Abstract
Myocarditis is an established but rare adverse event following administration of messenger RNA–based coronavirus disease 2019 (COVID-19) vaccines and is most common in male adolescents and young adults. Symptoms typically develop within a few days of vaccine administration. Most patients have mild abnormalities on cardiac imaging with rapid clinical improvement with standard treatment. However, longer term follow-up is needed to determine whether imaging abnormalities persist, to evaluate for adverse outcomes, and to understand the risk associated with subsequent vaccination. The purpose of the review is to evaluate the current literature related to myocarditis following COVID-19 vaccination, including the incidence, risk factors, clinical course, imaging findings, and proposed pathophysiologic mechanisms.
Keywords: COVID-19, Myocarditis, Vaccine, mRNA vaccine, Cardiac MRI
Key points
-
•
Myocarditis following messenger RNA–based COVID-19 vaccines is rare; however, adolescent and young adult men are at highest risk.
-
•
Chest pain is the most common symptom, with typical onset within a few days of vaccine administration.
-
•
Cardiac MRI plays an important role in the diagnosis of acute myocarditis following vaccination, with typical findings of subepicardial late gadolinium enhancement and co-localizing edema at the basal inferior lateral wall.
-
•
The disease course of myocarditis following COVID-19 vaccination is typically transient and mild, with resolution of symptoms within 1 to 3 weeks in most patients.
-
•
However, longer term follow-up is needed to determine whether imaging abnormalities persist, to evaluate for adverse outcomes, and to understand the risk associated with subsequent vaccination.
Abbreviations.
| MRI | Magnetic resonance imaging |
| CMR | Cardiac magnetic resonance imaging |
| COVID-19 | Coronavirus disease 2019 |
Introduction
Following the discovery of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in December 2019, there has been intense focus on the development of vaccines to limit the contagion and severity of coronavirus disease 2019 (COVID-19). On December 11, 2020, the US Food and Drug Administration (FDA) issued emergency use authorization for the Pfizer-BioNTech COVID-19 vaccine (BNT162b2 mRNA). Since then, the FDA has approved the Pfizer-BioNTech COVID-19 vaccine and has authorized 2 other vaccines for emergency use, Moderna (mRNA-1273) and Janssen/Johnson & Johnson (Ad26.COV2.S). The Janssen/Johnson & Johnson vaccine is an adenovirus vector vaccine, whereas the other 2 are messenger RNA (mRNA) vaccines. As of April 2022, more than 11.5 billion COVID-19 vaccine doses have been administered worldwide.
Several side effects have been reported after administration of COVID-19 vaccines, most of which are mild and self-limited. These side effects include pain at the injection site, lymphadenopathy ipsilateral to the site of injection, fever, chills, myalgias, headache, and fatigue.1 Serious side effects have been reported in a very small proportion of individuals following COVID-19 vaccination, which include thrombosis with thrombocytopenia syndrome following administration of viral vector vaccines and myocarditis and pericarditis following administration of mRNA-based vaccines.2, 3, 4
Myocarditis is an inflammatory disease of the myocardium without an ischemic cause, which can be diagnosed by histologic, immunologic, and imaging criteria.5 Pericarditis refers to nonischemic inflammation of the pericardium. The term myopericarditis is used when both the myocardium and pericardium are inflamed. Myocarditis following vaccination had been described very rarely before the introduction of COVID-19 vaccines, including following smallpox and anthrax vaccines.6 Although rare, there has been intense interest in myocarditis following COVID-19 vaccination, particularly given the higher incidence of this adverse event in young men and concerns about the potential for long-term sequelae.
The purpose of the review is to evaluate the current literature related to myocarditis following COVID-19 vaccination, including the incidence, risk factors, clinical presentation, imaging findings, proposed pathophysiologic mechanisms, treatment, and prognosis.
Definition of Vaccine-Associated Myocarditis
There is no test or investigation that can establish causality for vaccine-associated myocarditis or pericarditis. These associations are based on a close temporal relationship between vaccine administration and the onset of symptoms, usually defined as within 14 days although ranges of up to a month have been reported in the literature.7, 8, 9, 10 The US Centers of Disease Control and Prevention (CDC) has established case definitions of acute myocarditis and pericarditis, including probable or confirmed acute myocarditis and probable acute pericarditis, summarized in Table 1 .3
Table 1.
Centers for Disease Control and Prevention working case definitions for acute myocarditis and acute pericarditis
| CDC Working Case Definitions | ||
|---|---|---|
| Acute Myocarditis |
Acute Pericarditis |
|
| Confirmed Case | Probable Case | Probable Case |
|
|
|
Clinical symptoms are for adolescents and adults. Infants and children younger than 12 years night instead have greater than or equal to 2 of the following symptoms: irritability, vomiting, poor feeding, tachypnea, and lethargy. Individuals who lack the listed symptoms but who meet other criteria may be classified as subclinical myocarditis (probable or confirmed).
Using the Lake Lousie criteria.
To meet the ECG or rhythm monitoring criterion, a probable case must include at least one of the following: (1) ST-segment or T-wave abnormailities; (2) paroxysmal or sustained atrail, superventricular, or ventricular arrhythmias; or (3) AV nodal conduction delays or intraventricular conduction defects.
Adapted from Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices — United States, June 2021. MMWR Morb Mortal Wkly Rep 2021;70:977–982; and from Bozkurt B., Kamat I. and Hotez P.J., Myocarditis with COVID-19 mRNA vaccines, Circulation, 144 (6), 2021, 471–484.
Incidence
In the United States, most of the data on the incidence of adverse events following vaccination have been gleaned from the Vaccine Adverse Events Reporting System (VAERS). As of January 7, 2022, VAERS had received 2478 reports of myocarditis and 1900 reports of pericarditis following COVID-19 vaccination.11 Of note, the VAERS is a passive adverse event reporting system with limitations including reporting bias.
Postvaccine myocarditis has been documented worldwide, with no clear identified pattern of increased prevalence based on geographic region. Reported rates from different health care organizations across the world are similar, including large population-based studies from Israel and California.12 , 13 A recent systematic review estimated that the overall incidence of myopericarditis following COVID-19 vaccination is 18 per million vaccine doses.14 Compared with COVID-19 vaccines, the incidence of myopericarditis was higher following vaccination for small pox (132 per million vaccine doses) and did not differ significantly with the incidence of myopericarditis following influenza vaccination (1.3 per million vaccine dose) or other non-small pox vaccination (57 per million vaccine doses).14
Sex and age differences
The risk of myocarditis associated with mRNA-based COVID-19 vaccination is highest in men between 12 and 29 years of age following administration of the second dose.8 , 12 , 13 , 15 , 16 As of June 2021, crude reporting rates of myocarditis following COVID-19 vaccination based on VAERS data were 40.6 cases per million second doses among men aged 12 to 29 years and 4.2 cases per million second doses among women aged 12 to 29 years.17 The striking sex difference in adolescents and young adults has led to proposed pathophysiologic mechanisms related to sex hormones and has raised questions about the potential for underdiagnosis of myocarditis in women compared with men.8
Rates of myocarditis following COVID-19 vaccination are much lower in younger children between 5 and 11 years of age and adults older than 30 years.17 As of December 19, 2021, reported rates of myocarditis based on VAERS data per 1 million doses of Pfizer-BioNTech COVID-19 vaccines administered were only 4 for men aged 5 to 11 years compared with 46 for men aged 12 to 15 years and 70 for men aged 16 to 17 years after the second dose. For women, rates were 2, 4, and 8 in the same age ranges, respectively.18 The vaccine formulation of the Pfizer mRNA vaccine for ages 5 to 11 years of age is one-third the dose of the formulation for those aged 12 years and older, which raises the possibility of a weight-based dose–response relationship in younger adolescents who have lower body weight compared with adults.
Risk with different vaccinations
Among mRNA-based COVID-19 vaccines, the risk of myocarditis is higher following the Moderna vaccine compared with the Pfizer-BioNTech COVID-19 vaccine, although absolute cases numbers vary depending on local availability of each vaccine.7 , 19 A study of more than 2.5 million people who received the Pfizer-BioNTech COVID-19 vaccine demonstrated an estimated incidence of myocarditis of 2.1 cases per 100,000 persons,13 whereas a study of nearly 500,000 people vaccinated with the Moderna vaccine demonstrated an incidence of 4.2 cases per 100,000 persons.7 A large population-based study in Denmark found that the rates of myocarditis among individuals aged 12 to 39 years were 1.6 per 100,000 individuals for Pfizer-BioNTech and 5.7 per 100,000 individuals for Moderna.7
Myocarditis is much more common following the second dose compared with the first but has also been reported following the first dose particularly in those with a prior history of COVID-19 infection.4 There are limited data on the risk of myocarditis following third and subsequent booster doses.20 , 21 However, the risk after the third dose seems to be lower than following the second dose.14 , 22 History of prior exposure is likely relevant as well as shorter interval between doses.23 There are a few case reports of recurrent myocarditis following administration of mRNA-based COVID-19 vaccines.24 , 25 However, the risk of recurrence of myocarditis following receipt of additional doses of mRNA COVID-19 vaccines in individuals with a history of confirmed myocarditis is currently unknown.26 One preprint article suggests that the risk of myocarditis among individuals who received the Moderna vaccine for the second dose was higher for those who had a heterologous as opposed to homologous vaccine schedule (ie, higher in those who had received a COVID-19 vaccine other than the Moderna vaccine for their first dose).23
Risk Relative to COVID-19 Infection
Infection with SARS-CoV-2 can also result in myocardial inflammation, which is associated with adverse outcomes in hospitalized patients, and should be balanced against the risk of vaccine-related complications.27 Overall, the rates of myocarditis following SARS-CoV-2 infection are much higher than after vaccination.17 Data from the largest integrated health care organization in Israel indicate that SARS-CoV-2 infection is associated with an excess risk of myocarditis when compared with age- and risk-matched controls (risk ratio 18.3 and risk difference 11.0 events per 100,000 persons) that is much higher than the excess risk of myocarditis following administration of Pfizer-BioNTech COVID-19 vaccine (risk ratio 3.2 and risk difference 2.7 events per 100,000 persons).28 Similarly, higher risk of myocarditis after SARS-CoV-2 infection compared with COVID-19 vaccination was recently demonstrated in a study in England that evaluated more than 38 million individuals aged 16 years and older who had received at least one dose of a COVID-19 vaccine.9 The extent of abnormalities on cardiac MRI (CMR) is also less severe in myocarditis following COVID-19 vaccination compared with myocarditis following SARS-CoV-2 infection.29
Clinical Presentation
Symptoms
The clinical presentation of myocarditis following COVID-19 vaccination is similar to that of myocarditis due to other causes (see Table 1). Chest pain is the most frequently reported presenting symptom.19 , 30, 31, 32 However, as with myocarditis from other causes, myocarditis following COVID-19 vaccination can present with variable symptoms ranging from subclinical presentations to acute arrythmia, heart failure, or rarely cardiogenic shock.3 , 13 , 19
Timing after vaccination
Most patients with myocarditis following COVID-19 vaccination demonstrate symptom onset within the first week after vaccination, with the vast majority presenting within the first 4 days19 , 33, 34, 35; this is similar to myocarditis following non-COVID vaccination where symptom onset is usually less than 2 weeks after vaccine administration.11 , 12
Histopathology and Pathophysiology
Histopathology
Endomyocardial biopsy is not frequently performed in the setting of acute myocarditis but is indicated in patients with suspected myocarditis when diagnostic confirmation will alter therapy, in cases of hemodynamic instability or with clinical deterioration despite supportive care.36 A negative endomyocardial biopsy does not exclude the diagnosis of myocarditis, given sampling imprecision associated with spatial heterogeneity (patchiness) of the disease.3 , 36, 37, 38 However, it should also be noted that cardiac dysfunction mimicking myocarditis may result from a systemic increase in cytokine production, which does not have a histological correlate.37 , 39, 40, 41
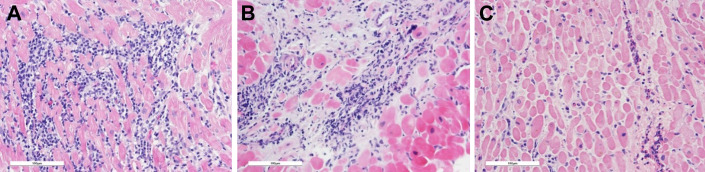
There are very few reports of histologically documented myocarditis following COVID-19 vaccination in the literature to date. Lymphocytic myocarditis is the predominant pattern observed in biopsied and autopsied hearts and occurs most frequently in adolescents and young adult men who present with mild, self-limited disease.3 , 41, 42, 43 In rare instances it may manifest as a fulminant, potentially fatal disease with no sex predilection.44, 45, 46 Depending on the timing of histologic assessment, a pattern of healing myocarditis may be present. Ameratunga and colleagues report a 57-year-old woman who died of fulminant necrotizing eosinophilic myocarditis 3 days after a first dose of the Pfizer-BioNTech COVID-19 vaccine.47 Interestingly, myocarditis following other viral vaccines, such as smallpox, measles-mumps-rubella, varicella, oral polio, yellow fever, influenza, hepatitis A and B, and human papillomavirus, is usually of the eosinophilic pattern, albeit not typically necrotizing.3 , 39 , 41 Different histologic patterns of myocarditis are summarized in Fig. 1 .
Fig. 1.
Examples of different histologic patterns of vaccine-associated myocarditis. (A) Lymphocytic myocarditis, the most common pattern reported in COVID-19 vaccination–associated myocarditis, is characterized by a dense mononuclear infiltrate and associated myocyte damage. (B) Healing myocarditis is more typically characterized by a loose and more mixed inflammatory infiltrate and with underlying damage already entering stages of repair (matrix formation). (C) Eosinophilic myocarditis, the pattern typically associated with other vaccinations, is characterized by a patchy infiltrate of eosinophils with relatively little cardiomyocyte damage. All images are hematoxylin and eosin (H&E)-stained slides, digital image capture (Leica DM2500 microscope, 200x original magnification with 10x Plan ocular and 20x FluorTar objective, OMAX 18MP camera, Toupview software, post-processing in GNU Image Manipulator Program 2.0); scale bar as indicated (100 μm).
Potential mechanisms of myocarditis following vaccination
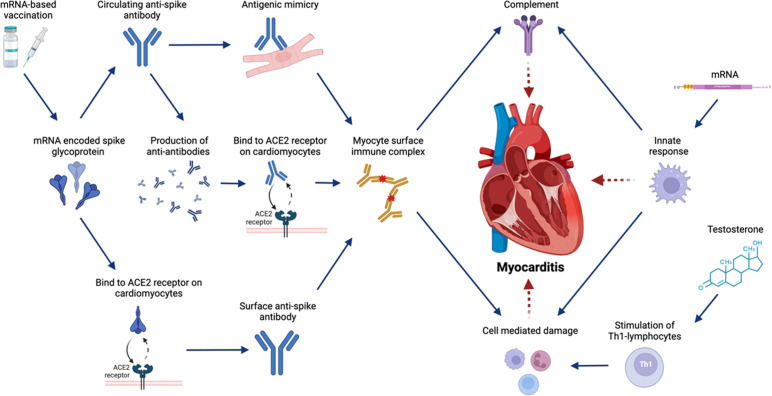
Several possible mechanisms for mRNA-related myocarditis have emerged (Fig. 2 ).3 , 33 , 41 , 48 Most of them focus on humoral (antibody-mediated) immunity, which is in keeping with the increased prevalence of myocarditis following the second dose of COVID-19 vaccines. One hypothesis involves molecular mimicry between the mRNA vaccine–encoded SARS-CoV2 spike glycoprotein and self-antigens in individuals with preexisting immune dysregulation, leading to polyclonal B-cell expansion, immune complex formation, and inflammation. Alternate explanations involve binding of mRNA vaccine–encoded viral spike glycoprotein to the surface of cardiomyocytes via angiotensin-converting enzyme 2 (ACE2) receptors or deposited immune complexes, thus directly acting as an antigenic trigger for inflammation. Yet another hypothesis involves production of antibodies targeting the antispike protein antibodies (ie, antiantibodies), which mimic the spike protein, bind cardiac ACE2 receptors, form immune complexes, and activate the classic complement pathway. Humoral mechanisms, however, fail to explain the case of a 40-year-old man with biopsy-proven lymphocytic myocarditis in the absence of serum SARS-CoV2 neutralizing antibodies 6 days after receiving a first dose of the Pfizer-BioNTech mRNA COVID-19 vaccine.42 Consequently, a subset of postvaccine myocarditis might be caused by an innate inflammatory response to the mRNA-encoded viral spike glycoprotein. Testosterone could play a role in the inhibition of antiinflammatory cells or the stimulation of immune responses mediated by Th1 lymphocytes.49
Fig. 2.
Potential mechanisms of myocarditis following COVID-19 mRNA vaccination. Overview of the potential mechanisms of myocarditis related to mRNA-based COVID-19 vaccination. ACE, angiotensin-converting enzyme; Th1, helper T cell. Created with BioRender.com.
Two additional mechanisms not described in the literature still warrant consideration. Theoretically, vaccine mRNA can enter circulation, rather than being directly expressed at the injection site, and could become expressed in cardiomyocytes and thus trigger direct cell-mediated responses against spike protein.50 In addition, despite chemical modifications and liposome delivery to prevent such, exogenous RNA can trigger the innate immune response, causing activation of the cellular inflammasome and acting as an adjuvant to antigen-mediated responses.51
Role of Cardiac Imaging
A summary of diagnostic test findings in acute myocarditis is provided in Table 2 . Cardiac imaging plays an important role in establishing a diagnosis of myocarditis and pericarditis, excluding other potential causes of symptoms, and risk-stratifying patients.
Table 2.
Diagnostic test findings in acute myocarditis
| Test | Typical Findings | Strengths | Limitations |
|---|---|---|---|
| Cardiac Imaging | |||
| CMR |
|
|
|
| Echocardiography |
|
|
|
| Cardiac CT |
|
|
|
| Cardiac PET |
|
|
|
| Chest radiography |
|
|
|
| Other Investigations | |||
| Troponin |
|
|
|
| BNP |
|
|
|
| ECG |
|
|
|
| Endomyocardial biopsy |
|
|
|
Abbreviations: BNP, brain natriuretic peptide; CMR, cardiac magnetic resonance; CT, computed tomography; ECG, echocardiography; ECV, extracellular volume; FDG, fluorodeoxyglucose; LGE, late gadolinium enhancement; PET, positron emission tomography.
Echocardiography
Transthoracic echocardiography is often the first cardiac imaging modality performed in the setting of suspected myocarditis, allowing for assessment of cardiac size and function and associated findings including the presence of a pericardial effusion. Although most findings in the setting of myocarditis are not specific, focal echogenicity, particularly in the lateral and inferior wall, and the presence of pericardial effusions have been shown to be sensitive for acute myopericarditis in young adults.52 Speckle tracking–based myocardial strain is also a potentially sensitive method to identify acute myocarditis with echocardiography.53 Impaired ventricular function is a predictor of poor outcomes, and echocardiography is also useful in follow-up to ensure recovery of function54
Cardiac computed tomography
Cardiac computed tomography (CT) is infrequently used in the evaluation of suspected myocarditis but can be helpful in excluding other potential causes of acute chest pain, including obstructive coronary artery disease. In the setting of an inability to perform CMR, late iodine enhancement on CT could be considered, given high sensitivity and positive predictive value for acute myocarditis.55
Cardiac positron emission tomography
Cardiac fluorodeoxyglucose PET (FDG-PET) is useful in the evaluation of cardiac inflammation and is most often used clinically in the setting of cardiac sarcoidosis.56 However, focal cardiac FDG uptake on PET has also been described in the setting of myocardial inflammation due to other causes including COVID-19 infection.57 Combined cardiac PET/MR adds complementary information and increases the sensitivity for mild or borderline myocarditis compared with CMR, although not widely available.57, 58, 59, 60
Cardiac magnetic resonance imaging
CMR is the most important imaging modality for diagnosis of acute myocarditis. The Lake Louise criteria are most commonly used for evaluation of suspected myocarditis on CMR. These criteria were initially established in 2009 and were revised in 2018 to incorporate parametric mapping.61 The revised criteria indicate a high likelihood of nonischemic myocardial inflammation when at least one of each of the T1- and T2-based criteria are met. T1-based criteria include increased native T1, increased extracellular volume (ECV), or presence of nonischemic pattern late gadolinium enhancement (LGE). T2-based criteria include increased native T2, regional T2 hyperintensity, or increased myocardial T2 signal intensity ratio compared with skeletal muscle on T2-weighted imaging. Regional and global left ventricular dysfunction and findings associated with pericarditis (including presence of a pericardial effusion and pericardial enhancement) are supportive criteria but are not required.
Although the presence of both T1- and T2-based criteria is associated with the highest specificity for active myocardial inflammation, the presence of only one criterion could still be consistent with a diagnosis of myocarditis, particularly if imaging was delayed after symptom onset or performed after immunosuppressive treatment is started (in which case edema might no longer be detectable).4 Given the importance of establishing a diagnosis of myocarditis, CMR should ideally be performed as soon as possible after the onset of symptoms; this may be particularly relevant in the setting of myocarditis following COVID-19 vaccination, given reports of very rapid resolution of symptoms and the potential implications of a diagnosis on recommendations for future vaccination.4
In acute myocarditis in general, LGE most commonly occurs in a subepicardial pattern and is often located at the basal inferior lateral wall. Mid-wall and septal patterns of LGE are less common but are associated with a worse prognosis.62 Myocardial edema is usually focal; however, diffuse changes have also been described.63 In the acute setting, LGE co-localizing with edema is often associated with functional recovery, as edema improves over time.64 However, isolated LGE without edema often reflects fibrosis, which is a risk marker for major cardiac events including sudden cardiac death and heart failure.64 Native T1, T2, and ECV allow for quantification of myocardial tissue changes and are all elevated in the setting of myocardial edema. On the other hand, elevation of T1 and ECV in the setting of normal T2 values suggests healed myocarditis with fibrosis but no active edema.4
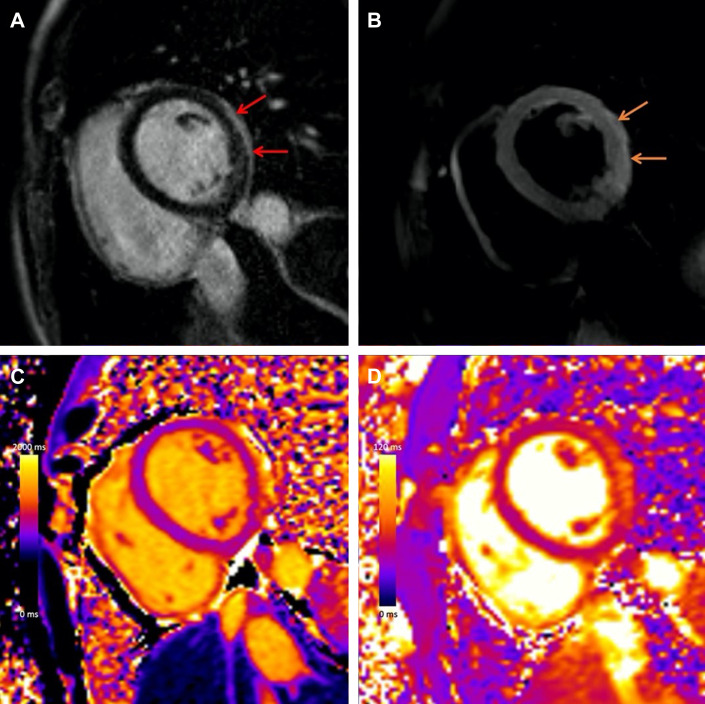
The pattern of CMR findings in myocarditis following COVID-19 vaccination is similar to myocarditis due to other causes, although the extent and severity of abnormalities tends to be milder.4 Typical findings in the acute phase include subepicardial LGE and co-localizing edema at the basal to mid-inferolateral wall, Fig. 3 . Compared with other causes of myocarditis, patients with vaccine-associated myocarditis have higher left ventricular ejection fraction, less extensive LGE, and less frequent involvement of the septum.29
Fig. 3.
COVID-19 vaccine–associated myocarditis. Short-axis 1.5 T MRI images of a young adult man with myocarditis following mRNA COVID-19 vaccine administration demonstrating (A) subepicardial late gadolinium enhancement (LGE) at the basal to mid-inferior lateral wall (red arrows), with corresponding (B) hyperintensity on T2-weighted imaging (orange arrows), (C) abnormal high native T1 (1274 ms, maximum region of interest), and (D) abnormal high native T2 (65 ms, maximum region of interest).
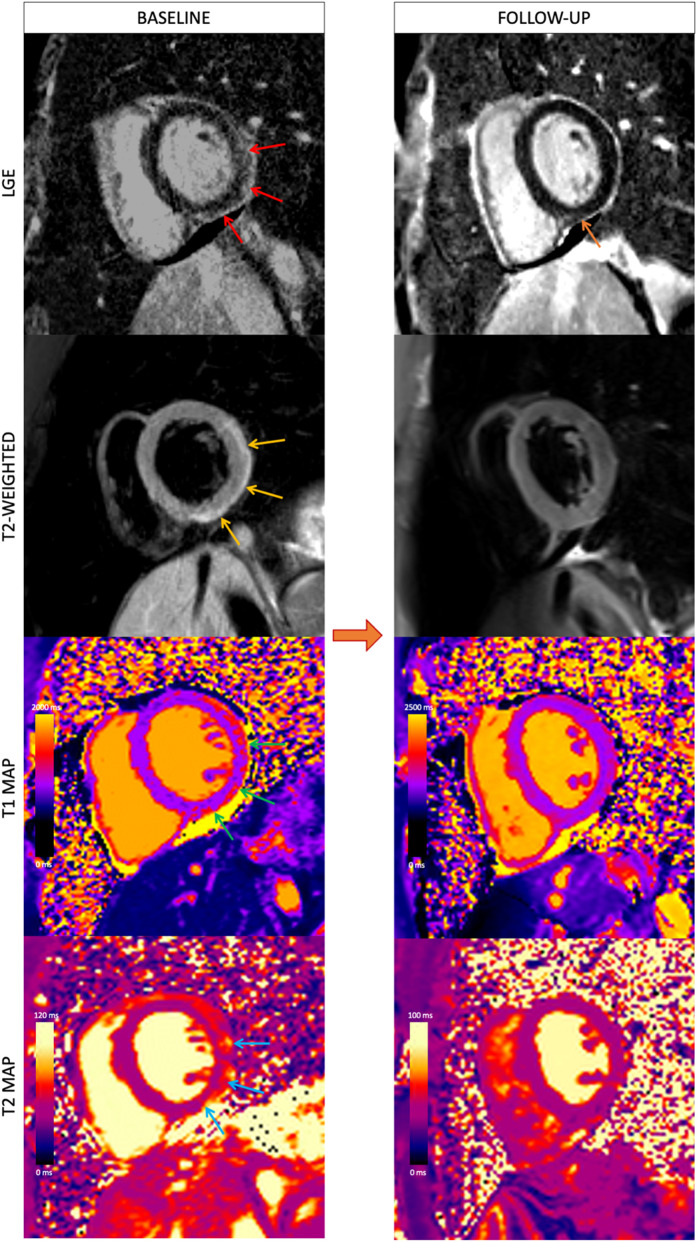
Follow-up CMR is often performed 3 to 6 months after acute myocarditis to evaluate for recovery of left ventricular function, resolution of edema, and any residual scarring, although there are no established guidelines defining the optimal timing of follow-up imaging.65 There are limited follow-up CMR data in myocarditis following vaccination.66, 67, 68, 69 In a case series of 13 adults with acute myocarditis following COVID-19 vaccination, intermediate-term follow-up CMR at a median of 5 months demonstrated resolution of myocardial edema, normalization of left ventricular function, and interval decrease in LGE extent (Fig. 4 ).66 However, minimal residual LGE without edema was present in 8 out of 13 patients at follow-up, likely reflecting myocardial fibrosis. Similarly, in a case series of 16 pediatric patients with myocarditis following COVID-19 vaccination, follow-up CMR at a median of 3 months demonstrated normalization of LVEF and interval decrease in LGE extent.67 However, minimal LGE persisted in 11 out of 16 and global longitudinal strain remained impaired in 12 out of 16. Longer-term follow-up in larger cohorts of patients is needed to understand the sequelae of myocarditis following vaccination and the ability of imaging abnormalities to identify patients who are at risk of adverse cardiac events.
Fig. 4.
Baseline and follow-up CMR in COVID-19 vaccine–associated myocarditis. Short-axis cardiac MRI images in a young adult man with myocarditis following the second dose of mRNA-1273. Baseline MRI at 1.5 T demonstrates subepicardial late gadolinium enhancement (LGE) at the basal inferior and inferolateral wall (red arrows) with corresponding high T2 signal in keeping with edema (yellow arrows), high regional native T1 (green arrows), and high regional T2 (blue arrows). Follow-up cardiac MRI performed 4 months later at 3 T demonstrates interval decrease in LGE extent (orange arrow) with resolution of edema and normalization of T1 and T2 values.
Other Investigations
Other diagnostic investigations used frequently in the setting of myocarditis and pericarditis include electrocardiography (ECG) and assessment of cardiac blood biomarkers. ECG findings in myocarditis are nonspecific but can include ST-segment elevation, T-wave inversions, and ectopic beats.31 , 34 , 70 Although these findings have low sensitivity for myocarditis, ECG is frequently used in patients with suspected myocarditis to evaluate for alternative causes of cardiac symptoms.70 In the setting of pericarditis, typical ECG changes include concave upward ST-segment elevation, upright T waves in the leads with ST-segment elevation, and PR depression. ECG findings in the setting of myocarditis and pericarditis following COVID-19 vaccination are similar to other causes.19 , 70
Although not specific to myocarditis, elevated troponin levels, indicating myocyte injury, are almost always present in the setting of acute myocarditis.3 , 19 , 30 , 71 The degree of troponin elevation varies and depends the severity of myocardial injury and timing of evaluation in relation to symptom onset. Elevated brain natriuretic peptide (BNP) levels indicate increased ventricular stretch and are elevated in the setting of heart failure. BNP is often assessed in patients with suspected myocarditis when heart failure symptoms are present. However, elevations of BNP are not included in the CDC working case definitions of probable or confirmed acute myocarditis, and values are not consistently evaluated or reported. Other testing, such as acute and convalescent viral testing (eg, SARS-CoV-2, coxsackievirus, and so forth), should be considered when clinically appropriate to exclude other potential causes of myocarditis.
Management
Currently, there are no specific management recommendations for myocarditis following COVID-19 vaccination. Care is largely supportive following guidelines for myocarditis due to other causes.39 Treatment is typically focused on addressing potential sequelae such as heart failure or arrhythmia as per guideline-directed medical therapy. For patients with very mild symptoms with rapid improvement, therapy can often be deferred.
Individuals are typically safe to return to their normal daily activities after their symptoms improve. However, the optimal duration of exercise restriction is unknown. The American Heart Association and American College of Cardiology Foundation have recommended 3 to 6 months of restriction from competitive sports following myocarditis. Repeat evaluation of serum biomarkers, 24-hour Holter monitor, and echocardiography is recommended before return to exercise in order to ensure normal ventricular function, resolution of active inflammation, and absence of arrhythmias.72
Although there is no clear evidence on risk associated with subsequent vaccination, current guidelines suggest that further doses of mRNA COVID-19 vaccines should be deferred among individuals who experienced myocarditis within 6 weeks of receiving a previous dose of an mRNA COVID-19 vaccine.26
Clinical Course and Adverse Outcomes
Myocarditis following COVID-19 vaccination is typically associated with a transient, mild course, with complete resolution of symptoms within 1 to 3 weeks in most of the patients.3 , 19 , 30 , 34 Patients with more severe disease with ventricular dysfunction might require hospitalization, although most reports indicate that patients who do require hospitalization typically require a short stay of less than 5 days.19 , 34 Patients rarely require intensive care unit admission or readmission following hospital discharge.12
Although most patients with myocarditis after COVID-19 vaccination have a mild course, there are limited long-term follow-up data, given the relatively recent introduction of these vaccines. Improvement or normalization of ejection fraction and resolution of symptoms in nearly all patients with myocarditis following COVID-19 vaccination has been demonstrated at short-interval follow-up.12 Limited intermediate term (∼5–6 months) follow-up data have demonstrated normalization of troponin levels, no residual cardiac symptoms or functional impairment, and no adverse cardiac events.66 , 68 Although these data are reassuring, further studies with long-term clinical and imaging follow-up are needed. Additional study is also needed to determine the risk with subsequent vaccine doses and other potential risk factors including prior history of myocarditis.
Summary
Myocarditis is an established but rare adverse event following administration of mRNA-based COVID-19 vaccines, with highest risk in male adolescents and young adults. Symptoms typically develop within a few days of vaccine administration. Most patients have mild abnormalities on cardiac imaging with rapid clinical improvement with standard treatment, which is reassuring. However, longer term follow-up is needed to determine whether imaging abnormalities persist, to evaluate for adverse outcomes, and to understand the risk associated with subsequent vaccination.
Clinics care points
-
•
Myocarditis following mRNA-based COVID-19 vaccines is rare; however, adolescent and young adult men are at highest risk.
-
•
Chest pain is the most common symptom, with typical onset within a few days of vaccine administration.
-
•
CMR plays an important role in the diagnosis of acute myocarditis following vaccination, with typical findings of subepicardial late gadolinium enhancement and co-localizing edema at the basal inferior lateral wall.
-
•
The disease course of myocarditis following COVID-19 vaccination is typically transient and mild, with resolution of symptoms within 1 to 3 weeks in most patients.
-
•
However, longer term follow-up is needed to determine whether imaging abnormalities persist, to evaluate for adverse outcomes, and to understand the risk associated with subsequent vaccination.
Funding
Dr P. Thavendiranathan is supported by a Canada Research Chair. Dr J.A. Udell is supported by a Department of Medicine, University of Toronto Merit Award and receives support from Ontario Ministry of Colleges and Universities Early Researcher Award (ER15-11-037).
Acknowledgments
Disclosure
Dr K. Hanneman has received speaker’s honorarium from Sanofi-Genzyme, Amicus, and Medscape. Dr P. Thavendiranathan has received speaker’s honorarium from Amgen, Boehringer Ingelheim-Lilly, and Takeda. Dr J.A. Udell has served as a consultant or speaker for AstraZeneca, Bayer, Boehringer Ingelheim-Lilly, Janssen, Merck, Novartis, and Sanofi and has received research grants from AstraZeneca, Amgen, Bayer, Boehringer Ingelheim-Lilly, and Janssen.
Footnotes
This article originally appeared in Cardiology Clinics, Volume 40, Issue 3, August 2022.
References
- 1.Hanneman K., Iwanochko R.M., Thavendiranathan P. Evolution of lymphadenopathy at PET/MRI after COVID-19 vaccination. Radiology. 2021;299(3):E282. doi: 10.1148/radiol.2021210386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.See I., Su J.R., Lale A., et al. US Case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325(24):2448–2456. doi: 10.1001/jama.2021.7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bozkurt B., Kamat I., Hotez P.J. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144(6):471–484. doi: 10.1161/CIRCULATIONAHA.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanchez Tijmes F., Thavendiranathan P., Udell J.A., et al. Cardiac MRI assessment of nonischemic myocardial inflammation: state of the art review and update on myocarditis associated with COVID-19 vaccination. Radiol Cardiothorac Imaging. 2021;3(6):e210252. doi: 10.1148/ryct.210252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caforio A.L., Pankuweit S., Arbustini E., et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the european society of cardiology working group on myocardial and pericardial diseases. Eur Heart J. 2013;34(33):2636–2648. doi: 10.1093/eurheartj/eht210. 48a-48d. [DOI] [PubMed] [Google Scholar]
- 6.Su J.R., McNeil M.M., Welsh K.J., et al. Myopericarditis after vaccination, vaccine adverse event reporting system (VAERS), 1990-2018. Vaccine. 2021;39(5):839–845. doi: 10.1016/j.vaccine.2020.12.046. [DOI] [PubMed] [Google Scholar]
- 7.Husby A., Hansen J.V., Fosbol E., et al. SARS-CoV-2 vaccination and myocarditis or myopericarditis: population based cohort study. BMJ. 2021;375:e068665. doi: 10.1136/bmj-2021-068665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mevorach D., Anis E., Cedar N., et al. Myocarditis after BNT162b2 mRNA vaccine against covid-19 in Israel. N Engl J Med. 2021;385(23):2140–2149. doi: 10.1056/NEJMoa2109730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patone M., Mei X.W., Handunnetthi L., et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2021;28(2):410–422. doi: 10.1038/s41591-021-01630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perez Y., Levy E.R., Joshi A.Y., et al. Myocarditis following COVID-19 mRNA vaccine: a case series and incidence rate determination. Clin Infect Dis. 2021;3:ciab926. doi: 10.1093/cid/ciab926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.United States Department of Health and Human Services (DHHS) Public health service (PHS), centers for disease control (CDC)/food and Drug administration (FDA), vaccine adverse event reporting system (VAERS) 1990 - 01/07/2022. http://wonder.cdc.gov/vaers CDC WONDER On-line Database [cited 2022 January 16]. Available at:
- 12.Simone A., Herald J., Chen A., et al. Acute myocarditis following COVID-19 mRNA vaccination in adults aged 18 years or older. JAMA Intern Med. 2021;181(12):1668–1670. doi: 10.1001/jamainternmed.2021.5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witberg G., Barda N., Hoss S., et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med. 2021;385(23):2132–2139. doi: 10.1056/NEJMoa2110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ling R.R., Ramanathan K., Tan F.L., et al. Myopericarditis following COVID-19 vaccination and non- COVID-19 vaccination: a systematic review and meta-analysis. Lancet Respir Med. 2022;11(22):S2213–S2600. doi: 10.1016/S2213-2600(22)00059-5. 00059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hajjo R., Sabbah D.A., Bardaweel S.K., et al. Shedding the light on post-vaccine myocarditis and pericarditis in COVID-19 and non-COVID-19 vaccine recipients. Vaccines. 2021;9(10) doi: 10.3390/vaccines9101186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oster M.E., Shay D.K., Su J.R., et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from december 2020 to august 2021. JAMA. 2022;327(4):331–340. doi: 10.1001/jama.2021.24110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gargano J.W., Wallace M., Hadler S.C., et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the advisory committee on immunization practices - United States, june 2021. MMWR Morb Mortal Wkly Rep. 2021;70(27):977–982. doi: 10.15585/mmwr.mm7027e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su J.R. COVID-19 vaccine safety updates: primary series in children and adolescents ages 5–11 and 12–15 years, and booster doses in adolescents ages 16–24 years 2022. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-01-05/02-covid-su-508.pdf [cited 2022 January 20]. Available at:
- 19.Montgomery J., Ryan M., Engler R., et al. Myocarditis following immunization with mRNA COVID-19 Vaccines in members of the US military. JAMA Cardiol. 2021;6(10):1202–1206. doi: 10.1001/jamacardio.2021.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aviram G., Viskin D., Topilsky Y., et al. Myocarditis associated with COVID-19 booster vaccination. Circ Cardiovasc Imaging. 2022;15(2):e013771. doi: 10.1161/CIRCIMAGING.121.013771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez Tijmes F., Zamorano A., Thavendiranathan P., et al. Imaging of myocarditis following mRNA COVID-19 booster vaccination. Radiol Cardiothorac Imaging. 2022;4(2):e220019. doi: 10.1148/ryct.220019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedensohn L., Levin D., Fadlon-Derai M., et al. Myocarditis following a third BNT162b2 vaccination dose in military recruits in Israel. JAMA. 2022;17:e224425. doi: 10.1001/jama.2022.4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buchan S.A., Seo C.Y., Johnson C., et al. Epidemiology of myocarditis and pericarditis following mRNA vaccines in Ontario, Canada: by vaccine product, schedule and interval. medRxiv. 2021:2021. doi: 10.1001/jamanetworkopen.2022.18505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minocha P.K., Better D., Singh R.K., et al. Recurrence of acute myocarditis temporally associated with receipt of the mRNA coronavirus disease 2019 (COVID-19) vaccine in a male adolescent. J Pediatr. 2021;238:321–323. doi: 10.1016/j.jpeds.2021.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Umei T.C., Kishino Y., Shiraishi Y., et al. Recurrence of myopericarditis following mRNA COVID-19 vaccination in a male adolescent. CJC Open. 2022;4(3):350–352. doi: 10.1016/j.cjco.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Summary of NACI advice on vaccination with COVID-19. https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/summary-advice-vaccination-covid-19-vaccines-following-myocarditis-with-without-pericarditis.html vaccines following myocarditis (with or without pericarditis) [cited 2022 January 28]. Available at:
- 27.Guo T., Fan Y., Chen M., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barda N., Dagan N., Ben-Shlomo Y., et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2021;385(12):1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fronza M.T., Thavendiranathan P., Chan V., et al. Myocardial injury pattern by MRI in COVID-19 vaccine associated myocarditis. Radiology. 2022;15:212559. doi: 10.1148/radiol.212559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engler R.J., Nelson M.R., Collins L.C., Jr., et al. A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination. PLoS One. 2015;10(3):e0118283. doi: 10.1371/journal.pone.0118283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Halsell J.S., Riddle J.R., Atwood J.E., et al. Myopericarditis following smallpox vaccination among vaccinia-naive US military personnel. JAMA. 2003;289(24):3283–3289. doi: 10.1001/jama.289.24.3283. [DOI] [PubMed] [Google Scholar]
- 32.Mei R., Raschi E., Forcesi E., et al. Myocarditis and pericarditis after immunization: gaining insights through the vaccine adverse event reporting system. Int J Cardiol. 2018;273:183–186. doi: 10.1016/j.ijcard.2018.09.054. [DOI] [PubMed] [Google Scholar]
- 33.Heymans S., Cooper L.T. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2022;19:75077. doi: 10.1038/s41569-021-00662-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Das B.B., Moskowitz W.B., Taylor M.B., et al. Myocarditis and pericarditis following mRNA COVID-19 vaccination: what do we know so far? Children (Basel) 2021;8(7):607. doi: 10.3390/children8070607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matta A., Kunadharaju R., Osman M., et al. Clinical presentation and outcomes of myocarditis post mRNA vaccination: a meta-analysis and systematic review. Cureus. 2021;13(11):e19240. doi: 10.7759/cureus.19240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leone O., Veinot J.P., Angelini A., et al. 2011 Consensus statement on endomyocardial biopsy from the association for european cardiovascular pathology and the society for cardiovascular pathology. Cardiovasc Pathol. 2012;21(4):245–274. doi: 10.1016/j.carpath.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 37.Baughman K.L. Diagnosis of myocarditis: death of Dallas criteria. Circulation. 2006;113(4):593–595. doi: 10.1161/CIRCULATIONAHA.105.589663. [DOI] [PubMed] [Google Scholar]
- 38.Chow L.H., Radio S.J., Sears T.D., et al. Insensitivity of right ventricular endomyocardial biopsy in the diagnosis of myocarditis. J Am Coll Cardiol. 1989;14(4):915–920. doi: 10.1016/0735-1097(89)90465-8. [DOI] [PubMed] [Google Scholar]
- 39.Luk A., Clarke B., Dahdah N., et al. Myocarditis and pericarditis after COVID-19 mRNA vaccination: practical considerations for care providers. Can J Cardiol. 2021;37(10):1629–1634. doi: 10.1016/j.cjca.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rali A.S., Ranka S., Shah Z., et al. Mechanisms of myocardial injury in coronavirus disease 2019. Card Fail Rev. 2020;6:e15. doi: 10.15420/cfr.2020.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Switzer C., Loeb M. Evaluating the relationship between myocarditis and mRNA vaccination. Expert Rev Vaccin. 2022;21(1):83–89. doi: 10.1080/14760584.2022.2002690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ehrlich P., Klingel K., Ohlmann-Knafo S., et al. Biopsy-proven lymphocytic myocarditis following first mRNA COVID-19 vaccination in a 40-year-old male: case report. Clin Res Cardiol. 2021;110(11):1855–1859. doi: 10.1007/s00392-021-01936-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jain S.S., Steele J.M., Fonseca B., et al. COVID-19 vaccination-associated myocarditis in adolescents. Pediatrics. 2021;148(5) doi: 10.1542/peds.2021-053427. [DOI] [PubMed] [Google Scholar]
- 44.Abbate A., Gavin J., Madanchi N., et al. Fulminant myocarditis and systemic hyperinflammation temporally associated with BNT162b2 mRNA COVID-19 vaccination in two patients. Int J Cardiol. 2021;340:119–121. doi: 10.1016/j.ijcard.2021.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lim Y., Kim M.C., Kim K.H., et al. Case report: acute fulminant myocarditis and cardiogenic shock after messenger RNA coronavirus disease 2019 vaccination requiring extracorporeal cardiopulmonary resuscitation. Front Cardiovasc Med. 2021;8:758996. doi: 10.3389/fcvm.2021.758996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verma A.K., Lavine K.J., Lin C.Y. Myocarditis after covid-19 mRNA vaccination. N Engl J Med. 2021;385(14):1332–1334. doi: 10.1056/NEJMc2109975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ameratunga R., Woon S.T., Sheppard M.N., et al. First identified case of fatal fulminant necrotizing eosinophilic myocarditis following the initial dose of the pfizer-biontech mRNA COVID-19 vaccine (BNT162b2, Comirnaty): an extremely rare idiosyncratic hypersensitivity reaction. J Clin Immunol. 2022;3:1–7. doi: 10.1007/s10875-021-01187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kadkhoda K. Post RNA-based COVID vaccines myocarditis: proposed mechanisms. Vaccine. 2022;40(3):406–407. doi: 10.1016/j.vaccine.2021.11.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lazaros G., Klein A.L., Hatziantoniou S., et al. The novel platform of mRNA COVID-19 vaccines and myocarditis: clues into the potential underlying mechanism. Vaccine. 2021;39(35):4925–4927. doi: 10.1016/j.vaccine.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rijkers G.T., Weterings N., Obregon-Henao A., et al. Antigen presentation of mRNA-based and virus-vectored SARS-CoV-2 vaccines. Vaccines (Basel) 2021;9(8) doi: 10.3390/vaccines9080848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Milano G., Gal J., Creisson A., et al. Myocarditis and COVID-19 mRNA vaccines: a mechanistic hypothesis involving dsRNA. Future Virol. 2022;17(3):191–196. doi: 10.2217/fvl-2021-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saricam E., Saglam Y., Hazirolan T. Clinical evaluation of myocardial involvement in acute myopericarditis in young adults. BMC Cardiovasc Disord. 2017;17(1):129. doi: 10.1186/s12872-017-0564-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hsiao J.F., Koshino Y., Bonnichsen C.R., et al. Speckle tracking echocardiography in acute myocarditis. Int J Cardiovasc Imaging. 2013;29(2):275–284. doi: 10.1007/s10554-012-0085-6. [DOI] [PubMed] [Google Scholar]
- 54.van den Heuvel F.M.A., Vos J.L., van Bakel B., et al. Comparison between myocardial function assessed by echocardiography during hospitalization for COVID-19 and at 4 months follow-up. Int J Cardiovasc Imaging. 2021;37(12):3459–3467. doi: 10.1007/s10554-021-02346-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bouleti C., Baudry G., Iung B., et al. Usefulness of late iodine enhancement on spectral CT in acute myocarditis. JACC Cardiovasc Imaging. 2017;10(7):826–827. doi: 10.1016/j.jcmg.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 56.Genovesi D., Bauckneht M., Altini C., et al. The role of positron emission tomography in the assessment of cardiac sarcoidosis. Br J Radiol. 2019;92(1100):20190247. doi: 10.1259/bjr.20190247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hanneman K., Houbois C., Schoffel A., et al. Combined cardiac fluorodeoxyglucose-positron emission tomography/magnetic resonance imaging assessment of myocardial injury in patients who recently recovered from COVID-19. JAMA Cardiol. 2022;7(3):298–308. doi: 10.1001/jamacardio.2021.5505. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen W., Jeudy J. Assessment of myocarditis: cardiac MR, PET/CT, or PET/MR? Curr Cardiol Rep. 2019;21(8):76. doi: 10.1007/s11886-019-1158-0. [DOI] [PubMed] [Google Scholar]
- 59.Cheung E., Ahmad S., Aitken M., et al. Combined simultaneous FDG-PET/MRI with T1 and T2 mapping as an imaging biomarker for the diagnosis and prognosis of suspected cardiac sarcoidosis. Eur J Hybrid Imaging. 2021;5(1):24. doi: 10.1186/s41824-021-00119-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hanneman K., Kadoch M., Guo H.H., et al. Initial experience with simultaneous 18F-FDG PET/MRI in the evaluation of cardiac sarcoidosis and myocarditis. Clin Nucl Med. 2017;42(7):e328–e334. doi: 10.1097/RLU.0000000000001669. [DOI] [PubMed] [Google Scholar]
- 61.Ferreira V.M., Schulz-Menger J., Holmvang G., et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72(24):3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 62.Grani C., Eichhorn C., Biere L., et al. Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J Am Coll Cardiol. 2017;70(16):1964–1976. doi: 10.1016/j.jacc.2017.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luetkens J.A., Isaak A., Zimmer S., et al. Diffuse myocardial inflammation in COVID-19 associated myocarditis detected by multiparametric cardiac magnetic resonance imaging. Circ Cardiovasc Imaging. 2020;13(5):e010897. doi: 10.1161/CIRCIMAGING.120.010897. [DOI] [PubMed] [Google Scholar]
- 64.Aquaro G.D., Ghebru Habtemicael Y., Camastra G., et al. Prognostic value of repeating cardiac magnetic resonance in patients with acute myocarditis. J Am Coll Cardiol. 2019;74(20):2439–2448. doi: 10.1016/j.jacc.2019.08.1061. [DOI] [PubMed] [Google Scholar]
- 65.Tschope C., Ammirati E., Bozkurt B., et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. 2021;18(3):169–193. doi: 10.1038/s41569-020-00435-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fronza M., Thavendiranathan P., Karur G.R., et al. Cardiac MRI and clinical follow-up in COVID-19 vaccine associated myocarditis. Radiology. 2022;May 3:220802. doi: 10.1148/radiol.220802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schauer J., Buddhe S., Gulhane A., et al. Persistent cardiac MRI findings in a cohort of adolescents with post COVID-19 mRNA vaccine myopericarditis. J Pediatr. 2022;26(22):S0022–S3476. doi: 10.1016/j.jpeds.2022.03.032. 00282-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rosner C.M., Atkins M., Saeed I.M., et al. Patients with myocarditis associated with COVID-19 vaccination. J Am Coll Cardiol. 2022;79(13):1317–1319. doi: 10.1016/j.jacc.2022.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cavalcante J.L., Shaw K.E., Gossl M. Cardiac magnetic resonance imaging midterm follow up of covid-19 vaccine–associated myocarditis. JACC Cardiovasc Imaging. 2022;16:2022. doi: 10.1016/j.jcmg.2022.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kindermann I., Barth C., Mahfoud F., et al. Update on myocarditis. J Am Coll Cardiol. 2012;59(9):779–792. doi: 10.1016/j.jacc.2011.09.074. [DOI] [PubMed] [Google Scholar]
- 71.Shaw K.E., Cavalcante J.L., Han B.K., et al. Possible association between COVID-19 vaccine and myocarditis: clinical and CMR findings. JACC Cardiovasc Imaging. 2021;14(9):1856–1861. doi: 10.1016/j.jcmg.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maron B.J., Udelson J.E., Bonow R.O., et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 3: hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy and other cardiomyopathies, and myocarditis: a scientific statement from the american heart association and american college of cardiology. Circulation. 2015;132(22):e273–e280. doi: 10.1161/CIR.0000000000000239. [DOI] [PubMed] [Google Scholar]