Abstract
BACKGROUND
Little attention has been paid to how well the American Heart Association's cardiovascular health (CVH) score predicts early‐onset diabetes in young adults. We investigated the association of CVH score with early‐ and later‐onset diabetes and with subsequent complications of diabetes.
METHODS AND RESULTS
Our sample included 4547 Black and White adults in the CARDIA (Coronary Artery Risk Development in Young Adults) study without diabetes at baseline (1985–1986; aged 18–30 years) with complete data on the CVH score at baseline, including smoking, body mass index, physical activity, diet quality, total cholesterol, blood pressure, and fasting blood glucose. Incident diabetes was determined based on fasting glucose, 2‐hour postload glucose, hemoglobin A1c, or self‐reported medication use throughout 8 visits for 30 years. Multinomial logistic regression was used to assess the association between CVH score and diabetes onset at age <40 years (early onset) versus age ≥40 years (later onset). Secondary analyses assessed the association between CVH score and risk of complications (coronary artery calcium, clinical cardiovascular disease, kidney function markers, diabetic retinopathy, and diabetic neuropathy) among a subsample with diabetes. We identified 116 early‐ and 502 later‐onset incident diabetes cases. Each 1‐point higher CVH score was associated with lower odds of developing early‐onset (odds ratio [OR], 0.64 [95% CI, 0.58–0.71]) and later‐onset diabetes (OR, 0.78 [95% CI, 0.74–0.83]). Lower estimates of diabetic complications were observed per 1‐point higher CVH score: 19% for coronary artery calcification≥100, 18% for cardiovascular disease, and 14% for diabetic neuropathy.
CONCLUSIONS
Higher CVH score in young adulthood was associated with lower early‐ and later‐onset diabetes as well as diabetic complications.
Keywords: cardiovascular health, early diabetes, prospective cohort, vascular complications, young adulthood
Subject Categories: Cardiovascular Disease, Epidemiology, Lifestyle, Diet and Nutrition
Nonstandard Abbreviations and Acronyms
- APDQS
A Priori Diet Quality Score
- CARDIA
Coronary Artery Risk Development in Young Adults
- IFG
impaired fasting glucose
- LS7
Life's Simple 7
JEL
Diabetes, Type 2Cardiovascular Disease Epidemiology Lifestyle Diet and Nutrition
Clinical Perspective.
What Is New?
A prospective cohort with a 30‐year follow‐up showed that a higher cardiovascular health score measured in young individuals aged 18 to 30 years was associated with lower early‐ and later‐onset diabetes as well as subsequent vascular complications of diabetes.
What Are the Clinical Implications?
The cardiovascular health score, a multifaceted assessment of cardiometabolic health, can be used as an indicator of future diabetes risk in generally healthy young adults.
The cardiovascular health score may help individuals in setting target goals for diabetes prevention and management.
Type 2 diabetes is becoming more prevalent among people aged <40 years in most regions of the world, including the United States. 1 Compared with later‐onset diabetes, early‐onset diabetes has a greater risk of micro‐ and macrovascular compilations as well as accelerated deterioration of glucose homeostasis. 1 , 2 , 3 , 4 Early‐onset diabetes risk factors include obesity, a sedentary lifestyle, a family history of diabetes, and low socioeconomic status. 5
The American Heart Association proposed cardiovascular health (CVH) metrics, Life's Simple 7 (LS7), which is an indicator of CVH that may be useful in reducing deaths from cardiovascular disease (CVD) and stroke. 6 The 7 metrics comprise physical activity, diet quality, smoking, body mass index (BMI), blood pressure (BP), fasting glucose, and total cholesterol. Each component is categorized as ideal, intermediate, or poor and is converted into a CVH score, which can then be used to assess how well individuals satisfy the LS7 goals.
Higher CVH score has been associated with a lower risk of diabetes in prospective cohort studies that followed middle‐aged or older adults, 7 , 8 , 9 , 10 but this association is largely understudied in young adulthood. If poor CVH status is formed throughout younger adulthood, the development of subclinical or clinically evident CVD and mortality may be more likely in midlife. 11 Furthermore, the relationship between CVH score and early‐onset diabetes has not been examined, despite the presumption that metabolic dysfunction phenotypes of early diabetes occurring earlier may be stronger than those that arise later in life. There are scarce data demonstrating whether CVH, especially lifestyle factors, is associated with a subsequent risk of vascular complications after diabetes onset in long‐term prospective studies beginning in young adulthood. 12 LS7 was constructed as a public health tool that was believed to promote individual CVD health. Many of the same variables are known to be related to future diabetes risk, but how well this multifaceted assessment of cardiometabolic health predicts diabetes that may have an earlier onset has been little studied.
Therefore, the present study tested the following hypotheses using >30 years of longitudinal data from the CARDIA (Coronary Artery Risk Development in Young Adults) study: (1) higher CVH score measured in young adulthood (an average age of 25 years) is associated with a lower odds of incident early‐onset and later‐onset diabetes, with the association being stronger for early‐onset diabetes; and (2) CVH score is inversely associated with the odds of incident micro‐ and macrovascular complications in those who develop diabetes.
METHODS
The data that support the findings of this study are available from the CARDIA Coordinating Center (https://www.cardia.dopm.uab.edu) on reasonable request.
Study Design and Population
The CARDIA study was established in 1985 and 1986 among 5115 Black and White men and women aged 18 to 30 years recruited from 4 urban communities (Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA). 13 By design, the following 4 factors were balanced at the center: age (18–24 years or 25–30 years), sex, self‐defined race (Black or White race), and education (high school graduate or lower versus higher education). After baseline, 8 examinations (years 2, 5, 7, 10, 15, 20, 25, and 30 [2015–2016]) were conducted at each center. Participants in the CARDIA study were contacted by phone, mail, or email every 6 months, and their interim medical histories and vital status were recorded annually with annual interviews to update health and vital status. Of the surviving participants, >71% attended the year 30 examination. The study protocol was approved by the institutional review boards at each institution, and all participants provided written informed consent at each study visit.
For the present study, we excluded participants who had a history of diabetes at or before baseline, were pregnant (year 0, n=34), lacked diabetes information (year 0, n=81), never attended a follow‐up clinic visit (n=153), lacked year 0 information on any of the 7 CVH components (n=395, primarily for not fasting before the blood draw or energy intake unacceptably large or small), or withdrew consent (n=1). The final analysis included 4547 participants. When compared with the included participants, the excluded participants were more likely to identify as Black race, have a lower educational level, and have a lower proportion of a list of the ideal CVH characteristics, such as smoking, diet quality, BP, and blood glucose. However, all population subgroups were well represented in those included.
Ascertainment of Diabetes
The primary outcome was incident diabetes, defined as meeting any of the following criteria for the first time from year 2 through year 30: fasting glucose ≥126 mg/dL (years 7–30), 2‐hour postload glucose ≥200 mg/dL (years 10, 20, and 25), hemoglobin A1c ≥6.5% (48 mmol/mol) at years 20 and 25, or self‐reported medication use for diabetes (per medication bottle brought to clinic). We were not able to distinguish between type 1 and type 2 diabetes, but type 1 onset is relatively rare in adulthood, and we excluded diabetes cases at year 0; therefore, we assume that the majority of cases are type 2 diabetes. According to the literature, diabetes onset at age <40 years was classified as early onset, and diabetes onset at age ≥40 years was described as later onset. 3 , 4 , 14
Ascertainment of Diabetes Complications
The secondary outcomes were 5 micro‐ and macrovascular complications of diabetes: coronary artery calcium (CAC), CVD, chronic kidney disease (CKD), diabetic retinopathy, and diabetic neuropathy.
Coronary Artery Calcium
CAC was assessed using chest computed tomography (CT) at years 15, 20, and 25. At years 15 and 20, 2 consecutive scans were performed and then averaged using electron beam CT (Chicago and Oakland centers) and multidetector CT (Birmingham and Minneapolis centers) scanners. At year 25, a single CT scan was performed using multidetector CT scanners. For each calcified lesion, a calcium score in Agatston units was determined, 15 and the values were summed for all lesions within a given artery and for all arteries (left anterior descending, left main, circumflex, and right coronary) to calculate the total calcium score.
CVD Outcomes
Clinical CVD outcomes were ascertained at annual follow‐up contacts and exams through August 31, 2019 (in the last 5 calendar years of the study, about 91% of CARDIA participants were successfully reached). All diagnoses of nonfatal CVD, including heart failure, were based on hospital records. Events were reported by participants during annual telephone, mail, or electronic contacts (with specific inquiry regarding hospitalizations), and deaths were identified on an ongoing basis from family contacts annually and queries of the National Death Index. With the approval of the next of kin, the death certificate, autopsy, and hospital documents were requested as necessary. Vital status follow‐up is thus virtually complete on all participants. Reported events were validated and adjudicated by 2 members of the CARDIA end points committee through medical record review using standard definitions. CVD was defined as a composite of myocardial infarction, non–myocardial infarction acute coronary syndrome, stroke, heart failure, carotid or peripheral artery disease, and deaths attributed to atherosclerotic coronary heart disease, other atherosclerotic disease, and nonatherosclerotic cardiac disease.
CKD Measures
Estimated glomerular filtration rate and urinary albumin‐to‐creatinine ratio were measured at years 10, 15, 20, 25, and 30. The serum creatinine‐based Chronic Kidney Disease Epidemiology Collaboration 2021 equation, 16 which does not consider race in its estimation, was used to determine the estimated glomerular filtration rate. Single untimed urine specimens taken soon after arrival at the clinic (mostly morning) were used to quantify urinary albumin and creatinine. Hospitalized or fatal kidney failure were ascertained in the annual contact up to August 31, 2019. CKD was defined as either estimated glomerular filtration rate <60 mL/min per 1.73 m2 or urinary albumin‐to‐creatinine ratio ≥30 mg/g. 17
Diabetic Retinopathy and Diabetic Neuropathy
The diagnoses of diabetic retinopathy and diabetic neuropathy were based on self‐reported medical history questionnaires administered only at exam years 25 and 30.
Assessment of CVH Score and Covariates
CVH score was calculated based on LS7 metrics at year 0: total cholesterol, BP, fasting blood glucose, smoking, BMI, physical activity, and diet. 6 Each component was classified as poor (0 points), intermediate (1 point), and ideal (2 points), and the total CVH score was calculated by summing all component scores, ranging from 1 to 14 points, as defined by American Heart Association criteria. The criteria for poor, intermediate, and ideal in each CVH component were defined in Table S1–S3. Because we excluded individuals with diabetes at baseline, the poor category of fasting glucose was not included. Total CVH score was categorized as poor (1–6 points), intermediate (7–8 points), or ideal (9–14 points).
Smoking
Cigarette smoking was assessed by self‐report. Participants were asked about smoking status and duration of smoking cessation.
Physical Activity
Leisure‐time physical activity was assessed using a validated interviewer‐administered CARDIA physical activity history questionnaire. 18 Participants reported the frequency of participation in 13 moderate or vigorous intensity physical activities during the past year. A total physical activity score (expressed as exercise units) was calculated by summing the weighted products of all activities (frequency in months×intensity of activity). For reference, 300 exercise units generally corresponds to the American College of Sports Medicine's guidelines for the quantity of exercise required to promote weight loss (5 sessions of 1260 kJ [300 kcal] of weekly energy expenditure). 19 A substudy in the Oakland clinic at year 25 showed that 300 exercise units corresponds to 150 min/week of moderate or vigorous physical activity. 20
Diet Quality
We used a more comprehensive diet quality score that is different from the diet component proposed by the American Heart Association. Diet was assessed using an interviewer‐administered diet history. The reliability and validity of the questionnaire were established previously. 21 Trained interviewers questioned participants about their food intake during the past month within 100 food categories and reported open‐ended responses regarding specific foods and beverages consumed, frequency of consumption, unit or serving sizes, and preparation methods. Total energy and nutrient intake were calculated based on the Nutrition Data System for Research (University of Minnesota, Minneapolis, MN). 22 About 950 items were collected at year 0 and were filtered down to 166 food groups based on the Nutrition Data System for Research's query system. Full scoring details are given in the supplement to a prior article (Table S2). 23 It was further collapsed into 46 food groups for the purpose of creating the A Priori Diet Quality Score (APDQS), a hypothesis‐driven diet quality index. The APDQS was composed of beneficially rated (n=20), adversely rated (n=13), and neutrally rated (n=13) food groups based on their presumed influence on CVD. Food intake was divided into quintiles according to servings per day consumed in each of the 46 food groups; beneficially rated food groups received positive scores (0 [lowest quintile]–4 [highest quintile]), and adversely rated food groups received reverse scores (4 [lowest quintile]–0 [highest quintile]). Neutrally rated food groups received a score of 0. The cut points used to define the quintiles for the individual 46 food groups are presented in Table S3. 24 For food groups with many recordings of 0 servings per day, the participants' values were grouped into 5 categories: category 1 (nonconsumers) and categories 2 to 5 (consumers divided into quartiles). For example, for avocado, the 0 is the first category, >0 to <0.06 is the second category, 0.06 to <0.14 is the third category, 0.14 to <0.31 is the fourth category, and ≥0.31 is the fifth category. The sum of the subscores for each food group's quintile category was used to compute the total APDQS (0–132). High APDQS represented a nutritionally rich, plant‐centered diet, given the number of points assigned to nutritionally rich plant foods. The APDQS has been validated with various clinical outcomes. 23 , 24 , 25 , 26
Body Mass Index
Participant height was measured to the closest 0.5 cm, and weight was measured to the closest 0.5 pound. BMI was computed by dividing the weight in kilograms by the height in meters squared.
Total Cholesterol
Venous blood was drawn after a 12‐hour fast and sent to a central laboratory. Total plasma cholesterol concentrations were measured using enzymatic reactions. Self‐reported lipid‐lowering medication use was collected. No one took cholesterol‐lowering medication at year 0.
Blood Pressure
Sitting BP was measured after a 5‐minute rest using a random zero sphygmomanometer 3 times at intervals of at least 30 seconds. For the analysis, the mean of the second and the third values of systolic and diastolic BP were used. Self‐reported BP‐lowering medication use was collected. A few people took BP medication at year 0.
Fasting Blood Glucose
Fasting glucose was determined in nonpregnant participants who reported fasting for ≥8 hours at baseline, and serum glucose was measured using the hexokinase ultraviolet method manufactured by American Bio‐Science Laboratories (Van Nuys, CA). Self‐reported glucose‐lowering medication use was collected. Although fasting blood glucose is part of the definition of our main outcome, it is appropriate to include it as a component of the CVH score because it is a risk factor; many people with glucose levels 100 to 125 mg/dL never progress to diabetes.
Other Covariates
Data on age, race, maximal educational attainment, and parental diabetes history were collected using a self‐reported standardized questionnaire. Parental history of diabetes was determined if the participant reported that either their mother or father had diabetes at years 0, 5, 10, or 25; unknown or missing history for 1 parent was classified as no history.
Statistical Analysis
Multinomial logistic regression models estimated the odds ratios (ORs) of early‐onset and later‐onset diabetes (versus no diabetes) according to 3 categories of total CVH score (poor, intermediate, and ideal [reference group]) as well as individual CVH components. The model was adjusted for baseline age, race (Black or White race), sex (male or female sex), total energy intake, maximal educational attainment (reported at each exam), and parental history of diabetes (yes or no). We tested the proportional odds assumption for total CVH score in the ordinal logistic regression models, and the assumption was rejected (P<0.001); therefore, we ran polytomous logistic regression models retaining early‐onset and later‐onset diabetes as separate outcome levels. A P value for trend was estimated entering the CVH score as a continuous variable into the logistic regression model. We included fasting blood glucose as a component of the CVH score, as we hypothesized that intermediate glucose level (100–125 mg/dL) predicted future incident diabetes, but many people with glucose levels 100 to 125 mg/dL never progress to diabetes. In our data, impaired fasting glucose (IFG) is a risk factor, as about 6% of people who never had an IFG at an exam ever converted to diabetes, whereas about 30% of those who ever had IFG at an exam ever converted to diabetes. IFG occurred once in about 21% and more than once in about 20% of participants. Thus, IFG is a risk factor, not a part of the outcome. As a sensitivity analysis, we repeated the analysis excluding the glucose component from the total score to confirm the consistency of the findings. To assess the relative importance of each component of CVH score and independent associations, we used forward stepwise logistic regression models, adjusting for the same covariates mentioned previously. Each component of the CVH score were fitted concurrently, and 3 (BMI, BP, and fasting serum glucose) were retained as the best model fit and statistically significant in the final step of the stepwise regression (thresholds for P value for both entry and removal <0.1). In the analysis of the second set of hypotheses, the associations between CVH score (per 1 point higher) and subsequent odds of each incident vascular complication were evaluated in a series of models among participants who developed diabetes (both early and later onset). For each of the complications including CAC≥100, CVD, CKD, diabetic retinopathy, and diabetic neuropathy, the first instance of each was used as an incident case using available data during the study. Multivariable Cox proportional hazards regression models were used for CAC≥100, CVD, and CKD, and multivariable logistic regression models for diabetic retinopathy and diabetic neuropathy, adjusting for the same covariates mentioned previously. The reason for not using Cox proportional hazards regression models for diabetic retinopathy and diabetic neuropathy was that time‐to‐event or censoring variables were not sufficiently precise.
Potential effect modification by race or sex was evaluated by testing the statistical significance of a multiplicative interaction term of CVH as a continuous variable with each respective modifier for each outcome of interest. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC). P values <0.05 were considered statistically significant.
RESULTS
Baseline Characteristics of Study Participants
Data were analyzed for 4547 participants free of diabetes without missing data at baseline for exposure, covariates, and outcomes. Of the participants, 55% were women and 49% were Black race. We identified 116 cases of early‐onset diabetes (age range, 26–39 years; mean±SD, 35.2±3.5 years) and 502 cases of later‐onset diabetes (age range, 40–61 years; mean±SD, 49.6±5.1 years) during 30 years of follow‐up. The Figure shows the distribution of the CVH scores for the nondiabetic, early‐onset diabetic, and late‐onset diabetic groups. Those with no diabetes tended to have the highest CVH scores, whereas those with early‐onset diabetes tended to have the lowest scores. Table 1 presents baseline characteristics (year 0) of participants according to diabetes status. Participants with early‐onset diabetes were more likely to be younger, self‐identified as Black race, had lower educational attainment, and had a higher proportion of parental history of diabetes than those without diabetes or with later‐onset diabetes. Regarding ideal CVH behaviors, lower proportions of individuals meeting ideal diet quality, BMI, total cholesterol, BP, and fasting serum glucose were observed in those with early‐onset diabetes compared with the no diabetes group and those with later‐onset diabetes. Participants who developed diabetes had a lower prevalence of ideal physical activity, regardless of early and later onset.
Figure . Figure. Histograms for the CVH score for the nondiabetic, early‐onset diabetic, and late‐onset diabetic groups.
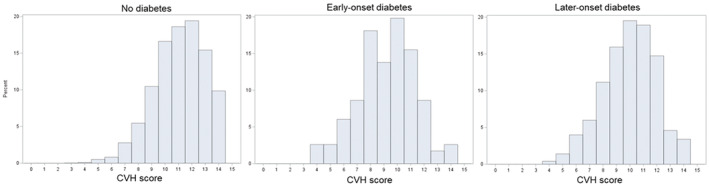
CVH indicates cardiovascular health.
Table 1.
Baseline Characteristics (Year 0) of the Participants According to Diabetes Status During 30 Years of Follow‐Up
| Diabetes Status | ||||
|---|---|---|---|---|
| No Diabetes | Early‐Onset Diabetes | Later‐Onset Diabetes | P value* | |
| Participants | 3929 (86.4) | 116 (2.6) | 502 (11.0) | |
| Total CVH score† | 11.1±1.9 | 9.2±2.2 | 9.9±2.0 | <0.001 |
| Age, y | 24.8±3.6 | 23.6±3.8 | 25.9±3.4 | <0.001 |
| Female sex | 2163 (55.1) | 65 (56.0) | 272 (54.2) | 0.91 |
| Black participants | 1835 (46.7) | 77 (66.4) | 322 (64.1) | <0.001 |
| Maximal educational attainment, grades‡ | 15.5±2.6 | 14.7±2.3 | 15.1±2.6 | <0.001 |
| Parental history of diabetes | 957 (24.4) | 62 (53.5) | 210 (41.8) | <0.001 |
| Energy intake, kcal/d | 2797±1334 | 2750±1268 | 2870±1326 | 0.47 |
| Ideal CVH category | ||||
| Never smoker or quit >12 mo | 2589 (65.9) | 74 (63.8) | 308 (61.4) | 0.07 |
| Physical activity§ | 2331 (59.3) | 62 (53.5) | 265 (52.8) | 0.002 |
| Healthy diet quality (APDQS)|| | 1416 (36.0) | 23 (19.8) | 134 (26.7) | <0.001 |
| BMI | 2748 (69.9) | 28 (24.1) | 207 (41.2) | <0.001 |
| Total cholesterol | 3080 (78.4) | 78 (67.2) | 363 (72.3) | 0.001 |
| BP | 3191 (81.2) | 66 (56.9) | 315 (62.8) | <0.001 |
| Fasting serum glucose | 3868 (98.5) | 102 (87.9) | 485 (96.6) | <0.001 |
| Mean CVH component | ||||
| Physical activity, EU§ | 420±294 | 395±297 | 376±277 | 0.006 |
| Healthy diet score (APDQS)|| | 63.4±13.1 | 58.4±11.8 | 60.5±12.1 | <0.001 |
| BMI, kg/m2 | 23.9±4.6 | 30.4±7.0 | 27.2±5.7 | <0.001 |
| Total cholesterol, mg/dL | 176±32.9 | 184±33.9 | 180±34.8 | 0.001 |
| SBP, mm Hg | 110±10.6 | 115±11.9 | 113±11.9 | <0.001 |
| DBP, mm Hg | 68.2±9.5 | 72.4±10.8 | 70.9±9.5 | <0.001 |
| Fasting serum glucose, mg/dL | 81.3±7.9 | 87.9±12.5 | 83.8±8.6 | <0.001 |
Values are reported as mean±SD or number (percentage). APDQS indicates A Priori Diet Quality Score; BP, blood pressure; BMI, body mass index; CARDIA, Coronary Artery Risk Development in Young Adults; CVH, cardiovascular health; DBP, diastolic blood pressure; EU, exercise unit; and SBP, systolic blood pressure.
Evaluated with χ2 tests for categorical variables and ANOVA for continuous variables.
CVH score was defined by Life's Simple 7 from the American Heart Association. For the present analysis, the metric score includes smoking, diet quality, physical activity, BMI, BP, total cholesterol, and blood glucose (exclusive of glucose ≥126 mg/dL).
For educational level, we used the maximum grades reported during 30 years of follow‐up to account for the fact that many participants were completing their schooling at year 0.
Physical activity score in EUs is derived from the CARDIA study physical activity history.
Diet quality was assessed using the APDQS. Total score of the ADOQS summed the 46 components (possible scores 0–132, with a range of 35–95 in these data), with higher scores representing a nutritionally rich, plant‐centered diet. A 1‐point increment represents a 1‐category shift in the presumed favorable direction.
Association Between CVH Score and Early‐ and Later‐Onset Diabetes
Each 1‐point higher CVH score was associated with 36% (95% CI, 0.58–0.71) lower odds of incident early‐onset diabetes (versus no diabetes) and a 22% (95% CI, 0.74–0.83) lower odds of incident later‐onset diabetes (versus no diabetes) (Table 2). In a sensitivity analysis where the modified CVH score excluding glucose was fitted, we found similar associations: 0.66 (95% CI, 0.60–0.72) for early‐onset diabetes and 0.79 (95% CI, 0.75–0.83) for later‐onset diabetes. In a single predictor model (Table 3), compared with ideal categories, poor categories of BMI (OR, 15.68 [95% CI, 9.62–25.55]), BP (OR, 7.26 [95% CI, 2.64–20.01]), and glucose (OR, 12.20 [95% CI, 6.40–23.50]) were associated with higher ORs of early‐onset diabetes, whereas no associations were found for smoking, physical activity, diet, and total cholesterol. A similar pattern of associations was observed between individual component and later‐onset diabetes. As opposed to the situation with early‐onset diabetes, poor diet quality category was related to later‐onset diabetes (OR, 1.38 [95% CI, 1.05–1.83]). In a stepwise logistic model in which all components were mutually adjusted and 3 variables were kept in the final model because they showed statistical significance, poor BMI (OR, 12.66 [95% CI, 7.65–20.95]) was the strongest predictor of early‐onset diabetes, followed by glucose (OR, 9.28 [95% CI, 4.64–18.54]) and BP (OR, 3.55 [95% CI, 1.21–10.44]). Similarly, poor BMI (OR, 3.57 [95% CI, 2.74–4.65]) and BP (OR, 2.18 [95% CI, 1.13–4.23]) were significantly associated with higher odds of later‐onset diabetes. The associations between CVH score and early‐ or later‐onset diabetes did not differ by race or sex (P for interaction >0.05).
Table 2.
Multivariable‐Adjusted ORs (95% CIs) of Incident Early‐ and Later‐Onset of Diabetes (Versus No Diabetes) According to CVH Score
| Poor (0–6 points) | Intermediate (7–8 points) | Ideal (9–14 points) | Continuous (per 1 point higher) | P trend† | |
|---|---|---|---|---|---|
| Participants, n | 98 | 440 | 4009 | ||
| Early‐onset diabetes (vs no diabetes) | |||||
| Unadjusted cumulative incidence, n (%) | 13 (13.3) | 31 (7.1) | 72 (1.8) | ||
| Unadjusted OR (95% CI) | 11.45 (5.99–21.86) | 4.73 (3.06–7.32) | 1.00 (reference) | 0.64 (0.59–0.70) | <0.001 |
| Multivariable OR (95% CI)* | 10.29 (5.14–20.62) | 4.20 (2.63–6.70) | 1.00 (reference) | 0.64 (0.58–0.71) | <0.001 |
| Later‐onset diabetes (vs no diabetes) | |||||
| Unadjusted cumulative incidence, n (%) | 29 (29.6) | 86 (19.6) | 387 (9.7) | ||
| Unadjusted OR (95% CI) | 4.75 (3.00–7.53) | 2.44 (1.88–3.17) | 1.00 (reference) | 0.76 (0.72–0.79) | <0.001 |
| Multivariable OR (95% CI)* | 3.33 (2.06–5.39) | 1.93 (1.47–2.55) | 1.00 (reference) | 0.78 (0.74–0.83) | <0.001 |
CVH indicates cardiovascular health; and OR, odds ratio.
Multinomial logistic regression was used to model outcome variables of no diabetes, early diabetes, and later diabetes. Model was adjusted for age, race (Black or White race), sex (male or female sex), total energy intake, maximal educational attainment, and parental history of diabetes (yes or no).
Estimated based on a continuous variable of CVH score.
Table 3.
Multivariable‐Adjusted ORs (95% CIs) of Incident Early‐ and Later‐Onset of Diabetes (Versus No Diabetes) According to Each Single Component of the CVH Score
| Separate Model for Each Component* | Mutually Adjusted Model*, † | |||||
|---|---|---|---|---|---|---|
| Individual Components of the CVH Score‡ | Poor (0 points) | Intermediate (1 point) | Ideal (2 points) | Poor (0 points) | Intermediate (1 point) | Ideal (2 points) |
| Early‐onset diabetes (vs no diabetes) | ||||||
| Smoking | 1318 | 258 | 2971 | |||
| Unadjusted cumulative incidence, n (%) | 37 (2.8) | 5 (1.9) | 74 (2.5) | |||
| Multivariable OR (95% CI) | 1.09 (0.71–1.67) | 0.82 (0.32–2.07) | 1.00 (reference) | NS | ||
| Physical activity | 505 | 1384 | 2658 | |||
| Unadjusted cumulative incidence, n (%) | 20 (4.0) | 34 (2.5) | 62 (2.3) | |||
| Multivariable OR (95% CI) | 1.42 (0.81–2.50) | 0.96 (0.62–1.50) | 1.00 (reference) | NS | ||
| Diet quality | 1424 | 1550 | 1573 | |||
| Unadjusted cumulative incidence, n (%) | 53 (3.7) | 40 (2.6) | 23 (1.5) | |||
| Multivariable OR (95% CI) | 1.52 (0.85–2.72) | 1.34 (0.77–2.33) | 1.00 (reference) | NS | ||
| BMI | 517 | 1047 | 2983 | |||
| Unadjusted cumulative incidence, n (%) | 58 (11.2) | 30 (2.9) | 28 (0.9) | |||
| Multivariable OR (95% CI) | 15.68 (9.62–25.55) | 3.41 (2.01–5.78) | 1.00 (reference) | 12.66 (7.65–20.95) | 2.94 (1.72–5.04) | 1.00 (reference) |
| Total cholesterol | 191 | 835 | 3521 | |||
| Unadjusted cumulative incidence, n (%) | 5 (2.6) | 33 (4.0) | 78 (2.2) | |||
| Multivariable OR (95% CI) | 1.26 (0.50–3.18) | 1.89 (1.23–2.89) | 1.00 (reference) | NS | ||
| BP | 55 | 920 | 3572 | |||
| Unadjusted cumulative incidence, n (%) | 5 (9.1) | 45 (4.9) | 66 (1.9) | |||
| Multivariable OR (95% CI) | 7.26 (2.64–20.01) | 3.11 (2.06–4.69) | 1.00 (reference) | 3.55 (1.21–10.44) | 1.94 (1.25–2.99) | 1.00 (reference) |
| Fasting serum glucose | 92 | 4455 | ||||
| Unadjusted cumulative incidence, n (%) | NA (by study design) | 14 (15.2) | 102 (2.3) | NA (by study design) | ||
| Multivariable OR (95% CI) | NA (by study design) | 12.20 (6.40–23.50) | 1.00 (reference) | NA (by study design) | 9.28 (4.64–18.54) | 1.00 (reference) |
| Later‐onset diabetes (vs no diabetes) | ||||||
| Smoking | 1318 | 258 | 2971 | |||
| Unadjusted cumulative incidence, n (%) | 159 (12.1) | 22 (8.5) | 289 (9.7) | |||
| Multivariable OR (95% CI) | 1.16 (0.94–1.44) | 0.85 (0.54–1.33) | 1.00 (reference) | NS | ||
| Physical activity | 505 | 1384 | 2658 | |||
| Unadjusted cumulative incidence, n (%) | 73 (14.5) | 151 (10.9) | 246 (9.3) | |||
| Multivariable OR (95% CI) | 1.34 (0.99–1.81) | 1.13 (0.91–1.41) | 1.00 (reference) | NS | ||
| Diet quality | 1424 | 1550 | 1573 | |||
| Unadjusted cumulative incidence, n (%) | 169 (11.9) | 176 (11.4) | 125 (8.0) | |||
| Multivariable OR (95% CI) | 1.38 (1.05–1.83) | 1.26 (0.98–1.62) | 1.00 (reference) | NS | ||
| BMI | 517 | 1047 | 2983 | |||
| Unadjusted cumulative incidence, n (%) | 126 (24.4) | 149 (14.2) | 195 (6.5) | |||
| Multivariable OR (95% CI) | 4.10 (3.17–5.32) | 2.26 (1.80–2.82) | 1.00 (reference) | 3.57 (2.74–4.65) | 2.13 (1.70–2.67) | 1.00 (reference) |
| Total cholesterol | 191 | 835 | 3521 | |||
| Unadjusted cumulative incidence, n (%) | 23 (12.0) | 106 (12.7) | 341 (9.7) | |||
| Multivariable OR (95% CI) | 1.24 (0.80–1.91) | 1.22 (0.96–1.54) | 1.00 (reference) | NS | ||
| BP | 55 | 920 | 3572 | |||
| Unadjusted cumulative incidence, n (%) | 14 (25.5) | 162 (17.6) | 294 (8.2) | |||
| Multivariable OR (95% CI) | 2.88 (1.51–5.49) | 2.11 (1.70–2.62) | 1.00 (reference) | 2.18 (1.13–4.23) | 1.70 (1.36–2.13) | 1.00 (reference) |
| Fasting serum glucose | 92 | 4455 | ||||
| Unadjusted cumulative incidence, n (%) | NA (by study design) | 14 (15.2) | 456 (10.2) | NA (by study design) | ||
| Multivariable OR (95% CI) | NA (by study design) | 2.10 (1.20–3.70) | 1.00 (reference) | NA (by study design) | 1.65 (0.93–2.95) | 1.00 (reference) |
BMI indicates body mass index; BP, blood pressure; CARDIA, Coronary Artery Risk Development in Young Adults; CVH, cardiovascular health; NA, not available; NS, not significant; and OR, odds ratio.
Multinomial logistic regression was used to model outcome variables of normal, early diabetes, and later diabetes. Model was adjusted for age, race (Black or White race), sex (male or female sex), total energy intake, maximal educational attainment, and parental history of diabetes (yes or no).
The individual components of the CVH score were fitted simultaneously in a stepwise logistic model, and 3 (BMI, BP, and fasting serum glucose) were kept in the best model fit and were statistically significant.
The criteria for poor, intermediate, and ideal in each CVH component were defined as the following: (1) smoking: current smoker, former smoker (quit within ≤12 months), and never smoker or quit within >12 months; (2) physical activity (CARDIA physical activity score): <100, 100–299, and ≥300 exercise units; (3) diet quality (APDQS): tertile 1 (median, 50), tertile 2 (median, 61), and tertile 3 (median, 76); (4) BMI: ≥30, 25–29.9, and <25.5 kg/m2; (5) total cholesterol: ≥240, 200–239 or <200 with medication, and <200 without medication; (6) BP: SBP≥140 mm Hg or DBP≥90 mm Hg, SBP 120–139 mm Hg or DBP 80–89 mm Hg or SBP<120 mm Hg and DBP<80 mm Hg with medication, and SBP<120 mm Hg and DBP<80 mm Hg without medication; (7) fasting serum glucose: ≥126 (NA by study design), 100–125, and <100 mg/dL without medication.
Association Between CVH Score and Subsequent Micro‐ and Macrovascular Complications After Diabetes Onset
In a subset of participants who developed diabetes during 30 years of follow‐up, every 1‐point higher CVH score was associated with 19% lower hazard for CAC≥100 Agatston units (95% CI, 0.70–0.94) and 18% lower hazard for CVD (95% CI, 0.72–0.93; Table 4). Each 1‐point higher CVH score was also associated with 14% lower odds of diabetic neuropathy (95% CI, 0.75–0.98). The inverse associations of CVH score with CKD and diabetic retinopathy did not achieve statistical significance. The associations between CVH score and this set of vascular complications was similar according to race or sex (P for interaction for each >0.05).
Table 4.
Association Between CVH Score and Subsequent Micro‐ and Macrovascular Complications in Those Who Developed Diabetes*
| Subsequent Vascular Complications | Unadjusted Cumulative Incidence, % (n/N) | CVH Score, per 1 Point Higher | P trend† |
|---|---|---|---|
| CAC≥100 Agatston units | 16.7 (43/257) | 0.81 (0.70–0.94) | 0.004 |
| CVD | 10.2 (61/597) | 0.82 (0.72–0.93) | 0.003 |
| CKD | 24.1 (90/373) | 0.93 (0.83–1.04) | 0.19 |
| Diabetic retinopathy | 7.1 (29/410) | 0.89 (0.72–1.09) | 0.27 |
| Diabetic neuropathy | 17.6 (72/410) | 0.86 (0.75–0.98) | 0.03 |
CAC indicates coronary artery calcium; CKD, chronic kidney disease; CVD, cardiovascular disease; and CVH, cardiovascular health.
Cox proportional hazards regression was used to estimate hazard ratios and 95% CIs for CAC≥100, CVD, and CKD. Logistic regression was used to estimate odds ratios and 95% CIs for diabetic retinopathy and diabetic neuropathy. Model was adjusted for age, race (Black or White race), sex (male or female sex), total energy intake, maximal educational attainment, and parental history of diabetes (yes or no).
Estimated based on continuous CVH score.
DISCUSSION
Our longitudinal cohort study adds to the growing body of evidence supporting the idea that CVH score is relevant to individuals at high risk of diabetes and is an easy‐to‐understand indicator of future diabetes risk. Higher CVH score, measured at an average age of 25 years, was associated with lower odds of incident early‐onset diabetes (<40 years) as well as later‐onset diabetes (≥40 years) in Black and White young adults free of diabetes at baseline. The association was stronger for early‐onset diabetes probably because the score variable used was closer to early‐onset diabetes. In addition, higher CVH score was associated with lower hazards for incident CAC≥100, incident CVD, and diabetic neuropathy after diabetes onset, which may help patients in setting target goals for diabetes management.
Our results are consistent with previous studies on the association between CVH score and diabetes. 7 , 8 , 9 , 10 , 27 Although adults diagnosed with diabetes before the age of 40 years have a greater risk of cardiovascular complications than adults diagnosed after the age of 40 years, 3 , 4 it has not been clearly demonstrated what factors are associated with the early development of diabetes and subsequent complications. A recent study showed that greater CVH score was related to a lower lifetime risk of diabetes, regardless of genetic susceptibility to diabetes. 28 Our data suggest that individuals who are at a higher risk of developing diabetes early in life can lower their risk by attaining and maintaining more LS7 goals at a younger age. We also observed that especially BMI appeared to be most strongly associated with diabetes, which is consistent with earlier research indicating that BMI had a larger estimate of effect size or changed significantly from the original score when BMI was removed from the total CVH score. 7 , 9 , 27 In individuals with obesity, increased levels of nonesterified fatty acids, glycerol, cytokines, proinflammatory markers, and other substances are observed. 29 Impairment of islet β cell function and insulin resistance caused by increased nonesterified fatty acids, in particular, led to an aggravated loss of blood glucose control. 29 These may help explain some of the probable pathways through which obesity leads to the development of diabetes.
Following BMI, the next most important CVH components were glucose and BP; however, total cholesterol was not associated with diabetes, which is consistent with previous studies. 7 , 9 , 27 The present study and Fretts et al 7 support a previous finding that demonstrated a higher risk of diabetes with increasing glucose level in the currently accepted normal range. 30 A substantial body of evidence has documented that high BP was associated with a greater risk of diabetes, but this association was attenuated in those with higher BMI and older age groups. 31 In contrast to our study and prior studies, 8 , 27 physical activity, smoking, and diet quality showed modest to weak associations with diabetes. Although our study and others did not find the association between the smoking component and diabetes, 7 , 27 smoking has been associated with higher diabetes risk. 9
Ideal diet quality showed a modest association in some studies, 9 including the present study, for later‐onset diabetes. Although there was no strong association between these behavioral factors of CVH and diabetes, substantial evidence suggests that these behavioral factors can influence clinical factors of CVH and diabetes. 23 , 32 , 33 , 34 , 35 , 36 CVH was created as a simple and approximate, but very informative, tool for understanding what an individual needs to know and do for CVD prevention. CVH score provides a multifaceted assessment of cardiometabolic health rather than focusing on an individual risk factor. 6 Our results indicate that BMI, blood glucose, and BP are most strongly associated with early‐onset diabetes; however, for a person with ideal levels of these factors, but suboptimal levels of the behavioral variables (diet, physical activity, smoking), the less‐than‐ideal score would serve as a warning of diabetes risk. Continuation of the unhealthy behaviors is likely to have an adverse effect on the clinical variables. 36 Clinical risk factors rarely exist in isolation, and many of these are related through common pathophysiology or causally related; for example, BMI seems causally related to insulin resistance and diabetes. 29 In addition, another important feature of the CVH score is that a given score can represent the sum of different individual score elements.
In comparison to primary prevention, there are relatively limited data on the effectiveness of diet and other lifestyle changes in preventing relevant complications in individuals with diabetes. 12 Importantly, our findings imply that a set of clinical and behavioral factors in young adulthood are important indicators of diabetes‐related complications. Our results support prior research demonstrating that lifestyle modifications in patients with diabetes were associated with a lower risk of cardiometabolic factors, including BMI, BP, and hemoglobin A1c. 37 Intensive control of BP, blood glucose, and weight were associated with a lower risk of incident micro‐ and macrovascular diseases. 38 , 39 , 40
Strengths of our study include the long‐term prospective study design with a high follow‐up rate, interviewer‐assisted measures of physical activity and diet, detailed measures of diet quality using the index composed of 46 food groups that is highly correlated with the Healthy Eating Index–2015 (r=0.73) 23 and that has good epidemiological validation with various outcomes, and objectively identified diabetes cases. In addition, our findings extend the predictive value of the CVH score for early‐onset diabetes in young Black and White populations. This study also had potential limitations. First, the observational nature of the study precludes inferring a causal relationship. Second, self‐reported diabetic neuropathy and diabetic retinopathy have unknown validity and reliability. Third, a single measurement of CVH at baseline cannot capture dynamic change in CVH score; however, the observed strong associations in this study suggest that young adulthood risk exposures may be a lifelong indicator for disease risk in later life. 11 Fourth, the relatively small number of diabetes cases, especially among those with early onset, and sparse time‐to‐event information for CAC and CKD, may have limited power for associations with individual CVH components and with diabetes complications. Lastly, the findings of our study may not be generalizable to other races, ethnicities, cultures, or adults with lower body weights (the mean BMI of all CARDIA participants was 24.4±5.0 kg/m2, close to the threshold for overweight).
In conclusion, the present study found that young adults with higher CVH scores who were free of diabetes at baseline had lower odds of incident early‐ and later‐onset diabetes as well as incident vascular complications after diabetes onset. Our findings support the use of the CVH score as an indicator of future diabetes risk in generally healthy young adults and as a target for the management of relevant vascular complications. More emphasis should be placed on maintaining ideal CVH for the prevention of diabetes and its complications.
Sources of Funding
The CARDIA (Coronary Artery Risk Development in Young Adults) study is conducted and supported by the National Heart, Lung, and Blood Institute in collaboration with the University of Alabama at Birmingham (HHSN268201800005I and HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), and Kaiser Foundation Research Institute (HHSN268201800004I). This article has been reviewed by CARDIA for scientific content.
Disclosures
None.
Supporting information
Table S1–S3
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.027558
For Sources of Funding and Disclosures, see page 11.
References
- 1. Lascar N, Brown J, Pattison H, Barnett AH, Bailey CJ, Bellary S. Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol. 2018;6:69–80. doi: 10.1016/S2213-8587(17)30186-9 [DOI] [PubMed] [Google Scholar]
- 2. TODAY Study Group . Longitudinal changes in cardiac structure and function from adolescence to young adulthood in participants with type 2 diabetes mellitus: the TODAY follow‐up study. Circ Heart Fail. 2020;13:e006685. doi: 10.1161/CIRCHEARTFAILURE.119.006685x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Al‐Saeed AH, Constantino MI, Molyneaux L, D'Souza M, Limacher‐Gisler F, Luo C, Wu T, Twigg SM, Yue DK, Wong J. An inverse relationship between age of type 2 diabetes onset and complication risk and mortality: The impact of youth‐onset type 2 diabetes. Diabetes Care. 2016;39:823–829. doi: 10.2337/dc15-0991 [DOI] [PubMed] [Google Scholar]
- 4. Sattar N, Rawshani A, Franzén S, Rawshani A, Svensson A‐M, Rosengren A, McGuire DK, Eliasson B, Gudbjörnsdottir S. Age at diagnosis of type 2 diabetes mellitus and associations with cardiovascular and mortality risks: findings from the Swedish National Diabetes Registry. Circulation. 2019;139:2228–2237. doi: 10.1161/CIRCULATIONAHA.118.037885 [DOI] [PubMed] [Google Scholar]
- 5. Wilmot E, Idris I. Early onset type 2 diabetes: Risk factors, clinical impact and management. Therapeutic Advances in Chronic Disease. 2014;5:234–244. doi: 10.1177/2040622314548679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, et al. Defining and setting National Goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 7. Fretts AM, Howard BV, McKnight B, Duncan GE, Beresford SAA, Mete M, Zhang Y, Siscovick DS. Life's simple 7 and incidence of diabetes among American Indians: the strong heart family study. Diabetes Care. 2014;37:2240–2245. doi: 10.2337/dc13-2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Effoe VS, Carnethon MR, Echouffo‐Tcheugui JB, Chen H, Joseph JJ, Norwood AF, Bertoni AG. The American Heart Association ideal cardiovascular health and incident type 2 diabetes mellitus among blacks: the Jackson heart study. J Am Heart Assoc. 2017;6:e005008. doi: 10.1161/JAHA.116.005008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Joseph JJ, Bennett A, Echouffo Tcheugui JB, Effoe VS, Odei JB, Hidalgo B, Dulin A, Safford MM, Cummings DM, Cushman M, et al. Ideal cardiovascular health, glycaemic status and incident type 2 diabetes mellitus: the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Diabetologia. 2019;62:426–437. doi: 10.1007/s00125-018-4792-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Climie RE, van Sloten TT, Périer M‐C, Tafflet M, Fayosse A, Dugravot A, Singh‐Manoux A, Empana J‐P. Change in cardiovascular health and incident type 2 diabetes and impaired fasting glucose: the Whitehall II study. Diabetes Care. 2019;42:1981–1987. doi: 10.2337/dc19-0379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spring B, Moller AC, Colangelo LA, Siddique J, Roehrig M, Daviglus ML, Polak JF, Reis JP, Sidney S, Liu K. Healthy lifestyle change and subclinical atherosclerosis in young adults: Coronary Artery Risk Development in young Adults (CARDIA) study. Circulation. 2014;130:10–17. doi: 10.1161/CIRCULATIONAHA.113.005445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Psaltopoulou T, Ilias I, Alevizaki M. The role of diet and lifestyle in primary, secondary, and tertiary diabetes prevention: a review of meta‐analyses. Rev Diabet Stud. 2010;7:26–35. doi: 10.1900/RDS.2010.7.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Liu K, Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7 [DOI] [PubMed] [Google Scholar]
- 14. Cha E, Pasquel FJ, Yan F, Jacobs DR, Dunbar SB, Umpierrez G, Choi Y, Shikany JM, Bancks MP, Reis JP, et al. Characteristics associated with early‐ vs. later‐onset adult diabetes: the CARDIA study. Diabetes Res Clin Pract. 2021;182:109144. doi: 10.1016/j.diabres.2021.109144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-T [DOI] [PubMed] [Google Scholar]
- 16. Delgado C, Baweja M, Crews DC, Eneanya ND, Gadegbeku CA, Inker LA, Mendu ML, Miller WG, Moxey‐Mims MM, Roberts GV, et al. A unifying approach for GFR estimation: recommendations of the NKF‐ASN task force on reassessing the inclusion of race in diagnosing kidney disease. JASN. 2021;32:2994–3015. doi: 10.1681/ASN.2021070988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO 2012 Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 18. Jacobs DR, Hahn LP, Haskell WL, Pirie P, Sidney S. Validity and reliability of short physical activity history: Cardia and the Minnesota heart health program. J Cardiopulm Rehabil. 1989;9:448–459. doi: 10.1097/00008483-198911000-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness in healthy adults. Position stand of the American College of Sports Medicine. Schweiz Z Sportmed. 1993;41:127–137. [PubMed] [Google Scholar]
- 20. Gabriel KP, Sidney S, Jacobs DR, Quesenberry CP, Reis JP, Jiang S‐F, Sternfeld B. Convergent validity of a brief self‐reported physical activity questionnaire. Med Sci Sports Exerc. 2014;46:1570–1577. doi: 10.1249/MSS.0000000000000278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu K, Slattery M, Jacobs D, Cutter G, McDonald A, Van Horn L, Hilner JE, Caan B, Bragg C, Dyer A. A study of the reliability and comparative validity of the cardia dietary history. Ethn Dis. 1994;4:15–27. [PubMed] [Google Scholar]
- 22. Schakel SF, Sievert YA, Buzzard IM. Sources of data for developing and maintaining a nutrient database. J Am Diet Assoc. 1988;88:1268–1271. doi: 10.1016/S0002-8223(21)07997-9 [DOI] [PubMed] [Google Scholar]
- 23. Choi Y, Larson N, Gallaher DD, Odegaard AO, Rana JS, Shikany JM, Steffen LM, Jacobs DR. A shift toward a plant‐centered diet from young to middle adulthood and subsequent risk of type 2 diabetes and weight gain: the coronary artery risk development in young adults (CARDIA) study. Diabetes Care. 2020;43:2796–2803. doi: 10.2337/dc20-1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Choi Y, Larson N, Steffen LM, Schreiner PJ, Gallaher DD, Duprez DA, Shikany JM, Rana JS, Jacobs DR. Plant‐centered diet and risk of incident cardiovascular disease during young to middle adulthood. J Am Heart Assoc. 2021;10:e020718. doi: 10.1161/JAHA.120.020718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Choi Y, Steffen LM, Chu H, Duprez DA, Gallaher DD, Shikany JM, Schreiner PJ, Shroff GR, Jacobs DR. A plant‐centered diet and markers of early chronic kidney disease during young to middle adulthood: findings from the coronary artery risk development in young adults (CARDIA) cohort. J Nutr. 2021;151:2721–2730. doi: 10.1093/jn/nxab155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mursu J, Steffen LM, Meyer KA, Duprez D, Jacobs DR. Diet quality indexes and mortality in postmenopausal women: the Iowa Women's health study. Am J Clin Nutr. 2013;98:444–453. doi: 10.3945/ajcn.112.055681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Joseph JJ, Echouffo‐Tcheugui JB, Carnethon MR, Bertoni AG, Shay CM, Ahmed HM, Blumenthal RS, Cushman M, Golden SH. The association of ideal cardiovascular health with incident type 2 diabetes mellitus: the multi‐ethnic study of atherosclerosis. Diabetologia. 2016;59:1893–1903. doi: 10.1007/s00125-016-4003-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang K, Kavousi M, Voortman T, Ikram MA, Ghanbari M, Ahmadizar F. Cardiovascular health, genetic predisposition, and lifetime risk of type 2 diabetes. Eur J Prev Cardiol. 2022;28:1850–1857. doi: 10.1093/eurjpc/zwab141 [DOI] [PubMed] [Google Scholar]
- 29. Al‐Goblan AS, Al‐Alfi MA, Khan MZ. Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes. 2014;7:587–591. doi: 10.2147/DMSO.S67400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nichols GA, Hillier TA, Brown JB. Normal fasting plasma glucose and risk of type 2 diabetes diagnosis. Am J Med. 2008;121:519–524. doi: 10.1016/j.amjmed.2008.02.026 [DOI] [PubMed] [Google Scholar]
- 31. Emdin CA, Anderson SG, Woodward M, Rahimi K. Usual blood pressure and risk of new‐onset diabetes: evidence from 4.1 million adults and a meta‐analysis of prospective studies. J Am Coll Cardiol. 2015;66:1552–1562. doi: 10.1016/j.jacc.2015.07.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Park S, Rink L, Wallace J. Accumulation of physical activity: Blood pressure reduction between 10‐min walking sessions. J Hum Hypertens. 2008;22:475–482. doi: 10.1038/jhh.2008.29 [DOI] [PubMed] [Google Scholar]
- 33. Primatesta P, Falaschetti E, Gupta S, Marmot MG, Poulter NR. Association between smoking and blood pressure: Evidence from the health survey for England. Hypertension. 2001;37:187–193. doi: 10.1161/01.HYP.37.2.187 [DOI] [PubMed] [Google Scholar]
- 34. Boniol M, Dragomir M, Autier P, Boyle P. Physical activity and change in fasting glucose and HbA1c: a quantitative meta‐analysis of randomized trials. Acta Diabetol. 2017;54:983–991. doi: 10.1007/s00592-017-1037-3 [DOI] [PubMed] [Google Scholar]
- 35. Gossett LK, Johnson HM, Piper ME, Fiore MC, Baker TB, Stein JH. Smoking intensity and lipoprotein abnormalities in active smokers. J Clin Lipidol. 2009;3:372–378. doi: 10.1016/j.jacl.2009.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Diabetes prevention program (DPP) Research Group . The diabetes prevention program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165–2171. doi: 10.2337/diacare.25.12.2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen L, Pei J‐H, Kuang J, Chen H‐M, Chen Z, Li Z‐W, Yang H‐Z. Effect of lifestyle intervention in patients with type 2 diabetes: a meta‐analysis. Metabolism. 2015;64:338–347. doi: 10.1016/j.metabol.2014.10.018 [DOI] [PubMed] [Google Scholar]
- 38. Reaven PD, Emanuele NV, Wiitala WL, Bahn GD, Reda DJ, McCarren M, Duckworth WC, Hayward RA, Investigators VADT. Intensive glucose control in patients with type 2 diabetes ‐ 15‐year follow‐up. N Engl J Med. 2019;380:2215–2224. doi: 10.1056/NEJMoa1806802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Grossman E, Messerli FH, Goldbourt U. High blood pressure and diabetes mellitus: are all antihypertensive drugs created equal? Arch Intern Med. 2000;160:2447–2452. doi: 10.1001/archinte.160.16.2447 [DOI] [PubMed] [Google Scholar]
- 40. Polemiti E, Baudry J, Kuxhaus O, Jäger S, Bergmann MM, Weikert C, Schulze MB. BMI and BMI change following incident type 2 diabetes and risk of microvascular and macrovascular complications: the EPIC‐Potsdam study. Diabetologia. 2021;64:814–825. doi: 10.1007/s00125-020-05362-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S3


