Abstract
Background
A previously underrecognized phenotype of left ventricular apical aneurysm (LVAA) has been increasingly identified in Fabry disease. This study explored LVAA's clinical prevalence and its prognostic implications over a long‐term follow‐up.
Methods and Results
We retrospectively analyzed 268 consecutive patients with Fabry disease at a tertiary medical center. Patients with increased left ventricular mass index were recognized as having left ventricular hypertrophy (LVH). LVAA was identified using either echocardiography or cardiovascular magnetic resonance imaging. Two patients with ischemic LVAA were excluded. The primary end point was a composite of cardiovascular events, including heart failure hospitalization, sustained ventricular tachycardia, ischemic stroke, and all‐cause mortality. Of 266 enrolled patients, 105 (39.5%) had LVH (age 58.5±11.9 years, 48.6% men), and 11 (10.5%) had LVAA. Over 49.3±34.8 months of follow‐up, 25 patients with LVH experienced composite events, including 9 heart failure hospitalizations, 4 sustained ventricular tachycardia, 6 ischemic strokes, and 15 mortalities. In patients with LVH, those with LVAA had a significantly higher risk of composite events and lower event‐free survival than those without LVAA (8 [72.7%] versus 17 [18.1%], log‐rank P<0.001). LVAA was independently associated with an increased risk of composite events (hazard ratio, 3.59 [95% CI, 1.30–9.91]; P=0.01) after adjusting for age, sex, advanced heart failure, renal function, dyslipidemia, atrial fibrillation, left ventricular ejection fraction, left ventricular diastolic function, and left ventricular mass index.
Conclusions
LVAA is present in approximately 10% of patients with Fabry disease and LVH. It is associated with an increased risk of adverse cardiovascular events and may necessitate aggressive treatment.
Keywords: cardiovascular magnetic resonance, echocardiography, Fabry disease, left ventricular apical aneurysm, left ventricular hypertrophy
Subject Categories: Cardiomyopathy, Echocardiography, Magnetic Resonance Imaging (MRI), Prognosis
Nonstandard Abbreviations and Acronyms
- ERT
enzyme replacement therapy
- HCM
hypertrophic cardiomyopathy
- LGE
late gadolinium enhancement
- LVAA
left ventricular apical aneurysm
Clinical Perspective.
What Is New?
Left ventricular apical aneurysm is present in approximately 10% of patients with Fabry disease with left ventricular hypertrophy.
Patients with Fabry disease and left ventricular apical aneurysm are at risk for more adverse cardiovascular events over a long‐term follow‐up period.
What Are the Clinical Implications?
Identification of apical aneurysm can be of clinical relevance for risk stratification in patients with Fabry disease.
Whether contemporary therapeutic interventions mitigate the cardiovascular risks derived from the aneurysm formation requires more investigation.
Fabry disease is an X‐linked inherited lysosomal storage disease caused by pathogenic variants of the GLA (α‐galactosidase A) gene. It results in deficient α‐Gal A (α‐galactosidase A) enzyme activity and accumulation of globotriaosylceramide in numerous tissues and organs. The cardiac manifestations of left ventricular hypertrophy (LVH), heart failure (HF), and arrhythmia are the major causes of low quality of life and premature death. 1 , 2 The administration of enzyme replacement therapy (ERT) has been demonstrated to be beneficial in Fabry disease. However, myocardial fibrosis, a crucial determinant of cardiovascular outcomes in Fabry cardiomyopathy, cannot be modified by ERT. 3 , 4 Hence, early recognition and identification of the high‐risk features are valuable for risk assessment and disease management.
LVH has been recognized as an important and common cardiovascular manifestation of Fabry disease. 1 , 5 Cardiac imaging enables the detection of various cardiac phenotypes and differentiation from other cardiomyopathies with LVH through the characterization of the myocardium. Thus, it has become essential for the prognostication and timely management of Fabry disease. 6 , 7 , 8 Distinct from the widely reported concentric LVH, a rare and novel phenotype of left ventricular apical aneurysm (LVAA) has been increasingly recognized in recent years. 9 , 10 This LVAA phenotype mimics hypertrophic cardiomyopathy (HCM) with apical hypertrophy or midventricular hypertrophy accompanied by LVAA. 9 , 10 , 11 Evidence indicates that patients with HCM with LVAA represent a subgroup at high risk of adverse cardiovascular events, including progressive HF and sudden cardiac death. 11 , 12 Nevertheless, the clinical importance and prognostic impact of LVAA remain unclear. Therefore, the present study aimed to investigate the clinical prevalence of LVAA and its prognostic implications among patients with Fabry disease.
METHODS
Study Population
This study was a retrospective cohort study conducted at a tertiary medical center in Taiwan, enrolling patients aged >20 years with Fabry disease from January 2010 through September 2020. Fabry disease was either identified from the pedigree of the index case diagnosed from the newborn screening program 13 , 14 or diagnosed upon clinical presentation of unexplained LVH. The diagnosis of Fabry disease was confirmed by the genetic sequencing of the GLA gene. 2 , 8 The identified pathogenic mutations were classified as either classic or late‐onset cardiac variants (eg, IVS4+919 G>A mutation, the predominant genotype in Taiwan). 13 , 15 Baseline characteristics were recorded, including body mass index, smoking status, functional capacity as categorized by the New York Heart Association functional classification, extracardiac symptoms, and comorbidities. Estimated glomerular filtration rate was calculated using the Modification of Diet in Renal Disease equation. 16 The presence of microalbuminuria was defined by the spot urine microalbumin‐to‐creatinine ratio of >0.03 upon the initial diagnosis. An ECG was recorded at baseline. ECG characteristics were determined by 2 independent researchers (H.‐C.C. and L.K.) who were blinded to the clinical data. ECG‐based diagnosis of LVH was performed according to the Sokolow‐Lyon criteria. Deep T‐wave inversion was defined as an inverted T wave of ≥5 mm. Poor R‐wave progression was defined as the absence of a QRS transition from a negative to positive deflection by lead V4. The present study was approved by the institutional review board of Taipei Veterans General Hospital, and written informed consent was obtained from each patient. The investigation also conformed to the principles outlined in the Declaration of Helsinki. The data underlying this article will be shared upon reasonable request to the corresponding author.
Imaging
Echocardiography
The transthoracic echocardiographic study was conducted according to the recommendations from the American Society of Echocardiography. 17 Left atrial dimension, interventricular septal thickness, and posterior wall thickness were measured using M‐mode tracing. Transmitral inflow parameters were measured using pulsed wave Doppler echocardiography, and early diastolic mitral annular velocity was measured through tissue Doppler echocardiography. Left ventricular ejection fraction (LVEF) was assessed using the modified Simpson's rule. Left ventricular (LV) mass was estimated using the area–length method and was divided by body surface area to yield LV mass index. LVH was defined as an increase in LV mass, with LV mass index >115 g/m2 in men or 95 g/m2 in women. LVAA was defined as the presence of a discrete, thin‐walled, dyskinetic, or akinetic segment at the LV apex. LVAA was identified by either echocardiography or cine images on cardiovascular magnetic resonance (CMR) imaging upon the initial diagnosis of Fabry disease. The diagnosis of LVAA was made by 2 independent investigators (H.‐C.C. and L.K.), who were both blind to data of clinical events on their respective review of the cardiac imaging and was confirmed by a third experienced cardiologist (W.‐C.Y.). The aneurysm size was measured as the maximal transverse dimension at end‐systole in the 4‐chamber view and was classified as small (<20 mm), medium (20–40 mm), or large (>40 mm). 12 Because obstructive atherosclerotic coronary disease can promote the formation of apical aneurysms, patients with ischemic LVAA were excluded. Atherosclerotic coronary disease was defined as either positive findings of noninvasive stress tests (either by exercise treadmill test or myocardial perfusion scan), history of acute coronary syndrome, or concomitant significant narrowing (≥50%) of the left anterior descending coronary artery by conventional coronary angiography.
CMR Imaging Acquisition Protocol and Late Gadolinium Enhancement Quantification
CMR imaging studies were performed on a 1.5T scanners (GE Optima MR450w; GE Healthcare, Waukesha, WI) and a 3T scanner (Discovery MR750; GE Healthcare), each with a cardiac phased‐array receiver surface coil and ECG gating. Cine imaging was performed using a steady‐state free precession sequence (echo time: 1.2–1.6 ms, repetition time: 3.2–3.6 ms) in a stack of 8‐mm‐thick short‐axis slices encompassing the whole ventricle as well as long‐axis slices. On the other hand, we used gradient echo sequence to minimize artifacts among patients with implantable devices. Late gadolinium enhancement (LGE) images were acquired 10 to 15 minutes after the intravenous administration of 0.15 mmol/kg gadobutrol (Gadovist, Bayer, Germany) as a contrast agent; an inversion‐recovery gradient echo pulse sequence was used to individually adjust the inversion time according to the result of inversion time scout scans to optimize the nulling of the normal myocardium (inversion time: 310–380 ms). 18 The field of view was 320×320 mm as standard but was altered depending on the patient size. The typical voxel size of the images was 1.6×2.0×8 mm, the echo time was 3.1 to 3.5 ms, and the repetition time was 6.2 to 7.6 ms for the 1.5T scanner. The echo time was 2.5 to 3.1 ms and the repetition time was 5.4 to 6.6 ms for the 3T scanner. All patients had obtained informed consent, and patient monitoring was performed according to the standard procedures of CMR imaging for patients with implanted devices. 19 The presence of LGE required confirmation in 2 spatial orientations, and the researcher was blinded to the clinical data. The extent of LGE was measured using CVI42 software (Circle Cardiovascular Imaging, Calgary, Canada), and signal thresholds of ≥5 SDs above the mean signal of the reference myocardium were applied to derive the values for total scar mass. Thereafter, each of those values was divided by the total LV mass to generate a percentage. 20
Study End Point and Follow‐Up
All of the patients were routinely evaluated every 3 months at our institution or a local clinic. The primary end point was a composite of cardiovascular events, including HF hospitalization, sustained ventricular tachycardia (VT), ischemic stroke, and all‐cause mortality. HF hospitalization was defined based on the presentation of typical HF symptoms and signs, chest radiograph findings, and elevated N‐terminal pro‐B‐type natriuretic peptide levels requiring hospitalization for intravenous therapies. Sustained VT was defined as ventricular beats at a rate of ≥100 bpm for >30 s, as detected either by continuous ECG strips, 24‐hour ambulatory Holter monitor studies routinely performed every year, or records from pacemakers or implantable cardioverter‐defibrillators. Ischemic stroke was defined as the sudden onset of a focal neurological deficit from a nontraumatic cause, as verified by brain magnetic resonance imaging or computed tomography.
Statistical Analysis
Continuous variables are expressed as mean±SD, and categorical variables are reported as count with percentage. One‐way analysis of variance, independent‐sample Student t‐test, χ2 test, and Fisher exact test were used to compare differences across groups, as appropriate. Bonferroni test was used for multiple comparison correction between groups. Among patients with LVH, logistic regression analysis was conducted to determine the characteristics associated with LVAA. Predictors of the primary end point were identified using Cox proportional hazards models by time‐to‐first‐event analysis. Multivariable Cox regression analysis with backward selection was conducted to assess the independence of the significant variables. At each follow‐up point, the proportion of patients not experiencing adverse events were estimated using the Kaplan‐Meier method. A 2‐tailed P value of <0.05 was considered indicative of statistical significance. All statistical analyses were performed using SPSS version 24.0 (IBM, Armonk, NY).
RESULTS
Baseline Characteristics
After excluding 2 patients with ischemic LVAA, we included a total of 266 patients (aged 50.2±15.4 years, 43.3% men) with Fabry disease into the analysis (Figure 1). Among them, 221 (83.1%) had the IVS4+919G>A mutation. Of the total study participants, 105 (39.5%) presented with LVH. Although none of the patients in the group of normal LV mass index had LVAA, 11 (10.5%) patients with LVH had LVAA, with sizes varying from 6.6 to 60 mm (median, 27 mm). LVAA could be identified using echocardiography in 6 patients, including 2 patients with large aneurysms and 4 patients with medium aneurysms; however, in 5 patients with medium or small aneurysms, LVAA could be only identified using CMR imaging.
Figure 1. Flowchart of the study population.
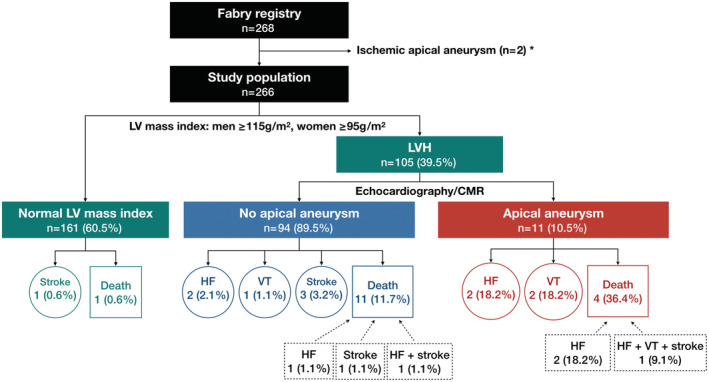
*Two patients with ischemic LVAA as indicated by coronary angiography showing significant lesions with ≥50% stenosis of the left anterior descending artery were excluded from analysis. CMR indicates cardiovascular magnetic resonance; HF, heart failure; LV, left ventricular; LVAA, left ventricular apical aneurysm; LVH, left ventricular hypertrophy; and VT, ventricular tachycardia.
The clinical and cardiac imaging characteristics are detailed in Table 1. In brief, patients with LVH were older and had more comorbidities such as hypertension, diabetes, dyslipidemia, atrial fibrillation, history of ischemic stroke, and advanced HF, as well as lower estimated glomerular filtration rate. There was no significant difference in the distribution of classical type or the manifestations of extracardiac symptoms among the 3 groups. Patients with LVAA had the lowest level of α‐Gal A activity, followed by the group of LVH without LVAA and the group with normal LV mass index. The ECGs suggest that prolonged PR interval and poor R‐wave progression were more prevalent in the patients with LVAA than in those without LVAA. A thicker posterior wall, greater LV mass index, larger left atrial dimension, and higher averaged ratio of early diastolic tansmitral inflow velocity (E) to early diastolic mitral annular veloctiy (e') (E/e') values were observed among patients with LVH and LVAA. However, the prevalence of LGE on CMR imaging was similar between the 2 groups. In regard to the medical treatment, there was no significant difference in the medical therapy or ERT between patients without and with LVAA, except that patients with LVAA were more likely on statins compared with those without LVAA.
Table 1.
Baseline Characteristics of the Study Population
| Variables | Normal LV mass index | LVH no apical aneurysm | LVH apical aneurysm | P1* value | P2† value |
|---|---|---|---|---|---|
| n=161 | n=94 | n=11 | |||
| Age, y | 44.8±15.0 | 57.5±11.8‡ | 67.5±9.5‡ | <0.001§ | 0.008§ |
| Men, n (%) | 72 (44.7) | 43 (45.7) | 8 (72.7) | 0.2 | 0.1 |
| Body mass index, kg/m2 | 24.0±3.7 | 24.5±4.5 | 24.9±4.0 | 0.5 | 0.8 |
| Smoking, active or quit, n (%) | 42 (26.1) | 17 (17.0) | 5 (45.5) | 0.06 | 0.04§ |
| Comorbidities | |||||
| Hypertension, n (%) | 40 (24.8) | 46 (48.9)‡ | 4 (36.4) | <0.001§ | 0.5 |
| Diabetes, n (%) | 11 (6.8) | 16 (17.0)‡ | 1 (9.1) | 0.04§ | 0.7 |
| Dyslipidemia, n (%) | 13 (8.1) | 15 (16.0) | 5 (45.5)‡ | 0.001§ | 0.03§ |
| Atrial fibrillation, n (%) | 2 (1.2) | 12 (12.8)‡ | 7 (63.6)‡ | <0.001§ | <0.001§ |
| Genetic mutation and clinical manifestations | |||||
| Classical type, n (%) | 12 (7.5) | 14 (14.9) | 1 (9.1) | 0.2 | 1 |
| IVS4+919G>A, n (%) | 142 (88.2) | 70 (75.3)‡ | 9 (81.8) | 0.03§ | 1 |
| α‐galactosidase A activity | 3.2±2.7 | 2.6±3.0 | 1.0±1.0‡ | 0.03§ | 0.001§ |
| NYHA class III/IV, n (%) | 1 (0.6) | 7 (7.4)‡ | 3 (27.3)‡ | <0.001§ | 0.07 |
| Angiokeratoma, n (%) | 1 (0.6) | 2 (2.2) | 0 (0) | NA | NA |
| Acroparesthesia, n (%) | 11 (6.8) | 8 (8.6) | 0 (0) | NA | NA |
| Abnormal sweating, n (%) | 2 (1.2) | 3 (3.2) | 0 (0) | NA | NA |
| eGFR, mL/min per 1.73 m2 | 99.2±22.9 | 80.3±26.3‡ | 79.9±18.7‡ | <0.001§ | 1 |
| Microalbuminuria, n (%) | 38 (23.6) | 33 (35.1) | 2 (18.2) | 0.1 | 0.3 |
| UACR, mg/g | 0.07±0.4 | 0.3±1.3 | 0.03±0.05 | 0.1 | 0.5 |
| Previous ischemic stroke, n (%) | 3 (1.9) | 8 (8.5)‡ | 1 (9.1) | 0.04§ | 1.0 |
| Electrocardiography | |||||
| PR interval, ms | 158.2±24.15 | 157.8±29.8 | 185.5±24.4‡ | 0.006§ | 0.005§ |
| LVH|| | 17 (10.7) | 38 (41.8)‡ | 5 (50)‡ | <0.001§ | 0.6 |
| Deep TWI | 6 (3.8) | 40 (44)‡ | 6 (60)‡ | <0.001§ | 0.3 |
| PRWP | 6 (3.8) | 7 (7.7) | 4 (40)‡ | <0.001§ | 0.002§ |
| Echocardiography | |||||
| LA dimension, mm | 30.6±5.1 | 36.2±6.7‡ | 43.3±8.6‡ | <0.001§ | 0.002§ |
| IVST, mm | 9.8±2.9 | 15.9±13.5‡ | 17.1±4.4‡ | <0.001§ | 0.8 |
| PWT, mm | 10.2±8.8 | 13.3±3.5‡ | 16.4±5.3‡ | <0.001§ | 0.008§ |
| E/A | 1.4±0.6 | 1.1±0.5‡ | 1.1±0.5 | <0.001§ | 0.6 |
| Average E/e′ | 8.2±2.8 | 12.8±5.6‡ | 18.9±6.7‡ | <0.001§ | 0.001§ |
| LVEF, % | 64.7±5.5 | 62.6±8.2 | 57.4±9.2‡ | <0.001§ | 0.05 |
| LVEF <40%, n (%) | 0 (0) | 1 (1.1) | 1 (9.1) | NA | 0.2 |
| LV mass index, g/m2 | 78.3±16.3 | 158.2±75.8‡ | 222.5±41.0‡ | <0.001§ | 0.007§ |
| CMR | |||||
| LGE, n (%), n=129 | 16 (21.3), n=75 | 21 (44.7),‡ n=47 | 5 (71.4),‡ n=7 | 0.002§ | 0.2 |
| Medication/treatment | |||||
| RAS‐i, n (%) | 18 (11.2) | 31 (33.0)‡ | 3 (27.3) | <0.001§ | 1 |
| β‐Blocker, n (%) | 8 (5.0) | 22 (23.4)‡ | 6 (54.5)‡ | <0.001§ | 0.06 |
| CCB, n (%) | 12 (7.5) | 20 (21.3)‡ | 1 (9.1) | 0.005§ | 0.7 |
| Diuretics, n (%) | 4 (2.5) | 13 (13.8)‡ | 2 (18.2)‡ | 0.001§ | 0.7 |
| MRA, n (%) | 0 (0) | 2 (2.1) | 1 (9.1) | NA | 0.3 |
| Statins, n (%) | 8 (5.0) | 11 (11.7)‡ | 5 (45.5)‡ | <0.001§ | 0.01§ |
| Aspirin, n (%) | 5 (3.1) | 12 (12.8)‡ | 2 (18.2)‡ | 0.005§ | 0.6 |
| Clopidogrel, n (%) | 0 (0) | 5 (5.3) | 1 (9.1) | NA | 0.5 |
| Warfarin, n (%) | 0 (0) | 0 (0) | 2 (18.2) | NA | NA |
| DOAC, n (%) | 1 (0.6) | 0 (0) | 1 (9.1) | NA | NA |
| ERT, n (%) | 52 (32.3) | 63 (67.0)‡ | 8 (72.7)‡ | <0.001§ | 1 |
CCB indicates calcium channel blocker; CMR, cardiac magnetic resonance; DOAC, direct oral anticoagulant; E/A, the ratio of of early to late diastolic transmitral flow velocity; E/e', the ratio of early diastolic tansmitral inflow velocity to early diastolic mitral annular velocity; eGFR, estimated glomerular filtration rate; ERT, enzyme replacement therapy; IVST, interventricular septal thickness; LA, left atrial; LGE, late gadolinium enhancement; LV, left ventricular; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; MRA, mineralocorticoid receptor antagonist; NA, not applicable; NYHA, New York Heart Association; PRWP, poor R wave progression; PWT, posterior wall thickness; RAS‐i, renin‐angiotensin system inhibitor; TWI, T wave inversion; and UACR, urine albumin‐to‐ceatinine ratio.
Comparison among the 3 groups.
Comparison between patients with LVH with and without apical aneurysm.
P<0.05, compared with patients with normal LV mass index after Bonferroni correction.
According to Sokolow‐Lyon criteria.
P<0.05.
Detailed clinical characteristics and LVAA morphology are presented in Figure 2. Of the 11 patients with LVAA, 9 (81.8%) had the IVS4 mutation. Only 1 patient with medium aneurysm had low LVEF of 33.5%. Of the 7 patients who underwent CMR imaging, 6 presented with LGE, and 1 with a small aneurysm displayed no signs of LGE. Representative instances of LGE revealed by gadolinium‐enhanced CMR imaging are presented in Figure 3. Among all the patients with LVH, older age, advanced‐stage HF (New York Heart Association functional class III or IV), comorbidity of atrial fibrillation, greater LV mass index, larger left atrial size, and higher average E/e′ were all significantly associated with the presence of LVAA (Table S1).
Figure 2. Clinical characteristics of the patients with LVAA.
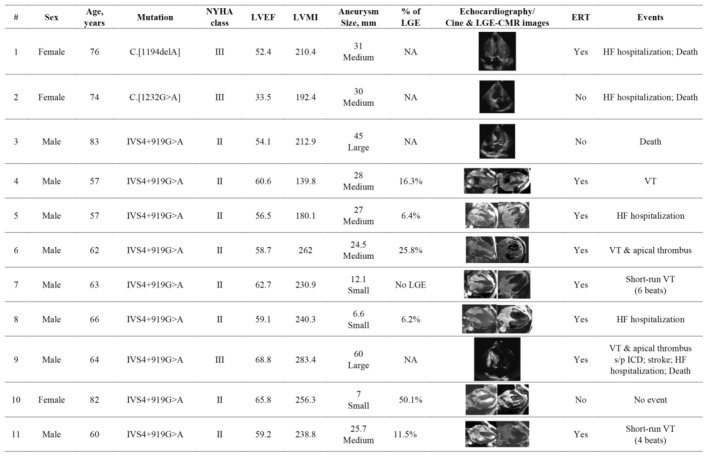
CMR indicates cardiovascular magnetic resonance; ERT, enzyme replacement therapy; HF, heart failure; ICD, implantable cardioverter‐defibrillator; LGE, late gadolinium enhancement; LVAA, left ventricular apical aneurysm; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; NA, not applicable; NYHA, New York Heart Association; and VT, ventricular tachycardia.
Figure 3. Representative LGE on gadolinium‐enhanced CMR images in patients with LVAA.
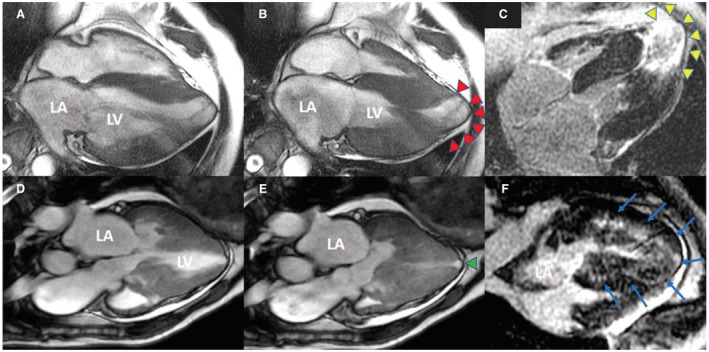
A 57‐year‐old man with a 2.7‐cm aneurysm (red arrowhead) in diastolic (A) and systolic phase (B), with midcavity obstruction visible in systole. Contrast‐enhanced CMR image reveals transmural LGE at the aneurysmal rim (C, yellow arrowhead). An 82‐year‐old woman with a small‐sized 0.7‐cm apical aneurysm (green arrowhead) in diastolic (D) and systolic phase (E); sigmoid hypertrophy (septum>lateral wall) with LVOT obstruction is visible. (F) The contrast‐enhanced CMR image displays diffused midwall LGE with extension to the apical aneurysm (blue arrows). CMR indicates cardiovascular magnetic resonance; LGE, late gadolinium enhancement; LVAA, left ventricular apical aneurysm; and LVOT, left ventricular outflow tract.
Survival Analysis
After a mean follow‐up of 51.9±37.5 months, 27 (10.2%) patients exhibited composite cardiovascular events, including 2 (1.2%) with normal LV mass index, 17 (18.1%) with LVH and without LVAA, and 8 (72.7%) with LVAA (Figure 1). Among the patients with LVH and LVAA, there was a total of 4 (36.4%) mortalities, 5 (45.5%) HF hospitalizations, 3 (27.3%) sustained VT, and 1 (9.1%) ischemic stroke over a mean follow‐up of 49.3±34.8 months. Both patients with large aneurysms, 5 of the 6 patients with medium aneurysms, and 1 of the 3 patients with small aneurysms experienced the primary end point. Of the 3 patients with LVAA who did not experience composite events, 1 had a medium aneurysm and the other 2 had small aneurysms. There was barely short‐run VT documented in these patients (Table 2).
Table 2.
Cox Regression Analysis on the Predictors of the Composite Outcomes in Patients With Fabry Disease Presenting With LVH
| Variables | Univariate analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Clinical characteristics | ||||||
| Age | 1.10 | 1.06–1.15 | <0.001* | 1.06 | 1.01–1.11 | 0.02* |
| Men | 2.07 | 0.91–4.68 | 0.08 | 2.37 | 0.96–5.89 | 0.06 |
| BMI | 0.93 | 0.84–1.04 | 0.2 | |||
| Smoking | 1.33 | 0.53–3.35 | 0.5 | |||
| NYHA class III/IV | 12.55 | 5.03–31.27 | <0.001* | 4.61 | 1.55–13.69 | 0.006* |
| eGFR | 0.98 | 0.96–0.99 | 0.002* | 0.98 | 0.96–1.00 | 0.03* |
| Cholesterol | 0.99 | 0.98–1.00 | 0.2 | |||
| Classical type | 0.84 | 0.29–2.48 | 0.8 | |||
| IVS4+919G>A | 1.95 | 0.66–5.72 | 0.2 | |||
| Hypertension | 1.43 | 0.64–3.20 | 0.4 | |||
| Diabetes | 1.18 | 0.44–3.16 | 0.7 | |||
| Dyslipidemia | 2.32 | 1.02–5.27 | 0.04* | 0.98 | 0.34–2.84 | 1.0 |
| AF | 3.8 | 1.70–8.50 | 0.001* | 0.83 | 0.25–2.75 | 0.8 |
| Echocardiography | ||||||
| LVEF <40% | 13.07 | 2.77–61.75 | 0.001* | 1.77 | 0.32–9.92 | 0.5 |
| LV mass index | 1.01 | 1.00–1.01 | 0.003* | 1.00 | 0.99–1.01 | 0.8 |
| LA dimension | 1.06 | 1.00–1.11 | 0.05 | |||
| Average E/e′ | 1.11 | 1.05–1.17 | <0.001* | 1.02 | 0.94–1.10 | 0.7 |
| Apical aneurysm | 8.41 | 3.46–20.42 | <0.001* | 3.59 | 1.30–9.91 | 0.01* |
| CMR | ||||||
| LGE | 3.68 | 0.99–13.73 | 0.05 | |||
| Treatment | ||||||
| ERT | 0.8 | 0.34–1.86 | 0.6 | |||
AF indicates atrial fibrillation; BMI, body mass index; CMR, cardiovascular magnetic resonance; E/e', the ratio of early diastolic tansmitral inflow velocity to early diastolic mitral annular velocity; eGFR, estimated glomerular filtration rate; ERT, enzyme replacement therapy; HR, hazard ratio; LA, left atrial; LGE, late gadolinium enhancement; LV, left ventricular; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; and NYHA, New York Heart Association.
P<0.05.
Notably, among the patients with LVH, the risk of composite cardiovascular events was 4‐fold higher (P<0.001) in the presence of LVAA (8 [72.7%]) than those without LVAA (17 [18.1%]). Similarly, the patients with LVAA were at 3‐fold higher risk of all‐cause mortality (4 [36.4%] versus 11 [11.7%]; P=0.049), 10‐fold higher risk of HF‐related hospitalization (5 [45.5%] versus 4 [4.3%]; P<0.001), and 25‐fold higher risk of VT (3 [27.3%] versus 1 [1.1%]; P=0.003). However, the proportion of ischemic stroke was similar between patients with and without LVAA (1 [9.1%] versus 5 [5.3%]; P=0.5; Figure 4). The comparison of the proportion of adverse cardiovascular events between the 3 groups of patients is shown in Figure S1.
Figure 4. Comparison of the proportion of adverse cardiovascular events between the groups of LVH without LVAA and LVH with LVAA.
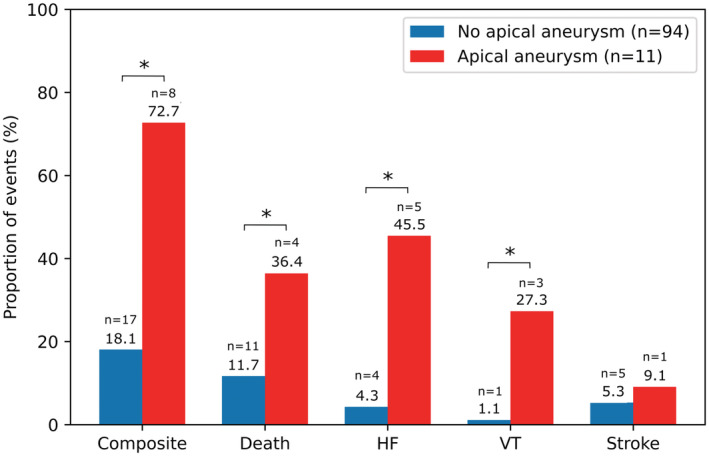
*P<0.05. HF indicates heart failure; LVAA, left ventricular apical aneurysm; LVH, left ventricular hypertrophy; and VT, ventricular tachycardia.
Table 2 summarizes the prognostic implications of each parameter for predicting the long‐term primary cardiovascular events by univariable and multivariable Cox proportional hazards models. Univariable predictors of composite cardiovascular events were older age, advanced HF, low estimated glomerular filtration rate, comorbidities of dyslipidemia and atrial fibrillation, LVEF of <40%, high LV mass index, and high average E/e′, as well as LVAA. Although the trend of a higher risk of adverse events in the patients with LGE than in those without was observed, the difference was not significant (hazard ratio [HR], 3.68 [95% CI, 0.99–13.73]; P=0.05). After multivariable adjustment, LVAA remained an independent predictor of cardiovascular events in the patients with LVH (adjusted HR, 3.59 [95% CI, 1.30–9.91]; P=0.01). In the Kaplan‐Meier survival analysis, patients with LVH and LVAA registered a shorter composite event‐free survival compared with the group with LVH without LVAA and the group with normal LV mass index (log‐rank P<0.001; Figure 5).
Figure 5. Kaplan‐Meier analysis of composite adverse cardiovascular events in patients with Fabry disease at 5‐year follow‐up.
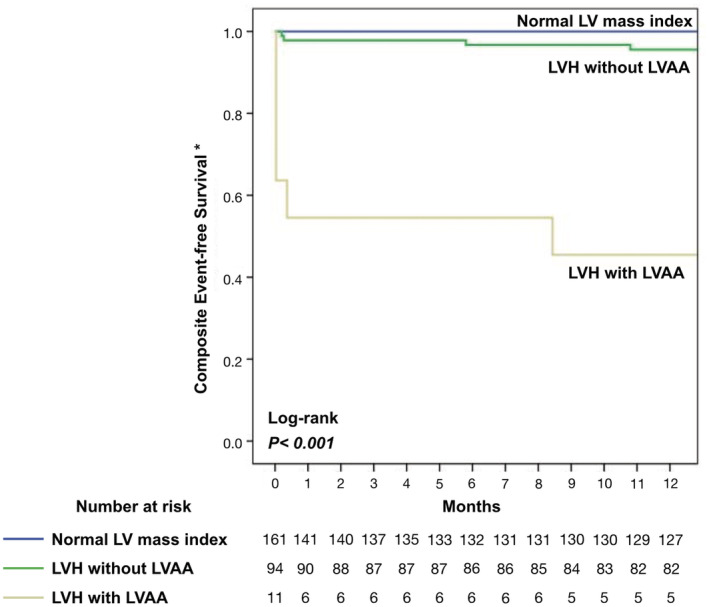
*Composite events include heart failure hospitalization, sustained ventricular tachycardia, ischemic stroke, and all‐cause mortality. LV indicates left ventricular; LVAA, left ventricular apical aneurysm; and LVH, left ventricular hypertrophy.
DISCUSSION
To the best of our knowledge, this is the first study to assess the clinical prevalence and prognostic implications of LVAA in patients with Fabry disease. The present study revealed that approximately 10% of patients with Fabry disease with LVH were present with LVAA and were characterized by older age, advanced HF, comorbidity of atrial fibrillation, increased LV mass index, enlarged left atrial dimensions, and LV diastolic dysfunction. In addition, LVAA was independently associated with an increased risk of adverse cardiovascular events, including HF hospitalization, VT, and all‐cause mortality.
Mechanisms of LVAA Formation
Although LVAAs have been discovered in numerous nonischemic cardiomyopathies, 9 , 21 , 22 , 23 the underlying mechanisms of LVAA formation can be variable. Two potential hypotheses have been proposed for patients with Fabry disease: (1) decreased myocardial perfusion at the LV apex and (2) local inflammation. The hypothesis of decreased myocardial perfusion has been derived from the observations of patients with HCM, considering the similar hypertrophic morphologies. 12 , 22 The peculiar hypertrophic morphology manifesting as LV midchamber hypertrophy with systolic midventricular obstruction can cause increased pressure at the LV apex, which leads to reduced coronary perfusion. The subsequent mismatch between myocardial oxygen supply and demand can result in microvascular dysfunction, further decreased coronary reserve, and the genesis of LVAA. The hypothesis of local inflammation can be inferred from Chagas cardiomyopathy 24 , 25 and cardiac sarcoidosis. 23 , 26 , 27 , 28 Recent studies have proposed the detection of cardiac involvement in patients with Fabry disease by using 18F‐fluorodeoxyglucose‐positron emission tomography and CMR imaging to characterize local myocardial inflammation, edema, and fibrosis. 29 , 30 In addition, a previous study reported that the aberrant accumulation of lipid antigens and the subsequent activation of immature natural killer T cells may cause autoimmunity and myocardial inflammation. 31 Although the formation of LVAA might be logically attributed to local inflammation, further investigation is required to verify the hypothesis of inflammation being involved in the formation of LVAA in patients with Fabry disease.
Thromboembolic Events
Another critical issue for LVAA is the increased risk of thromboembolic events. Nunes et al demonstrated that the prevalence of ischemic stroke in patients with Chagas disease was 20%, and apical aneurysm and intracavitary thrombus of the LV were independently associated with ischemic stroke. 21 Papanastasioua et al conducted a meta‐analysis that included a total of 2382 patients with HCM and discovered that LVAA was significantly associated with a high risk of thromboembolic events and sudden cardiac death. 32 However, in our study cohort, a significantly higher risk of ischemic stroke was not observed in the patients with LVAA compared with those without LVAA (9.1% versus 5.3%, P=0.5). Notably, this result was concluded according to the limited stroke event rate in the present study. Further investigation of thromboembolic events in a larger patient cohort is warranted to determine whether the prophylactic use of anticoagulants helps to prevent ischemic stroke in patients with LVAA.
Prognosis and Treatment
Notably, in the present study, LVAA was identified as an independent risk factor for poor cardiovascular outcomes, regardless of reduced LVEF or increased LV mass index, both of which are well‐known predictors of adverse cardiovascular events in patients with various cardiomyopathies. 33 , 34 , 35 , 36 Hanneman et al demonstrated that risk of adverse cardiovascular events increased with the extent of LGE in patients with Fabry cardiomyopathy. 37 It is noteworthy that in our cohort, 3 patients with the IVS4+919G>A mutation presented with small LVAAs, preserved LVEF, and severe LVH but variable LGE burden (Figure 2; patients 7, 8, and 10). These patients exhibited unique cardiovascular outcomes. For example, the oldest woman had extensive LGE, but no adverse cardiovascular events developed during a 3‐year follow‐up, even without ERT. Notably, because of the lack of serial CMR follow‐up to evaluate LVAA progression, the effect of ERT on LVAA remains unclear. Another concern is the size of the LVAA and the relationship with subsequent adverse events. Rowin et al reported the high risk of arrhythmic sudden cardiac death in patients with HCM regardless of LVAA size. 12 Limited by the nature of the observational study design and the case number of LVAA in the present study, the impact of the size of the LVAA or ERT on the adverse cardiovascular events could not be thoroughly assessed.
Study Limitations
The present study has some limitations. First, this study could be limited by its single‐center setting and retrospective analysis. The low case number of LVAAs could reduce the statistical power, increase the margin of error, and affect the interpretability of the result. However, because Fabry disease is considered a rare disease, our study represented a large cohort with collectively the most cases of LVAAs and an extended follow‐up duration. Second, there were only 8 of 11 patients with LVAA suffering from adverse cardiovascular events during the follow‐up period. Therefore, the overfitness of the multivariable Cox regression model may be a concern. Nevertheless, a type II error is the main issue on the problem of a small number of events in the regression model, which is not the case in this study. 38 Third, because of the limited nature of the retrospective cohort study, not all the patients in the cohort underwent CMR imaging. Because the detection of small aneurysms could be hindered by the acoustic window of echocardiography at the LV apex, the prevalence of LVAA could be underestimated, and selection bias might be present. 12 In addition, we could not evaluate the severity of sphingolipid accumulation by parametric native T1 mapping, and the LGE quantification was hindered in some studies because of suboptimal imaging quality. To further explore the natural course and the cause of LVAA formation, longitudinal follow‐up by echocardiography and CMR imaging at the early stage of Fabry disease could be necessary. 8 Fourth, natriuretic peptide was not routinely obtained as a consistent protocol in our cohort; therefore, its impact on the adverse cardiovascular events could not be fairly assessed in our study. Furthermore, whether the contemporary management strategies, such as early initiation of ERT or primary prevention with ICD, could effectively mitigate the higher risk of LVAA‐related complications was unclear and warrants further exploratory investigation.
CONCLUSIONS
Patients with Fabry disease and LVH are at risk of adverse cardiovascular events and premature deaths. Among them, at least 10% present with LVAA, which represents an even higher risk factor for complications, including HF‐related hospitalization, VT, and all‐cause mortality, and is independent of the severity of LVH or LV diastolic dysfunction. Further mechanistic studies are required to explore the mechanisms of LVAA formation in the context of disease progression. As a clinically relevant risk marker, identification of LVAA is potentially critical for risk stratification in patients with Fabry disease. Further exploratory studies are required to elucidate the potential benefits of applying contemporary treatment modalities to this vulnerable subgroup.
Sources of Funding
This study was supported by the intramural grants from the Taipei Veterans General Hospital (grant numbers: V101C‐187, V103C‐166, V104C‐175, V104E14‐003‐MY3‐2, V104D14‐003‐MY3‐3, V105C‐189, V106C‐186, and V107C‐174).
Disclosures
None.
Supporting information
Table S1
Figure S1
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.027041
For Sources of Funding and Disclosures, see page 10.
References
- 1. Linhart A, Kampmann C, Zamorano JL, Sunder‐Plassmann G, Beck M, Mehta A, Elliott PM, European FOSI. Cardiac manifestations of Anderson‐Fabry disease: results from the international Fabry outcome survey. Eur Heart J. 2007;28:1228–1235. doi: 10.1093/eurheartj/ehm153 [DOI] [PubMed] [Google Scholar]
- 2. Pieroni M, Moon JC, Arbustini E, Barriales‐Villa R, Camporeale A, Vujkovac AC, Elliott PM, Hagege A, Kuusisto J, Linhart A, et al. Cardiac involvement in Fabry disease: JACC review topic of the week. J Am Coll Cardiol. 2021;77:922–936. doi: 10.1016/j.jacc.2020.12.024 [DOI] [PubMed] [Google Scholar]
- 3. Weidemann F, Niemann M, Breunig F, Herrmann S, Beer M, Stork S, Voelker W, Ertl G, Wanner C, Strotmann J. Long‐term effects of enzyme replacement therapy on fabry cardiomyopathy: evidence for a better outcome with early treatment. Circulation. 2009;119:524–529. doi: 10.1161/CIRCULATIONAHA.108.794529 [DOI] [PubMed] [Google Scholar]
- 4. Kramer J, Niemann M, Stork S, Frantz S, Beer M, Ertl G, Wanner C, Weidemann F. Relation of burden of myocardial fibrosis to malignant ventricular arrhythmias and outcomes in Fabry disease. Am J Cardiol. 2014;114:895–900. doi: 10.1016/j.amjcard.2014.06.019 [DOI] [PubMed] [Google Scholar]
- 5. Wu JC, Ho CY, Skali H, Abichandani R, Wilcox WR, Banikazemi M, Packman S, Sims K, Solomon SD. Cardiovascular manifestations of Fabry disease: relationships between left ventricular hypertrophy, disease severity, and alpha‐galactosidase a activity. Eur Heart J. 2010;31:1088–1097. doi: 10.1093/eurheartj/ehp588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hsu TR, Hung SC, Chang FP, Yu WC, Sung SH, Hsu CL, Dzhagalov I, Yang CF, Chu TH, Lee HJ, et al. Later onset Fabry disease, cardiac damage progress in silence: experience with a highly prevalent mutation. J Am Coll Cardiol. 2016;68:2554–2563. doi: 10.1016/j.jacc.2016.09.943 [DOI] [PubMed] [Google Scholar]
- 7. Perry R, Shah R, Saiedi M, Patil S, Ganesan A, Linhart A, Selvanayagam JB. The role of cardiac imaging in the diagnosis and management of Anderson‐Fabry disease. JACC Cardiovasc Imaging. 2019;12:1230–1242. doi: 10.1016/j.jcmg.2018.11.039 [DOI] [PubMed] [Google Scholar]
- 8. Hung CL, Wu YW, Lin CC, Lai CH, Jyh‐Ming Juang J, Chao TH, Kuo L, Sung KT, Wang CY, Wang CL, et al. 2021 TSOC expert consensus on the clinical features, diagnosis, and clinical management of cardiac manifestations of Fabry disease. Acta Cardiol Sin. 2021;37:337–354. doi: 10.6515/ACS.202107_37(4).20210601A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Agarwal A, Malik A, DeFranco AC, Tajik AJ. Left ventricular apical aneurysm: a novel phenotype of Fabry's disease. Eur Heart J Cardiovasc Imaging. 2014;15:585. doi: 10.1093/ehjci/jet197 [DOI] [PubMed] [Google Scholar]
- 10. Poulin MF, Shah A, Trohman RG, Madias C. Advanced Anderson‐Fabry disease presenting with left ventricular apical aneurysm and ventricular tachycardia. World J Clin Cases. 2015;3:519–524. doi: 10.12998/wjcc.v3.i6.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maron MS, Finley JJ, Bos JM, Hauser TH, Manning WJ, Haas TS, Lesser JR, Udelson JE, Ackerman MJ, Maron BJ. Prevalence, clinical significance, and natural history of left ventricular apical aneurysms in hypertrophic cardiomyopathy. Circulation. 2008;118:1541–1549. doi: 10.1161/CIRCULATIONAHA.108.781401 [DOI] [PubMed] [Google Scholar]
- 12. Rowin EJ, Maron BJ, Haas TS, Garberich RF, Wang W, Link MS, Maron MS. Hypertrophic cardiomyopathy with left ventricular apical aneurysm: implications for risk stratification and management. J Am Coll Cardiol. 2017;69:761–773. doi: 10.1016/j.jacc.2016.11.063 [DOI] [PubMed] [Google Scholar]
- 13. Lin HY, Chong KW, Hsu JH, Yu HC, Shih CC, Huang CH, Lin SJ, Chen CH, Chiang CC, Ho HJ, et al. High incidence of the cardiac variant of Fabry disease revealed by newborn screening in the Taiwan Chinese population. Circ Cardiovasc Genet. 2009;2:450–456. doi: 10.1161/CIRCGENETICS.109.862920 [DOI] [PubMed] [Google Scholar]
- 14. Liao HC, Chiang CC, Niu DM, Wang CH, Kao SM, Tsai FJ, Huang YH, Liu HC, Huang CK, Gao HJ, et al. Detecting multiple lysosomal storage diseases by tandem mass spectrometry—a national newborn screening program in Taiwan. Clin Chim Acta. 2014;431:80–86. doi: 10.1016/j.cca.2014.01.030 [DOI] [PubMed] [Google Scholar]
- 15. Hwu WL, Chien YH, Lee NC, Chiang SC, Dobrovolny R, Huang AC, Yeh HY, Chao MC, Lin SJ, Kitagawa T, et al. Newborn screening for Fabry disease in Taiwan reveals a high incidence of the later‐onset GLA mutation c.936+919G>a (IVS4+919G>a). Hum Mutat. 2009;30:1397–1405. doi: 10.1002/humu.21074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F; Chronic Kidney Disease Epidemiology C . Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004 [DOI] [PubMed] [Google Scholar]
- 17. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 18. Simonetti OP, Kim RJ, Fieno DS, Hillenbrand HB, Wu E, Bundy JM, Finn JP, Judd RM. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218:215–223. doi: 10.1148/radiology.218.1.r01ja50215 [DOI] [PubMed] [Google Scholar]
- 19. Nazarian S, Hansford R, Rahsepar AA, Weltin V, McVeigh D, Gucuk Ipek E, Kwan A, Berger RD, Calkins H, Lardo AC, et al. Safety of magnetic resonance imaging in patients with cardiac devices. N Engl J Med. 2017;377:2555–2564. doi: 10.1056/NEJMoa1604267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moravsky G, Ofek E, Rakowski H, Butany J, Williams L, Ralph‐Edwards A, Wintersperger BJ, Crean A. Myocardial fibrosis in hypertrophic cardiomyopathy: accurate reflection of histopathological findings by CMR. JACC Cardiovasc Imaging. 2013;6:587–596. doi: 10.1016/j.jcmg.2012.09.018 [DOI] [PubMed] [Google Scholar]
- 21. Nunes MC, Kreuser LJ, Ribeiro AL, Sousa GR, Costa HS, Botoni FA, de Souza AC, Gomes Marques VE, Fernandez AB, Teixeira AL, et al. Prevalence and risk factors of embolic cerebrovascular events associated with Chagas heart disease. Glob Heart. 2015;10:151–157. doi: 10.1016/j.gheart.2015.07.006 [DOI] [PubMed] [Google Scholar]
- 22. Yang K, Song YY, Chen XY, Wang JX, Li L, Yin G, Zheng YC, Wei MD, Lu MJ, Zhao SH. Apical hypertrophic cardiomyopathy with left ventricular apical aneurysm: prevalence, cardiac magnetic resonance characteristics, and prognosis. Eur Heart J Cardiovasc Imaging. 2020;21:1341–1350. doi: 10.1093/ehjci/jeaa246 [DOI] [PubMed] [Google Scholar]
- 23. Kosuge H, Noda M, Kakuta T, Kishi Y, Isobe M, Numano F. Left ventricular apical aneurysm in cardiac sarcoidosis. Jpn Heart J. 2001;42:265–269. doi: 10.1536/jhj.42.265 [DOI] [PubMed] [Google Scholar]
- 24. Oliveira JS, Mello De Oliveira JA, Frederigue U Jr, Lima Filho EC. Apical aneurysm of Chagas's heart disease. Br Heart J. 1981;46:432–437. doi: 10.1136/hrt.46.4.432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garg G, Cohen S, Neches R, Travin MI. Cardiac (18)F‐FDG uptake in chagas disease. J Nucl Cardiol. 2016;23:321–325. doi: 10.1007/s12350-015-0218-0 [DOI] [PubMed] [Google Scholar]
- 26. Pedrotti P, Ammirati E, Bonacina E, Roghi A. Ventricular aneurysms in cardiac sarcoidosis: from physiopathology to surgical treatment through a clinical case presenting with ventricular arrhythmias. Int J Cardiol. 2015;186:294–296. doi: 10.1016/j.ijcard.2015.03.256 [DOI] [PubMed] [Google Scholar]
- 27. Ferrans VJ, Hibbs RG, Black WC, Walsh JJ, Burch GE. Myocardial degeneration in cardiac sarcoidosis: histochemical and electron microscopic studies. Am Heart J. 1965;69:159–172. doi: 10.1016/0002-8703(65)90033-5 [DOI] [PubMed] [Google Scholar]
- 28. Nanno T, Kobayashi S, Yoshitomi R, Fujii S, Kajii T, Kohno M, Ishiguchi H, Okuda S, Okada M, Suga K, et al. Detection of active inflammation status around ventricular aneurysms in patients with cardiac sarcoidosis. Circ J. 2019;83:2494–2504. doi: 10.1253/circj.CJ-19-0248 [DOI] [PubMed] [Google Scholar]
- 29. Nappi C, Altiero M, Imbriaco M, Nicolai E, Giudice CA, Aiello M, Diomiaiuti CT, Pisani A, Spinelli L, Cuocolo A. First experience of simultaneous PET/MRI for the early detection of cardiac involvement in patients with Anderson‐Fabry disease. Eur J Nucl Med Mol Imaging. 2015;42:1025–1031. doi: 10.1007/s00259-015-3036-3 [DOI] [PubMed] [Google Scholar]
- 30. Spinelli L, Imbriaco M, Nappi C, Nicolai E, Giugliano G, Ponsiglione A, Diomiaiuti TC, Riccio E, Duro G, Pisani A, et al. Early cardiac involvement affects left ventricular longitudinal function in females carrying alpha‐galactosidase a mutation: role of hybrid positron emission tomography and magnetic resonance imaging and speckle‐tracking echocardiography. Circ Cardiovasc Imaging. 2018;11:e007019. doi: 10.1161/CIRCIMAGING.117.007019 [DOI] [PubMed] [Google Scholar]
- 31. Mauhin W, Lidove O, Masat E, Mingozzi F, Mariampillai K, Ziza JM, Benveniste O. Innate and adaptive immune response in Fabry disease. JIMD Rep. 2015;22:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Papanastasiou CA, Zegkos T, Kokkinidis DG, Parcharidou D, Karamitsos TD, Efthimiadis GK. Prognostic role of left ventricular apical aneurysm in hypertrophic cardiomyopathy: a systematic review and meta‐analysis. Int J Cardiol. 2021;339:108. doi: 10.1016/j.ijcard.2021.07.025 [DOI] [PubMed] [Google Scholar]
- 33. Marstrand P, Han L, Day SM, Olivotto I, Ashley EA, Michels M, Pereira AC, Wittekind SG, Helms A, Saberi S, et al. Hypertrophic cardiomyopathy with left ventricular systolic dysfunction: insights from the SHaRe registry. Circulation. 2020;141:1371–1383. doi: 10.1161/CIRCULATIONAHA.119.044366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Olivotto I, Maron MS, Autore C, Lesser JR, Rega L, Casolo G, De Santis M, Quarta G, Nistri S, Cecchi F, et al. Assessment and significance of left ventricular mass by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2008;52:559–566. doi: 10.1016/j.jacc.2008.04.047 [DOI] [PubMed] [Google Scholar]
- 35. Grogan M, Scott CG, Kyle RA, Zeldenrust SR, Gertz MA, Lin G, Klarich KW, Miller WL, Maleszewski JJ, Dispenzieri A. Natural history of wild‐type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol. 2016;68:1014–1020. doi: 10.1016/j.jacc.2016.06.033 [DOI] [PubMed] [Google Scholar]
- 36. Patel MR, Cecchi F, Cizmarik M, Kantola I, Linhart A, Nicholls K, Strotmann J, Tallaj J, Tran TC, West ML, et al. Cardiovascular events in patients with fabry disease natural history data from the fabry registry. J Am Coll Cardiol. 2011;57:1093–1099. doi: 10.1016/j.jacc.2010.11.018 [DOI] [PubMed] [Google Scholar]
- 37. Hanneman K, Karur GR, Wasim S, Wald RM, Iwanochko RM, Morel CF. Left ventricular hypertrophy and late gadolinium enhancement at cardiac MRI are associated with adverse cardiac events in Fabry disease. Radiology. 2020;294:42–49. doi: 10.1148/radiol.2019191385 [DOI] [PubMed] [Google Scholar]
- 38. Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and cox regression. Am J Epidemiol. 2007;165:710–718. doi: 10.1093/aje/kwk052 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figure S1


