Short abstract
Evidence supporting the use of balloon pulmonary angioplasty #BPA, which has drastically changed management of non-operable chronic thromboembolic pulmonary hypertension #CTEPH, and rationale for a future multimodal approach with combined medical therapy https://bit.ly/3F7ccKD
The management of chronic thromboembolic pulmonary hypertension (CTEPH) has continued to evolve since the establishment of pulmonary endarterectomy (PEA) as a curative therapy for carefully selected patients with surgically accessible disease. Over the past two decades, the development of balloon pulmonary angioplasty (BPA) and, more recently, the publication of data supporting its potential combination with pulmonary vasodilator therapy is drastically changing the treatment of non-operable or recurrent/residual CTEPH.
BPA is an interventional endovascular technique where specific appropriately sized balloons are used to dilate stenotic segments, webs or slit lesions in pulmonary arteries (figures 1 and 2). Rigorous patient evaluation is crucial, and both computed tomographic pulmonary angiography (CTPA) and conventional pulmonary angiography may be required to appropriately stratify vascular lesions and determinate BPA accessibility. BPA should only be performed in expert centres due to the complexity of the procedure and the significant learning curve effect seen with the procedure.
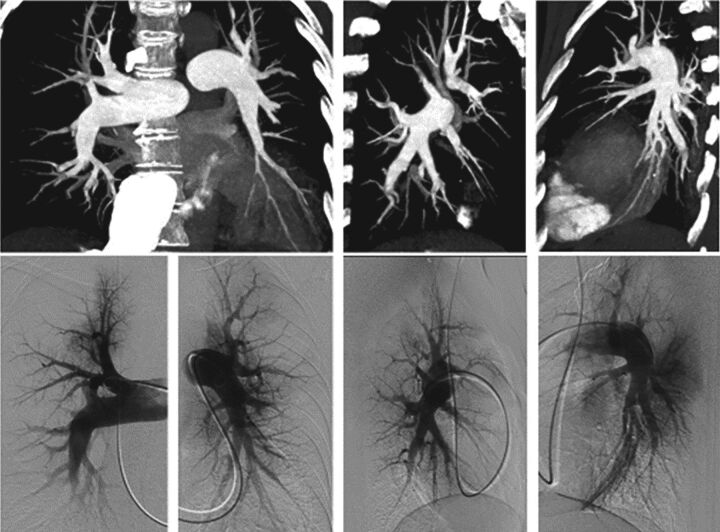
FIGURE 1.
Obstruction of distal pulmonary arteries accessible to balloon pulmonary angioplasty. The top row shows a computed tomography pulmonary angiogram with maximum intensity projection reconstructions, and the bottom row shows pulmonary angiography.
FIGURE 2.
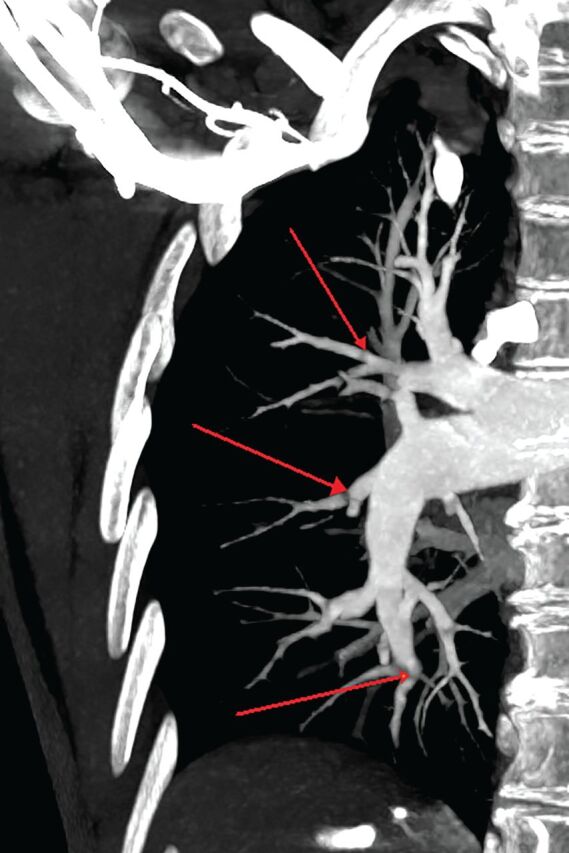
Computed tomography pulmonary angiogram of balloon pulmonary angioplasty-accessible lesions with maximum intensity projection reconstructions. Arrows indicate stenosis and webs.
The process of BPA development began in the 1980s. The original application of BPA was related to non-atherosclerotic arterial stenoses [1]. The method was also used in the treatment of congenital valvular disease [2]. It was not until 1988 that Voorburg et al. [3] published a case report in Chest, which was the first report on the treatment of a patient with “pulmonary hypertension after pulmonary embolism” using this interventional method. Mean pulmonary arterial pressure (mPAP) was reduced from 46 to 35 mmHg by applying BPA in three sessions [3]. In 2001, Feinstein et al. [4] published the first larger cohort including 18 patients that underwent BPA treatment. The average number of procedures was 2.6 (range from 1 to 5) and the average number of dilations was 6 (range from 1 to 12). After a follow-up of 36 months, the authors reported significant improvement in functional parameters (New York Heart Association class reduced from 3.3 to 1.8 (p<0.001) and 6-min walking distance (6MWD) increased from 191.11 to 454.45 m (p<0.0001)), as well as a significant reduction in mPAP from 43.0±12.1 to 33.7±10.2 mmHg (p=0.007) [4]. Due to complications after the intervention, predominantly reperfusion pulmonary oedema but also the need for endotracheal intubation and death (61.1%, 16.6% and 5.5%, respectively), as well as insufficient data in general, BPA was not included in the therapeutic recommendations in the 2009 European Society of Cardiology (ESC)/European Respiratory Society (ERS) guidelines for the diagnosis and treatment of pulmonary hypertension [4, 5].
The wide acceptance of BPA in recent years is largely due to the pioneering work of several Japanese research groups, who refined and improved the procedure. Mizoguchi et al. [6] contributed to the development of the procedure by being the first to use intravascular ultrasound to determine the optimal balloon size. Apart from the mentioned study, the results of two more cohorts from Japan were published in 2012 by Kataoka et al. [7] and Sugimura et al. [8]. A total of 109 patients were included in the interventional treatment, on average 3.6 sessions were performed per patient and reperfusion pulmonary oedema occurred in 103 out of 318 sessions [6–8]. The results indicated a significant improvement in World Health Organization functional class (WHO-FC), 6MWD and mPAP with an acceptable risk profile. In the 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension, BPA garnered a IIb recommendation, meaning that it could be considered as a treatment option in non-operable CTEPH patients [9].
The procedure started to be widely used and several case series were reported from CTEPH expert centres throughout Europe. A German series with 56 patients showed improvements in 6MWD, and a reduction of mPAP and pulmonary vascular resistance (PVR) [10]. Subsequently, a French series of consecutive BPA procedures with 1006 interventions in 184 patients reported a reduction of mPAP and PVR of 26% and 43%, respectively [11]. Similar reports were described in the Japanese registry including patients from seven centres with a 43% decrease in mPAP and a significant reduction of pulmonary arterial hypertension-targeted medical therapy [12]. Available Japanese data indicate a persistence of haemodynamic benefits and survival in the long term [13]. In 2019, Taniguchi et al. [14] showed that the arrival of BPA has significantly improved the survival of non-operable CTEPH. The 1- and 3-year survival rates after implementation of BPA were 91.6% and 85.0%, respectively, compared with 89.0% and 74.3% prior to the availability of the procedure. Thanks to these developments, BPA has assumed an important role in the management of CTEPH patients who are deemed technically inoperable [15].
Complications related to BPA are common but observed total peri-procedural mortality is low and acceptable. In the two largest published series, the French report and the multicentre Japanese registry, lung injury occurred in 9.1% and 17.8% of all sessions and the BPA mortality rates were 3.8% and 3.9%, respectively (table 1 and figure 3) [11, 12]. Brenot et al. [11] reported that peri-procedural complications decreased from 13.3% to 5.9% from the initial period to the last period of their observation, confirming the important learning curve effect with BPA. High baseline mPAP and PVR were detected as predictors of BPA-related adverse events [16].
TABLE 1.
Balloon pulmonary angioplasty complications
| During the procedure | After the procedure |
| Vascular injury with/without haemoptysis | Lung injury (radiographic opacity with/without haemoptysis, with/without hypoxaemia) |
| Wire perforation | Renal dysfunction |
| Balloon over-dilatation | Access site problems |
| High-pressure contrast injection | |
| Vascular dissection | |
| Allergic reaction to contrast |
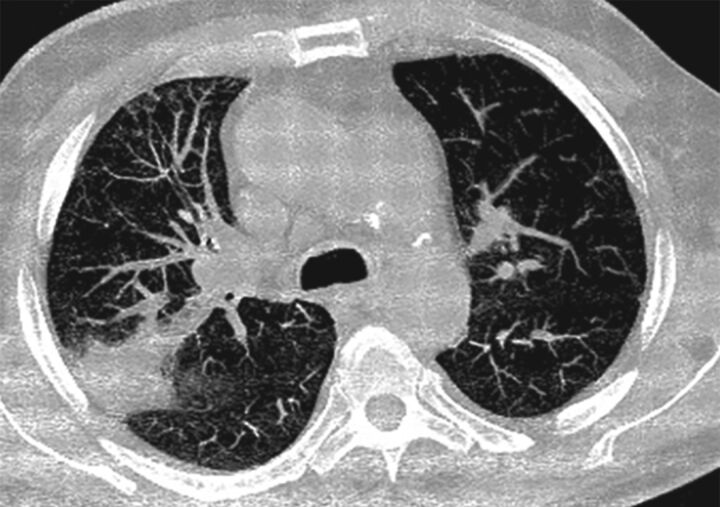
FIGURE 3.
Balloon pulmonary angioplasty-related adverse event. An example of acute lung injury.
While BPA has taken a more important role in the management of patients with non-operable CTEPH, randomised control trials comparing BPA to medical therapy were not available. The pulmonary hypertension community identified this gap in knowledge and just this year two large, randomised controlled trials have been published: the RACE study by Jaïs et al. [17] and the MR BPA study by Kawakami et al. [18]. Both trials compare BPA versus riociguat in non-operable CTEPH. Riociguat, a soluble guanylate cyclase stimulator evaluated in the CHEST-1 and CHEST-2 randomised controlled trials, is an established orally administered medication in patients with non-operable CTEPH that targets the cyclic guanosine monophosphate (cGMP) pathway [19, 20]. As the targets of BPA and riociguat are different, comparing the two treatments as well as assessing the potential for combined therapy is important for clinical management.
The RACE study enrolled 105 patients that were randomly assigned to either riociguat or BPA [17]. At week 26, PVR reduction was more pronounced with BPA than with riociguat. Geometric mean PVR decreased to 39.9% of baseline PVR, corresponding to a mean decrease from baseline of 458.4 dyn·s·cm−5, in the BPA group versus 66.7%, corresponding to a mean decrease from baseline of 200.8 dyn·s·cm−5, for the riociguat group. Patients with persistent symptomatic CTEPH after week 26 had the possibility to receive add-on BPA after first-line riociguat or add-on riociguat after first-line BPA. In patients who remained symptomatic after week 26, add-on therapy with riociguat or BPA allowed further PVR reduction. Treatment-related serious adverse events were more common in the BPA group versus the riociguat group, 42% versus 9% of patients, respectively. Interestingly, after week 26, the incidence of serious adverse events related to BPA was lower in patients who were pretreated with riociguat (14% versus 42%). The pretreated group was significantly less severe in terms of PVR, WHO-FC and 6MWD. Among patients who had BPA at any time, piecewise logistic regression identified mPAP higher than 45 mmHg as a predictive factor associated with BPA-related adverse events.
The MR BPA study enrolled 61 patients that were randomly assigned to BPA or riociguat [18]. The study demonstrated the superiority of BPA in reducing mPAP compared with riociguat. At 12 months, mPAP and PVR decreased by −16.3 mmHg and −332.4 dyn·s·cm−5 in the BPA group and −7 mmHg and −228.3 dyn·s·cm−5 in the riociguat group from baseline. This trial did not add BPA or riociguat during the follow-up period. The greater reduction in mPAP with BPA was likely related to the relief of the macrovascular obstruction by the procedure. The choice of primary endpoint was different between these two trials, decrease of mPAP for MR BPA and of PVR for RACE. Haemosputum, haemoptysis or pulmonary haemorrhage were the most frequently observed adverse events as seen in the RACE trial.
These trials demonstrate that both BPA and riociguat are efficacious treatments for non-operable CTEPH. Sequential add-on therapy appears to be superior to monotherapy and in selected patients pretreatment with riociguat appears to reduce the risk of BPA-related serious adverse events. As the authors have stated, BPA should not replace medical treatment but may be safely used in conjunction with medical treatment. Patients with severe haemodynamic parameters are more at risk for BPA-related complications and these patients may be discussed for a multimodal approach. Thanks to the acquired experience over time and the emerging trials (figure 4), in the most recent 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension, BPA has become an established treatment and garnered a I recommendation [21]. Furthermore, the guidelines suggest using medical treatment before BPA in patients with a PVR >4 Wood units to improve pulmonary haemodynamics and safety of the procedure, although this recommendation is conditional due to the low certainty of the evidence. It is important to specify that these results and safety findings are not generalisable and may only be reproducible in expert centres due to the complexity of CTEPH management. As a next step, the exact definition and treatment sequence for a multimodal approach needs to be defined and some studies are ongoing to give us answers to these questions, including a phase 2b trial evaluating initial dual oral combination therapy versus initial oral monotherapy prior to BPA in non-operable CTEPH (ClinicalTrials.gov identifier: NCT04780932; www.clinicaltrials.gov/ct2/show/NCT04780932). And thinking one step ahead, we need to study de-escalation strategies in patients with normalised haemodynamics after multimodal therapy which includes BPA.
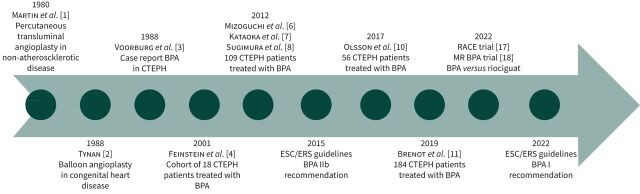
FIGURE 4.
Balloon pulmonary angioplasty (BPA) landmark papers. CTEPH: chronic thromboembolic pulmonary hypertension; ESC: European Society of Cardiology; ERS: European Respiratory Society; RACE: BPA versus riociguat for the treatment of inoperable CTEPH: a multicentre, phase 3, open-label, randomised controlled trial and ancillary follow-up study; MR BPA: BPA versus riociguat in inoperable CTEPH: an open-label, randomised controlled trial.
In patients with chronic thromboembolic pulmonary disease without pulmonary hypertension, BPA should be considered for non-operable lesions (IIa recommendation) [21]. These patients should be selected with a complete assessment of exertional dyspnoea and further studies are needed to understand long-term evolution of this disease.
In conclusion, the treatment of non-operable CTEPH has changed significantly over time since the introduction of BPA. In the future, it is possible that a multimodal approach will become the preferred treatment strategy, but further clinical trials are needed.
Key points
• BPA has become over time an established treatment for patients with inoperable CTEPH or persistent/recurrent pulmonary hypertension after PEA.
• Severe haemodynamic parameters are a risk for BPA-related complications and medical therapy should be considered prior to the procedure.
• Due to a significant learning curve, BPA should be performed in expert high-volume CTEPH centres.
Footnotes
Conflict of interest: T. Gille reports personal fees from Roche S.A.S., other fees from Oxyvie (oxygen provider), other fees from Vivisol France (oxygen provider), other fees from Menarini France, outside the submitted work. L. Bertoletti reports grants or contracts from MSD and Janssen, outside the submitted work; support for attending meetings and/or travel from Janssen. The remaining authors have nothing to disclose.
References
- 1.Martin EC, Diamond NG, Casarella WJ. Percutaneous transluminal angioplasty in non-atheroscklerotic disease. Radiology 1980; 135: 27–33. doi: 10.1148/radiology.135.1.6127750 [DOI] [PubMed] [Google Scholar]
- 2.Tynan M. Balloon angioplasty in congenital heart disease. Herz 1988; 13: 59–70. [PubMed] [Google Scholar]
- 3.Voorburg JA, Cats VM, Buis B, et al. Balloon angioplasty in the treatment of pulmonary hypertension caused by pulmonary embolism. Chest 1988; 94: 1249–1253. doi: 10.1378/chest.94.6.1249 [DOI] [PubMed] [Google Scholar]
- 4.Feinstein JA, Goldhaber SZ, Lock JE, et al. Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation 2001; 103: 10–13. doi: 10.1161/01.CIR.103.1.10 [DOI] [PubMed] [Google Scholar]
- 5.Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2009; 30: 2493–2537. doi: 10.1093/eurheartj/ehp297 [DOI] [PubMed] [Google Scholar]
- 6.Mizoguchi H, Ogawa A, Munemasa M, et al. Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012; 5: 748–755. doi: 10.1161/CIRCINTERVENTIONS.112.971077 [DOI] [PubMed] [Google Scholar]
- 7.Kataoka M, Inami T, Hayashida K, et al. Percutaneous transluminal pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012; 5: 756–762. doi: 10.1161/CIRCINTERVENTIONS.112.971390 [DOI] [PubMed] [Google Scholar]
- 8.Sugimura K, Fukumoto Y, Satoh K, et al. Percutaneous transluminal pulmonary angioplasty markedly improves pulmonary hemodynamics and long-term prognosis in patients with chronic thromboembolic pulmonary hypertension. Circ J 2012; 76: 485–488. doi: 10.1253/circj.CJ-11-1217 [DOI] [PubMed] [Google Scholar]
- 9.Galiè N, Humbert M, Vachiery J-L, et al. 2015. ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2015; 46: 903–975. doi: 10.1183/13993003.01032-2015 [DOI] [PubMed] [Google Scholar]
- 10.Olsson KM, Wiedenroth CB, Kamp J-C, et al. Balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension: the initial German experience. Eur Respir J 2017; 49: 1602409. doi: 10.1183/13993003.02409-2016 [DOI] [PubMed] [Google Scholar]
- 11.Brenot P, Jaïs X, Taniguchi Y, et al. French experience of balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Eur Respir J 2019; 53: 1802095. doi: 10.1183/13993003.02095-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogawa A, Satoh T, Fukuda T, et al. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: results of a multicenter registry. Circ Cardiovasc Qual Outcomes 2017; 10: e004029. doi: 10.1161/CIRCOUTCOMES.117.004029 [DOI] [PubMed] [Google Scholar]
- 13.Inami T, Kataoka M, Yanagisawa R, et al. Long-term outcomes after percutaneous transluminal pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Circulation 2016; 134: 2030–2032. doi: 10.1161/CIRCULATIONAHA.116.024201 [DOI] [PubMed] [Google Scholar]
- 14.Taniguchi Y, Jaïs X, Jevnikar M, et al. Predictors of survival in patients with not-operated chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant 2019; 38: 833–842. doi: 10.1016/j.healun.2019.04.006 [DOI] [PubMed] [Google Scholar]
- 15.Delcroix M, Torbicki A, Gopalan D, et al. ERS statement on chronic thromboembolic pulmonary hypertension. Eur Respir J 2021; 57: 2002828. doi: 10.1183/13993003.02828-2020 [DOI] [PubMed] [Google Scholar]
- 16.Gerges C, Friewald R, Gerges M, et al. Efficacy and safety of percutaneous pulmonary artery subtotal occlusion and chronic total occlusion intervention in chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2021; 14: e010243. doi: 10.1161/CIRCINTERVENTIONS.120.010243 [DOI] [PubMed] [Google Scholar]
- 17.Jaïs X, Brenot P, Bouvaist H, et al. Balloon pulmonary angioplasty versus riociguat for the treatment of inoperable chronic thromboembolic pulmonary hypertension (RACE): a multicentre, phase 3, open-label, randomised controlled trial and ancillary follow-up study. Lancet Respir Med 2022; 10: 961–971. doi: 10.1016/S2213-2600(22)00214-4 [DOI] [PubMed] [Google Scholar]
- 18.Kawakami T, Matsubara H, Shinke T, et al. Balloon pulmonary angioplasty versus riociguat in inoperable chronic thromboembolic pulmonary hypertension (MR BPA): an open-label, randomised controlled trial. Lancet Respir Med 2022; 10: 949–960. doi: 10.1016/S2213-2600(22)00171-0 [DOI] [PubMed] [Google Scholar]
- 19.Ghofrani H-A, Simonneau G, Rubin LJ, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med 2013; 369: 330–340. doi: 10.1056/NEJMoa1209655 [DOI] [PubMed] [Google Scholar]
- 20.Simonneau G, D'Armini AM, Ghofrani HA, et al. Predictors of long-term outcomes in patients treated with riociguat for chronic thromboembolic pulmonary hypertension: data from the CHEST-2 open-label, randomised, long-term extension trial. Lancet Respir Med 2016; 4: 372–380. doi: 10.1016/S2213-2600(16)30022-4 [DOI] [PubMed] [Google Scholar]
- 21.Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2022; 43: 3618–3731. doi: 10.1093/eurheartj/ehac237 [DOI] [PubMed] [Google Scholar]