% of patients with ANCA antibody positivity have detectable anti-GBM antibodies and 30% of patients with anti-GBM antibodies are ANCA positive [6]. MPO-ANCA antibodies are more common than PR3-ANCA [39]. The typical age of onset is later in life (median age of 62 years), in keeping with the demographics of AAV [40]. Clinical presentation is more in keeping with that seen in anti-GBM disease with prevalent renal involvement [23, 41]. There is an inverse relationship between renal recovery and titres of antibodies [42]. Patients typically have a significant risk of relapse compared with single-positive disease [37].
ANCA-negative diseases
Pulmonary renal syndrome is very rare in ANCA-negative systemic vasculitis, but has been described in a number of diseases.
Systemic lupus erythematosus (SLE)
SLE is responsible for 80% of the immune complex-mediated diseases that cause pulmonary renal syndrome. It is caused by antibodies directed against double-stranded DNA (dsDNA) resulting in the formation and deposition of immune complexes in the glomerulus and activation of a pro-inflammatory cascade [8]. Pulmonary and pleural disease manifest in up to 70% of patients with SLE [43]; however, pulmonary renal syndrome is rare, reported in 2–4% of cases [9]. DAH is associated with a 50% mortality rate when present [44]. It is unusual for DAH to be the presenting feature as most patients have established lupus nephritis prior to DAH [45]. DAH should be distinguished from acute lupus pneumonitis, which presents similarly and is frequently the initial presentation of SLE [23].
Antiphospholipid syndrome (APS)
APS is caused by antibodies that target phospholipids inhibiting activated protein C, antithrombin 3 and fibrinolysis, thus creating a pro-coagulant state [9]. It is associated with thrombosis, valvular heart disease, cognitive dysfunction and fetal loss. It is more common in men and patients present between the ages of 40 and 60 years old [46]. It can be associated with other autoimmune conditions, classically SLE. Catastrophic APS is an accelerated form of the disease, developing over days or weeks which is characterised by widespread thromboses in small and large vessels within at least three organ systems [47]. While renal disease occurs in <10% of patients with primary APS, it is common in catastrophic APS where it often presents with DAH and is associated with a high mortality [48].
Thrombotic thrombocytopenia purpura (TTP)
Pulmonary renal syndrome has been described in TTP. The classical presentation is with thrombocytopenia, microangiopathic haemolytic anaemia and ischaemic manifestations. Studies suggest that the insufficiency of ADAM metallopeptidase with thrombospondin type 1 motif 13 (ADAMTS-13), a metalloprotease responsible for cleaving von Willebrand factor, leads to platelet aggregation in the microcirculation. Pregnancy, neoplasm and chemotherapy are considered pre-disposing factors [49].
IgA vasculitis
IgA vasculitis is a small vessel vasculitis commonly associated with IgA nephropathy. It is more common in younger patients, with 90% of cases reported in children <10 years old. Clinical presentation consists of a triad of abdominal pain, purpuric rash and arthritis. DAH is a rare complication more commonly seen in adults, and occurs when IgA immune complexes are deposited within the alveoli leading to haemorrhage [8].
Cryoglobulinaemia
Cryoglobulinaemia is a rare cause of pulmonary renal syndrome. Monoclonal antibody production causes abnormal immunoglobulins to precipitate at lower temperatures leading to an immune complex-mediated vasculitis. It is frequently associated with chronic hepatitis C viral infection but a similar presentation can occur with malignancies, such as lymphoma, so a careful workup is needed. Clinically a triad of arthralgia, purpura and neurological weakness is seen. In severe cases, alveolar haemorrhage is seen, with DAH reported in 3% of cases. Renal involvement is more common with over 90% of patients developing some degree of glomerulonephritis [8].
Drug-associated DAH
Drug-associated DAH is seen more commonly in young women. It presents similarly to MPA with skin involvement [30] and is associated with ANCA positivity. Implicated drugs include propylthiouracil, hydralazine, d-penicillamine, allopurinol, sulfasalazine and cocaine [9]. It generally has a more benign course than an ANCA-positive pulmonary renal syndrome. Cessation of the offending agent typically leads to disease regression [50].
Other causes
Pulmonary renal syndrome is a rare complication of systemic sclerosis. It is seen more often in patients with systemic sclerosis-related fibrotic lung disease [51]. 5% of patients with rheumatoid arthritis (RA) have rheumatoid vasculitis, where the clinical manifestations of both RA and vasculitis are seen. DAH is uncommon. Renal involvement is typically in the form of renal amyloidosis and glomerulonephritis [6, 52]. Mixed connective tissue disease is an autoimmune disease with clinical features of SLE and RA. Pulmonary renal syndrome is rare [53]. Post-streptococcal glomerulonephritis usually presents 10 days after a streptococcal pharyngitis. Pulmonary syndrome has been reported but is very rare [6]. DAH is a rare feature of polymyositis and dermatomyositis [53].
Clinical features
Clinical features vary depending on the underlying aetiology. However, DAH and glomerulonephritis are the unifying features. Haemoptysis is the most common manifestation of DAH, but it is absent in up to 30% of cases [54, 55]. Haemoptysis is usually mild but can be large volume. Other common symptoms include cough, dyspnoea and low-grade pyrexia. Acute respiratory failure requiring intubation occurs in ∼50% of cases [23]. DAH is more common in GPA (42% of cases), compared with MPA (29%) and EGPA (3%).
Glomerulonephritis should be suspected when haematuria, proteinuria and active urinary sediment are seen. It can progress to end-stage renal failure requiring renal replacement therapy [9].
Clinical features of pulmonary renal syndrome are nonspecific and, thus, a high index of suspicion is required. Pulmonary renal syndrome should be considered in those with bilateral pulmonary infiltrates, falling haemoglobin levels and renal failure. It should also be considered in patients with unexplained sinusitis, mononeuritis multiplex, polyarthralgia, asthma, pericarditis, cerebral ischaemia, purpura, and congestive heart failure [9].
Diagnostic evaluation
Establishing the diagnosis promptly is crucial as respiratory failure and end-stage renal failure can occur rapidly.
Radiology
Chest radiograph typical demonstrates bilateral airspace opacities, with or without air bronchograms. These are typically in a perihilar distribution, predominantly affecting the middle and lower zones; however, the chest radiograph can be normal in up to 25% of cases [8]. The typical features on chest computed tomography (CT) are mixed areas of consolidation and ground glass (figure 1). When interlobular septal thickening is present it is usually coarse. Cavitating nodules/masses can be seen in GPA, while airway wall thickening and small non-cavitating nodules are common in EGPA. Multiphase CT angiography can help localise an active bleeding source in large volume haemoptysis, and define the bronchial artery anatomy. Adenopathy is an uncommon finding and should prompt consideration of infection or malignancy if seen [56].
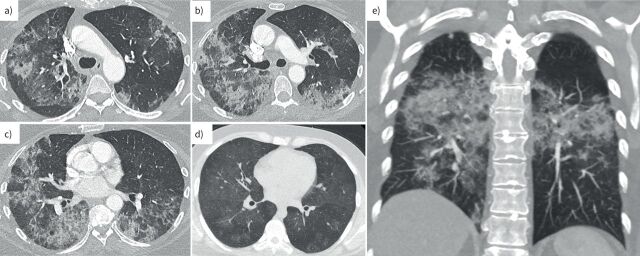
FIGURE 1.
High-resolution computed tomography (CT) images of the chest from patients with diffuse alveolar haemorrhage (DAH) in pulmonary renal syndrome. a–c) The axial CT slices show extensive bilateral mixed consolidative and ground-glass opacities with a mid to lower zone predominance admixed with coarsened interlobular septa, appearances typical for DAH. d) Milder disease is shown on an axial CT slice with patchy ground-glass opacities bilaterally. e) A coronal CT chest image highlights the mid to lower zone predominance in DAH.
Bronchoscopy
Bronchoscopy can be useful in diagnosing DAH. Bronchoalveolar lavage (BAL) fluid shows increasing blood-stained aspirates in sequential samples with haemosiderin-laden macrophages on cytology (figure 2). The upper limit of normal for haemosiderin-laden macrophages in BAL fluid is 5% [6]. Transbronchial biopsy may be considered for histology but often an alternate site, such as renal biopsy, may confer a lower risk. Bronchoscopy can also be valuable in excluding infection [9].
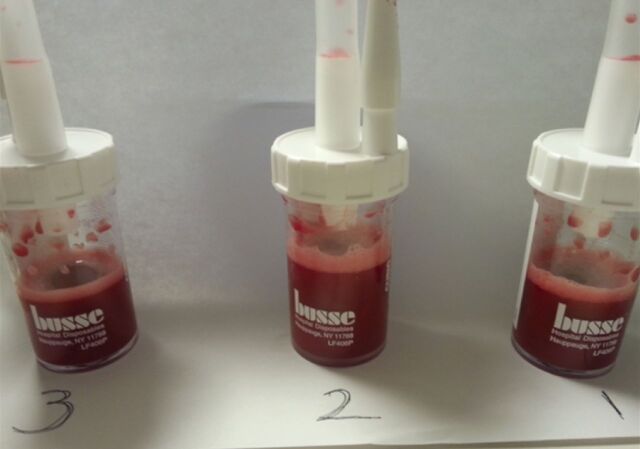
FIGURE 2.
Bronchoalveolar lavage (BAL) fluid specimens from a patient with diffuse alveolar haemorrhage (DAH) in pulmonary renal syndrome. Progressive haemorrhagic BAL can be noted in the serial samples (number 1 is the first BAL, number 2 is the second BAL, and number 3 is the third BAL).
Laboratory testing
A reduction in serum haemoglobin and haematocrit should raise suspicion for DAH especially when acute in nature. Typically, a normochromic normocytic anaemia is seen, which is out of proportion to the renal failure. An elevated urea and creatinine can signify acute renal injury suggestive of glomerulonephritis. Abnormal platelet count and coagulation studies may indicate a bleeding diathesis as a cause of DAH. Peripheral eosinophilia is suggestive of EGPA. A Coombs’ negative haemolytic anaemia with schistocytes or fragmented red cells with thrombocytopenia is suggestive of TTP. Respiratory viral and atypical bacteria PCR along with blood cultures should be evaluated to assess for an infectious process [57].
Urine analysis is essential; proteinuria is always present but rarely in the nephrotic range. Urinalysis can demonstrate dysmorphic red cells, red cell casts and fragments. Proteinuria is more common than haematuria; however, when both are present this is highly indicative of glomerulonephritis [58, 59].
Serology is useful in determining the cause; however, the pre-test probability is vital when it comes to the interpretation [8, 9, 12]. ANCA antibodies can be directed against PR3 and against MPO. GPA is more commonly associated with PR3-ANCA, whereas MPA is associated with MPO-ANCA [30]. EGPA is more often ANCA negative than positive [8, 9]. Anti-GBM disease is associated with anti-GBM antibodies, which are 95–100% sensitive and 90–100% specific [9, 12].
Anti-dsDNA, anti-Smith and anti-C1q antibodies are associated with lupus nephritis. Lupus anticoagulant and anti-cardiolipin antibodies are associated with APS. Rheumatoid factor and anti-CCP are seen in RA. Anti-centromere and anti-Scl70 antibodies are associated with systemic sclerosis. Anti-RNP is seen in mixed connective tissue disease. Anti-Jo1 is associated with myositis. Positive hepatitis serology and cryoglobulins are seen in cryoglobulinaemia (table 2) [60].
TABLE 2.
Serology testing in pulmonary renal syndromes
| ANCA-associated vasculitis | |
| Granulomatosis with polyangiitis (GPA) | ANCA positive 90% [61]; PR3 positive in 75% |
| Microscopic polyangiitis (MPA) | ANCA positive 60% [61]; MPO positive in 65% |
| Eosinophilic granulomatosis with polyangiitis (EGPA) | ANCA positive 30–70% [61]; PR3 positive in 5%, MPO positive in 45% [62] |
| Anti-GBM disease | Anti-GBM antibodies are 95–100% sensitive and 90–100% specific [10, 63, 64] |
| ANCA-negative vasculitis | |
| IgA disease | Nil specific |
| Cryoglobulinaemia | Hepatitis serology; serum cryoglobulins [65] |
| Autoimmune connective tissue disease | |
| Systemic lupus erythematosus (SLE) | Anti-dsDNA, anti-Smith, anti-C1q antibodies |
| Antiphospholipid syndrome (APS) | Anti-cardiolipin, lupus anticoagulant antibodies |
| Rheumatoid arthritis (RA) | Rheumatoid factor, anti-cyclic citrullinated peptides |
| Mixed connective tissue disease (MCTD) | Anti-RNP |
| Polymyositis and dermatomyositis | Anti-Jo1, anti-Ro antibodies |
| Systemic sclerosis | Anti-centromere, anti-Scl70 [60, 66] |
Positive serology can aid in narrowing the diagnosis in pulmonary renal syndrome. Differentiating between ANCA-positive and ANCA-negative serology is often the first step in determining the cause. ANCA: anti-neutrophil cytoplasm antibodies; GBM: glomerular basement membrane; PR3: proteinase-3; MPO: myeloperoxidase; dsDNA: double-stranded DNA.
Pulmonary function testing
Extravascular blood increases the uptake of carbon monoxide [61]. If this blood is present in the alveoli, clearance of the carbon monoxide radioisotope will be reduced, resulting in a low transfer coefficient [62]. Thus, the ratio of uptake to clearance can indicate recent pulmonary haemorrhage [6]. However, practically it can be difficult to perform in a patient with respiratory compromise and most patients will not have a baseline value to compare.
Histology
A positive biopsy is strongly supportive of a diagnosis of AAV and is recommended to assist in establishing a new diagnosis [63]. Biopsy options for histological confirmation include percutaneous renal biopsy (typically the preferred site), transbronchial, thoracoscopic or surgical lung biopsy. Lung biopsy carries considerable risk and should only be used if diagnosis cannot be achieved by an alternate site [8].
Lung biopsy indicating alveolar haemorrhage typically demonstrates intra-alveolar fibrin with haemosiderin-laden alveolar macrophages. Haemosiderin appears 48 h after haemorrhage, thus is useful in delineating DAH from surgical trauma [23]. Capillaritis is the most common accompanying histopathological condition seen in 63% of cases [6], particularly in the presence of vasculitis. The histological features include fibrin thrombi occluding capillaries and fibrinoid necrosis of the capillary wall, with interstitial accumulation of fragmented neutrophils [23]. Diffuse alveolar damage can also be seen supported by evidence of alveolar oedema, capillary congestion, microvascular thrombi and hyaline membrane formation [45, 64, 65].
There are several variations in renal pathological findings depending on the aetiology [8]. Renal biopsies typically demonstrate a focal segmental necrotic glomerulonephritis in anti-GBM and AAV disease where crescent formation with normal glomerular segments intermixed is seen in >90% of cases. This is in contrast to immune-complex disease, where a membranoproliferative glomerulonephritis is seen. Necrosis and crescent formation are typically absent; if crescent formation is present, it typically affects less than 50% of the glomerulus [66]. Furthermore, renal histology can be divided into three immunohistochemical patterns: type 1 antibody-mediated as seen in anti-GBM disease, type 2 immune complex-mediated as seen in SLE and type 3 pauci-immune as seen in AAV [67].
In addition, specific findings can indicate the underlying aetiology. Anti-GBM disease can be confirmed by linear deposition of IgG on renal biopsy [68]. Granulomas with necrosis on lung biopsy indicates GPA. Necrotising vasculitis, eosinophilic tissue infiltration and extravascular granulomas on lung biopsy indicates EGPA [9]. Lupus nephritis has six phenotypes, generally demonstrating immunofluorescence strongly positive for immunoglobulins and complement in a granular pattern [69]. In AAV, immunofluorescence typically reveals minimal antibody deposits hence the term “pauci-immune”.
The American College of Rheumatology and European Alliance of Associations for Rheumatology have validated criteria for the classification of EGPA, MPA and GPA based on clinical, biochemical and histological features which are highly sensitive and specific [70–72].
General management
Treatment should be initiated promptly as mortality and morbidity in pulmonary renal syndrome is high [4, 45, 73]. Optimal treatment is based on the underlying disease process; hence it is best considered on an individual basis (figure 3). Initial treatment typically involves a combination of glucocorticoids, immunosuppression and plasmapheresis. Supportive measures such as transfusion, mechanical ventilation and renal replacement therapy are implemented when needed [15]. Broad spectrum antimicrobial cover is often given until further workup is performed to exclude infection [48].
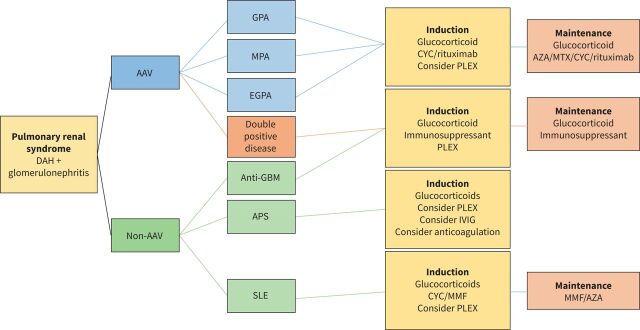
FIGURE 3.
Treatment algorithm in pulmonary renal syndrome. Treatment depends on the underlying cause; however, it is often a combination of glucocorticoid and immunosuppressant. Plasmapheresis can be considered in certain groups. Treatment typically consists of an induction phase followed by a maintenance phase. DAH: diffuse alveolar haemorrhage; AAV: ANCA-associated vasculitis; ANCA: anti-neutrophil cytoplasm antibodies; GPA: granulomatosis with polyangiitis; MPA: microscopic polyangiitis; EGPA: eosinophilic granulomatosis with polyangiitis; GBM: glomerular basement membrane; APS: antiphospholipid syndrome; SLE: systemic lupus erythematosus; CYC: cyclophosphamide; PLEX: plasmapheresis; IVIG: intravenous immunoglobulin; MMF: mycophenolate mofetil; AZA: azathioprine; MTX: methotrexate.
Disease-specific management
AAV
AAV is generally treated with a combination of glucocorticoids and immunosuppressive therapy. Treatment is divided into an induction phase, typically 6–12 months, followed by a maintenance phase of 18–24 months.
Glucocorticoids are the cornerstone of treatment. Standard induction regimens include pulsed methylprednisolone for 3–5 days followed by a taper to 1 mg·kg−1 for the first month and further taper over the subsequent months [74]. Rapid tapering of glucocorticoids with cyclophosphamide and rituximab induction therapy showed no difference in disease relapse or remission compared with a slow taper and patients had less complications [75, 76]. Furthermore, the PEXIVAS trial indicated that a low-dose glucocorticoid regimen was non-inferior to the standard regimen [76], which has been reflected in clinical guidelines [74]. Glucocorticoids are typically continued at low dose for a minimum of 18 months alongside a steroid-sparing agent. Patients with PR3-ANCA positivity typically complete an extended 24−36-month course given the higher rate of disease relapse [63, 77, 78].
A combination of immunosuppressive therapy and glucocorticoids are recommended in organ-threatening disease [63]. Rituximab, rather than cyclophosphamide, is the preferred agent of the American College of Rheumatology as it is considered less toxic [74]. Rituximab has been shown to be non-inferior to glucocorticoids plus oral cyclophosphamide and azathioprine [79, 80]. Rituximab is as effective as cyclophosphamide in AAV with renal involvement and in relapsed disease [81]. Pulsed intravenous cyclophosphamide achieves a lower cumulative dose with less toxicity, while achieving similar efficacy to daily oral cyclophosphamide [82]. The most common complications include sepsis, malignancy, haemorrhagic cystitis and cytopenia [83]. Cyclophosphamide is not recommended as maintenance treatment because of dose-related complications. For maintenance treatment, low-dose glucocorticoids with azathioprine, rituximab, methotrexate or mycophenolate mofetil are recommended [63]. The American College of Rheumatology recommends rituximab for maintenance given the lower risk of relapse compared with methotrexate and azathioprine [74]. In Europe, most centres switch to a maintenance regime after 3–6 months of induction therapy [8, 13].
Plasmapheresis had been the mainstay of induction therapy in pulmonary renal syndrome secondary to AAV with RPGN or DAH. The mechanism of action of plasmapheresis is largely unknown; it likely reduces ANCA titres and removes a large fraction of pro-inflammatory cytokines, complement and coagulation factors from the systemic circulation. Prior studies suggested that it reduced the risk of end-stage renal disease [84] and reduced dependence on dialysis by 50% at 12 months in severe renal vasculitis [85]. However, the PEXIVAS trial did not identify any additional benefit of the addition of plasmapheresis to standard therapy in patients with severe AAV. While none of the patients in this study underwent a renal biopsy, the study made a compelling case against the routine administration of plasmapheresis as part of induction in patients with AAV and renal involvement. There is still a subset of patients with severe RPGN who may derive benefit from plasmapheresis, especially those with markedly elevated ANCA titres or those approaching renal replacement therapy. The American College of Rheumatology recommend against routine use of plasmapheresis in patients with glomerulonephritis but advise that it should be considered in those at higher risk of progression to end-stage renal disease [74]. The evidence for plasmapheresis in DAH is limited [13, 85, 86]. Trials have suggested that the risk of death from pulmonary haemorrhage was reduced [87]; however, there is no proven long-term survival benefit and certainly very little evidence for its role in the treatment of less severe disease [85]. In the PEXIVAS trial, there was no observed treatment effect. However, there was a possible benefit of plasmapheresis in the subgroup analysis in patients with DAH [76]. The American College of Rheumatology recommend against the use of plasmapheresis in DAH; however, they suggest consideration in those who are critically ill or failed to respond to the recommended remission induction therapies [74].
Anti-GBM disease
Anti-GBM disease is treated aggressively due to the high mortality and morbidity. Plasmapheresis to remove the circulating antibodies is the gold standard treatment, usually performed for 2 weeks or until the anti-GBM antibodies are undetectable. There is evidence to suggest earlier commencement has a beneficial effect on long-term renal recovery [36]. In general, the disease does not relapse, and immunosuppressive agents can be safely withdrawn within a few months [88]. The largest experience in anti-GBM disease used high-dose prednisolone tapered over 6 months, oral cyclophosphamide for 2–3 months, and daily plasma exchange until anti-GBM antibodies were no longer detectable [89].
Anti-GBM disease and AAV
Patients who are double-seropositive behave similarly to those with an isolated anti-GBM disease in the initial disease phase and so should be treated in a similar manner with plasmapheresis [36, 90]. The subsequent disease course is more similar to AAV [88], and as such, they should then receive maintenance immunosuppression to prevent relapse, as used in patients with AAV.
APS
Catastrophic APS is treated with a combination of anticoagulation, glucocorticoids, intravenous immunoglobulin and plasmapheresis [91]. Treatments are based on observational data and case series as there are no randomised controlled studies to date. DAH in the setting of APS is treated with intravenous pulsed methylprednisolone. Anticoagulation should be used with caution once haemorrhage is controlled. Plasmapheresis and intravenous immunoglobulin should be used as adjuncts in steroid unresponsive disease. The use of cyclophosphamide is associated with increased mortality and thus should be avoided [48].
SLE
As with other organ-threatening manifestations of SLE, the treatment of DAH is with high-dose glucocorticoids with an additional immunosuppressive agent [92]. A retrospective series supported the use of cyclophosphamide in conjunction with glucocorticoids [45]. Plasmapheresis can also be used in DAH; however, variable treatment responses have been seen [93, 94]. Proliferative lupus nephritis is typically treated with an induction phase with either mycophenolate or cyclophosphamide in conjunction with glucocorticoids [95]. Mycophenolate or azathioprine are used as maintenance therapy, with lower relapses seen in the mycophenolate group. Plasmapheresis conferred no additional benefit [96].
Cryoglobulinaemia
Treatment usually includes a combination of glucocorticoids, plasmapheresis and cyclophosphamide [6]. It is critical to identify and treat the underlying condition. Patients with HIV or hepatitis B viral infection should receive antiviral therapy prior to immunosuppressive therapy and those with hepatitis C viral infection can receive immunosuppression prior to receiving antivirals.
Other diseases
Glucocorticoids are the first-line therapy in IgA vasculitis-related pulmonary renal syndrome [97]. Pulsed intravenous cyclophosphamide is recommended if the patient develops respiratory failure. Systemic sclerosis is treated with glucocorticoids and cyclophosphamide [51] after careful consideration of the disease subtype; glucocorticoids can worsen and trigger thrombotic microangiopathy, where plasmapheresis should be considered instead [98]. In rheumatoid disease the mainstay of treatment is steroids and no treatment has been shown to be superior in terms of survival benefit [6, 52].
Natural history and prognosis
If left untreated pulmonary renal syndrome can follow a fulminant course. With treatment, improvement is typically seen within the first few days of initiation. If a patient deteriorates following treatment, sepsis must be considered given the level of immunosuppression. In a series of 26 patients with necrotising vasculitis admitted to ICU, 75% died of sepsis [99]. Refractory pulmonary renal syndrome and adverse drug effects should also be considered.
In AAV, 85% of patients achieve disease remission [77, 100]. DAH and renal function at presentation are independently associated with induction refractory disease [101]. Relapse will occur in 30–50% of patients over 5 years [102], requiring re-induction therapy with aggressive immunosuppression. Recurrence is rare in patients with anti-GBM disease, reported in <3% of cases [36]. In SLE, disease recurrence after treatment is frequent [95].
The 5-year mortality of pulmonary renal syndrome with combination therapy is 12% [103]. The 5-year survival rates for GPA, MPA and EGPA are estimated to be 74–91%, 45–76% and 60–97%, respectively [78]. DAH usually heralds severe vasculitis and mortality remains high. DAH is associated with a worse prognosis regardless of the underlying aetiology [104]. Renal recovery is less common in patients who require renal replacement therapy. Despite treatment, almost 66% of patients with pulmonary renal syndrome will need renal transplantation within 4 years of presentation [12]. In AAV, age and serum creatinine at presentation remain the strongest predictors of both survival and renal outcome [105]. In anti-GBM disease, renal replacement therapy at presentation heralds poor prognosis for renal recovery [36, 106]. Patients who presented with a creatinine concentration <500 μmol·L−1 had 100% patient survival and 95% renal survival at 1 year. In contrast, patients requiring dialysis at presentation had 65% patient survival and 8% renal survival at 1 year [36].
Key points
The causes of pulmonary renal syndrome can broadly be divided into ANCA-associated vasculitis (AAV) and immune complex-mediated vasculitis, which includes anti-GBM disease and ANCA-negative diseases.
Haemoptysis is the most common manifestation of DAH, but it is absent in 30–35% of cases so a high index of suspicion is needed in a patient in undifferentiated respiratory failure.
Chest imaging typically demonstrates bilateral ground-glass opacities and consolidation in a perihilar distribution, with a middle and lower zone predominance.
Treatment involves a combination of glucocorticoids, immunosuppression and plasmapheresis with supportive measures such as transfusion, mechanical ventilation and renal replacement therapy when needed.
Self-evaluation questions
- A 54-year-old woman presents to the emergency department with a 3-month history of progressive dyspnoea on a background of asthma and sinusitis. While awaiting assessment she has an episode of bright red haemoptysis. On examination she is vitally stable with oxygen saturations of 95% on room air. There are crackles in the right lung base on auscultation. Chest radiography reveals perihilar bilateral ground-glass opacification. Which of the following blood tests would be most likely to make the correct diagnosis?
- Rheumatoid factor
- Anti-RNP antibodies
- Anti-Jo1 antibodies
- ANCA testing
- dsDNA levels
- A 26-year-old male, ex-smoker, is admitted with haematuria and scant haemoptysis. He is haemodynamically stable and remains on 2 L of oxygen via nasal prongs to maintain oxygen saturations >95%. He is diagnosed with pulmonary renal syndrome due to anti-GBM disease. Which of the following would be the most appropriate initial management step?
- Administer a saline nebuliser
- Glucocorticoids and cyclophosphamide
- Commence renal replacement therapy
- Plasmapheresis with glucocorticoids and cyclophosphamide
- High-dose glucocorticoids alone
Suggested answers
1. d.
2. d.
Footnotes
Conflict of interest: M.P. Keane has received consulting fees from Boehringer and Roche, outside the submitted work; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Boehringer, outside the submitted work; and support for attending meets and/or travel from Boehringer, AstraZeneca and Roche, outside the submitted work. C. McCarthy has received grants and speaker fees from Boehringer Ingelheim, and speaker fees from Roche Ltd. He is on the scientific advisory boards of the LAM Foundation and the European Pulmonary Fibrosis Federation. He has a consulting contract with Savara Pharmaceuticals as part of their clinical advisory board. C. McCarthy is a current member of the Breathe editorial board. The remaining authors have nothing to disclose.
References
- 1.Goodpasture EW. The significance of certain pulmonary lesions in relation to the etiology of influenza. Am J Med Sci 1919; 158: 863. doi: 10.1097/00000441-191911000-00012 [DOI] [PubMed] [Google Scholar]
- 2.Stanton MC, Tange JD. Goodpasture's syndrome (pulmonary haemorrhage associated with glomerulonephritis). Australas Ann Med 1958; 7: 132–144. doi: 10.1111/imj.1958.7.2.132 [DOI] [PubMed] [Google Scholar]
- 3.Lerner R. The role of anti-glomerular basement membrane antibody in the pathogenesis of human glomerulonephritis. J Am Soc Nephrol 1999; 10: 1389–1404. [PubMed] [Google Scholar]
- 4.Gallagher H, Kwan JT, Jayne DR. Pulmonary renal syndrome: a 4-year, single-center experience. Am J Kidney Dis 2002; 39: 42–47. doi: 10.1053/ajkd.2002.29876 [DOI] [PubMed] [Google Scholar]
- 5.Saladi L, Shaikh D, Saad M, et al. . Pulmonary renal syndrome: a case report of diffuse alveolar hemorrhage in association with ANCA negative pauci-immune glomerulonephritis. Medicine (Baltimore) 2018; 97: e10954. doi: 10.1097/MD.0000000000010954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.West SC, Arulkumaran N, Ind PW, et al. . Pulmonary-renal syndrome: a life threatening but treatable condition. Postgrad Med J 2013; 89: 274–283. doi: 10.1136/postgradmedj-2012-131416 [DOI] [PubMed] [Google Scholar]
- 7.Jennette JC, Falk RJ, Bacon PA, et al. . 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Arthritis Rheum 2013; 65: 1–11. doi: 10.1002/art.37715 [DOI] [PubMed] [Google Scholar]
- 8.McCabe C, Jones Q, Nikolopoulou A, et al. . Pulmonary-renal syndromes: an update for respiratory physicians. Respir Med 2011; 105: 1413–1421. doi: 10.1016/j.rmed.2011.05.012 [DOI] [PubMed] [Google Scholar]
- 9.Papiris SA, Manali ED, Kalomenidis I, et al. . Bench-to-bedside review: pulmonary–renal syndromes – an update for the intensivist. Crit Care 2007; 11: 213. doi: 10.1186/cc5778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarz MI, Brown KK. Small vessel vasculitis of the lung. Thorax 2000; 55: 502–510. doi: 10.1136/thorax.55.6.502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franks TJ, Koss MN. Pulmonary capillaritis. Curr Opin Pulm Med 2000; 6: 430–435. doi: 10.1097/00063198-200009000-00008 [DOI] [PubMed] [Google Scholar]
- 12.Lee RW, D'Cruz DP. Pulmonary renal vasculitis syndromes. Autoimmun Rev 2010; 9: 657–660. doi: 10.1016/j.autrev.2010.05.012 [DOI] [PubMed] [Google Scholar]
- 13.Jayne D, Rasmussen N, Andrassy K, et al. . A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med 2003; 349: 36–44. doi: 10.1056/NEJMoa020286 [DOI] [PubMed] [Google Scholar]
- 14.Csernok E. Anti-neutrophil cytoplasmic antibodies and pathogenesis of small vessel vasculitides. Autoimmun Rev 2003; 2: 158–164. doi: 10.1016/S1568-9972(03)00010-7 [DOI] [PubMed] [Google Scholar]
- 15.Brusselle GG. Pulmonary-renal syndromes. Acta Clinica Belgica 2007; 62: 88–96. doi: 10.1179/acb.2007.016 [DOI] [PubMed] [Google Scholar]
- 16.Banerjee P, Jain A, Kumar U, et al. . Epidemiology and genetics of granulomatosis with polyangiitis. Rheumatol Int 2021; 41: 2069–2089. doi: 10.1007/s00296-021-05011-1 [DOI] [PubMed] [Google Scholar]
- 17.Gibelin A, Maldini C, Mahr A. Epidemiology and etiology of Wegener granulomatosis, microscopic polyangiitis, Churg-Strauss syndrome and Goodpasture syndrome: vasculitides with frequent lung involvement. Semin Respir Crit Care Med 2011; 32: 264–273. doi: 10.1055/s-0031-1279824 [DOI] [PubMed] [Google Scholar]
- 18.Ntatsaki E, Watts RA, Scott DG. Epidemiology of ANCA-associated vasculitis. Rheumatic Dis Clin 2010; 36: 447–461. doi: 10.1016/j.rdc.2010.04.002 [DOI] [PubMed] [Google Scholar]
- 19.Cotch MF, Hoffman GS, Yerg DE, et al. . The epidemiology of Wegener's granulomatosis. Estimates of the five-year period prevalence, annual mortality, and geographic disease distribution from population-based data sources. Arthritis Rheum 1996; 39: 87–92. doi: 10.1002/art.1780390112 [DOI] [PubMed] [Google Scholar]
- 20.Langford CA, Hoffman GS. Rare diseases. 3: Wegener's granulomatosis. Thorax 1999; 54: 629–637. doi: 10.1136/thx.54.7.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iudici M, Quartier P, Terrier B, et al. . Childhood-onset granulomatosis with polyangiitis and microscopic polyangiitis: systematic review and meta-analysis. Orphanet J Rare Dis 2016; 11: 141. doi: 10.1186/s13023-016-0523-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson G, Coles E, Crane M, et al. . Wegener's granuloma. A series of 265 British cases seen between 1975 and 1985. A report by a sub-committee of the British Thoracic Society Research Committee. QJM 1992; 83: 427–438. [PubMed] [Google Scholar]
- 23.Collard HR, Schwarz MI. Diffuse alveolar hemorrhage. Clin Chest Med 2004; 25: 583–592. doi: 10.1016/j.ccm.2004.04.007 [DOI] [PubMed] [Google Scholar]
- 24.Haworth S, Savage C, Carr D, et al. . Pulmonary haemorrhage complicating Wegener's granulomatosis and microscopic polyarteritis. Br Med J (Clin Res Ed) 1985; 290: 1775–1778. doi: 10.1136/bmj.290.6484.1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Travis WD, Carpenter HA, Lie J. Diffuse pulmonary hemorrhage. An uncommon manifestation of Wegener's granulomatosis. Am J Surg Pathol 1987; 11: 702–708. doi: 10.1097/00000478-198709000-00006 [DOI] [PubMed] [Google Scholar]
- 26.Mohammad AJ, Jacobsson LTH, Mahr AD, et al. . Prevalence of Wegener's granulomatosis, microscopic polyangiitis, polyarteritis nodosa and Churg–Strauss syndrome within a defined population in southern Sweden. Rheumatology 2007; 46: 1329–1337. doi: 10.1093/rheumatology/kem107 [DOI] [PubMed] [Google Scholar]
- 27.Chung SA, Seo P. Microscopic polyangiitis. Rheum Dis Clin 2010; 36: 545–558. doi: 10.1016/j.rdc.2010.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohammad AJ. An update on the epidemiology of ANCA-associated vasculitis. Rheumatology (Oxford) 2020; 59: Suppl. 3, iii42–iii50. doi: 10.1093/rheumatology/keaa089 [DOI] [PubMed] [Google Scholar]
- 29.Brown KK. Pulmonary vasculitis. Proc Am Thorac Soc 2006; 3: 48–57. doi: 10.1513/pats.200511-120JH [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitching AR, Anders H-J, Basu N, et al. . ANCA-associated vasculitis. Nat Rev Dis Primers 2020; 6: 71. doi: 10.1038/s41572-020-0204-y [DOI] [PubMed] [Google Scholar]
- 31.Jones J, Lawler P, Crawley J, et al. . Increased alveolar epithelial permeability in cigarette smokers. Lancet 1980; 315: 66–68. doi: 10.1016/S0140-6736(80)90493-6 [DOI] [PubMed] [Google Scholar]
- 32.Rees A, Peters D, Compston D, et al. . Strong association between HLA-DRW2 and antibody-mediated Goodpasture's syndrome. Lancet 1978; 311: 966–968. doi: 10.1016/S0140-6736(78)90252-0 [DOI] [PubMed] [Google Scholar]
- 33.Rees AJ, Peters DK, Amos N, et al. . The influence of HLA-linked genes on the severity of anti-GBM antibody-mediated nephritis. Kidney Int 1984; 26: 444–450. doi: 10.1038/ki.1984.194 [DOI] [PubMed] [Google Scholar]
- 34.Perl S, Pussell B, Charlesworth J, et al. . Goodpasture's (anti-GBM) disease and HLA-DRw2. N Engl J Med 1981; 305: 463–464. doi: 10.1056/nejm198108203050818 [DOI] [PubMed] [Google Scholar]
- 35.Wilson CB, Smith RC. Goodpasture's syndrome associated with influenza A2 virus infection. Ann Intern Med 1972; 76: 91–94. doi: 10.7326/0003-4819-76-1-91 [DOI] [PubMed] [Google Scholar]
- 36.Levy JB, Turner AN, Rees AJ, et al. . Long-term outcome of anti–glomerular basement membrane antibody disease treated with plasma exchange and immunosuppression. Ann Intern Med 2001; 134: 1033–1042. doi: 10.7326/0003-4819-134-11-200106050-00009 [DOI] [PubMed] [Google Scholar]
- 37.McAdoo SP, Pusey CD. Anti-glomerular basement membrane disease. Clin J Am Soc Nephrol 2017; 12: 1162–1172. doi: 10.2215/CJN.01380217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Philip R, Dumont A, Martin Silva N, et al. . ANCA and anti-glomerular basement membrane double-positive patients: a systematic review of the literature. Autoimmun Rev 2021; 20: 102885. doi: 10.1016/j.autrev.2021.102885 [DOI] [PubMed] [Google Scholar]
- 39.Rutgers A, Slot M, van Paassen P, et al. . Coexistence of anti-glomerular basement membrane antibodies and myeloperoxidase-ANCAs in crescentic glomerulonephritis. Am J Kidney Dis 2005; 46: 253–262. doi: 10.1053/j.ajkd.2005.05.003 [DOI] [PubMed] [Google Scholar]
- 40.Hellmark T, Niles JL, Collins AB, et al. . Comparison of anti-GBM antibodies in sera with or without ANCA. J Am Soc Nephrol 1997; 8: 376–385. doi: 10.1681/ASN.V83376 [DOI] [PubMed] [Google Scholar]
- 41.Henderson SR, Salama AD. Diagnostic and management challenges in Goodpasture's (anti-glomerular basement membrane) disease. Nephrol Dial Transplant 2018; 33: 196–202. doi: 10.1093/ndt/gfx057 [DOI] [PubMed] [Google Scholar]
- 42.Bosch X, Mirapeix E, Font J, et al. . Prognostic implication of anti-neutrophil cytoplasmic autoantibodies with myeloperoxidase specificity in anti-glomerular basement membrane disease. Clin Nephrol 1991; 36: 107–113. [PubMed] [Google Scholar]
- 43.Hunninghake GW, Fauci AS. Pulmonary involvement in the collagen vascular diseases. Am Rev Respir Dis 1979; 119: 471–503. doi: 10.1164/arrd.1979.119.3.471 [DOI] [PubMed] [Google Scholar]
- 44.Schwarz MI, Zamora MR, Hodges TN, et al. . Isolated pulmonary capillaritis and diffuse alveolar hemorrhage in rheumatoid arthritis and mixed connective tissue disease. Chest 1998; 113: 1609–1615. doi: 10.1378/chest.113.6.1609 [DOI] [PubMed] [Google Scholar]
- 45.Zamora MR, Warner ML, Tuder R, et al. . Diffuse alveolar hemorrhage and systemic lupus erythematosus. Clinical presentation, histology, survival, and outcome. Medicine (Baltimore) 1997; 76: 192–202. doi: 10.1097/00005792-199705000-00005 [DOI] [PubMed] [Google Scholar]
- 46.Ford HJ, Roubey RA. Pulmonary manifestations of the antiphospholipid antibody syndrome. Clin Chest Med 2010; 31: 537–545. doi: 10.1016/j.ccm.2010.05.005 [DOI] [PubMed] [Google Scholar]
- 47.Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N Engl J Med 2002; 346: 752–763. doi: 10.1056/NEJMra002974 [DOI] [PubMed] [Google Scholar]
- 48.Cervera R, Bucciarelli S, Plasín MA, et al. . Catastrophic antiphospholipid syndrome (CAPS): descriptive analysis of a series of 280 patients from the “CAPS Registry”. J Autoimmun 2009; 32: 240–245. doi: 10.1016/j.jaut.2009.02.008 [DOI] [PubMed] [Google Scholar]
- 49.Panoskaltsis N, Derman MP, Perillo I, et al. . Thrombotic thrombocytopenic purpura in pulmonary–renal syndromes. Am J Hematol 2000; 65: 50–55. doi: [DOI] [PubMed] [Google Scholar]
- 50.Bonaci-Nikolic B, Nikolic MM, Andrejevic S, et al. . Antineutrophil cytoplasmic antibody (ANCA)-associated autoimmune diseases induced by antithyroid drugs: comparison with idiopathic ANCA vasculitides. Arthritis Res Ther 2005; 7: R1072–R1081. doi: 10.1186/ar1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bar J, Ehrenfeld M, Rozenman J, et al. . Pulmonary–renal syndrome in systemic sclerosis. Seminars in arthritis and rheumatism. Semin Arthritis Rheum 2001; 30: 403–410. doi: 10.1053/sarh.2001.21904 [DOI] [PubMed] [Google Scholar]
- 52.Genta MS, Genta RM, Gabay C, ed. Systemic rheumatoid vasculitis: a review. Seminars in arthritis and rheumatism. Semin Arthritis Rheum 2006; 36: 88–98. doi: 10.1016/j.semarthrit.2006.04.006 [DOI] [PubMed] [Google Scholar]
- 53.Taha R, Feteih M. Pulmonary manifestations of connective tissue diseases. In: Almoallim H, Cheikh M, eds. Skills in Rheumatology. Singapore, Springer, 2021; pp. 139–175. Doi: 10.1007/978-981-15-8323-0_7 [DOI] [PubMed] [Google Scholar]
- 54.de Prost N, Parrot A, Cuquemelle E, et al. . Diffuse alveolar hemorrhage in immunocompetent patients: etiologies and prognosis revisited. Respir Med 2012; 106: 1021–1032. doi: 10.1016/j.rmed.2012.03.015 [DOI] [PubMed] [Google Scholar]
- 55.Lara AR, Schwarz MI. Diffuse alveolar hemorrhage. Chest 2010; 137: 1164–1171. doi: 10.1378/chest.08-2084 [DOI] [PubMed] [Google Scholar]
- 56.Ioachimescu OC, Stoller JK. Diffuse alveolar hemorrhage: diagnosing it and finding the cause. Cleve Clin J Med 2008; 75: 258–280. doi: 10.3949/ccjm.75.4.258 [DOI] [PubMed] [Google Scholar]
- 57.Lopez Ruiz A, Niven A. Management of pulmonary-renal syndrome. 2020. www.reliasmedia.com/articles/147185-management-of-pulmonary-renal-syndrome
- 58.Harel Z, Simel DL, Wald R. Urinalysis in the evaluation of proliferative glomerulonephritis. JAMA 2017; 318: 1276–1277. doi: 10.1001/jama.2017.14482 [DOI] [PubMed] [Google Scholar]
- 59.Lau KK, Wyatt RJ. Glomerulonephritis. Adolesc Med Clin 2005; 16: 67–85. doi: 10.1016/j.admecli.2004.09.008 [DOI] [PubMed] [Google Scholar]
- 60.Didier K, Bolko L, Giusti D, et al. . Autoantibodies associated with connective tissue diseases: what meaning for clinicians? Front Immunol 2018; 9: 541. doi: 10.3389/fimmu.2018.00541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rees AJ. Pulmonary injury caused by anti-basement membrane antibodies. Semin Respir Crit Care Med 1984; 5: 264–272. doi: 10.1055/s-2007-1011461 [DOI] [Google Scholar]
- 62.Ewan PW, Jones HA, Rhodes CG, et al. . Detection of intrapulmonary hemorrhage with carbon monoxide uptake. Application in Goodpasture's syndrome. N Engl J Med 1976; 295: 1391–1396. doi: 10.1056/NEJM197612162952502 [DOI] [PubMed] [Google Scholar]
- 63.Yates M, Watts RA, Bajema IM, et al. . EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis 2016; 75: 1583–1594. doi: 10.1136/annrheumdis-2016-209133 [DOI] [PubMed] [Google Scholar]
- 64.Leatherman JW. Immune alveolar hemorrhage. Chest 1987; 91: 891–897. doi: 10.1378/chest.91.6.891 [DOI] [PubMed] [Google Scholar]
- 65.Mark E, Ramirez J. Pulmonary capillaritis and hemorrhage in patients with systemic vasculitis. Arch Pathol Lab Med 1985; 109: 413–418. [PubMed] [Google Scholar]
- 66.Jennette JC. Implications for pathogenesis of patterns of injury in small- and medium-sized-vessel vasculitis. Cleve Clin J Med 2002; 69: Suppl. 2, SII-33–SII-38. doi: 10.3949/ccjm.69.Suppl_2.SII33 [DOI] [PubMed] [Google Scholar]
- 67.Kambham N. Crescentic glomerulonephritis: an update on pauci-immune and anti-GBM diseases. Adv Anat Pathol 2012; 19: 111–124. doi: 10.1097/PAP.0b013e318248b7a1 [DOI] [PubMed] [Google Scholar]
- 68.Wilson CB, Dixon FJ. Anti-glomerular basement membrane antibody-induced glomerulonephritis. Kidney Int 1973; 3: 74–89. doi: 10.1038/ki.1973.14 [DOI] [PubMed] [Google Scholar]
- 69.Weening JJ, D'Agati VD, Schwartz MM, et al. . The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int 2004; 65: 521–530. doi: 10.1111/j.1523-1755.2004.00443.x [DOI] [PubMed] [Google Scholar]
- 70.Grayson PC, Ponte C, Suppiah R, et al. . 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for eosinophilic granulomatosis with polyangiitis. Arthritis Rheumatol 2022; 74: 386–392. doi: 10.1002/art.41982 [DOI] [PubMed] [Google Scholar]
- 71.Suppiah R, Robson JC, Grayson PC, et al. . 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for microscopic polyangiitis. Arthritis Rheumatol 2022; 74: 400–406. doi: 10.1002/art.41983 [DOI] [PubMed] [Google Scholar]
- 72.Robson JC, Grayson PC, Ponte C, et al. . 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for granulomatosis with polyangiitis. Arthritis Rheumatol 2022; 74: 393–399. doi: 10.1002/art.41986 [DOI] [PubMed] [Google Scholar]
- 73.Holguin F, Ramadan B, Gal AA, et al. . Prognostic factors for hospital mortality and ICU admission in patients with ANCA-related pulmonary vasculitis. Arthritis Rheumatol 2008; 336: 321–326. doi: 10.1097/MAJ.0b013e31816805fa [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chung SA, Langford CA, Maz M, et al. . 2021 American College of Rheumatology/Vasculitis Foundation guideline for the management of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol 2021; 73: 1366–1383. doi: 10.1002/art.41773 [DOI] [PubMed] [Google Scholar]
- 75.Pepper RJ, McAdoo SP, Moran SM, et al. . A novel glucocorticoid-free maintenance regimen for anti-neutrophil cytoplasm antibody-associated vasculitis. Rheumatology (Oxford) 2019; 58: 260–268. doi: 10.1093/rheumatology/key288 [DOI] [PubMed] [Google Scholar]
- 76.Walsh M, Merkel PA, Peh C-A, et al. . Plasma exchange and glucocorticoids in severe ANCA-associated vasculitis. N Engl J Med 2020; 382: 622–631. doi: 10.1056/NEJMoa1803537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hogan SL, Falk RJ, Chin H, et al. . Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis. Ann Intern Med 2005; 143: 621–631. doi: 10.7326/0003-4819-143-9-200511010-00005 [DOI] [PubMed] [Google Scholar]
- 78.Mukhtyar C, Flossmann O, Hellmich B, et al. . Outcomes from studies of antineutrophil cytoplasm antibody associated vasculitis: a systematic review by the European League Against Rheumatism systemic vasculitis task force. Ann Rheum Dis 2008; 67: 1004–1010. doi: 10.1136/ard.2007.071936 [DOI] [PubMed] [Google Scholar]
- 79.Specks U, Merkel PA, Seo P, et al. . Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med 2013; 369: 417–427. doi: 10.1056/NEJMoa1213277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jones RB, Cohen Tervaert JW, Hauser T, et al. . Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 2010; 363: 211–220. doi: 10.1056/NEJMoa0909169 [DOI] [PubMed] [Google Scholar]
- 81.Stone JH, Merkel PA, Spiera R, et al. . Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010; 363: 221–232. doi: 10.1056/NEJMoa0909905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Groot KD, Adu D, Savage CO. The value of pulse cyclophosphamide in ANCA-associated vasculitis: meta-analysis and critical review. Nephrol Dial Transplant 2001; 16: 2018–2027. doi: 10.1093/ndt/16.10.2018 [DOI] [PubMed] [Google Scholar]
- 83.West CP, Dyrbye LN, Rabatin JT, et al. . Intervention to promote physician well-being, job satisfaction, and professionalism: a randomized clinical trial. JAMA Intern Med 2014; 174: 527–533. doi: 10.1001/jamainternmed.2013.14387 [DOI] [PubMed] [Google Scholar]
- 84.Walsh M, Catapano F, Szpirt W, et al. . Plasma exchange for renal vasculitis and idiopathic rapidly progressive glomerulonephritis: a meta-analysis. Am J Kidney Dis 2011; 57: 566–574. doi: 10.1053/j.ajkd.2010.10.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jayne DR, Gaskin G, Rasmussen N, et al. . Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol 2007; 18: 2180–2188. doi: 10.1681/ASN.2007010090 [DOI] [PubMed] [Google Scholar]
- 86.Jayne D. Review article: Progress of treatment in ANCA-associated vasculitis. Nephrology (Carlton) 2009; 14: 42–48. doi: 10.1111/j.1440-1797.2009.01101.x [DOI] [PubMed] [Google Scholar]
- 87.Klemmer PJ, Chalermskulrat W, Reif MS, et al. . Plasmapheresis therapy for diffuse alveolar hemorrhage in patients with small-vessel vasculitis. Am J Kidney Dis 2003; 42: 1149–1153. doi: 10.1053/j.ajkd.2003.08.015 [DOI] [PubMed] [Google Scholar]
- 88.Pusey CD. Anti-glomerular basement membrane disease. Kidney Int 2003; 64: 1535–1550. doi: 10.1046/j.1523-1755.2003.00241.x [DOI] [PubMed] [Google Scholar]
- 89.Salama AD, Levy JB, Lightstone L, et al. . Goodpasture's disease. Lancet 2001; 358: 917–920. doi: 10.1016/S0140-6736(01)06077-9 [DOI] [PubMed] [Google Scholar]
- 90.Cortazar FB, Niles JL. The fate of plasma exchange and glucocorticoid dosing in ANCA-associated vasculitis after PEXIVAS. Am J Kidney Dis 2020; 76: 595–597. doi: 10.1053/j.ajkd.2020.03.010 [DOI] [PubMed] [Google Scholar]
- 91.Garcia D, Erkan D. Diagnosis and management of the antiphospholipid syndrome. N Engl J Med 2018; 378: 2010–2021. doi: 10.1056/NEJMra1705454 [DOI] [PubMed] [Google Scholar]
- 92.Ednalino C, Yip J, Carsons SE. Systematic review of diffuse alveolar hemorrhage in systemic lupus erythematosus: focus on outcome and therapy. J Clin Rheumatol 2015; 21: 305–310. doi: 10.1097/RHU.0000000000000291 [DOI] [PubMed] [Google Scholar]
- 93.Al-Adhoubi NK, Bystrom J. Systemic lupus erythematosus and diffuse alveolar hemorrhage, etiology and novel treatment strategies. Lupus 2020; 29: 355–363. doi: 10.1177/0961203320903798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aguirre-Valencia D, Naranjo-Escobar J, Posso-Osorio I, et al. . Therapeutic plasma exchange as management of complicated systemic lupus erythematosus and other autoimmune diseases. Autoimmune Dis 2019; 2019: 5350960. doi: 10.1155/2019/5350960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fanouriakis A, Kostopoulou M, Alunno A, et al. . 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 2019; 78: 736–745. doi: 10.1136/annrheumdis-2019-215089 [DOI] [PubMed] [Google Scholar]
- 96.Tunnicliffe DJ, Palmer SC, Henderson L, et al. . Immunosuppressive treatment for proliferative lupus nephritis. Cochrane Database Syst Rev 2018; 6: CD002922. doi: 10.1002/14651858.CD002922.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pego-Reigosa JM, Medeiros DA, Isenberg DA. Respiratory manifestations of systemic lupus erythematosus: old and new concepts. Best Pract Res Clin Rheumatol 2009; 23: 469–480. doi: 10.1016/j.berh.2009.01.002 [DOI] [PubMed] [Google Scholar]
- 98.Naniwa T, Banno S, Sugiura Y, et al. . Pulmonary-renal syndrome in systemic sclerosis: a report of three cases and review of the literature. Mod Rheumatol 2007; 17: 37–44. doi: 10.3109/s10165-006-0540-0 [DOI] [PubMed] [Google Scholar]
- 99.Cruz B, Ramanoelina J, Mahr A, et al. . Prognosis and outcome of 26 patients with systemic necrotizing vasculitis admitted to the intensive care unit. Rheumatology (Oxford) 2003; 42: 1183–1188. doi: 10.1093/rheumatology/keg322 [DOI] [PubMed] [Google Scholar]
- 100.Tesar V, Rihova Z, Jancova E, et al. . Current treatment strategies in ANCA-positive renal vasculitis – lessons from European randomized trials. Nephrol Dial Transplant 2003; 18: Suppl. 5, v2–v4. doi: 10.1093/ndt/gfg1032 [DOI] [PubMed] [Google Scholar]
- 101.Seror R, Pagnoux C, Ruivard M, et al. . Treatment strategies and outcome of induction-refractory Wegener's granulomatosis or microscopic polyangiitis: analysis of 32 patients with first-line induction-refractory disease in the WEGENT trial. Ann Rheum Dis 2010; 69: 2125–2130. doi: 10.1136/ard.2010.131953 [DOI] [PubMed] [Google Scholar]
- 102.Robson J, Doll H, Suppiah R, et al. . Damage in the ANCA-associated vasculitides: long-term data from the European Vasculitis Study group (EUVAS) therapeutic trials. Ann Rheum Dis 2015; 74: 177–184. doi: 10.1136/annrheumdis-2013-203927 [DOI] [PubMed] [Google Scholar]
- 103.Fauci AS, Haynes BF, Katz P, et al. . Wegener's granulomatosis: prospective clinical and therapeutic experience with 85 patients for 21 years. Ann Intern Med 1983; 98: 76–85. doi: 10.7326/0003-4819-98-1-76 [DOI] [PubMed] [Google Scholar]
- 104.Saxena R, Bygren P, Arvastson B, et al. . Circulating autoantibodies as serological markers in the differential diagnosis of pulmonary renal syndrome. J Intern Med 1995; 238: 143–152. doi: 10.1111/j.1365-2796.1995.tb00912.x [DOI] [PubMed] [Google Scholar]
- 105.Hoffman GS, Kerr GS, Leavitt RY, et al. . Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992; 116: 488–498. doi: 10.7326/0003-4819-116-6-488 [DOI] [PubMed] [Google Scholar]
- 106.Lazor R, Bigay-Gamé L, Cottin V, et al. . Alveolar hemorrhage in anti-basement membrane antibody disease: a series of 28 cases. Medicine (Baltimore) 2007; 86: 181–193. doi: 10.1097/md.0b013e318067da56 [DOI] [PubMed] [Google Scholar]