Abstract
Eosinophilic granulomatosis with polyangiitis (EGPA), previously known as Churg–Strauss syndrome, is a multisystem disorder characterised by asthma, blood and tissue eosinophilia and small-vessel vasculitis. Eosinophilic tissue infiltration and extravascular granuloma formation can lead to damage in any organ, but it is classically seen to cause pulmonary infiltrates, sino-nasal disease, peripheral neuropathy, renal and cardiac involvement, and rashes.
EGPA is part of the anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis syndromes, with the antibody being detected in ∼30–40% of cases and mostly against myeloperoxidase. Two genetically and clinically distinct phenotypes, defined by the presence or absence of ANCA have been identified. Treatment for EGPA focuses on inducing and maintaining disease remission. To date, oral corticosteroids remain first-line agents whilst second-line treatments include immunosuppressants such as cyclophosphamide, azathioprine, methotrexate, rituximab and mycophenolate mofetil. However, long-term steroid usage results in multiple and well-known adverse health effects and new insights into the pathophysiology of EGPA have allowed for the development of targeted biologic therapies, like the anti-eosinophilic, anti-interleukin-5 monoclonal antibodies.
Short abstract
EGPA is a rare, multisystem disorder. Whilst effective, treatment with corticosteroids is associated with a high burden of side-effects. Deeper understanding of the pathophysiology is allowing for the development of novel therapeutic options. https://bit.ly/3Dv4nMa
Educational aims
To review and summarise EGPA and its aetiology.
To equip clinicians with a better understanding of clinical presentations to aid diagnosis.
To understand the developments in novel pharmacological therapies.
Case report
A 41-year-old female patient was referred to the severe asthma clinic with a diagnosis of Samter's triad (asthma, aspirin-exacerbated respiratory disease and rhinosinusitis) made 18 years ago. Despite treatment with and optimal adherence to high dose inhaled corticosteroids/long-acting β-agonist and long-acting muscarinic antagonist inhalers and montelukast, she had suffered from recurrent exacerbations needing three courses of oral corticosteroid therapy in the preceding 12 months.
At her first clinic review she had a peripheral blood eosinophil count of 0.9×109 cells per L, total IgE 55 kU·L−1 with specific IgE positive to house dust mite 22.5 kUA·L−1. She had a forced expiratory volume in 1 s (FEV1) of 2.56 L (84%) and evidence of airway inflammation based on the fraction of exhaled nitric oxide (FENO) of 122 ppb. On computer tomography (CT) images of the chest there was mild bronchial wall thickening, a CT of the sinuses showed mild to moderate mucosal thickening.
Given a recurrent need for prednisolone therapy, she commenced on the anti-interleukin-5 receptor biologic benralizumab 30 mg subcutaneously, to good effect. She remained exacerbation free with excellent symptom control for the following 11 months.
1 year after starting benralizumab, she contacted the centre. She reported loss of asthma control (breathlessness, wheeze and chest tightness) and worsening sino-nasal disease for the preceding month. She felt fatigued. She had noticed a widespread vasculitic rash over her arms and peripheral legs. Eosinophils were noted to be 0.15×109 cells per L for the first time since starting benralizumab. On assessment her oxygen saturations were 99%, FEV1 2.21 L (76%) and FENO 106 ppb. Troponin and renal function were normal as was the ECG. Her last benralizumab injection was 7 weeks ago.
The patient was treated with a course of oral prednisolone 40 mg per day for 5 days and asked to attend for a review on the day of her next benralizumab injection in a week's time or anytime sooner should her symptoms worsen.
On review 7 days later, the patient reported to feel only a little better; the rash had resolved. The blood eosinophil count was 3.08×109 cells per L pre-benralizumab injection, dropping to 0.1×109 cells per L 4 h after injection. Troponin and renal function were normal. Total IgE was 116 kU·L−1. Anti-neutrophil antibodies against proteinase-3 (PR3) and myeloperoxidase (MPO) were negative. A CT of the chest showed widespread severe bronchial wall thickening and areas of resolving dense consolidation with some ground-glass changes seen bilaterally. CT of the sinuses showed progression to severe sino-nasal mucosal thickening and polyposis including complete opacification of the intranasal olfactory recesses. A cardiovascular magnetic resonance scan was normal.
A further high-dose course of prednisolone was prescribed, which was tapered over the following weeks to continue on 5 mg daily; benralizumab was continued and the patient has remained well and exacerbation free over the course of the following months.
Introduction
Eosinophilic granulomatosis with polyangiitis (EGPA) is a rare multisystem disorder, which was first identified in 1951 by the pathologists Jacob Churg and Lotte Strauss [1]. Asthma, blood and tissue eosinophilia, and vasculitic inflammation defined a condition that has remained complex and heterogeneous [2, 3]. EGPA is part of the anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis syndromes (AAV). It is one of the rarest AAV, with an incidence of 0.8–4 per million persons and a prevalence of around 8.1–22 per million persons [4]. Due to its low prevalence and limited epidemiological data, it remains challenging to diagnose and treat [5–7].
The mean age at diagnosis has been reported between 48 and 55 years [8–10]; however, patients have been diagnosed as young as 15 years of age [11, 12]. Through the advent of novel monoclonal antibodies targeting cytokines responsible for upregulation and proliferation of eosinophils, the clinical prognosis of patients has improved as has diagnostic accuracy [13–15].
Pathogenesis
The pathogenesis underpinning EGPA remains largely unknown; it is widely thought that different genetic and environmental factors come into play. Environmental factors including medications such as omalizumab and leukotriene receptor antagonists (LTRA), or irritants such a silica have been investigated as possible triggers. However, the evidence is weak [16] and in the case of omalizumab or LTRAs more likely the effect of steroid tapering rather than directly drug induced.
EGPA is a T-helper (Th)2 cell associated disease; Th2 cells mediate the activation and maintenance of humoral, or antibody-mediated, immune responses through the production of cytokines. Biopsy samples from affected tissues are rich in Th2 related markers such as CD294 [17] and eosinophilic selective chemokines and cytokines such as eotaxin-3, CCL17 and interleukin (IL)-4, IL-5 and IL-13, which induce the maturation and delayed apoptosis of eosinophils [18]. Hyper-eosinophilia is a cardinal feature of the condition. Organ damage can be caused directly by eosinophilic infiltration as seen in cardiac disease, or by the release of proinflammatory cytotoxic granule proteins such as major basic protein, eosinophilic cationic protein and eosinophil peroxidase [19]. The identification of these cytokines, especially IL-5, has allowed for novel biologic therapies to be developed in the first instance for severe eosinophilic asthma (SEA) and, more recently, for use in a broader range of hyper-eosinophilic conditions.
There is further evidence of Th17 involvement due to high levels of IL-17 later in the disease process [20]. Humoral immunity is also felt to play a role in the pathogenesis of EGPA. Elevation of IgE [21] and serum IgG4 is noted in EGPA; these are propagated by cytokines such as IL-4 and IL-5. The role of IgG4 in the pathogenesis remains unclear, the levels of IgG4 have not correlated with disease severity in current studies [22].
ANCA
ANCA are identified in ∼40% of patients [2]. Immunofluorescence assays show a perinuclear pattern of ANCA. In most cases of EGPA, ANCA is directed against MPO rather than PR3 [23]. Our knowledge about their pathogenic role in inflammation and organ damage is developing constantly.
A recent genome-wide association study has identified loci associated with the condition and has recognised two clinically and genetically distinct subgroups, shown in table 1. MPO ANCA+ was associated with HLA-DQ, whereas the ANCA− subset was associated with IL5/IRF1 and the barrier protein GPA33, both of which were not associated with MPO+ subsets [24].
TABLE 1.
Clinical and genetic subset of eosinophilic granulomatosis with polyangiitis and anti-neutrophil cytoplasmic antibody (ANCA) status
| ANCA positive | ANCA negative | |
| Genetic association | HLA DQA1 and HLA DRB1 | GPA33 and IL-5/IRF1 |
| Pathogenesis | Vasculitis Similar to other ANCA-associated vasculitis syndromes |
Eosinophilic infiltration Similar to asthma |
| Organ involvement | Glomerulonephritis Alveolar haemorrhage Peripheral neuropathy |
Pulmonary infiltrates Cardiomyopathy Purpura |
These aren't completely distinct entities, with clinical findings overlapping in patients; cardiomyopathy and neuropathy can develop as a result of eosinophilic infiltration and vasculitis, so both processes are not mutually exclusive [24–26].
Clinical presentation
Clinical findings and course
Most patients experience a classic three phases pattern of symptoms and signs as shown in figure 1. EGPA may not necessarily manifest itself in such a defined order and distinct phases, with some patients only exhibiting some of these conditions. The mean time for the progression of patients from the initial prodromal allergic phase to the terminal vasculitic phase can take between 3 and 9 years [27].
FIGURE 1.
Three clinical phases of eosinophilic granulomatosis with polyangiitis.
Organ involvement
Respiratory
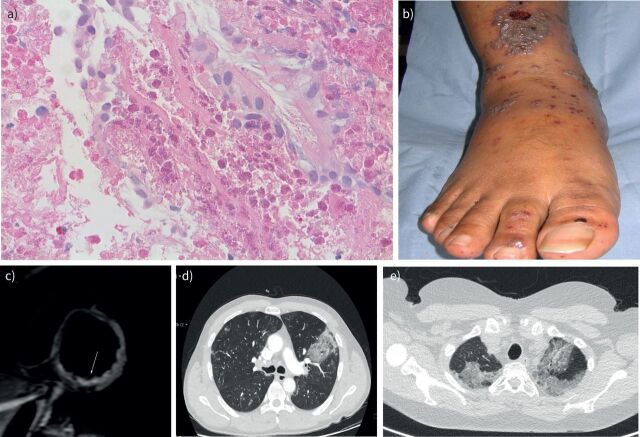
Asthma occurs in all cases of EGPA and tends to be severe with most patients becoming reliant on frequent courses or maintenance oral corticosteroids. A retrospective study looking at 157 patients reported that asthma preceded other systemic manifestations by a mean of 11.8 years and the severity of asthma increased 3–6 months before the onset of systemic disease. Patients were followed up for a mean of 7.4 years and 27% of patients went onto develop non-reversible obstructive symptoms [28]. Eosinophilic lung tissue infiltration was seen in 58% of patients including ground-glass changes, consolidation, pulmonary nodules and pleural effusions (figure 2d, e).
FIGURE 2.
a) Eosinophilic tissue infiltration. b) Vasculitic rash in a patient with eosinophilic granulomatosis with polyangiitis (EGPA). c) Cardiac magnetic resonance imaging (MRI), T2W MRI showing an area of high signal in an acutely injured, inflamed inferior wall in a patient with EGPA (image courtesy of Tevfik Ismail, Consultant Cardiologist, Guy's and St Thomas' NHS Trust). d, e) Computed tomography (CT) of the chest showing peribronchovascular and patchy ground-glass opacification in a 45-year-old male patient (d) and 48-year-old female (e) patient with EGPA. Images in panels a, b, d and e are the authors’ own, all images are published with patient consent.
Sinus
Up to 75% of patients present with chronic rhinosinusitis. ∼55% of patients tend to have a background of nasal polyposis, with a past history of surgery or frequent use of oral corticosteroids [28].
Skin
Patients may present with palpable purpura or petechiae (especially of lower limbs); however, other lesions such as subcutaneous nodules can also occur (figure 2b) [26].
Cardiac
Cardiac manifestations range widely; they are caused both by eosinophilic infiltration and vasculitis. Cardiac disease is the primary cause of mortality in patients with EGPA. Coronary arteritis can lead to myocardial infarction and myocarditis, leading to fibrosis and thus restrictive cardiomyopathy. Untreated, this can lead to heart failure with associated morbidity and mortality. Other presentations include pericarditis, arrhythmias and pericardial effusions. Troponin at baseline and during flares is a useful and easily available blood marker. ECG and echocardiography are recommended for all patients. Cardiac magnetic resonance imaging (MRI) is the most sensitive diagnostic technique and a valuable tool to detect cardiac involvement early (figure 2c) [29, 30].
Gastrointestinal
Eosinophilic infiltration in the gastrointestinal tract leads to abdominal pain, diarrhoea and nausea. This may present before or in conjunction with the vasculitis phase. Patients present with mucosal ulceration especially affecting the duodenum, rectal bleeding and ischaemic bowel. Emergency presentations can also occur such as bowel obstructions and perforation necessitating surgery [31].
Peripheral neuropathy
Peripheral neuropathy can present in 75–80% of patients, with central nervous system involvement in 10–39%. Patients present initially with sensory impairment, followed by motor deficits. The most common presentation is mononeuritis multiplex, with axonal derangement, which leads to paraesthesia and pain. The most common areas tend to be the dermatomes supplied by the common peroneal nerves and tibial nerves. In the upper limb the tibial, ulnar and median nerves are usually affected. Nerve conduction studies can aid with the diagnosis of patients [32].
Renal
Renal involvement occurs in ∼25% patients and the most common presentation is with necrotising crescentic glomerulonephritis. This is commonly found in ANCA positive cases. Clinical features can vary from isolated proteinuria, isolated haematuria to rapidly progressive glomerulonephritis [26]. Although it was initially thought that renal involvement in EGPA was benign; increasingly, studies have reported that nearly 20% of patients can develop end-stage renal disease in 4 years from first presentation, warranting regular monitoring and early management of renal disease [33, 34].
Clinical outcomes
The five-factor score (FFS), created by the French Vasculitis Study Group, can be used at diagnosis to assess the severity of EGPA and to predict prognosis. Initially proposed in 1996, it underwent revision in 2009 and assigns scores depending on organ involvement, clinical and biological parameters. One point each is attributed to older age (>65 years), renal insufficiency, cardiac insufficiency and significant gastrointestinal involvement, as well as for the absence of ENT (ear, nose and throat) manifestations.
It has been reported that survival rate is improved when stratifying treatment according to the FFS and that the FFS score at diagnosis can help to predict the chance of relapse [35–37]. 2009 FFS values of 0, 1, and 2 are associated with respective 5-year mortality rates of 9%, 21%, and 40%; [37]. Factors which influence the frequency of relapse have noted to be lower eosinophil count at baseline and MPO-positivity [25, 26, 35].
A study looking at 50 patients reported that higher doses of steroids were needed to maintain remission in ANCA positive patients [38]. This was supported by another study which observed more frequent relapses in patients with MPO targeted ANCA [35]. Low-dose, long-term corticosteroid treatment seems to reduce relapse risk; however, this needs to be balanced against corticosteroid-related side-effects [34].
The most commonly reported sequelae in the disease are asthma and neurological symptoms [35]. Cardiac manifestations in the form of myocardial infarction, myocarditis and coronary arteritis have been demonstrated to affect survival [26], as did age of onset ≥65 years [39]. Biologic therapies are expected to affect survival and relapse rates positively in the future.
Classification of EGPA
ANCA-associated vasculitides pose as a diagnostic challenge for many clinicians. Various diagnostic and classification criteria have been published. The 1984 Lanham criteria, the American College of Rheumatology classification criteria, the 2012 revised Chapel Hill Consensus conference and the EGPA consensus task force have helped to define and identify patients for clinical trials [5, 40–42].
The 2022 American College of Rheumatology/European Alliance of Associations classification criteria is a newly validated tool used to assist in diagnosing EGPA (table 2) [41]. It is applied to patients who have a confirmed diagnosis of a small- or medium-vessel vasculitis. It allocates different scores to different sub-criteria; a score ≥6 is needed for a diagnosis of EGPA.
TABLE 2.
The 2022 American College of Rheumatology/European Alliance of Associations eosinophilic granulomatosis with polyangiitis classification criteria
| Clinical criteria | Obstructive airway disease | +3 |
| Nasal polyps | +3 | |
| Mononeuritis multiplex | +1 | |
| Laboratory criteria | Blood eosinophil count ≥1×109 cells per L | +5 |
| Biopsy evidence of extravascular eosinophilic inflammation | +2 | |
| Positive test for cytoplasmic antineutrophilic cytoplasmic antibodies (cANCA) or anti-proteinase 3 (PR3) antibodies | −3 | |
| Haematuria | −1 |
Reproduced from [41] with permission.
This criterion was altered as previous versions led to frequent overlaps between different AAV. The criteria were validated with a group of 119 EGPA cases and 437 comparators; the sensitivity was found to be 85% (95% CI 77–91%) and specificity 99% (95% CI 98–100%) [41].
The European Respiratory Society formed the EGPA consenus task force which made 22 recommendations for the diagnosis and management of EGPA (table 3) [5]. This involved guidance around the diagnostic criteria for the condition. These criteria encompass the varying nature of patients’ presentations, whilst also setting the requirement of having clinical and serological proof of EGPA.
TABLE 3.
The European Respiratory Society eosinophilic granulomatosis with polyangiitis diagnostic criteria
| Asthma |
| Blood eosinophil count ≥1×109 cells per L |
| And at least two of the following: |
| ANCA positivity (myeloperoxidase or proteinase 3) |
| Palpable purpura |
| Alveolar haemorrhage |
| Glomerulonephritis |
| Cardiomyopathy |
| Histopathological evidence of eosinophilic vasculitis |
| Sino-nasal disease |
| Non-fixed pulmonary infiltrates |
| Mono or polyneuropathy |
Information from [13].
Relationship between SEA and EGPA
Respiratory manifestations, especially in the form of asthma, tend to present several years prior to diagnosis of EGPA [28]. SEA is considered to be a prodromal phase of EGPA for some patients (see the case report). With initial respiratory tract symptoms affecting the upper and lower airways, patients then develop organ involvement due to eosinophilic tissue infiltration and vasculitis [43]. Due to the similarity in the pathogenesis of the two conditions, driven by hyper-eosinophilia, similar treatments have been and continue to be trialled and tested. This includes anti-IL-5 biological agents, which have acted as corticosteroid-sparing treatments in both SEA [44, 45] and EGPA [13, 46].
Diagnostic work-up
There are currently no reliable biomarkers that can be used to identify and diagnose EGPA [47]; however, a comprehensive work-up of patients is essential to affirm diagnosis and set out treatment plans. This includes IgE titre levels, IgG and subsets, rheumatoid factor, C-reactive protein, erythrocyte sedimentation rate, antinuclear antibodies (ANCA), troponin, renal function, tryptase and vitamin B12. Stool and serology sampling for parasites ought to be considered depending on the clinical and travel history, as should a thorough drug and toxin review [7]. Organ specific testing should be utilised when appropriate. To assess pulmonary health, functional testing alongside radiological tests such as CT scans and chest radiographs are to be used. Echocardiogram or preferentially cardiac MRIs should be used to rule out involvement. Urinalysis should be sued to assess for haematuria and bone marrow biopsy to rule out haematological causes for eosinophilia [3, 5, 40].
Biopsy of affected organ systems can aid in diagnosing EGPA and monitoring relapses. Biopsies may illustrate eosinophilic infiltrates, small-to-medium sized vessel vasculitis and extravascular granulomas. The vasculitis consists of fibroid necrosis of the vasculature wall and can be associated with eosinophilic infiltrates and palisading granulomas [6, 41]. An in-depth assessment will help to evaluate inflammatory activity and differentiate the disease from other hyper-eosinophilic conditions.
Differential diagnoses
Vasculitic and eosinophilic disorders primarily form the differential diagnoses for EGPA. Other small vessel AAVs, such as granulomatosis with polyangiitis and microscopic polyangiitis, may present with similar clinical features to EGPA. However, the latter is characterised by elevated blood and tissue eosinophils and the presence of asthma [40], whilst the others are not.
Chronic eosinophilic pneumonia (CEP) commonly presents with symptoms of breathlessness, cough, fatigue and low-grade fever as well as pulmonary infiltrates and hyper-eosinophilia and it can as such be difficult to distinguish from EGPA, especially EGPA in its early phase. Asthma can precede or accompany CEP in up to 50% of cases. However, EGPA is a vasculitic multisystem disease whereas CEP is not [48].
Non-myeloid neoplasms, including Hodgkin Lymphoma, T-cell neoplasms and some solid tumours, may be associated with hyper-eosinophilia and should be excluded [49].
Hyper-eosinophilic syndrome (HES) comprises of serum hyper-eosinophilia and tissue eosinophilic infiltration. This leads to organ damage and dysfunction [50, 51]. Clinically, both HES and EGPA can present with similar symptoms due to the organs affected. Patients present with sino-nasal disease and eosinophilic pneumonia; however, in HES patients do not frequently present with asthma [52]. HES are heterogeneous with idiopathic or overlap syndromes most commonly presenting with pulmonary infiltrations; myeloid HES sees clonal eosinophilic involvement, such as FIP1L1/PDGFRA; in lymphoid-variant HES there is a clonal or phenotypically aberrant lymphoid population [50, 51].
Management
Scoring tools
There are currently no specific assessment and monitoring tools that exist for EGPA. General vasculitis assessment tools such as the Birmingham Vasculitis Activity Score (BVAS) and asthma-specific tools such as the Asthma Control Questionnaire (ACQ) can be utilised. These can be used alongside clinical presentation and biochemistry to assess for severity of disease and monitor for flares. The BVAS tool looks at 56 organ-based symptoms and scores these depending on if they are new or worsening in the past 4 weeks [53]. It is not specific for EGPA and is more commonly used in other AAV. The ACQ focuses on the past 1 week and has seven items which patients respond to, including spirometry results. This, alongside other biomarkers allows for treatment to be started or escalated as needed [54]. A recent retrospective study looked at 119 patients with EGPA and compared accuracy of using the FFS and BVAS in assessing survival and concluded that the 2009 FFS had the best prognostic accuracy for survival [36].
Treatment: induction regimes
Corticosteroids
Due to the rarity of the condition, there is a paucity of randomised controlled trials looking at gold-standard remission induction and remission treatments for patients.
In patients without poor prognostic factors, i.e. FFS score of 0, or limited disease corticosteroids alone are used to achieve remission. Corticosteroids can be given intravenously (usually methylprednisolone 500–1000 mg for 1–3 days in more severe cases) or orally 1 mg·kg−1·day−1[55]. Due to the well-documented side-effects of corticosteroids, the dose ought to be tapered to the lowest dose possible. In patients experiencing disease relapse when the dose is tapered down [12], steroid-sparing agents, such as immunosuppressants, or anti-IL-5 biologics are used to maintain remission.
Immunosuppressants
Patients with an FFS score of 0 are found to enter remission with just corticosteroids; however, patients with FFS ≥1 have a worse prognosis and are usually treated with glucocorticoid and immunosuppressants.
Cyclophosphamide
Cyclophosphamide has traditionally been used to induce remission whilst azathioprine, methotrexate and mycophenolate mofetil (MMF) are used to maintain remission alongside oral corticosteroids.
Cyclophosphamide has successfully been used to induce remission in a randomised controlled trial comparing six and 12 cyclophosphamide pulses given with corticosteroids to 67 patients. Both groups achieved remission and were equally effective; however, relapses were more common in the six pulses group [35]. In granulomatosis with polyangiitis and microscopic polyangiitis vasculitides, cyclophosphamide was equally effective when given as continuous oral therapy versus intravenous pulses with a lower risk of adverse effects in the intravenous group. Side-effects of cyclophosphamide include bone marrow suppression and increased risk of cancer, as well as ovarian failure and sperm abnormalities [56].
The usefulness of other immunosuppressants for EGPA lacks prospective, EGPA-focussed trial data and their effectiveness remains controversial. A randomised controlled trial (CHUSPAN2) which looked at different non-severe AAV including EGPA, concluded that azathioprine was not superior to placebo in preventing relapse, inducing remission or reducing exacerbation rate of asthma when administered for 12 months [57]. A retrospective study of 188 Japanese patients with EGPA suggested that over a median follow-up period of 56 months, azathioprine may be an independent factor for lower relapse rates [25]. Azathioprine has been compared to other treatments and in patients with AAV it was found to be equally effective as methotrexate but with lower efficacy when compared with rituximab [58, 59].
In a prospective trial of 28 patents with EGPA, methotrexate, when given alongside prednisolone, achieved remission in 72% but relapse occurred in 50% within 1 year [60]. A small observational study of 15 patients with newly diagnosed EGPA reported remission in 67% of patients who received MMF together with prednisolone at 12 months [61]. However, MMF was found to be less effective than azathioprine in non-EGPA AAVs when used for remission maintenance [62]. Similarly, in patients with relapsing and refractory EGPA, MMF was found to be less effective than rituximab [63]. Immunosuppressants require regular blood and lung function testing to monitor for organ toxicity.
Rituximab
Rituximab is an anti-CD20 chimeric mouse-human monoclonal IgG antibody that induces B-cell depletion. Case series and cohort studies report success rates in inducing remission in up to 80% of patients although lower rates are reported in ANCA negative patients [64, 65].
The REOVAS trial, a double-blind controlled trial of rituximab in EGPA randomised patients with an FFS score of 0 into rituximab+corticosteroids versus corticosteroids and patients with FFS score of ≥1 to rituximab+corticosteroids versus cyclophosphamide+corticosteroids. Rituximab was not found to be superior in either subgroup. Results of a trial looking at rituximab versus azathioprine as maintenance of remission are expected in due course (MAINRITSEG study; ClinicalTrials.gov identifier: NCT03164473). Earlier studies are presented in table 4.
TABLE 4.
Summary of trials investigating the use of anti-IgE and anti-IL-5/5R monoclonal antibodies in EGPA
| Name of biologic and target | First author [ref.], year | Study type |
Number of patients,
other immunosuppresants |
Remission or response definition and end-points |
Results |
| Rituximab, anti-CD20 | Teixeira [66], 2019 | Retrospective cohort study | 69 patients; other immunosuppressants allowed but not specified | Vasculitic end-point: BVAS of 0 with <5 mg·day−1 of oral glucocorticoid |
58% of patients achieved remission with 7% being in remission with no corticosteroid need. 54% of patients relapsed by 24 months. ANCA-positive patients had a significantly longer relapse-free survival time than ANCA-negative. The median prednisolone dose reduced at 6, 12 and 24 months from 12.5 to 7, 7.5 and 5 mg·day−1. |
| Thiel [64], 2017 | Retrospective cohort study | 14 patients; methotrexate, azathioprine, MMF | Vasculitic end-point: BVAS of 0 and ≤7.5 mg·day−1 of prednisolone |
28 patients with severe organ threatening EGPA previously refractory to other immunosuppressive treatment regimens were treated with rituximab (n=14) or cyclophosphamide (n=14). 36% on rituximab and 29% on cyclophosphamide achieved complete remission at 48 months. Both groups had a significant decrease in median prednisolone dose. | |
| Mohammad [63], 2016 | Retrospective cohort study |
41 patients; MMF, azathioprine, cyclophosphamide, methotrexate, i.v. immunoglobulin | Vasculitic end-point: BVAS of 0 with <7.5 mg·day−1 of oral glucocorticoid |
49% of patients achieved remission with 5% not requiring glucocorticoids at 12 months. 10% of patients relapsed after achieving remission. The average oral glucocorticoid requirement decreased. ANCA-positive patients had a higher remission rate at 12 months. | |
| Omalizumab, anti-IgE | Celebi Sozener[67], 2018 | Retrospective cohort study |
18 patients; other immunosuppressants allowed but not specified |
Eosinophilic end-point: Absence of asthma and/or ENT exacerbations with a prednisolone dosage of ≤7.5 mg day−1 |
Full response in 55.6% of patients, no response in 38.9%. Daily OCS dose and asthma exacerbations reduced over 52 weeks. |
| Jachiet [68], 2016 | Retrospective cohort study |
17 patients; MMF, azathioprine, methotrexate |
Eosinophilic and vasculitic end-points: Response: absence of asthma and/or sino-nasal exacerbations with prednisolone dose <7.5 mg·day−1 BVAS and CRP levels |
Full response in 35%, partial response in 30% of patients on ≥7.5 mg·day−1 of prednisolone. Reducing OCS was potentially associated with severe EGPA flares. | |
| Reslizumab, anti-IL-5 | Manka [69], 2021 | Open-label pilot study | 10 patients; MMF, azathioprine, methotrexate |
Eosinophilic and vasculitic end-points: Worsening symptoms needing increased mOCS, physiological and blood markers |
Reduction in OCS dose. At the end of the trial, 75% of patients were receiving OCS ≤5 mg and 37.5% of patients received 0 mg. 67.25% of patients were able to taper their OCS dose without having an exacerbation of their symptoms. Improvement of BVAS. |
| Kent [70], 2020 | Prospective cohort study | 9 patients; continuation of other immunosuppressants not mentioned | Eosinophilic and vasculitic end-points: ACQ-7 score, mini-AQLQ, FEV1, BVAS, peripheral eosinophil count |
Significant reduction in OCS dose from 23.4 mg·day−1 to 5.9 mg·day−1 at 48 months. All patients had a ≥50% reduction of median OCS dose. Significant improvement in mini-AQLQ score but not in ACQ-7 score, BVAS and spirometry. | |
| Mepolizumab, anti-IL-5 | Bettiol [15], 2022 | Retrospective cohort study |
191 patients; MMF, azathioprine, methotrexate, cyclosporine, rituximab, i.v. immunoglobulin |
Vasculitic and eosinophilic end-point: Response: No disease activity (BVAS=0) and prednisolone or prednisone dose (or equivalent) of ≤4 mg·day−1 Relapse includes worsening asthma symptoms |
30.4% and 35.7% of patients achieved complete response at 12 and 24 months. 31% of patients relapsed after remission for a median of 6 months. |
| Steinfeld [71], 2019 | Randomised, placebo-controlled double-blind parallel-group trial | 68 patients; other immunosuppressants allowed but not specified | Vasculitic and eosinophilic end-points: Remission: No disease activity (BVAS=0) and a prednisolone or prednisone dose (or equivalent) of ≤4 mg·day−1 Relapse: BVAS >0 or worsening ACQ-6 |
53% of patients achieved remission in the mepolizumab group versus 19% in the placebo group. 57% of patients in the mepolizumab group were able to reduce their OCS dose by ≥50% compared with 21% in the placebo group. 18% of patients were relapse-free in the placebo group compared with 44% in the mepolizumab group. | |
| Wechsler [13], 2017 | Double-blind, parallel-group phase 3 trial | 68 patients; other immunosuppressants allowed but not specified | Vasculitic and eosinophilic end-points: Remission: No disease activity (BVAS=0) and a prednisolone or prednisone dose (or equivalent) of ≤4 mg·day−1 Relapse includes worsening asthma symptoms |
28% of patients receiving mepolizumab and 3% of placebo patients experienced ≥24 weeks of accrued remission. 32% of patients in the mepolizumab group versus 3% in the placebo group achieved remission at both 36 weeks and 48 weeks. Remission didn't occur in 47% of patients in the mepolizumab and 81% of patients in the placebo group. The relapse rate was 1.14 in the mepolizumab group and 2.27 in the placebo group. | |
| Benralizumab, anti-IL-5R | Guntur [46], 2021 | Prospective open-label pilot study | 10 patients; MMF, azathioprine, methotrexate |
Eosinophilic and vasculitic end-points: Worsening symptoms needing increase in mOCS, ACQ, AQLQ, BVAS, physiological and blood markers | Mean OCS dose reduced at the end of the trial period from 15 mg·day−1 to 2 mg·day−1. Mean annualised exacerbation rate was lowest during treatment period compared with the pre- and post-treatment period. No difference in BVAS was noted at the beginning and end of the study. |
| Nanzer [14], 2020 | Retrospective study | 11 patients; continuation of other immunosuppressants not mentioned | Eosinophilic and vasculitic end-points: ACQ-7 score, mini-AQLQ, FEV1, BVAS, peripheral eosinophil count |
At 24 weeks, the median reduction in OCS dose was 50%. 73% of patients reduced their dose by over 50%. At 48 weeks, the median reduction in OCS dose was 65%. 89% of patients were able to reduce their dose by over 50%. A significant improvement was noted in BVAS at 24 and 48 weeks. |
IL: interleukin; EGPA: eosinophilic granulomatosis with polyangiitis; BVAS: Birmingham Vasculitis Activity Score; ANCA: anti-neutrophil cytoplasmic antibody; MMF: mycophenolate mofetil; ENT: ear, nose and throat; OCS: oral corticosteroid; CRP: C-reactive protein; mOCS: maintenance OCS; ACQ: Asthma Control Questionnaire; AQLQ: Asthma Quality of Life Questionnaire; FEV1: forced expiratory volume in 1 s.
Treatment: anti-eosinophilic biologic therapies
Monoclonal antibodies, including therapies which specifically target eosinophils through suppressing Th2 inflammation, have been trialled and proven effective in SEA, HES and more recently, EGPA. They have proven most successful in airways dominant EGPA with effective steroid-sparing activity. The results of recent studies are summarised in the table 4.
IL-5 plays a vital role in the proliferation and differentiation of eosinophils and in preventing apoptosis. Monoclonal antibodies against IL-5 or the IL-5 receptor (anti-IL5/5R) have proven effective in disease control and steroid-sparing activity in asthma [72]. In EGPA, so far only mepolizumab has been tested in a randomised controlled trial [13], whereas the effect of benralizumab and reslizumab has been reported in small observational studies (table 4). A multicentre active controlled phase 3 study comparing the efficacy and safety of benralizumab versus mepolizumab in patients with relapsing or refractory EGPA is currently ongoing (MANDARA study; ClinicalTrials.gov identifier: NCT04157348), as are trials with reslizumab and benralizumab.
Biologics may be a means through which complete remission can be achieved with reduced exposure to corticosteroids for patients. However, larger randomised controlled trials with longer follow-up periods are required to better understand the optimal use of these medications.
Conclusion
EGPA remains a rare but complex and heterogeneous multisystem disease. In the future, outcomes may improve through early diagnosis, updates in classification criteria and the use of novel biologic agents. Corticosteroids remain effective in inducing remission and maintenance of it, but their long-term side-effects can be deleterious. The dichotomy between ANCA positive versus ANCA negative EGPA shapes clinical presentation, diagnosis, monitoring and, importantly, treatments and outcomes. A deeper understanding of the pathophysiology of EGPA and its subgroups will support the development of effective, safe and personalised treatments.
Key points
Respiratory manifestations, in the form of asthma and sino-nasal disease, tend to precede other clinical presentations in patients by several years.
No reliable biomarkers have been identified for the identification and diagnosis of EGPA; however, a thorough work-up using different laboratory and radiological techniques should be carried out in a patient suspected of having the condition.
Monoclonal antibodies targeting IL-5 have proven effective in small cohort studies for disease remission and as steroid-sparing agents, with larger randomised controlled trials being undertaken currently.
Self-evaluation questions
- According to a genome-wide association study, which genetic associations were identified with the MPO ANCA positive subgroup?
- GPA33 and IL5/IRF1
- HLA-DQA1 and IL5/IRF1
- HLA-DQA1 and HLA-DRB1
- HLA-DRB1 and GPA33
- Which scoring tool can be utilised to assess severity and estimate prognosis at diagnosis?
- Birmingham Vasculitis Activity Score
- Five-factor score
- Asthma Quality of Life Questionnaire
- Asthma Control Questionnaire
- Which class of biologic agents does benralizumab belong to?
- Anti-CD20
- Anti-IgE
- Anti-IL-5R
- Anti-IL-4
- Involvement of which organ system is the primary cause of mortality in patients with EGPA?
- Cardiac
- Respiratory
- Gastrointestinal
- Renal
Suggested answers
1. c.
2. b.
3. c.
4. a.
Footnotes
Conflict of interest: V. Alam has nothing to disclose.
Conflict of interest: A.M. Nanzer has received speaker's fees and conference support from AstraZeneca, Chiesi, Teva and Napp, outside the submitted work.
References
- 1.Churg J, Strauss L. Allergic granulomatosis, allergic angiitis, and periarteritis nodosa. Am J Pathol 1951; 27: 277–301. [PMC free article] [PubMed] [Google Scholar]
- 2.Fagni F, Bello F, Emmi G. Eosinophilic granulomatosis with polyangiitis: dissecting the pathophysiology. Front Med (Lausanne) 2021; 8: 627776. doi: 10.3389/fmed.2021.627776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldini C, Talarico R, Della Rossa A, et al. Clinical manifestations and treatment of Churg–Strauss syndrome. Rheum Dis Clin North Am 2010; 36: 527–543. doi: 10.1016/j.rdc.2010.05.003 [DOI] [PubMed] [Google Scholar]
- 4.Mohammad AJ. An update on the epidemiology of ANCA-associated vasculitis. Rheumatology (Oxford) 2020; 59: Suppl. 3, iii42–iii50. doi: 10.1093/rheumatology/keaa089 [DOI] [PubMed] [Google Scholar]
- 5.Groh M, Pagnoux C, Baldini C, et al. Eosinophilic granulomatosis with polyangiitis (Churg–Strauss) (EGPA) Consensus Task Force recommendations for evaluation and management. Eur J Intern Med 2015; 26: 545–553. doi: 10.1016/j.ejim.2015.04.022 [DOI] [PubMed] [Google Scholar]
- 6.Lie JT. Illustrated histopathologic classification criteria for selected vasculitis syndromes. American College of Rheumatology Subcommittee on Classification of Vasculitis. Arthritis Rheum 1990; 33: 1074–1087. doi: 10.1002/art.1780330804 [DOI] [PubMed] [Google Scholar]
- 7.Taweesedt PT, Nordstrom CW, Stoeckel J, et al. Pulmonary manifestations of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome: a systematic review. Biomed Res Int 2019; 2019: 7863815. doi: 10.1155/2019/7863815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sada KE, Amano K, Uehara R, et al. A nationwide survey on the epidemiology and clinical features of eosinophilic granulomatosis with polyangiitis (Churg-Strauss) in Japan. Mod Rheumatol 2014; 24: 640–644. doi: 10.3109/14397595.2013.857582 [DOI] [PubMed] [Google Scholar]
- 9.Mouthon L, Dunogue B, Guillevin L. Diagnosis and classification of eosinophilic granulomatosis with polyangiitis (formerly named Churg-Strauss syndrome). J Autoimmun 2014; 48–49: 99–103. doi: 10.1016/j.jaut.2014.01.018 [DOI] [PubMed] [Google Scholar]
- 10.Moosig F, Bremer JP, Hellmich B, et al. A vasculitis centre based management strategy leads to improved outcome in eosinophilic granulomatosis and polyangiitis (Churg–Strauss, EGPA): monocentric experiences in 150 patients. Ann Rheum Dis 2013; 72: 1011–1017. doi: 10.1136/annrheumdis-2012-201531 [DOI] [PubMed] [Google Scholar]
- 11.Gendelman S, Zeft A, Spalding SJ. Childhood-onset eosinophilic granulomatosis with polyangiitis (formerly Churg–Strauss syndrome): a contemporary single-center cohort. J Rheumatol 2013; 40: 929–935. doi: 10.3899/jrheum.120808 [DOI] [PubMed] [Google Scholar]
- 12.Ribi C, Cohen P, Pagnoux C, et al. Treatment of Churg–Strauss syndrome without poor-prognosis factors: a multicenter, prospective, randomized, open-label study of seventy-two patients. Arthritis Rheum 2008; 58: 586–594. doi: 10.1002/art.23198 [DOI] [PubMed] [Google Scholar]
- 13.Wechsler ME, Akuthota P, Jayne D, et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med 2017; 376: 1921–1932. doi: 10.1056/NEJMoa1702079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nanzer AM, Dhariwal J, Kavanagh J, et al. Steroid-sparing effects of benralizumab in patients with eosinophilic granulomatosis with polyangiitis. ERJ Open Res 2020; 6: 00451-2020. doi: 10.1183/23120541.00451-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bettiol A, Urban ML, Dagna L, et al. Mepolizumab for eosinophilic granulomatosis with polyangiitis: a European multicenter observational study. Arthritis Rheumatol 2022; 74: 295–306. doi: 10.1002/art.41943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keogh KA. Leukotriene receptor antagonists and Churg–Strauss syndrome: cause, trigger or merely an association? Drug Saf 2007; 30: 837–843. doi: 10.2165/00002018-200730100-00003 [DOI] [PubMed] [Google Scholar]
- 17.Dallos T, Heiland GR, Strehl J, et al. CCL17/thymus and activation-related chemokine in Churg–Strauss syndrome. Arthritis Rheum 2010; 62: 3496–3503. doi: 10.1002/art.27678 [DOI] [PubMed] [Google Scholar]
- 18.Jakiela B, Szczeklik W, Plutecka H, et al. Increased production of IL-5 and dominant Th2-type response in airways of Churg–Strauss syndrome patients. Rheumatology (Oxford) 2012; 51: 1887–1893. doi: 10.1093/rheumatology/kes171 [DOI] [PubMed] [Google Scholar]
- 19.Wechsler ME, Munitz A, Ackerman SJ, et al. Eosinophils in health and disease: a state-of-the-art review. Mayo Clin Proc 2021; 96: 2694–2707. doi: 10.1016/j.mayocp.2021.04.025 [DOI] [PubMed] [Google Scholar]
- 20.Kiene M, Csernok E, Müller A, et al. Elevated interleukin-4 and interleukin-13 production by T cell lines from patients with Churg–Strauss syndrome. Arthritis Rheum 2001; 44: 469–473. doi: [DOI] [PubMed] [Google Scholar]
- 21.Manger BJ, Krapf FE, Gramatzki M, et al. IgE-containing circulating immune complexes in Churg–Strauss vasculitis. Scand J Immunol 1985; 21: 369–373. doi: 10.1111/j.1365-3083.1985.tb01443.x [DOI] [PubMed] [Google Scholar]
- 22.Dejaco C, Oppl B, Monach P, et al. Serum biomarkers in patients with relapsing eosinophilic granulomatosis with polyangiitis (Churg–Strauss). PLoS One 2015; 10: e0121737. doi: 10.1371/journal.pone.0121737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Austin K, Janagan S, Wells M, et al. ANCA associated vasculitis subtypes: recent insights and future perspectives. J Inflamm Res 2022; 15: 2567–2582. doi: 10.2147/JIR.S284768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyons PA, Peters JE, Alberici F, et al. Genome-wide association study of eosinophilic granulomatosis with polyangiitis reveals genomic loci stratified by ANCA status. Nat Commun 2019; 10: 5120. doi: 10.1038/s41467-019-12515-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saku A, Furuta S, Hiraguri M, et al. Long-term outcomes of 188 Japanese patients with eosinophilic granulomatosis with polyangiitis. J Rheumatol 2018; 45: 1159–1166. doi: 10.3899/jrheum.171352 [DOI] [PubMed] [Google Scholar]
- 26.Comarmond C, Pagnoux C, Khellaf M, et al. Eosinophilic granulomatosis with polyangiitis (Churg–Strauss): clinical characteristics and long-term followup of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheum 2013; 65: 270–281. doi: 10.1002/art.37721 [DOI] [PubMed] [Google Scholar]
- 27.Chakraborty RK, Aeddula NR. Churg Strauss Syndrome. Treasure Island, StatPearls Publishing, 2022. [Google Scholar]
- 28.Cottin V, Bel E, Bottero P, et al. Respiratory manifestations of eosinophilic granulomatosis with polyangiitis (Churg–Strauss). Eur Respir J 2016; 48: 1429–1441. doi: 10.1183/13993003.00097-2016 [DOI] [PubMed] [Google Scholar]
- 29.Liu S, Guo L, Zhang Z, et al. Cardiac manifestations of eosinophilic granulomatosis with polyangiitis from a single-center cohort in China: clinical features and associated factors. Ther Adv Chronic Dis 2021; 12: 2040622320987051. doi: 10.1177/2040622320987051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knockaert DC. Cardiac involvement in systemic inflammatory diseases. Eur Heart J 2007; 28: 1797–1804. doi: 10.1093/eurheartj/ehm193 [DOI] [PubMed] [Google Scholar]
- 31.Kawasaki K, Nakamura S, Esaki M, et al. Gastrointestinal involvement in patients with vasculitis: IgA vasculitis and eosinophilic granulomatosis with polyangiitis. Endosc Int Open 2019; 7: E1333–E1343. doi: 10.1055/a-0977-2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang W, Zhou G, Shi Q, et al. Clinical analysis of nervous system involvement in ANCA-associated systemic vasculitides. Clin Exp Rheumatol 2009; 27: Suppl. 52, S65–S69. [PubMed] [Google Scholar]
- 33.Doreille A, Buob D, Bay P, et al. Renal involvement in eosinophilic granulomatosis with polyangiitis. Kidney Int Rep 2021; 6: 2718–2721. doi: 10.1016/j.ekir.2021.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durel CA, Sinico RA, Teixeira V, et al. Renal involvement in eosinophilic granulomatosis with polyangiitis (EGPA): a multicentric retrospective study of 63 biopsy-proven cases. Rheumatology (Oxford) 2021; 60: 359–365. doi: 10.1093/rheumatology/keaa416 [DOI] [PubMed] [Google Scholar]
- 35.Samson M, Puéchal X, Devilliers H, et al. Long-term outcomes of 118 patients with eosinophilic granulomatosis with polyangiitis (Churg–Strauss syndrome) enrolled in two prospective trials. J Autoimmun 2013; 43: 60–69. doi: 10.1016/j.jaut.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 36.Solans-Laque R, Rodriguez-Carballeira M, Rios-Blanco JJ, et al. Comparison of the Birmingham vasculitis activity score and the five-factor score to assess survival in antineutrophil cytoplasmic antibody-associated vasculitis: a study of 550 patients from Spain (REVAS registry). Arthritis Care Res (Hoboken) 2020; 72: 1001–1010. doi: 10.1002/acr.23912 [DOI] [PubMed] [Google Scholar]
- 37.Kim DS, Song JJ, Park YB, et al. Five factor score of more than 1 is associated with relapse during the first 2 year-follow up in patients with eosinophilic granulomatosis with polyangiitis. Int J Rheum Dis 2017; 20: 1261–1268. doi: 10.1111/1756-185X.13056 [DOI] [PubMed] [Google Scholar]
- 38.Sokolowska BM, Szczeklik WK, Wludarczyk AA, et al. ANCA-positive and ANCA-negative phenotypes of eosinophilic granulomatosis with polyangiitis (EGPA): outcome and long-term follow-up of 50 patients from a single Polish center. Clin Exp Rheumatol 2014; 32: Suppl. 82, S41–S47. [PubMed] [Google Scholar]
- 39.Tsurikisawa N, Oshikata C, Kinoshita A, et al. Longterm prognosis of 121 patients with eosinophilic granulomatosis with polyangiitis in Japan. J Rheumatol 2017; 44: 1206–1215. doi: 10.3899/jrheum.161436 [DOI] [PubMed] [Google Scholar]
- 40.Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised international Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum 2013; 65: 1–11. doi: 10.1002/art.37715 [DOI] [PubMed] [Google Scholar]
- 41.Grayson PC, Ponte C, Suppiah R, et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for eosinophilic granulomatosis with polyangiitis. Ann Rheum Dis 2022; 81: 309–314. doi: 10.1136/annrheumdis-2021-221794 [DOI] [PubMed] [Google Scholar]
- 42.Lanham JG, Elkon KB, Pusey CD, et al. Systemic vasculitis with asthma and eosinophilia: a clinical approach to the Churg–Strauss syndrome. Medicine (Baltimore) 1984; 63: 65–81. doi: 10.1097/00005792-198403000-00001 [DOI] [PubMed] [Google Scholar]
- 43.Ricciardi L, Soler DG, Bennici A, et al. Case report: severe eosinophilic asthma associated with ANCA-negative EGPA in a young adult successfully treated with benralizumab. Front Pharmacol 2022; 13: 858344. doi: 10.3389/fphar.2022.858344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Domingo Ribas C, Carrillo Díaz T, Blanco Aparicio M, et al. Real world effectiveness and safety of mepolizumab in a multicentric Spanish cohort of asthma patients stratified by eosinophils: the REDES study. Drugs 2021; 81: 1763. doi: 10.1007/s40265-021-01597-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kavanagh JE, Hearn AP, Dhariwal J, et al. Real-world effectiveness of benralizumab in severe eosinophilic asthma. Chest 2021; 159: 496–506. doi: 10.1016/j.chest.2020.08.2083 [DOI] [PubMed] [Google Scholar]
- 46.Guntur VP, Manka LA, Denson JL, et al. Benralizumab as a steroid-sparing treatment option in eosinophilic granulomatosis with polyangiitis. J Allergy Clin Immunol Pract 2021; 9: 1186–1193.e1. doi: 10.1016/j.jaip.2020.09.054 [DOI] [PubMed] [Google Scholar]
- 47.Grayson PC, Monach PA, Pagnoux C, et al. Value of commonly measured laboratory tests as biomarkers of disease activity and predictors of relapse in eosinophilic granulomatosis with polyangiitis. Rheumatology (Oxford) 2015; 54: 1351–1359. doi: 10.1093/rheumatology/keu427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marchand E, Reynaud-Gaubert M, Lauque D, et al. Idiopathic chronic eosinophilic pneumonia. A clinical and follow-up study of 62 cases. The Groupe d'Etudes et de Recherche sur les Maladies ‘Orphelines’ Pulmonaires (GERM‘O’P). Medicine (Baltimore) 1998; 77: 299–312. doi: 10.1097/00005792-199809000-00001 [DOI] [PubMed] [Google Scholar]
- 49.Valent P, Klion AD, Horny HP, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol 2012; 130: 607–612.e9. doi: 10.1016/j.jaci.2012.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams KW, Ware J, Abiodun A, et al. Hypereosinophilia in children and adults: a retrospective comparison. J Allergy Clin Immunol Pract 2016; 4: 941–947.e1. doi: 10.1016/j.jaip.2016.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ogbogu PU, Bochner BS, Butterfield JH, et al. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol 2009; 124: 1319–1325.e3. doi: 10.1016/j.jaci.2009.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kahn JE, Groh M, Lefevre G. (A critical appraisal of) Classification of hypereosinophilic disorders. Front Med (Lausanne) 2017; 4: 216. doi: 10.3389/fmed.2017.00216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yumura W, Kobayashi S, Suka M, et al. Assessment of the Birmingham vasculitis activity score in patients with MPO-ANCA-associated vasculitis: sub-analysis from a study by the Japanese Study Group for MPO-ANCA-associated vasculitis. Mod Rheumatol 2014; 24: 304–309. doi: 10.3109/14397595.2013.854075 [DOI] [PubMed] [Google Scholar]
- 54.Juniper EF, Bousquet J, Abetz L, et al. Identifying ‘well-controlled' and ‘not well-controlled' asthma using the Asthma Control Questionnaire. Respir Med 2006; 100: 616–621. doi: 10.1016/j.rmed.2005.08.012 [DOI] [PubMed] [Google Scholar]
- 55.Schleimer RP, Bochner BS. The effects of glucocorticoids on human eosinophils. J Allergy Clin Immunol 1994; 94: 1202–1213. doi: 10.1016/0091-6749(94)90333-6 [DOI] [PubMed] [Google Scholar]
- 56.de Groot K, Harper L, Jayne DR, et al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med 2009; 150: 670–680. doi: 10.7326/0003-4819-150-10-200905190-00004 [DOI] [PubMed] [Google Scholar]
- 57.Puechal X, Pagnoux C, Baron G, et al. Adding azathioprine to remission-induction glucocorticoids for eosinophilic granulomatosis with polyangiitis (Churg-Strauss), microscopic polyangiitis, or polyarteritis nodosa without poor prognosis factors: a randomized, controlled trial. Arthritis Rheumatol 2017; 69: 2175–2186. doi: 10.1002/art.40205 [DOI] [PubMed] [Google Scholar]
- 58.Guillevin L, Pagnoux C, Karras A, et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med 2014; 371: 1771–1780. doi: 10.1056/NEJMoa1404231 [DOI] [PubMed] [Google Scholar]
- 59.Pagnoux C, Mahr A, Hamidou MA, et al. Azathioprine or methotrexate maintenance for ANCA-associated vasculitis. N Engl J Med 2008; 359: 2790–2803. doi: 10.1056/NEJMoa0802311 [DOI] [PubMed] [Google Scholar]
- 60.Metzler C, Hellmich B, Gause A, et al. Churg Strauss syndrome--successful induction of remission with methotrexate and unexpected high cardiac and pulmonary relapse ratio during maintenance treatment. Clin Exp Rheumatol 2004; 22: Suppl. 36, S52–S61. [PubMed] [Google Scholar]
- 61.Philobos M, Perkins A, Karabayas M, et al. A real-world assessment of mycophenolate mofetil for remission induction in eosinophilic granulomatosis with polyangiitis. Rheumatol Int 2021; 41: 1811–1814. doi: 10.1007/s00296-021-04961-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hiemstra TF, Walsh M, Mahr A, et al. Mycophenolate mofetil vs azathioprine for remission maintenance in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized controlled trial. JAMA 2010; 304: 2381–2388. doi: 10.1001/jama.2010.1658 [DOI] [PubMed] [Google Scholar]
- 63.Mohammad AJ, Hot A, Arndt F, et al. Rituximab for the treatment of eosinophilic granulomatosis with polyangiitis (Churg–Strauss). Ann Rheum Dis 2016; 75: 396–401. doi: 10.1136/annrheumdis-2014-206095 [DOI] [PubMed] [Google Scholar]
- 64.Thiel J, Troilo A, Salzer U, et al. Rituximab as induction therapy in eosinophilic granulomatosis with polyangiitis refractory to conventional immunosuppressive treatment: a 36-month follow-up analysis. J Allergy Clin Immunol Pract 2017; 5: 1556–1563. doi: 10.1016/j.jaip.2017.07.027 [DOI] [PubMed] [Google Scholar]
- 65.Akiyama M, Kaneko Y, Takeuchi T. Rituximab for the treatment of eosinophilic granulomatosis with polyangiitis: a systematic literature review. Autoimmun Rev 2021; 20: 102737. doi: 10.1016/j.autrev.2020.102737 [DOI] [PubMed] [Google Scholar]
- 66.Teixeira V, Mohammad AJ, Jones RB, et al. Efficacy and safety of rituximab in the treatment of eosinophilic granulomatosis with polyangiitis. RMD Open 2019; 5: e000905. doi: 10.1136/rmdopen-2019-000905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Celebi Sozener Z, Gorgulu B, Mungan D, et al. Omalizumab in the treatment of eosinophilic granulomatosis with polyangiitis (EGPA): single-center experience in 18 cases. World Allergy Organ J 2018; 11: 39. doi: 10.1186/s40413-018-0217-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jachiet M, Samson M, Cottin V, et al. Anti-IgE monoclonal antibody (omalizumab) in refractory and relapsing eosinophilic granulomatosis with polyangiitis (Churg–Strauss): data on seventeen patients. Arthritis Rheumatol 2016; 68: 2274–2282. doi: 10.1002/art.39663 [DOI] [PubMed] [Google Scholar]
- 69.Manka LA, Guntur VP, Denson JL, et al. Efficacy and safety of reslizumab in the treatment of eosinophilic granulomatosis with polyangiitis. Ann Allergy Asthma Immunol 2021; 126: 696–701.e1. doi: 10.1016/j.anai.2021.01.035 [DOI] [PubMed] [Google Scholar]
- 70.Kent BD, d'Ancona G, Fernandes M, et al. Oral corticosteroid-sparing effects of reslizumab in the treatment of eosinophilic granulomatosis with polyangiitis. ERJ Open Res 2020; 6: 00311-2019. doi: 10.1183/23120541.00311-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Steinfeld J, Bradford ES, Brown J, et al. Evaluation of clinical benefit from treatment with mepolizumab for patients with eosinophilic granulomatosis with polyangiitis. J Allergy Clin Immunol 2019; 143: 2170–2177. doi: 10.1016/j.jaci.2018.11.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hearn AP, Kent BD, Jackson DJ. Biologic treatment options for severe asthma. Curr Opin Immunol 2020; 66: 151–160. doi: 10.1016/j.coi.2020.10.004 [DOI] [PubMed] [Google Scholar]