Abstract
Background
Guidelines recommend that coronary slow flow phenomenon (CSFP), defined as corrected thrombolysis in myocardial infarction frame count (CTFC) 27, can diagnose coronary microvascular dysfunction (CMD) in patients with angina and nonobstructed coronary arteries. CSFP has also historically been regarded as a sign of coronary endothelial dysfunction (CED). We sought to validate the utility of CTFC, as a binary classifier of CSFP and as a continuous variable, to diagnose CMD and CED.
Methods and Results
Patients with angina and nonobstructed coronary arteries had simultaneous coronary pressure and flow velocity measured using a dual sensor‐tipped guidewire during rest, adenosine‐mediated hyperemia, and intracoronary acetylcholine infusion. CMD was defined as the inability to augment coronary blood flow in response to adenosine (coronary flow reserve <2.5) and CED in response to acetylcholine (acetylcholine flow reserve ≤1.5); 152 patients underwent assessment using adenosine, of whom 82 underwent further acetylcholine testing. Forty‐six patients (30%) had CSFP, associated with lower flow velocity and higher microvascular resistance as compared with controls (16.56.9 versus 20.26.9 cm/s; P=0.001 and 6.261.83 versus 5.361.83 mm Hg/cm/s; P=0.009, respectively). However, as a diagnostic test, CSFP had poor sensitivity and specificity for both CMD (26.7% and 65.2%) and CED (21.1% and 56.0%). Furthermore, on receiver operating characteristics analyses, CTFC could not predict CMD or CED (area under the curve, 0.41 [95% CI, 0.32%–0.50%] and 0.36 [95% CI, 0.23%–0.49%], respectively).
Conclusions
In patients with angina and nonobstructed coronary arteries, CSFP and CTFC are not diagnostic of CMD or CED. Guidelines supporting the use of CTFC in the diagnosis of CMD should be revisited.
Keywords: angina, endothelial dysfunction, microvascular dysfunction, TIMI frame count
Subject Categories: Catheter-Based Coronary and Valvular Interventions, Diagnostic Testing, Exercise Testing
Clinical Perspective
What Is New?
Coronary slow flow on angiography has poor sensitivity and specificity to diagnose microvascular dysfunction in patients with angina and unobstructed coronary arteries.
Corrected thrombolysis in myocardial infarction frame count cannot predict any of the indices of endothelium‐independent and endothelium‐dependent coronary microvascular function.
What Are the Clinical Implications?
Guidelines supporting the use of thrombolysis in myocardial infarction frame count to diagnose coronary microvascular dysfunction should be revisited.
Upcoming angiographic technologies to assess coronary microvascular function warrant similar validation studies before clinical use.
Nonstandard Abbreviations and Acronyms
- ANOCA
angina with nonobstructed coronary arteries
- AChFR
acetylcholine flow reserve
- APV
average peak velocity
- CED
coronary endothelial dysfunction
- CMD
coronary microvascular dysfunction
- CFR
coronary flow reserve
- CSFP
coronary slow flow phenomenon
- CTFC
corrected TIMI frame count
- hMR
hyperemic microvascular resistance
Delayed progression of contrast medium in the absence of a significant epicardial stenosis is a common angiographic finding, observed in roughly 7% of angiograms. 1 It was first proposed as a primary mechanism of angina by Tambe et al in 1972, who suggested that this finding most likely represents elevated microvascular resistance. 2 Subsequent studies coined the term coronary slow flow phenomenon (CSFP) and defined it using the corrected thrombolysis in myocardial infarction frame count (CTFC >27). 3 Though initially proposed to assess antegrade flow and microvascular obstruction in the acute revascularization setting, CTFC more broadly is an angiographic surrogate for coronary blood flow, 4 and its use has now been extrapolated to the evaluation of angina with nonobstructed coronary arteries (ANOCA).
Recommendations by COVADIS (Coronary Vasomotion Disorders International Study) recognize CSFP as evidence of impaired microvascular function, commensurate with a diagnosis of coronary microvascular dysfunction (CMD). 5 , 6 , 7 Furthermore, CSFP has historically been attributed to coronary endothelial dysfunction (CED), 8 , 9 which is known to carry a risk of major adverse cardiac events. 10 , 11 Guidewire‐based assessment of coronary reactivity to pharmacological vasodilators (ie, adenosine and acetylcholine) remains the gold standard for diagnosing both CMD and CED in patients with ANOCA. 12 , 13 Despite CSFP being the most widely accessible method of assessing microvascular dysfunction within the COVADIS criteria, its diagnostic utility has not been formally evaluated against invasive standards. This study therefore aims to (1) test the null hypothesis that patients with CSFP have similar clinical characteristics and invasive physiology to those without CSFP and (2) evaluate the diagnostic utility of CSFP and CTFC to identify patients with CMD and CED defined by invasive methodology.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
We enrolled consecutive patients who underwent elective diagnostic angiography followed by intracoronary physiology assessment using a dual pressure and Doppler sensor‐tipped wire, ComboWire (Combowire, Philips, CA), for clinical assessment of typical angina between 2017 and 2022. Inclusion criteria were preserved left ventricular ejection fraction (>50%) and unobstructed coronary arteries (ie, <50% diameter stenosis or fractional flow reserve >0.80). Exclusion criteria were intolerance to adenosine or acetylcholine, chronic kidney disease (estimated glomerular filtration rate <30 mL/min/m2), significant valvular disease, recent acute coronary syndrome, or cardiomyopathy. Institutional review board approval was obtained by the UK National Research Ethics Service (17/LO/0203 and 20/LO/1294), and all patients provided written informed consent.
Intracoronary Physiology Assessment
All patients received 1 mg intravenous midazolam, 200 μg intracoronary glyceryl trinitrate, and 70 U/kg unfractionated heparin before angiography and physiology assessment. Our catheterization laboratory protocol for interrogating ANOCA has been described in full previously. 12 , 14 A 0.014‐inch intracoronary guidewire was sited in the distal left anterior descending artery for continuous monitoring of distal coronary pressure and average peak flow velocity (APV). Aortic pressure was measured via the guide catheter. We first assessed endothelium‐independent microvascular function using intravenous adenosine (140 μg/kg/min), followed by endothelium‐dependent microvascular function using graded intracoronary infusions of acetylcholine (18.2 μg/mL at 1 mL/min for 2 minutes followed by 2 mL/min for 2 minutes).
Off‐Line Physiology Data Analysis
Signals were sampled at 200 Hz, with data exported into a custom‐made study manager program (Academic Medical Centre, University of Amsterdam, Netherlands) and analyzed on custom‐made software: Cardiac Waves (Kings College London, UK). Coronary flow reserve (CFR) was calculated as hyperemic APV/basal APV (bAPV), and CMD was defined as CFR <2.5. 5 , 12 Microvascular resistance was calculated as distal coronary pressure/APV at base (bMR) and hyperemia (hMR). For measurement of acetylcholine flow reserve (AChFR), quantitative coronary angiography was performed to measure vessel diameter 5‐mm distal to the tip of the guidewire. Coronary blood flow (CBF) was given by CBF=cross‐sectional area × APV × 0.5. AChFR was calculated as (CBFACh/CBFrest), with impaired AChFR defined as AChFR ≤1.5. 12 , 15 Patients with normal CFR (2.5) and AChFR (1.5) were classed as reference groups for comparisons.
Calculation of CTFC
Diagnostic angiograms were retrospectively analyzed by an independent observer masked to patient characteristics and physiology data to calculate CTFC, as described previously. 4 Briefly, number of frames were counted (at 15 frames/sec) for contrast to transit between standardized proximal and distal landmarks in the left anterior descending artery. This value was multiplied by 2 to obtain thrombolysis in myocardial infarction frame count (equivalent to 30 frames/sec). CTFC is thereafter given by TFC/1.7 to correct for the length of the left anterior descending artery. 4 , 16 Patients were classified as having CSFP where CTFC >27. To calculate intra‐ and interobserver variability of CTFC calculation, 50 randomly selected angiograms were reviewed 4 weeks later by the same observer and a second observer, both masked to previous results.
Statistical Analysis
Continuous data are presented as mean±SD and compared using independent samples Student t‐test. Categorical variables are presented as n (%) and compared using ‐test. Diagnostic statistics were calculated by cross‐tabulating presence/absence of CSFP (CTFC >27) with CMD (CFR <2.5) and CED (AChFR ≤1.5) and presented as percentages (with Clopper‐Pearson 95% CIs). Intra‐ and interobserver variability of CTFC calculation were calculated as mean absolute differences, presented as mean±SD. The correlation between CTFC and Doppler‐derived indices was analyzed by Pearson coefficient (r). Receiver operating characteristic curve analysis was performed to assess the discriminator function of CTFC with respect to the following invasive classifications of microvascular dysfunction: CFR <2.0, CFR <2.5, hMR >2.5, and AChFR ≤1.5. 5 , 12 , 17 P values were calculated as 2‐tailed, with <0.05 considered statistically significant. All graphs, calculations, and statistical analyses were performed using SPSS 27.0 (IBM, NY) or GraphPad prism software version 9.0 for Mac (GraphPad Software, San Diego, CA).
RESULTS
Study Population
A total of 152 patients underwent intracoronary physiology assessment with intravenous adenosine, of whom 82 patients underwent further intracoronary acetylcholine testing. Baseline demographic, physiological, and angiographic characteristics are shown in Table 1. These characteristics are dichotomized and compared by impaired versus normal CFR and AChFR in Table S1. Our patient cohort was predominantly women (73%), with similar prevalence of cardiovascular risk factors between patients with normal and impaired CFR and AChFR. bAPV was higher in impaired CMD and impaired AChFR groups, as compared with their respective reference groups. Mean CTFC and proportion of CSFP were similar between CMD and reference groups (21.1±8.3 versus 23.6±8.6; P=0.08 and 27% versus 35%; P=0.28). Mean CTFC and prevalence of CSFP were lower in the CED group as compared with the reference group (20.5±8.1 versus 24.9±8.3; P=0.03 and 21% versus 44%; P=0.03).
Table 1.
Baseline Characteristics
| Characteristic | Total cohort (n=152) | Acetylcholine subgroup (n=82) |
|---|---|---|
| Patient demographics | ||
| Age, y | 58±10 | 57±10 |
| Women | 111 (73) | 58 (71) |
| Hypertension | 78 (52) | 41 (50) |
| Diabetes | 33 (25) | 20 (24) |
| Hypercholesterolemia | 81 (53) | 41 (50) |
| Smoking history | 42 (29) | 22 (27) |
| Doppler‐derived indices | ||
| Pd/Pa | 0.95±0.03 | 0.95±0.03 |
| FFR | 0.91±0.05 | 0.92±0.05 |
| CFR | 2.46±0.76 | 2.51±0.75 |
| CMD | 86 (57) | 43 (52) |
| AChFR | N/A | 1.31±0.64 |
| CED | N/A | 57 (70) |
| Angiographic indices | ||
| CTFC | 22.2±8.5 | 21.9±8.4 |
| CSFP | 46 (30) | 23 (28) |
Values are mean±SD or n (%).
AChFR indicates acetylcholine flow reserve; CED, coronary endothelial function; CFR, coronary flow reserve; CMD, coronary microvascular dysfunction; CSFP, coronary slow flow phenomenon; CTFC, corrected thrombolysis in myocardial infarction frame count; FFR, fractional flow reserve; and Pd/Pa, distal coronary pressure/aortic pressure.
Characterization of CSFP
Fifty‐six of 152 patients (30%) had CSFP. There were no demographic differences between CSFP or control groups. The CSFP group had a lower mean bAPV (16.5±4.9 versus 20.2±6.9 cm/s; P=0.001) and higher mean bMR (6.62±1.83 versus 5.36±1.83 mm Hg/cm/s; P=0.009) (Table 2). However, there were no differences in mean CFR or proportion of CMD between CSFP and control groups (2.59±0.80 versus 2.40±0.74; P=0.16 and 56% versus 62%; P=0.53, respectively). Mean AChFR was similar between groups (1.47±0.66 versus 1.24±0.82; P=0.17), with a lower prevalence of CED in the CSFP group as compared with the reference group (52% versus 75%; P=0.03). Findings were identical in an alternative analysis using CTFC >25 as the dichotomous cutoff point (see Tables S2 and S3).
Table 2.
Characterization of Patients With CSFP (CTFC >27)
| CTFC | CSFP (n=46) | Controls (n=106) | P value |
|---|---|---|---|
| 32.9±5.6 | 17.6±4.3 | <0.001* | |
| Demographic characterization | |||
| Age, y | 60±10 | 58±10 | 0.12 |
| Women | 31 (67) | 80 (76) | 0.30 |
| Hypertension | 27 (59) | 51 (48) | 0.23 |
| Diabetes | 13 (28) | 26 (25) | 0.63 |
| Hypercholesterolemia | 25 (54) | 56 (53) | 0.86 |
| Smoking history | 11 (24) | 31 (29) | 0.50 |
| Invasive physiology assessment | |||
| Pd/Pa | 0.95±0.03 | 0.95±0.03 | 0.73 |
| FFR | 0.91±0.05 | 0.91±0.05 | 0.46 |
| bAPV, cm/s | 16.5±4.9 | 20.2±6.9 | 0.001* |
| bMR, mm Hg/cm/s | 6.26±1.83 | 5.36±1.83 | 0.009* |
| hMR, mm Hg/cm/s | 2.21±0.61 | 2.08±0.76 | 0.34 |
| CFR | 2.59±0.80 | 2.40±0.74 | 0.16 |
| AChFR† | 1.47±0.66 | 1.24±0.82 | 0.17 |
| CMD, % | 23/46 (50) | 63/106 (59) | 0.28 |
| CED†, % | 12/23 (52) | 45/59 (76) | 0.03* |
Values are mean±SD or n (%).
AChFR indicates acetylcholine flow reserve; bAPV, basal average peak velocity; bMR, basal microvascular resistance; CED, coronary endothelial function; CFR, coronary flow reserve; CMD, coronary microvascular dysfunction; CSFP, coronary slow flow phenomenon; CTFC, corrected thrombolysis in myocardial infarction frame count; FFR, fractional flow reserve; and hMR, hyperemic microvascular resistance.
Significant difference from controls (P<0.05).
n=82.
Diagnostic Utility of CSFP
Diagnostic statistics for CSFP as a test for CMD and CED are shown in Table 3. Overall, CSFP had poor diagnostic accuracy for both CMD and CED (43.4% [95% CI, 35.4%–51.7%] and 31.7% [95% CI, 21.9%–42.9%], respectively), with particularly poor sensitivity (26.7% [95% CI, 17.8%–37.4%] and 21.1% [95% CI, 11.4%–33.9%], respectively). Specificity was only slightly higher: 65.2% (95% CI, 52.4%–76.5%) for CMD and 56.0% (95% CI, 34.9%–75.6%) for CED.
Table 3.
Diagnostic Utility of CSFP (CTFC >27)
| CMD (CFR <2.5) | CED (AChFR ≤1.5) | |
|---|---|---|
| Diagnostic accuracy | 43.4 (35.4–51.7) | 31.7 (21.9–42.9) |
| Sensitivity | 26.7 (17.8–37.4) | 21.1 (11.4–33.9) |
| Specificity | 65.2 (52.4–76.5) | 56.0 (34.9–75.6) |
| Positive predictive value | 50.0 (38.2–61.8) | 52.2 (35.8–68.1) |
| Negative predictive value | 40.6 (35.4–45.9) | 23.7 (17.7–31.1) |
| Positive likelihood ratio | 0.77 (0.47–1.24) | 0.48 (0.24–0.93) |
| Negative likelihood ratio | 1.12 (0.90–1.40) | 1.41 (0.97–2.05) |
Values are presented as % (95% CIs). AChFR indicates acetylcholine flow reserve; CED, coronary endothelial function; CFR, coronary flow reserve; CMD, coronary microvascular dysfunction; and CTFC, corrected thrombolysis in myocardial infarction frame count.
Prediction of Microvascular Function Using CTFC
Intra‐ and interobserver mean absolute differences in CTFC calculation were 2.20±2.81 frames and 3.74±2.94 frames, respectively. Figure 1 shows modest correlation of CTFC with bAPV (r=−0.320, P<0.001) and bMR (r=0.229; P=0.006). There is especially poor capture of CMD by CSFP among patients with high bAPV and low bMR. There was no correlation between CTFC and CFR (r=0.141; P=0.08), hMR (r=0.087; P=0.29), or AChFR (r=0.020; P=0.86). Furthermore, receiver operating characteristics analyses were performed to assess whether a higher CTFC can predict any accepted indices of impaired microvascular function (Figure 2). CTFC lacked discriminator function (ie, area under the curve [AUC] was <0.5) for both CFR <2 and <2.5 (AUC, 0.37 [0.27–0.46] and 0.41 [0.32–0.50], respectively), hMR ≥2.5 mm Hg/cm/s (AUC, 0.53 [0.43–0.64]), as well as AChFR ≤1.5 (AUC, 0.34 [0.22–0.46]). We also performed an exploratory analysis to assess whether a lower CTFC could predict CMD or CED; this demonstrated a similarly poor diagnostic accuracy (AUC, 0.59 and 0.66, respectively; see Table S4).
Figure 1. Correlation of corrected thrombolysis in myocardial infarction frame count with basal average peak flow velocity (A) and microvascular resistance (B).
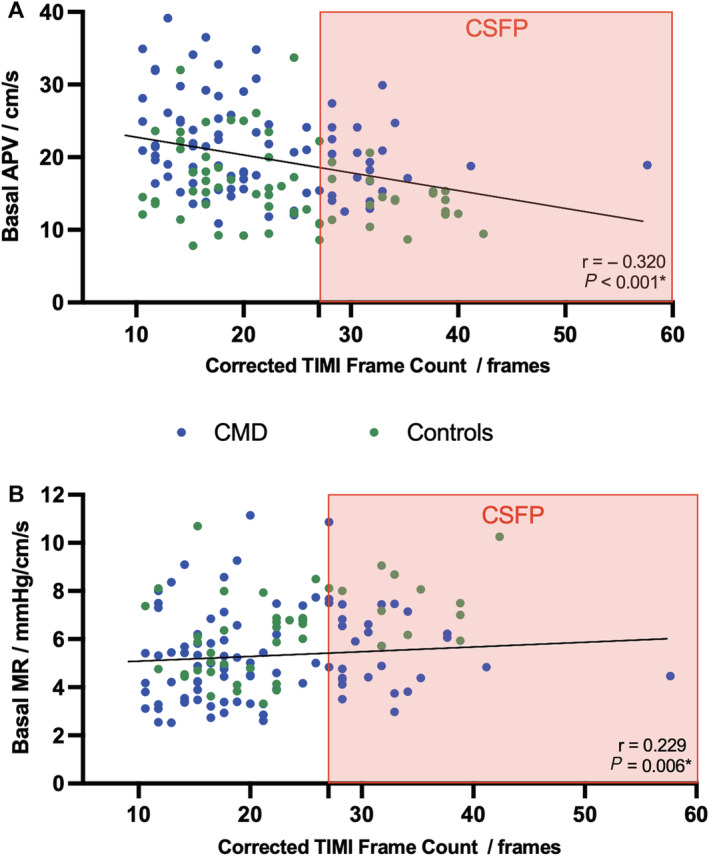
Corrected thrombolysis in myocardial infarction frame count had weak negative correlation with basal average peak velocity (r=−0.320, P<0.001) and weak positive correlation with basal microvascular resistance (r=0.229, P=0.006). Patients identified by coronary slow flow phenomenon (corrected thrombolysis in myocardial infarction frame count >27) are shaded in the red box, showing poor capture of patients with coronary microvascular dysfunction (coronary flow reserve <2.5; blue). Controls (ie, coronary flow reserve ≥2.5) are shown in green. APV indicates average peak velocity; CMD, coronary microvascular dysfunction; CSFP, coronary slow flow phenomenon; MR, basal microvascular resistance; and TIMI, thrombolysis in myocardial infarction.
Figure 2. Receiver operating characteristic curve analysis.
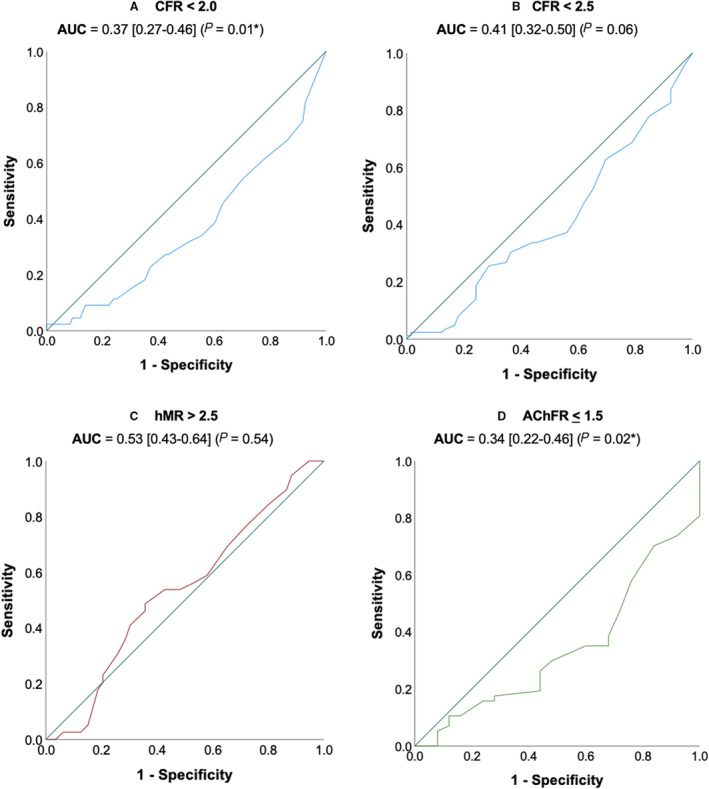
Receiver operating characteristic curve analysis of higher corrected thrombolysis in myocardial infarction frame count to predict impaired coronary flow reserve (<2.0 or <2.5), (A and B), elevated hyperemic microvascular resistance (>2.5) (C) and impaired acetylcholine flow reserve (≤1.50) (D). Diagonal line represents area under the curve=0.5 (ie, no discriminatory ability). AChFR indicates acetylcholine flow reserve; AUC, area under the curve; CFR, coronary flow reserve; and hMR, hyperemic microvascular resistance.
DISCUSSION
To our knowledge, this is the largest invasive validation study of CSFP to date that has assessed both endothelium‐independent and endothelium‐dependent coronary microvascular function. Our main finding is that CTFC (whether considered as a continuous variable or a binary classifier of CSFP) poorly predicts CMD or CED and therefore warrants reconsideration of current recommendations for diagnosing CMD (Figure 3).
Figure 3. Visual summary of key findings.
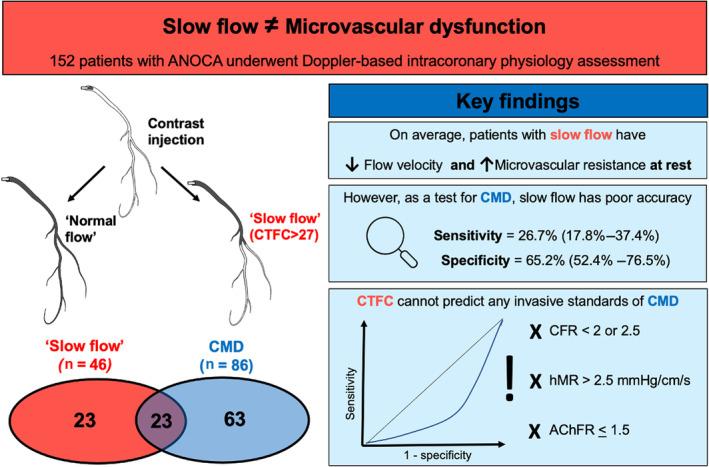
Coronary "slow flow" (corrected thrombolysis in myocardial infarction frame count >27) identifies patients with lower coronary flow velocity and higher microvascular resistance at rest. However, as a diagnostic test, it has poor sensitivity and specificity for detecting invasively defined coronary microvascular dysfunction (ie, coronary flow reserve <2.5). As a continuous variable on receiver operating characteristics analyses, corrected thrombolysis in myocardial infarction frame count could not reliably predict any invasively defined standard of coronary microvascular dysfunction (coronary flow reserve <2 or <2.5, or hyperemic microvascular resistance >2.5 mm Hg/cm/s, or acetylcholine flow reserve ≤1.5). AChFR indicates acetylcholine flow reserve; ANOCA, angina with nonobstructed coronary arteries; CFR, coronary flow reserve; CMD, coronary microvascular dysfunction; CTFC, corrected thrombolysis in myocardial infarction frame count; and hMR, hyperemic microvascular resistance.
Diagnosis of CMD
Intracoronary physiology assessment in patients with ANOCA can uncover underlying coronary vasomotor abnormalities, enabling stratified medical management and improvement of patient‐centered outcomes. 18 , 19 This invasive standard is, however, resource‐intensive and requires specialist expertise. Indeed, the appeal of using CTFC lies in its wide availability, favorable safety profile, and reasonable intra‐ and interobserver reproducibility. 20 The recognition of CSFP as evidence of elevated microvascular resistance and by extension, CMD, is primarily founded on evidence from histopathology studies, demonstrating hyperplastic fibromuscular thickening, endothelial degeneration cells, and luminal narrowing. 2 , 21 , 22 However, these studies have exceedingly small sample sizes, are without comparator arms, and have not been corroborated with in vivo evidence of microvascular dysfunction. To the extent that CSFP is meant to identify patients with "slow flow," we found that these patients on average had a lower bAPV and, correspondingly, a higher bMR.
However, our evaluation revealed that both CSFP and CTFC have very limited utility in predicting invasively defined CMD. The explanation for the poor sensitivity of CSFP as a diagnostic test for CMD is likely 2‐fold. First, a significant proportion of patients with impaired CFR, especially those with concurrently normal hMR, are now known to have elevated coronary blood flow at rest 23 , 24 and are therefore unlikely to present with "slow flow" on angiography. Second, the correlation between CTFC and in vivo parameters was weak, with a classifier of CTFC >27 identifying patients with hugely variable bAPV and bMR. Moreover, as a standalone resting index, CTFC was unable to reliably predict any indices that incorporate flow in response to adenosine or acetylcholine (hMR, CFR, or AChFR). This may largely explain why as a diagnostic test, CSFP only had modest specificity and diagnostic accuracy for CMD and CED, both conditions being defined by the ratio of resting to hyperemic flow.
In this context, a dynamic angiographic index, based on CTFC at rest as well as hyperemia, frame count reserve, 25 might theoretically fare better at predicting CMD, although the weak correlation between CTFC and bAPV undermines this assertion. Our findings would suggest that using CSFP as a point‐of‐care test risks not only missing a large proportion of patients with underlying CMD but may also lead to significant rates of misdiagnosis in patients with ANOCA. Chugh et al have previously shown that there is no correlation between CTFC and CFR in the setting of percutaneous coronary intervention 26 ; our findings similarly show that CTFC cannot predict invasive indices of microvascular function and should therefore not be used to interrogate microvascular function in patients with ANOCA.
Coronary Slow Flow Phenomenon
Several groups have proposed CSFP as a distinct "cardiac syndrome Y," characterized by recurrent episodes of chest pain at rest, most commonly in young, male, smokers with metabolic syndrome. 1 , 27 , 28 Longitudinal data from the Women's Ischemia Syndrome Evaluation study revealed that in patients with ANOCA, CTFC was an independent predictor of hospitalizations for angina, although rates of major adverse cardiovascular events and all‐cause mortality were similar between normal and slow flow groups. 29 Using the intracoronary thermodilution method, Fineschi et al found that patients with CSFP had elevated resting MR but normal CFR in a small study of only 15 patients. 30 Our study however found similar mean CFR and burden of CMD in both groups.
Whether CSFP represents a distinct pathogenic phenotype within ANOCA remains unanswered. It has previously been suggested that CSFP may be a consequence of CED. 9 , 31 Flow‐mediated dilation studies in the brachial artery suggested that patients with CSFP may have a higher burden of endothelial dysfunction. 32 Radial artery applanation tonometry, however, found a similar endothelium‐dependent response to salbutamol between patients with CSFP and healthy, age‐matched controls. 33 Ours is the first study to assess coronary endothelial function in patients with CSFP using intracoronary acetylcholine, demonstrating similar mean AChFR between CSFP and control groups as well as the inability of CTFC to predict an impaired response to acetylcholine. Together, these findings provide strong evidence that slow flow on angiography should not be interpreted as evidence of CED. Although our study is unable to offer a unifying physiological explanation for CSFP or corroborate the expected demographic profile, our findings demonstrate that CSFP should not be considered synonymous with CMD.
Angiographic Indices in Coronary Physiology
There is significant interest in the use of angiographic indices to predict coronary physiology, for reasons discussed earlier. We found that CTFC is unable to predict invasive indices of microvascular function and overall correlates poorly with flow and resistance in vivo. Similar poor correlation between CTFC and bAPV has been reported previously, 34 as well as recent demonstrations of normal absolute resting flow despite slow flow on angiography. 35 This may be because CTFC is influenced by a number of operator‐related variables (injection speed 36 and phase of cardiac cycle in which contrast is injected 20 ), as well as patient‐specific factors (age, sex, heart rate, systemic arterial pressure 20 , 37 ). This discordance may also in part explain the disparity between fractional flow reserve and quantitative flow ratio, as the latter relies on CTFC to predict proximal and distal pressures in vivo. 38 The angiography‐derived index of microvascular resistance (given by aortic pressure quantitative flow ratio thrombolysis in myocardial infarction frame count/30fps) will require similar validation before it can be used to diagnose or endotype CMD. 39
Study Limitations
Our study has limitations. First, this was a single‐center retrospective study with patients enrolled based on symptom adjudication rather than evidence of ischemia on prior noninvasive imaging. However, this is in keeping with real world practice and therefore applicable to routine clinical practice. Second, our method of measuring microvascular resistance is an approximation (given by distal coronary pressure/APV), as there is no clinically accepted gold standard. This is especially pertinent with respect to the observed higher bMR in the CSFP group, which may be secondary to the difference in bAPV. Third, angiography was not performed during adenosine‐ or acetylcholine‐induced hyperemia (as this is not standard practice) and hence our study is unable to validate the utility of frame count reserve. 25
CONCLUSIONS
In patients with ANOCA, CSFP on invasive coronary angiography has poor diagnostic accuracy for identification of CMD and CED. Moreover, CTFC is a poor discriminator of both endothelium‐independent and endothelium‐dependent microvascular dysfunction. Recommendations supporting the use of CTFC in the diagnosis of CMD should, therefore, be revisited.
Sources of Funding
The authors' work is supported by grants from the Medical Research Council (MR/T029390/1), British Heart Foundation (FS/16/49/32320), and the UK National Institute for Health Research (through the Biomedical Research Centre award to King's College London and Guy's and St Thomas' Hospital).
Disclosures
None.
Supporting information
Tables S1–S4
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.027664
For Sources of Funding and Disclosures, see page 8.
U. Dutta and A. Sinha are joint first authors.
REFERENCES
- 1. Beltrame JF, Limaye SB, Horowitz JD. The coronary slow flow phenomenon—a new coronary microvascular disorder. Cardiology. 2002;97:197–202. doi: 10.1159/000063121 [DOI] [PubMed] [Google Scholar]
- 2. Tambe AA, Demany MA, Zimmerman HA, Mascarenhas E. Angina pectoris and slow flow velocity of dye in coronary arteries‐‐a new angiographic finding. Am Heart J. 1972;84:66–71. doi: 10.1016/0002-8703(72)90307-9 [DOI] [PubMed] [Google Scholar]
- 3. Beltrame JF. Defining the coronary slow flow phenomenon. Circ J. 2012;76:818–820. doi: 10.1253/circj.CJ-12-0205 [DOI] [PubMed] [Google Scholar]
- 4. Gibson CM, Cannon CP, Daley WL, Dodge JT Jr, Alexander B Jr, Marble SJ, McCabe CH, Raymond L, Fortin T, Poole WK, et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996;93:879–888. doi: 10.1161/01.CIR.93.5.879 [DOI] [PubMed] [Google Scholar]
- 5. Ong P, Camici PG, Beltrame JF, Crea F, Shimokawa H, Sechtem U, Kaski JC, Bairey Merz CN, Coronary Vasomotion Disorders International Study G . International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018;250:16–20. doi: 10.1016/j.ijcard.2017.08.068 [DOI] [PubMed] [Google Scholar]
- 6. Kunadian V, Chieffo A, Camici PG, Berry C, Escaned J, Maas A, Prescott E, Karam N, Appelman Y, Fraccaro C, et al. An EAPCI expert consensus document on Ischaemia with non‐obstructive coronary arteries in collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by coronary vasomotor disorders international study group. Eur Heart J. 2020;41:3504–3520. doi: 10.1093/eurheartj/ehaa503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Erdogan D, Caliskan M, Gullu H, Sezgin AT, Yildirir A, Muderrisoglu H. Coronary flow reserve is impaired in patients with slow coronary flow. Atherosclerosis. 2007;191:168–174. doi: 10.1016/j.atherosclerosis.2006.03.016 [DOI] [PubMed] [Google Scholar]
- 8. Pekdemir H, Polat G, Cin VG, Camsari A, Cicek D, Akkus MN, Doven O, Katircibasi MT, Muslu N. Elevated plasma endothelin‐1 levels in coronary sinus during rapid right atrial pacing in patients with slow coronary flow. Int J Cardiol. 2004;97:35–41. doi: 10.1016/j.ijcard.2003.06.025 [DOI] [PubMed] [Google Scholar]
- 9. Turhan H, Erbay AR, Yasar AS, Bicer A, Sasmaz H, Yetkin E. Impaired coronary blood flow in patients with metabolic syndrome: Documented by thrombolysis in myocardial infarction (TIMI) frame count method. Am Heart J. 2004;148:789–794. doi: 10.1016/j.ahj.2004.05.016 [DOI] [PubMed] [Google Scholar]
- 10. Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR Jr, Lerman A. Long‐term follow‐up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. doi: 10.1161/01.CIR.101.9.948 [DOI] [PubMed] [Google Scholar]
- 11. AlBadri A, Bairey Merz CN, Johnson BD, Wei J, Mehta PK, Cook‐Wiens G, Reis SE, Kelsey SF, Bittner V, Sopko G, et al. Impact of abnormal coronary reactivity on long‐term clinical outcomes in women. J Am Coll Cardiol. 2019;73:684–693. doi: 10.1016/j.jacc.2018.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rahman H, Demir OM, Ryan M, McConkey H, Scannell C, Ellis H, Webb A, Chiribiri A, Perera D. Optimal use of vasodilators for diagnosis of microvascular angina in the cardiac catheterization laboratory. Circ Cardiovasc Interv. 2020;13:e009019. doi: 10.1161/CIRCINTERVENTIONS.120.009019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck‐Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425 [DOI] [PubMed] [Google Scholar]
- 14. Rahman H, Corcoran D, Aetesam‐Ur‐Rahman M, Hoole SP, Berry C, Perera D. Diagnosis of patients with angina and non‐obstructive coronary disease in the catheter laboratory. Heart. 2019;105:1536–1542. doi: 10.1136/heartjnl-2019-315042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hasdai D, Gibbons RJ, Holmes DR Jr, Higano ST, Lerman A. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation. 1997;96:3390–3395. doi: 10.1161/01.CIR.96.10.3390 [DOI] [PubMed] [Google Scholar]
- 16. Kunadian V, Harrigan C, Zorkun C, Palmer AM, Ogando KJ, Biller LH, Lord EE, Williams SP, Lew ME, Ciaglo LN, et al. Use of the TIMI frame count in the assessment of coronary artery blood flow and microvascular function over the past 15 years. J Thromb Thrombolysis. 2009;27:316–328. doi: 10.1007/s11239-008-0220-3 [DOI] [PubMed] [Google Scholar]
- 17. Demir OM, Rahman H, van de Hoef TP, Escaned J, Piek JJ, Plein S, Perera D. Invasive and non‐invasive assessment of ischaemia in chronic coronary syndromes: translating pathophysiology to clinical practice. Eur Heart J. 2022;43:105–117. doi: 10.1093/eurheartj/ehab548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ford TJ, Stanley B, Good R, Rocchiccioli P, McEntegart M, Watkins S, Eteiba H, Shaukat A, Lindsay M, Robertson K, et al. Stratified medical therapy using invasive coronary function testing in angina: the CorMicA trial. J Am Coll Cardiol. 2018;72:2841–2855. doi: 10.1016/j.jacc.2018.09.006 [DOI] [PubMed] [Google Scholar]
- 19. Lee BK, Lim HS, Fearon WF, Yong AS, Yamada R, Tanaka S, Lee DP, Yeung AC, Tremmel JA. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation. 2015;131:1054–1060. doi: 10.1161/CIRCULATIONAHA.114.012636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abaci A, Oguzhan A, Eryol NK, Ergin A. Effect of potential confounding factors on the thrombolysis in myocardial infarction (TIMI) trial frame count and its reproducibility. Circulation. 1999;100:2219–2223. doi: 10.1161/01.CIR.100.22.2219 [DOI] [PubMed] [Google Scholar]
- 21. Mangieri E, Macchiarelli G, Ciavolella M, Barilla F, Avella A, Martinotti A, Dell'Italia LJ, Scibilia G, Motta P, Campa PP. Slow coronary flow: clinical and histopathological features in patients with otherwise normal epicardial coronary arteries. Cathet Cardiovasc Diagn. 1996;37:375–381. [DOI] [PubMed] [Google Scholar]
- 22. Mosseri M, Yarom R, Gotsman MS, Hasin Y. Histologic evidence for small‐vessel coronary artery disease in patients with angina pectoris and patent large coronary arteries. Circulation. 1986;74:964–972. doi: 10.1161/01.CIR.74.5.964 [DOI] [PubMed] [Google Scholar]
- 23. Nardone M, McCarthy M, Ardern CI, Nield LE, Toleva O, Cantor WJ, Miner SES. Concurrently low coronary flow reserve and low index of microvascular resistance are associated with elevated resting coronary flow in patients with chest pain and nonobstructive coronary arteries. Circ Cardiovasc Interv. 2022;15:e011323. [DOI] [PubMed] [Google Scholar]
- 24. Rahman H, Ryan M, Lumley M, Modi B, McConkey H, Ellis H, Scannell C, Clapp B, Marber M, Webb A, et al. Coronary microvascular dysfunction is associated with myocardial ischemia and abnormal coronary perfusion during exercise. Circulation. 2019;140:1805–1816. doi: 10.1161/CIRCULATIONAHA.119.041595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stoel MG, Zijlstra F, Visser CA. Frame count reserve. Circulation. 2003;107:3034–3039. doi: 10.1161/01.CIR.0000074279.44131.DE [DOI] [PubMed] [Google Scholar]
- 26. Chugh SK, Koppel J, Scott M, Shewchuk L, Goodhart D, Bonan R, Tardif JC, Worthley SG, DiMario C, Curtis MJ, et al. Coronary flow velocity reserve does not correlate with TIMI frame count in patients undergoing non‐emergency percutaneous coronary intervention. J Am Coll Cardiol. 2004;44:778–782. doi: 10.1016/j.jacc.2004.05.048 [DOI] [PubMed] [Google Scholar]
- 27. Fineschi M, Gori T. Coronary slow flow: description of a new "cardiac Y" syndrome. Int J Cardiol. 2009;137:308–310. doi: 10.1016/j.ijcard.2008.05.076 [DOI] [PubMed] [Google Scholar]
- 28. Goel PK, Gupta SK, Agarwal A, Kapoor A. Slow coronary flow: a distinct angiographic subgroup in syndrome X. Angiology. 2001;52:507–514. doi: 10.1177/000331970105200801 [DOI] [PubMed] [Google Scholar]
- 29. Petersen JW, Johnson BD, Kip KE, Anderson RD, Handberg EM, Sharaf B, Mehta PK, Kelsey SF, Merz CN, Pepine CJ. TIMI frame count and adverse events in women with no obstructive coronary disease: a pilot study from the NHLBI‐sponsored Women's ischemia syndrome evaluation (WISE). PLoS One. 2014;9:e96630. doi: 10.1371/journal.pone.0096630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fineschi M, Bravi A, Gori T. The "slow coronary flow" phenomenon: evidence of preserved coronary flow reserve despite increased resting microvascular resistances. Int J Cardiol. 2008;127:358–361. doi: 10.1016/j.ijcard.2007.06.010 [DOI] [PubMed] [Google Scholar]
- 31. Aparicio A, Cuevas J, Moris C, Martin M. Slow coronary blood flow: pathogenesis and clinical implications. Eur Cardiol. 2022;17:e08. doi: 10.15420/ecr.2021.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sezgin AT, Sigirci A, Barutcu I, Topal E, Sezgin N, Ozdemir R, Yetkin E, Tandogan I, Kosar F, Ermis N, et al. Vascular endothelial function in patients with slow coronary flow. Coron Artery Dis. 2003;14:155–161. doi: 10.1097/00019501-200304000-00008 [DOI] [PubMed] [Google Scholar]
- 33. Kopetz V, Kennedy J, Heresztyn T, Stafford I, Willoughby SR, Beltrame JF. Endothelial function, oxidative stress and inflammatory studies in chronic coronary slow flow phenomenon patients. Cardiology. 2012;121:197–203. doi: 10.1159/000336948 [DOI] [PubMed] [Google Scholar]
- 34. Tanedo JS, Kelly RF, Marquez M, Burns DE, Klein LW, Costanzo MR, Parrillo JE, Hollenberg SM. Assessing coronary blood flow dynamics with the TIMI frame count method: comparison with simultaneous intracoronary doppler and ultrasound. Catheter Cardiovasc Interv. 2001;53:459–463. doi: 10.1002/ccd.1203 [DOI] [PubMed] [Google Scholar]
- 35. Gallinoro E, Paolisso P, Bermpeis K, Tino Bertolone D, Esposito G, De Bruyne B. When "slow flow" is not "low flow." JACC Cardiovasc Interv. 2022;15:e119–e121. doi: 10.1016/j.jcin.2022.02.015 [DOI] [PubMed] [Google Scholar]
- 36. Dodge JT Jr, Rizzo M, Nykiel M, Altmann J, Hobkirk K, Brennan M, Gibson CM. Impact of injection rate on the thrombolysis in myocardial infarction (TIMI) trial frame count. Am J Cardiol. 1998;81:1268–1270. doi: 10.1016/S0002-9149(98)00138-6 [DOI] [PubMed] [Google Scholar]
- 37. Faile BA, Guzzo JA, Tate DA, Nichols TC, Smith SC, Dehmer GJ. Effect of sex, hemodynamics, body size, and other clinical variables on the corrected thrombolysis in myocardial infarction frame count used as an assessment of coronary blood flow. Am Heart J. 2000;140:308–314. doi: 10.1067/mhj.2000.108003 [DOI] [PubMed] [Google Scholar]
- 38. Tanigaki T, Emori H, Kawase Y, Kubo T, Omori H, Shiono Y, Sobue Y, Shimamura K, Hirata T, Matsuo Y, et al. QFR versus FFR derived from computed tomography for functional assessment of coronary artery stenosis. JACC Cardiovasc Interv. 2019;12:2050–2059. doi: 10.1016/j.jcin.2019.06.043 [DOI] [PubMed] [Google Scholar]
- 39. Scarsini R, Shanmuganathan M, Kotronias RA, Terentes‐Printzios D, Borlotti A, Langrish JP, Lucking AJ, Ox AMISI, Ribichini F, Ferreira VM, et al. Angiography‐derived index of microcirculatory resistance (IMRangio) as a novel pressure‐wire‐free tool to assess coronary microvascular dysfunction in acute coronary syndromes and stable coronary artery disease. Int J Cardiovasc Imaging. 2021;37:1801–1813. doi: 10.1007/s10554-021-02254-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4


