Abstract
Myocardial injury is common in patients with COVID-19 and is associated with an adverse prognosis. Cardiac troponin (cTn) is used to detect myocardial injury and assist with risk stratification in this population. SARS-CoV-2 infection can play a role in the pathogenesis of acute myocardial injury due to both direct and indirect damage to the cardiovascular system. Despite the initial concerns about an increased incidence of acute myocardial infarction (MI), most cTn increases are related to chronic myocardial injury due to comorbidities and/or acute nonischemic myocardial injury. This review will discuss the latest findings on this topic.
Keywords: COVID-19, Cardiac troponin, High sensitivity cardiac troponin, Myocardial injury, Risk stratification, Prognosis
Key points
-
•
For patients with COVID-19 infection, myocardial injury is diagnosed when cardiac troponin (cTn) concentrations exceed the 99th percentile upper-reference limit.
-
•
Although myocardial injury is common, cTn increases are usually modest and criteria for myocardial infarction (MI) are infrequently met.
-
•
While both direct and indirect mechanisms of myocardial damage play a role in acute myocardial injury during COVID-19, chronic myocardial injury related to comorbidities is frequently present.
-
•
Myocardial injury has adverse short-term prognostic implications, with more data needed on long-term outcomes. The magnitude of cTn increases is also prognostic.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic caused by the SARS-CoV-2 infection continues to have a severe global impact. Since the earliest reports from China,1, 2, 3 it has been clear that cardiac involvement is frequent in patients with COVID-19, especially in those with concomitant cardiovascular comorbidities. The early studies had limitations due in part to arbitrary definitions for cardiac involvement.4 Numerous studies have documented the value of cardiac troponin (cTn) to detect myocardial injury and for risk stratification. This review will discuss the latest information about cardiac involvement with an emphasis on the use of cTn.
Definition of myocardial injury
Per the Fourth Universal Definition of Myocardial Infarction (4UDMI),5 cTn is the biomarker of choice for the detection of myocardial injury and, in the proper clinical situation, the diagnosis of myocardial infarction (MI). If available, high-sensitivity (hs-cTn) cTn assays are preferred.6 An assay is defined as high sensitivity if (a) the 99th percentile can be measured with analytical imprecision ≤10% and (b) the assay measures cTn concentrations above the limit of detection (LOD) in ≥50% of both healthy men and women.7
Myocardial injury is defined as any cTn increase above the assay-specific 99th percentile upper reference limit (URL) of a healthy population. When acute myocardial injury occurs, defined as a dynamic rising and/or falling pattern of cTn concentrations with at least one cTn concentration above the 99th percentile, and there are signs and/or symptoms of acute myocardial ischemia, a diagnosis of MI is made. Due to the increased sensitivity of hs-cTn assays, myocardial injury is detected far more frequently in a variety of clinical situations not related to myocardial ischemia than in those with MI.5 It is often challenging for clinicians to identify the specific reason for hs-cTn elevations, as it can often occur in the critically ill. COVID-19 infections can induce alterations in myocardial oxygen consumption and contribute to ischemia but are also associated with pulmonary embolism (PE), critical illness, myocarditis, as well as the direct effects of SARS-CoV-2 on the myocardium and perhaps the microvasculature, making it challenging for clinicians to determine a discrete etiology.
Etiologies myocardial injury in COVID-19
There are multiple mechanisms that link COVID-19 disease to myocardial injury but also with other forms of cardiac involvement like heart failure (HF) with reduced ejection fraction and arrhythmias.8 While clinicians often associate cTn increases in COVID-19 to direct effects, many patients often have clear antecedent causes for chronic injury like chronic cardiovascular disease that explain such elevations. In this section, we will analyze potential mechanisms of cardiac involvement that can lead to myocardial injury in this setting.
Direct Damage of SARS-CoV-2 in the Cardiovascular System
One possible mechanism for direct damage is the cytotoxic effect of SARS-CoV-2 on the endothelium which can cause diffuse microthrombosis.9 , 10 At postmortem evaluation, nonocclusive fibrin microthrombi (without ischemic injury) are common (12/15 patients with COVID-19).11
Another potential mechanism is direct virus-induced myocardial injury and the potential for myocarditis. SARS-CoV-2 has been detected in the myocardium12 and, in a multicenter autopsy study,13 increased interstitial myocardial macrophages were identified in most of the cases but lymphocytic myocarditis in only a small fraction. Clinical studies suggest that myocarditis caused by SARS-CoV-2 is uncommon.14
Other hypotheses for direct damage include the possibility of infection and replication of virus within noncontractile cells in the heart such as endothelial cells, fibroblasts, and pericytes with matrix inflammation and fibrosis. There also are other speculative hypotheses.9
Nondirect Effects of SARS-CoV-2 in the Cardiovascular System
Nondirect effects of SARS-CoV-2 could be related to angiotensin-converting enzyme 2 (ACE2) downregulation/shedding with a subsequent hyperactive renin–angiotensin–aldosterone system (RAAS). Moreover, SARS-CoV-2 infection induces the activation of the innate immune system, leading to elevated levels of proinflammatory cytokines, including interleukin-6 (IL-6), interleukin-1, interleukin-2, tumor necrosis factor alpha, and interferon-c.9
Furthermore, SARS-CoV-2 can activate a cascade of thrombotic mechanisms through hyperactivated monocytes, platelets, and neutrophils generating neutrophil extracellular traps (NETs).9 Indeed, hypercoagulation with diffuse microthrombi is considered the main cause of organ failure in severe cases.11 , 13
Viral Load and Myocardial Injury
There may be a relationship between viral load and myocardial injury. In one study,15 all patients with detectable SARS-CoV-2 viral load had quantifiable (≥6 ng/L) hs-cTnT concentrations, and 76% of them had concentrations above the assay-specific 99th percentile indicative of myocardial injury. While those without viremia also had quantifiable hs-cTnT concentrations (59% of cases) and myocardial injury (38%),15 these abnormalities were significantly more common in those with viremia. Another report16 evaluating both groups, however, concluded that there was no significant difference in the incidence of myocardial injury in patients with low compared with elevated viral load. Nonetheless, both myocardial injury and an elevated viral load were independent predictors of in-hospital mortality.16 Finally, a study of symptomatic hospitalized patients suggest that patients with COVID-19 and viremia have higher concentrations of inflammatory markers (such as IL-6, C-reactive protein, procalcitonin, and ferritin), but similar levels of cTnT and NT-proBNP to patients without viremia.17
Classification of myocardial injury in COVID-19
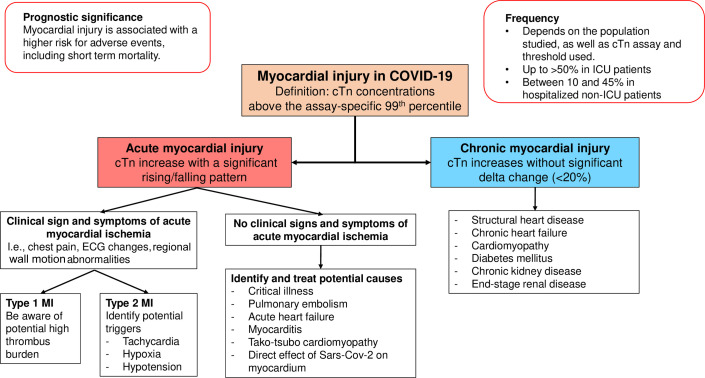
As suggested previously,4 each cTn increase greater than the 99th percentile URL should be classified as chronic myocardial injury, acute nonischemic myocardial injury, or acute MI. Fig. 1 summarizes this classification and some of the possible mechanisms of myocardial injury in patients with COVID-19.
Fig. 1.
Classification of myocardial injury and its possible pathogenetic mechanisms in patients with COVID-19.
Chronic Myocardial Injury
Chronic myocardial injury is defined as stable increases (<20% variation) above the 99th percentile of cTn concentrations.5 Patients with COVID-19 are frequently affected by chronic cardiovascular comorbidities, such as hypertension, diabetes, coronary artery disease, HF, and chronic kidney disease (CKD),1 , 3 , 18 all of which can be associated with cTn increases above the 99th percentile. Structural heart disease and HF are often associated with chronic cTn increases which portend an adverse prognosis.5 , 19, 20, 21 Similarly, an elevated cTn in patients with diabetes and CKD identifies patients at higher risk of cardiovascular events.22 , 23
Studies in patients with COVID-19 with serial cTn measurements indicate that from 13% to 26% have stable and thus chronic increases in cTn.24, 25, 26 In our multicenter Mayo Clinic health system study,27 we adjudicated every hs-cTnT increase above the sex-specific 99th percentile among patients with COVID-19. Most hs-cTnT elevations were modest, with a median value of 12 ng/L, and significantly higher in men than in women (15 vs 9 ng/L). About half of the increases were associated with conditions such as HF, cardiomyopathy, or CKD. These data support the hypothesis that, in significant proportions of patients with COVID-19, myocardial injury is chronic and not due to effects directly related to COVID-19.
Acute Nonischemic Myocardial Injury
Acute nonischemic myocardial injury is defined as a significant rise and/or fall in cTn concentrations with at least one cTn concentration above the 99th percentile without clinical signs and symptoms of acute myocardial ischemia.5 These occur often in critically ill patients4 , 8 and are not specific to COVID-19. A recent study28 comparing COVID-19 with influenza patients showed that, despite a higher absolute risk of death in patients with COVID-19, myocardial injury was frequent and increased the risk of death in both diseases. Moreover, acute myocardial injury is common in critically ill patients,29 in those with acute respiratory distress,30 and sepsis.31 In our COVID-19 study,27 we found that critical illness and sepsis could be identified as drivers of cTn increases in about 40% of patients. Metkus and colleagues32 compared the frequency of myocardial injury in intubated patients with COVID-19 with patients with other causes of acute respiratory distress syndrome (ARDS) and reported that the rate of myocardial injury was similar (51% in COVID-19 compared with 49.6% in ARDS). They concluded that myocardial injury in severe COVID-19 is related to baseline comorbidities, advanced age, and multisystem organ dysfunction, like what happens in traditional ARDS. In addition to the multiorgan dysfunction and hemodynamic impairment that can lead to cTn increases, patients with severe sepsis and septic shock may manifest abnormal systolic function and impaired myocardial relaxation.33 An echocardiography study in patients with COVID-19 reported that those with myocardial injury more frequently manifested left ventricular (LV) dysfunction detected by global longitudinal strain (GLS) and right ventricular (RV) dysfunction, which only partially resolved during follow-up.34 Similarly, another study reported that patients with myocardial injury more frequently manifest global LV dysfunction, regional wall motion abnormalities, diastolic dysfunction, RV dysfunction, and pericardial effusions.35
Other causes of acute nonischemic myocardial injury include RV pressure overload related to PE36 , 37 and/or microthrombi in the pulmonary circulation.13 In a retrospective study37 of 1240 patients with COVID-19, PE was identified in 8.3% by computed tomography. Male gender, higher C-reactive protein levels, and longer hospitalization were associated with higher risk of PE while anticoagulation (both at prophylactic and therapeutic dose) were protective. A meta-analysis36 of 7178 patients with COVID-19 reported a pooled incidence of acute PE in 15% of patients hospitalized in general wards and in 23% of ICU patients.
Data on endomyocardial biopsy (EMB)/autopsy tissue characterization in suspected COVID-19 are scarce38 but myocardial inflammation (without necrosis) caused by macrophages and T cells is common in noninfectious and in COVID-19 related deaths but usually without histologic criteria for myocarditis.39 There are, however, a few cases of EMB/autopsy-proven histologic and immuno-histological active myocarditis but only 3 tested positive for SARS-CoV-2 by polymerase chain reaction on heart tissue suggesting the hypothesis that a virus-negative form, possibly triggered by the infection, might be etiologic.38 In our report,27 myocarditis was rare. There was clinical suspicion in 3 patients, but none had confirmatory testing performed.
Finally, features compatible with Takotsubo cardiomyopathy have been identified in 2% to 4%40 , 41 of patients with COVID-19 undergoing transthoracic echocardiogram. It could develop from catecholamine-induced microvascular dysfunction or secondary to the metabolic, inflammatory, and emotional impairment associated with COVID-19.41
Type 1 and type 2 Myocardial Infarction
When reports demonstrated a high incidence of myocardial injury in patients with COVID-19, there were concerns about a possible high incidence of type 1 MI related to the prothrombotic state or, in those critically ill, type 2 MI. In our study27 which used systematic adjudication5 of all hs-cTnT increases, only a minority (5%) met MI criteria. Among those with type 2 MI, the most frequent triggers were hypoxia, hypotension, and/or tachyarrhythmias. Salbach and colleagues26 reported a similarly low incidence. Differences in the frequency of type 2 MI in nonadjudicated studies are likely related to patient selection and less rigor in applying criteria establishing the presence of acute myocardial ischemia. One potential difference is that42 in patients with COVID-19, oxygen demand-supply imbalance is often secondary to hypoxemia, increased heart rate, inflammatory status, and/or decompensated HF, whereas in most type 2 MIs, tachyarrhythmias and anemia are often prevalent mechanisms. Conventional treatment strategies seem appropriate but individualized care is warranted given the heterogeneous presentations and mechanisms. It is worth noting that in those with STEMI,43 there seems to be a higher thrombus burden, and these patients can have worse outcomes.
Frequency of myocardial injury in patients with COVID-19
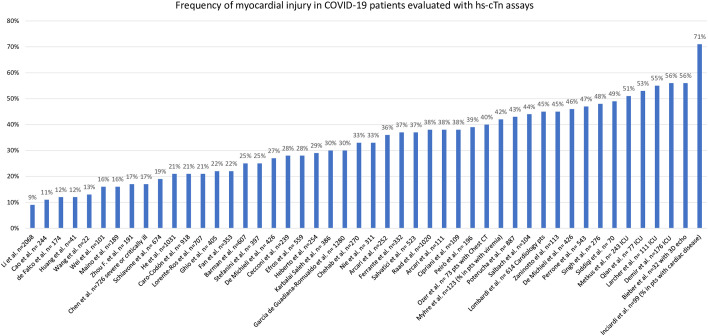
Many studies in this area have used arbitrary definitions and cutoffs to define myocardial injury2 , 44 and others have been based on non-high sensitivity cTn assays.45 Table 1 tabulates the frequency of myocardial injury based on hs-cTn concentrations above the 99th percentile URL or above specified thresholds. As shown in Fig. 2 , the frequency of myocardial injury varies widely probably in relation to patient selection. In studies of patients admitted to intensive care units (ICU), the frequency of myocardial injury is as high as or greater than 50%.32 , 46 , 47 Studies that include a broader spectrum of patients suggest a frequency that ranges from 10%48 , 49 to more than 45%.26 , 27 , 50, 51, 52 This variation is likely related to the specific assay and/or threshold used, patient selection, and the population baseline characteristics. Only a small number of studies (see Table 1) applied sex-specific 99th percentiles as recommended.5
Table 1.
Frequency of myocardial injury based on hs-cTn concentrations above the 99th percentile URL or above specified thresholds
| Study | Location | Population | Cardiac Troponin Assay | Cutoffs Used | Frequency of Myocardial Injury |
|---|---|---|---|---|---|
| Cao et al,63 2020 | Wuhan, China | 244 COVID-19 admitted patients w/o CV disease or CKD | ADVIA Centaur XP, Siemens Healthcare Diagnostics, Erlangen, Germany). | >40 ng/L | 11% |
| Li et al,48 2020 | Wuhan, China | 2068 COVID-19 admitted patients | Hs-cTnI, other details NR | >34.2 pg/mL | 8.8% total, 2.3% in non-critically ill 30% in critically ill |
| Lorente-Ros et al,64 2020 | Spain | 707 COVID-19 admitted patients | Abbott hs-cTnI | > 14 ng/L | 20.9% |
| Huang et al,44 2020 | Wuhan, China. | 41 COVID-19 admitted patients | Hs-cTnI, other details NR. | >28 ng/L | All: 12% ICU: 31% Non-ICU: 4% |
| Zhou F. et al,3 2020 | Wuhan, China. | 191 COVID-19 admitted patients | Hs-cTnI, other details NR | >28 pg/mL | All: 17% Non-survivor: 46% Survivor: 1% |
| Inciardi et al,50 2020 | Brescia, Italy | 99 COVID-19 admitted patients | Hs-TnT | >14 ng/L | 71% of patients with cardiac disease, 47% without cardiac disease |
| Cecconi et al,65 2020 | Milano, Italy | 239 COVID-19 admitted patients | Troponin I, other details NR | >19.8 ng/L | 27.7% overall |
| Nie et al,66 2020 | Huazhong, China | 311 COVID-19 admitted patients | hs-cTnI, ARCHITECT STAT, Abbott | >99th URL | 33.1% |
| Wei et al,67 2020 | China | 101 COVID-19 admitted patients | hs-TnT | >14 ng/L | 15.8% |
| Wang et al,68 2020 | Wuhan, China | 22 COVID-19 admitted patients with severe pneumonia | hs-TnI, other details NR | >34.2 pg/mL | 13% |
| Heberto et al,69 2020 | Mexico | 254 COVID-19 admitted patients | hs-cTnI Beckman Coulter | >17.5 ng/L | 28.7% |
| Raad et al,70 2020 | Southeast Michigan, USA |
1020 COVID-19 admitted patients | hs-cTnI Beckman-Coulter | >18 ng/L | 38% |
| Stefanini et al,71 2020 | Milan, Italy | 397 COVID-19 admitted patients | hs-TnI Beckman Coulter | ≥19.6 ng/L | 25% |
| Schiavone et al,72 2020 | Italy | 674 COVID-19 admitted patients | Hs-cTn, other details NR | >99th URL | 43.8% in CCS 14.4% without CCS |
| Arcari et al,73 2020 | Rome, Italy | 111 COVID-19 admitted patients | Hs-Troponin T Hs-Troponin I (other details NR) |
< 14 pg/mL < 35 pg/mL |
38% |
| Ghio et al,74 2020 | Pavia, Italy | 405 COVID-19 admitted patients | Hs-cTnI (other details NR) | 99th URL | 74/340 (22%) |
| Karbalai Saleh et al,75 2020 | Tehran, Iran | 386 COVID-19 admitted patients | hs-cTnI, other details NR | >26 ng/mL for men >11 ng/L for women |
29.8% |
| Lombardi et al,51 2020 | Italy, multicentric | 614 COVID-19 patients admitted to Cardiology Units | Hs-cTnI or hs-cTnT, other details NR | >99th URL | 45% |
| Salvatici et al,76 2020 | Milan, Italy | 523 COVID-19 admitted patients | hs-TnI Beckman Coulter | 11.6 ng/L for women 19.8 ng/L for men | 37.3% |
| Singh et al,52 2020 | Chicago USA | 276 COVID-19 admitted patients | Hs-TnT | 17 ng/L (median in their population) | 48% |
| Fan et al,77 2020 | Wuhan china | 353 COVID-19 admitted patients | Hs-cTnI STAT High Sensitive Troponin-I Abbott | >34.2 pg/mL for men >15.6 pg/mL for women |
22.4% |
| He et al,78 2020 | Wuhan china | 1031 COVID-19 admitted patients | Hs-cTnI, other details NR | >99th URL | 20.7% |
| Zaninotto el al.24 2020 | Padova, Italy | 113 COVID-19 admitted patients | Hs-cTnI Architect i2000, Abbott Diagnostics | 16 ng/L for women 34 ng/L for men |
45% |
| Ferrante et al,79 2020 | Milano. Italy | 332 COVID-19 admitted patients with chest CT | Hs-cTnI, other details NR | >20 ng/L | 37% |
| Chen et al,80 2020 | Wuhan china | 726 COVID-19 admitted patients severe or critically ill | Hs-cTnI Architect i2000, Abbott Diagnostics | >28 ng/L | 37.4% in critical patients 10.4% in severe patients |
| Poterucha et al,81 2021 | New York, USA | 887 COVID-19 admitted patients with ECG | Hs-cTnT | ≥20 ng/L | 43% |
| Perrone et al,82 2021 | Italy, multicentric | 543 COVID-19 admitted patients | hs-cTnT | >14 ng/L | 47% |
| Metkus et al,32 2021 | Baltimore, USA | 243 COVID-19 admitted patients intubated. | Hs-cTnI and hs-cTnT | >99th URL | 51% |
| Peirò et al,83 2021 | Tarragona, Spain | 196 COVID-19 patients ED/hospital | Hs-cTn I Assay, Advia Centaur, Siemens | >21 ng/L | 39.3% |
| Efros et al,84 2021 | Tel-Aviv, Israel | 559 COVID-19 admitted patients | hs-TnT | >99th URL | 28.4% |
| Cipriani et al,85 2021 | Padova, Italy | 109 COVID-19 admitted patients | Hs-cTnI Architect i2000, Abbott Diagnostics | 16 ng/L for women 34 ng/L for men |
38% |
| Qian et al,86 2021 | Wuhan china | 77 ICU COVID-19 patients | Hs-cTnI, other details NR | >28 ng/L | 53% |
| De Michieli et al,54 2021 | Padova, Italy | 426 ED COVID-19 patients | Hs-cTnI Architect i2000, Abbott Diagnostics | 16 ng/L for women 34 ng/L for men |
27.2% |
| Siddiqi et al,15 2021 | Boston, USA | 70 COVID-19 admitted patients | Hs-cTnT | >14 ng/L | 16/21 (76%) Pts with viremia 18/49 (38%) w/o viremia |
| Larcher et al,47 2021 | France | 111 ICU COVID-19 patients | Hs-cTnT | >14 ng/L | 55% |
| Demir et al,46 2021 | London, UK | 176 ICU COVID-19 pts with cTn | Hs-cTnT | >14 ng/L | 56% |
| Myhre et al,17 2021 | Akershus University Hospital Norway |
123 COVID-19 admitted patients | Hs-cTnT | >10 ng/L for women >15 ng/L for men |
42% in pts with viremia 33% in pts w/o |
| Bieber et al,34 2021 | Munich, Germany | 32 COVID-19 admitted patients with 3D echo | Hs-cTnT | >14 ng/L | 56% |
| Garcia de Guadiana-Romualdo et al,87 2021 | Spain, multicenter | 1280 ED COVID-19 patients | Hs-cTnT cTnI Siemens Atellica cTnI Siemens Advia Centaur cTn I Siemens Dimension EXL cTnI Abbott Architect cTn I Beckman DxI 800/Access |
>99th URL | 26.9% w/o sex-specific cutoffs 30% with sex-specific cutoffs |
| de Falco et al,49 2021 | Naples, Italy | 174 COVID-19 admitted patients | Hs-cTnI Architect i2000, Abbott Diagnostics | 16 ng/L for women 34 ng/L for men |
11.5% |
| Barman et al,58 2021 | Turkey | 607 COVID-19 admitted patients | Hs-cTnI, other details NR | >14 pg/mL | 24.7% |
| De Michieli et al,27 2021 | USA, multicenter | 367 COVID-19 admitted patients with cTn measured | Hs-cTnT | >10 ng/L for women, >15 ng/L for men |
46% |
| Ozer et al,88 2021 | Turkey | 73 COVID-19 admitted patients with Chest CT | Abbott, ARCHITECT STAT High Sensitive Troponin-I |
>11.5 ng/L | 39.7% |
| Caro-Codón et al,89 2021 | Madrid, Spain | 918 patients with COVID-19 ED with cTn measured | Atellica Solution IM1600, Siemens Healthineers hs-cTnI | > 34.1 ng/L > 53.5 ng/L | 20.7% |
| Maino et al,90 2021 | Rome, Italy | 189 COVID-19 admitted patients | hs-TnI Advia Centaur Siemens |
57 ng/L For men 37 ng/L for women |
16% overall 9.7% in mild 29.0% in severe 61.3% in critical |
| Chehab et al,16 2021 | Detroit, USA | 270 COVID-19 admitted patients with cTn | Hs-cTnI Beckman Coulter | 100 ng/L (not URL) | 32.6% |
| Arcari et al,91 2021 | Italy, multicenter | 252 COVID-19 admitted patients, 229 with cTn | Hs-Troponin T hs-Troponin I, other details NR | 14 pg/mL 35 pg/mL |
36% |
| Salbach et al,26 2021 | Heildeberg, Germany | 104 COVID-19 admitted patients | Hs-cTnT | >14 ng/L | 44.2% |
Fig. 2.
Frequency of myocardial injury in multiple studies based on hs-cTn values. Details about different studies’ population, the assays used, and a complete list of references are available on Table 1.
The use of cardiac troponin in patients with COVID-19
Using high-sensitivity cTn assays, following guideline recommendations, sex-specific 99th percentile URLs should be used to define myocardial injury.5 The use of uniform criteria will allow reporting in a comparable way between studies. Moreover, the prognostic significance of myocardial injury as defined by cTn concentrations greater than 99th percentile URL has been demonstrated repeatedly in the COVID-19 population. Irrespective of etiology, myocardial injury is associated with adverse events and increased mortality in patients with COVID-19.2 , 45 , 51
Single Sample Versus Serial Samples
Most studies only report values at baseline. Limited data exist addressing serial samples. Kini and colleagues25 evaluated hs-cTnI measurements between 72h before and 48h after the COVID19 diagnosis and classified patients as suffering from chronic myocardial injury or acute myocardial injury (>20% or >50% delta with elevated or normal baseline cTn, respectively). They found that both types of myocardial injury were associated with increased mortality at 30 days and 6 months even after multivariable adjustment. However, among patients less than 65 years and those without known coronary artery disease, acute myocardial injury was associated with a worse prognosis at 6 months. It was associated with a more pronounced inflammatory status, more ischemic risk factors such as intracoronary thrombosis and more oxygen supply–demand imbalance due to sepsis, but also more nonischemic conditions, like myocarditis, PE, and Takotsubo syndrome. In contrast, patients with chronic myocardial injury had more chronic comorbidities, including CKD and HF. Nuzzi and colleagues53 evaluated hs-cTn measurements (either T or I) within 24 h of admission and, subsequently, again between 24 and 48 h. They categorized patients in 4 groups: normal (troponin <99th URL at both assessments), normal-elevated (normal cTn at admission and elevated thereafter), elevated-normal or elevated (ie, cTn>99th URL at both measurements). Patients with incident myocardial injury, with persistent elevated cTn, and with elevated cTn only at admission had a higher risk of death compared with those with normal cTn at both evaluations. By multivariable analysis, patients that developed myocardial injury had the highest mortality risk. A smaller study24 showed that patients with significant variation in concentrations of hs-cTnI (delta ≥ 20%), and at least one value ≥ 99th sex-specific URLs had longer hospital stays, more aggressive disease, and more often needed admission to ICU. Therefore, the data seem to indicate an adjunctive prognostic role for serial sampling although the populations that benefit most from this monitoring are a matter of debate.
Adjunctive Role of Cardiac Troponin in Risk Stratification
The role of very low hs-cTn concentrations to facilitate the identification of low-risk patients with a favorable prognosis has been demonstrated for both hs-cTnT27 and hs-cTnI.54 Patients with very low values at presentation (<6 ng/L for Roche hs-cTnT and < 5 ng/L for Abbott hs-cTnI) are at low risk for mortality and adverse events. Particularly, a single hs-cTnT less than 6 ng/L identified 26% of patients with COVID-19 without mortality and a low risk of major adverse events among patients presenting to the ED.27 Similarly, an initial hs-cTnI less than 5 ng/L identified 33% of patients at low risk with 97.8% sensitivity and 99.2% negative predictive value in a hospitalized cohort.54 These findings are similar to what is suggested for ruling-out MI, and likely occur because very low hs-cTn concentrations represent an objective measure to identify younger patients with fewer comorbidities.
Conversely, whether cTn increases enhance risk stratification in patients with COVID-19 remains a matter of debate. Omland and colleagues55 reported that in multivariable models adjusting for clinical variables and a severity of illness score, only ferritin and lactate dehydrogenase (but not cTn) were significant predictors of a composite outcome of hospital mortality and admission to the ICU for mechanical ventilation and lasting greater than 24 hours in consecutive unselected COVID-19 patients. In our Padova study,54in patients with COVID-19 presenting through the ED, hs-cTnI was a significant predictor of mortality for patients with lower Acute Physiology and Chronic Health Evaluation II (APACHE II) score but not in those with higher (>13) APACE score. One could argue that in those that are more critically ill, the adjunctive role of cTn in predicting outcomes is more limited. However, hs-cTn can help to identify those who are less severely ill but are also at risk. Moreover, its use may be more clinically convenient than a more complex multivariable model. It may also be the case that many studies were based on cTn concentrations obtained for clinical reasons, potentially biasing the analysis.
When to Measure Cardiac Troponin and What to Do if It Is Elevated?
The European Society of Cardiology Study Group on Biomarkers in Cardiology of the Acute Cardiovascular Care Association developed a document discussing the significance and the proper use of cTn in COVID-19.56 There is a paucity of evidence regarding the appropriate response to finding an increased hs-cTn concentration. If a type 1 MI is suspected, established diagnostic algorithms for rule-out and/or rule-in of MI should be deployed according to current guidelines.56 However, given that in most patients with COVID-19 a type 1 MI is not present, these individuals rarely undergo coronary angiography. Indeed, in critically ill patients with septic shock and/or ARDS, cTn increases are more likely due to critical illness with or without hemodynamic impairment, resulting in myocardial injury or, if ischemia is present, type 2 MI.56 Data on the appropriate therapy for type 2 MI in the critically ill are scarce and this is even more true for patients with COVID-19, constituting an important research gap.57
Prognostic implications
Most studies have correlated myocardial injury with a poor in hospital outcome and short term mortality, regardless of the presence of known concomitant cardiovascular disease.58 , 59 Conversely, cTn concentrations remain within the normal range in most survivors.56 The incidence of myocardial injury increases with greater severity of illness and with the development of ARDS.56 Regarding the consequences of myocardial injury in COVID-19, Kotecha and colleagues60 performed cardiac magnetic resonance (CMR) in 148 patients with such injury who recovered from severe COVID-19 after a median of 68 days. They found late gadolinium enhancement and/or ischemia in 54% of patients. This included myocarditis-like scar in 26%, infarction and/or ischemia in 22%, and dual pathology in 6%. Myocarditis-like injury was limited in extent and had minimal functional consequences; however, in 30% signs of active myocarditis persisted. Of the patients with an ischemic injury pattern, 66% had no history of coronary disease suggesting pre-existing silent disease or de novo COVID-19-related changes. Puntmann and colleagues61 performed CMR after a median of 71 days in 100 recovered patients with COVID-19 (including two-thirds of patients that recovered at home). hs-TnT was detectable in 71 patients and elevated (>13.9 pg/mL) in 5 patients. CMR revealed cardiac involvement in 78 patients and ongoing myocardial inflammation in 60. Hs-cTnT was significantly correlated with native T1 mapping, native T2 mapping, and LV mass.
Data regarding long-term consequences of myocardial injury in those who survived COVID-19 are scarce. A prospective exercise echocardiographic evaluation of 48 patients 6 months after COVID-19 disease (some of whom had experienced myocardial injury62) revealed that exercise induced a significant increase in the average E/e′ ratio and systolic pulmonary artery pressure in those who had suffered myocardial injury.
Summary
Myocardial injury, defined as cTn increases above the assay-specific 99th percentile, is frequent in patients with COVID-19. It correlates with adverse events and short-term mortality. Most increases seem related to chronic cardiovascular conditions and acute nonischemic myocardial injury, similarly to that reported in severely ill patients. However, some studies with advanced cardiac imaging and long-term follow-up indicate that myocardial injury might be associated with long-term structural abnormalities and worse cardiac performance. Except for patients suffering from type 1 MI, the appropriate treatment of patients with COVID-19 with myocardial injury remains case-specific and further investigations are necessary to understand how to improve outcomes in this population.
Clinics care points
-
•
Myocardial injury is common in patients with COVID-19 infection, but its frequency varies widely based on the population studied and the cTn assay and threshold used.
-
•
Even though COVID-19 patients can present with type 1 or type 2 MI, acute and chronic myocardial injury (cTn increases above the 99th percentile without clinical evidence of acute myocardial ischemia) are the most common reasons for cTn increases.
-
•
Regardless of the mechanism, myocardial injury, and the magnitude of cTn increases have prognostic significance.
Acknowledgments
Disclosure
Dr Y. Sandoval has previously served on the Advisory Boards for Roche Diagnostics and Abbott Diagnostics without personal compensation. He has also been a speaker without personal financial compensation for Abbott Diagnostics. Dr A.S. Jaffe has consulted or presently consults for most of the major diagnostics companies, including Beckman-Coulter, Abbott, Siemens, Ortho Diagnostics, ET Healthcare, Roche, Radiometer, Sphingotec, RCE, and Amgen and Novartis. Dr L. De Michieli has nothing to disclose.
Footnotes
This article originally appeared in Cardiology Clinics, Volume 40, Issue 3, August 2022.
References
- 1.Guo T., Fan Y., Chen M., et al. Cardiovascular implications of Fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi S., Qin M., Shen B., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandoval Y., Januzzi J.L., Jaffe A.S. Cardiac troponin for assessment of myocardial injury in COVID-19. J Am Coll Cardiol. 2020;76(10):1244–1258. doi: 10.1016/j.jacc.2020.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thygesen K., Alpert J.S., Jaffe A.S., et al. Fourth Universal definition of myocardial infarction (2018) Circulation. 2018;138(20) doi: 10.1161/CIR.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 6.Sandoval Y., Jaffe A.S. Using high-sensitivity cardiac troponin T for acute cardiac care. Am J Med. 2017;130(12):1358–1365.e1. doi: 10.1016/j.amjmed.2017.07.033. [DOI] [PubMed] [Google Scholar]
- 7.Apple F.S., Jaffe A.S., Collinson P., et al. IFCC educational materials on selected analytical and clinical applications of high sensitivity cardiac troponin assays. Clin Biochem. 2015;48(4–5):201–203. doi: 10.1016/j.clinbiochem.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 8.Jaffe A.S., Cleland J.G.F., Katus H.A. Myocardial injury in severe COVID-19 infection. Eur Heart J. 2020;41(22):2080–2082. doi: 10.1093/eurheartj/ehaa447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pesce M., Agostoni P., Bøtker H.E., et al. COVID-19-related cardiac complications from clinical evidences to basic mechanisms: opinion paper of the ESC Working Group on Cellular Biology of the Heart. Cardiovasc Res. 2021;117(10):2148–2160. doi: 10.1093/cvr/cvab201. [DOI] [PubMed] [Google Scholar]
- 10.Libby P., Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020;41(32):3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bois M.C., Boire N.A., Layman A.J., et al. COVID-19–Associated Nonocclusive fibrin microthrombi in the heart. Circulation. 2021;143(3):230–243. doi: 10.1161/CIRCULATIONAHA.120.050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tavazzi G., Pellegrini C., Maurelli M., et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22(5):911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basso C., Leone O., Rizzo S., et al. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J. 2020;41(39):3827–3835. doi: 10.1093/eurheartj/ehaa664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozieranski K., Tyminska A., Jonik S., et al. Clinically suspected myocarditis in the course of severe acute respiratory syndrome novel coronavirus-2 infection: Fact or Fiction? J Card Fail. 2021;27(1):92–96. doi: 10.1016/j.cardfail.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siddiqi H.K., Weber B., Zhou G., et al. Increased prevalence of myocardial injury in patients with SARS-CoV-2 viremia. Am J Med. 2021;134(4):542–546. doi: 10.1016/j.amjmed.2020.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chehab O., El Zein S., Kanj A., et al. SARS-CoV-2 viral load and myocardial injury: independent and Incremental predictors of adverse outcome. Mayo Clinic Proc Innov Qual Outcomes. 2021;5(5):891–897. doi: 10.1016/j.mayocpiqo.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myhre P.L., Prebensen C., Jonassen C.M., et al. SARS-CoV-2 viremia is associated with inflammatory, but not cardiovascular biomarkers, in patients hospitalized for COVID-19. JAHA. 2021;10(9) doi: 10.1161/JAHA.120.019756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu C., Chen X., Cai Y., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Lemos J.A., Drazner M.H., Omland T., et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304(22):2503. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takashio S., Yamamuro M., Izumiya Y., et al. Coronary microvascular dysfunction and diastolic load correlate with cardiac troponin T Release measured by a highly sensitive assay in patients with nonischemic heart failure. J Am Coll Cardiol. 2013;62(7):632–640. doi: 10.1016/j.jacc.2013.03.065. [DOI] [PubMed] [Google Scholar]
- 21.Myhre P.L., Claggett B., Ballantyne C.M., et al. Association between Circulating troponin concentrations, left ventricular systolic and diastolic functions, and incident heart failure in Older Adults. JAMA Cardiol. 2019;4(10):997. doi: 10.1001/jamacardio.2019.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dillmann W.H. Diabetic cardiomyopathy: what is it and can it Be Fixed? Circ Res. 2019;124(8):1160–1162. doi: 10.1161/CIRCRESAHA.118.314665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel P.C., Ayers C.R., Murphy S.A., et al. Association of Cystatin C with left ventricular structure and function: the Dallas heart study. Circ Heart Fail. 2009;2(2):98–104. doi: 10.1161/CIRCHEARTFAILURE.108.807271. [DOI] [PubMed] [Google Scholar]
- 24.Zaninotto M., Mion M.M., Padoan A., et al. Cardiac troponin I in SARS-CoV-2-patients: the additional prognostic value of serial monitoring. Clin Chim Acta. 2020;511:75–80. doi: 10.1016/j.cca.2020.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kini A., Cao D., Nardin M., et al. Types of myocardial injury and mid-term outcomes in patients with COVID-19. Eur Heart J - Qual Care Clin Outcomes. 2021;7(5):438–446. doi: 10.1093/ehjqcco/qcab053. [DOI] [PubMed] [Google Scholar]
- 26.Salbach C., Mueller-Hennessen M., Biener M., et al. Interpretation of myocardial injury subtypes in COVID-19 disease per fourth version of Universal Definition of Myocardial Infarction. Biomarkers. 2021;26(5):401–409. doi: 10.1080/1354750X.2021.1921031. [DOI] [PubMed] [Google Scholar]
- 27.De Michieli L., Ola O., Knott J.D., et al. High-sensitivity cardiac troponin T for the detection of myocardial injury and risk stratification in COVID-19. Clin Chem. 2021;67(8):1080–1089. doi: 10.1093/clinchem/hvab062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biasco L., Klersy C., Beretta G.S., et al. In: Comparative frequency and prognostic impact of myocardial injury in hospitalized patients with COVID-19 and Influenza. Bäck M., editor. 2021. (European heart Journal Open). oeab025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Babuin L., Vasile V.C., Rio Perez J.A., et al. Elevated cardiac troponin is an independent risk factor for short- and long-term mortality in medical intensive care unit patients. Crit Care Med. 2008;36(3):759–765. doi: 10.1097/CCM.0B013E318164E2E4. [DOI] [PubMed] [Google Scholar]
- 30.Vasile V.C., Chai H.S., Khambatta S., et al. Significance of elevated cardiac troponin T levels in critically ill patients with acute respiratory disease. Am J Med. 2010;123(11):1049–1058. doi: 10.1016/j.amjmed.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 31.Vasile V.C., Chai H.S., Abdeldayem D., et al. Elevated cardiac troponin T levels in critically ill patients with sepsis. Am J Med. 2013;126(12):1114–1121. doi: 10.1016/j.amjmed.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 32.Metkus T.S., Sokoll L.J., Barth A.S., et al. Myocardial injury in severe COVID-19 compared with non–COVID-19 acute respiratory distress syndrome. Circulation. 2021;143(6):553–565. doi: 10.1161/CIRCULATIONAHA.120.050543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landesberg G., Jaffe A.S., Gilon D., et al. Troponin elevation in severe sepsis and septic shock: the role of left ventricular diastolic dysfunction and right ventricular Dilatation. Crit Care Med. 2014;42(4):790–800. doi: 10.1097/CCM.0000000000000107. [DOI] [PubMed] [Google Scholar]
- 34.Bieber S., Kraechan A., Hellmuth J.C., et al. Left and right ventricular dysfunction in patients with COVID-19-associated myocardial injury. Infection. 2021;49(3):491–500. doi: 10.1007/s15010-020-01572-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giustino G., Croft L.B., Stefanini G.G., et al. Characterization of myocardial injury in patients with COVID-19. J Am Coll Cardiol. 2020;76(18):2043–2055. doi: 10.1016/j.jacc.2020.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roncon L., Zuin M., Barco S., et al. Incidence of acute pulmonary embolism in COVID-19 patients: systematic review and meta-analysis. Eur J Intern Med. 2020;82:29–37. doi: 10.1016/j.ejim.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fauvel C., Weizman O., Trimaille A., et al. Pulmonary embolism in COVID-19 patients: a French multicentre cohort study. Eur Heart J. 2020;41(32):3058–3068. doi: 10.1093/eurheartj/ehaa500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caforio A.L.P., Baritussio A., Basso C., et al. Clinically suspected and biopsy-proven myocarditis Temporally associated with SARS-CoV-2 infection. Annu Rev Med. 2022;73(1) doi: 10.1146/annurev-med-042220-023859. annurev-med-042220-023859. [DOI] [PubMed] [Google Scholar]
- 39.Kawakami R., Sakamoto A., Kawai K., et al. Pathological evidence for SARS-CoV-2 as a cause of myocarditis. J Am Coll Cardiol. 2021;77(3):314–325. doi: 10.1016/j.jacc.2020.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dweck M.R., Bularga A., Hahn R.T., et al. Global evaluation of echocardiography in patients with COVID-19. Eur Heart J - Cardiovasc Imaging. 2020;21(9):949–958. doi: 10.1093/ehjci/jeaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giustino G., Croft L.B., Oates C.P., et al. Takotsubo cardiomyopathy in COVID-19. J Am Coll Cardiol. 2020;76(5):628–629. doi: 10.1016/j.jacc.2020.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Talanas G., Dossi F., Parodi G. Type 2 myocardial infarction in patients with coronavirus disease 2019. J Cardiovasc Med (Hagerstown) 2021;22:603–605. doi: 10.2459/JCM.0000000000001136. [DOI] [PubMed] [Google Scholar]
- 43.Choudry F.A., Hamshere S.M., Rathod K.S., et al. High thrombus burden in patients with COVID-19 presenting with ST-Segment elevation myocardial infarction. J Am Coll Cardiol. 2020;76(10):1168–1176. doi: 10.1016/j.jacc.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lala A., Johnson K.W., Januzzi J.L., et al. Prevalence and Impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76(5):533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Demir O.M., Ryan M., Cirillo C., et al. Impact and determinants of high-sensitivity cardiac troponin-T concentration in patients with COVID-19 admitted to critical care. Am J Cardiol. 2021;147:129–136. doi: 10.1016/j.amjcard.2021.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Larcher R., Besnard N., Akouz A., et al. Admission high-sensitive cardiac troponin T level increase is independently associated with higher mortality in critically ill patients with COVID-19: a multicenter study. JCM. 2021;10(8):1656. doi: 10.3390/jcm10081656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li C., Jiang J., Wang F., et al. Longitudinal correlation of biomarkers of cardiac injury, inflammation, and coagulation to outcome in hospitalized COVID-19 patients. J Mol Cell Cardiol. 2020;147:74–87. doi: 10.1016/j.yjmcc.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Falco R., Vargas M., Palma D., et al. B-type natriuretic peptides and high-sensitive troponin I as COVID-19 survival factors: which one is the best performer? JCM. 2021;10(12):2726. doi: 10.3390/jcm10122726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Inciardi R.M., Adamo M., Lupi L., et al. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur Heart J. 2020;41(19):1821–1829. doi: 10.1093/eurheartj/ehaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lombardi C.M., Carubelli V., Iorio A., et al. Association of troponin levels with mortality in Italian patients hospitalized with coronavirus disease 2019: Results of a multicenter study. JAMA Cardiol. 2020;5(11):1274. doi: 10.1001/jamacardio.2020.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singh N., Anchan R.K., Besser S.A., et al. High sensitivity Troponin-T for prediction of adverse events in patients with COVID-19. Biomarkers. 2020;25(8):626–633. doi: 10.1080/1354750X.2020.1829056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nuzzi V., Merlo M., Specchia C., et al. The prognostic value of serial troponin measurements in patients admitted for COVID-19. ESC Heart Fail. 2021;8:3504–3511. doi: 10.1002/ehf2.13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Michieli L., Babuin L., Vigolo S., et al. Using high sensitivity cardiac troponin values in patients with SARS-CoV-2 infection (COVID-19): the Padova experience. Clin Biochem. 2021;90:8–14. doi: 10.1016/j.clinbiochem.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Omland T., Prebensen C., Røysland R., et al. Established cardiovascular biomarkers Provide limited prognostic information in unselected patients hospitalized with COVID-19. Circulation. 2020;142(19):1878–1880. doi: 10.1161/CIRCULATIONAHA.120.050089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mueller C., Giannitsis E., Jaffe A.S., et al. Cardiovascular biomarkers in patients with COVID-19. Eur Heart J Acute Cardiovasc Care. 2021;10(3):310–319. doi: 10.1093/ehjacc/zuab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bularga A., Chapman A.R., Mills N.L. Mechanisms of myocardial injury in COVID-19. Clin Chem. 2021;67(8):1044–1046. doi: 10.1093/clinchem/hvab111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barman H.A., Atici A., Sahin I., et al. Prognostic significance of cardiac injury in COVID-19 patients with and without coronary artery disease. Coron Artery Dis. 2021;32(5):359–366. doi: 10.1097/MCA.0000000000000914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Çınar T., Hayıroğlu M.İ., Çiçek V., et al. Prognostic significance of cardiac troponin level in Covid-19 patients without known cardiovascular risk factors. Am J Emerg Med. 2021;45:595–597. doi: 10.1016/j.ajem.2020.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kotecha T, Knight DS, Razvi Y, et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur Heart J 2021;42(19):1866-1878. doi:10.1093/eurheartj/ehab075. [DOI] [PMC free article] [PubMed]
- 61.Puntmann V.O., Carerj M.L., Wieters I., et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(11):1265. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fayol A., Livrozet M., Boutouyrie P., et al. Cardiac performance in patients hospitalized with COVID-19: a 6 month follow-up study. ESC Heart Fail. 2021;8(3):2232–2239. doi: 10.1002/ehf2.13315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cao J., Zheng Y., Luo Z., et al. Myocardial injury and COVID-19: Serum hs-cTnI level in risk stratification and the prediction of 30-day fatality in COVID-19 patients with no prior cardiovascular disease. Theranostics. 2020;10(21):9663–9673. doi: 10.7150/thno.47980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lorente-Ros A., Ruiz J.M.M., Rincón L.M., et al. Myocardial injury determination improves risk stratification and predicts mortality in COVID-19 patients. Cardiol J. 2020;27(4):8. doi: 10.5603/CJ.a2020.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cecconi M., Piovani D., Brunetta E., et al. Early predictors of clinical Deterioration in a cohort of 239 patients hospitalized for Covid-19 infection in Lombardy. Italy JCM. 2020;9(5):1548. doi: 10.3390/jcm9051548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nie S.F., Yu M., Xie T., et al. Cardiac troponin I is an independent predictor for mortality in hospitalized patients with coronavirus disease 2019. Circulation. 2020;120 doi: 10.1161/CIRCULATIONAHA.120.048789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wei J.F., Huang F.Y., Xiong T.Y., et al. Acute myocardial injury is common in patients with COVID-19 and impairs their prognosis. Heart. 2020;106(15):1154–1159. doi: 10.1136/heartjnl-2020-317007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y., Zheng Y., Tong Q., et al. Cardiac injury and clinical course of patients with coronavirus disease 2019. Front Cardiovasc Med. 2020;7:147. doi: 10.3389/fcvm.2020.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heberto A.B., Carlos P.C.J., Antonio C.R.J., et al. Implications of myocardial injury in Mexican hospitalized patients with coronavirus disease 2019 (COVID-19) IJC Heart & Vasculature. 2020;30:100638. doi: 10.1016/j.ijcha.2020.100638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Raad M., Dabbagh M., Gorgis S., et al. Cardiac injury patterns and Inpatient outcomes among patients admitted with COVID-19. Am J Cardiol. 2020;133:154–161. doi: 10.1016/j.amjcard.2020.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stefanini G.G., Chiarito M., Ferrante G., et al. Early detection of elevated cardiac biomarkers to optimise risk stratification in patients with COVID-19. Heart. 2020;106(19):1512–1518. doi: 10.1136/heartjnl-2020-317322. [DOI] [PubMed] [Google Scholar]
- 72.Schiavone M., Gasperetti A., Mancone M., et al. Redefining the prognostic value of high-sensitivity troponin in COVID-19 patients: the importance of concomitant coronary artery disease. JCM. 2020;9(10):3263. doi: 10.3390/jcm9103263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arcari L., Luciani M., Cacciotti L., et al. Incidence and determinants of high-sensitivity troponin and natriuretic peptides elevation at admission in hospitalized COVID-19 pneumonia patients. Intern Emerg Med. 2020;15(8):1467–1476. doi: 10.1007/s11739-020-02498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ghio S., Baldi E., Vicentini A., et al. Cardiac involvement at presentation in patients hospitalized with COVID-19 and their outcome in a tertiary referral hospital in Northern Italy. Intern Emerg Med. 2020;15(8):1457–1465. doi: 10.1007/s11739-020-02493-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karbalai Saleh S., Oraii A., Soleimani A., et al. The association between cardiac injury and outcomes in hospitalized patients with COVID-19. Intern Emerg Med. 2020;15(8):1415–1424. doi: 10.1007/s11739-020-02466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Salvatici M., Barbieri B., Cioffi S.M.G., et al. Association between cardiac troponin I and mortality in patients with COVID-19. Biomarkers. 2020;25(8):634–640. doi: 10.1080/1354750X.2020.1831609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fan Q., Zhu H., Zhao J., et al. Risk factors for myocardial injury in patients with coronavirus disease 2019 in China. ESC Heart Fail. 2020;7(6):4108–4117. doi: 10.1002/ehf2.13022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.He X., Wang L., Wang H., et al. Factors associated with acute cardiac injury and their effects on mortality in patients with COVID-19. Sci Rep. 2020;10(1):20452. doi: 10.1038/s41598-020-77172-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ferrante G., Fazzari F., Cozzi O., et al. Risk factors for myocardial injury and death in patients with COVID-19: insights from a cohort study with chest computed tomography. Cardiovasc Res. 2020;116(14):2239–2246. doi: 10.1093/cvr/cvaa193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen H., Li X., Marmar T., et al. Cardiac Troponin I association with critical illness and death risk in 726 seriously ill COVID-19 patients: a retrospective cohort study. Int J Med Sci. 2021;18(6):1474–1483. doi: 10.7150/ijms.53641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Poterucha T.J., Elias P., Jain S.S., et al. Admission cardiac diagnostic testing with Electrocardiography and troponin measurement Prognosticates increased 30-day mortality in COVID-19. JAHA. 2021;10(1) doi: 10.1161/JAHA.120.018476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Perrone M.A., Spolaore F., Ammirabile M., et al. The assessment of high sensitivity cardiac troponin in patients with COVID-19: a multicenter study. IJC Heart & Vasculature. 2021;32:100715. doi: 10.1016/j.ijcha.2021.100715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peiró Ó.M., Carrasquer A., Sánchez-Gimenez R., et al. Biomarkers and short-term prognosis in COVID-19. Biomarkers. 2021;26(2):119–126. doi: 10.1080/1354750X.2021.1874052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Efros O., Barda N., Meisel E., et al. Myocardial injury in hospitalized patients with COVID-19 infection—risk factors and outcomes. Ai T., editor. PLoS ONE. 2021;16(2) doi: 10.1371/journal.pone.0247800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cipriani A., Capone F., Donato F., et al. Cardiac injury and mortality in patients with Coronavirus disease 2019 (COVID-19): insights from a mediation analysis. Intern Emerg Med. 2021;16(2):419–427. doi: 10.1007/s11739-020-02495-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Qian H., Gao P., Tian R., et al. Myocardial injury on admission as a risk in critically ill COVID-19 patients: a retrospective in-ICU study. J Cardiothorac Vasc Anesth. 2021;35(3):846–853. doi: 10.1053/j.jvca.2020.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.García de Guadiana-Romualdo L., Morell-García D., Rodríguez-Fraga O., et al. Cardiac troponin and COVID-19 severity: Results from BIOCOVID study. Eur J Clin Invest. 2021;51(6) doi: 10.1111/eci.13532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Özer S., Bulut E., Özyıldız A.G., et al. Myocardial injury in COVID-19 patients is associated with the thickness of epicardial adipose tissue. Kardiologiia. 2021;61(8):48–53. doi: 10.18087/cardio.2021.8.n1638. [DOI] [PubMed] [Google Scholar]
- 89.Caro-Codón J., Rey J.R., Buño A., et al. Characterization of myocardial injury in a cohort of patients with SARS-CoV-2 infection. Medicina Clínica. 2021;157(6):274–280. doi: 10.1016/j.medcli.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maino A., Di Stasio E., Grimaldi M.C., et al. Prevalence and characteristics of myocardial injury during COVID-19 pandemic: a new role for high-sensitive troponin. Int J Cardiol. 2021;338:278–285. doi: 10.1016/j.ijcard.2021.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Arcari L., Luciani M., Cacciotti L., et al. Coronavirus disease 2019 in patients with cardiovascular disease: clinical features and implications on cardiac biomarkers assessment. J Cardiovasc Med. 2021;22(11):832–839. doi: 10.2459/JCM.0000000000001252. [DOI] [PubMed] [Google Scholar]