Abstract
Background
The Ross operation appears to restore normal survival in young and middle‐aged adults with aortic valve disease. However, there are limited data comparing it with conventional aortic valve replacement. Herein, we compared outcomes of the Ross procedure with mechanical and bioprosthetic aortic valve replacement (M‐AVR and B‐AVR, respectively).
Methods and Results
MEDLINE and EMBASE were searched through March 2022 to identify randomized controlled trials and propensity score–matched studies that investigated outcomes of patients aged ≥16 years undergoing the Ross procedure, M‐AVR, or B‐AVR. The systematic literature search identified 2 randomized controlled trials and 8 propensity score–matched studies involving a total of 4812 patients (Ross: n=1991; M‐AVR: n=2019; and B‐AVR: n=802). All‐cause mortality was significantly lower in the Ross procedure group compared with M‐AVR (hazard ratio [HR] [95% CI], 0.58 [0.35–0.97]; P=0.035) and B‐AVR (HR [95% CI], 0.32 [0.18–0.59]; P<0.001) groups. The reintervention rate was lower after the Ross procedure and M‐AVR compared with B‐AVR, whereas it was higher after the Ross procedure compared with M‐AVR. Major bleeding rate was lower after the Ross procedure compared with M‐AVR. Long‐term stroke rate was lower following the Ross procedure compared with M‐AVR and B‐AVR. The rate of endocarditis was also lower after the Ross procedure compared with B‐AVR.
Conclusions
Improved long‐term outcomes of the Ross procedure are demonstrated compared with conventional M‐AVR and B‐AVR options. These results highlight a need to enhance the recognition of the Ross procedure and revisit current guidelines on the optimal valve substitute for young and middle‐aged patients.
Keywords: aortic valve substitute, bioprosthetic aortic valve replacement, mechanical aortic valve replacement, Ross procedure, surgical aortic valve replacement
Subject Categories: Cardiovascular Surgery
Nonstandard Abbreviations and Acronyms
- B‐AVR
bioprosthetic aortic valve replacement
- M‐AVR
mechanical aortic valve replacement
- PSM
propensity score matched
- SAVR
surgical aortic valve replacement
- TAVR
transcatheter aortic valve replacement
Clinical Perspective.
What Is New?
Our meta‐analysis of 2 randomized control trials and 8 propensity score–matched studies showed that the Ross procedure was associated with improved late clinical end points, including all‐cause mortality and stroke, compared with conventional mechanical and bioprosthetic aortic valve replacement.
The Ross procedure was associated with lower rates of endocarditis compared with bioprosthetic aortic valve replacement and lower rates of permanent pacemaker implantation and bleeding compared with mechanical aortic valve replacement.
What Are the Clinical Implications?
Our results highlight a need to enhance the recognition of the Ross procedure and revisit the optimal valve substitute selection in the guidelines for young and middle‐aged patients needing an aortic valve replacement.
The landscape of aortic valve therapy has evolved substantially over the past few decades. Transcatheter aortic valve replacement (TAVR) has become an established alternative to bioprosthetic surgical aortic valve replacement (SAVR) for patients with severe aortic stenosis and appropriate anatomical features, irrespective of patient risk profile. 1 , 2 , 3 , 4 In addition, further expansion of TAVR indications now includes bicuspid pathology. 5 However, the ideal aortic valve substitute for young and middle‐aged adults remains debated.
Among the available aortic valve substitute options, the conventional option has been to use either a mechanical or a bioprosthetic valve, although the use of bioprostheses has increased substantially in the past 2 decades. 6 , 7 Bioprosthetic SAVR or TAVR for young/middle‐aged individuals may be regarded as a noncurative measure, as it inevitably requires at least ≥1 reoperations in the future. Mechanical prostheses have been implanted for the benefit of durability at the expense of more major bleeding or thromboembolic events. However, the use of mechanical prostheses does not eliminate the risk of reoperation. It is associated with reoperation rates ranging between 7% and 10% by 15 years after implantation. 6 , 8 , 9 The Ross procedure, also known as the pulmonary autograft procedure, is a surgical technique in which the diseased aortic valve is removed and replaced with the patient's own pulmonary valve, and a human homograft valve is attached where the pulmonary valve was removed. Several long‐term observational studies of the Ross procedure, some of which contain series of contemporary modifications of surgical techniques, have shown favorable results. 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 Despite the large body of cumulative evidence demonstrating restored survival after the Ross procedure, the recognition of the Ross procedure in our community remains sparse. In the 2020 American College of Cardiology/American Heart Association valve guidelines, there is a class IIb recommendation for use of the Ross procedure in patients aged <50 years, 19 whereas there is no description of the Ross procedure in the 2021 European guidelines. 20 One of the reasons for the poor recognition of the Ross procedure despite the uniformly excellent long‐term outcomes includes an inadequate number of prospective randomized controlled trials (RCTs), scattered reports with inconsistent study designs with or without control groups, and perhaps limited availability of the Ross procedure.
In this context, we compared the Ross procedure with mechanical and bioprosthetic aortic valve replacement (M‐AVR and B‐AVR, respectively) by applying the latest methods of network meta‐analysis to provide insights for the choice of valve options in young and middle‐aged adults.
METHODS
Given the nature of the study, this study was deemed exempt from institutional review board review or written informed consent for publication by all participating institutions. The data that support the findings of this study are available from the corresponding author on reasonable request. Network meta‐analysis performed in this study follows the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis statement. 21
Protocol and Registration
The study protocol was registered on PROSPERO (identifier=319471).
Eligibility Criteria
Included studies met the following criteria: the research was peer reviewed with comparative study designs and included either RCTs or propensity score–matched (PSM) studies that reported clinical outcomes in patients aged ≥16 years who underwent SAVR or aortic root replacement using the Ross procedure, M‐AVR, or B‐AVR. Patients who received an aortic homograft (n=108) 10 were assigned to the B‐AVR group.
Information Source and Search
All RCTs and PSM studies that investigated clinical outcomes in patients aged ≥16 years who underwent the Ross procedure, M‐AVR, or B‐AVR were identified using a 2‐level strategy. First, a database, including MEDLINE and EMBASE, was searched through March 19, 2022, using Web‐based search engines (PubMed and OVID). Search terms included the following: “Ross procedure” or “autograft” and “mechanical valve” or “bioprosthetic valve” or “homograft” and “aortic valve replacement” or “aortic root replacement” and “randomized” or “randomize” or “propensity” or “propensity‐score”. Language restriction was not applied.
Study Selection and Data Collection Process
Relevant studies were identified through a manual search of secondary sources, including references of initially identified articles, reviews, and commentaries. All references were downloaded for consolidation, elimination of duplicates, and further analyses. Two independent and blinded authors (Y.Y. and S.F.) reviewed the search results separately to select the studies based on present inclusion and exclusion criteria. Disagreements were resolved by consensus.
Data Items
We sought the data according to the following PICOS: P (population), patients aged ≥16 years with aortic valve diseases; I (intervention), the Ross procedure; C (comparison), M‐AVR and B‐AVR; O (outcome), short‐ and long‐term outcomes; and S (study type), RCTs and PSM studies.
Risk of Bias in Individual Studies
Study quality was assessed by 2 independent and blinded authors (Y.Y. and S.F.) using the Cochrane Collaboration risk of bias 2.0 tool for an RCT 22 and the Newcastle‐Ottawa Scale for observational studies. 23 Disagreements were resolved by consensus.
Summary Measures
The outcomes of interest were short‐term outcomes, including 30‐day mortality and rates of stroke, myocardial infarction, permanent pacemaker implantation, new‐onset atrial fibrillation, and reoperation for bleeding; and long‐term outcomes, including all‐cause mortality and rates of reintervention, major bleeding, long‐term stroke, and infectious endocarditis. Reinterventions include both aortic and pulmonary valve reinterventions for the Ross procedure group. The definition of each outcome was applied according to each study protocol. We extracted the risk ratios (RRs) for short‐term outcomes and hazard ratios (HRs) for long‐term outcomes from each study. If HR was not described in a study, HR was calculated from a Kaplan‐Meier curve 24 using the spreadsheet programmed to estimate the overall HR with 95% CI with an inverse variance‐weighted average, which was provided by Tierney et al 25 based on standard statistical methods reported by Parmar et al 26 and Williamson et al. 27 If a Kaplan‐Meier curve was not provided in a study, RRs were calculated from the event number and the patient number.
Synthesis of Results and Risk of Bias Across Studies
We performed a network meta‐analysis using “netmeta” 3.6.2 package (R Foundation for Statistical Computing, Vienna, Austria). Within the framework, I2 and the Q statistics, which represent the proportion of total variation in study estimates attributable to heterogeneity, were used to quantify heterogeneity. The I2 statistic represents the proportion of variability that is not attributable to chance. We used the random‐effects model for the analysis. The procedures were ranked using P scores of 0% to 100%, where higher scores indicate more effective or safer procedures compared with those with lower scores. Funnel plot asymmetry, suggesting publication bias, was assessed using Egger linear regression test. 28
RESULTS
Study Selection
The database search identified 102 articles that were reviewed on the basis of the title and abstract. Of those, 71 articles were excluded on the basis of the titles and abstracts. In addition, 21 articles were excluded with the following reasons: n=10: no comparison or control groups; n=4: neither randomized nor PSM; n=3: pediatric patients aged <16 years; n=2: review article; n=1: no outcomes of interest reported; and n=1: trial protocol. Ten articles met the inclusion criteria and were assessed for the systematic review and the meta‐analysis 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 29 (Figure S1). Of those, 2 were RCTs 10 , 29 and 8 were PSM studies, 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 which enrolled a total of 4812 patients who received the Ross procedure (n=1991), M‐AVR (n=2019), or B‐AVR (n=802).
Study Characteristics
Study profiles and patient characteristics are summarized in the Table. Three articles were from Canada, 12 , 14 , 18 2 each were from Germany 11 , 29 and the United Kingdom, 10 , 13 and 1 each was from Australia, 15 Czech Republic, 16 and the United States. 18 Six articles were single‐center studies, 10 , 12 , 14 , 15 , 18 , 29 and 4 were registry‐based studies. 11 , 13 , 16 , 17 Although 30‐day mortality was reported in 7 articles, 10 , 12 , 14 , 15 , 16 , 18 , 29 no events were observed in 4 studies. Therefore, we could not analyze the 30‐day mortality. The number of articles that provided each outcome are follows: stroke, 6 10 , 12 , 14 , 16 , 17 , 18 ; myocardial infarction, 5 12 , 14 , 16 , 17 , 18 ; permanent pacemaker implantation, 4 10 , 14 , 15 , 16 ; new‐onset atrial fibrillation, 5 10 , 12 , 14 , 17 , 18 ; reoperation for bleeding, 5 10 , 14 , 16 , 18 , 29 ; all‐cause mortality, 9 10 , 11 , 12 , 13 , 15 , 16 , 17 , 18 , 29 ; reintervention, 7 10 , 11 , 12 , 16 , 17 , 18 , 29 ; major bleeding, 5 10 , 11 , 12 , 17 , 29 ; long‐term stroke, 5 10 , 11 , 12 , 17 , 18 ; and endocarditis, 5. 10 , 11 , 12 , 17 , 18 The definition of each outcome was shown in Table S1. Inclusion age criteria and exclusion criteria were summarized in Table S2. Variables used in PSM studies were summarized in Table S3.
Table 1.
Patient Characteristics and Study Profiles
| Author | Doss 29 | El‐Hamamsy 10 | Mokhles 11 | Mazine 12 | Sharabiani 13 | Bouhout 14 | Buratto 15 | Gofus 16 | El‐Hamamsy 17 | Mazine 18 |
|---|---|---|---|---|---|---|---|---|---|---|
| Year | 2005 | 2010 | 2011 | 2016 | 2016 | 2017 | 2018 | 2022 | 2022 | 2022 |
| Periods | 1999–2001 | 1994–2001 | 1994–2008 | 1990–2014 | 2000–2012 | 2007–2015 | 1992–2016 | 2009–2020 | 1997–2014 | 1990–2014 |
| Follow‐up | 1 y | 10.2±3.2 y | 5.1 vs 6.3 y | 14.2±6.5 y | 5.3 (2.1–8.6) y | 30 d | 10±7 y | 4.1 vs 6.1 y | 12.5 (9.3–15.7) y | 14.5±7.2 y |
| Design | RCT | RCT | PSM study | PSM study | PSM study | PSM study | PSM study | PSM study | PSM study | PSM study |
| Patients, n | 40 | 216 | 506 | 416 | 844 | 140 | 550 | 582 | 1302 | 216 |
| Ross, n | 20 | 108 | 253 | 208 | 224 | 70 | 275 | 291 | 434 | 108 |
| Mechanical, n | 20 | N/A | 253 | 208 | 468 | 70 | 275 | 291 | 434 | N/A |
| Bioprosthetic, n | N/A | 108 | N/A | N/A | 152 | N/A | N/A | N/A | 434 | 108 |
| Median age, y | 49 | N/A | 47.6±9.8 | 37±10 | N/A | 52±10 | 44±11 | 42 | 36±9 | 41 (34–47) |
| Men, % | 57 | 88 | 75 | 63 | N/A | 71 | 72 | 76 | 75 | 69 |
| Hypertension, % | 33 | 23 | 33 | 20 | N/A | 22 | 21 | 38 | 18 | 18 |
| Dyslipidemia, % | N/A | 3.2 | N/A | 11 | N/A | 7.8 | N/A | 20 | N/A | 13 |
| Diabetes, % | N/A | 1.4 | 4 | 1.9 | N/A | 19 | 1 | 1.7 | 0.2 | 2.5 |
| COPD, % | N/A | N/A | 2.8 | 1.2 | N/A | 6.4 | 5 | 6 | 4 | 0.9 |
| CAD, % | N/A | N/A | N/A | N/A | N/A | 2.1 | N/A | 7 | N/A | N/A |
| CKD, % | N/A | 6 | N/A | N/A | N/A | 4.3 | N/A | N/A | N/A | N/A |
| CVD, % | N/A | N/A | N/A | 4.1 | N/A | N/A | 5 | 2.6 | 0.3 | 1.9 |
| Afib, % | 2.5 | 2.8 | N/A | 2.2 | N/A | 2.1 | N/A | N/A | 4 | 0.9 |
| Pacemaker, % | N/A | 2.3 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| AS, % | 100 | 30 | 32 | 45 | N/A | 69 | 46 | 28 | N/A | 52 |
| AR, % | 0 | 44 | 18 | 37 | N/A | 14 | 34 | 34 | N/A | 26 |
| Mixed AS/AR, % | N/A | 26 | 47 | 18 | N/A | 17 | 20 | 38 | N/A | 22 |
| Aneurysm, % | N/A | 1.4 | N/A | N/A | N/A | 36 | N/A | N/A | N/A | N/A |
| Degenerative, % | N/A | 44 | 80 | 65 | N/A | N/A | N/A | 3.5 | N/A | N/A |
| Congenital, % | N/A | 50 | N/A | 9.3 | N/A | N/A | N/A | 89 | N/A | N/A |
| Rheumatic, % | N/A | 6 | 7 | 7.2 | N/A | N/A | N/A | 0 | N/A | N/A |
| Endocarditis, % | 0 | N/A | N/A | 2.2 | N/A | N/A | 0 | 6 | N/A | N/A |
| Previous intervention, % | N/A | 43 | 3.2 | 20 | N/A | 0 | 9 | 9 | N/A | N/A |
| Elective, % | 100 | 88 | N/A | N/A | N/A | 100 | 100 | N/A | N/A | N/A |
| Urgent/emergent, % | 0 | 12 | N/A | N/A | N/A | 0 | 0 | 1.9 | N/A | N/A |
| Concomitant CABG, % | 2.5 | N/A | 13 | 2.9 | N/A | N/A | N/A | N/A | N/A | N/A |
Follow‐up periods are expressed as numbers, means ± standard deviations, or medians (interquartile range).
Afib indicates atrial fibrillation; AR, aortic regurgitation; AS, aortic stenosis; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVD, cerebrovascular disease; N/A, not applicable; PSM, propensity score matched; and RCT, randomized control trial.
Risk of Bias Within Studies
The quality of observational studies was shown in Figure S2 and Table S4. All the PSM studies were considered as having low risk of bias.
Short‐Term Outcomes
In the network meta‐analysis of 2 studies comparing the Ross and B‐AVR, 3 studies for the Ross and M‐AVR, and 1 study for B‐AVR and M‐AVR, the rate of permanent pacemaker implantation was significantly lower in the Ross procedure compared with M‐AVR (RR [95% CI], 0.53 [0.31–0.91]; P=0.021; I2=66.6%) (Figure 1). There was no inconsistency (P=0.93). No significant difference was observed between the Ross and B‐AVR or between M‐AVR and B‐AVR. Other short‐term outcomes, including rates of stroke, myocardial infarction, new‐onset atrial fibrillation, and reoperation for bleeding, were similar among the groups (Figure S3). There was no inconsistency for each outcome (P=0.36, P=0.55, and P=0.18 for stroke, myocardial infarction, and new‐onset atrial fibrillation, respectively).
Figure 1. Forest plots of rates of permanent pacemaker implantation among treatment strategies (random‐effects model).
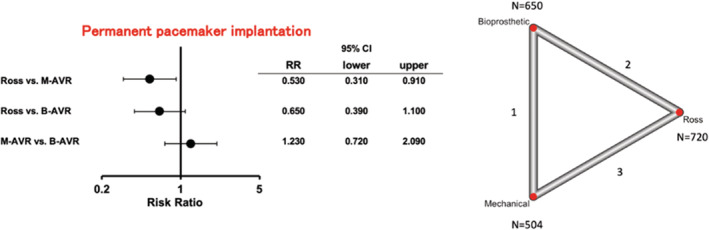
The horizontal lines represent the values within the 95% CI of the underlying effects. The vertical line indicates an incident risk ratio (RR) of 1. B‐AVR indicates bioprosthetic aortic valve replacement; and M‐AVR, mechanical aortic valve replacement.
Long‐Term Outcomes
Mean follow‐up periods were 7.4 years (range, 1–14.5 years).
All‐cause mortality (4 studies for the Ross versus B‐AVR and 6 studies for the Ross versus M‐AVR) was significantly lower in patients with the Ross procedure compared with M‐AVR (HR [95% CI], 0.58 [0.35–0.97]; P=0.035) and B‐AVR (HR [95% CI], 0.32 [0.18–0.59]; P<0.001; I2=44.7%) (Figure 2).
Figure 2. Forest plots of long‐term all‐cause mortality among treatment strategies (random‐effects model).
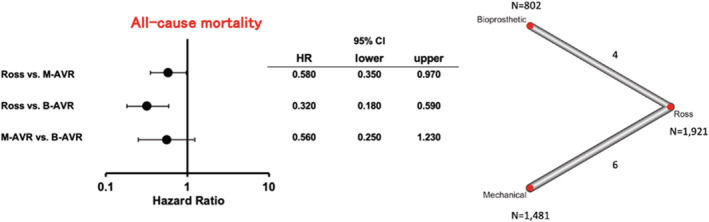
The horizontal lines represent the values within the 95% CI of the underlying effects. The vertical line indicates an incident hazard ratio (HR) of 1. B‐AVR indicates bioprosthetic aortic valve replacement; and M‐AVR, mechanical aortic valve replacement.
The reintervention rate (3 studies for the Ross versus B‐AVR and 5 studies for the Ross versus M‐AVR) was lower in the Ross procedure and M‐AVR groups compared with the B‐AVR group (HR [95% CI], 0.31 [0.15–0.65]; P=0.002; I2=66.6%; and HR [95% CI], 0.15 [0.05–0.41]; P<0.001; I2=66.6%, respectively), whereas it was higher in the Ross procedure group compared with the M‐AVR group (HR [95% CI], 2.12 [1.04–4.33]; P=0.039; I2=66.6%) (Figure 3A).
Figure 3. Forest plots of rates of long‐term reintervention (A) and long‐term major bleeding (B) among treatment strategies (random‐effects model).
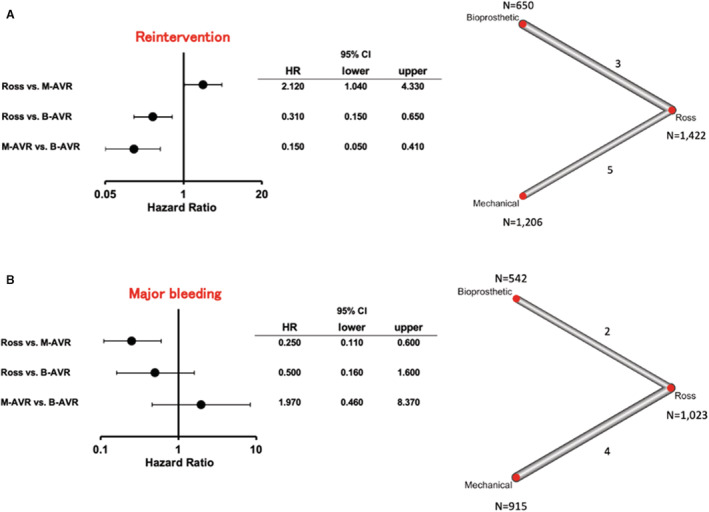
The horizontal lines represent the values within the 95% CI of the underlying effects. The vertical line indicates an incident hazard ratio (HR) of 1. B‐AVR indicates bioprosthetic aortic valve replacement; and M‐AVR, mechanical aortic valve replacement.
The rate of major bleeding event (2 studies for the Ross versus B‐AVR and 4 studies for the Ross versus M‐AVR) was lower in the Ross procedure compared with M‐AVR (HR [95% CI], 0.25 [0.11–0.60]; P=0.0014; I2=11.6%) (Figure 3B). No significant differences were observed in the other comparison pairs.
The rate of long‐term stroke (3 studies for the Ross versus B‐AVR and 3 studies for the Ross versus M‐AVR) was lower in the Ross procedure compared with M‐AVR (HR [95% CI], 0.34 [0.12–0.98]; P=0.044; I2=37.6%) and B‐AVR (HR [95% CI], 0.33 [0.12–0.87]; P=0.028; I2=37.6%) (Figure 4A).
Figure 4. Forest plots of rates of long‐term stroke (A) and infectious endocarditis (B) among treatment strategies (random‐effects model).
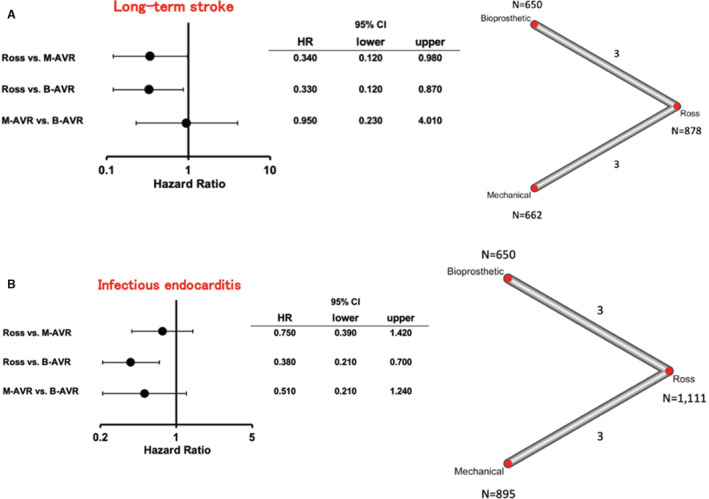
The horizontal lines represent the values within the 95% CI of the underlying effects. The vertical line indicates an incident hazard ratio (HR) of 1. B‐AVR indicates bioprosthetic aortic valve replacement; and M‐AVR, mechanical aortic valve replacement.
The rate of infectious endocarditis (3 studies for the Ross versus B‐AVR and 3 studies for the Ross versus M‐AVR) was lower in the Ross procedure compared with B‐AVR (HR [95% CI], 0.38 [0.21–0.70]; P=0.002; I2=0%) (Figure 4B). No significant difference was observed between the Ross and M‐AVR or M‐AVR and B‐AVR.
Direct and indirect comparisons for each outcome were presented in Figure S4.
Ranking of the Treatment Strategies
For short‐term outcomes, the Ross procedure was the most effective for reducing risks of stroke and permanent pacemaker implantation (P scores: 64.3% for stroke and 96.8% for permanent pacemaker implantation). M‐AVR was best for reducing myocardial infarction and atrial fibrillation events (P scores: 88.4% for myocardial infarction and 70.5% for atrial fibrillation), whereas B‐AVR was the best procedure for reducing reoperation for bleeding (P score: 92.9%) (Figure S5).
For long‐term outcomes, the Ross procedure was ranked as the best procedure for 4 of 5 outcomes (P scores: 99.0% for all‐cause mortality, 83.9% for major bleeding, 98.2% for long‐term stroke, and 90.7% for infectious endocarditis), except for reintervention rates, for which M‐AVR was ranked as the best (P score: 99.0%) (Figure S6).
Risk of Bias Across Studies
Publication bias was assessed using funnel plots (Figure S7).
DISCUSSION
To the best of our knowledge, this is the first network meta‐analysis, involving exclusively RCTs and PSM studies, to compare the outcomes of the Ross procedure with M‐AVR and B‐AVR in adults. We observed that the Ross procedure was associated with improved late clinical end points, including mortality, stroke, and endocarditis. Although reintervention was higher after the Ross procedure compared with M‐AVR, this did not impact long‐term patient survival.
Since the original description of the Ross procedure >5 decades ago, 30 its role in our clinical practice in adults remains controversial, despite the cumulative evidence of proven benefits. 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 29 The fundamental driving force for the clinical advantage of the Ross operation is not only the long‐term advantages of survival, avoidance of anticoagulant therapy, rare endocarditis, and infrequent reintervention, but also the fact that the autograft is alive: the leaflets retain the native valve physiology and contractile and neurohumoral responsiveness, with resultant superior hemodynamics and quality of life. 31
Selecting the optimal substitute for young and middle‐aged patients has been an ongoing debate. The advent and expansion of TAVR has heavily shifted the choice of substitute toward bioprostheses with expected future valve‐in‐valve TAVR. 7 However, despite the worldwide trend of favoring B‐AVRs, valve‐in‐valve TAVR options remain limited to high‐risk cohorts with limited long‐term data. It is of critical importance to note that patients in the B‐AVR group demonstrated worse long‐term clinical outcomes across the board, including all‐cause mortality, reintervention, long‐term stroke, and endocarditis, compared with patients undergoing the Ross procedure in the present study. Irrespective of receiving a mechanical or a biological prosthesis, a reduction in life expectancy compared with an age‐ and sex‐matched population was previously observed in large series, and this trend appeared more evident for younger populations. 6 , 32 In contrast, considerable data with the Ross procedure have accumulated worldwide in recent years. These long‐term studies demonstrated restored late survival in the Ross procedure recipients up to 25 years compared with a matched general population. 18 , 33
A few factors need to be considered when interpreting the reintervention rate data among the described SAVR options. Reinterventions in the Ross group are a result of 2 valves rather than 1, representing the Achilles heel of the Ross procedure. However, the mortality of post‐Ross reoperations in experienced hands, regardless of autograft or right ventricular outflow tract, is low, 34 , 35 and autograft valve‐sparing operations are frequently achievable. 34 Furthermore, the Ross operation has been modified to include specific technical elements: trimming of any excess muscle off the autograft; trimming of any excess autograft above the neosinotubular junction; placement of the autograft deep into the left ventricular outflow tract by meticulous attention to each suture (intra‐annular implantation for external annulus support); and providing external supports of the autograft annulus and the neosinotubular junction using various materials, particularly in patients with aortic insufficiency or large aortic annulus. 36 In addition to these technical aspects, the role of tight postoperative blood pressure regulation should not be understated. These modifications with various meticulous refinements have become standard of care in current practice and are associated with reduction of late autograft dilatation and improved durability. 34 Although reintervention rates were higher with the Ross procedure compared with M‐AVR, the upper limit of CI was approaching 1; therefore, further follow‐up is necessary considering recent surgical and medical modifications. As for right‐side reinterventions after the Ross procedure, percutaneous therapies have emerged as the first‐line therapy for failed homografts or prostheses in the pulmonary position, 37 although the feasibility of these transcatheter approaches is not guaranteed, and the risk of procedure‐related complications, such as coronary compression, 38 and late complications, such as transcatheter pulmonary valve endocarditis, 39 exists. M‐AVR appears favorable for the reintervention rate alone, but the reintervention risk is not trivial, with an estimated cumulative risk of 0.5% per year, mostly attributable to nonstructural valve dysfunction and endocarditis. 40 Furthermore, reinterventions following M‐AVR mandate a redo open heart surgery. As for reinterventions following B‐AVR, most patients in the present study were before or at the beginning of the TAVR era. Therefore, the long‐term outcomes following B‐AVR may be different in the contemporary series in the presence of valve‐in‐valve TAVR options in selected patients.
Meta‐analyses comparing the Ross procedure with prosthetic SAVR have been conducted in the past. Mazine et al compared the Ross procedure with M‐AVR using 1 RCT and 17 observational studies, 41 which showed similar results to our analysis (ie, lower mortality and higher rates of reintervention in Ross procedure). However, the included 7 studies were unmatched/unadjusted observational studies; therefore, there remains a concern for significant selection bias. McClure et al performed a meta‐analysis comparing the Ross procedure with conventional aortic valve replacement with 2 RCTs, 6 matched observational studies, and 7 unmatched/unadjusted observational studies, which showed decreased mortality in the Ross procedure. 42 However, the control group of the study was conventional aortic valve replacement by combining both M‐AVR and B‐AVR, and the follow‐up was only 2.6 years. Our meta‐analysis is the first network meta‐analysis that compared the Ross procedure with M‐AVR and B‐AVR separately, exclusively using RCTs or PSM studies. Furthermore, we reported the important outcome measures, such as rates of pacemaker implantation and infectious endocarditis, which were not reported in the previous meta‐analyses.
Study Limitations
Our study contains several limitations. First, one of the included studies compared the Ross procedure with homograft in the aortic position (n=108), which was included within the B‐AVR group, representing 13.5% of the B‐AVR group. However, homografts and conventional bioprostheses are known to offer similar survival and freedom from reoperation. 43 Second, the details of reintervention procedures were not available in most studies, and the breakdowns of percutaneous versus conventional open approach among reinterventions, which may provide additional insights, were unable to be described. Furthermore, we were unable to separately compare the reintervention rate on autograft alone because of lack of available data. Third, this study analyzed data sets from multiple studies. Although fundamental study conclusions should not be affected, small discrepancies in the definitions of relevant clinical variables exist. Fourth, mean follow‐up periods were 7.4 years in this analysis; therefore, longer follow‐up is necessary to conclude the optimal valve substitute in this population. Fifth, we exclusively included RCTs or PSM studies; however, this does not completely alleviate selection bias. Sixth, our analysis included a few comparisons between M‐AVR and B‐AVR; thus, the outcomes comparing those 2 methods were derived mainly from indirect comparisons. Last, the Ross procedure is generally performed only at selected centers with experience. Although most conventional SAVRs in this analysis were also performed in high‐volume SAVR centers, surgeons' experience could have influenced the outcomes. In addition, differences in surgical techniques could not be accounted for in the present analysis. The lack of RCTs remains one of the major criticisms of the Ross procedure that has not highly impacted the guideline recommendations. Major barriers to conducting RCTs include lack of equipoise on surgeon experience, clinical features obviously favoring one particular therapy to the others (such as younger age and extremely small annulus), and necessity of long‐term follow‐up. As a result, clinical trial feasibility and patient enrollment are subject to be challenged in such circumstances.
In summary, excellent long‐term clinical outcomes of the Ross procedure are demonstrated in the present network meta‐analysis compared with conventional M‐AVR and B‐AVR options. Future studies should include even longer follow‐up and breakdowns of reintervention approaches (open versus transcatheter) with corresponding outcomes. Our results highlight a need to enhance the recognition of the Ross procedure and revisit the optimal valve substitute selection in the guidelines for young and middle‐aged patients needing an aortic valve replacement.
Sources of Funding
None.
Disclosures
Dr Fukuhara serves as a consultant for Terumo Aortic, Artivion, and Medtronic Inc. Dr Ouzounian serves as a consultant for Terumo Aortic and Medtronic Inc. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S4
Figures S1–S3
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.027715
For Sources of Funding and Disclosures, see page 9.
Contributor Information
Toshiki Kuno, Email: tkuno@montefiore.org, Email: kuno-toshiki@hotmail.co.jp.
Shinichi Fukuhara, Email: fukuhara@med.umich.edu.
References
- 1. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, et al. Transcatheter or surgical aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2016;374:1609–1620. doi: 10.1056/NEJMoa1514616 [DOI] [PubMed] [Google Scholar]
- 2. Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Søndergaard L, Mumtaz M, Adams DH, Deeb GM, Maini B, Gada H, et al. Surgical or transcatheter aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2017;376:1321–1331. doi: 10.1056/NEJMoa1700456 [DOI] [PubMed] [Google Scholar]
- 3. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, et al. Transcatheter aortic‐valve replacement with a balloon‐expandable valve in low‐risk patients. N Engl J Med. 2019;380:1695–1705. doi: 10.1056/NEJMoa1814052 [DOI] [PubMed] [Google Scholar]
- 4. Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O'Hair D, Bajwa T, Heiser JC, Merhi W, Kleiman NS, et al. Transcatheter aortic‐valve replacement with a self‐expanding valve in low‐risk patients. N Engl J Med. 2019;380:1706–1715. doi: 10.1056/NEJMoa1816885 [DOI] [PubMed] [Google Scholar]
- 5. Forrest JK, Ramlawi B, Deeb GM, Zahr F, Song HK, Kleiman NS, Chetcuti SJ, Michelena HI, Mangi AA, Skiles JA, et al. Transcatheter aortic valve replacement in low‐risk patients with bicuspid aortic valve stenosis. JAMA Cardiol. 2021;6:50–57. doi: 10.1001/jamacardio.2020.4738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goldstone AB, Chiu P, Baiocchi M, Lingala B, Patrick WL, Fischbein MP, Woo YJ. Mechanical or biologic prostheses for aortic‐valve and mitral‐valve replacement. N Engl J Med. 2017;377:1847–1857. doi: 10.1056/NEJMoa1613792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tam DY, Rocha RV, Wijeysundera HC, Austin PC, Dvir D, Fremes SE. Surgical valve selection in the era of transcatheter aortic valve replacement in the Society of Thoracic Surgeons Database. J Thorac Cardiovasc Surg. 2020;159:416–427.e8. doi: 10.1016/j.jtcvs.2019.05.081 [DOI] [PubMed] [Google Scholar]
- 8. Chiang YP, Chikwe J, Moskowitz AJ, Itagaki S, Adams DH, Egorova NN. Survival and long‐term outcomes following bioprosthetic vs mechanical aortic valve replacement in patients aged 50 to 69 years. JAMA. 2014;312:1323–1329. doi: 10.1001/jama.2014.12679 [DOI] [PubMed] [Google Scholar]
- 9. Sotade OT, Falster MO, Pearson SA, Jorm LR, Sedrakyan A. Comparison of long‐term outcomes of bioprosthetic and mechanical aortic valve replacement in patients younger than 65 years. J Thorac Cardiovasc Surg. 2022;S0022‐5223(22)00086–1. doi: 10.1016/j.jtcvs.2022.01.016 [DOI] [PubMed] [Google Scholar]
- 10. El‐Hamamsy I, Eryigit Z, Stevens LM, Sarang Z, George R, Clark L, Melina G, Takkenberg JJ, Yacoub MH. Long‐term outcomes after autograft versus homograft aortic root replacement in adults with aortic valve disease: a randomised controlled trial. Lancet. 2010;376:524–531. doi: 10.1016/S0140-6736(10)60828-8 [DOI] [PubMed] [Google Scholar]
- 11. Mokhles MM, Körtke H, Stierle U, Wagner O, Charitos EI, Bogers AJ, Gummert J, Sievers HH, Takkenberg JJ. Survival comparison of the Ross procedure and mechanical valve replacement with optimal self‐management anticoagulation therapy: propensity‐matched cohort study. Circulation. 2011;123:31–38. doi: 10.1161/CIRCULATIONAHA.110.947341 [DOI] [PubMed] [Google Scholar]
- 12. Mazine A, David TE, Rao V, Hickey EJ, Christie S, Manlhiot C, Ouzounian M. Long‐term outcomes of the Ross procedure versus mechanical aortic valve replacement: propensity‐matched cohort study. Circulation. 2016;134:576–585. doi: 10.1161/CIRCULATIONAHA.116.022800 [DOI] [PubMed] [Google Scholar]
- 13. Sharabiani MT, Dorobantu DM, Mahani AS, Turner M, Peter Tometzki AJ, Angelini GD, Parry AJ, Caputo M, Stoica SC. Aortic valve replacement and the Ross operation in children and young adults. J Am Coll Cardiol. 2016;67:2858–2870. doi: 10.1016/j.jacc.2016.04.021 [DOI] [PubMed] [Google Scholar]
- 14. Bouhout I, Noly PE, Ghoneim A, Stevens LM, Cartier R, Poirier N, Bouchard D, Demers P, El‐Hamamsy I. Is the Ross procedure a riskier operation? Perioperative outcome comparison with mechanical aortic valve replacement in a propensity‐matched cohort. Interact Cardiovasc Thorac Surg. 2017;24:41–47. doi: 10.1093/icvts/ivw325 [DOI] [PubMed] [Google Scholar]
- 15. Buratto E, Shi WY, Wynne R, Poh CL, Larobina M, O'Keefe M, Goldblatt J, Tatoulis J, Skillington PD. Improved survival after the Ross procedure compared with mechanical aortic valve replacement. J Am Coll Cardiol. 2018;71:1337–1344. doi: 10.1016/j.jacc.2018.01.048 [DOI] [PubMed] [Google Scholar]
- 16. Gofus J, Fila P, Drabkova S, Zacek P, Ondrasek J, Nemec P, Sterba J, Tuna M, Jarkovsky J, Vojacek J. Ross procedure provides survival benefit over mechanical valve in adults: a propensity‐matched nationwide analysis. Eur J Cardiothorac Surg. 2022;61:1357–1365. doi: 10.1093/ejcts/ezac013 [DOI] [PubMed] [Google Scholar]
- 17. El‐Hamamsy I, Toyoda N, Itagaki S, Stelzer P, Varghese R, Williams EE, Erogova N, Adams DH. Propensity‐matched comparison of the Ross procedure and prosthetic aortic valve replacement in adults. J Am Coll Cardiol. 2022;79:805–815. doi: 10.1016/j.jacc.2021.11.057 [DOI] [PubMed] [Google Scholar]
- 18. Mazine A, David TE, Stoklosa K, Chung J, Lafreniere‐Roula M, Ouzounian M. Improved outcomes following the Ross procedure compared with bioprosthetic aortic valve replacement. J Am Coll Cardiol. 2022;79:993–1005. doi: 10.1016/j.jacc.2021.12.026 [DOI] [PubMed] [Google Scholar]
- 19. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP III, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2021;143:e72–e227. doi: 10.1161/CIR.0000000000000923 [DOI] [PubMed] [Google Scholar]
- 20. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561–632. doi: 10.1093/eurheartj/ehab395 [DOI] [PubMed] [Google Scholar]
- 21. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle‐Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. The Ottawa Hospital Research Institute. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed January 28, 2022.
- 24. Yokoyama Y, Kuno T, Takagi H. Meta‐analysis of phase‐specific survival after elective endovascular versus surgical repair of abdominal aortic aneurysm from randomized controlled trials and propensity score‐matched studies. J Vasc Surg. 2020;72:1464–1472.e1466. doi: 10.1016/j.jvs.2020.03.041 [DOI] [PubMed] [Google Scholar]
- 25. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(SICI)1097‐0258(19981230)17:24<2815::AID‐SIM110>3.0.CO;2‐8 [DOI] [PubMed] [Google Scholar]
- 27. Williamson PR, Smith CT, Hutton JL, Marson AG. Aggregate data meta‐analysis with time‐to‐event outcomes. Stat Med. 2002;21:3337–3351. doi: 10.1002/sim.1303 [DOI] [PubMed] [Google Scholar]
- 28. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Doss M, Wood JP, Martens S, Wimmer‐Greinecker G, Moritz A. Do pulmonary autografts provide better outcomes than mechanical valves? A prospective randomized trial. Ann Thorac Surg. 2005;80:2194–2198. doi: 10.1016/j.athoracsur.2005.06.006 [DOI] [PubMed] [Google Scholar]
- 30. Ross DN. Replacement of aortic and mitral valves with a pulmonary autograft. Lancet. 1967;2:956–958. doi: 10.1016/S0140-6736(67)90794-5 [DOI] [PubMed] [Google Scholar]
- 31. Ibrahim M, Fukuhara S. More data "In support" of the Ross. Ann Thorac Surg. 2022;114:509–510. doi: 10.1016/j.athoracsur.2021.09.058 [DOI] [PubMed] [Google Scholar]
- 32. Glaser N, Persson M, Jackson V, Holzmann MJ, Franco‐Cereceda A, Sartipy U. Loss in life expectancy after surgical aortic valve replacement: SWEDEHEART study. J Am Coll Cardiol. 2019;74:26–33. doi: 10.1016/j.jacc.2019.04.053 [DOI] [PubMed] [Google Scholar]
- 33. Aboud A, Charitos EI, Fujita B, Stierle U, Reil JC, Voth V, Liebrich M, Andreas M, Holubec T, Bening C, et al. Long‐term outcomes of patients undergoing the Ross procedure. J Am Coll Cardiol. 2021;77:1412–1422. doi: 10.1016/j.jacc.2021.01.034 [DOI] [PubMed] [Google Scholar]
- 34. Abeln KB, Schäfers S, Ehrlich T, Federspiel JM, Schäfers HJ. Ross operation with autologous external autograft stabilization‐long‐term results. Ann Thorac Surg. 2022;114:502–509. doi: 10.1016/j.athoracsur.2021.09.017 [DOI] [PubMed] [Google Scholar]
- 35. Stelzer P, Mejia J, Williams EE. Outcomes of reoperations after Ross procedure. Ann Cardiothorac Surg. 2021;10:491–498. doi: 10.21037/acs-2021-rp-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mazine A, El‐Hamamsy I. The Ross procedure is an excellent operation in non‐repairable aortic regurgitation: insights and techniques. Ann Cardiothorac Surg. 2021;10:463–475. doi: 10.21037/acs-2021-rp-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nordmeyer J, Lurz P, Tsang VT, Coats L, Walker F, Taylor AM, Khambadkone S, de Leval MR, Bonhoeffer P. Effective transcatheter valve implantation after pulmonary homograft failure: a new perspective on the Ross operation. J Thorac Cardiovasc Surg. 2009;138:84–88. doi: 10.1016/j.jtcvs.2008.08.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fraisse A, Assaidi A, Mauri L, Malekzadeh‐Milani S, Thambo JB, Bonnet D, Iserin L, Mancini J, Boudjemline Y. Coronary artery compression during intention to treat right ventricle outflow with percutaneous pulmonary valve implantation: incidence, diagnosis, and outcome. Catheter Cardiovasc Interv. 2014;83:E260–E268. doi: 10.1002/ccd.25471 [DOI] [PubMed] [Google Scholar]
- 39. McElhinney DB, Zhang Y, Aboulhosn JA, Morray BH, Biernacka EK, Qureshi AM, Torres AJ, Shahanavaz S, Goldstein BH, Cabalka AK. Multicenter study of endocarditis after transcatheter pulmonary valve replacement. J Am Coll Cardiol. 2021;78:575–589. doi: 10.1016/j.jacc.2021.05.044 [DOI] [PubMed] [Google Scholar]
- 40. Korteland NM, Etnel JRG, Arabkhani B, Mokhles MM, Mohamad A, Roos‐Hesselink JW, Bogers AJJC, Takkenberg JJM. Mechanical aortic valve replacement in non‐elderly adults: meta‐analysis and microsimulation. Eur Heart J. 2017;38:3370–3377. doi: 10.1093/eurheartj/ehx199 [DOI] [PubMed] [Google Scholar]
- 41. Mazine A, Rocha RV, El‐Hamamsy I, Ouzounian M, Yanagawa B, Bhatt DL, Verma S, Friedrich JO. Ross procedure vs mechanical aortic valve replacement in adults: a systematic review and meta‐analysis. JAMA Cardiol. 2018;3:978–987. doi: 10.1001/jamacardio.2018.2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McClure GR, Belley‐Cote EP, Um K, Gupta S, Bouhout I, Lortie H, Alraddadi H, Alsagheir A, McIntyre WF, Dorobantu DM, et al. The Ross procedure versus prosthetic and homograft aortic valve replacement: a systematic review and meta‐analysis. Eur J Cardiothorac Surg. 2019;55:247–255. doi: 10.1093/ejcts/ezy247 [DOI] [PubMed] [Google Scholar]
- 43. Yanagawa B, Mazine A, Tam DY, Jüni P, Bhatt DL, Spindel S, Puskas JD, Verma S, Friedrich JO. Homograft versus conventional prosthesis for surgical management of aortic valve infective endocarditis: a systematic review and meta‐analysis. Innovations (Phila). 2018;13:163–170. doi: 10.1097/imi.0000000000000510 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4
Figures S1–S3


