Abstract
Background
Left atrial substrate may have mechanistic relevance for ablation of atrial fibrillation (AF). We sought to analyze the relationship between low‐voltage zones (LVZs), transition zones, and AF recurrence in patients undergoing pulmonary vein isolation.
Methods and Results
We conducted a prospective multicenter study on consecutive patients undergoing pulmonary vein isolation‐only approach. LVZs and transition zones (0.5–1 mV) were analyzed offline on high‐density electroanatomical maps collected before pulmonary vein isolation. Overall, 262 patients (61±11 years, 31% female) with paroxysmal (130 pts) or persistent (132 pts) AF were included. After 28 months of follow‐up, 73 (28%) patients experienced recurrence. An extension of more than 5% LVZ in paroxysmal AF and more than 15% in persistent AF was associated with recurrence (hazard ratio [HR], 4.4 [95% CI, 2.0–9.8], P<0.001 and HR, 1.9 [95% CI, 1.1–3.7], P=0.04, respectively). Significant association was found between LVZs and transition zones and between LVZs and left atrial volume index (LAVI) (both P<0.001). Thirty percent of patients had significantly increased LAVI without LVZs. Eight percent of patients had LVZs despite normal LAVI. Older age, female sex, oncological history, and increased AF recurrence characterized the latter subgroup.
Conclusions
In patients undergoing first pulmonary vein isolation, the impact of LVZs on outcomes occurs with lower burden in paroxysmal than persistent AF, suggesting that not all LVZs have equal prognostic implications. A proportional area of moderately decreased voltages accompanies LVZs, suggesting a continuous substrate instead of the dichotomous division of healthy or diseased tissue. LAVI generally correlates with LVZs, but a small subgroup of patients may present with disproportionate atrial remodeling, despite normal LAVI.
Keywords: atrial fibrillation, atrial volume, fibrosis, low‐voltage zones, pulmonary vein isolation, scar, transition zone
Subject Categories: Arrhythmias, Atrial Fibrillation, Electrophysiology, Catheter Ablation and Implantable Cardioverter-Defibrillator
Clinical Perspective.
What Is New
The clinical relevance of spontaneous low‐voltage zones is different between patients with paroxysmal and persistent atrial fibrillation, likely reflecting the different underlying pathophysiological mechanisms: atrial myopathy versus electrical remodeling.
Ten percent of patients with atrial fibrillation have a high scar burden in spite of normal atrial volume. This subset of patients is often female, with advanced age, previous stroke, normal left ventricular filling pressure, and low success rate after pulmonary vein isolation.
A proportional area of moderately decreased voltages (transition zone) accompanies low‐voltage zones, suggesting a continuous substrate instead of the dichotomous division of healthy or diseased tissue.
What Are the Clinical Implications?
Not all low‐ and intermediate‐voltage zones carry the same arrhythmogenic properties. Better characterization of the atrial substrate is needed to improve the outcomes of substrate‐based ablation.
Overcoming the dichotomous division between healthy/diseased tissue and accepting the concept of continuous substrate is necessary to move forward in the field of substrate‐based ablation.
Nonstandard Abbreviations and Acronyms
- LAVI
left atrial volume index
- LVZ
low‐voltage zone
- PVI
pulmonary vein isolation
- TrZ
transition zone
Atrial fibrosis plays a role in atrial fibrillation (AF) pathophysiology through promoting inhomogeneous slow conduction and AF susceptibility. 1 Low‐voltage zones (LVZs) identified by electroanatomical voltage mapping have been used as a surrogate for atrial fibrosis. Multiple observational studies have described a correlation between the presence of atrial scarring and high recurrence rate after pulmonary vein isolation (PVI). 1 , 2 , 3 , 4 Based on these observations, in an attempt to improve AF ablation outcomes, multiple strategies based on fibrosis ablation (encircling, homogenization or modification of fibrotic regions) have been proposed. After the initial promising results derived from observational studies and small‐size single‐center randomized controlled trials, 2 , 5 , 6 , 7 , 8 , 9 more recent larger multicenter trials have cast doubts over the efficacy of this approach. 10 , 11 , 12 , 13 In order to better understand the mechanistic relevance of atrial substrate in AF, better insights are urgently needed.
The LVZ burden threshold for prognostic relevance is unknown. The natural history of patients with different burdens of LVZ or transition zone (TrZ) undergoing the PVI‐only approach has not been well characterized. Additionally, left atrial (LA) enlargement has been associated with worse outcome. Yet, the exact relation between atrial dilation and LVZ burden is incompletely understood.
Our aims were (1) to characterize the relationship between LVZ burden and AF recurrences after first procedural PVI‐only approach in paroxysmal and persistent AF; (2) to analyze the relationship between LVZ and TrZ regions and their impact on outcomes; and (3) to analyze the relationship between LVZ and LA volume.
Methods
Study Design and Study Population
We conducted a multicenter prospective study of consecutive patients undergoing first procedure PVI‐only approach between 2017 and 2019 at the University Hospital of Antwerp (Belgium), the Complexo Hospitalario Universitario de Santiago de Compostela (Spain), and the Houston Methodist Hospital (Houston, TX). High‐density electroanatomical maps were prospectively collected before PVI and analyzed offline. The study flow chart is shown in Figure 1. The study protocol conformed to the ethical guidelines of the Declaration of Helsinki. All patients provided informed consent to participate, and the study protocol was approved by the ethical committee of the participating centers. Data used in the analysis will be made available upon reasonable request, pending the agreement of all the authors.
Figure 1. Study flow‐chart.

Study population stratified according to AF pattern and underlying rhythm during the mapping. AF indicates atrial fibrillation; AFL, atrial flutter; Parox, paroxysmal AF; Pers, persistent AF; PVI, pulmonary vein isolation; SR, sinus rhythm; and TOT, total.
High‐Density Electroanatomical Voltage Mapping and Pulmonary Vein Isolation
The mapping procedure and the map analysis workflow are described in detail in Data S1. Briefly, all patients underwent high‐density automatic bipolar voltage mapping with multipolar catheters. Three different cutoffs were used for LVZ, according to the underlying rhythm. In sinus rhythm, mapping LVZ cutoff was set at <0.5 mV, 14 and TrZ range was between 0.5 and 1 mV. 15 TrZ was evaluated only if mapping was in sinus rhythm. If mapping was in AF, LVZ cutoff was <0.24 mV and in atrial flutter <0.3 mV. 16 LVZ was identified as an area of at least 1 cm2 in area containing ≥3 neighboring points with ≤10 mm distance. LA volume index (LAVI) was calculated by indexing the final LA volume on the electroanatomical map to the body surface area.
The catheter ablation technique used for PVI (wide antral circumferential ablation) has been previously described in detail and is discussed in the Data S1 and exemplary cases are shown in Figure S1. 17 Briefly, all patients underwent ipsilateral circumferential wide antral PVI with the use of contact force sensing irrigated tip ablation catheter and automatic ablation annotation module. The primary outcome of the study was freedom from any arrhythmia recurrence (including AF, atrial flutter, and atrial tachycardia) lasting at least 30 s after a blanking period of 3 months. 18 During follow‐up, patients were evaluated at 3, 6, and 12 months in the first year and subsequently every 6 to 12 months or if symptoms developed. At each visit, ambulatory 24‐ to 48‐hour Holter monitoring was performed. The follow‐up strategy was the same in the 3 participating centers.
Statistical Analysis
The study power was calculated based on previous studies. We calculated the sample size assuming a distribution ratio of 3 to 1 of no‐scar (LVZ <5%) to scar (LVZ >5%). The AF recurrence rate in patients with scar was set at 40% and 20% among patients without scar. It was calculated that the inclusion of 224 patients would allow for a statistical power of 80% with a type I error of 0.05 to detect an absolute difference of 20%. In order to account for dropout and patient missing at follow‐up, a 10% margin was adopted, which led to the expected final population size of 246 patients. Continuous variables are presented as mean ± SD or median and interquartile range as appropriate. Comparisons between groups were undertaken with parametric (Student's t test) or nonparametric (Mann–Whitney U test) test, respectively. The comparison between categorical variables was performed with the χ2 test and Fisher's exact test. Event‐free survival was estimated by the Kaplan–Meier method using the log‐rank test. Variables significantly related to AF recurrence according to previous literature were entered in a univariate and multivariable analysis (after testing for Cox proportional hazards assumption in the general population). Variance inflation factor was used to assess collinearity, and in case this was present, univariate standardized hazard ratios (HRs) were calculated for comparison (methods of centering the variables). Thereafter, the variable with the greatest association was kept in the regression model and the other variables were excluded. Correlation between variables was assessed via Spearman or Pearson tests. Comparison between the 4 volume/LVZ groups was performed via 1‐way ANOVA, and Bonferroni method was subsequently used to identify variables that characterized subgroup G4. The SPSS 23.0 software (IBM Corp, Armonk, NY) was used for all statistical analysis.
Results
Baseline Clinical Characteristics
A total of 262 patients (61 ± 11years, 31.3% female) formed the final study population. The baseline clinical and procedural characteristics are shown in Table 1. Patients with <5% LVZ burden were significantly younger and more often male, had more frequently paroxysmal AF, lower CHA2DS2‐VASc score, and smaller atrium (Table 1).
Table 1.
Baseline Characteristics
| Total | LVZ <5% | LVZ >5% | P value | ||||
| n=262 | n=190 | 72.5% | n=72 | 27.4% | |||
| Age, y | 61.2±10.9 | 58.8±11.0 | 67.3±7.8 | <0.001 | |||
| Sex, female | 82 | 31.3% | 52 | 27.4% | 30 | 41.6% | 0.04 |
| Hypertension | 150 | 57.3% | 76 | 40.0% | 34 | 47.2% | 0.3 |
| Diabetes | 30 | 11.4% | 19 | 10.0% | 11 | 15.2% | 0.3 |
| Previous transient ischemic attack or stroke | 12 | 4.6% | 6 | 3.2% | 6 | 8.3% | 0.1 |
| Coronary artery disease | 45 | 17.2% | 30 | 15.7% | 15 | 20.8% | 0.4 |
| CHA2DS2‐VASc | 1 | 0–2 | 1 | 0–2 | 2 | 1–3 | <0.001 |
| Body mass index | 28.6±4.8 | 28.5±4.7 | 28.8±5.0 | 0.5 | |||
| Estimated glomerular filtration rate <30 mL | 12 | 4.6% | 15 | 7.8% | 8 | 11.3% | 0.3 |
| Obstructive sleep apnea | 23 | 8.8% | 7 | 3.7% | 5 | 7.0% | 0.4 |
| Persistent AF | 132 | 50.4% | 82 | 43.2% | 50 | 69.4% | <0.001 |
| Years of AF history | 1.9 | 0.7–4.7 | 1.5 | 0.7–4.4 | 2.5 | 1.1–5.6 | 0.08 |
| Ejection fraction, % | 58.4±11.2 | 59.1±9.7 | 56.1±14.4 | 0.09 | |||
| Left atrium diameter, mm | 41.3±12.1 | 42.1±8.2 | 45.3±8.4 | 0.01 | |||
| Electroanatomical voltage mapping data | |||||||
| Left atrial volume index, mL/m2 | 67.3±16.5 | 64.6±16.4 | 74.6±14.7 | <0.001 | |||
| Sinus rhythm at the beginning of procedure | 174 | 66.4% | 133 | 70.0% | 41 | 56.9% | 0.04 |
| % Area of TrZ | 7.0 | 2.6–14.3 | 5.7 | 1.5–9.8 | 21.8 | 11.1–28.2 | <0.001 |
| Area of TrZ, cm2 | 8.9 | 2.8–17.6 | 6.4 | 1.9–11.6 | 25.8 | 14.2–35.0 | <0.001 |
Continuous variables are shown as mean ± SD or median and interquartile range. Discrete variables are presented as numbers and percentages (%).
AF indicates atrial fibrillation; LVZ, low‐voltage zone (<0.5 mV in sinus rhythm, <0.3 mV in atrial flutter, <0.24 mV in AF); and TrZ, transition zone (0.5–1 mV for maps in sinus rhythm). Years of AF history stands for “years since the first diagnosis of AF.”
LVZ and Recurrence
Two patients (0.7%) were lost to follow‐up. Over a median follow‐up of 28 (interquartile range 16–46) months, 73 (28.1%) of the 260 patients experienced recurrence (Table S1). Kaplan–Meier analysis (Figure 2) shows higher recurrence rate for patients with LVZ >5%, as compared with <5%, in the overall population (Figure 2A and 2B) and in patients with paroxysmal AF (Figure 2C and 2F) but not in patients with persistent AF (Figure 2D). In multivariable analysis, in the overall population, after adjustment for body mass index, obstructive sleep apnea, age, AF pattern, atrial volume, and rhythm at the beginning of procedure, only LVZ <5% was independently and significantly associated with AF/atrial flutter/atrial tachycardia recurrence (HR, 0.6 [95% CI, 0.3–0.9], P=0.04) (Table 2). In patients with paroxysmal AF, female sex and >5% LVZ burden were associated with recurrence, at univariate analysis (Table 3). In patients with persistent AF, >15% LVZ burden and not >5% LVZ burden was significantly associated with the primary outcome, in univariate analysis (Table 3, Figure 2D and 2E). Analyzing the pattern of AF recurrence, patients who recurred with paroxysmal AF had mean LVZ of 8.9% ± 1.3 as compared with 17.0% ± 3.0 among patients who recurred with persistent AF (P=0.004). Additionally, among patients with LVZ <5%, 70% of the recurrences were paroxysmal AF, as compared with patients with LVZ >5%, who experienced persistent AF recurrence in 58% of the cases (P<0.001). No center‐related difference was found in terms of AF‐recurrence free survival and distribution of persistent/paroxysmal AF (Figure S2).
Figure 2. Atrial arrhythmia recurrence‐free survival in the overall population and subgroups.

A, Kaplan–Meier analysis comparing patients with and without low‐voltage zones (LVZ ≥ or <5%), in the overall population. B, Kaplan–Meier analysis comparing patients with LVZ <5%, LVZ between 5–15%, and LVZ >15%, in the overall population. C, Kaplan–Meier analysis comparing patients with and without LVZ <5%, in patients with paroxysmal atrial fibrillation (AF). D, Kaplan–Meier analysis comparing patients with and without LVZ < 5%, in patients with persistent AF. E, Kaplan–Meier analysis comparing patients with and without LVZ <15%, in patients with persistent AF. F, Kaplan–Meier analysis comparing patients with and without LVZ <5% between paroxysmal and persistent AF illustrates the similar outcome between patients with persistent AF and paroxysmal AF with LVZ >5%. AF indicates atrial fibrillation; AFL, atrial flutter; AT, atrial tachycardia; and HR, hazard ratio.
Table 2.
Overall Population, Predictors of Recurrence
| HR | 95% CI | P value | HR | 95% CI | P value | |||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | |||||
| Body mass index | 1.02 | 0.97 | 1.07 | 0.391 | 1.01 | 0.96 | 1.07 | 0.621 |
| Obstructive sleep apnea | 1.81 | 0.89 | 3.89 | 0.814 | 1.21 | 0.55 | 2.67 | 0.634 |
| Age | 1.02 | 1.00 | 1.05 | 0.041 | 1.01 | 0.98 | 1.02 | 0.311 |
| Persistent AF | 2.13 | 1.48 | 3.07 | <0.001 | 1.76 | 0.99 | 3.12 | 0.052 |
| Left atrial volume index, mL/m2 | 1.02 | 1.00 | 1.03 | 0.010 | 1.01 | 0.99 | 1.02 | 0.931 |
| % left ventricle | 1.02 | 1.01 | 1.03 | <0.001 | — | … | … | … |
| area LVZ, cm2 | 1.02 | 1.01 | 1.03 | <0.001 | — | … | … | … |
| Sinus rhythm at the beginning of procedure | 0.47 | 0.30 | 0.75 | <0.001 | 0.65 | 0.39 | 1.11 | 0.114 |
| %LVZ <5% | 0.4 | 0.25 | 0.63 | <0.001 | 0.57 | 0.33 | 0.96 | 0.042 |
| %LVZ >15% | 2.94 | 1.7 | 5.1 | <0.001 | — | … | … | … |
| Standardized HR for related variables | ||||||||
| LVZ% | 1.27 | 1.09 | 1.47 | 0.001 | … | … | … | … |
| LVZ area | 1.29 | 1.12 | 1.49 | <0.001 | … | … | … | … |
| LVZ> 5% | 1.52 | 1.87 | 1.23 | <0.001 | … | … | … | … |
| LVZ> 15% | 1.44 | 1.19 | 1.73 | <0.001 | … | … | … | … |
Univariate (column on the left) and multivariable (on the right) Cox regression analysis for predictors of AF recurrence after pulmonary vein isolation.
AF indicates atrial fibrillation; HR, hazard ratio; and LVZ, low‐voltage zone (<0.5 mV in sinus rhythm, <0.3 mV in atrial flutter, <0.24 mV in AF). Years of AF stands for “years since the first diagnosis of AF.”
Table 3.
Predictors of Recurrence According to AF Pattern
| Univariate analysis | ||||
|---|---|---|---|---|
| HR | 95% CI | P value | ||
| Lower | Upper | |||
| Paroxysmal AF | ||||
| Female sex | 2.26 | 1.04 | 4.88 | 0.041 |
| BMI | 1.00 | 0.92 | 1.07 | 0.942 |
| Age | 1.02 | 0.98 | 1.06 | 0.254 |
| LAVI, mL/m2 | 0.99 | 0.96 | 1.02 | 0.625 |
| %LVZ | 1.08 | 1.04 | 1.12 | <0.001 |
| Area LVZ | 1.07 | 1.04 | 1.10 | <0.001 |
| %LVZ >5% | 4.39 | 1.96 | 9.80 | <0.001 |
| %LVZ >15% | 3.99 | 1.36 | 11.73 | 0.012 |
| Persistent AF | ||||
| Female sex | 1.16 | 0.61 | 2.21 | 0.642 |
| BMI | 1.02 | 0.96 | 1.09 | 0.425 |
| Age | 1.02 | 0.99 | 1.05 | 0.156 |
| Long‐standing persistent AF | 1.53 | 0.64 | 3.63 | 0.326 |
| LAVI | 1.01 | 0.99 | 1.03 | 0.083 |
| %LVZ | 1.01 | 0.99 | 1.02 | 0.313 |
| Area LVZ | 1.01 | 0.99 | 1.01 | 0.243 |
| %LVZ <5% | 0.70 | 0.39 | 1.25 | 0.233 |
| %LVZ >15% | 1.93 | 1.01 | 3.66 | 0.041 |
Univariate (column on the left) and multivariable (on the right) Cox regression analysis for predictors of AF recurrence after pulmonary vein isolation.
AF indicates atrial fibrillation; BMI, body mass index; HR, hazard ratio; LAVI, left atrial volume index; and LVZ, low‐voltage zone (<0.5 mV in sinus rhythm, <0.3 mV in atrial flutter, <0.24 mV in AF). Years of AF history stands for “years since the first diagnosis of AF.”
LVZs and TrZs
A significant correlation was found between the area of LVZ and TrZ (Spearman correlation=0.74; P<0.001). Both LVZ and TrZ (area and percentage) were significantly associated with the primary outcome in univariate analysis (HR, 1.03 [95% CI, 1.01–1.05], P=0.001; HR, 1.04 [95% CI, 1.01–1.06], P=0.003; respectively). When corrected for LVZ via multivariable analysis, TrZ burden was nonsignificantly associated with recurrence (P=0.3). In a subanalysis of patients with <5% LVZ (171 patients), the presence of TrZ was not useful to further stratify patients at higher risk of recurrence (HR, 1.03, P=0.1).
LVZs and LAVI
There was a weak but significant correlation between burden of LVZ and LAVI (Spearman correlation=0.27; P<0.01) (Figure 3A). According to the presence or absence of >5% LVZ and the dimension of LAVI, the study population was divided into 4 groups. A discordant pattern between LAVI and % LVZ area (figure 3A) was observed in 100 (38%) patients. Comparing G1, G2, and G3 subgroups there was a significant stepwise increase in age, CHA2DS2‐VASc score, grade of diastolic dysfunction, proportion of patients with hypertension, persistent AF, and increased left ventricle filling pressure (Table 4 and Figure 4). In contrast, G4 patients were more likely to be female, older, with higher CHA2DS2‐VASc score and normal ventricular filling pressure. Kaplan–Meier analysis showed significantly higher recurrence rate in G3 and G4 patients as compared with G1 and G2 patients (Figure 3B).
Figure 3. Relationship between low voltages and atrial volume.
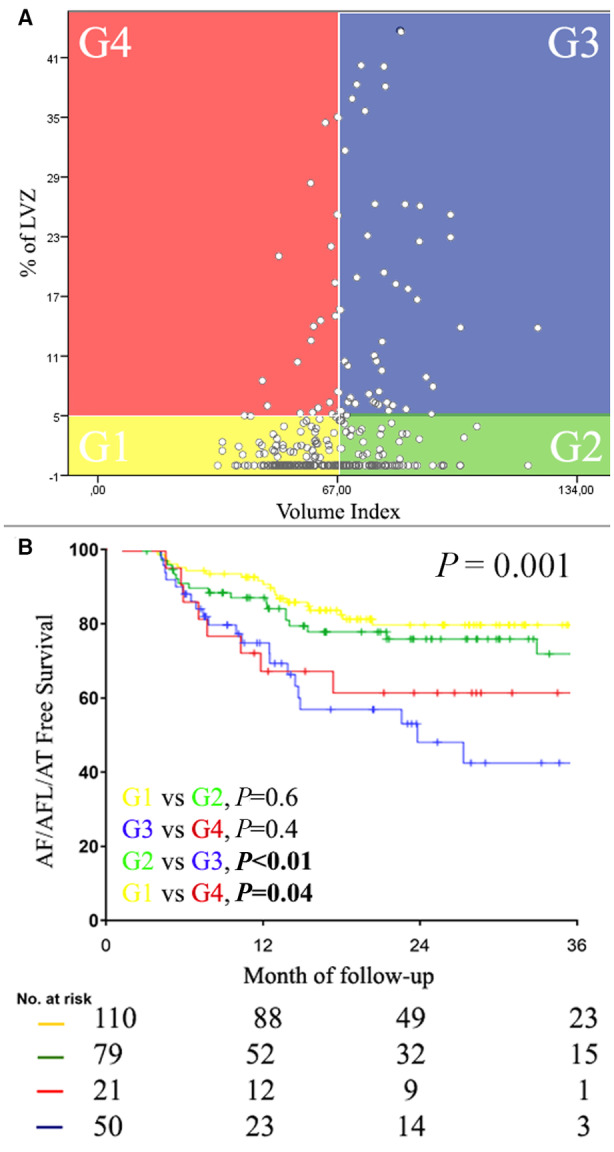
A, The overall population was divided into 4 groups according to the presence or absence of LVZ >5% and significant left atrial dilation. G1 and G3 show a concordant pattern between LVZ and LAVI, whereas G2 and G4 show a discordant pattern: significant LA dilatation without LVZ or LVZ without significant LA dilatation. B, The Kaplan–Meier analysis in the lower panel shows how the outcome is entirely driven by the presence or absence of LVZ, more than the dimension of the LAVI. AFL indicates atrial flutter; AF, atrial fibrillation; AT, atrial tachycardia; LA, left atrial; LAVI, left atrial volume index; and LVZ, low‐voltage zone.
Table 4.
Comparison of Baseline Clinical Characteristics Between 4 Subgroups of Patients With or Without >5% LVZ and With or Without Significantly Dilated Left Atrium (G1, G2, G3, G4)
| G1 < 5% LVZ and nonsignificantly dilated LAVI | G2 < 5% LVZ and significantly dilated LAVI | G3 > 5%LVZ and significantly dilated LAVI | G4 > 5%LVZ and nonsignificantly dilated LAVI | P value | |||||
|---|---|---|---|---|---|---|---|---|---|
| n=111 (42.4%) | n=79 (30.1%) | n=51 (19.5%) | n=21 (8.0%) | ||||||
| Age, | 57.4±12.6* | 60.8±8.0 | 67.4±7.8 | 67.4±8.2 | <0.001 | ||||
| Sex, male | 77 | 69.4% | 61 | 77.2%* | 33 | 64.7% | 9 | 42.8% | 0.02 |
| Body mass index | 28.1±4.6 | 29.0±4.9 | 29.5±4.6 | 27.4±5.6 | 0.2 | ||||
| Hypertension | 39 | 35.1% | 37 | 46.8% | 28 | 54.9% | 6 | 28.6% | 0.03 |
| Diabetes | 11 | 9.9% | 8 | 10.1% | 7 | 13.7% | 4 | 19.0% | 0.5 |
| CHA2DS2‐VASc | 1 | 0–2* | 1 | 0–2 | 2 | 1–4 | 2 | 1–3 | <0.001 |
| eGFR <30 mL | 3 | 2.7% | 4 | 5.1% | 3 | 5.9% | 2 | 9.5% | 0.5 |
| Obstructive sleep apnea | 5 | 4.5% | 10 | 12.6% | 7 | 13.7% | 1 | 4.7% | 0.1 |
| Persistent AF | 37 | 33.3% | 45 | 56.9% | 39 | 76.4% | 11 | 5.23% | <0.001 |
| Years of AF History | 1.6 | 0.6–4.6 | 1.5 | 0.7–4.5 | 2.3 | 1.0–5.7 | 4.0 | 1.4–7.1 | 0.8 |
| Oncological History | 3 | 2.7%* | 2 | 2.5%* | 2 | 3.9%* | 5 | 23.8% | <0.001 |
| e’ | 8.0±2.3 | 6.6±1.9 | 6.2±1.5 | 7.6±1.6 | <0.001 | ||||
| E/e' | 10.9±3.9 | 13.6±4.7 | 16.5±7.0* | 11.2±3.4 | <0.001 | ||||
| E/A | 1.5±0.5 | 1.7±0.6 | 2.1±0.8 | 1.6±0.7 | <0.001 | ||||
| Grade of Diastolic Dysfunction | 0.9±0.9 | 1.2±0.9 | 2.0±0.4* | 1.2±1.0 | <0.001 | ||||
| Increased LV Filling Pressure | 21 | 27.2% | 33 | 58.1%* | 35 | 83.2%* | 4 | 22.3% | <0.001 |
| EF | 59%±9.7 | 59%±9.8 | 54%±13.8 | 59%±14.4 | 0.2 | ||||
| EF <50%, n° | 10 | 9.0% | 4 | 5.0% | 10 | 19.6% | 2 | 11.9% | 0.2 |
| %LVZ | 0.9±1.3* | 0.9±1.5* | 19.4±16.2 | 18.1±16.1 | <0.001 | ||||
The table reports a comparison between 4 groups, identified according to the presence/absence of significant LVZ and the presence/absence of atrial dilation. A P value (which is the result of the ANOVA analysis) <0.05 signifies that there is a significant difference between at least 2 of the 4 groups. A further subanalysis, via Bonferroni method, allowed identification of the variable that significantly differed from G4. These variables are marked with *. AF indicates atrial fibrillation; EF, ejection fraction; LAVI, left atrial volume index; LV, left ventricle; and LVZ, low‐voltage zone (<0.5 mV in sinus rhythm, <0.3 mV in atrial flutter, <0.24 mV in AF). Years of AF stands for “years since the first diagnosis of AF.”
Figure 4. Two fibrotic pathways.
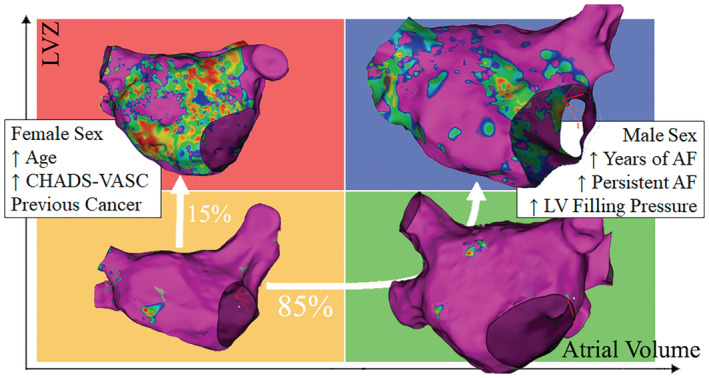
The 2 different hypothetical “fibrotic pathways” are shown. On one side, in the majority of patients, first progressive atrial dilatation occurs and is followed later by extensive fibrosis. On the other side, progressive fibrosis occurs without preceding left atrial dilatation. AF indicates atrial fibrillation; LV, left ventricle; and LVZ, low‐voltage zones.
Discussion
Our study is the largest systematic prospective detailed evaluation of LA substrate using standardized high‐density multipolar bipolar voltage mapping in patients undergoing first PVI‐only catheter ablation. Our main results are as follows: (1) the presence of >5% LVZ area is a strong and independent predictor of recurrence in patients with paroxysmal AF; (2) in patients with persistent AF, only the presence of high‐burden (>15%) LVZ is predictive of recurrence; (3) a correlation exists between the amount of LVZ and TrZ, and in patients without LVZ, the presence of TrZ does not predict recurrence; (4) a weak correlation exists between the extent of LVZ and indexed LA volume: in more than one‐third of patients, LVZ occurs without significant LA dilatation or, despite significant LA dilatation, there is no LVZ; and (5) in a small subgroup of older, mostly female patients LVZ is present without significant LA dilatation, and this group is characterized by high recurrence following PVI.
LVZ as a Marker of Recurrence Risk Following PVI
In line with previous studies, 3 , 4 , 13 our study confirms that the presence of LVZ in patients with paroxysmal AF is relatively infrequent (17%) but is associated with a 4 times higher risk of recurrence after first PVI‐only procedure. The majority of previous studies have used the presence of LVZ as a binary parameter with cutoff values ranging from 0 to 5 cm2 LVZ area. 3 , 4 We report in the current study that 5% of LA surface area LVZ is likely the best cutoff in patients with paroxysmal AF for predicting recurrence after first PVI procedure. Above this value, the presence of LVZ is a strong predictor of lower ablation success. The presence of LVZ in paroxysmal AF patients is clinically relevant, as PVI‐only approach performs poorly in these patients, with a recurrence rate similar to persistent AF. Our results confirm previous studies and suggest that patients with paroxysmal AF and LVZ deserve to be included in trials evaluating additional treatment strategies.
Our study shows for the first time that the extent of LVZ predictive of higher recurrence following PVI‐only approach is different in patients with persistent and paroxysmal AF. In persistent AF only high‐burden LVZ (with cutoff >15% of LA area) stratifies adequately the risk of AF recurrence following PVI‐only approach. A possible explanation for the difference may be that LVZ in paroxysmal AF is the marker of an underlying diffuse atrial myopathy, which continues to progress despite PVI. Conversely, in persistent AF, LVZ may be partially owing to the AF itself caused by reversible electrical remodeling. It is plausible that LVZ regions in patients with persistent AF overestimate the underlying irreversible structural myopathy. Our results have important clinical implications. It implies that targeting all LVZ areas in patients with low‐ and moderate‐burden LVZ in persistent AF will likely not improve outcomes and that some LVZ areas are less arrhythmogenic than others. It may also explain the negative results of the recent randomized trial of LVZ targeted ablation. 11 We confirm in the current study that in patients with persistent AF without LVZ (62% of the patients), the recurrence‐free survival after PVI‐only approach is still suboptimal (67%). Despite absence of LVZ and similar structural atrial substrate to patients with paroxysmal AF, these patients experience more than double the rate of AF recurrence (15% versus 33%), suggesting that alternative targets should be evaluated.
Relation Between LVZs and TrZs
Yagishita et al conducted a study in 100 patients with paroxysmal AF. Based on a control group of 6 patients without AF, they defined the TrZ as a region with voltages between 0.5 mV and 1.1 mV. 15 However, the relationship between the amount of LVZ and TrZ has not been further analyzed previously. In the current study, for the TrZ we used a definition of bipolar voltage cutoff values from 0.5 mV to 1 mV. We report that for every cm2 of LVZ, there is a large accompanying TrZ. Although the used voltage cutoffs are arbitrary, our finding suggests that the presence of LVZ could be seen as the tip of the “fibrosis iceberg” and as a marker of diffuse underlying atrial substrate. It is likely that a discrete bipolar voltage threshold to dichotomize normal tissue from tissue with atrial fibrosis does not exist. Atrial fibrosis is likely a continuum heterogeneously also present in normal hearts and by increasing with age and other upstream triggers (including AF) may reach a level in certain regions when it starts to be associated with measurable bipolar voltage decrease. Although the arrhythmogenicity of different types and extent of fibrosis is largely unknown, encircling LVZ regions may leave large areas of surrounding TrZ untreated. Yagishita et al reported that the extent of TrZ in patients without LVZ predicts recurrence in patients with paroxysmal AF. We report contrary findings; in the current study the presence of TrZ without LVZ did not predict worse outcome. The reasons behind this discrepancy may be the differences between the catheters used for mapping with different electrode size and interelectrode spacing and PVI techniques.
Relationship Between LA LVZ Extension and Atrial Dilatation
Several previous studies have shown an association between larger LA size and presence of LVZ. 1 , 19 However, no study analyzed the exact relationship yet. Based on our findings, there seem to be 2 different “fibrotic pathways” present in patients with AF. In the majority of patients, during the time course of AF a stepwise dilation of the atrium is present, followed later by diffuse fibrosis. It is still unclear if progression in AF is a bystander marker of this process or has a casual role. On the other side, it seems that in a small subgroup of frequently older female patients sometimes with oncological history, extensive fibrosis precedes significant LA dilatation. These patients have high recurrence rate following PVI. It is interesting to notice that patients with LVZ >5% and nonsignificantly dilated LAVI have a significantly lower prevalence of diastolic dysfunction and lower filling pressure, as compared with patients with dilated LAVI (Table 4). This might point toward a different etiological process causing atrial remodeling. This observation is limited by the low number of patients in G4 and should be further investigated.
Our findings have important clinical implications. They may suggest that in younger, especially male, patients with a significantly dilated left atrium, traditionally considered poor candidates for PVI, the procedure may have better than expected outcomes. On the contrary, in older female patients with a high CHA2DS2‐VASc score, extensive fibrosis may already be present, even with a nonsignificantly dilated left atrium, and outcome may be worse.
Limitations
Spontaneous low‐voltage regions are a surrogate marker for atrial fibrosis, but histological validation is missing. Spatial distribution and extent of LVZ depend largely on spontaneous rhythm and site and frequency of atrial pacing, as well as the mapping catheter and interelectrode distance. However, all our patients were mapped in spontaneous rhythm without pacing, using catheters with same electrode size, interelectrode distance, and automatic acquisition setting validated in our previous studies. As the correlation between bipolar voltage in AF and sinus rhythm becomes less accurate above 0.5 mV, to analyze the impact of the TrZ, only sinus maps were used. Multipolar catheters may be prone to suboptimal contact in several LA regions. However, meticulous attempts were made to ensure that only points in close proximity to the anatomical shell were collected. We acknowledge that the 68 mL/m2 cutoff used to identify patients with significantly dilated atrium based on the median LAVI on electroanatomical mapping is arbitrary. The LAVI cutoff for significant LA dilatation may appear larger than the correspondent conventional echocardiographic parameter. A more adequate comparison for electroanatomical mapping LAVI may be the upper limit of normal LAVI in cardiac computer tomography examination reported at 63 mL/m2. 20
Conclusions
In patients undergoing first PVI, the impact of LVZ on outcomes occurs with lower LVZ burden in paroxysmal (>5%) than persistent AF (>15%), suggesting that not all LVZs have prognostic implications in persistent AF. A proportional area of moderately decreased voltage zone is present around LVZ, suggesting the presence of a more widespread atrial myopathy associated with commonly visualized LVZ regions and arguing against the dichotomous definition of healthy/diseased tissue. The significance of moderately decreased voltage zones in the absence of LVZ remains uncertain. In general, LA dilatation correlates with the presence of LVZ. However, in more than one‐third of patients, significantly dilated left atrium is present without LVZ or LVZ is present without significant LA dilation. In the majority of male patients LA dilatation seem to precede extensive fibrosis. In a small subgroup of frequently older female patients, fibrosis is present without significant LA dilatation and predicts high recurrence following PVI.
Sources of Funding
The study has been financially supported by an investigator‐initiated research grant by Biosense Webster (Study ID – IIS‐532) (M.R.M.).
Disclosures
None.
Supporting information
Data S1
Table S1
Figures S1–S2
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.027795
For Sources of Funding and Disclosures, see page 9.
References
- 1. Verma A, Wazni OM, Marrouche NF, Martin DO, Kilicaslan F, Minor S, Schweikert RA, Saliba W, Cummings J, Burkhardt JD, et al. Pre‐existent left atrial scarring in patients undergoing pulmonary vein antrum isolation: an independent predictor of procedural failure. J Am Coll Cardiol. 2005;45:285–292. doi: 10.1016/j.jacc.2004.10.035 [DOI] [PubMed] [Google Scholar]
- 2. Rolf S, Kircher S, Arya A, Eitel C, Sommer P, Richter S, Gaspar T, Bollmann A, Altmann D, Piedra C, et al. Tailored atrial substrate modification based on low‐voltage areas in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2014;7:825–833. doi: 10.1161/CIRCEP.113.001251 [DOI] [PubMed] [Google Scholar]
- 3. Masuda M, Fujita M, Iida O, Okamoto S, Ishihara T, Nanto K, Kanda T, Tsujimura T, Matsuda Y, Okuno S, et al. Left atrial low‐voltage areas predict atrial fibrillation recurrence after catheter ablation in patients with paroxysmal atrial fibrillation. Int J Cardiol. 2018;257:97–101. doi: 10.1016/j.ijcard.2017.12.089 [DOI] [PubMed] [Google Scholar]
- 4. Vlachos K, Efremidis M, Letsas KP, Bazoukis G, Martin R, Kalafateli M, Lioni L, Georgopoulos S, Saplaouras A, Efremidis T, et al. Low‐voltage areas detected by high‐density electroanatomical mapping predict recurrence after ablation for paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2017;28:1393–1402. doi: 10.1111/jce.13321 [DOI] [PubMed] [Google Scholar]
- 5. Jadidi AS, Lehrmann H, Keyl C, Sorrel J, Markstein V, Minners J, Park C‐I, Denis A, Jaïs P, Hocini M, et al. Ablation of persistent atrial fibrillation targeting low‐voltage areas with selective activation characteristics. Circ Arrhythm Electrophysiol. 2016;9. [DOI] [PubMed] [Google Scholar]
- 6. Schreiber D, Rieger A, Moser F, Kottkamp H. Catheter ablation of atrial fibrillation with box isolation of fibrotic areas: Lessons on fibrosis distribution and extent, clinical characteristics, and their impact on long‐term outcome. J Cardiovasc Electrophysiol. 2017;28:971–983. doi: 10.1111/jce.13278 [DOI] [PubMed] [Google Scholar]
- 7. Kircher S, Arya A, Altmann D, Rolf S, Bollmann A, Sommer P, Dagres N, Richter S, Breithardt O‐A, Dinov B, et al. Individually tailored vs. standardized substrate modification during radiofrequency catheter ablation for atrial fibrillation: a randomized study. Europace. 2018;20:1766–1775. [DOI] [PubMed] [Google Scholar]
- 8. Kottkamp H, Berg J, Bender R, Rieger A, Schreiber D. Box isolation of fibrotic areas (BIFA): a patient‐tailored substrate modification approach for ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2016;27:22–30. doi: 10.1111/jce.12870 [DOI] [PubMed] [Google Scholar]
- 9. Yang G, Yang B, Wei Y, Zhang F, Ju W, Chen H, Li M, Gu K, Lin Y, Wang B, et al. Catheter ablation of nonparoxysmal atrial fibrillation using electrophysiologically guided substrate modification during sinus rhythm after pulmonary vein isolation. Circ Arrhythm Electrophysiol. 2016;9:e003382. [DOI] [PubMed] [Google Scholar]
- 10. Lee JM, Shim J, Park J, Yu HT, Kim T‐H, Park J‐K, Uhm J‐S, Kim J‐B, Joung B, Lee M‐H, et al. The electrical isolation of the left atrial posterior wall in catheter ablation of persistent atrial fibrillation. JACC Clin Electrophysiol. 2019;5:1253–1261. doi: 10.1016/j.jacep.2019.08.021 [DOI] [PubMed] [Google Scholar]
- 11. Yang B, Jiang C, Lin Y, Yang G, Chu H, Cai H, Lu F, Zhan X, Xu J, Wang X, et al. STABLE‐SR (electrophysiological substrate ablation in the left atrium during sinus rhythm) for the treatment of nonparoxysmal atrial fibrillation: a prospective, multicenter randomized clinical trial. Circ Arrhythm Electrophysiol. 2017;10:e005405. [DOI] [PubMed] [Google Scholar]
- 12. Bisbal F, Benito E, Teis A, Alarcón F, Sarrias A, Caixal G, Villuendas R, Garre P, Soto N, Cozzari J, et al. Magnetic resonance imaging‐guided fibrosis ablation for the treatment of atrial fibrillation. Circ Arrhythmia Electrophysiol. 2020;13:e008707. doi: 10.1161/CIRCEP.120.008707 [DOI] [PubMed] [Google Scholar]
- 13. Masuda M, Asai M, Iida O, Okamoto S, Ishihara T, Nanto K, Kanda T, Tsujimura T, Matsuda Y, Okuno S, et al. Additional low‐voltage‐area ablation in patients with paroxysmal atrial fibrillation: results of the randomized controlled VOLCANO trial. J Am Heart Assoc. 2020;9:e015927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sim I, Bishop M, O'Neill M, Williams SE. Left atrial voltage mapping: defining and targeting the atrial fibrillation substrate. J Interv Card Electrophysiol. 2019;56:213–227. doi: 10.1007/s10840-019-00537-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yagishita A, Sparano D, Cakulev I, Gimbel JR, Phelan T, Mustafa H, De Oliveira S, Mackall J, Arruda M. Identification and electrophysiological characterization of early left atrial structural remodeling as a predictor for atrial fibrillation recurrence after pulmonary vein isolation. J Cardiovasc Electrophysiol. 2017;28:642–650. doi: 10.1111/jce.13211 [DOI] [PubMed] [Google Scholar]
- 16. Rodríguez‐Mañero M, Valderrábano M, Baluja A, Kreidieh O, Martínez‐Sande JL, García‐Seara J, Saenen J, Iglesias‐Álvarez D, Bories W, Villamayor‐Blanco LM, et al. Validating left atrial low voltage areas during atrial fibrillation and atrial flutter using multielectrode automated electroanatomic mapping. JACC Clin Electrophysiol. 2018;4:1541–1552. doi: 10.1016/j.jacep.2018.08.015 [DOI] [PubMed] [Google Scholar]
- 17. Tijskens M, Bergonti M, Spera F, Ascione C, Saenen J, Huybrechts W, Miljoen H, Riva S, Wittock A, Heidbuchel H, et al. Etiology and outcome of catheter ablation in patients with onset of atrial fibrillation <45 years of age. Am J Cardiol. 2022;166:45–52. doi: 10.1016/j.amjcard.2021.11.030 [DOI] [PubMed] [Google Scholar]
- 18. Calkins H, Hindricks G, Cappato R, Kim Y‐H, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2018;20:e1–e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kirstein B, Neudeck S, Gaspar T, Piorkowski J, Wechselberger S, Kronborg MB, Zedda A, Hankel A, El‐Armouche A, Tomala J, et al. Left atrial fibrosis predicts left ventricular ejection fraction response after atrial fibrillation ablation in heart failure patients: the fibrosis‐HF study. Europace. 2020;22:1812–1821. doi: 10.1093/europace/euaa179 [DOI] [PubMed] [Google Scholar]
- 20. Fuchs A, Mejdahl MR, Kühl JT, Stisen ZR, Nilsson EJP, Køber LV, Nordestgaard BG, Kofoed KF. Normal values of left ventricular mass and cardiac chamber volumes assessed by 320‐detector computed tomography angiography in the Copenhagen general population study. Eur Hear J Cardiovasc Imaging. 2016;17:1009–1017. doi: 10.1093/ehjci/jev337 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Table S1
Figures S1–S2


