Abstract
Background
Larger epicardial fat volume (EFV) has been associated with increased risks of cardiovascular disease and atrial fibrillation. Yet, evidence on the association of EFV with cardiac function and incident heart failure (HF) remains scarce.
Methods and Results
We included 2103 participants (mean age, 68 years; 54.4% women) from the prospective population‐based RS (Rotterdam Study) with computed tomography–based EFV and repeated echocardiography‐based assessment of left ventricular (LV) systolic and diastolic function. Linear mixed effects and Cox‐proportional hazard regression models, adjusted for cardiovascular risk factors, were used to assess the associations of EFV with repeated measurements of echocardiographic parameters and with incident HF. During a median follow‐up of 9.7 years, 124 HF events occurred (incidence rate, 6.37 per 1000 person‐years). For LV systolic function, 1‐SD larger EFV was associated with 0.76 (95% CI, 0.54–0.98) mm larger LV end‐diastolic dimension, 0.66 (95% CI, 0.47–0.85) mm larger LV end‐systolic dimension, and 0.56% (95% CI, −0.86% to −0.27%) lower LV ejection fraction. Interactions between EFV and time were small. For LV diastolic function, 1‐SD larger EFV was associated with 1.02 (95% CI, 0.78–1.27) mm larger left atrial diameter. Larger EFV was also associated with incident HF (hazard ratio per 1‐SD increase in EFV, 1.34 [95% CI, 1.07–1.68] per 1‐SD larger EFV).
Conclusions
We report an independent association between EFV with new‐onset HF in the general population. EFV seems to exert its influence on HF through different pathways contributing to deteriorations in systolic function and larger left atrial size in part, likely through mechanical restraint and hypertrophy.
Keywords: cardiac function, ectopic fat, epicardial fat volume, heart failure, left ventricular diastolic function, left ventricular systolic function
Subject Categories: Heart Failure, Echocardiography, Imaging, Aging, Epidemiology
Nonstandard Abbreviations and Acronyms
- EFV
epicardial fat volume
- E / A
E wave/A wave
- LVM
left ventricular mass
- RS
Rotterdam Study
Clinical Perspective.
What Is New?
Larger volumes of epicardial fat were associated with worsening in repeated echocardiographic parameters of left ventricular systolic function and larger left atrial size in an elderly population free of heart failure.
During follow‐up, larger epicardial fat volume also showed an independent association with new‐onset heart failure, independent from coronary heart disease and atrial fibrillation.
What Are the Clinical Implications?
Further research into mechanisms linking epicardial fat to heart failure may assist in the treatment of cardiometabolic disease by pharmacological modulation of epicardial fat.
Heart failure (HF) is a life‐threatening syndrome with substantial morbidity and mortality and increasing incidence at older ages. 1 , 2 Adiposity plays a role in the pathophysiology of HF through inflammation. 3 Beyond overall adiposity, obesity also prompts ectopic fat accumulation in tissues and around internal organs. 4 Epicardial fat is an ectopic visceral fat depot, located in the atrioventricular and interventricular grooves of the heart, extending to its apex. 5 Sharing microcirculation with the myocardium facilitates harmful changes induced by epicardial fat to coronary arteries and myocardium via local inflammatory and mechanical effects. 5 , 6 This way, ectopic fat deposition within the heart may impact cardiovascular function. 4
Clinical studies have shown infiltration of epicardial adipose tissue to the myocardium and increased risk of coronary artery disease and atrial fibrillation (AF). 3 , 4 The association of epicardial fat with hypertension, diabetes, and metabolic syndrome further supports the potential role of epicardial fat in the pathophysiology of HF. 7 In this regard, epicardial fat around the proximal coronary arteries has been prospectively associated with higher risk of HF in community‐dwelling men and women. 7 However, few studies in small selected groups of participants with HF 3 , 8 , 9 , 10 have shown a link between larger epicardial fat volumes (EFVs) with worse left ventricular (LV) diastolic function 8 or hemodynamic balance, 11 relating EFV to a phenotype within the HF spectrum. 3 The effect of epicardial fat on LV systolic contractility is still unclear. 3 On the basis of these studies, epicardial fat has shown incongruous roles in different defined phenotypes of HF, 12 and the role of this ectopic fat in cardiac dysfunction requires further investigation.
To further understand the role of this ectopic fat in the pathophysiology of HF, we investigated the association of EFV with longitudinal changes in echocardiographic parameters of cardiac LV systolic and diastolic function in the general elderly population. We subsequently examined the association between EFV and new‐onset HF.
METHODS
The data that support the findings of our study are available from the corresponding author on reasonable request.
Study Design
This study was embedded within the RS (Rotterdam Study). The RS is a prospective population‐based cohort study performed in the Ommoord district, in the city of Rotterdam, the Netherlands. Details of the RS have been described previously. 13 The RS started in 1989 (RS‐I; n=7983) and was extended twice: in 2000 (RS‐II; n=3011) and 2006 (RS‐III; n=3932). Baseline examination took place at the beginning of each cohort, with a follow‐up every 3 to 4 years. Between 2003 and 2006 (RS‐I‐4 and RS‐II‐2), participants underwent noncontrast computed tomography (CT) scan of the heart as part of a project on the visualization of arterial calcification. Our study included individuals with data on CT‐based EFV and echocardiography measurements from the fourth examination of the original cohort (RS‐I‐4) and the second examination of the extended cohort (RS‐II‐2). From 2370 participants with available EFV measurements, 2365 also had echocardiography measurements available. Participants with no follow‐up or consent for follow‐up, those with a history of HF, AF, or coronary heart disease (CHD), and participants with outlying EFV measures were excluded. Thereafter, 2103 participants with available EFV and echocardiographic measurements remained for the current analyses (Figure 1).
Figure 1. Flowchart of the study population.
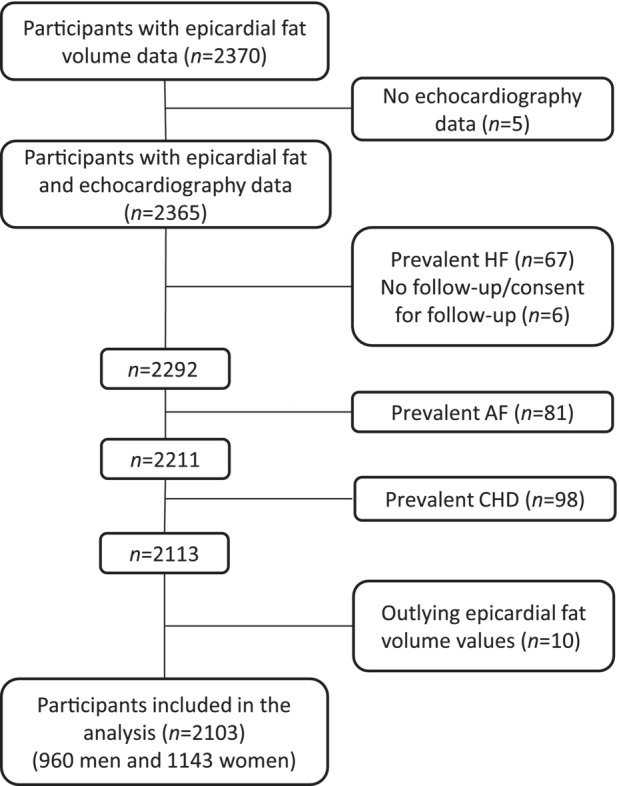
AF indicates atrial fibrillation; CHD, coronary heart disease; and HF, heart failure.
The RS has been approved by the Medical Ethics Committee of Erasmus Medical Center (registration No. MEC02.1015) and by the Ministry of Health, Welfare and Sport of the Netherlands (Population Screening Act (Wet op het Bevolkingsonderzoek): license No. 1071272‐159 521‐PG). All participants provided written informed consent to participate in the study and to have their information obtained from their physicians.
Assessment of EFV
At baseline (RSI‐4 and RSII‐2), participants went through noncontrast multidetector CT, and EFV was measured by either a 16‐ or a 64‐slice multidetector CT scanner (Somatom Sensation; Siemens, Forchheim, Germany). Participants received a cardiac scan and a scan of the aortic arch with the carotid arteries. Detailed information on imaging parameters has been described previously. 14 We used a fully automatic algorithm to quantify EFV in milliliters, which has previously been described in detail. 15
Assessment of LV Systolic and Diastolic Function Parameters
From RSI‐4 and RSII‐2 onwards, individuals went through transthoracic 2‐dimensional, M‐mode and Doppler echocardiography at every examination performed by commercially available ultrasonography systems (AU3 Partner, Esaote Biomedica, with a 3.5/2.5‐MHz transducer or Acuson Cypress, with a 3V2c transducer). As of January 2009, a Vivid I (GE Healthcare, Little Chalfont, UK), with a 3 S‐RS Sector Array probe (1.5–3.6 MHz) was used. (Number of individuals with echocardiography data in the RS were RSI‐4 and RSII‐2=5301; RSI‐5 and RSII‐3=3378; RSI‐6 and RSII‐4=1669). Left systolic function indexes included LV end‐diastolic dimension and LV end‐systolic dimension, in millimeters. To quantify LV systolic function, LV ejection fraction (LVEF) was calculated using LV end‐systolic and end‐diastolic volumes based on the Teichholz formula. 16 Indexes of LV diastolic function included mitral E wave/A wave (E/A) ratio and mitral valve deceleration time in m/s. Left atrial (LA) anteroposterior diameter was measured from the 2‐ and 4‐chamber parasternal echocardiography views in millimeters. LV mass (LVM) indexed by body surface area was calculated using the cube formula.
Assessment of New‐Onset HF During Follow‐Up
Details of determining HF in participants of the RS have been described previously. 17 Prevalent HF at entry of the original cohorts was based on clinical information from medical records and by using a validated score, using a combination of clinical symptoms or signs of HF from medical records, such as breathlessness at rest or during exertion, ankle edema, and pulmonary crepitation, as confirmed by imaging and by a specialist, similar to the definition of HF by the European Society of Cardiology. 18 , 19 In the subsequent cohorts, medical records of participants were screened for prevalent HF at entry. Thereafter, incidence of HF during follow‐up was defined on the basis of clinical information continuously collected from medical records. HF diagnosis was ascertained by a clinician. 19 In this study, participants with a history of HF at entry to the RS cohorts or a recorded incident HF event before the baseline of our study (RSI‐4 and RSII‐2 examination) were defined as having prevalent HF. Follow‐up time was calculated as the time difference between the examination date and the date of HF diagnosis for those with incident HF or last date of follow‐up for HF for the censored participants. Follow‐up for incident HF was complete until January 1, 2016.
Cardiovascular Risk Factors
Information on covariates was collected through home interviews, obtained by using an extensive questionnaire, or obtained at the examination rounds. 13 Body mass index (BMI) was calculated by dividing body weight (kilograms) by height (meters) squared. Blood pressure was measured twice at the right arm with a random‐zero sphygmomanometer, and the average of the 2 measurements was used. 20 Prevalence of CHD was defined as (fatal and nonfatal) myocardial infarction and fatal CHD. 19 Serum total and high‐density lipoprotein cholesterol (mmol/L) were measured using standard techniques. Prevalent diabetes was defined as a fasting blood glucose concentration of ≥7.0 mmol/L or use of blood glucose–lowering medication. Smoking status (current) and use of antihypertensive and lipid‐lowering medication were assessed by questionnaires.
Statistical Analysis
Continuous variables were presented as mean (SD), and categorical variables were presented as numbers (percentages). Variables between men and women were compared using Student t‐test or χ2 test based on their distribution. We used multiple imputation for missing values on covariates (highest proportion of missing was 1.6%). Parameter estimates were obtained by pooling 5 imputed data sets using Rubin rules. 21
To study the association between EFV (per SD increase) and repeated measurements of echocardiographic parameters of LV systolic and diastolic function (maximum of 3 measurements) during follow‐up, we used linear mixed effects models with random intercepts and slopes and an unstructured variance‐covariance matrix. In the fixed‐effect part, analyses were first adjusted for sex, age (time varying) at examination, and cohort (model 1). Then, models were further adjusted for baseline (fixed) prevalent diabetes, BMI, smoking, systolic blood pressure, diastolic blood pressure, use of antihypertensive medication, serum total and high‐density lipoprotein cholesterol, and use of lipid‐lowering medication (model 2). To account for changes in the associations between EFV and repeated measurements of cardiac function during follow‐up, we repeated the analyses also adding the interaction between EFV and time in the fixed effects part of the analyses.
The association of EFV with incident HF was investigated with Cox‐proportional hazard regression models. The Schoenfeld test of residuals using the Kaplan‐Meier estimate of the survival function was used to assess the proportionality of the models. Analyses were first adjusted for age, sex, and cohort (model 1). Next, we additionally adjusted the analyses for prevalent diabetes, BMI, smoking, systolic blood pressure, diastolic blood pressure, use of antihypertensive medication, serum total and high‐density lipoprotein cholesterol, and use of lipid‐lowering medication (model 2). To further assess if the associations were also independent from CHD and AF during follow‐up and before incident HF, survival analyses were additionally adjusted for CHD and AF events during follow‐up as time‐varying covariates. All analyses were also performed among women and men, separately.
We examined the nonlinearity of age and EFV using restricted cubic splines and possible interactions between EFV and covariates in the analyses using χ 2 test (P<0.2 was considered significant).
In sensitivity analyses, analyses were adjusted for waist circumference instead of BMI. All analyses were also performed with further adjustment for coronary artery calcification (Agatston score). Finally, we adjusted all analyses between epicardial fat and cardiac function with LVM index and the survival analyses additionally with LVM index and LA size to assess possible modification of analyses by heart size.
Analyses were performed with R software version 3.6.1 (packages: rms, nlme, survival, and ggplot2).
RESULTS
Characteristics of the study population are shown in Table 1. Mean (SD) age was 68 (±6.3) years in the total population, and 1147 (54.4%) were women. Mean (SD) EFV was 105.6 (37.5) mL in the total population. Mean (SD) of LVEF in the total population was 65.7% (6.67%). Mean E/A ratio and LA diameter were 0.91 (0.27) and 40.2 (5.29) mm, respectively. Men had larger EFV than women (mean [SD]: 122.2 [39.9] mL in men and 91.7 [28.9] mL in women) (Table S1).
Table 1.
Characteristics of the Study Population at Baseline
| Characteristic | Total population (N=2103) |
|---|---|
| Women, N (%) | 1143 (54.4) |
| Age, y | 68 (6.33) |
| Waist circumference, cm | 93.7 (11.4) |
| BMI, kg/m2 | 27.6 (3.96) |
| SBP, mm Hg | 147 (19.9) |
| DBP, mm Hg | 80.6 (10.7) |
| Antihypertensive use, N (%) | 745 (36) |
| Smoking, N (%) | |
| Current | 609 (29.6) |
| Never | 1117 (54.4) |
| Former | 329 (16.0) |
| Total cholesterol, mmol/L | 5.75 (0.96) |
| HDL cholesterol, mmol/L | 1.46 (0.40) |
| Lipid‐lowering medication use, N (%) | 443 (21.0) |
| Prevalent diabetes, N (%) | 251 (11.9) |
| Prevalent CHD, N (%) | |
| EFV, mL | 105.6 (37.5) |
| Echocardiographic parameters | |
| LVEDD, mm | 51.8 (4.7) |
| LVESD, mm | 30.7 (4.5) |
| LVEF, % | 65.7 (6.67) |
| LA diameter, mm | 40.2 (5.29) |
| E/A ratio | 0.91 (0.27) |
| DT, ms | 212.7 (42.8) |
Data are mean (SD) for continuous variables and number (percentage) for categorical variables. BMI indicates body mass index; CHD, coronary heart disease; DBP, diastolic blood pressure; DT, deceleration time; E/A, E wave/A wave; EFV, epicardial fat volume; HDL, high‐density lipoprotein; LA diameter, left atrial anteroposterior diameter; LVEDD, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic dimension; and SBP, systolic blood pressure.
Association of EFV With Cardiac Function
Associations of EFV with LV end‐diastolic dimension, LV end‐systolic dimension, and LVEF were linear. In the multivariate‐adjusted mixed effects models, 1‐SD higher EFV was associated with 0.76 (95% CI, 0.54–0.98) and 0.66 (95% CI, 0.47–0.85) mm larger LV end‐diastolic dimension and LV end‐systolic dimension, respectively. The 1‐SD higher EFV was associated with 0.56% (95% CI, −0.86% to −0.27%) lower mean LVEF (Table 2). We also checked for changes in these associations with time; interaction terms between EFV and time were small (Table S2). Figure 2 shows the changes in LV end‐diastolic dimension, LV end‐systolic dimension, and LVEF during follow‐up for the 25th, 50th, and 75th percentile values of EFV. We observed similar results in the multivariable analyses in men and women (Table S3).
Table 2.
Association of EFV With Repeated Measures of Echocardiographic Parameters of Cardiac Function in the Total Population
| Echocardiographic parameters | Model 1 | Model 2 | ||
|---|---|---|---|---|
| β (95% CI) | P value | β (95% CI) | P value | |
| LVEDD | 1.27 (1.09 to 1.46) | <0.001 | 0.76 (0.54 to 0.98) | <0.001 |
| LVESD | 1.04 (0.88 to 1.21) | <0.001 | 0.66 (0.47 to 0.85) | <0.001 |
| LVEF | −0.77 (−1.02 to −0.52) | <0.001 | −0.56 (−0.86 to −0.27) | <0.001 |
| LA diameter | 1.83 (1.61 to 2.04) | <0.001 | 1.02 (0.78 to 1.27) | <0.001 |
| E/A ratio | −0.01 (−0.02 to −0.001) | 0.035 | 0.001 (−0.01 to 0.01) | 0.916 |
| DT | 0.33 (−1.28 to 1.94) | 0.689 | 0.04 (−1.88 to 1.96) | 0.964 |
Results are based on linear mixed effects models. Model 1 is adjusted for age (time varying), sex, and cohort. Model 2 is additionally adjusted for baseline values of prevalent diabetes, body mass index, smoking, systolic blood pressure, diastolic blood pressure, use of antihypertensive medication, total and high‐density lipoprotein cholesterol, and use of lipid‐lowering medication. The number of individuals with available data on outcomes was 2065 for LVEDD, 2037 for LVESD, 2045 for LVEF, 2089 for LA diameter, 2074 for E/A ratio, and 2045 for DT. DT indicates deceleration time; E/A, E wave/A wave; EFV, epicardial fat volume; LA diameter, left atrial anteroposterior diameter; LVEDD, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction; and LVESD, left ventricular end‐systolic dimension.
Figure 2. Longitudinal changes in repeated echocardiographic parameters of cardiac function for percentiles of epicardial fat volume (EFV).
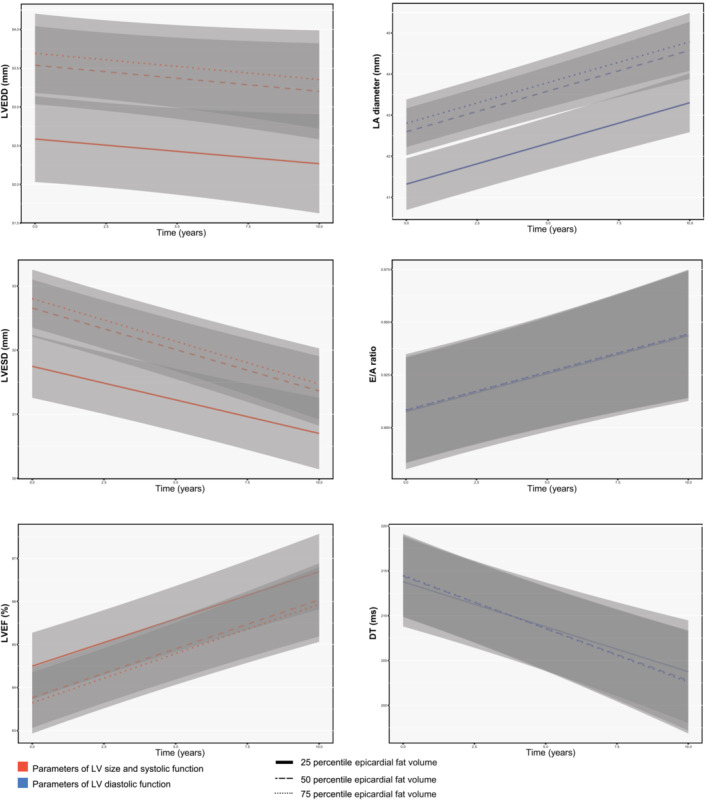
Changes in cardiac function and 95% CIs (gray area) are depicted for the 25th (simple line), 50th (dashed line), and 75th (dotted line) percentiles of EFV from the multivariable‐adjusted model, including interaction term between EFV and time. DT indicates deceleration time; E/A, E wave/A wave; LA, left atrial; LV, left ventricular; LVEDD, LV end‐diastolic dimension; LVEF, LV ejection fraction; and LVESD, LV end‐systolic dimension.
Associations between EFV and parameters of LV diastolic function were also linear. In the multivariate‐adjusted linear mixed effects models, 1‐SD larger EFV was associated with 1.02 (0.78 to 1.27) mm larger LA. During follow‐up, small changes were observed toward increasing LA diameter in the analyses (interaction between EFV and time: 0.05 [0.03 to 0.08]; Table S2). However, the associations with E/A ratio and deceleration time were not significant (Table 2). Figure 2 shows changes in LA diameter, E/A ratio, and deceleration time during follow‐up for the 25th, 50th, and 75th percentile values of EFV. Among men and women, EFV was also similarly associated with LA diameter, but not with E/A ratio or deceleration time (Tables S3 and S4).
Association of EFV With Incident HF
During a median follow‐up time of 9.7 years, 124 incident HF events occurred (incidence rate, 6.37 per 1000 person‐years of follow‐up). The incidence rate of HF was 6.41 and 6.34 per 1000 person‐years in men and women, respectively. The association between nonlinear EFV (restricted cubic splines) and incident HF was linear (P‐nonlinearity: 0.336). In multivariate model (model 2), 1‐SD increase in EFV showed a linear hazard ratio (HR) (95% CI) of 1.34 (1.07–1.68) in the total population (Table 3). The association between EFV and HF did not differ in men and women (HRs [95% CIs]: 1.30 [0.97–1.76] among men and 1.36 [0.96–1.93] among women; P for interaction: 0.897) (Table S5). We did not find interactions between EFV and covariates in the analysis (P‐interactions were >0.2).
Table 3.
Association of EFV With Incident HF in the Total Population
| Model | HR (95% CI) | P value |
|---|---|---|
| 1 | 1.37 (1.15–1.65) | 0.001 |
| 2 | 1.34 (1.07–1.68) | 0.010 |
| 3 | 1.32 (1.01–1.72) | 0.041 |
| 4 | 1.33 (1.02–1.74) | 0.036 |
Model 1 is adjusted for age, sex, and cohort. Model 2 is additionally adjusted for baseline values of prevalent diabetes, body mass index, smoking, systolic blood pressure, diastolic blood pressure, use of antihypertensive medication, total and high‐density lipoprotein cholesterol, and use of lipid‐lowering medication. Model 3 is model 2 plus additional adjustment for coronary heart disease as a time‐varying covariate. Model 4 is model 3 plus additional adjustment for atrial fibrillation as a time‐varying covariate. EFV indicates epicardial fat volume; HF, heart failure; and HR, hazard ratio.
After adjusting for CHD (86 events) and AF (134 events) during follow‐up, the association of EFV and incident HF in the total population did not change (HR [95% CI]: 1.32 [1.01–1.72] and 1.33 [1.02–1.74] for adjustment with CHD and AF, respectively) (Table 3). This was also the case in the sex‐stratified analyses (Table S5), although the associations were not significant anymore in women. In ancillary analyses, adjustment with waist circumference instead of BMI and further adjustment for coronary artery calcification did not change our results (data not shown). After adjusting our analyses with LVM index, our results did not drastically change, but the estimates for cardiac function parameters and HF incidence were reduced. Addition of LA diameter to LVM index in the survival analyses did not further change the findings (Tables S6 and S7).
DISCUSSION
Larger volumes of epicardial fat were associated with worsening in echocardiographic parameters of LV systolic function and larger LA size in an elderly population free of HF. During follow‐up, larger EFV also showed an independent association with new‐onset HF. Our results suggest that larger EFV could be associated with deterioration in cardiac function leading to HF, partly by its mechanical component.
Studies on the association between epicardial fat with HF or cardiac function in the general population are sparse and have not often examined longitudinal clinical outcomes. Previous cross‐sectional reports have mostly included small, selected samples of individuals with HF. 3 , 8 , 10 , 12 , 22 On the basis of these studies, epicardial fat has shown divergent possible roles in the pathophysiology of different HF profiles. 12 In one study, larger EFV was more common in 64 individuals with HF and LVEF >40% compared with controls. 3 However, decreased volumes of EFV were reported in individuals with HF and LVEF <35%. 10 This is while more recent studies have shown increased epicardial fat tissue in individuals with reduced or preserved LVEF (HF with reduced ejection fraction or HF with preserved ejection fraction [HFpEF]), and one has also reported reduced thickness in both profiles. 12 Following these studies, in hypothesis‐generating analyses, a prospective study showed a possible higher HR of HF with incident preserved LVEF, midrange LVEF but not HF with reduced ejection fraction, suggesting different pathways relating epicardial fat to HF profiles. 7 However, their findings may have been affected by a reduced number of events in their study. 7
Among individuals with HF and LVEF >50%, higher EFV was associated with parameters of LV diastolic function among women aged >60 years. 8 And epicardial adipose tissue showed a moderate correlation with diastolic dysfunction among 127 hypertensive individuals with normal LV systolic function. 22 Epicardial fat thickness has also been significantly correlated with LVEF and E/A ratio. 23 These findings have mainly suggested a link between epicardial fat with diastolic dysfunction in HFpEF. In a more recent study, lower epicardial fat tissue correlated with worse LV systolic function in HF with reduced ejection fraction, whereas increased epicardial fat was related to ventriculo‐atrial uncoupling and hemodynamic changes in HFpEF. 11 This is while increased EFV has also been associated with worse LV strain in HFpEF and diastolic dysfunction 3 and with cardiac contractile function in participants with HF and diabetes. 24 Active changes in function lead to unique trajectories and a spectrum of phenotypes with overlapping, distinct characteristics in HF. 25 Thus, different profiles of HF have overlapping epidemiological and pathophysiological features. 25 Bidirectional LVEF changes in HF, use of different cutoffs and LVEF‐based classification separating overlapping groups of patients, and the cross‐sectional nature of most of these studies may have resulted in different findings. 25 , 26
To study the role of EFV in the spectral nature of HF in more detail, we investigated its association with continuous indexes of cardiac function changes in the general population before HF presentation. Systolic dysfunction is caused by decreased pump function with reduced LVEF and an enlarged end‐diastolic chamber volume. 27 , 28 And diastolic dysfunction is a result of any pathophysiological condition accompanied by LV stiffness, characterized by an increased resistance to filling of the ventricles. 27 , 28 , 29 The effect of epicardial fat on cardiac mechanics mediated by pericardial restraint and remodeling can lead to systolic dysfunction. 30 Larger EFV may contribute to diastolic dysfunction by decreasing coronary flow reserve by secreting mediators that affect myocardial tissue and coronary arteries 31 ; hemodynamic derangements have also played a role. 30 In our study among community‐dwelling individuals free of HF, larger EFV was strongly related to worsening of systolic function during follow‐up. This implies that increase in epicardial fat may be more closely associated with deteriorations in the cardiac systolic function, likely through mechanical obstruction and hypertrophy. In addition, larger volumes of epicardial fat were associated with larger LA diameters during follow‐up. Increase in epicardial fat thickness has shown correlations with enlarged atria and impaired LV diastolic filling. 32 Functional LA changes become evident at the earliest stages of LV diastolic dysfunction, and LA metrics may improve the evaluation of diastolic dysfunction and, as a result, also HFpEF. 26 , 33
At the same time, larger epicardial fat volume was associated with higher risk of new‐onset HF in our study. Epicardial fat may have an important role in the cardiovascular physiology and pathophysiology through its mechanical, metabolic, and thermogenic functions. 4 , 6 Modulating epicardial fat with pharmacological agents has been suggested as a potential strategy in cardiometabolic disease treatment. 6 Increased epicardial fat may result in myocardial dysfunction and remodeling via fatty degeneration of the myocardium or extension between myocardial bundles, lead to coronary atherosclerosis and cardiomyopathy via paracrine or vasocrine pathways, which could, in turn, result in HF. 3 , 5 , 6 The association of epicardial fat with hypertension, diabetes, and altered hemodynamics 11 and diastolic, but not systolic, dysfunction has also been suggested. 7 Epicardial fat also might have direct effects on cardiac mechanics mediated by pericardial restraint. 30 On the basis of our study, the link to cardiac dysfunction in HF may be via both systolic and diastolic dysfunction. Increased LA size is also an established predictor of AF. 34 As such, increases in EFV could show arrhythmogenic effects contributing to HF. 32 , 35 The magnitude of epicardial fat might additionally pose a noticeable restraint in cardiac expansion, altering cardiac function in the long‐term, resulting in clinical HF. 3 , 36 The specific location of increased epicardial fat might also influence the profile of HF. 37
HF pathophysiology might differ between men and women, 38 and the incidence of HF is lower in women than in men. 39 Sex‐based differences in the amount of this ectopic fat are also unclear. 7 We did not observe substantial differences in the association of EFV with cardiac function and incident HF between men and women. In our population, EFV was larger in men than women. Our findings are in line with a recent prospective study in men and women from the MESA (Multi‐Ethnic Study of Atherosclerosis). 7 However, in another study, epicardial fat thickness did not differ between men and women aged <60 years with HF but was greater only in women aged >60 years and was associated with LV function only in postmenopausal women, 8 grounding the findings on the increase in visceral adiposity attributable to deficiency of estrogen.
Epicardial fat has shown associations with AF and CHD, 3 , 4 , 35 2 underlying conditions contributing to HF. In the total population, after taking CHD or AF during follow‐up into account, the association between EFV and incident HF did not change. This could further highlight the mechanical restrictive impact of EFV on cardiac structure and function above its possible impact of EFV on LA size in connection with AF. In sex‐stratified analyses, similar results were observed, although the associations lost significance in women. Among women, the impact of EFV on HF might be partly explained through its arrhythmogenic impact and AF, also observed as its association with increasing LA size. Mean EFV was also smaller in women. This could imply that the impact of epicardial fat through AF and CHD, mostly related to its paracrine functions, is more pronounced in them and larger volumes of epicardial fat are required to have the proper impact on cardiac structure and function among women.
Visceral adiposity, more than excess fat per se, could play a role in the changes in LV morphology. 40 Whether echocardiographic epicardial fat can be associated with intramyocardial fat accumulation and fatty degeneration is a subject of investigation. 5 Epicardial fat has shown to be associated with higher LV mass. 40 , 41 , 42 Further adjustment with LV mass did not hinder our findings but was accompanied by reduced associations, which further advocates that changes in epicardial fat possibly impose on LV structure and morphology and may mediate the observed associations.
Strengths of our study are its population‐based design and access to detailed information on cardiovascular risk factors. Availability of well‐adjudicated HF events and detailed follow‐up information allowed us to investigate the longitudinal impact of epicardial fat on new‐onset HF among men and women from general population. Our findings with increased risk of HF are in line with previous finding in a prospective study 7 and benefit from a fully automatic of the whole heart, CT‐based assessment that is also a superior method in accurate quantification of EFV. 43 Application of robust mixed effects models enabled us to use repeated measurements of cardiac function parameters. There are also limitations. We used LVEF quantified by the Teichholz formula, which could result in higher estimation of LVEF in case of LV wall motion abnormalities. 16 However, participants with a history of myocardial infarction were excluded from the study, which would minimize this issue. Changes in cardiac function associated with EFV observed in our study were small, which may reduce the clinical relevance of these findings despite the observed increase in the risk of incident HF. This could be attributable to use of continuous linear echocardiographic measures of cardiac function. Also, about 75% of epicardial fat resides over the right ventricle. Because the impact of epicardial fat depends on its location, 37 we believe investigating changes in right ventricular filling pressure and function may show further impact of epicardial fat on cardiac function. Also, mean changes in cardiac function in an aging population and in an observational setting may not be of a magnitude to capture a true clinical estimate in changes of cardiac function associated with EFV increase. Thus, further studies are needed to replicate our findings and to study the effect of EFV on cardiac dysfunction in a randomized clinical setting. Moreover, measures of LV systolic or diastolic function at the time of HF diagnosis were not present in the RS for further assessment of the link between epicardial fat and HF classifications. Because different profiles of HF share pathophysiological characteristics regardless of LVEF‐based profiles, 25 we believe studying the impact of EFV on cardiac function could help with a better understanding of its role in HF syndrome. Advancing knowledge on the role of epicardial fat in cardiac dysfunction and its influence on risk stratification for primary prevention of HF warrants further research. Our population consists of middle‐aged and elderly participants of European ancestry. Therefore, our findings might not be generalizable to younger populations and other ethnicities.
CONCLUSIONS
Larger EFV was associated with worsening of echocardiographic parameters of LV systolic function and LA size. Furthermore, larger EFV showed independent association with new‐onset HF in the general population, which was independent from CHD and AF. Our results suggest that larger volumes of epicardial fat may also be associated with deteriorations in the cardiac systolic function, most likely through changes in LV morphology and mechanical restrictions.
Sources of Funding
The RS (Rotterdam Study) is funded by Erasmus Medical Center and Erasmus University, Rotterdam, Netherlands Organization for the Health Research and Development, the Research Institute for Diseases in the Elderly, the Ministry of Education, Culture and Science, the Ministry for Health, Welfare and Sports, the European Commission, and the Municipality of Rotterdam. This study is further supported by the Senior Scientist Grant from the Dutch Heart Foundation (03‐004‐2021‐T050).
Disclosures
None.
Supporting information
Table S1–S7
Acknowledgments
We gratefully acknowledge the dedication, commitment, and contribution of the inhabitants, general practitioners, and pharmacists of the Ommoord district to the RS (Rotterdam Study). Author contributions: Dr Arshi has been involved in conceptualization, data curation, analysis, investigation, methods, visualization, writing, revising, and editing. Dr Aliahmad has been involved in conceptualization, data curation, investigation, writing, and editing. Dr Ikram has contributed to data curation, funding acquisition, methods, and reviewing. Dr Bos has been involved in data collection, methods, administration, writing, and reviewing. Dr Kavousi has contributed in conceptualization, investigation, methods, administration, supervision, writing, and reviewing.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.026197
For Sources of Funding and Disclosures, see page 9.
References
- 1. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 2. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2013;128:1810–1852. doi: 10.1161/CIR.0b013e31829e8807 [DOI] [PubMed] [Google Scholar]
- 3. van Woerden G, Gorter TM, Westenbrink BD, Willems TP, van Veldhuisen DJ, Rienstra M. Epicardial fat in heart failure patients with mid‐range and preserved ejection fraction. Eur J Heart Fail. 2018;20:1559–1566. doi: 10.1002/ejhf.1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Iacobellis G, Bianco AC. Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends Endocrinol Metab. 2011;22:450–457. doi: 10.1016/j.tem.2011.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iacobellis G, Willens HJ. Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr. 2009;22:1311–1319. doi: 10.1016/j.echo.2009.10.013 [DOI] [PubMed] [Google Scholar]
- 6. Iacobellis G. Epicardial fat: a new cardiovascular therapeutic target. Curr Opin Pharmacol. 2016;27:13–18. doi: 10.1016/j.coph.2016.01.004 [DOI] [PubMed] [Google Scholar]
- 7. Kenchaiah S, Ding J, Carr JJ, Allison MA, Budoff MJ, Tracy RP, Burke GL, McClelland RL, Arai AE, Bluemke DA. Pericardial fat and the risk of heart failure. J Am Coll Cardiol. 2021;77:2638–2652. doi: 10.1016/j.jacc.2021.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim S‐A, Kim M‐N, Shim W‐J, Park S‐M. Epicardial adipose tissue is related to cardiac function in elderly women, but not in men. Nutr Metab Cardiovasc Dis. 2017;27:41–47. doi: 10.1016/j.numecd.2016.11.001 [DOI] [PubMed] [Google Scholar]
- 9. Khawaja T, Greer C, Chokshi A, Chavarria N, Thadani S, Jones M, Schaefle K, Bhatia K, Collado JE, Shimbo D. Epicardial fat volume in patients with left ventricular systolic dysfunction. Am J Cardiol. 2011;108:397–401. doi: 10.1016/j.amjcard.2011.03.058 [DOI] [PubMed] [Google Scholar]
- 10. Doesch C, Haghi D, Flüchter S, Suselbeck T, Schoenberg SO, Michaely H, Borggrefe M, Papavassiliu T. Epicardial adipose tissue in patients with heart failure. J Cardiovasc Magn Reson. 2010;12:1–9. doi: 10.1186/1532-429X-12-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pugliese NR, Paneni F, Mazzola M, De Biase N, Del Punta L, Gargani L, Mengozzi A, Virdis A, Nesti L, Taddei S. Impact of epicardial adipose tissue on cardiovascular haemodynamics, metabolic profile, and prognosis in heart failure. Eur J Heart Fail. 2021;23:1858–1871. doi: 10.1002/ejhf.2337 [DOI] [PubMed] [Google Scholar]
- 12. Tromp J, Bryant JA, Jin X, van Woerden G, Asali S, Yiying H, Liew OW, Ching JCP, Jaufeerally F, Loh SY. Epicardial fat in heart failure with reduced versus preserved ejection fraction. Eur J Heart Fail. 2021;23:835–838. doi: 10.1002/ejhf.2156 [DOI] [PubMed] [Google Scholar]
- 13. Ikram MA, Brusselle G, Ghanbari M, Goedegebure A, Ikram MK, Kavousi M, Kieboom BCT, Klaver CCW, de Knegt RJ, Luik AI. Objectives, design and main findings until 2020 from the Rotterdam study. Eur J Epidemiol. 2020;35:483–517. doi: 10.1007/s10654-020-00640-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Odink AE, van der Lugt A, Hofman A, Hunink MGM, Breteler MMB, Krestin GP, Witteman JCM. Association between calcification in the coronary arteries, aortic arch and carotid arteries: the Rotterdam study. Atherosclerosis. 2007;193:408–413. doi: 10.1016/j.atherosclerosis.2006.07.007 [DOI] [PubMed] [Google Scholar]
- 15. Shahzad R, Bos D, Metz C, Rossi A, Kirişli H, van der Lugt A, Klein S, Witteman J, de Feyter P, Niessen W. Automatic quantification of epicardial fat volume on non‐enhanced cardiac CT scans using a multi‐atlas segmentation approach. Med Phys. 2013;40:091910. doi: 10.1118/1.4817577 [DOI] [PubMed] [Google Scholar]
- 16. Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic‐angiographic correlations in the presence or absence of asynergy. Am J Cardiol. 1976;37:7–11. doi: 10.1016/0002-9149(76)90491-4 [DOI] [PubMed] [Google Scholar]
- 17. Mosterd A, Hoes AW, De Bruyne MC, Deckers JW, Linker DT, Hofman A, Grobbee DE. Prevalence of heart failure and left ventricular dysfunction in the general population: the Rotterdam Study. Eur Heart J. 1999;20:447–455. doi: 10.1053/euhj.1998.1239 [DOI] [PubMed] [Google Scholar]
- 18. Swedberg K, Cleland J, Dargie H, Drexler H, Follath F, Komajda M, Tavazzi L, Smiseth OA, Gavazzi A, Haverich A. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005) the task force for the diagnosis and treatment of chronic heart failure of the European Society of Cardiology. Eur Heart J. 2005;26:1115–1140. doi: 10.1093/eurheartj/ehi204 [DOI] [PubMed] [Google Scholar]
- 19. Leening MJG, Kavousi M, Heeringa J, van Rooij FJA, Verkroost‐van Heemst J, Deckers JW, Mattace‐Raso FUS, Ziere G, Hofman A, Stricker BHC. Methods of data collection and definitions of cardiac outcomes in the Rotterdam study. Eur J Epidemiol. 2012;27:173–185. doi: 10.1007/s10654-012-9668-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen Q‐J, Lai H‐M, Chen B‐D, Li X‐M, Zhai H, He C‐H, Pan S, Luo J‐Y, Gao J, Liu F. Appropriate LDL‐C‐to‐HDL‐C ratio cutoffs for categorization of cardiovascular disease risk factors among Uygur adults in Xinjiang, China. Int J Environ Res Public Health. 2016;13:235. doi: 10.3390/ijerph13020235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Van Buuren S, Groothuis‐Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–6. [Google Scholar]
- 22. Çetin M, Kocaman SA, Durakoğlugil ME, Erdoğan T, Ergül E, Dogan S, Çanga A. Effect of epicardial adipose tissue on diastolic functions and left atrial dimension in untreated hypertensive patients with normal systolic function. J Cardiol. 2013;61:359–364. doi: 10.1016/j.jjcc.2012.12.015 [DOI] [PubMed] [Google Scholar]
- 23. Young LR, Backus RC. Serum 25‐hydroxyvitamin D3 and 24R, 25‐dihydroxyvitamin D3 concentrations in adult dogs are more substantially increased by oral supplementation of 25‐hydroxyvitamin D3 than by vitamin D3. J Nutr Sci. 2017;6:e30. doi: 10.1017/jns.2017.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Levelt E, Pavlides M, Banerjee R, Mahmod M, Kelly C, Sellwood J, Ariga R, Thomas S, Francis J, Rodgers C. Ectopic and visceral fat deposition in lean and obese patients with type 2 diabetes. J Am Coll Cardiol. 2016;68:53–63. doi: 10.1016/j.jacc.2016.03.597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Triposkiadis F, Butler J, Abboud FM, Armstrong PW, Adamopoulos S, Atherton JJ, Backs J, Bauersachs J, Burkhoff D, Bonow RO. The continuous heart failure spectrum: moving beyond an ejection fraction classification. Eur Heart J. 2019;40:2155–2163. doi: 10.1093/eurheartj/ehz158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beale AL, Meyer P, Marwick TH, Lam CSP, Kaye DM. Sex differences in cardiovascular pathophysiology: why women are overrepresented in heart failure with preserved ejection fraction. Circulation. 2018;138:198–205. doi: 10.1161/CIRCULATIONAHA.118.034271 [DOI] [PubMed] [Google Scholar]
- 27. Federmann M, Hess OM. Differentiation between systolic and diastolic dysfunction. Eur Heart J. 1994;15:2–6. doi: 10.1093/eurheartj/15.suppl_D.2 [DOI] [PubMed] [Google Scholar]
- 28. Hess OM. Hamodynamik bei herzinsuffizienz: systolische und diastolische dysfunktion. Ther Umsch. 1993;50:414–418. [PubMed] [Google Scholar]
- 29. Satpathy C, Mishra TK, Satpathy R, Satpathy HK, Barone EJ. Diagnosis and management of diastolic dysfunction and heart failure. Am Family Physician. 2006;73:841–846. [PubMed] [Google Scholar]
- 30. Koepp KE, Obokata M, Reddy YNV, Olson TP, Borlaug BA. Hemodynamic and functional impact of epicardial adipose tissue in heart failure with preserved ejection fraction. Heart Fail. 2020;8:657–666. doi: 10.1016/j.jchf.2020.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brahimaj A, Ahmadizar F, Vernooij MW, Ikram MK, Ikram MA, van Walsum T, Dehghan A, Franco Duran OH, Bos D, Kavousi M. Epicardial fat volume and the risk of cardiometabolic diseases among women and men from the general population. Eur J Prev Cardiol. 2021;28:e14–e16. [DOI] [PubMed] [Google Scholar]
- 32. Iacobellis G, Zaki MC, Garcia D, Willens HJ. Epicardial fat in atrial fibrillation and heart failure. Horm Metab Res. 2014;46:587–590. doi: 10.1055/s-0034-1367078 [DOI] [PubMed] [Google Scholar]
- 33. Thomas L, Marwick TH, Popescu BA, Donal E, Badano LP. Left atrial structure and function, and left ventricular diastolic dysfunction: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2019;73:1961–1977. doi: 10.1016/j.jacc.2019.01.059 [DOI] [PubMed] [Google Scholar]
- 34. Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH. Atrial fibrillation: epidemiology, pathophysiology, and clinical outcomes. Circ Res. 2017;120:1501–1517. doi: 10.1161/CIRCRESAHA.117.309732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bos D, Vernooij MW, Shahzad R, Kavousi M, Hofman A, van Walsum T, Deckers JW, Ikram MA, Heeringa J, Franco OH. Epicardial fat volume and the risk of atrial fibrillation in the general population free of cardiovascular disease. JACC: Cardiovasc Imaging. 2017;10:1405–1407. doi: 10.1016/j.jcmg.2016.12.005 [DOI] [PubMed] [Google Scholar]
- 36. Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136:6–19. doi: 10.1161/CIRCULATIONAHA.116.026807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu CK, Lee JK, Hsu JC, Su MYM, Wu YF, Lin TT, Lan CW, Hwang JJ, Lin LY. Myocardial adipose deposition and the development of heart failure with preserved ejection fraction. Eur J Heart Fail. 2020;22:445–454. doi: 10.1002/ejhf.1617 [DOI] [PubMed] [Google Scholar]
- 38. Rueda‐Ochoa OL, Smiderle‐Gelain MA, Rizopoulos D, Dhana K, van den Berge J‐K, Echeverria LE, Ikram MA, Deckers JW, Franco OH, Kavousi M. Risk factors for longitudinal changes in left ventricular diastolic function among women and men. Heart. 2019;105:1414–1422. doi: 10.1136/heartjnl-2018-314487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016;13:368–378. doi: 10.1038/nrcardio.2016.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Iacobellis G, Ribaudo MC, Zappaterreno A, Iannucci CV, Leonetti F. Relation between epicardial adipose tissue and left ventricular mass. Am J Cardiol. 2004;94:1084–1087. doi: 10.1016/j.amjcard.2004.06.075 [DOI] [PubMed] [Google Scholar]
- 41. Iacobellis G, Petramala L, Barbaro G, Kargi AY, Serra V, Zinnamosca L, Colangelo L, Marinelli C, Ciardi A, De Toma G. Epicardial fat thickness and left ventricular mass in subjects with adrenal incidentaloma. Endocrine. 2013;44:532–536. doi: 10.1007/s12020-013-9902-5 [DOI] [PubMed] [Google Scholar]
- 42. Erdoğan T, Çetin M, Kocaman S, DurakoĞLugİL M, Ergul E, Ugurlu Y, Çanga A. Epicardial adipose tissue is independently associated with increased left ventricular mass in untreated hypertensive patients: an observational study. Anadolu Kardiyol Derg. 2013;13:320–327. [DOI] [PubMed] [Google Scholar]
- 43. Nichols JH, Samy B, Nasir K, Fox CS, Schulze PC, Bamberg F, Hoffmann U. Volumetric measurement of pericardial adipose tissue from contrast‐enhanced coronary computed tomography angiography: a reproducibility study. J Cardiovasc Comput Tomogr. 2008;2:288–295. doi: 10.1016/j.jcct.2008.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S7


