Abstract
Background
Neutrophil‐to‐lymphocyte ratio (NLR) and platelet‐to‐lymphocyte ratio (PLR) are novel inflammation markers. Their combined usefulness for estimating the prognosis of patients with heart failure with preserved ejection fraction (HFpEF) admitted for acute decompensated heart failure remains elusive.
Methods and Results
We investigated 1026 patients registered in the Prospective Multicenter Observational Study of Patients with Heart Failure with Preserved Ejection Fraction. Both NLR and PLR values were measured at the time of admission. Comorbidity burden was defined as the number of occurrences of 8 common comorbidities of HFpEF. The primary end point was cardiac death. The patients were stratified into 3 groups based on the optimal cut‐off values of NLR and PLR on the receiver operating characteristic curve analysis for predicting cardiac death (low NLR and PLR, either high NLR or PLR, and both high NLR and PLR). After a median follow‐up of 429 days, 195 patients died, with 85 of these deaths attributed to cardiac causes. An increased comorbidity burden was significantly associated with a higher proportion of patients with high NLR (>4.5) or PLR (>193), or both. High NLR and PLR values were independently associated with cardiac death, and a combination of both values was the strongest predictor (hazard ratio, 2.66 [95% CI, 1.51–4.70], P=0.0008). A significant difference was found in the rate of cardiac death among the 3 groups stratified by NLR and PLR values.
Conclusions
The combination of NLR and PLR is useful for the prediction of postdischarge cardiac death in patients with acute HFpEF.
Registration
URL: ClinicalTrials.gov; Unique identifier: UMIN000021831.
Keywords: cardiac death, heart failure with preserved ejection fraction, inflammation, prognosis
Subject Categories: Heart Failure, Inflammation, Biomarkers
Nonstandard Abbreviations and Acronyms
- ADHF
acute decompensated heart failure
- E
early transmitral flow velocity
- e'
early diastolic septal mitral annular velocity
- HFpEF
heart failure with preserved ejection fraction
- NLR
neutrophil‐to‐lymphocyte ratio
- PLR
platelet‐to‐lymphocyte ratio
- PV
plasma volume
Clinical Perspective.
What Is New?
A higher comorbidity burden was associated with high neutrophil‐to‐lymphocyte ratio, high platelet‐to‐lymphocyte ratio, or both, possibly reflecting the systemic inflammation caused by comorbidities in patients with heart failure with preserved ejection fraction (HFpEF).
The combination of high neutrophil‐to‐lymphocyte ratio and platelet‐to‐lymphocyte ratio was useful for the identification of patients at risk of cardiac and all‐cause death in patients with HFpEF who were admitted with acute decompensated heart failure.
What Are the Clinical Implications?
Neutrophil‐to‐lymphocyte ratio and platelet‐to‐lymphocyte ratio can help refine the clinical diagnosis of HFpEF and select patients at high risk of poor clinical outcome in patients with HFpEF admitted for acute decompensated heart failure.
These biomarkers may help identify the patients with proinflammatory state who might benefit from anti‐inflammatory therapy in patients with acute HFpEF.
The rate of hospitalization for acute decompensated heart failure (ADHF) is increasing, largely driven by acute heart failure (HF) with preserved ejection fraction (HFpEF). 1 Systemic inflammation resulting from comorbidities, such as obesity, diabetes, and arterial hypertension, has been postulated to be responsible for the pathogenesis of myocardial structural and functional changes in HFpEF. 2 , 3 Furthermore, specific biomarker profiles in HFpEF are mainly related to inflammation, suggesting a larger pathophysiological role of inflammation in patients with HFpEF than in those with HF with reduced ejection fraction or mildly reduced ejection fraction. 4 , 5 , 6 , 7
Neutrophil‐to‐lymphocyte ratio (NLR) and platelet‐to‐lymphocyte ratio (PLR) are novel, cost‐effective, easily obtainable, and widely available markers of inflammation. They are elevated in several comorbidities of HFpEF, including hypertension, coronary artery disease, and diabetes. 8 , 9 , 10 They can also be used for the risk stratification of patients with HF. 11 , 12 , 13 , 14 , 15 , 16 Although an association between inflammation at admission and poor clinical outcome has already been reported in patients with ADHF, 11 , 13 , 15 , 17 , 18 no information is available on the combined usefulness of NLR and PLR in patients with HFpEF admitted for ADHF. In addition, little is known about the relationship between comorbidity burden and the extent of inflammation evaluated using NLR, PLR, or the combination of NLR and PLR, in patients with ADHF with HFpEF. Accordingly, we sought to evaluate the combined usefulness of NLR and PLR as a predictor of prognosis and the association between comorbidity burden and these indices in patients with ADHF with HFpEF.
METHODS
The authors declare that all supporting data are available within the article.
Study Patients and Data Collection
PURSUIT‐HFpEF (Prospective Multicenter Observational Study of Patients with Heart Failure with Preserved Ejection Fraction) is a prospective, multicenter, observational study in which collaborating hospitals in the Osaka urban area record clinical, echocardiographic, and outcome data from patients with ADHF with preserved left ventricular ejection fraction (University Hospital Medical Information Network Clinical Trials Registry ID: UMIN000021831). 19 Consecutive patients with ADHF and preserved ejection fraction were prospectively registered and agreed to be followed up for the collection of outcome data. The anonymized data were transferred to the data center of Osaka University Hospital for analysis. Inclusion criteria were acute decompensated HFpEF diagnosed using the Framingham criteria 20 and the following: (1) left ventricular ejection fraction ≥50% and (2) NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) ≥400 ng/L or brain natriuretic peptide ≥100 ng/L on admission. Exclusion criteria were age <20 years, severe valvular disease (aortic stenosis, aortic regurgitation, mitral stenosis, or mitral regurgitation), acute coronary syndrome on admission, life expectancy of <6 months because of noncardiac diseases, and previous heart transplantation. All patients provided written informed consent for participation in this study. The ethics committee of each participating hospital approved the study protocol. This study conformed to the ethical guidelines outlined in the Declaration of Helsinki.
Details of the data collection have been described elsewhere. 19 In brief, basic patient characteristics, echocardiography, laboratory tests, and lists of medications were obtained on admission, at discharge, and 1 year after discharge. In this analysis, echocardiography and laboratory data obtained at the time of admission were used. NLR and PLR values at discharge and 1 year after discharge were also obtained. Echocardiography was performed according to standard techniques using a commercially available machine, as previously reported. 19 , 21 Blood samples were obtained and used to measure complete blood count—serum levels of sodium, creatinine, blood urea nitrogen, NT‐proBNP, uric acid, and albumin—and CRP (C‐reactive protein) levels. NLR and PLR were calculated by dividing the absolute neutrophil and platelet counts by the absolute lymphocyte count using the same blood samples, respectively. Anemia was defined as a hemoglobin level of <13.0 g/dL in men and <12.0 g/dL in women on admission, according to the World Health Organization criteria. 22 The estimated glomerular filtration rate was calculated using the modified isotope dilution mass spectrometry traceable modification of diet in renal disease study equation with a Japanese coefficient. 23 Chronic kidney disease was defined by an estimated glomerular filtration rate of ≤60 mL/min per 1.73 m2. Comorbidity burden was defined as the number of occurrences of the following comorbidities: atrial fibrillation, hypertension, diabetes, coronary artery disease, chronic kidney disease, chronic obstructive pulmonary disease, anemia, and obesity (body mass index >30 kg/m2). 24 In addition, plasma volume status was calculated as an index of congestion, which was defined as follows: actual plasma volume (PV) = (1−hematocrit) × [a+(b×body weight)] (a=1530 in men and 864 in women, b=41.0 in men and 47.9 in women); ideal PV=c×body weight (c=39 in men and 40 in women); and plasma volume status =[(actual PV−ideal PV)/ideal PV]×100 (%). 25
End Point and Follow‐Up
The primary and secondary end points of this study were cardiac and all‐cause death, respectively. All patients were followed up after discharge. Survival data were obtained by dedicated coordinators and investigators by 1 of the following methods: direct contact with patients and their physicians at the hospital; in an outpatient setting, by a telephone interview with the patient's families, or by mail. The duration of the follow‐up period was calculated from the day of admission until the end point.
Statistical Analysis
Data are presented as medians and interquartile ranges of 25% to 75% for continuous variables and as percentages for categorical variables. The Mann–Whitney U test or the Kruskal−Wallis rank sum test was used to compare the differences in continuous variables, with the results being presented as medians and interquartile ranges. The χ2 test was used to compare the differences in categorical variables. The predictive values of NLR and PLR for the end point were evaluated using receiver operating characteristic curve analysis, and the results are expressed in terms of the area under the curve and its 95% CI. Statistical trends among groups were evaluated with the Cochran−Armitage trend test. A multivariable logistic regression model was created to identify clinical characteristics associated with high NLR or PLR values, in which 19 clinically relevant factors were selected a priori, including patient demographics, comorbidities, medical history, and oral medications, and were simultaneously forced to enter the model. The prognostic value of the baseline characteristics and serial NLR and PLR measurements were assessed using Cox proportional hazards regression analysis. A multivariate Cox model for the end points was adjusted for 8 characteristics—age, sex, hypertension, diabetes, coronary artery disease, hemoglobin, estimated glomerular filtration rate, and NT‐proBNP level—which were speculated to be clinically important or were previously demonstrated to have prognostic significance. 26 , 27 , 28 The NT‐proBNP level was log10 transformed before its inclusion in the Cox model. The event‐free survival rate was calculated using the Kaplan–Meier method, and differences in survival rates were compared among groups using the log‐rank test. The χ2 test was performed to compare sensitivity, specificity, positive and negative predictive values, and predictive accuracy, which meant the proportion of all test results—both positive and negative—that were correct among the different criteria for prediction of outcome. Statistical significance was set at P<0.05. Statistical analysis was performed using MedCalc statistical software (version 20.026; MedCalc Software Ltd, Ostend, Belgium).
RESULTS
Among the 1095 consecutive patients admitted from June 2016 to December 2020 and enrolled in the PURSUIT‐HFpEF study, those with chronic kidney disease on dialysis (n=17) and missing NLR or PLR data (n=52) were excluded. Finally, data from 1026 patients were analyzed in this study.
After a median follow‐up of 429 days, 195 patients died; 85 of these deaths were attributed to cardiac causes (exacerbation of HF, n=51; fatal arrhythmia or sudden cardiac death, n=10; myocardial infarction, n=3; and other causes of death, n=21), and 110 were attributed to noncardiac causes (infection, n=41; cancer, n=12; renal failure, n=9; stroke, n=6; and other causes of death, n=42). Patients with cardiac death had significantly higher NLR and PLR values on admission than those without (5.6 [3.5–7.9] versus 3.8 [2.5–6.1], P<0.0001, and 200 [135–301] versus 162 [108–244], P=0.0016, respectively). Receiver operating characteristic curve analysis revealed that an NLR of 4.5 on admission (area under the curve, 0.64 [95% CI, 0.60–0.66, P<0.0001]; sensitivity 65% and specificity 61%) and a PLR of 193 on admission (area under the curve, 0.60 [95% CI, 0.57–0.63, P=0.0009]; sensitivity 56% and specificity 62%) were fair discriminators for cardiac death.
Baseline Characteristics
The baseline characteristics of 1026 patients are summarized in Table 1. The patients were stratified into 3 groups based on the optimal cut‐off values of NLR and PLR on admission on receiver operating characteristic curve analysis for the detection of cardiac death: (1) low NLR (≤4.5) and PLR (≤193) (n=492); (2) either high NLR (>4.5) or PLR (>193) (n=242); and (3) both high NLR and PLR (n=292). Patients with high NLR and PLR values were older and had a higher prevalence of chronic obstructive pulmonary disease and anemia. In addition, left ventricular ejection fraction, early transmitral flow velocity (E), tricuspid regurgitation pressure gradient, pulmonary artery systolic pressure, plasma volume status, white blood cell, neutrophil, and platelet counts, and serum blood urea nitrogen, NT‐proBNP, and CRP levels were higher in patients with both high NLR and PLR. Systolic blood pressure, lymphocyte count, hemoglobin level, serum sodium and albumin levels, and estimated glomerular filtration rate were lower in patients with both high NLR and PLR. Significant differences were also found in diastolic blood pressure among the 3 groups.
Table 1.
Baseline Characteristics of the Patients
| Total | Low NLR and PLR | Either High NLR or PLR | Both High NLR and PLR | P value | |
|---|---|---|---|---|---|
| Characteristics | (N=1026) | (N=492) | (N=242) | (N=292) | |
| Age, y | 83 (77–87) | 82 (77–86) | 83 (77–88) | 84 (78–89) | 0.0053 |
| Female sex | 55% | 54% | 52% | 61% | 0.1042 |
| Comorbidities | |||||
| Atrial fibrillation | 46% | 46% | 48% | 45% | 0.8624 |
| Hypertension | 85% | 84% | 83% | 86% | 0.7423 |
| Diabetes | 32% | 30% | 34% | 34% | 0.4003 |
| Dyslipidemia | 42% | 43% | 37% | 43% | 0.2450 |
| Hyperuricemia | 33% | 34% | 29% | 35% | 0.2840 |
| Coronary artery disease | 17% | 18% | 15% | 19% | 0.5255 |
| Chronic kidney disease | 39% | 37% | 40% | 43% | 0.2718 |
| COPD | 8% | 6% | 7% | 11% | 0.0835 |
| Anemia | 72% | 67% | 75% | 79% | 0.0008 |
| Prior HF‐related hospitalization | 25% | 22% | 27% | 28% | 0.1020 |
| Within 6 mo | 5% | 4% | 6% | 6% | 0.4097 |
| Body mass index, kg/m2 | 24 (21–27) | 24 (21–27) | 24 (21–27) | 24 (21–27) | 0.8928 |
| Heart rate, beats/min | 82 (67–100) | 81 (65–100) | 83 (68–100) | 84 (70–100) | 0.1272 |
| Systolic blood pressure, mm Hg | 146 (127–169) | 150 (129–170) | 146 (125–168) | 142 (128–163) | 0.0385 |
| Diastolic blood pressure, mm Hg | 79 (66–93) | 81 (67–98) | 76 (65–91) | 78 (66–90) | 0.0145 |
| Echocardiographic data | |||||
| LVEDD, mm | 46 (42–50) | 46 (42–51) | 46 (41–51) | 45 (42–49) | 0.2053 |
| LVEF, % | 62 (57–68) | 62 (56–68) | 62 (57–68) | 64 (58–70) | 0.0229 |
| LAD, mm | 44 (40–50) | 45 (40–50) | 44 (40–51) | 44 (39–50) | 0.8509 |
| E, m/s | 0.96 (0.75–1.17) | 0.92 (0.72–1.13) | 0.96 (0.75–1.20) | 1.02 (0.81–1.20) | 0.0018 |
| e', m/s | 0.059 (0.047–0.073) | 0.058 (0.047–0.071) | 0.059 (0.046–0.075) | 0.060 (0.047–0.074) | 0.6904 |
| E/e' ratio | 16.1 (12.0–20.8) | 15.7 (11.4–20.0) | 16.2 (11.7–21.5) | 16.7 (13.1–22.0) | 0.0750 |
| TRPG, mm Hg | 36 (28–45) | 34 (27–43) | 35 (29–44) | 39 (31–48) | <0.0001 |
| PASP, mm Hg | 44 (34–54) | 41 (33–53) | 43 (34–53) | 46 (39–56) | <0.0001 |
| Plasma volume status, % | 8.4 (−0.5 to 16.8) | 5.4 (−2.4 to 14.0) | 10.4 (1.5–18.9) | 11.6 (2.7–18.8) | <0.0001 |
| Laboratory data | |||||
| White blood cell, ×103/μL | 6.50 (5.10–8.60) | 6.10 (4.90–7.70) | 6.40 (4.90–9.00) | 7.25 (5.65–9.30) | <0.0001 |
| Neutrophil, ×103/μL | 4.50 (3.28–6.16) | 3.92 (2.93–4.94) | 4.68 (3.33–7.03) | 5.85 (4.38–7.70) | <0.0001 |
| Lymphocyte, ×103/μL | 1.12 (0.78–1.52) | 1.49 (1.18–2.02) | 0.99 (0.80–1.26) | 0.71 (0.52–0.89) | <0.0001 |
| Platelet, ×103/μL | 188 (148–240) | 176 (141–218) | 184 (136–246) | 211 (174–261) | <0.0001 |
| NLR | 3.9 (2.6–6.3) | 2.6 (1.8–3.4) | 4.7 (3.7–6.1) | 7.8 (5.9–12.2) | <0.0001 |
| PLR | 166 (111–247) | 117 (85–147) | 189 (142–234) | 300 (243–425) | <0.0001 |
| Hemoglobin, g/dL | 11 (10–13) | 12 (10–13) | 11 (10–12) | 11 (9–12) | <0.0001 |
| Sodium, mEq/L | 140 (137–142) | 141 (138–143) | 140 (137–142) | 139 (136–142) | <0.0001 |
| Creatinine, mg/dL | 1.1 (0.8–1.5) | 1.0 (0.8–1.4) | 1.1 (0.8–1.5) | 1.1 (0.8–1.6) | 0.0519 |
| BUN, mg/dL | 22 (16–31) | 21 (16–28) | 23 (16–32) | 25 (17–36) | <0.0001 |
| eGFR, mL/min per 1.73 m2 | 45 (30–59) | 45 (33–60) | 44 (30–58) | 40 (27–57) | 0.0056 |
| NT‐proBNP, pg/mL | 3210 (1698–6218) | 2584 (1390–5015) | 3583 (2038–6740) | 4180 (2403–7550) | <0.0001 |
| Uric acid, mg/dL | 6.1 (5.1–7.4) | 5.9 (5.1–7.3) | 6.2 (5.1–7.5) | 6.2 (5.0–7.5) | 0.6081 |
| Albumin, mg/dL | 3.5 (3.2–3.8) | 3.6 (3.3–3.9) | 3.4 (3.1–3.8) | 3.4 (3.1–3.7) | <0.0001 |
| CRP, mg/dL | 0.54 (0.19–2.04) | 0.32 (0.11–0.87) | 0.93 (0.20–2.92) | 1.12 (0.38–4.31) | <0.0001 |
| Oral medications | |||||
| Loop diuretic | 50% | 48% | 54% | 52% | 0.2195 |
| ACE inhibitor/ARB | 50% | 50% | 51% | 50% | 0.9472 |
| β‐blocker | 46% | 45% | 46% | 47% | 0.8923 |
| Aldosterone antagonist | 21% | 21% | 20% | 23% | 0.7322 |
| SGLT2 inhibitor | 2% | 2% | 2% | 1% | 0.8881 |
| Statin | 30% | 31% | 28% | 30% | 0.7380 |
Values are presented as median (interquartile range) or %. High NLR, NLR>4.5; low NLR, NLR ≤4.5; high PLR, PLR>193; low PLR, PLR ≤193. ACE indicates angiotensin‐converting enzyme; ARB, angiotensin II type 1 receptor blocker; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; E, early transmitral flow velocity; e', septal mitral annular early diastolic velocity; eGFR, estimated glomerular filtration rate; HF, heart failure; LAD, left atrial dimension; LVEDD, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction; NLR, neutrophil‐to‐lymphocyte ratio; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PASP, pulmonary artery systolic pressure; PLR, platelet‐to‐lymphocyte ratio; SGLT2, sodium‐glucose cotransporter type 2; and TRPG, tricuspid regurgitation pressure gradient.
Comorbidity Burden and Factors Associated With NLR or PLR
The associations between comorbidity burden and proportion of patients with high NLR or PLR, and both high NLR and PLR are shown in Figure 1. Increased comorbidity burden was significantly associated with a higher proportion of patients with high NLR or PLR, and both high NLR and PLR.
Figure 1. Association of comorbidity burden with NLR, PLR, and the combination of NLR and PLR.
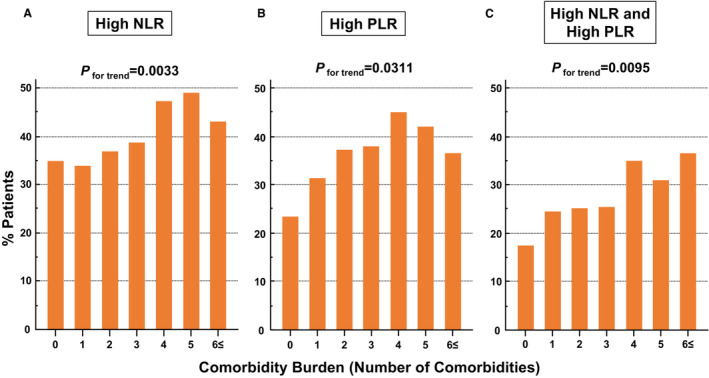
Association between comorbidity burden and the proportions of patients with high NLR (>4.5) (A), high PLR (>193) (B), or both high NLR and PLR (C). NLR indicates neutrophil‐to‐lymphocyte ratio; and PLR, platelet‐to‐lymphocyte ratio.
Among the 19 clinically relevant factors—patient demographics, comorbidities, medical history, and oral medications—diabetes and chronic obstructive pulmonary disease were independently associated with high NLR, while female sex and anemia were independently associated with high PLR (Table 2).
Table 2.
Factors Associated With High NLR or High PLR by Logistic Regression Analysis
| High NLR | High PLR | |||
|---|---|---|---|---|
| Adjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value | |
| Age ≥80 y | 1.22 (0.92–1.62) | 0.1624 | 1.29 (0.97–1.73) | 0.0829 |
| Female sex | 1.09 (0.83–1.43) | 0.5237 | 1.57 (1.19–2.07) | 0.0013 |
| Atrial fibrillation | 0.93 (0.72–1.21) | 0.5982 | 1.05 (0.81–1.37) | 0.7107 |
| Hypertension | 1.08 (0.74–1.58) | 0.6754 | 0.99 (0.68–1.44) | 0.9417 |
| Diabetes | 1.38 (1.03–1.84) | 0.0289 | 1.10 (0.82–1.48) | 0.5249 |
| Dyslipidemia | 0.84 (0.60–1.18) | 0.3221 | 1.11 (0.79–1.57) | 0.5384 |
| Hyperuricemia | 1.22 (0.91–1.63) | 0.1791 | 0.75 (0.56–1.02) | 0.0627 |
| Coronary artery disease | 1.01 (0.71–1.43) | 0.9618 | 1.00 (0.70–1.44) | 0.9929 |
| Chronic kidney disease | 1.09 (0.82–1.44) | 0.5605 | 1.07 (0.80–1.43) | 0.6402 |
| COPD | 1.65 (1.02–2.67) | 0.0396 | 1.62 (0.99–2.64) | 0.0530 |
| Anemia | 1.26 (0.94–1.71) | 0.1234 | 1.92 (1.40–2.62) | <0.0001 |
| Prior HF‐related hospitalization | 1.18 (0.86–1.63) | 0.3013 | 1.20 (0.87–1.66) | 0.2771 |
| Body mass index >30 kg/m2 | 1.12 (0.74–1.68) | 0.6024 | 1.09 (0.71–1.67) | 0.6820 |
| Loop diuretic | 0.85 (0.63–1.15) | 0.2929 | 1.22 (0.90–1.64) | 0.1996 |
| ACE inhibitor/ARB | 0.99 (0.76–1.29) | 0.9376 | 0.96 (0.74–1.26) | 0.7888 |
| β‐blocker | 0.95 (0.73–1.23) | 0.6876 | 1.10 (0.84–1.44) | 0.5082 |
| Aldosterone antagonist | 1.12 (0.81–1.56) | 0.4968 | 0.90 (0.64–1.26) | 0.5397 |
| SGLT2 inhibitor | 0.79 (0.29–2.17) | 0.6432 | 0.74 (0.25–2.26) | 0.6030 |
| Statin | 1.12 (0.79–1.59) | 0.5343 | 0.70 (0.49–1.01) | 0.0584 |
High NLR, NLR>4.5; high PLR, PLR>193. ACE indicates angiotensin‐converting enzyme; ARB, angiotensin II type 1 receptor blocker; COPD, chronic obstructive pulmonary disease; HF, heart failure; NLR, neutrophil‐to‐lymphocyte ratio; OR, odds ratio; PLR, platelet‐to‐lymphocyte ratio; and SGLT2, sodium‐glucose cotransporter type 2.
Prognostic Analysis
Multivariate Cox analysis demonstrated that high NLR and PLR on admission were independently associated with cardiac and all‐cause death and that their combination was the strongest predictor of cardiac and all‐cause death (Table 3). A significant difference was noted in the cardiac and all‐cause death rates among the 3 groups stratified by NLR and PLR values on admission (Figure 2).
Table 3.
Cox Proportional Hazard Analysis for Cardiac and All‐Cause Death Using NLR and PLR Values on Admission
| Multivariate analysis* | ||||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Model 1 | Model 2 | Model 3 | |||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Cardiac death | ||||||||
| High NLR | 2.78 (1.78–4.35) | <0.0001 | 2.49 (1.51–4.10) | 0.0003 | … | … | ||
| High PLR | 2.12 (1.38–3.26) | 0.0006 | … | 1.76 (1.09–2.84) | 0.0215 | … | ||
| Low NLR and Low PLR | Reference | … | … | Reference | ||||
| Either High NLR or High PLR | 1.55 (0.85–2.83) | 0.1536 | … | … | 1.30 (0.66–2.53) | 0.4465 | ||
| High NLR and High PLR | 3.23 (1.96–5.34) | <0.0001 | … | … | 2.66 (1.51–4.70) | 0.0008 | ||
| All‐cause death | ||||||||
| High NLR | 1.97 (1.48–2.61) | <0.0001 | 1.75 (1.27–2.42) | 0.0006 | … | … | ||
| High PLR | 1.83 (1.38–2.42) | <0.0001 | … | 1.47 (1.06–2.03) | 0.0198 | … | ||
| Low NLR and Low PLR | Reference | … | … | Reference | ||||
| Either High NLR or High PLR | 1.34 (0.92–1.96) | 0.1316 | … | … | 1.10 (0.72–1.69) | 0.6532 | ||
| High NLR and High PLR | 2.35 (1.70–3.24) | <0.0001 | … | … | 1.90 (1.31–2.76) | 0.0007 | ||
HR indicates hazard ratio; NLR, neutrophil‐to‐lymphocyte ratio; and PLR, platelet‐to‐lymphocyte ratio.
Multivariate models were adjusted for age, sex, hypertension, diabetes, coronary artery disease, hemoglobin, estimated glomerular filtration rate, and N‐terminal pro‐B‐type natriuretic peptide level. High NLR, NLR>4.5; low NLR, NLR ≤4.5; high PLR, PLR>193; low PLR, PLR ≤193. Model 1 included high NLR; Model 2, high PLR; Model 3, the groups stratified by NLR and PLR values.
Figure 2. Kaplan–Meier estimates stratified by NLR, PLR, and the combination of NLR and PLR.
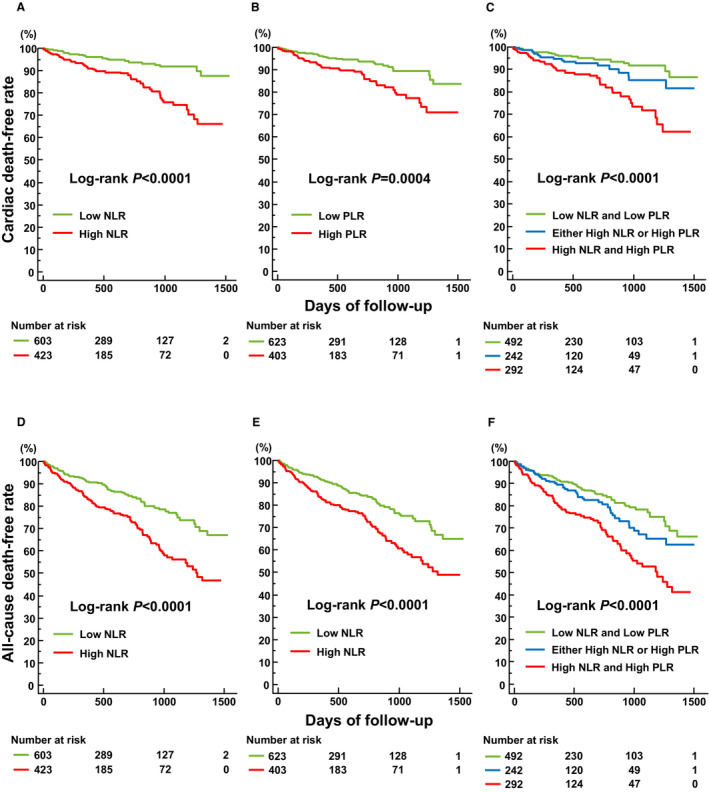
Kaplan–Meier estimates of freedom from cardiac death stratified by NLR (A), PLR (B), and NLR and PLR (C), and all‐cause death stratified by NLR (D), PLR (E), and NLR and PLR (F). High NLR, NLR>4.5; low NLR, NLR ≤4.5; high PLR, PLR>193; low PLR, PLR≤193. NLR indicates neutrophil‐to‐lymphocyte ratio; and PLR, platelet‐to‐lymphocyte ratio.
Prediction of Cardiac and All‐Cause Death
Prediction of cardiac and all‐cause death with NLR and PLR values on admission is shown in Table 4. Specificity and predictive accuracy for cardiac and all‐cause death significantly increased by the combination of high NLR and PLR, whereas sensitivity was significantly lower than in high NLR.
Table 4.
Prediction of Cardiac Death and All‐Cause Death Using a Combination of NLR and PLR Values on Admission
| High NLR | High PLR | High NLR and High PLR | |
|---|---|---|---|
| Cardiac death | |||
| Sensitivity, % | 65 (55/85) | 56 (48/85) | 49* (42/85) |
| Specificity, % | 61 (573/941) | 62 (586/941) | 73† (691/941) |
| Positive predictive value, % | 13 (55/423) | 12 (48/403) | 14 (42/292) |
| Negative predictive value, % | 95 (573/603) | 94 (586/623) | 94 (691/734) |
| Predictive accuracy, % | 61 (628/1026) | 62 (634/1026) | 71† (733/1026) |
| All‐cause death | |||
| Sensitivity, % | 56 (110/195) | 53 (103/195) | 43‡ (84/195) |
| Specificity, % | 62 (518/831) | 64 (531/831) | 75† (623/831) |
| Positive predictive value, % | 26 (110/423) | 26 (103/403) | 29 (84/292) |
| Negative predictive value, % | 86 (518/603) | 85 (531/623) | 85 (623/734) |
| Predictive accuracy, % | 61 (628/1026) | 62 (634/1026) | 69§ (707/1026) |
The numbers in parentheses are patient numbers. High NLR, NLR>4.5; high PLR, PLR>193. NLR indicates neutrophil‐to‐lymphocyte ratio; and PLR, platelet‐to‐lymphocyte ratio.
P<0.05 vs high NLR.
P<0.0001 vs high NLR and high PLR.
P<0.01 vs high NLR.
P<0.001 vs high NLR and high PLR.
CRP Level and Postdischarge Outcomes
Cox analysis for cardiac and all‐cause death using CRP levels on admission is shown in Table 5. Patients were divided into tertiles based on CRP levels on admission: first tertile (<0.28 mg/dL), second tertile (0.28–1.22 mg/dL), and third tertile (>1.22 mg/dL). Although CRP levels in the third tertile were associated with cardiac death in univariate analysis, multivariate analysis showed no association between cardiac death and any CRP tertiles. In contrast, both second and third CRP tertiles were associated with all‐cause death in univariate and multivariate analysis.
Table 5.
Cox Proportional Hazard Analysis for Cardiac Death and All‐Cause Death Using Admission CRP tertiles
| Univariate analysis | Multivariate analysis* | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Cardiac death | ||||
| First tertile | Reference | Reference | ||
| Second tertile | 1.29 (0.72–2.29) | 0.3947 | 1.54 (0.82–2.90) | 0.1794 |
| Third tertile | 1.99 (1.16–3.43) | 0.0126 | 1.70 (0.95–3.05) | 0.0747 |
| All‐cause death | ||||
| First tertile | Reference | Reference | ||
| Second tertile | 1.63 (1.11–2.39) | 0.0119 | 1.88 (1.23–2.86) | 0.0035 |
| Third tertile | 2.02 (1.39–2.94) | 0.0002 | 1.62 (1.07–2.45) | 0.0229 |
Multivariate models were adjusted for age, sex, hypertension, diabetes, coronary artery disease, hemoglobin, estimated glomerular filtration rate, and N‐terminal pro‐B‐type natriuretic peptide level. CRP indicates C‐reactive protein; and HR, hazard ratio.
Serial NLR and PLR Values and Postdischarge Outcomes
NLR and PLR values at discharge and 1 year after discharge were obtained in 983 and 580 patients, respectively. NLR and PLR at discharge were 2.3 (1.6–3.4) and 154 (112–218), respectively, and NLR and PLR 1 year after discharge were 2.6 (1.9–3.6) and 139 (99–191), respectively.
Multivariate Cox analysis demonstrated that the combination of high NLR and PLR on admission and at discharge was independently associated with cardiac and all‐cause death (Table 6). Similarly, the combination of high NLR and PLR on admission and 1 year after discharge had a significant association with cardiac and all‐cause death on multivariate analysis. There was a significant difference in the cardiac and all‐cause death rates between the 2 groups stratified by serial NLR and PLR values (Figure 3).
Table 6.
Cox Proportional Hazard Analysis for Cardiac and All‐Cause Death Using Serial NLR and PLR Values
| Univariate analysis | Multivariate analysis* | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Cardiac death | ||||
| High NLR and PLR on admission and at discharge | 2.76 (1.38–5.54) | 0.0043 | 2.71 (1.33–5.53) | 0.0061 |
| High NLR and PLR on admission and 1 year after discharge | 3.66 (1.41–9.52) | 0.0079 | 4.10 (1.41–11.88) | 0.0094 |
| All‐cause death | ||||
| High NLR and PLR on admission and at discharge | 2.56 (1.58–4.12) | 0.0001 | 2.43 (1.46–4.06) | 0.0007 |
| High NLR and PLR on admission and 1 year after discharge | 2.74 (1.36–5.53) | 0.0047 | 2.55 (1.10–5.94) | 0.0298 |
HR indicates hazard ratio; NLR, neutrophil‐to‐lymphocyte ratio; and PLR, platelet‐to‐lymphocyte ratio.
Multivariate models were adjusted for age, sex, hypertension, diabetes, coronary artery disease, hemoglobin, estimated glomerular filtration rate, and N‐terminal pro‐B‐type natriuretic peptide level. High NLR, NLR>4.5; high PLR, PLR>193.
Figure 3. Kaplan–Meier estimates stratified by serial NLR and PLR values.
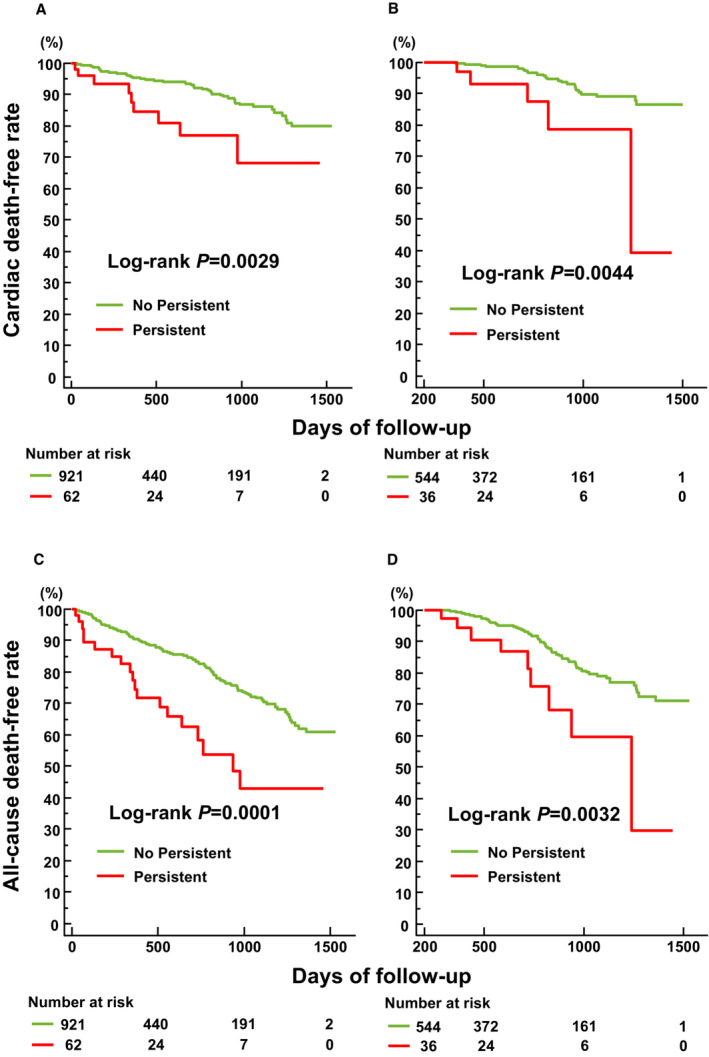
Kaplan–Meier estimates of freedom from cardiac death stratified by the presence of a combination of persistent high NLR (>4.5) and PLR (>193) values on admission and discharge (A) and on admission and 1 year after discharge (B), and all‐cause death stratified by the presence of a combination of persistent high NLR and PLR values on admission and discharge (C) and on admission and 1 year after discharge (D). NLR indicates neutrophil‐to‐lymphocyte ratio; and PLR, platelet‐to‐lymphocyte ratio.
DISCUSSION
NLR and PLR values are cost effective and easily calculated and accessible inflammatory biomarkers that are associated with the severity and prognosis of numerous cardiac and noncardiac diseases. 8 , 9 , 10 , 29 , 30 , 31 An elevated NLR indicates an imbalance between the innate and acquired immune response and an increased PLR indicates inflammation and platelet activation. In this study, a higher comorbidity burden was associated with high NLR or PLR, or both, possibly reflecting the systemic inflammation induced by comorbidities, which can cause myocardial structural and functional damage in HFpEF. Moreover, high NLR and PLR were associated with a higher risk of not only cardiac death but also all‐cause death. The combination of high NLR and PLR was also useful for identifying patients at risk of both end points. To the best of our knowledge, this study is the first to demonstrate the association between comorbidity burden and NLR and PLR and their combined usefulness for the prognostication of patients with ADHF with HFpEF. Furthermore, our results also indicated that the serial evaluation of NLR and PLR has clinical value for the prognostication of patients with ADHF with HFpEF.
The current paradigm on the underlying pathophysiology of HFpEF suggests that a systemic proinflammatory state driven by a plethora of comorbidities causes coronary microvascular endothelial inflammation. This inflammation is responsible for the stiffening of cardiomyocytes and interstitial fibrosis, leading to cardiac stiffening and increased left ventricular filling pressure. 2 , 3 Biomarkers in HFpEF are predominantly associated with inflammation. 4 , 5 Furthermore, the unique biomarker profiles in HFpEF are mainly related to inflammation compared with the other HF subtypes, which implies its larger pathophysiological role in patients with HFpEF than in patients with heart failure with reduced ejection fraction or heart failure with mildly reduced ejection fraction. 6 , 7 Considering the prominent pathophysiological and prognostic role of inflammation in patients with ADHF, 17 , 18 identifying patients in comorbidity burden‐induced inflammatory states and at risk of poor clinical outcomes in acute HFpEF seems particularly important. 6 Although several biomarkers, such as pentraxin‐3 and receptor for advanced glycation end product, are specific markers of the inflammatory pathway in patients with acute HFpEF, 6 they are not available in daily clinical practice. NLR and PLR indices are advantageous in that they are widely available and cost‐effective biomarkers and do not require specialized equipment for measurement. Moreover, NLR and PLR values are less likely to be influenced by several physiological conditions, such as dehydration and exercise, which may affect the absolute numbers of neutrophils, platelets, and lymphocytes. 29
A previous report showed that plasma CRP levels progressively increased with the increasing number of comorbidities in individual patients with HFpEF. 24 In addition, circulating inflammatory biomarkers mediate the association between comorbidity burden and echocardiographic parameters of poor left ventricular diastolic function, including increased E velocity, its ratio to early diastolic septal mitral annular velocity (e'), and tricuspid regurgitation velocity. 32 In line with these findings, we observed a significant association between comorbidity burden and a higher proportion of patients with high NLR and PLR. Moreover, E velocity, tricuspid regurgitation pressure gradient, and pulmonary artery systolic pressure were higher in patients with high NLR and PLR, although no significant difference was found in the E/e' ratio among the groups. An increased plasma volume status in patients with high NLR and PLR might have reflected the systemic and pulmonary congestion caused by cardiac diastolic dysfunction. 25 Previous reports have shown the utility of NLR or PLR indices for risk stratification of patients with chronic HF. 12 , 14 , 16 Several studies have also demonstrated the prognostic values of NLR and PLR in patients with ADHF, although most of them included not only patients with HFpEF but also those with heart failure with reduced ejection fraction and heart failure with mildly reduced ejection fraction, used relatively old databases, or were performed in small cohorts of patients. 11 , 13 , 15 Our findings expand on these earlier reports by not only demonstrating the association between comorbidity burden and NLR and PLR but also the prognostic value of the combination of NLR and PLR in a large contemporary cohort of patients with ADHF with HFpEF. In this study, we could not find any significant association between the risk of cardiac death and high CRP levels in multivariate Cox analysis, although higher tertiles of CRP levels were associated with all‐cause death, suggesting that the prognostic value of the combined use of NLR and PLR for cardiac death would be higher than CRP. This discrepancy between our result and that from previous reports may be because we did not exclude patients with severe infection, considering that the prognostic impact of CRP in patients with ADHF is weakened by the presence of an infectious complication. 33
The precise mechanisms responsible for the increase in NLR and PLR in patients with HF are not fully understood; however, an increase in neutrophil and platelets because of systemic inflammation, and lymphopenia caused by elevated cytokines, 34 splanchnic congestion, 35 and increased endogenous cortisol and sympathetic tone, 36 , 37 seem to play a role. A significant association has recently been reported between NLR and coronary microvascular dysfunction in patients with type 2 diabetes. 38 In contrast, platelet activation is a biological process unique to HFpEF. 7 Moreover, our results have shown that high NLR and PLR have strong associations with diabetes, chronic obstructive pulmonary disease, and anemia, all of which are well‐known noncardiac comorbidities that are prevalent and have strong prognostic impact, especially in patients with HFpEF. 39 Considering these, the combination of NLR and PLR may be suitable for the evaluation of a proinflammatory state induced by comorbidities, which characterize the pathophysiology of HFpEF. Independently associated factors were different between high NLR and PLR, suggesting that the inflammatory response reflected by NLR is different from that by PLR. This may explain the significant improvement of predictive accuracy for cardiac and all‐cause death by NLR and PLR combination.
Recently, we reported that the high‐density lipoprotein cholesterol to CRP ratio on admission is a simple and useful biomarker for the prediction of clinical outcomes in patients with HFpEF admitted for ADHF. 40 In this study, we demonstrated that the combination of NLR and PLR is another potential candidate for a simple inflammatory marker that can be used for the risk stratification of patients with ADHF with HFpEF. Inflammatory biomarkers can help refine the clinical diagnosis of HFpEF and select patients at high risk. Furthermore, HFpEF is a highly heterogeneous syndrome with numerous underlying causes and pathophysiological abnormalities. Patients with acute HFpEF can be divided into several phenotypes with distinct characteristics and clinical outcomes. 41 , 42 Prognostic inflammatory biomarkers may help identify the phenogroup of patients with proinflammatory state and provide appropriate and inflammatory phenotype‐specific therapies in acute HFpEF. 43
This study has a few limitations. First, the empirically chosen sample size and relatively short follow‐up period are major limitations. Second, because this study utilized a multicenter prospective East‐Asian HFpEF registry, possible ethnic differences should be considered when attempting to generalize the results to non‐Japanese populations. Third, because we lacked detailed data on the biomarker profiles of the study patients, an investigation on the association and comparison of prognostic values among NLR, PLR, and other biomarkers of inflammation, myocyte stress, or fibrosis, which were measured in previous reports, 4 , 5 , 6 , 7 was not performed. Although the predictive accuracy for cardiac and all‐cause death was significantly improved by the combination of NLR and PLR, its sensitivity was relatively lower, and area under the curve for the prediction of the end point was not significantly improved by the combination of NLR and PLR (data not shown). In addition, sensitivity and specificity of high NLR and PLR for the prediction of cardiac and all‐cause death were not high enough. Therefore, NLR and PLR might not be sufficient to represent several inflammatory pathways that are involved in the heterogeneous pathophysiology of HFpEF. 32 Analysis using detailed biomarker profiles would be needed to elucidate this point. Fourth, we did not exclude patients with concomitant infection, cancer, and chronic systemic inflammatory disorders such as autoimmune diseases and asthma, which may influence the interpretation of the results and might have caused relatively low specificity of high NLR and PLR for the prediction of cardiac and all‐cause death in this study. Fifth, because we included only the patients with HFpEF, the prognostic value of the combination of NLR and PLR in patients with ADHF with heart failure with reduced ejection fraction or heart failure with mildly reduced ejection fraction remains unknown. Finally, because of the observational nature of this study, whether NLR and PLR are merely markers of disease severity or therapeutic targets remains unknown. Furthermore, the question of whether patient management using the combination of NLR and PLR leads to better prognosis in patients with acute HFpEF should be addressed in future studies.
In conclusion, this prospective multicenter East‐Asian HFpEF registry showed that the combination of NLR and PLR values is useful for the prediction of postdischarge outcomes in patients with HFpEF admitted for ADHF. Further investigation is warranted to confirm our results and improve our understanding of the pathophysiological significance of inflammation in patients with ADHF with HFpEF.
Sources of Funding
This work was funded by Roche Diagnostics K.K. and Fuji Film Toyama Chemical Co. Ltd.
Disclosures
Dr Nakatani has received honoraria from Roche Diagnostics. Dr Hikoso has received personal fees from Daiichi Sankyo Company, Bayer, Astellas Pharma, Pfizer Pharmaceuticals, Boehringer Ingelheim Japan, and Novartis, and grants from Roche Diagnostics, FUJIFILM Toyama Chemical, and Actelion Pharmaceuticals. Dr Sotomi has received research grants from Abbott Medical Japan and speaker honoraria from Abbott Medical Japan, Boston Scientific Japan, TERUMO, Japan Lifeline, Biosensors, and Medtronic, and is an endowed chair funded by TOA EIYO. Dr Sakata has received personal fees from Otsuka Pharmaceutical, Ono Pharmaceutical, Daiichi Sankyo Company, Mitsubishi Tanabe Pharma Corporation, and Actelion Pharmaceuticals, and grants from Roche Diagnostic, FUJIFILM Toyama Chemical, Abbott Medical, Japan, Otsuka Pharmaceutical, Daiichi Sankyo Company, Mitsubishi Tanabe Pharma Corporation, and Biotronik. The other authors have no conflicts of interest to disclose.
Supporting information
Appendix S1
Acknowledgments
The authors thank Nagisa Yoshioka, Kyoko Tatsumi, Satomi Kishimoto, Noriko Murakami, and Sugako Mitsuoka for their excellent assistance with data collection.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.026326
For Sources of Funding and Disclosures, see page 12.
References
- 1. Chang PP, Wruck LM, Shahar E, Rossi JS, Loehr LR, Russell SD, Agarwal SK, Konety SH, Rodriguez CJ, Rosamond WD. Trends in hospitalizations and survival of acute decompensated heart failure in four US communities (2005‐2014): ARIC study community surveillance. Circulation. 2018;138:12–24. doi: 10.1161/CIRCULATIONAHA.117.027551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092 [DOI] [PubMed] [Google Scholar]
- 3. Paulus WJ, Zile MR. From systemic inflammation to myocardial fibrosis: the heart failure with preserved ejection fraction paradigm revisited. Circ Res. 2021;128:1451–1467. doi: 10.1161/CIRCRESAHA.121.318159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sanders‐van Wijk S, van Empel V, Davarzani N, Maeder MT, Handschin R, Pfisterer ME, Brunner‐La Rocca HP; TIME‐CHF Investigators . Circulating biomarkers of distinct pathophysiological pathways in heart failure with preserved vs. reduced left ventricular ejection fraction. Eur J Heart Fail. 2015;17:1006–1014. doi: 10.1002/ejhf.414 [DOI] [PubMed] [Google Scholar]
- 5. Tromp J, Khan MA, Klip IT, Meyer S, de Boer RA, Jaarsma T, Hillege H, van Veldhuisen DJ, van der Meer P, Voors AA. Biomarker profiles in heart failure patients with preserved and reduced ejection fraction. J Am Heart Assoc. 2017;6:e003989. doi: 10.1161/JAHA.116.003989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tromp J, Khan MAF, Mentz RJ, O'Connor CM, Metra M, Dittrich HC, Ponikowski P, Teerlink JR, Cotter G, Davison B, et al. Biomarker profiles of acute heart failure patients with a mid‐range ejection fraction. JACC Heart Fail. 2017;5:507–517. doi: 10.1016/j.jchf.2017.04.007 [DOI] [PubMed] [Google Scholar]
- 7. Tromp J, Westenbrink BD, Ouwerkerk W, van Veldhuisen DJ, Samani NJ, Ponikowski P, Metra M, Anker SD, Cleland JG, Dickstein K, et al. Identifying pathophysiological mechanisms in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2018;72:1081–1090. doi: 10.1016/j.jacc.2018.06.050 [DOI] [PubMed] [Google Scholar]
- 8. Verdoia M, Schaffer A, Barbieri L, Aimaretti G, Marino P, Sinigaglia F, Suryapranata H, De Luca G; Novara Atherosclerosis Study Group (NAS) . Impact of diabetes on neutrophil‐to‐lymphocyte ratio and its relationship to coronary artery disease. Diabetes Metab. 2015;41:304–311. doi: 10.1016/j.diabet.2015.01.001 [DOI] [PubMed] [Google Scholar]
- 9. Jhuang YH, Kao TW, Peng TC, Chen WL, Li YW, Chang PK, Wu LW. Neutrophil to lymphocyte ratio as predictor for incident hypertension: a 9‐year cohort study in Taiwan. Hypertens Res. 2019;42:1209–1214. doi: 10.1038/s41440-019-0245-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Qiu Z, Jiang Y, Jiang X, Yang R, Wu Y, Xu Y, Cheng X. Relationship between platelet to lymphocyte ratio and stable coronary artery disease: meta‐analysis of observational studies. Angiology. 2020;71:909–915. doi: 10.1177/0003319720943810 [DOI] [PubMed] [Google Scholar]
- 11. Uthamalingam S, Patvardhan EA, Subramanian S, Ahmed W, Martin W, Daley M, Capodilupo R. Utility of the neutrophil to lymphocyte ratio in predicting long‐term outcomes in acute decompensated heart failure. Am J Cardiol. 2011;107:433–438. doi: 10.1016/j.amjcard.2010.09.039 [DOI] [PubMed] [Google Scholar]
- 12. Benites‐Zapata VA, Hernandez AV, Nagarajan V, Cauthen CA, Starling RC, Tang WH. Usefulness of neutrophil‐to‐lymphocyte ratio in risk stratification of patients with advanced heart failure. Am J Cardiol. 2015;115:57–61. doi: 10.1016/j.amjcard.2014.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cho JH, Cho HJ, Lee HY, Ki YJ, Jeon ES, Hwang KK, Chae SC, Baek SH, Kang SM, Choi DJ, et al. Neutrophil‐lymphocyte ratio in patients with acute heart failure predicts in‐hospital and long‐term mortality. J Clin Med. 2020;9:557. doi: 10.3390/jcm9020557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Curran FM, Bhalraam U, Mohan M, Singh JS, Anker SD, Dickstein K, Doney AS, Filippatos G, George J, Metra M, et al. Neutrophil‐to‐lymphocyte ratio and outcomes in patients with new‐onset or worsening heart failure with reduced and preserved ejection fraction. ESC Heart Fail. 2021;8:3168–3179. doi: 10.1002/ehf2.13424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ye GL, Chen Q, Chen X, Liu YY, Yin TT, Meng QH, Liu YC, Wei HQ, Zhou QH. The prognostic role of platelet‐to‐lymphocyte ratio in patients with acute heart failure: a cohort study. Sci Rep. 2019;9:10639. doi: 10.1038/s41598-019-47143-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dahlen B, Schulz A, Gobel S, Trobs SO, Schwuchow‐Thonke S, Spronk HM, Prochaska JH, Arnold N, Lackner KJ, Gori T, et al. The impact of platelet indices on clinical outcome in heart failure: results from the MyoVasc study. ESC Heart Fail. 2021;8:2991–3001. doi: 10.1002/ehf2.13390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mueller C, Laule‐Kilian K, Christ A, Brunner‐La Rocca HP, Perruchoud AP. Inflammation and long‐term mortality in acute congestive heart failure. Am Heart J. 2006;151:845–850. doi: 10.1016/j.ahj.2005.06.046 [DOI] [PubMed] [Google Scholar]
- 18. Minami Y, Kajimoto K, Sato N, Hagiwara N, Takano T; ATTEND Study Investigators . C‐reactive protein level on admission and time to and cause of death in patients hospitalized for acute heart failure. Eur Heart J Qual Care Clin Outcomes. 2017;3:148–156. doi: 10.1093/ehjqcco/qcw054 [DOI] [PubMed] [Google Scholar]
- 19. Suna S, Hikoso S, Yamada T, Uematsu M, Yasumura Y, Nakagawa A, Takeda T, Kojima T, Kida H, Oeun B, et al. Study protocol for the PURSUIT‐HFpEF study: a prospective, multicenter, observational study of patients with heart failure with preserved ejection fraction. BMJ Open. 2020;10:e038294. doi: 10.1136/bmjopen-2020-038294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601 [DOI] [PubMed] [Google Scholar]
- 21. Nakagawa A, Yasumura Y, Yoshida C, Okumura T, Tateishi J, Yoshida J, Abe H, Tamaki S, Yano M, Hayashi T, et al. Prognostic importance of right ventricular‐vascular uncoupling in acute decompensated heart failure with preserved ejection fraction. Circ Cardiovasc Imaging. 2020;13:e011430. doi: 10.1161/CIRCIMAGING.120.011430 [DOI] [PubMed] [Google Scholar]
- 22. McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993−2005. Public Health Nutr. 2009;12:444–454. doi: 10.1017/S1368980008002401 [DOI] [PubMed] [Google Scholar]
- 23. Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034 [DOI] [PubMed] [Google Scholar]
- 24. DuBrock HM, AbouEzzeddine OF, Redfield MM. High‐sensitivity C‐reactive protein in heart failure with preserved ejection fraction. PLoS One. 2018;13:e0201836. doi: 10.1371/journal.pone.0201836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ling HZ, Flint J, Damgaard M, Bonfils PK, Cheng AS, Aggarwal S, Velmurugan S, Mendonca M, Rashid M, Kang S, et al. Calculated plasma volume status and prognosis in chronic heart failure. Eur J Heart Fail. 2015;17:35–43. doi: 10.1002/ejhf.193 [DOI] [PubMed] [Google Scholar]
- 26. Beale AL, Meyer P, Marwick TH, Lam CSP, Kaye DM. Sex differences in cardiovascular pathophysiology: why women are overrepresented in heart failure with preserved ejection fraction. Circulation. 2018;138:198–205. doi: 10.1161/CIRCULATIONAHA.118.034271 [DOI] [PubMed] [Google Scholar]
- 27. Sotomi Y, Hikoso S, Nakatani D, Mizuno H, Okada K, Dohi T, Kitamura T, Sunaga A, Kida H, Oeun B, et al. Sex differences in heart failure with preserved ejection fraction. J Am Heart Assoc. 2021;10:e018574. doi: 10.1161/JAHA.120.018574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nakagawa A, Yasumura Y, Yoshida C, Okumura T, Tateishi J, Yoshida J, Abe H, Tamaki S, Yano M, Hayashi T, et al. Prognostic importance of pulmonary arterial capacitance in acute decompensated heart failure with preserved ejection fraction. J Am Heart Assoc. 2021;10:e023043. doi: 10.1161/JAHA.121.023043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bhat T, Teli S, Rijal J, Bhat H, Raza M, Khoueiry G, Meghani M, Akhtar M, Costantino T. Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Rev Cardiovasc Ther. 2013;11:55–59. doi: 10.1586/erc.12.159 [DOI] [PubMed] [Google Scholar]
- 30. Afari ME, Bhat T. Neutrophil to lymphocyte ratio (NLR) and cardiovascular diseases: an update. Expert Rev Cardiovasc Ther. 2016;14:573–577. doi: 10.1586/14779072.2016.1154788 [DOI] [PubMed] [Google Scholar]
- 31. Balta S, Ozturk C. The platelet‐lymphocyte ratio: a simple, inexpensive and rapid prognostic marker for cardiovascular events. Platelets. 2015;26:680–681. doi: 10.3109/09537104.2014.979340 [DOI] [PubMed] [Google Scholar]
- 32. Sanders‐van Wijk S, Tromp J, Beussink‐Nelson L, Hage C, Svedlund S, Saraste A, Swat SA, Sanchez C, Njoroge J, Tan RS, et al. Proteomic evaluation of the comorbidity‐inflammation paradigm in heart failure with preserved ejection fraction: results from the PROMIS‐HFpEF study. Circulation. 2020;142:2029–2044. doi: 10.1161/CIRCULATIONAHA.120.045810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lourenco P, Paulo Araujo J, Paulo C, Mascarenhas J, Frioes F, Azevedo A, Bettencourt P. Higher C‐reactive protein predicts worse prognosis in acute heart failure only in noninfected patients. Clin Cardiol. 2010;33:708–714. doi: 10.1002/clc.20812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. von Haehling S, Schefold JC, Jankowska E, Doehner W, Springer J, Strohschein K, Genth‐Zotz S, Volk HD, Poole‐Wilson P, Anker SD. Leukocyte redistribution: effects of beta blockers in patients with chronic heart failure. PLoS One. 2009;4:e6411. doi: 10.1371/journal.pone.0006411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Battin DL, Ali S, Shahbaz AU, Massie JD, Munir A, Davis RC Jr, Newman KP, Weber KT. Hypoalbuminemia and lymphocytopenia in patients with decompensated biventricular failure. Am J Med Sci. 2010;339:31–35. doi: 10.1097/MAJ.0b013e3181bfc83f [DOI] [PubMed] [Google Scholar]
- 36. Maisel AS, Knowlton KU, Fowler P, Rearden A, Ziegler MG, Motulsky HJ, Insel PA, Michel MC. Adrenergic control of circulating lymphocyte subpopulations. Effects of congestive heart failure, dynamic exercise, and terbutaline treatment. J Clin Invest. 1990;85:462–467. doi: 10.1172/JCI114460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thomson SP, McMahon LJ, Nugent CA. Endogenous cortisol: a regulator of the number of lymphocytes in peripheral blood. Clin Immunol Immunopathol. 1980;17:506–514. doi: 10.1016/0090-1229(80)90146-4 [DOI] [PubMed] [Google Scholar]
- 38. Chen Y, Chai Q, Wang Q, Zhang Z, Shan Y, Lu D, Liu M, Wu W. Neutrophil‐to‐lymphocyte ratio is associated with coronary microvascular dysfunction in type 2 diabetes mellitus patients. Diabetes Res Clin Pract. 2021;178:108983. doi: 10.1016/j.diabres.2021.108983 [DOI] [PubMed] [Google Scholar]
- 39. Streng KW, Nauta JF, Hillege HL, Anker SD, Cleland JG, Dickstein K, Filippatos G, Lang CC, Metra M, Ng LL, et al. Non‐cardiac comorbidities in heart failure with reduced, mid‐range and preserved ejection fraction. Int J Cardiol. 2018;271:132–139. doi: 10.1016/j.ijcard.2018.04.001 [DOI] [PubMed] [Google Scholar]
- 40. Yano M, Nishino M, Ukita K, Kawamura A, Nakamura H, Matsuhiro Y, Yasumoto K, Tsuda M, Okamoto N, Tanaka A, et al. High density lipoprotein cholesterol/C reactive protein ratio in heart failure with preserved ejection fraction. ESC Heart Fail. 2021;8:2791–2801. doi: 10.1002/ehf2.13350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sotomi Y, Hikoso S, Komukai S, Sato T, Oeun B, Kitamura T, Nakagawa A, Nakatani D, Mizuno H, Okada K, et al. Phenotyping of acute decompensated heart failure with preserved ejection fraction. Heart. 2022;108:1553–1561. doi: 10.1136/heartjnl-2021-320270 [DOI] [PubMed] [Google Scholar]
- 42. Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, Bonow RO, Huang CC, Deo RC. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;131:269–279. doi: 10.1161/CIRCULATIONAHA.114.010637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Everett BM, Cornel JH, Lainscak M, Anker SD, Abbate A, Thuren T, Libby P, Glynn RJ, Ridker PM. Anti‐inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation. 2019;139:1289–1299. doi: 10.1161/CIRCULATIONAHA.118.038010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


