Abstract
Background
The effectiveness of vascular closure devices (VCDs) to reduce bleeding after transfemoral percutaneous coronary intervention remains unsettled.
Methods and Results
Participants in the REGULATE‐PCI (Effect of the REG1 anticoagulation system versus bivalirudin on outcomes after percutaneous coronary intervention) trial who underwent transfemoral percutaneous coronary intervention with VCD implantation were compared with those who underwent manual compression. The primary effectiveness end point was type 2, 3, or 5 Bleeding Academic Research Consortium access site bleeding at day 3. Univariate and multivariate analyses were adjusted by the inverse probability weighting method using propensity score. Time to hemostasis and time to ambulation were compared between groups. Of the 1580 patients who underwent transfemoral percutaneous coronary intervention, 1004 (63.5%) underwent VCD implantation and 576 (36.5%) had manual compression. The primary effectiveness end point occurred in 64 (6.4%) participants in the VCD group and in 38 (6.6%) participants in the manual compression group (inverse probability weighting–adjusted odds ratio, 1.02 [95% CI, 0.77–1.36]; P=0.89). There were statistically significant 2‐way interactions between VCD use and female sex, chronic kidney disease, and use of high‐potency P2Y12 inhibition (ticagrelor or prasugrel) (P<0.05 for all) with less bleeding with VCD use in these high‐risk subgroups. Median time to hemostasis and time to ambulation were shorter in the VCD versus the manual compression group (P<0.01 for both).
Conclusions
Following transfemoral percutaneous coronary intervention, VCD use is associated with a shorter time to hemostasis and time to ambulation but not less bleeding. Further study of patients with high‐bleeding risk is required, including women, patients with chronic kidney disease, and those using high‐potency P2Y12 inhibitors.
Registration
URL: https://clinicaltrials.gov/ct2/show/NCT01848106; Unique identifier: NCT01848106.
Keywords: arterial access, bleeding, outcomes, percutaneous coronary intervention, radial
Subject Categories: Catheter-Based Coronary and Valvular Interventions, Percutaneous Coronary Intervention
Nonstandard Abbreviations and Acronyms
- BARC
Bleeding Academic Research Consortium
- VCD
vascular closure device
Clinical Perspective.
What Is New?
Contemporary evidence on the effectiveness and safety of femoral vascular closure devices (VCDs) in patients undergoing percutaneous coronary intervention is scarce, especially in subgroups at higher risk of vascular complications.
In this secondary analysis of the REGULATE‐PCI (Effect of the REG1 anticoagulation system versus bivalirudin on outcomes after percutaneous coronary intervention) trial including 1580 patients, access site bleeding at day 3 after percutaneous coronary intervention was similar in patients with and without VCD use after multivariable adjustment, but time to hemostasis and to ambulation was shorter.
VCDs were, however, associated with a significant reduction in access site bleeding at day 3 in the following high‐risk subgroups: female patients, patients with chronic kidney disease, and patients using ticagrelor or prasugrel.
What Are the Clinical Implications?
This study does not support the use of VCD to reduce bleeding in all‐comer patients undergoing percutaneous coronary intervention from a transfemoral approach.
The study supports the recommendation that VCDs can be used to reduce time to hemostasis and time to ambulation.
The observation that female patients, patients with chronic kidney disease, and patients using ticagrelor or prasugrel may benefit from VCDs is hypothesis generating and calls for future randomized trials targeting these high‐risk populations.
The radial artery access is increasingly the default site of vascular access used for coronary angiography and percutaneous coronary intervention (PCI) globally, but femoral artery remains the most commonly used approach in the United States. 1 , 2 Although hemostasis following femoral sheath removal is traditionally obtained using manual compression of the common femoral arteriotomy site, vascular closure devices (VCDs) have been developed as an alternative strategy. These devices are currently recommended to achieve faster hemostasis and earlier ambulation (class IIa, level of evidence B) but not for decreasing vascular complications or bleeding following coronary angiography and/or PCI (class III, level of evidence B). 3 , 4
Large contemporary randomized trials showed that VCDs decrease the risk of access site complications following coronary angiography, mostly driven by lower rates of hematoma ≥5 mm, but not of major bleeding or of other non–bleeding‐related complications. 5 , 6 However, these randomized trials excluded patients at higher risk of periprocedural bleeding because of the use of more potent anticoagulation and antiplatelet therapy. Current clinical practice is thus limited by the little contemporary evidence on the effectiveness and safety of VCDs in patients undergoing PCI. Evidence is also limited by the absence of data on populations at higher risk for vascular complications, such as women, patients with peripheral artery disease, and patients with chronic kidney disease (CKD), in whom the effect size of the use of VCD is expected to be higher than in all‐comers undergoing coronary angiography. Indeed, patients at higher risk of complications have been identified as an area of focus by scientific societies for future studies assessing the safety and efficacy of VCDs. 3 , 4
The objectives of this study are to compare the risk of bleeding, time to hemostasis, time to ambulation, and periprocedural complications with VCDs versus manual compression following transfemoral PCI, overall and specifically in high‐risk subgroups.
METHODS
The data, methods used in the analysis, and materials used to conduct the research will be available to any researcher for purposes of reproducing the results or replicating the procedure on reasonable request. REGULATE‐PCI was a multicenter, international, open‐label, randomized controlled trial conducted from September 2013 to June 2014 in 225 sites in Europe and North America, designed to evaluate REG1 (a combination of the factor IXa inhibitor pegnivacogin and its complementary controlling agent anivamersen) or bivalirudin for prevention of ischemic complications during PCI. The trial was stopped early after enrollment of 3232 patients because of severe allergic reactions reported in 10 patients randomized to REG1. The methods and results of the trial have been published previously. 7 In brief, patients aged ≥18 years undergoing a PCI were randomized 1:1 to REG1 or bivalirudin. Key exclusion criteria were acute ST‐segment–elevation myocardial infarction within 48 hours, clinical instability, contraindication to anticoagulation, and recent use of bivalirudin, fibrinolysis, or glycoprotein IIb/IIIa inhibitors. In the REG1 arm, pegnivacogin (1 mg/kg) was administered intravenously before PCI was performed, and the reversal agent anivamersen (0.5 mg/kg) was given at completion of PCI to achieve near complete reversal of factor IXa inhibition. 8 A second bolus of anivamersen could be administered at the discretion of the operator as needed if bleeding occurred. In the bivalirudin group, patients received an intravenous bolus of 0.75 mg/kg, followed by an infusion at a rate of 1.75 mg/kg per hour, which was stopped on completion of PCI. Arterial access site, choice of P2Y12 inhibitor, and use of VCD were left to the operator's discretion but were prestipulated before randomization. For the purpose of this post hoc analysis, patients enrolled in the REGULATE‐PCI trial who underwent PCI through a femoral access were included. Participants were divided into 2 groups according to whether they underwent closure with VCD (VCD group) or not (manual compression group). All patients provided informed consent to participate in the REGULATE‐PCI trial, and the appropriate national and institutional regulatory and ethical boards approved the protocol.
Outcomes
The primary effectiveness end point of this analysis is type 2, 3, or 5 Bleeding Academic Research Consortium (BARC) access site bleeding at day 3. 9 Type 2 BARC bleeding is defined as any overt, actionable sign of hemorrhage (eg, more bleeding than would be expected for a clinical circumstance, including bleeding found by imaging alone) that does not fit the criteria for type 3, 4, or 5 but does meet at least 1 of the following criteria: (1) requiring nonsurgical, medical intervention by a health care professional, (2) leading to hospitalization or increased level of care, or (3) prompting evaluation. Type 3a BARC bleeding is defined as overt bleeding plus hemoglobin decrease of 3 to 5 g/dL (provided hemoglobin decrease is related to bleed), or any transfusion with overt bleeding. Type 3b BARC bleeding is defined as overt bleeding plus hemoglobin decrease of 5 g/dL (provided hemoglobin decrease is related to bleed), cardiac tamponade, bleeding requiring surgical intervention for control (excluding dental/nasal/skin/hemorrhoid), or bleeding requiring intravenous vasoactive agents. Type 3c BARC bleeding is defined as intracranial hemorrhage (does not include microbleeds or hemorrhagic transformation, does include intraspinal), subcategories confirmed by autopsy, imaging, or lumbar puncture, or intraocular bleed compromising vision. Type 5 BARC bleeding is fatal bleeding (type 5a: probable fatal bleeding, no autopsy or imaging confirmation but clinically suspicious; type 5b: definite fatal bleeding, overt bleeding, or autopsy or imaging confirmation). Prespecified secondary effectiveness end points include type 2, 3, or 5 non–coronary artery bypass graft–related access or nonaccess site bleeding, BARC type 2 access, and/or nonaccess site bleeding, BARC type 3 or 5 access and/or nonaccess site bleeding at day 3; the same bleeding end points at 30 days; time to hemostasis (defined as time of hemostasis minus time of end of procedure); and time to ambulation (defined as time of ambulation to time of end of procedure). Type 3 and 5 BARC bleeding events were reviewed by an adjudication committee. All other end points were investigator reported. Prespecified safety end points include investigator‐reported pseudoaneurysm, access site pain, and iliac and femoral perforation.
Statistical Analysis
The statistical analysis plan was prespecified before the analyses were conducted but after the data were collected. Continuous data are expressed as median with interquartile range (IQR), and nominal/ordinal data are expressed as count with percentages. Comparisons of categorical baseline characteristics of the VCD and manual compression groups were made using a Fisher exact test or a χ2 test. For continuous variables, the means were compared using a t‐test when the data in each group were approximately normally distributed with the homogeneous variances. When these criteria were not met, the nonparametric Wilcoxon rank‐sum test was performed.
Closure devices were used by physician choice and were not randomized. To address potential confounding attributable to imbalance in patient baseline characteristics between VCD and manual compression groups, all analyses comparing these 2 groups were adjusted by the inverse probability weighting (IPW) method using propensity score. 10 The propensity of getting VCD was estimated through a multivariable logistic model. The following covariates were recommended by the clinicians based on clinical relevance and study design characteristics: age; sex; region; body mass index; prior myocardial infarction; prior PCI; prior peripheral vascular disease; renal insufficiency (creatinine clearance <60 mL/min); clopidogrel, heparin, prasugrel, or ticagrelor use within 48 hours before randomization; size of the index PCI sheath; and indication for index PCI. Covariates were included in the model in their original form, and there were no interaction terms. Standardized weights were generated on the basis of the propensity scores. Standard mean differences between VCD and manual compression groups were calculated for each covariate. Compared with the original observations, the standardized mean differences were significantly reduced in the weighted observations; the largest of these differences had an 85% reduction and was 0.041 in absolute value (prior peripheral vascular disease), which was far less than the recommended upper limit of 0.25. 11
Associations between VCD use and bleeding outcomes are assessed through univariate and multivariable analyses. Unadjusted and adjusted odds ratio (ORs) with 95% CIs and P values were reported. Covariates included in the multivariable model were randomized treatment, female sex, acute coronary syndrome as indication for index PCI (unstable angina or myocardial infarction during the previous 7 days), peripheral vascular disease, CKD, chronic liver disease, use of glycoprotein IIb/IIIa inhibitors, sheath size, use of heparin within 48 hours of randomization, and use of ticagrelor or prasugrel (versus clopidogrel). Two‐way interactions between VCD use and the following high‐risk subgroups for the primary end point were evaluated in a prespecified manner: sex, peripheral vascular disease, CKD, sheath size (6F versus bigger), and use of ticagrelor or prasugrel (versus clopidogrel) within 48 hours before randomization.
Time to hemostasis and time to ambulation were compared between VCD use and randomized treatment. The log‐rank tests were performed. P<0.05 was considered significant through the analyses. Results were not adjusted for multiple comparisons.
RESULTS
Of the 3232 REGULATE‐PCI trial participants, 1580 underwent PCI with femoral access, of whom 1004 (63.5%) underwent VCD implantation, and 576 (36.5%) underwent manual compression. In both groups, 51% of participants were randomized to REG1, and 49% were randomized to bivalirudin. Patients in the VCD group were more likely to have a history of PCI (60.2% versus 54.7%; P=0.034) and of peripheral artery disease (21.7% versus 11.3%; P<0.01) (Table 1). A non–acute coronary syndrome presentation was more frequent in the VCD group (P<0.01). Larger sheath sizes were used in the VCD group, with 13.9% using a sheath >6F compared with 11.6% in the manual compression group (P<0.01). The proportion of participants from North America was higher among patients who used a VCD (87.9%) versus no VCD (78.1%) (P<0.01).
Table 1.
Baseline and Procedural Characteristics
| Characteristic | Vascular closure device (N=1004) | Manual compression (N=576) | P value |
|---|---|---|---|
| Baseline characteristics | |||
| Age, y | 67 (58–74) | 67 (58–74) | 0.67 |
| Male sex | 706 (70.3) | 432 (75.0) | 0.05 |
| Race | 0.31 | ||
| White | 959 (95.9) | 542 (94.4) | |
| Black | 27 (2.7) | 23 (4.0) | |
| American Indian or Alaska Native | 3 (0.3) | 1 (0.2) | |
| Asian | 6 (0.6) | 6 (1.0) | |
| Native Hawaiian or other Pacific Islander | 5 (0.5) | 1 (0.2) | |
| Multiracial | 0 (0.0) | 1 (0.2) | |
| Region | <0.01 | ||
| North America | 883 (87.9) | 450 (78.1) | |
| East European Union | 44 (4.4) | 80 (13.9) | |
| West European Union | 77 (7.7) | 46 (8.0) | |
| Weight, kg/m2 | 86.0 (75.2–98.3) | 87.0 (75.0–100.0) | 0.52 |
| Body mass index, kg/m2 | 29.2 (25.8–32.9) | 28.7 (25.6–32.9) | 0.55 |
| Diabetes | 371 (37.0) | 220 (38.2) | 0.62 |
| Renal insufficiency (CrCl <60 mL/min) | 143 (14.2) | 77 (13.4) | 0.63 |
| Prior MI | 365 (36.4) | 237 (41.1) | 0.06 |
| PCI | 604 (60.2) | 315 (54.7) | 0.03 |
| Prior CABG | 253 (25.2) | 155 (26.9) | 0.46 |
| Peripheral artery disease | 218 (21.7) | 65 (11.3) | <0.01 |
| Prior stroke | 43 (4.3) | 24 (4.2) | 0.91 |
| Left ventricular ejection fraction | 0.35 | ||
| Normal (>55%) | 571 (60.4) | 302 (56.9) | |
| Mild dysfunction (40%–55%) | 272 (28.8) | 176 (33.1) | |
| Moderate dysfunction (25%–39%) | 85 (9.0) | 45 (8.5) | |
| Severe dysfunction (<25%) | 18 (1.9) | 8 (1.5) | |
| Smoking status | 0.36 | ||
| Current smoker | 196 (19.5) | 118 (20.5) | |
| Former smoker | 434 (43.2) | 228 (39.6) | |
| Never smoked | 374 (37.3) | 230 (39.9) | |
| Medications 48 h before randomization | |||
| Glycoprotein IIb/IIIa inhibitors | 0 (0.0) | 1 (0.2) | 0.37 |
| Clopidogrel | 580 (57.8) | 297 (51.6) | 0.02 |
| Ticagrelor | 77 (7.7) | 49 (8.5) | 0.55 |
| Prasugrel | 99 (9.9) | 55 (9.5) | 0.84 |
| Index PCI sheath size | <0.01 | ||
| 5F | 10 (1.0) | 31 (5.4) | |
| 6F | 854 (85.1) | 478 (83.0) | |
| 7F | 122 (12.2) | 60 (10.4) | |
| 8F | 17 (1.7) | 7 (1.2) | |
| Indication for index PCI | <0.01 | ||
| MI within 7 d | 115 (11.5) | 75 (13.0) | |
| MI >7 d | 31 (3.1) | 25 (4.3) | |
| Unstable angina | 244 (24.3) | 210 (36.5) | |
| Stable angina | 547 (54.5) | 215 (37.3) | |
| Asymptomatic ischemia | 67 (6.7) | 51 (8.9) | |
Continuous variables are presented as median (interquartile range). Other data are given as number (percentage). CABG indicates coronary artery bypass graft; CrCl, creatinine clearance; MI, myocardial infarction; and PCI, percutaneous coronary intervention.
BARC type 2, 3, or 5 access site bleeding through day 3 occurred in 64 (6.4%) participants in the VCD group and in 38 (6.6%) participants in the manual compression group (IPW‐adjusted OR, 1.02 [95% CI, 0.77–1.36]; P=0.89) (Table 2). There was no difference between both groups for all secondary bleeding end points (P>0.05 for all). There was no BARC type 3 or 5 access site bleeding at day 3 and at day 30. On multivariate analysis, there was no difference between VCD and manual compression for the primary end point (adjusted OR, 1.04 [95% CI, 0.78–1.39]; P=0.79) (Table 3).
Table 2.
IPW Analyses of Bleeding End Points at Day 3 and Day 30
| Variable | Vascular closure device (N=1004)* | Manual compression (N=576)* | OR (95% CI)† | P value |
|---|---|---|---|---|
| Day 3 | ||||
| BARC 2, 3, or 5 access site bleeding (primary end point) | 64 (6.4) | 38 (6.6) | 1.02 (0.77–1.36) | 0.89 |
| BARC 3 or 5 access site bleeding | 0 (0) | 0 (0) | NA | NA |
| BARC 2 access site bleeding | 64 (6.4) | 38 (6.6) | 1.02 (0.77–1.36) | 0.89 |
| BARC 2, 3, or 5 bleeding | 74 (7.4) | 42 (7.3) | 1.07 (0.81–1.40) | 0.64 |
| BARC 3 or 5 bleeding | 3 (0.3) | 2 (0.3) | 0.82 (0.23–2.90) | 0.76 |
| BARC 2 bleeding | 71 (7.1) | 40 (6.9) | 1.08 (0.82–1.42) | 0.59 |
| Day 30 | ||||
| BARC 2, 3, or 5 access site bleeding | 66 (6.6) | 41 (7.1) | 0.98 (0.74–1.20) | 0.87 |
| BARC 3 or 5 access site bleeding | 0 (0) | 0 (0) | NA | NA |
| BARC 2 access site bleeding | 66 (6.6) | 41 (7.1) | 0.98 (0.74–1.20) | 0.87 |
| BARC 2, 3, or 5 bleeding | 82 (8.2) | 50 (8.7) | 1.00 (0.77–1.29) | 0.98 |
| BARC 3 or 5 bleeding | 4 (0.4) | 6 (1.0) | 0.41 (0.16–1.08) | 0.06 |
| BARC 2 bleeding | 78 (7.8) | 44 (7.6) | 1.07 (0.82–1.40) | 0.61 |
BARC indicates Bleeding Academic Research Consortium; IPW, inverse probability weighting; NA, not applicable; and OR, odds ratio.
Data are given as number (percentage).
Weighted by the inverse probability of getting vascular closure device or manual compression.
Table 3.
Multivariable Analysis of BARC Type 2, 3, or 5 Access Site Bleeding at Day 3
| Variable | OR (95% CI)* | P value |
|---|---|---|
| VCD vs manual compression | 1.04 (0.78–1.39) | 0.79 |
| Randomized treatment REG1 (vs bivalirudin) | 0.98 (0.82–1.17) | 0.82 |
| Age (per 1‐y increase) | 1.04 (1.02–1.05) | <0.01 |
| Male sex (vs female sex) | 0.67 (0.49–0.91) | 0.01 |
| ACS within 7 d before randomization | 1.04 (0.76–1.43) | 0.79 |
| Peripheral vascular disease | 0.41 (0.25–0.69) | <0.01 |
| Renal insufficiency (creatinine clearance <60 mL/min) | 1.09 (0.71–1.66) | 0.70 |
| Chronic liver disease | 2.44 (1.02–5.85) | 0.05 |
| Medication 48 h before randomization | ||
| Use of heparin (vs bivalirudin) | 2.04 (1.42–2.92) | <0.01 |
| Use of ticagrelor or prasugrel (vs clopidogrel) | 1.64 (1.17–2.31) | <0.01 |
| Index PCI sheath size | ||
| 7F vs 5F or 6F | 1.77 (1.19–2.62) | <0.01 |
ACS indicates acute coronary syndrome; BARC, Bleeding Academic Research Consortium; OR, odds ratio; PCI, percutaneous coronary intervention; REG1, a combination of the factor IXa inhibitor pegnivacogin and its complementary controlling agent anivamersen; and VCD, vascular closure device.
Weighted by the inverse probability of getting VCD or manual compression.
In a prespecified analysis of patients at high risk of bleeding, female patients, patients with CKD, and patients who received ticagrelor or prasugrel, but not other subgroups, exhibited lower rates of bleeding with VCD use compared with manual compression (P for interaction <0.05 for all) (Figure).
Figure . Prespecified analysis of subgroups at high risk of bleeding for BARC 2, 3, or 5 access site bleeding at day 3.
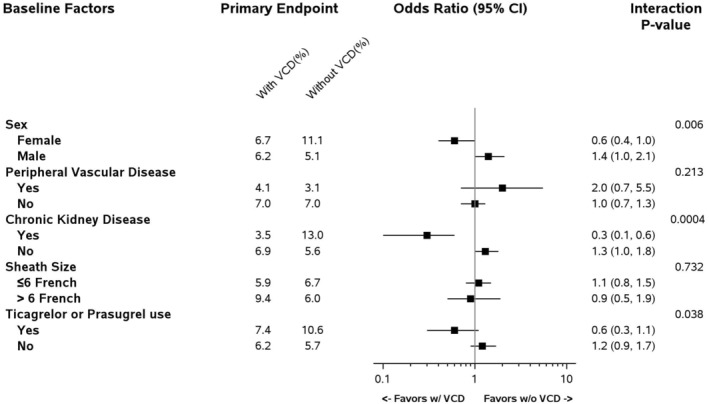
No BARC 3 or 5 access site bleeding at day 3 was reported in the trial. BARC indicates Bleeding Academic Research Consortium; and VCD, vascular closure device.
Median (25th–75th percentile) time to hemostasis was 2 (IQR, 0–7) minutes in the VCD group, and 20 (IQR, 10.0–31.0) minutes in the manual compression group (P<0.01). Median time to ambulation was 6 hours in the VCD group (IQR, 4.2–8.5 hours), and 7 hours (IQR, 5.1–14.0 hours) in the manual compression group (P<0.01). In both the randomized groups (REG1 and bivalirudin), time to hemostasis and time to ambulation were shorter with VCD versus manual compression (P<0.01) (Table 4).
Table 4.
Time to Hemostasis and Time to Ambulation According to Closure Device Group and Randomized Treatment Group
| REG1 | Bivalirudin | ||||
|---|---|---|---|---|---|
| Variable | VCD (N=510) | Manual compression (N=489) | VCD (N=293) | Manual compression (N=280) | P value* |
| Time to hemostasis, min | 1 (0.0–7.0) | 19 (10.0–30.0) | 2 (0.0–9.0) | 20 (13.0–32.0) | <0.01 |
| Time to ambulation, h | 5 (4.0–8.0) | 7 (5.0–13.0) | 6 (4.0–9.0) | 7 (5.0–15.0) | <0.01 |
Data are presented as median (interquartile range). REG1 indicates a combination of the factor IXa inhibitor pegnivacogin and its complementary controlling agent anivamersen; and VCD, vascular closure device.
Jonckheere‐Terpstra test weighted by the inverse probability of getting VCD or manual compression.
The risk of pseudoaneurysm was lower with VCD than manual compression (3 [0.3%] versus 7 [1.2%], respectively; IPW‐adjusted OR, 0.35 [95% CI, 0.17–0.70]). Patients in the VCD group experienced access site pain more frequently (36 [3.6%] versus 11 [1.9%], respectively; IPW‐adjusted OR, 1.63 [95% CI, 1.20–2.22]). One case of iliac/femoral perforation was reported in a patient who used a VCD (<0.1%).
DISCUSSION
This analysis of the REGULATE‐PCI trial represents one of the largest comparisons of VCD versus manual compression in patients undergoing PCI through femoral access, with bleeding end points adjudicated by a blinded committee using standardized BARC definitions. The main finding is that there was no statistical evidence that VCD was associated with fewer bleeding complications in this population, whereas VCDs were associated with shorter time to hemostasis and time to ambulation. These observations support the current recommendations that VCDs be used to decrease the latter, but not with the intent of reducing bleeding risk. 3 , 4 Although the present analysis studied the role of VCDs in a PCI population at higher risk of access site bleeding compared with the available previous randomized trials, in which only patients scheduled for a diagnostic angiogram were included, there was no major (BARC 3–5) access site bleeding event in our cohort. Consequently, the risk of bleeding events may have been too low in the REGULATE‐PCI population to capture a significant treatment effect of VCD compared with manual compression.
Current evidence guiding the clinical use of VCD is composed of studies predominantly enrolling low‐ or medium‐risk patients. However, exploratory subgroup analyses in our study suggest that VCD may be associated with improved bleeding outcomes in some higher‐risk subgroups. Female patients, patients with CKD, and patients who received ticagrelor or prasugrel appeared more likely to benefit from a VCD to reduce the risk of access site bleeding. Although it remains premature to establish a causal relationship between VCD use and bleeding end point reduction in these subgroups, this novel finding is hypothesis generating and calls for future randomized trials targeting these patients given the prognostic impact of post‐PCI bleeding. Indeed, previous randomized trials may have not captured the benefits of VCDs because the magnitude of the treatment effect was too low in populations who were not at risk of bleeding.
The 2 largest clinical trials designed to evaluate the efficacy of VCDs, the ISAR‐CLOSURE (instrumental sealing of arterial puncture site closure device versus manual compression trial) and CLOSE‐UP (comparison of the femoseal arterial closure device to manual compression after coronary angiography) trials, which evaluated the Exoseal and the FemoSeal devices, respectively, showed that VCDs were noninferior to manual compression in terms of vascular access site complications and reduced the rate of femoral hematomas in patients undergoing coronary angiography. 5 , 6 In addition, an instrumental variable analysis of the CathPCI Registry, including 2 056 585 PCIs, suggested that VCDs were associated with a small magnitude but statistically significant reduction in access site complications (absolute 0.36% reduction) and in bleeding (absolute 0.73% reduction). 1 However, end points were not adjudicated by an independent committee in the latter observational study. In a secondary analysis of the HORIZONS‐AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trial, including 642 patients with primary PCI who underwent hemostasis with the use of a VCD compared with 642 matched control patients, VCD use was associated with a significant reduction in the rate of non–coronary artery bypass graft–related major bleeding (5.0% versus 8.1%; hazard ratio, 0.61 [95% CI, 0.39–0.94]; P=0.02). 12 A holistic interpretation of the previous literature is, however, limited by the fact that end point definitions were not standardized across studies and that populations were heterogeneous. The use of clinically relevant, standardized, and validated outcomes, such as BARC bleeding types, which were used in our study, should be favored in future VCD trials.
Our finding that VCD is associated with reduced time to hemostasis and time to ambulation in the PCI population, independently of randomized treatment assignation, is consistent with previous randomized trials conducted in patients undergoing diagnostic procedures alone. A subanalysis of the ISAR‐CLOSURE trial showed a similar reduction in time to hemostasis with VCD use. 13 A meta‐analysis of 14 401 patients randomized to VCD or to manual compression after diagnostic or interventional procedures also demonstrated that VCD was associated with shorter time to hemostasis, ambulation, and discharge, 14 which led to a 13% reduction in overall costs. 14 Future guidelines focusing on post‐PCI length of stay should consider suggesting the use of VCDs in routine clinical care to facilitate same‐day discharge. 15
In the REGULATE‐PCI trial, nonbleeding access site complications, such as pseudoaneurysms, were rare in both study groups. These findings are in line with the ISAR‐CLOSURE and the CLOSE‐UP trials, revealing low and similar rates of access site complications between VCDs and manual complication. 5 , 6 The increased rate of access site pain in the VCD group observed in our study is clinically relevant for our patients, but the intensity and duration of the pain were not recorded. In the CLOSE‐UP trial, closure of femoral access with VCD was associated with more pain and discomfort immediately during the closure procedure, but no difference in pain and discomfort was found between groups at follow‐up. 16 Pain and discomfort are investigator reported in the current literature, and their degree and duration are rarely available. Future trials should aim to examin systematically examine the impact of VCDs on access site pain using standardized and validated tools to better evaluate the impact of pain and discomfort after femoral PCI in a patient‐oriented perspective.
Limitations
Assignment to VCD or to manual compression was not randomized, and associations might be confounded by unmeasured variables that biased the choice of hemostasis strategy (including the operator's experience and familiarity with the devices as well as their local availability), despite the use of IPW and of multivariable adjustment. No adjudicated major access site bleeding was reported in our study, suggesting that despite the antithrombotic treatment administered during PCI (anticoagulants and antiplatelet agents), the study population may not have reflected a truly high‐risk population, and the potential of VCDs to reduce bleeding risk may have been underestimated. Finally, different types of VCDs were not analyzed separately, because the device type was not reported by the investigators, and whether our results apply to patients treated with other anticoagulants, such as heparin, during the PCI is unknown.
CONCLUSIONS
Compared with manual compression, femoral VCDs are not associated with a reduction of access or nonaccess site bleeding following PCI but are safe and are associated with a significant reduction in time to hemostasis and time to ambulation. Alternative risk mitigation techniques may better prevent bleeding in patients undergoing PCI. Exploratory analysis suggested potential benefits of VCD in prespecified subgroups at particularly high risk for bleeding, including female patients, patients with CKD, and patients who were pretreated with ticagrelor or prasugrel. Randomized trials in populations enriched with these high‐bleeding risk subgroups might further explore the impact of VCDs on patient‐oriented end points, standardized clinical end points, and health care costs.
Sources of Funding
None.
Disclosures
RM: Dr. Mehran reports institutional research payments from Abbott, Abiomed, Alleviant Medical, Amgen, AM‐Pharma, Arena, AstraZeneca, AtriCure, Bayer, Biosensors, Biotronik, Boston Scientific, Bristol‐Myers Squibb, CardiaWave, CeloNova, Chiesi, Concept Medical, CSL Behring, Cytosorbents, Daiichi Sankyo, Element Science, Faraday, Filterlex Medical, Humacyte, Idorsia, Janssen, Magenta, Mediasphere, Medtelligence, Medtronic, Novartis, OrbusNeich, Penumbra, PhaseBio, Philips, Pi‐Cardia, PLx Pharma, Protembis, RenalPro, RM Global, Shockwave, Vivasure, Zoll; personal fees from Cine‐Med Research, Ionis Pharmaceuticals, Novartis, Vectura, WebMD; Equity 〈1% in Applied Therapeutics, Elixir Medical, Stel, ControlRad (spouse); Scientific Advisory Board for AMA, ACC (BOT Member), SCAI (Women in Innovations Committee Member), JAMA Cardiology Associate Editor; Faculty CRF (no fee). PGS: Research grants: Amarin, Bayer, Sanofi, and Servier Clinical Trials (SC, DMC, CEC): Amarin, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Idorsia, Novartis, Pfizer, Regado, Sanofi, Servier Consulting or speaking: Amarin, Amgen, BMS/Myokardia, Novo‐Nordisk, Regeneron Senior Associate Editor at Circulation. AML: Reseach grants from AbbVie, Astra Zeneca, CSL Behring, ELi Lilly, Esperion, and Novartis. Consulting agreements with Ardelyx, Becton‐Dickson, Eli Lilly, Endologix, Fibrogen, Glaxo SmithKline, Nono Nordisk, and Provention Bio. JHA: Research Grants from Artivion/CryoLife, Bayer, Bristol‐Myers Squibb, CSL Behring, Ferring, U.S. FDA, Humacyte, U.S. NIH, and XaTek and Advisory Board/Consulting payments from AbbVie, Akros, Artivion/CryoLife, AtriCure, Bayer, Bristol‐Myers Squibb, Ferring, GlaxoSmithKline, Janssen, Pfizer, Portola, and Quantum Genomics. TJP: Research grants: CSL Behring, Xylocor Therapeutics. Consultant: NovoNordisk, AstraZeneca, Biocardia, Boehringer Ingelheim. Associate Editor: Circulation: CV Interventions. All others nothing to disclose.
Presented in part at ACC.22: American College of Cardiology's 71st Annual Scientific Session and Expo in Washington, DC, April 2 to 4, 2022, and published in abstract form (J Am Coll Cardiol. 2022;79:831 or https://doi.org/10.1016/S0735‐1097[22]01822‐8).
See Editorial by Thakker et al.
For Sources of Funding and Disclosures, see page 8.
References
- 1. Wimmer NJ, Secemsky EA, Mauri L, Roe MT, Saha‐Chaudhuri P, Dai D, McCabe JM, Resnic FS, Gurm HS, Yeh RW. Effectiveness of arterial closure devices for preventing complications with percutaneous coronary intervention: an instrumental variable analysis. Circ Cardiovasc Interv. 2016;9:1–9. doi: 10.1161/CIRCINTERVENTIONS.115.003464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Masoudi FA, Ponirakis A, de Lemos JA, Jollis JG, Kremers M, Messenger JC, Moore JWM, Moussa I, Oetgen WJ, Varosy PD, et al. Trends in U.S. cardiovascular care: 2016 report from 4 ACC national cardiovascular data registries. J Am Coll Cardiol. 2017;69:1427–1450. doi: 10.1016/j.jacc.2016.12.005 [DOI] [PubMed] [Google Scholar]
- 3. Patel MR, Jneid H, Derdeyn CP, Klein LW, Levine GN, Lookstein RA, White CJ, Yeghiazarians Y, Rosenfield K. Arteriotomy closure devices for cardiovascular procedures. Circulation. 2010;122:1882–1893. doi: 10.1161/CIR.0b013e3181f9b345 [DOI] [PubMed] [Google Scholar]
- 4. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SE, Guyton RA, Hollenberg SM, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574–e651. doi: 10.1161/CIR.0b013e31823ba622 [DOI] [PubMed] [Google Scholar]
- 5. Holm NR, Sindberg B, Schou M, Maeng M, Kaltoft A, Bottcher M, Krusell LR, Hjort J, Thuesen L, Terkelsen CJ, et al. Randomised comparison of manual compression and FemoSeal™ vascular closure device for closure after femoral artery access coronary angiography: the CLOSure dEvices Used in everyday Practice (CLOSE‐UP) study. EuroIntervention. 2014;10:183–190. doi: 10.4244/EIJV10I2A31 [DOI] [PubMed] [Google Scholar]
- 6. Schulz‐Schüpke S, Helde S, Gewalt S, Ibrahim T, Linhardt M, Haas K, Hoppe K, Bottiger C, Groha P, Bradaric C, et al. Comparison of vascular closure devices vs manual compression after femoral artery puncture: the ISAR‐CLOSURE randomized clinical trial. JAMA. 2014;312:1981–1987. doi: 10.1001/jama.2014.15305 [DOI] [PubMed] [Google Scholar]
- 7. Lincoff AM, Mehran R, Povsic TJ, Zelenkofske SL, Huang Z, Armstrong PW, Steg PG, Bode C, Cohen MG, Buller C, et al. Effect of the REG1 anticoagulation system versus bivalirudin on outcomes after percutaneous coronary intervention (REGULATE‐ PCI): a randomised clinical trial. Lancet. 2016;387:349–356. doi: 10.1016/S0140-6736(15)00515-2 [DOI] [PubMed] [Google Scholar]
- 8. Chan MY, Cohen MG, Dyke CK, Myles SK, Aberle LG, Lin M, Walder J, Steinhubl SR, Gilchrist IC, Kleiman NS, et al. Phase 1b randomized study of antidote‐controlled modulation of factor IXa activity in patients with stable coronary artery disease. Circulation. 2008;117:2865–2874. doi: 10.1161/CIRCULATIONAHA.107.745687 [DOI] [PubMed] [Google Scholar]
- 9. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nokolsky E, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the bleeding academic research consortium. Circulation. 2011;123:2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449 [DOI] [PubMed] [Google Scholar]
- 10. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–3679. doi: 10.1002/sim.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rubin DB. Using propensity scores to help design observational studies: application to the tobacco litigation. Health Serv Outcomes Res Methodol. 2001;2:169–188. doi: 10.1023/A:1020363010465 [DOI] [Google Scholar]
- 12. Sanborn TA, Tomey MI, Mehran R, Genereux P, Witzenbichler B, Brener SJ, Kirtane AJ, McAndrew TC, Kornowski R, Dudek D, et al. Femoral vascular closure device use, bivalirudin anticoagulation, and bleeding after primary angioplasty for STEMI: results from the HORIZONS‐AMI trial. Catheter Cardiovasc Interv. 2015;85:371–379. doi: 10.1002/ccd.25663 [DOI] [PubMed] [Google Scholar]
- 13. Mankerious N, Mayer K, Gewalt SM, Helde SM, Ibrahim T, Bott‐Flugel L, Laugwitz KL, Schunkert H, Kastrati A, Schupke S. Comparison of the FemoSeal vascular closure device with manual compression after femoral artery puncture ‐ post‐hoc analysis of a large‐scale, randomized clinical trial. J Invasive Cardiol. 2018;30:235–239. [PubMed] [Google Scholar]
- 14. Cox T, Blair L, Huntington C, Lincourt A, Sing R, Heniford BT. Systematic review of randomized controlled trials comparing manual compression to vascular closure devices for diagnostic and therapeutic arterial procedures. Surg Technol Int. 2015;27:32–44. [PubMed] [Google Scholar]
- 15. Seto AH, Shroff A, Abu‐Fadel M, Blankenship JC, Boudoulas KD, Cigarroa JE, Dehmer GJ, Feldman DN, Kolansky DM, Lata K, et al. Length of stay following percutaneous coronary intervention: an expert consensus document update from the society for cardiovascular angiography and interventions. Catheter Cardiovasc Interv. 2018;92:717–731. doi: 10.1002/ccd.27637 [DOI] [PubMed] [Google Scholar]
- 16. Sindberg B, Schou M, Hansen L, Christiansen KJ, Jorgensen KS, Soltoft M, Holm NR, Maeng M, Kristensen SD, Lassen JF. Pain and discomfort in closure of femoral access coronary angiography. The CLOSuredEvices Used in everyday Practice (CLOSE‐UP) pain sub study. Eur J Cardiovasc Nurs. 2014;13:221–226. doi: 10.1177/1474515113482809 [DOI] [PubMed] [Google Scholar]


