Abstract
Background
The fractional excretion of urea nitrogen (FEUN) has been used as a renal blood flow index related to cardiac output, and the estimated plasma volume status (ePVS) as a body fluid volume index. However, the usefulness of their combination in acute decompensated heart failure (HF) management is unclear. We investigated the effect of 4 hemodynamic categories according to the high and low FEUN and ePVS values at discharge on the long‐term prognosis of patients with acute decompensated HF.
Methods and Results
Between April 2011 and December 2018, we retrospectively identified 466 patients with acute decompensated HF with FEUN and ePVS values at discharge. Primary end point was postdischarge all‐cause death. Secondary end points were (1) the composite of all‐cause death and HF readmission, and (2) HF readmission in a time‐to‐event analysis. The patients were divided into 4 groups according to the high/low FEUN (≥35%, <35%) and ePVS (>5.5%, ≤5.5%) values at discharge: high‐FEUN/low‐ePVS, high‐FEUN/high‐ePVS, low‐FEUN/low‐ePVS, and low‐FEUN/high‐ePVS groups. During a median follow‐up period of 28.1 months, there were 173 all‐cause deaths (37.1%), 83 cardiovascular deaths (17.8%), and 121 HF readmissions (26.0%). The Kaplan–Meier curve analysis showed that the high‐FEUN/low‐ePVS group had a better prognosis than the other groups (log‐rank test, P<0.001). In the multivariable Cox regression analysis, the low‐FEUN/high‐ePVS group had a higher mortality than the high‐FEUN/low‐ePVS group (hazard ratio, 2.92 [95% CIs, 1.73–4.92; P<0.001]).
Conclusions
The new classification of the 4 hemodynamic profiles using the FEUN and ePVS values may play an important role in improving outcomes in patients with stable acute decompensated HF.
Keywords: ADHF, ePVS, FEUN, heart failure
Subject Categories: Heart Failure
Nonstandard Abbreviations and Acronyms
- ADHF
acute decompensated heart failure
- ePVS
estimated plasma volume status
- FEUN
fractional excretion of urea nitrogen
Clinical Perspective.
What Is New?
The fractional excretion of urea nitrogen (FEUN) has been used as renal blood flow index related to cardiac output, and the estimated plasma volume status (ePVS) as body fluid volume index; however, the usefulness of their combination in acute decompensated heart failure (ADHF) management is unclear.
We provide new insights into the ADHF management based on imprecise cardiac output and volume status markers, including symptom improvement, physical and laboratory examination findings, urine output, and weight loss.
Low‐FEUN/high‐ePVS was independently associated with poor prognosis and may play an important role in improving outcomes of patients with ADHF.
What Are the Clinical Implications?
Our study presents a new classification of 4 hemodynamic profiles using the FEUN and ePVS values that is both cost‐effective and noninvasive, to properly understand the condition of patients with ADHF.
Using FEUN and ePVS as markers for long‐term prognosis in patients with ADHF has not been researched enough, and we hope that further research is conducted to verify our findings and study the correlation between the 4 FEUN/ePVS hemodynamic profiles using the values at discharge and long‐term prognosis.
Knowledge of cardiac output and volume status is essential for the implementation of an effective treatment strategy in acute decompensated heart failure (ADHF), in which congestion is one of the main causes of hospitalization. Furthermore, insufficient decongestion at discharge has been associated with higher rehospitalization rates and death in patients with ADHF. 1 , 2 However, hypoperfusion is also recognized as a poor prognostic factor. During the treatment of heart failure (HF), overcorrections because of excess fluid removal arising from the use of diuretics have been associated with adverse events, including worsening renal function, increased activity of the renin angiotensin system and sympathetic nervous system, and death. 3 , 4 , 5 Therefore, international guidelines have suggested the assessment of the “volume status” and “perfusion” required to maintain an euvolemic state, by controlling the diuretic doses appropriately. 6 , 7 However, in some clinical cases, it can be difficult to relieve the congestion while avoiding hypovolemic conditions arising from excessive diuresis. Therefore, objective indicators are needed to guide the adjustments of HF medications.
As a diagnostic approach to acute kidney injury, the fractional excretion of urea nitrogen (FEUN) is frequently used to diagnose renal or prerenal failure, and is more useful than the fractional excretion of sodium, which is affected strongly by diuretic use. 8 , 9 , 10 A low FEUN indicates prerenal failure, which is because of the dehydration that occurs in general conditions and the dehydration and low cardiac output that occurs in HF. In other words, the FEUN may be a novel surrogate marker of perfusion in patients with ADHF. Recently, we identified a lower FEUN as a predictor of the prognosis in patients with ADHF, especially in those with estimated glomerular filtration rate (eGFR) <60 mL/min per 1.73 m2. 11 However, we were unable to determine whether the low FEUN was because of the low cardiac function or the decrease in the circulating plasma volume.
The estimated plasma volume status (ePVS), which was derived from the hemoglobin and hematocrit values, showed a good correlation with the measured plasma volume. 12 Previous studies have demonstrated that a high ePVS was associated with a poor prognosis, and proposed a threshold of >5.5 mL/g (high ePVS) to indicate excessive congestion in patients with ADHF. 13 , 14
We hypothesized that the combination of the FEUN and ePVS is useful clinically to determine the perfusion and volume status. Therefore, the present study aimed to investigate the effect of the 4 hemodynamic categories according to the high and low FEUN and ePVS values at discharge on the long‐term prognosis of patients with ADHF, and whether the usefulness of the 4 hemodynamic categories in patients with ADHF depends on renal function.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design and Patients
The NARA‐HF Study 4 (Nara Registry and Analyses for Heart Failure Study 4) is a prospective cohort study that comprises 1012 consecutive patients admitted as emergency cases to our department or the coronary care unit of our hospital with documented ADHF (either acute new‐onset or acute‐on‐chronic HF) between April 2011 and December 2018. The diagnosis of HF was based on the Framingham Criteria. 15 Patients with acute myocardial infarction, acute myocarditis, or acute HF with acute pulmonary embolism were excluded. Of the patients enrolled in the NARA‐HF Study 4, 466 (excluding patients who died during hospitalization, on dialysis, or without measured urine urea nitrogen at discharge) were included in the present study. We divided the 466 patients with ADHF into 4 groups according to the high/low FEUN (≥35%, <35%) and ePVS (>5.5%, ≤5.5%) values at discharge: high‐FEUN/low‐ePVS, high‐FEUN/high‐ePVS, low‐FEUN/low‐ePVS, and low‐FEUN/high‐ePVS groups (Figure 1).
Figure 1. The 4 hemodynamic profiles based on the combined assessment of fractional excretion of urea nitrogen and estimated plasma volume status values at discharge.
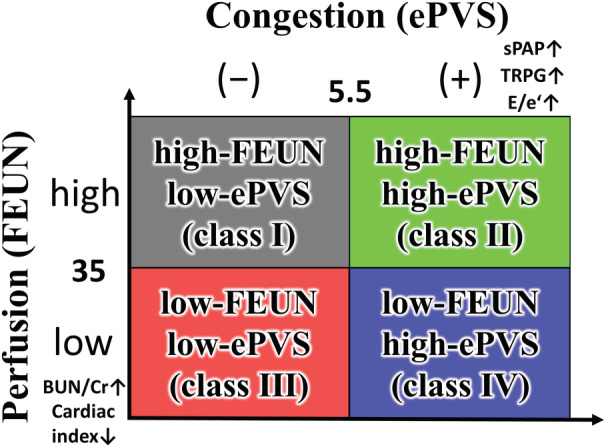
BUN/Cr indicates blood urea nitrogen/creatinine; E/e`, early mitral inflow velocity to early diastolic mitral annular velocity; ePVS, estimated plasma volume status; FEUN, fractional excretion of urea nitrogen; sPAP, systolic pulmonary artery pressure; and TRPG, tricuspid regurgitation pressure gradient.
We investigated the impact of the combined assessment of the FEUN and ePVS values on the prognosis of ADHF. The study protocol was approved by the Ethics Committee of the Nara Medical University (approval number 624). Written informed consent was obtained from all the patients according to the Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects.
Data Collection and Definitions
The laboratory parameters including hemoglobin, hematocrit, albumin, blood urea nitrogen (BUN), creatinine, eGFR according to the diet modifications of the renal disease method, cystatin C, serum electrolytes (sodium and potassium), B‐type natriuretic peptide (BNP), renin, aldosterone, urine electrolytes (sodium and potassium), and urine urea nitrogen (from the urine samples that were collected) were measured in all patients at discharge. The vital signs, including heart rate and blood pressure at discharge, were recorded.
The FEUN was calculated according to its well‐defined formula 8 , 16 , 17 :
The ePVS was calculated using the Strauss‐derived Duarte formula with the hematocrit and hemoglobin values 12 , 18 :
For loop diuretics other than furosemide, we converted the dose to furosemide equivalent doses: 4 mg of torasemide and 30 mg of azosemide were both considered to be equivalent to 20 mg of furosemide. 19 , 20
Outcomes
The primary end point was postdischarge all‐cause death in a time‐to‐event analysis. The secondary end points were (1) the composite of all‐cause death and HF readmission, and (2) HF readmission in a time‐to‐event analysis. The statuses of all the patients were surveyed, and the information on outcomes was obtained from the patients’ medical records and the participating cardiologists. When this information was unavailable in the medical records, the clinicians sent letters to the patients’ homes or telephoned the patients or their families to request the data.
Statistical Analysis
The data were expressed as means and SDs for the normally distributed data, and as medians with interquartile ranges for the non‐normally distributed data. The Kolmogorov–Smirnov test was performed to assess for normality. The categorical data were expressed as numbers and percentages. The differences between the 4 groups were tested using the ANOVA for the normally distributed variables and the Kruskal–Wallis test for the non‐normally distributed variables. The Chi‐square test was used to compare categorical variables.
To evaluate the association between the combined assessment of the FEUN and ePVS values at discharge and outcomes, Kaplan–Meier analyses with log‐rank tests, and univariate and multivariate Cox proportional hazard analyses were performed between groups. We selected the FEUN value (35%) that is used commonly as an indicator of prerenal failure in patients with acute kidney injury, 8 and the ePVS value (5.5%) that is used commonly as an indicator of excessive congestion in patients with ADHF. 12 In the multivariate analysis, the following variables were selected as pre‐existing and known prognostic factors for HF: age, sex, the New York Heart Association functional classification, diabetes, BNP, left ventricular ejection fraction at discharge, BUN, creatinine, serum sodium, systolic blood pressure at discharge, angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, beta‐blockers, and aldosterone antagonists. 11 In addition to the Cox proportional hazard analysis, a competing‐risk analysis using the Fine and Gray model was used to analyze the risk of HF readmission. Finally, subgroup analyses in Kaplan–Meier analyses with log‐rank tests for postdischarge all‐cause death were conducted by eGFR (<60 mL/min per 1.73 m2, ≥60 mL/min per 1.73 m2).
The results were reported as hazard ratios (HR), 95% CI, and P values. A value of P<0.05 was considered to be significant for the individual comparisons. All the statistical analyses were performed using the R software version 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patient Characteristics
The median age was 76 (67–83) years, and 55.8% of patients were men. Among them, the high‐FEUN/low‐ePVS, high‐FEUN/high‐ePVS, low‐FEUN/low‐ePVS, and low‐FEUN/high‐ePVS groups comprised 134 (28.8%), 108 (23.2%), 99 (21.2%), and 125 (26.8%) patients, respectively (Figure 2).
Figure 2. Flowchart of the study cohort.
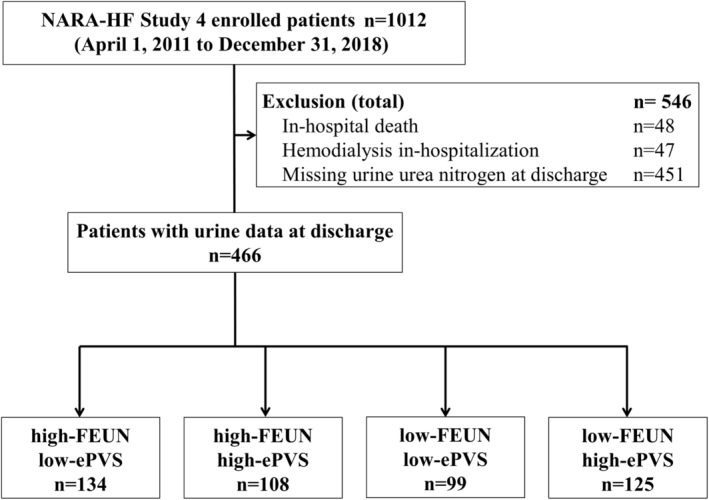
ePVS indicates estimated plasma volume status; FEUN, fractional excretion of urea nitrogen; and NARA‐HF Study 4, Nara Registry and Analyses for Heart Failure Study 4.
There were no significant differences in the sex, systolic blood pressure, heart rate levels, and the grade of New York Heart Association, at discharge among the 4 groups (Table 1). Age in the high‐FEUN/high‐ePVS and low‐FEUN/high‐ePVS groups was significantly higher compared with that of the high‐FEUN/low‐ePVS and low‐FEUN/low‐ePVS groups. The proportion of diabetes in the low‐FEUN/high‐ePVS group was significantly higher compared with that of the high‐FEUN/low‐ePVS group (Table 1).
Table 1.
Baseline Characteristics
| High‐FEUN low‐ePVS (n=134) | High‐FEUN high‐ePVS (n=108) | Low‐FEUN low‐ePVS (n=99) | Low‐FEUN high‐ePVS (n=125) | P value | |
|---|---|---|---|---|---|
| Age, y | 73 (61–81) | 78 (71–84) | 73 (64–80) | 80 (71–85) |
<0.05 ‖ |
| Men, % | 85 (63.4) | 54 (50.0) | 59 (59.6) | 62 (49.6) | 0.067 |
| BMI, kg/m2 | 21.8 (19.2–24.3) | 20.1 (17.8–22.5) | 21.6 (19.0–24.0) | 20.4 (17.5–22.8) | <0.05† , § |
| SBP, mm Hg | 108 (98–120) | 110 (99–126) | 109 (100–120) | 108 (96–119) | 0.431 |
| DBP, mm Hg | 63 (58–70) | 60 (50–67) | 61 (56–68) | 58 (50–64) |
<0.001 § |
| HR, beats/min | 70 (64–78) | 71 (61–78) | 70 (60–83) | 71 (64–80) | 0.856 |
| NYHA at discharge, % | 0.339 | ||||
| 1 | 49 (36.6) | 32 (29.6) | 42 (54.5) | 30 (24.0) | |
| 2 | 83 (61.9) | 72 (66.7) | 54 (54.5) | 88 (70.4) | |
| 3 | 2 (1.5) | 4 (3.7) | 2 (2.0) | 7 (5.6) | |
| 4 | 0 (0) | 0 (0) | 1 (1.0) | 0 (0) | |
| Medical history, % | |||||
| Hypertension | 93 (69.4) | 87 (80.6) | 73 (73.7) | 88 (70.4) | 0.215 |
| Dyslipidemia | 55 (41.0) | 52 (48.1) | 33 (33.3) | 60 (48.0) | 0.093 |
| Diabetes | 37 (27.6) | 47 (43.5) | 38 (38.4) | 62 (49.6) | <0.01§ |
| Cerebrovascular disease | 24 (17.9) | 23 (21.3) | 8 (8.1) | 19 (15.2) | 0.062 |
| CKD | 91 (67.9) | 87 (80.6) | 72 (72.7) | 105 (84.0) | 0.025§ |
| COPD | 22 (16.4) | 19 (17.6) | 6 (6.1) | 14 (11.2) | 0.048‖ |
| Current or ex‐smoker | 90 (67.2) | 55 (50.9) | 63 (63.6) | 69 (55.2) | 0.042† |
| Atrial fibrillation | 60 (44.8) | 40 (37.0) | 45 (45.5) | 59 (47.2) | 0.431 |
| Myocardial infarction | 34 (25.4) | 24 (22.2) | 13 (13.1) | 35 (28.0) | 0.052 |
| Medication at discharge, % | |||||
| ACE‐I or ARB | 124 (92.5) | 87 (80.6) | 92 (92.9) | 110 (88.0) | 0.012† , ‖ |
| Beta blocker | 111 (82.8) | 75 (69.4) | 86 (86.9) | 94 (75.2) | 0.009‖ |
| Aldosterone antagonist | 72 (53.7) | 44 (40.7) | 55 (55.6) | 52 (41.6) | 0.040‖ |
| Statin | 53 (39.6) | 48 (44.4) | 39 (39.4) | 51 (40.8) | 0.863 |
| Diuretic | 107 (79.9) | 80 (74.1) | 81 (81.8) | 104 (83.2) | 0.351 |
| Loop diuretic | 104 (77.6) | 78 (72.2) | 77 (77.8) | 97 (77.6) | 0.726 |
| Loop diuretic dose, mg | 22.5±17.8 | 22.7±18.1 | 24.0±18.1 | 32.0±27.8 |
<0.05 # |
| Tolvaptan | 8 (6.0) | 12 (11.1) | 4 (4.0) | 16 (12.8) | 0.057 |
| Nondrug therapy | |||||
| Pacemaker | 7 (5.2) | 10 (9.3) | 3 (3.0) | 8 (6.4) | 0.302 |
| ICD | 3 (2.2) | 3 (2.8) | 4 (4.0) | 2 (1.6) | 0.711 |
| CRT | 1 (0.7) | 2 (1.9) | 0 (0) | 4 (3.2) | 0.228 |
| Laboratory data | |||||
| Hemoglobin, g/dL | 13.3 (12.4–14.5) | 10.3 (9.6–11.0) | 13.3 (12.4–14.4) | 10.3 (9.7–11.0) | <0.001† , § , ‖ , # |
| Hematocrit, % | 41.1 (38.0–44.5) | 32.4 (30.1–34.0) | 40.5 (38.3–44.5) | 32.2 (29.6–33.9) | <0.001† , § , ‖ , # |
| ePVS, mL/g | 4.4 (3.8–5.0) | 6.5 (6.0–7.3) | 4.5 (3.9–4.9) | 6.6 (6.0–7.3) | <0.001† , § , ‖ , # |
| Albumin, g/dL | 3.8 (3.7–4.0) | 3.4 (3.2–3.8) | 3.9 (3.6–4.2) | 3.6 (3.3–3.8) | <0.001† , § , ‖ , # |
| BUN, mg/dL | 19.5 (15.0–25.0) | 24.0 (16.0–35.0) | 26.0 (20.5–36.5) | 39.0 (24.0–52.0) |
<0.05 † |
| Creatinine, mg/dL | 1.02 (0.82–1.30) | 1.21 (0.86–1.80) | 1.05 (0.82–1.36) | 1.42 (1.01–1.93) |
<0.05 † |
| BUN/creatinine | 18.4 (15.7–23.2) | 17.6 (13.7–22.6) | 26.0 (22.3–31.7) | 25.5 (21.4–32.7) | <0.001‡ , § , ‖ , ¶ |
| eGFR, mL/min per 1.73 m2 | 51.3 (38.6–62.8) | 38.9 (23.9–56.1) | 48.9 (36.0–62.4) | 34.5 (23.5–48.5) |
<0.01 d |
| Cystatin C, mg/L | 1.33 (1.07–1.72) | 1.80 (1.37–2.54) | 1.47 (1.18–1.85) | 2.08 (1.49–2.84) |
<0.01 ‖ |
| Serum sodium, mEq/L | 139 (137–141) | 139 (136–141) | 139 (136–141) | 138 (135–140) | 0.136 |
| Serum potassium, mEq/L | 4.2 (3.9–4.6) | 4.3 (4.0–4.6) | 4.2 (3.8–4.7) | 4.3 (4.0–4.6) | 0.702 |
| BNP, pg/mL | 259 (135–438) | 328 (161–577) | 203 (124–380) | 242 (129–529) | <0.05‖ |
| Renin, ng/ml per h | 3.8 (1.4–9.5) | 1.9 (0.9–6.7) | 5.9 (2.4–15.5) | 5.4 (1.5–13.8) |
<0.001 ‖ <0.01 ¶ |
| Aldosterone, pg/mL | 136.3 (88.9–200.3) | 81.6 (61.6–133.8) | 109.3 (84.3–162.5) | 87.9 (63.2–123.9) | |
| AVP, pg/mL* | 2.3 (1.4–3.2) | 1.9 (1.1–3.6) | 4.0 (2.3–5.8) | 2.4 (1.4–4.1) |
<0.01 # |
| Urine sodium, mEq/L | 67 (50–81) | 65 (44–83) | 72 (54–90) | 67 (49–81) | 0.143 |
| Urine potassium, mEq/L | 23 (17–28) | 15 (12–22) | 24 (20–33) | 18 (14–27) |
<0.01 ¶ |
| FENa, % | 0.72 (0.53–1.10) | 1.26 (0.85–1.92) | 0.63 (0.42–1.20) | 1.14 (0.67–2.07) | <0.001† , § , ‖ , # |
| FEUN, % | 42.0 (37.8–46.9) | 42.1 (38.4–45.9) | 29.0 (25.7–32.6) | 28.2 (23.9–31.6) | <0.001‡, § , ‖ , ¶ |
P value refers to comparisons of the means, median, or proportions among the groups by the 1‐way ANOVA, Kruskal–Wallis, and Pearson Chi‐square tests with Bonferroni post hoc analysis. ACE indicates angiotensin‐converting enzyme; ARB, angiotensin II receptor blocker; AVP, arginine vasopressin; BMI, body mass index; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; ePVS, estimated plasma volume status; FENa, fractional excretion of sodium; FEUN, fractional excretion of urea nitrogen; HR, heart rate; ICD, implantable cardioverter defibrillator; NYHA, New York Heart Association; and SBP, systolic blood pressure.
Values are n (%) or median [interquartile range]. The body mass index is the weight in kilograms divided by the square of the height in meters.
Data on arginine vasopressin were available for 246 patients (high‐FEUN/low‐ePVS: 116 patients, high‐FEUN/high‐ePVS: 75 patients, low‐FEUN/low‐ePVS: 71 patients, low‐FEUN/high‐ePVS: 81 patients).
High‐FEUN/low‐ePVS vs high‐FEUN/high‐ePVS.
High‐FEUN/low‐ePVS vs low‐FEUN/low‐ePVS.
High‐FEUN/low‐ePVS vs low‐FEUN/high‐ePVS.
High‐FEUN/high‐ePVS vs low‐FEUN/low‐ePVS.
High‐FEUN/high‐ePVS vs low‐FEUN/high‐ePVS.
Low‐FEUN/low‐ePVS vs low‐FEUN/high‐ePVS.
The proportion of patients treated with angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers in the high‐FEUN/low‐ePVS and low‐FEUN/low‐ePVS groups was significantly higher compared with that of the high‐FEUN/high‐ePVS group. The proportion of patients treated with beta‐blockers and aldosterone antagonist in the low‐FEUN/low‐ePVS group was significantly higher compared with that of the high‐FEUN/high‐ePVS group. The dose of loop diuretic in the low‐FEUN/high‐ePVS group was significantly higher compared with that of the other 3 groups (Table 1).
With regard to the laboratory parameters, hemoglobin, hematocrit, and albumin in the high‐FEUN/low‐ePVS and low‐FEUN/low‐ePVS groups were significantly higher compared with those of the high‐FEUN/high‐ePVS and low‐FEUN/high‐ePVS groups. BUN in the low‐FEUN/high‐ePVS group was significantly higher compared with that of the other 3 groups. Creatinine in the high‐FEUN/high‐ePVS and low‐FEUN/high‐ePVS groups were significantly higher compared with that of the high‐FEUN/low‐ePVS group. BUN/creatinine ratio in the low‐FEUN/low‐ePVS and low‐FEUN/high‐ePVS groups was significantly higher compared with that of the high‐FEUN/low‐ePVS and high‐FEUN/high‐ePVS groups. Cystatin C in the high‐FEUN/low‐ePVS and low‐FEUN/low‐ePVS groups were significantly lower compared with those of the high‐FEUN/high‐ePVS and low‐FEUN/high‐ePVS groups. BNP in the high‐FEUN/high‐ePVS group was significantly higher compared with that of the low‐FEUN/low‐ePVS group (Table 1).
In the echocardiographic parameters, the levels of the left ventricular end‐diastolic diameter and left ventricular end‐systolic diameter in the high‐FEUN/low‐ePVS group were significantly higher compared with those of the high‐FEUN/high‐ePVS and low‐FEUN/high‐ePVS groups. The levels of the left ventricular end‐diastolic volume and left ventricular end‐systolic volume in the high‐FEUN/low‐ePVS group were significantly higher compared with those of the other 3 groups. The levels of transtricuspid pressure gradient, early mitral inflow velocity to early diastolic mitral annular velocity (E/e′), and systolic pulmonary artery pressure in the high‐FEUN/high‐ePVS and low‐FEUN/high‐ePVS groups were significantly higher compared with those of the low‐FEUN/low‐ePVS group. The levels of left ventricular ejection fraction in the high‐FEUN/high‐ePVS and low‐FEUN/high‐ePVS groups was significantly higher compared with that of the high‐FEUN/low‐ePVS and low‐FEUN/low‐ePVS groups. The levels of stroke volume, cardiac output, and cardiac index in the high‐FEUN/low‐ePVS and high‐FEUN/high‐ePVS groups were significantly higher compared with those of the low‐FEUN/low‐ePVS and low‐FEUN/high‐ePVS groups (Table 2).
Table 2.
Echocardiographic Data at Discharge in the 4 Groups
| High‐FEUN low‐ePVS (n=134) | High‐FEUN high‐ePVS (n=108) | Low‐FEUN low‐ePVS (n=99) | Low‐FEUN high‐ePVS (n=125) | P value | |
|---|---|---|---|---|---|
| IVS, mm | 10 (9–12) | 11 (9–12) | 10 (9–12) | 10 (9–12) | 0.848 |
| LVPW, mm | 10 (9–11) | 10 (9–11) | 10 (9–12) | 10 (9–12) | 0.635 |
| AOD, mm | 32 (29–34) | 31 (29–33) | 31 (29–34) | 31 (29–34) | 0.635 |
| LAD, mm | 44 (38–48) | 43 (40–46) | 42 (38–47) | 44 (40–50) | 0.102 |
| LVEDD, mm | 54 (48–62) | 50 (45–56) | 52 (47–59) | 51 (45–57) |
<0.01* <0.05 ‡ |
| LVESD, mm | 44 (34–53) | 36 (30–44) | 41 (34–48) | 36 (29–46) |
<0.001* <0.01 ‡ <0.05 § |
| LVEDV, mL | 140 (102–191) | 122 (97–146) | 104 (83–136) | 104 (70–137) | |
| LVEDVi, mL/m2 | 96.7 (73.1–116.0) | 84.5 (68.8–106.9) | 69.2 (55.8–90.2) | 72.3 (51.5–96.4) | |
| LVESV, mL | 83 (47–125) | 55 (35–88) | 60 (40–95) | 52 (29–82) |
<0.001 ‡ <0.01* <0.05 † |
| LVESVi, mL/m2 | 55.3 (32.3–77.7) | 39.3 (23.6–61.8) | 40.3 (28.1–59.6) | 34.5 (20.6–57.5) |
<0.001 ‡ |
| Valvular regurgitation | |||||
| MR severity grade | 2.0±1.1 | 1.9±1.0 | 1.9±1.0 | 2.1±1.0 | 0.470 |
| AR, severity grade | 1.0±1.1 | 1.1±1.0 | 0.9±1.0 | 1.1±1.1 | 0.449 |
| TR, severity grade | 1.6±0.8 | 1.8±0.9 | 1.6±0.9 | 1.9±1.0 | <0.05¶ |
| TRPG, mm Hg | 22 (16–30) | 27 (21–33) | 22 (17–29) | 27 (21–36) |
<0.001 ¶ |
| E/A | 0.90 (0.66–1.37) | 0.73 (0.60–1.20) | 0.80 (0.60–1.10) | 0.80 (0.66–1.20) | 0.348 |
| E/e′ | 15.4 (12.5–19.0) | 18.8 (12.7–24.6) | 15.2 (11.5–20.0) | 17.8 (13.5–22.7) | <0.05§ , ¶ |
| e′ | 4.6 (3.6–5.7) | 4.2 (3.2–5.4) | 4.6 (3.5–6.0) | 4.1 (3.3–5.3) | 0.205 |
| DCT, ms | 207 (163–248) | 224 (180–263) | 199 (175–236) | 219 (174–262) | 0.117 |
| LVEF, % | 39 (29–52) | 53 (39–64) | 41 (32–50) | 48 (36–64) |
<0.001* <0.05 ¶ |
| Stroke volume, ml | 57 (45–70) | 60 (49–72) | 42 (33–51) | 47 (36–61) |
<0.01 ‡ |
| SVI, mL/m2 | 36.6 (29.5–43.9) | 41.2 (35.2–48.5) | 27.9 (22.7–32.0) | 32.0 (25.6–43.3) | |
| Heart rate at echocardiography | 77 (66–90) | 71 (63–79) | 65 (56–72) | 62 (55–70) | |
| Cardiac output, L/min | 4.33 (3.40–5.21) | 4.08 (3.32–4.96) | 2.60 (2.12–3.29) | 2.85 (2.13–3.99) | <0.001†,‡,§,‖ |
| Cardiac index, L/min/m2 | 2.73 (2.17–3.57) | 2.84 (2.40–3.35) | 1.73 (1.41–2.17) | 1.99 (1.54–2.65) | <0.001†,‡,§,‖ |
| RVDd, mm | 35 (32–40) | 34 (32–37) | 34 (31–38) | 33 (29–37) | 0.059 |
| Maximum IVC diameter, mm | 14.9±4.1 | 15.1±4.6 | 14.4±4.2 | 15.5±5.6 | 0.400 |
| IVC collapsibility index | 0.46±0.11 | 0.48±0.13 | 0.46±0.14 | 0.49±0.13 | 0.282 |
| sPAP, mm Hg | 27 (21–34)) | 32 (25–39) | 27 (20–33) | 33 (25–42) |
<0.001 <0.01 ‡ <0.05 § |
P value refers to comparisons of the means or median among the groups by 1‐way ANOVA and Kruskal–Wallis tests with Bonferroni post hoc analysis. AOD, aorta diameter; AR, aortic regurgitation; DCT, deceleration time; e`, early diastolic mitral annular velocity; E/A, early mitral inflow velocity; E/e`, early mitral inflow velocity to early diastolic mitral annular velocity; IVC, inferior vena cava; IVS, interventricular septum; LAD, left atrial diameter; LVEDD, left ventricular end‐diastolic diameter; LVEDV, left ventricular end‐diastolic volume; LVEDVi, left ventricular end‐diastolic volume index; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic diameter; LVESV, left ventricular end‐systolic volume; LVESVi, left ventricular end‐systolic volume index; LVPW, left ventricular posterior wall; MR, mitral regurgitation; RVDd, right ventricular diastolic diameter; sPAP, systolic pulmonary artery pressure; SVI, stroke volume index; TR, tricuspid regurgitation; and TRPG, tricuspid regurgitation pressure gradient.
High‐FEUN/low‐ePVS vs high‐FEUN/high‐ePVS.
High‐FEUN/low‐ePVS vs low‐FEUN/low‐ePVS.
High‐FEUN/low‐ePVS vs low‐FEUN/high‐ePVS.
High‐FEUN/high‐ePVS vs low‐FEUN/low‐ePVS.
High‐FEUN/high‐ePVS vs low‐FEUN/high‐ePVS.
Low‐FEUN/low‐ePVS vs low‐FEUN/high‐ePVS.
Clinical Outcomes
During a median follow‐up period of 28.1 months, there were 173 all‐cause deaths (37.1%), 83 cardiovascular deaths (17.8%), and 121 HF readmissions (26.0%). The incidences of both all‐cause deaths and HF readmissions were the highest in the low‐FEUN/high‐ePVS group (58.4% [n=73] and 34.4% [n=43], respectively) and the lowest in the high‐FEUN/low‐ePVS group (17.1% [n=23] and 18.7% [n=25], respectively) (Table 3).
Table 3.
Incidence of All‐Cause Death and HF Readmission After Discharge in the 4 Groups
| High‐FEUN low‐ePVS (n=134) | High‐FEUN high‐ePVS (n=108) | Low‐FEUN low‐ePVS (n=99) | Low‐FEUN high‐ePVS (n=125) | P value | |
|---|---|---|---|---|---|
| All‐cause death, % | 23 (17.1) | 47 (43.6) | 30 (30.3) | 73 (58.4) | <0.001 * , † , § |
| Cardiovascular death, % | 11 (8.2) | 30 (27.8) | 14 (14.1) | 28 (22.4) |
<0.001 * <0.05 † |
| Infection, % | 2 (1.5) | 8 (7.4) | 3 (3.0) | 14 (11.2) | <0.05† |
| Malignancy, % | 3 (2.2) | 6 (5.6) | 5 (5.1) | 10 (8.0) | 0.196 |
| Others, % | 7 (5.2) | 3 (2.8) | 8 (8.1) | 21 (16.8) |
<0.01 ‡ <0.05 † |
| HF readmission, % | 25 (18.7) | 32 (29.6) | 21 (21.2) | 43 (34.4) | <0.05† |
P value refers to comparisons of the proportions among the groups by the Pearson Chi‐square tests with Bonferroni post hoc analysis. e`, early diastolic mitral annular velocity; E/A, early mitral inflow velocity; E/e`, early mitral inflow velocity to early diastolic mitral annular velocity; ePVS indicates estimated plasma volume status; FEUN, fractional excretion of urea nitrogen; and HF, heart failure.
High‐FEUN/low‐ePVS vs high‐FEUN/high‐ePVS.
High‐FEUN/low‐ePVS vs low‐FEUN/high‐ePVS.
High‐FEUN/high‐ePVS vs low‐FEUN/high‐ePVS.
Low‐FEUN/low‐ePVS vs low‐FEUN/high‐ePVS.
The Kaplan–Meier curve analyses showed that the low‐FEUN/high‐ePVS group had much higher rates of all‐cause death (log‐rank test, P<0.001) and composite end points (log‐rank test, P<0.001) than the high‐FEUN/low‐ePVS and low‐FEUN/low‐ePVS groups, and HF readmissions (log‐rank test, P=0.003) than the high‐FEUN/low‐ePVS group overall (Figure 3A through 3C). In patients with eGFR<60 mL/min per 1.73 m2, the Kaplan–Meier curve analyses showed that the low‐FEUN/high‐ePVS group had much higher rates of all‐cause death than the other 3 groups (log‐rank test with Bonferroni post hoc analysis, versus the high‐FEUN/low‐ePVS group: P<0.001, versus the low‐FEUN/low‐ePVS group: P=0.002, versus the high‐FEUN/high‐ePVS group: P=0.032) (Figure S1–S2A). In patients with eGFR ≥60 mL/min per 1.73 m2, the Kaplan–Meier curve analyses showed that there were no significant differences in the all‐cause death between the low‐FEUN/high‐ePVS group and the other 3 groups (Figure S1–S2B).
Figure 3. Kaplan–Meier analyses of the 4 hemodynamic profiles based on the combined assessment of FEUN and ePVS values at discharge for postdischarge all‐cause mortality, composite end point, and heart failure readmission.
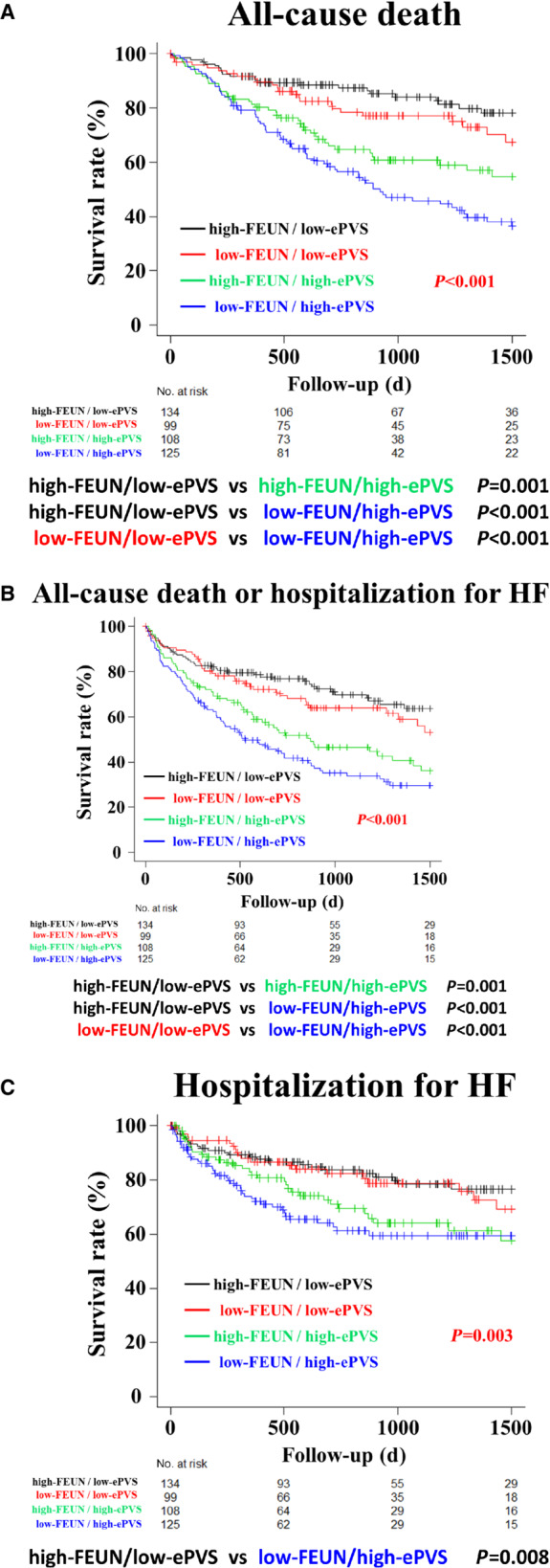
Kaplan–Meier curve analyses showed that the low‐FEUN/high‐ePVS group had much higher rates of all‐cause death (log‐rank test, P<0.001) and composite end points (log‐rank test, P<0.001) than the high‐FEUN/low‐ePVS and low‐FEUN/low‐ePVS groups, and HF readmissions (log‐rank test, P=0.003) than the high‐FEUN/low‐ePVS group in overall (A through C). ePVS indicates estimated plasma volume status; FEUN, fractional excretion of urea nitrogen; and HF, heart failure.
A competing‐risk analysis was performed to assess the effect of death as a competing risk, and a similar result was observed (Gray test, P=0.022) (Figure S2).
In the univariate Cox regression analyses, the low‐FEUN/low‐ePVS, high‐FEUN/high‐ePVS, and low‐FEUN/high‐ePVS groups were associated with a higher all‐cause mortality than the high‐FEUN/low‐ePVS group (Table 4). In the multivariable Cox regression models adjusted for established prognostic factors for ADHF (age, sex, New York Heart Association functional classification, diabetes, BNP, left ventricular ejection fraction, BUN, creatinine, serum sodium, systolic blood pressure at discharge, angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, beta‐blockers, and aldosterone antagonists at discharge), the low‐FEUN/low‐ePVS, high‐FEUN/high‐ePVS, and low‐FEUN/high‐ePVS groups were associated with a higher all‐cause mortality than the high‐FEUN/low‐ePVS group (HR, 1.89 [95% CI, 1.07–3.33; P=0.029]; HR, 2.51 [95% CI, 1.46–4.26; P<0.001]; HR, 2.92 [95% CI, 1.73–4.92; P<0.001], respectively) (Table 4).
Table 4.
Independent Predictors of All‐Cause Death, All‐Cause Death or HF Readmission, and HF Readmission After Discharge
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95%CI) | P value | HR (95%CI) | P value | |
| All‐cause death | ||||
| High‐FEUN/low‐ePVS | 1 (reference) | 1 (reference) | ||
| Low‐FEUN/low‐ePVS | 1.88 (1.09–3.24) | 0.023 | 1.89 (1.07–3.33) | 0.029 |
| High‐FEUN/high‐ePVS | 3.00 (1.82–4.94) | <0.001 | 2.51 (1.46–4.26) | <0.001 |
| Low‐FEUN/high‐ePVS | 4.16 (2.60–6.65) | <0.001 | 2.92 (1.73–4.92) | <0.001 |
| All‐cause death or HF readmission | ||||
| High‐FEUN/low‐ePVS | 1 (reference) | 1 (reference) | ||
| Low‐FEUN/low‐ePVS | 1.40 (0.90–2.18) | 0.132 | 1.47 (0.93–2.33) | 0.102 |
| High‐FEUN/high‐ePVS | 2.22 (1.49–3.31) | <0.001 | 1.92 (1.25–2.94) | 0.003 |
| Low‐FEUN/high‐ePVS | 2.94 (2.02–4.29) | <0.001 | 2.15 (1.41–3.28) | <0.001 |
| HF readmission | ||||
| High‐FEUN/low‐ePVS | 1 (reference) | 1 (reference) | ||
| Low‐FEUN/low‐ePVS | 1.21 (0.67–2.15) | 0.527 | 1.29 (0.70–2.36) | 0.410 |
| High‐FEUN/high‐ePVS | 1.85 (1.10–3.13) | 0.021 | 1.58 (0.90–2.77) | 0.112 |
| Low‐FEUN/high‐ePVS | 2.34 (1.43–3.83) | <0.001 | 1.79 (1.02–3.14) | 0.043 |
Univariate and multivariate Cox proportional hazard analyses were performed among groups adjusted for age, sex, the New York Heart Association functional classification, diabetes, BNP, left ventricular ejection fraction at discharge, BUN, creatinine, serum sodium, systolic blood pressure at discharge, angiotensin‐converting enzyme inhibitor or angiotensin receptor blockers, beta‐blockers, and aldosterone antagonist. BNP indicates brain natriuretic peptide; BUN, blood urea nitrogen; ePVS, estimated plasma volume status; FEUN, fractional excretion of urea nitrogen; HF, heart failure; HR, hazard ratio; and SBP, systolic blood pressure.
Similarly, the low‐FEUN/high‐ePVS group was associated with the composite outcome and HF readmissions (Table 4).
DISCUSSION
This study investigated the effect of the 4 hemodynamic categories according to the high and low FEUN and ePVS values at discharge and long‐term prognosis in patients with ADHF. The main findings of the present study were that (1) the all‐cause mortality was lower in the high‐FEUN/low‐ePVS group than in any other groups, and (2) low‐FEUN/high‐ePVS group was associated independently with higher HF readmissions than the high‐FEUN/low‐ePVS group. To the best of our knowledge, this was the first report that investigated the use of the combined assessment of the FEUN and ePVS values at discharge to predict the long‐term prognosis of patients with ADHF. These findings may be applied to clinical practice.
In patients with ADHF, it has been shown that persistent congestion before discharge is associated with a higher risk of HF readmission and mortality. 21 Furthermore, low cardiac output attributable to primary cardiac dysfunction has also been shown to lead to a life‐threatening condition of tissue hypoperfusion, which can lead to multiple organ failure and death. 22 Previous studies have shown that a classification based on congestion and perfusion status provides clinically relevant information for targeted strategies that may improve the outcomes. 21 , 23
The hemodynamic classification in HF was proposed originally by Forrester and Waters 24 , 25 and then adapted clinically by Nohria et al. 26 The Forrester classification is easy for everyone to understand because it is based on objective values, such as pulmonary artery wedge pressure and cardiac index measured using the Swan‐Ganz catheter; however, it has the disadvantage of requiring invasive procedures, whereas the Nohria–Stevenson classification uses the 4 hemodynamic profiles according to physical assessments and has the advantage that it can be assessed easily at the bedside for all patients. However, different clinicians may vary in their physical assessments. In addition, it is difficult to assess mild congestion or hypoperfusion that is not clinically apparent; therefore, it is used mainly in the worsening phase of HF and may not be suitable for use in the predischarge assessment. 26 , 27
In the present study, we focused on the ePVS that is associated with congestion 12 and the FEUN that is associated with renal perfusion in patients with ADHF. 8 , 11 These indices are minimally invasive and do not vary in interpretation among clinicians because they are objective results that are measured using blood or urine tests. However, no studies have evaluated the association between the combined assessment of the FEUN and ePVS values at discharge and the long‐term prognosis in patients with ADHF. In the present study, the BNP level, transtricuspid pressure gradient, and E/e′ were significantly higher in the high‐ePVS group than in the low‐ePVS group, and the cardiac index was significantly lower in the low‐FEUN group than in the high‐FEUN group. In short, these findings suggested that a high‐ePVS value may represent an excessive congestive status, and a low‐FEUN value may represent a hypoperfusion status. Therefore, in the present study, we categorized 4 hemodynamic profiles as follows: (1) high‐FEUN/low‐ePVS; (2) high‐FEUN/high‐ePVS; (3) low‐FEUN/low‐ePVS; and (4) low‐FEUN/high‐ePVS (Figure 1).
The Kaplan–Meier curves and the adjusted Cox proportional hazards regression model showed that the lowest rates of all‐cause death were observed in the patients classified as having “high‐FEUN/low‐ePVS”. Based on this result, it is important to transfer the patients from the other 3 groups to the high‐FEUN/low‐ePVS group. Therefore, it is necessary to understand the status of each group correctly before considering treatment methods. In the present study, the BUN/creatinine ratio, plasma renin activity, and vasopressin levels as the indicators of hypoperfusion were significantly higher and the cardiac index was significantly lower in the low‐FEUN/low‐ePVS group compared with the high‐FEUN/low‐ePVS group. This suggested that the low‐FEUN/low‐ePVS profile may indicate hypoperfusion because of excessive dehydration. Therefore, in the low‐FEUN/low‐ePVS group, it may be necessary to reduce the use of diuretics to transfer the patients to the high‐FEUN/low‐ePVS profile. Moreover, the transtricuspid pressure gradient, E/e′, and systolic pulmonary artery pressure as indicators of congestion were significantly higher in the high‐FEUN/high‐ePVS group compared with the high‐FEUN/low‐ePVS group. This suggested that the high‐FEUN/high‐ePVS profile may indicate residual clinical congestion. Therefore, in the high‐FEUN/high‐ePVS group, we should remove residual congestion more aggressively to transfer the patients to the high‐FEUN/low‐ePVS profile. Furthermore, in the present study, the BUN/creatinine ratio, transtricuspid pressure gradient, E/e′, and systolic pulmonary artery pressure values were significantly higher, and the cardiac index was significantly lower in the low‐FEUN/high‐ePVS group compared with the high‐FEUN/low‐ePVS group. These findings suggested that the low‐FEUN/high‐ePVS profile may represent a combination of hypoperfusion and congestion, that is, the state of Forester IV group. To improve the outcomes of patients who belong to this profile, intensive treatment such as medical therapies that include not only cardioprotective and vasoactive agents, but also invasive procedures such as mechanical circulatory support, may be necessary.
Subgroup analyses of all‐cause death showed that the low‐FEUN/high‐ePVS group had much higher rates of all‐cause death than the high‐FEUN/high‐ePVS group in patients with eGFR <60 mL/min per 1.73 m2, but not in patients with eGFR ≥60 mL/min per 1.73 m2. These results were similar to the previous study showing that FEUN was more useful in patients with eGFR <60 mL/min per 1.73 m2. 11 However, given the small number of patients with eGFR ≥60 mL/min per 1.73 m2 in the present study, it is not yet clear whether the differences of the renal function affect the combined assessment of the FEUN and ePVS values. Therefore, generalizing our results to all patients with ADHF might be limited.
The hemodynamic classification using the combined assessment of the FEUN and ePVS values at discharge will lead to an understanding of the status of the patients with HF, before treatment methods are considered. However, the impact of the 4 hemodynamic categories according to the combined assessment of the FEUN and ePVS values should be further evaluated in prospective studies.
This study had several potential limitations that should be acknowledged. First, this was a single‐center study with a relatively small number of patients with ADHF and it has not been validated in an independent cohort. Second, we excluded a large number of patients with missing data on FEUN and ePVS values, patients who died in‐hospital because we targeted postdischarge prognosis, and patients on hemodialysis because we used urinary data. These aspects of the study could be both selection and sampling bias. Third, we could not directly evaluate the association between the combined assessment of the FEUN and ePVS values at discharge and the invasive hemodynamic measurements, because we did not routinely perform right heart catheterization using the Swan‐Ganz catheter at discharge. Finally, in the present study, we only included stable patients with ADHF at discharge because both FEUN and ePVS are clearly dynamic through the course of cardiorenal illness. Therefore, we need to be careful in our interpretation if the combined assessment of the FEUN and ePVS values is used in the unstable general condition, such as immediately after HF hospitalization. This new classification might be more appropriate for predischarged patients with eGFR <60 mL/min per 1.73 m2 than those with preserved GFR.
CONCLUSIONS
Classifying patients with ADHF according to the combined assessment of the FEUN and ePVS values at discharge enabled the identification of significant differences in all‐cause mortality and HF readmissions among the profiles. The new classification of the 4 hemodynamic profiles using the FEUN and ePVS values may play an important role in the implementation of targeted strategies to improve outcomes.
Sources of Funding
None.
Disclosures
Saito Y. has received: research funds from Otsuka Pharmaceutical Co., Ltd., OnoPharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Bristol‐Myers Squibb Company, Actelion Pharmaceuticals Japan Ltd., Kyowa Kirin Co., Ltd., Kowa Pharmaceutical Co., Ltd, Shionogi & Co., Ltd, Dainippon Sumitomo Pharma Co., Ltd., Teijin Pharma Ltd., Chugai Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Nihon Medi‐Physics Co., Ltd., Novartis Pharma K.K., Pfizer Japan Inc., and Fuji Yakuhin Co., Ltd.; research expenses from Novartis Pharma K.K., Roche Diagnostics K.K., Amgen Inc., Bayer Yakuhin, Ltd., Astellas Pharma Inc., and Actelion Pharmaceuticals Japan Ltd.; speakers' bureau/honorarium from Alnylam Japan K.K., AstraZeneca K.K., Otsuka Pharmaceutical Co., Ltd., Kowa Pharmaceutical Co., Ltd, Daiichi Sankyo Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Tsumura & Co., Teijin Pharma Ltd., Toa Eiyo Ltd., Nippon Shinyaku Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K.K., Bayer Yakuhin Ltd., Pfizer Japan Inc., Bristol‐Myers Squibb Company, and Mochida Pharmaceutical Co., Ltd.; and consultation fees from Ono Pharmatical Co., Ltd. and Novartis Pharma K.K. The other authors have no financial conflicts of interest to disclose.
Supporting information
Figures S1–S2
Acknowledgments
This work was supported in part by MEXT KAKENHI Grant Number JP19155855 (Grants‐in‐aid from the Ministry of Education, Culture, Sports, Science), Health Labour Sciences Research Grant Number 19189094 and 17933459 (Technology and the Ministry of Health, Labor, and Welfare of Japan (Comprehensive Research on Life‐Style Related Disease including Cardiovascular Disease and Diabetes Mellitus)), and AMED under Grant Number JP19ek0210080, JP19ek0210118, JP19ek0210121, JP19ek0210115 (Practical Research Project for Life‐Style related Diseases including Cardiovascular Diseases and Diabetes Mellitus), AMED under Grant Number JP19ek0109367, JP19ek0109406 (Practical Research Project for Rare/Intractable Diseases) and AMED under Grant Number JP19km0405009 (Platform Program for Promotion of Genome Medicine).
See Editorial by Rao.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.025596
For Sources of Funding and Disclosures, see page 12.
REFERENCES
- 1. Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC, Traver B, Maggioni AP, Cook T, Swedberg K, Burnett JC Jr, et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J. 2013;34:835–843. doi: 10.1093/eurheartj/ehs444 [DOI] [PubMed] [Google Scholar]
- 2. Rubio‐Gracia J, Demissei BG, Ter Maaten JM, Cleland JG, O'Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Davison BA, et al. Prevalence, predictors and clinical outcome of residual congestion in acute decompensated heart failure. Int J Cardiol. 2018;258:185–191. doi: 10.1016/j.ijcard.2018.01.067 [DOI] [PubMed] [Google Scholar]
- 3. Forman DE, Butler J, Wang Y, Abraham WT, O'Connor CM, Gottlieb SS, Loh E, Massie BM, Rich MW, Stevenson LW, et al. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol. 2004;43:61–67. doi: 10.1016/j.jacc.2003.07.031 [DOI] [PubMed] [Google Scholar]
- 4. Damman K, Navis G, Voors AA, Asselbergs FW, Smilde TD, Cleland JG, van Veldhuisen DJ, Hillege HL. Worsening renal function and prognosis in heart failure: systematic review and meta‐analysis. J Card Fail. 2007;13:599–608. doi: 10.1016/j.cardfail.2007.04.008 [DOI] [PubMed] [Google Scholar]
- 5. Ueda T, Kawakami R, Sugawara Y, Okada S, Nishida T, Onoue K, Soeda T, Okayama S, Takeda Y, Watanabe M, et al. Worsening of renal function during 1 year after hospital discharge is a strong and independent predictor of all‐cause mortality in acute decompensated heart failure. J Am Heart Assoc. 2014;3:e001174. doi: 10.1161/JAHA.114.001174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H, Butler J, Celutkiene J, Chioncel O, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 7. Writing C, Maddox TM, Januzzi JL Jr, Allen LA, Breathett K, Butler J, Davis LL, Fonarow GC, Ibrahim NE, Lindenfeld J, et al. 2021 Update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the american college of cardiology solution set oversight committee. J Am Coll Cardiol. 2021;77:772–810. doi: 10.1016/j.jacc.2020.11.022 [DOI] [PubMed] [Google Scholar]
- 8. Carvounis CP, Nisar S, Guro‐Razuman S. Significance of the fractional excretion of urea in the differential diagnosis of acute renal failure. Kidney Int. 2002;62:2223–2229. doi: 10.1046/j.1523-1755.2002.00683.x [DOI] [PubMed] [Google Scholar]
- 9. Diskin CJ, Stokes TJ, Dansby LM, Radcliff L, Carter TB. The comparative benefits of the fractional excretion of urea and sodium in various azotemic oliguric states. Nephron Clin Pract. 2010;114:c145–c150. doi: 10.1159/000254387 [DOI] [PubMed] [Google Scholar]
- 10. A A Kaplan OFK. Fractional excretion of urea as a guide to renal dysfunction. Am J Nephrol. 1992;12:49–54. doi: 10.1159/000168417 [DOI] [PubMed] [Google Scholar]
- 11. Nogi K, Kawakami R, Ueda T, Nogi M, Ishihara S, Nakada Y, Hashimoto Y, Nakagawa H, Nishida T, Seno A, et al. Prognostic value of fractional excretion of urea nitrogen at discharge in acute decompensated heart failure. J Am Heart Assoc. 2021;10:e020480. doi: 10.1161/JAHA.120.020480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kobayashi M, Girerd N, Duarte K, Chouihed T, Chikamori T, Pitt B, Zannad F, Rossignol P. Estimated plasma volume status in heart failure: clinical implications and future directions. Clin Res Cardiol. 2021;110:1159–1172. doi: 10.1007/s00392-020-01794-8 [DOI] [PubMed] [Google Scholar]
- 13. Kobayashi M, Rossignol P, Ferreira JP, Aragao I, Paku Y, Iwasaki Y, Watanabe M, Fudim M, Duarte K, Zannad F, et al. Prognostic value of estimated plasma volume in acute heart failure in three cohort studies. Clin Res Cardiol. 2019;108:549–561. doi: 10.1007/s00392-018-1385-1 [DOI] [PubMed] [Google Scholar]
- 14. Huang CY, Lin TT, Wu YF, Chiang FT, Wu CK. Long‐term prognostic value of estimated plasma volume in heart failure with preserved ejection fraction. Sci Rep. 2019;9:14369. doi: 10.1038/s41598-019-50427-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ho KKL, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993;22:A6–A13. doi: 10.1016/0735-1097(93)90455-A [DOI] [PubMed] [Google Scholar]
- 16. Lima C, Macedo E. Urinary biochemistry in the diagnosis of acute kidney injury. Dis Markers. 2018;2018:4907024. doi: 10.1155/2018/4907024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pepin MN, Bouchard J, Legault L, Ethier J. Diagnostic performance of fractional excretion of urea and fractional excretion of sodium in the evaluations of patients with acute kidney injury with or without diuretic treatment. Am J Kidney Dis. 2007;50:566–573. doi: 10.1053/j.ajkd.2007.07.001 [DOI] [PubMed] [Google Scholar]
- 18. Duarte K, Monnez JM, Albuisson E, Pitt B, Zannad F, Rossignol P. Prognostic value of estimated plasma volume in heart failure. JACC Heart Fail. 2015;3:886–893. doi: 10.1016/j.jchf.2015.06.014 [DOI] [PubMed] [Google Scholar]
- 19. Kruck F, Bablok W, Besenfelder E, Betzien G, Kaufmann B. Clinical and pharmacological investigations of the new saluretic azosemid. Eur J Clin Pharmacol. 1978;14:153–161. doi: 10.1007/BF02089953 [DOI] [PubMed] [Google Scholar]
- 20. Diez J, Coca A, de Teresa E, Anguita M, Castro‐Beiras A, Conthe P, Cobo E, Fernandez E, Group TI . TORAFIC study protocol: torasemide prolonged release versus furosemide in patients with chronic heart failure. Expert Rev Cardiovasc Ther. 2009;7:897–904. doi: 10.1586/erc.09.74 [DOI] [PubMed] [Google Scholar]
- 21. Chioncel O, Mebazaa A, Harjola VP, Coats AJ, Piepoli MF, Crespo‐Leiro MG, Laroche C, Seferovic PM, Anker SD, Ferrari R, et al. Clinical phenotypes and outcome of patients hospitalized for acute heart failure: the ESC heart failure long‐term registry. Eur J Heart Fail. 2017;19:1242–1254. doi: 10.1002/ejhf.890 [DOI] [PubMed] [Google Scholar]
- 22. Chioncel O, Parissis J, Mebazaa A, Thiele H, Desch S, Bauersachs J, Harjola VP, Antohi EL, Arrigo M, Gal TB, et al. Epidemiology, pathophysiology and contemporary management of cardiogenic shock ‐ a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2020;22:1315–1341. doi: 10.1002/ejhf.1922 [DOI] [PubMed] [Google Scholar]
- 23. Chioncel O, Mebazaa A, Maggioni AP, Harjola VP, Rosano G, Laroche C, Piepoli MF, Crespo‐Leiro MG, Lainscak M, et al. Acute heart failure congestion and perfusion status ‐ impact of the clinical classification on in‐hospital and long‐term outcomes; insights from the ESC‐EORP‐HFA heart failure long‐term registry. Eur J Heart Fail. 2019;21:1338–1352. doi: 10.1002/ejhf.1492 [DOI] [PubMed] [Google Scholar]
- 24. Forrester JS, Diamond G, Chatterjee K, Swan HJ. Medical therapy of acute myocardial infarction by application of hemodynamic subsets (first of two parts). N Engl J Med. 1976;295:1356–1362. doi: 10.1056/NEJM197612092952406 [DOI] [PubMed] [Google Scholar]
- 25. Forrester JS, Diamond G, Chatterjee K, Swan HJ. Medical therapy of acute myocardial infarction by application of hemodynamic subsets (second of two parts). N Engl J Med. 1976;295:1404–1413. doi: 10.1056/NEJM197612162952505 [DOI] [PubMed] [Google Scholar]
- 26. Nohria A, Tsang SW, Fang JC, Lewis EF, Jarcho JA, Mudge GH, Stevenson LW. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol. 2003;41:1797–1804. doi: 10.1016/S0735-1097(03)00309-7 [DOI] [PubMed] [Google Scholar]
- 27. Binanay C, Califf RM, Hasselblad V, O'Connor CM, Shah MR, Sopko G, Stevenson LW, Francis GS, Leier CV, Miller LW. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294:1625–1633. doi: 10.1001/jama.294.13.1625 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S2


