Abstract
Background
Oral anticoagulation reduces stroke and disability in atrial fibrillation (AF) but is underused. We evaluated the effects of a novel patient‐clinician shared decision‐making (SDM) tool in reducing oral anticoagulation patient's decisional conflict as compared with usual care.
Methods and Results
We designed and evaluated a new digital decision aid in a multicenter, randomized, comparative effectiveness trial, ENHANCE‐AF (Engaging Patients to Help Achieve Increased Patient Choice and Engagement for AF Stroke Prevention). The digital AF shared decision‐making toolkit was developed using patient‐centered design with clear health communication principles (eg, meaningful images, limited text). Available in English and Spanish, the toolkit included the following: (1) a brief animated video; (2) interactive questions with answers; (3) a quiz to check on understanding; (4) a worksheet to be used by the patient during the encounter; and (5) an online guide for clinicians. The study population included English or Spanish speakers with nonvalvular AF and a CHA2DS2‐VASc stroke score ≥1 for men or ≥2 for women. Participants were randomized in a 1:1 ratio to either usual care or the shared decision‐making toolkit. The primary end point was the validated 16‐item Decision Conflict Scale at 1 month. Secondary outcomes included Decision Conflict Scale at 6 months and the 10‐item Decision Regret Scale at 1 and 6 months as well as a weighted average of Mann–Whitney U‐statistics for both the Decision Conflict Scale and the Decision Regret Scale. A total of 1001 participants were enrolled and followed at 5 different sites in the United States between December 18, 2019, and August 17, 2022. The mean patient age was 69±10 years (40% women, 16.9% Black, 4.5% Hispanic, 3.6% Asian), and 50% of participants had CHA2DS2‐VASc scores ≥3 (men) or ≥4 (women). The primary end point at 1 month showed a clinically meaningful reduction in decisional conflict: a 7‐point difference in median scores between the 2 arms (16.4 versus 9.4; Mann–Whitney U‐statistics=0.550; P=0.007). For the secondary end point of 1‐month Decision Regret Scale, the difference in median scores between arms was 5 points in the direction of less decisional regret (P=0.078). The treatment effects lessened over time: at 6 months the difference in medians was 4.7 points for Decision Conflict Scale (P=0.060) and 0 points for Decision Regret Scale (P=0.35).
Conclusions
Implementation of a novel shared decision‐making toolkit (afibguide.com; afibguide.com/clinician) achieved significantly lower decisional conflict compared with usual care in patients with AF.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT04096781.
Keywords: anticoagulants, anticoagulation, atrial fibrillation, decisional conflict, digital health, shared decision making, stroke
Subject Categories: Arrhythmias, Atrial Fibrillation, Anticoagulants, Digital Health
Nonstandard Abbreviations and Acronyms
- CE
composite end point
- DCS
Decision Conflict Scale
- DRS
Decision Regret Scale
- ENHANCE‐AF
Engaging Patients to Help Achieve Increased Patient Choice and Engagement for AF Stroke Prevention
- SDM
shared decision‐making
- UC
usual care
- WMW
Wilcoxon‐Mann–Whitney
Clinical Perspective
What Is New?
A new, multiplatform atrial fibrillation digital tool was developed in English and Spanish to enhance the process of patient‐clinician shared decision making for stroke prevention with oral anticoagulants.
The use of this digital toolkit by patients before a visit and by clinicians during the visit empowered participant involvement through a sequence of important low‐health literacy messages presented in an animated video with graphical illustrations of information.
As compared with a usual care group, the use of a digital toolkit improved patients' knowledge of atrial fibrillation and their assessment of the decision‐making process as reflected by lowered decisional conflict.
What Are the Clinical Implications?
This digital toolkit can be used by clinicians in their decision‐making discussions with patients centered around oral anticoagulation for stroke prevention in atrial fibrillation.
This patient‐ and clinician‐centered end‐user approach to the design of a digital shared decision‐making toolkit has potential application to other clinical contexts, particularly technically complex issues where patient preference can affect the balance between benefits and harms.
Atrial fibrillation (AF) is associated with an increased risk of thromboembolism and stroke. Oral anticoagulation diminishes this risk, improves clinical outcomes, and reduces major disability in patients with AF at increased risk of stroke. 1 , 2 However, oral anticoagulation is both underused (with less than half of high‐risk patients receiving anticoagulation) 3 , 4 and adhered to inadequately. A failure to adequately involve patients in discussions centered around anticoagulation contributes to these shortfalls. A large number of patients with AF are perplexed about the risk–benefit ratio of oral stroke prevention therapy and continue to have significant decisional conflict related to oral anticoagulation. 5 , 6 , 7 A sound decision‐making process relies on patients having a sufficient knowledge of oral anticoagulation for treatment as well as adequate motivation to participate in making decisions. 8 , 9 Patient knowledge about the disease process, available therapeutic options, and their personal values and preferences are essential for success. Even modest increases in decisional regret have been shown to be associated with increased likelihood to change one's mind or for the provider to be blamed. 10 Shared decision‐making (SDM) processes have been introduced in a wide range of clinical settings and may facilitate communication about anticoagulation and thereby improve outcomes. 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 Patients who make decisions consistent with their goals generally have increased levels of patient satisfaction and engagement and frequently have greater patient adherence scores. 6 This patient‐centered approach results in increased clinician responsiveness to patient's individual preferences and values. 6 Although clinicians generally assume that all patients are equally “ready” to make decisions about their health, there are several critical barriers to successful patient decision making. 19 , 20 , 21 , 22 , 23 We, therefore, hypothesize that the use of our novel patient–clinician shared decision‐making (SDM) toolkit will reduce anticoagulation‐related decisional conflict as compared with usual care (UC).
METHODS
Design of SDM Tool: A Patient‐Centered Approach
The digital toolkit, a web‐based application, plays a central part in the shared decision‐making pathway and was developed through a patient‐centered design approach. Our goal was to develop a tool that would address many of the educational, socioeconomic, and health literacy barriers in shared decision making. We collaborated with Daylight Design (San Francisco, CA), to use design thinking, a human‐centered design approach, deep patient interviews and observations, and wireframe mockups for concept generation to create animated and highly graphical videos with limited use of text.
Throughout the tool‐making process, patients provided input iteratively to identify needed changes in the toolkit design, including usability and understandability of content. Blackbird Studios (San Francisco, CA) developed the tool's software, with a strong focus on robust performance across different hardware platforms and varying levels of Internet connectivity, including the ability to function offline, which may be particularly important in underresourced settings.
In addition to an animated video, other tools included (1) interactive questions with answers, (2) a quiz to check on patient understanding, (3) a worksheet for the patient to record questions for the clinician visit (https://afibguide.com), and (4) an online guide that the clinician may use to illustrate key messages about anticoagulation for AF stroke prevention (https://afibguide.com/clinician). Figure 1 depicts key elements of our tool.
Figure 1. A, Screenshot of patient web app navigation screen leading to 4 parts of the video: (1) patient video; (2) frequently asked questions; (3) brief quiz; (4) worksheet.
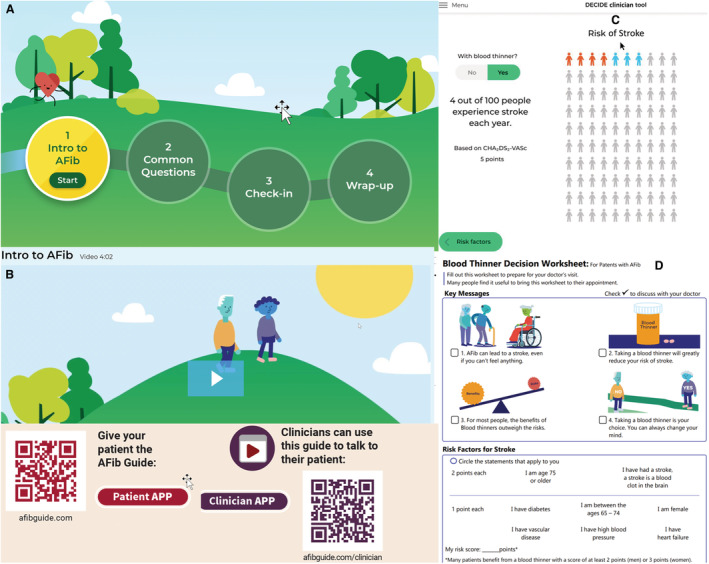
B, Screenshot of opening scene of patient video with animated characters who take the journey to decide about anticoagulation for atrial fibrillation stroke prevention. C, Screenshot of an example of stroke reduction estimate based on atrial fibrillation stroke risk that clinician may use via the clinician web app. D, First page of the worksheet given to the patient to indicate key messages. The second page (not shown) has space for questions that the patient has for the clinician visit. E, QR codes to the patient tool and clinician tool.
Design of Clinical Trial
A randomized comparative effectiveness trial was performed to evaluate the effectiveness of the SDM Pathway. The design of the trial has been published previously. 24
The study population included English or Spanish speakers ≥18 years of age with nonvalvular AF and a CHA2DS2‐VASc score ≥1 for men or ≥2 for women. Patients with moderate to severe mitral stenosis, mechanical valve replacement, absolute contraindications to anticoagulation, left atrial appendage exclusion (by surgery or device placement), or any indication for anticoagulation therapy for a condition other than AF were not eligible for the study.
Intervention and UC Arms
In the intervention arm (SDM), the participant used a digital SDM tool with minimal assistance of a research study coordinator. The web‐based application was administered using a tablet. The study research coordinator instructed the patient on the use of the web‐based application and stayed with the patient while the patient used the toolkit. Patient training was uniform and not tailored to the patient's age and the like.
After using the tool, the participant completed a worksheet to record any additional questions for the clinician visit. As part of the office visit, the clinician used a clinician version of the web‐based app tool to highlight key AF anticoagulation learning points to the participant. Clinicians were trained in the use of the clinician tool, which included videos about AF stroke risk, a risk calculator, and pictures of the available anticoagulant medications. In the control arm (UC), the participants and the clinicians were not provided with the digital SDM tool and, therefore, followed usual clinical practice. Each participant received their assigned intervention 1 time during their baseline clinical visit. In both arms of the trial, at the conclusion of the visit, the study coordinator administered outcome scales to the participants. The study coordinator arranged follow‐up telephone or video visits 1 and 6 months after the index visit to administer selected outcome scales and acquire information regarding participants' anticoagulant usage, clinical events, and other adverse events.
Participants were randomized in a 1:1 ratio to either (1) usual care (UC) or (2) our SDM Pathway stratified by study site, prior anticoagulation history, and CHA2DS2‐VASc score. A random allocation sequence was generated a priori by the trial statistician through a computer‐generated system using blocks of randomization sizes randomly selected from 4 or 6.
Participating Sites, Clinical Coordination, Data Coordination, and Funding
Five US sites participated in this trial, including Stanford University (Stanford, CA), Ochsner Medical Center (New Orleans, LA), East Carolina University (Greenville, NC), Cleveland Clinic (Cleveland, OH), and Cooper HealthCare (Camden, NJ). Stanford Center for Clinical Research served as the clinical coordinating center. The Stanford Center for Innovative Study Design served as the data coordinating center. Research materials consisted of electronic case report forms entered by the study site coordinators into a secured Research Electronic Data Capture relational database at Stanford University. An independent clinical monitor with experience in the conduct of clinical trials and AF studies reviewed study safety information, monitored data quality every 6 months, and reported these findings to the trial leadership. The report included adverse events; significant adverse events; clinical outcomes including strokes, transient ischemic attacks, bleeding, and death; and aggregated trial status, including protocol deviations, participant enrollment numbers, participant withdrawal and lost to follow‐up numbers, data completeness, and data quality measures.
The study was approved by the Stanford Institutional Review Board, and participants gave their written informed consent.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study End Points
The primary end point was the Decision Conflict Scale (DCS) score at 1 month after the clinical visit when anticoagulation decisions were made and immediate postvisit outcome surveys were completed. This primary end point was selected to reflect the participants' priority of what is most important to them. We administered a Decision‐making and Choices to Inform Dialogue and Empower A‐Fib Patients (DECIDE) Outcome Questionnaire to 100 potential participants. Of these participants, 77 (77%) considered the DCS 25 to be more relevant to them than the Decision Regret Scale (DRS). Two key secondary end points were proposed: the DRS and a composite end point of DCS and DRS (designated as CE) that integrated patients' preferences at 1 month after decision. The composite end point of DCS and DRS was constructed according to the method in Lu et al. 26 Specifically, it was a weighted average of Mann–Whitney U‐statistics for DCS (denoted as U1) and DRS (denoted as U2), mathematically, CE=0.77U1+0.23U2, where the weight of 0.77 and 0.23 was derived from the Outcome Questionnaire Study. The rationale for this end point is to consider not only the preference of the majority (77%) but also the minority (23%) of participants who prefer the DRS, thus accounting for heterogeneity of the participant population. We developed an 8‐item AF knowledge scale (range, 0–8) assessing participant understanding of AF, stroke risk, and anticoagulation. Anticoagulation medication persistence and adherence, based on participant self‐reported missed doses, were also prespecified secondary outcomes and assessed by questionnaires. Secondary safety end points covered postbaseline clinical end points, including major bleeding, stroke, transient ischemic attack, deep venous thrombosis or pulmonary embolus, and death. Major bleeding was defined as requiring a transfusion of ≥2 units modified on the basis of criteria from the Control of Anticoagulation Subcommittee of the International Society on Thrombosis and Hemostasis. 27 Additional secondary end points reported in this paper included the DCS, DRS, and the composite end point for other visits; Preparation for Decision Making 28 postbaseline; changes in AF knowledge from baseline; duration of clinical visit; duration of anticoagulation medication discussion during the visit; anticoagulation medication decisions reported by clinicians and participants and their agreements; and quality of communication.
Statistical Analysis
The Consolidated Standards of Reporting Trials guidelines were used for reporting trial results. Statistical analyses were performed using RStudio (2022.07.1 Build 554, Boston, MA). All clinical trial data were entered directly from the participating sites into the Research Electronic Data Capture database. Data monitoring and quality control followed a prespecified data monitoring plan. The statistical analysis was performed for the intent‐to‐treat population. Treatment effects were evaluated for significance (P<0.05, 2‐sided).
Sample Size
Based on prior studies 10 and work by Kunneman et al, 5 we targeted an effect size above 31% for DCS. Using the Wilcoxon‐Mann–Whitney (WMW) U‐statistics, the corresponding U statistic equals 58.68%. Since there is a lack of literature on the treatment effect of the DRS, we targeted an effect size above 20%, a U‐statistic of 55.60%. Using the sample size formula given by Shieh 29 and a R‐package WMWPOW version 0.1.2, we planned a sample size of 1000 participants with an anticipated 5% lost to follow‐up, leading to a total of 950 evaluable participants. The corresponding powers to reject the null hypothesis of no treatment differences for DCS, DRS, and CE at the 1‐month visit were 99.7%, 84.8%, and 98.7%, respectively.
Analysis Methods
Key baseline and demographic characteristics were summarized for all participants by treatment arms. The means and SDs were computed for continuous variables, and frequency and percentages were presented for categorical variables. Median and range were used to summarize ordinal outcome scales. No formal statistical hypothesis testing was performed to compare the baseline characteristics, as the patients were randomly assigned into treatment arms. There were no treatment arm–specific statistics for CE because this end point was valid only in comparing treatment arms.
We used the Wilcoxon rank test to compare DCS an DRS between arms. A permutation test of 200 000 times was used to determine the significance of CE under the null hypothesis of CE=0.5. To control for type I error attributable to multiple tests, the primary end point was tested first, and 2 key secondary end points were tested only when the primary end point reached statistical significance. The Holm–Bonferroni method was used to adjust for type I errors of the 2 key secondary end points. The protocol indicated that no multiple comparison adjustments would be used for other secondary end points. A Wilcoxon test was used for ordinal variables and Fisher's exact test for categorical variables. To evaluate the interactions between treatment and a few prespecified subgroups, we used the aligned rank transform ANOVA and R‐package ARTool to calculate their significance levels.
Missing Data and Sensitivity Analysis
Sensitivity analyses were performed for the primary end point of the trial. A total of 5.8% (58/1001) of participants missed the primary end point, which was close to the anticipated 5% dropout rate. The chance of missing was associated with baseline characteristics of high risk of stroke, high blood pressure, and age groups, and the missing at‐random assumption was violated. We used the inverse probability of observed data to correct for missing data in the primary sensitivity analysis. Logistic regressions were performed to estimate the probability of observing the primary end point for each treatment arm using all baseline characteristics. The weight of the inverse probability was used to derive a weighted WMW U‐statistic, and a permutation test was performed to determine its significance. We further performed 2 additional sensitivity analyses. First, we imputed the missing data in the UC arm by the median DCS from the SDM arm and the missing data in the SDM arm by the median DCS from the UC arm. A Wilcoxon test was then used to compare treatment arms. Second, we included all participants in the calculation of WMW U‐statistic. When comparing a participant with a missing primary end point with another participant from the opposite arm, a tie score (0.5) was assigned. A permutation test was then used to determine the significance. The full results of these sensitivity analyses are presented in Table S1.
RESULTS
Trial participants were enrolled and followed‐up between 12/18/2019 to 08/17/2022. The Consolidated Standards of Reporting Trials diagram (Figure 2) indicates the flow of patients who were screened and randomized into an intervention arm and the UC arm. Of 1620 patients who were screened, 1099 were found eligible, and 1001 (91%) consented and were randomized into the trial. The demographic characteristics are shown in Table 1.
Figure 2. Consolidated standards of reporting trials diagram.
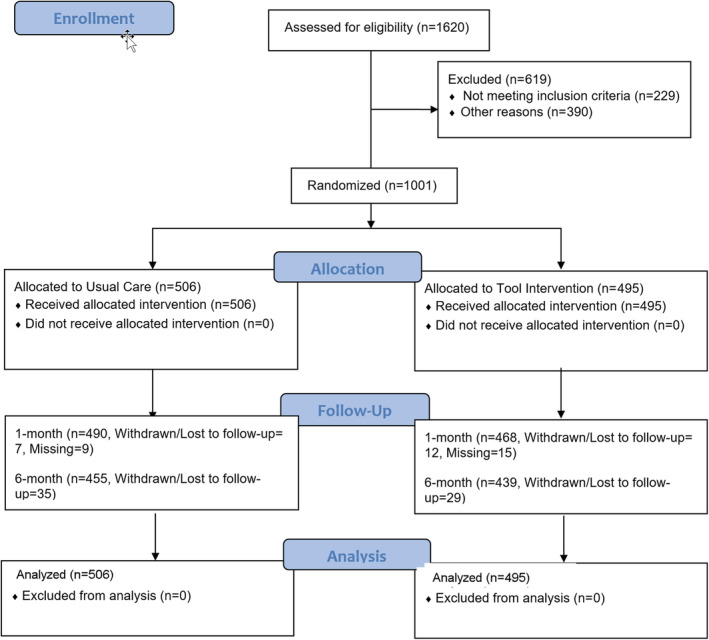
Table 1.
Patient Demographic and Baseline Variables
| Usual care (N=506) | Tool intervention (N=495) | |
|---|---|---|
| Age, mean (SD) | 69.0 (±10.0) | 68.9 (±10.5) |
| Sex, male, n (%) | 308 (60.%) | 294 (59.4) |
| Race and ethnicity, n (%) | ||
| Non‐Hispanic White | 356 (70.4) | 378 (76.4) |
| Hispanic or Latino | 33 (6.5) | 12 (2.4) |
| Asian | 14 (2.8) | 22 (4.4) |
| Black or African American | 92 (18.2) | 77 (15.6) |
| American Indian or Alaskan Native | 0 (0.0) | 1 (0.2) |
| Native Hawaiian or other Pacific Islander | 1 (0.2) | 2 (0.4) |
| Other or multiple | 10 (2.0) | 3 (0.6) |
| Highest level of education, n (%) | ||
| No college | 156 (30.8) | 172 (34.7) |
| College | 239 (47.2) | 222 (44.8) |
| Postgraduate | 95 (18.8) | 86 (17.4) |
| Decline to state | 16 (3.2) | 15 (3.0) |
| Anticoagulant use duration ≤6 months or never been on, n (%) | 244 (48.2) | 241 (48.7) |
| CHA2DS2VASc score, mean (SD) | 3.4 (±1.5) | 3.4 (±1.6) |
| Low AF stroke risk, n (%)* | 250 (49.4) | 248 (50.1) |
| Diabetes, n (%) | 125 (24.7) | 136 (27.5) |
| Hypertension, n (%) | 410 (81.0) | 397 (80.2) |
| Prior stroke/TIA, n (%) | 62 (12.3) | 64 (12.9) |
| Vascular disease, n (%) | 165 (32.6) | 160 (32.3) |
| Age, y, 65–74, n (%) | 237 (46.8) | 208 (42.0) |
| Age, y, >75, n (%) | 142 (28.1) | 150 (30.3) |
| Atrial Fibrillation Severity Scale total burden | ||
| n (%) | 496 (98) | 483 (98) |
| Median (min, max) | 14.0 (3.0, 30.0) | 13.0 (3.0, 30.0) |
| AF knowledge, median (min, max) | ||
| n (%) | 505 (>99) | 493 (>99) |
| Median (min, max) | 6.0 (0.0, 8.0) | 6.0 (0.0, 8.0) |
AF indicates atrial fibrillation; and TIA, transient ischemic attack.
Low risk: Male CHA2DS2‐VASc score <3 or female CHA2DS2‐VASc score <4.
The mean age of these 1001 participants age was 69.0±10.2 years, with 60% of them men (n=602/1001), and they were all randomized into either the intervention arm (495 participants: 68.9±10.5 years; 294 (59.4%) men) or the UC arm (506 participants: 69±10.0 years; 308 (60.9%) men). Overall, 73% participants were non‐Hispanic White, 4% Hispanic, 17% Black, and 4% Asian.
About 64% of participants had education through college or beyond. Approximately 48% of patients had either no history of anticoagulation use or were on it for <6 months. The mean and SD of CHA2DS2VASc scores was 3.4±1.5. At baseline, participants had high prevalence of diabetes (26%), hypertension (81%), prior stroke or transient ischemic event (13%), and vascular disease (32%). Twenty‐nine percent of participants were ≥75 years of age. The median AF knowledge at baseline was 6.0 with interquartile range between 5.0 and 7.0. Atrial Fibrillation Severity Scale total burden had a median of 13.0 with an interquartile range between 9.0 and 19.0.
The baseline demographic and clinical characteristics were comparable between the 2 arms except for race and ethnicity. There were more non‐Hispanic White Americans in SDM and more Hispanic and Black participants in UC. Since randomization was assigned according to blinded and predeveloped sequence, racial and ethnic difference was attributable to chance.
Clinical Visits and Postintervention Outcomes
Table 2 shows results of participant and clinician surveys of their clinical visits and immediate postvisit outcome scales. Participants in the SDM arm observed a nonsignificantly shorter visit (median for UC, 21–30 minutes; and for SDM, 11–20 minutes; P=0.330). During this visit, participants in the SDM arm spent more time with their clinicians discussing anticoagulation strategies (median, 0–10 minutes for both arms; P=0.015; percentage of 10 minutes+, 31% UC and 39% SDM). More participants in the SDM arm reported better quality of communication with their clinicians (median for both arms was “Yes, definitely”; P=0.069) and that clinicians listened carefully (median for both arms was “Yes, definitely”; P=0.004). There was no difference with regard to clinicians' respect for what participants had to say (P=0.24). The most common decisions made in the visit regarding anticoagulation medication reported by participants were apixaban, rivaroxaban, dabigatran, or edoxaban, with 87% reported by clinicians in both arms, 86% in the UC arm, and 88% in the SDM arm. The participants and clinicians were in high agreement (96% in both arms) with their anticoagulant use decisions (P=0.78).
Table 2.
Intervention Visit and Postintervention Outcome
| Usual Care (N=506) | Tool Intervention (N=495) | P value | |
|---|---|---|---|
| Duration of patient visit, n (%) | 0.33 | ||
| 0–10 min | 61 (12.1) | 43 (8.7) | |
| 11–20 min | 188 (37.2) | 209 (42.2) | |
| 21–30 min | 128 (25.3) | 89 (18.0) | |
| 31–60 min | 106 (20.9) | 135 (27.3) | |
| >60 min | 19 (3.8) | 17 (3.4) | |
| Missing | 4 (0.8) | 2 (0.4) | |
| Duration of explanation/discussion of anticoagulation during visit, n (%) | 0.015 | ||
| Did not explain/discuss | 2 (0.4) | 2 (0.4) | |
| 0–10 min | 345 (68.2) | 300 (60.6) | |
| 11–20 min | 125 (24.7) | 160 (32.3) | |
| 21–30 min | 17 (3.4) | 15 (3.0) | |
| 31–60 min | 10 (2.0) | 13 (2.6) | |
| >60 min | 3 (0.6) | 3 (0.6) | |
| Missing | 4 (0.8) | 2 (0.4) | |
| Clinician reported decision of anticoagulation medication, n (%) | 0.77 | ||
| Warfarin (Coumadin) | 33 (6.5) | 26 (5.3) | |
| Apixaban, dabigatran, edoxaban, or rivaroxaban | 439 (86.8) | 432 (87.3) | |
| To not take a blood thinner medication | 16 (3.2) | 19 (3.8) | |
| To start taking aspirin or other antiplatelet agent for the purpose of stroke prevention | 6 (1.2) | 4 (0.8) | |
| The decision about anticoagulation medication was not made today | 9 (1.8) | 12 (2.4) | |
| Missing | 3 (0.6) | 2 (0.4) | |
| Patient reported decision of anticoagulation medication, n (%) | 0.37 | ||
| Warfarin (Coumadin) | 35 (6.9) | 24 (4.8) | |
| Apixaban, dabigatran, edoxaban, or rivaroxaban | 434 (85.8) | 433 (87.5) | |
| To not take a blood thinner medication | 16 (3.2) | 20 (4.0) | |
| To start taking aspirin or other antiplatelet agent for the purpose of stroke prevention | 9 (1.8) | 4 (0.8) | |
| The decision about anticoagulation medication was not made today | 9 (1.8) | 10 (2.0) | |
| Missing | 3 (0.6) | 4 (0.8) | |
| Agreement between patient and clinician reported decision, n (%) | 0.78 | ||
| Yes | 483 (95.5) | 475 (96.0) | |
| No | 19 (3.8) | 15 (3.0) | |
| Missing | 4 (0.8) | 5 (1.0) | |
| Quality of communication—did clinician explain things,* n (%) | 0.069 | ||
| Yes, definitely | 475 (93.9) | 475 (96.0) | |
| Yes, somewhat | 23 (4.5) | 14 (2.8) | |
| No | 4 (0.8) | 1 (0.2) | |
| Missing | 4 (0.8) | 5 (1.0) | |
| Quality of communication—did clinician listen carefully, n (%) | 0.004 | ||
| Yes, definitely | 474 (93.7) | 480 (97.0) | |
| Yes, somewhat | 24 (4.7) | 9 (1.8) | |
| No | 4 (0.8) | 1 (0.2) | |
| Missing | 4 (0.8) | 5 (1.0) | |
| Quality of communication—did the clinician show respect, n (%)† | 0.24 | ||
| Yes, definitely | 487 (96.2) | 481 (97.2) | |
| Yes, somewhat | 14 (2.8) | 7 (1.4) | |
| No | 1 (0.2) | 2 (0.4) | |
| Missing | 4 (0.8) | 5 (1.0) | |
| DCS | 0.0001 | ||
| n (%) | 502 (99) | 489 (99) | |
| Median (min, max) | 12.5 (0.0, 76.6) | 6.2 (0.0, 54.7) | |
| DRS | 0.016 | ||
| n (%) | 503 (99) | 490 (99) | |
| Median (min, max) | 10.0 (0, 65.0) | 5.0 (0, 60.0) | |
| DCS and DRS composite end point | 0.0001 | ||
| Preparation for decision making | <0.0001 | ||
| n (%) | 503 (99) | 490 (99) | |
| Median (min, max) | 70.0 (0, 100) | 82.5 (0, 100) | |
| AF knowledge | <0.0001 | ||
| n (%) | 502 (99) | 490 (99) | |
| Median (min, max) | 6.0 (2.00, 8.00) | 7.0 (2.00, 8.00) | |
AF indicates atrial fibrillation; DCS, Decision Conflict Scale; and DRS, Decision Regret Scale.
Did the clinician explain things in a way that was easy to understand?
Did the clinician show respect for what patient had to say?
In the immediate postvisit outcome evaluation, participants in the SDM arm had significantly lower DCS score (median, 12.5 for UC and 6.2 for SDM; P<0.001), significantly lower DRS score (median, 10 for UC and 5 for SDM; P=0.016), significantly higher AF knowledge (median, 6.0 for UC and 7.0 for SDM; P<0.001), significantly higher preparation for decision making (median, 70.0 for UC and 82.5 for SDM; P<0.0001) and favorable CE.
Primary and Selected Secondary End Points at 1‐ and 6‐Month Follow‐Up
Table 3 shows the primary and selected secondary outcomes at 1‐ and 6‐month follow‐up visits. The primary end point of 1‐month DCS achieved a 7‐point difference in median scores between 2 arms (median, 16.4 versus 9.4; U=0.550; P=0.007), indicating a clinically relevant degree of decreased decisional conflict in participants in the intervention arm (see Discussion for impact of each unit of change). For the secondary end point of 1‐month DRS, the difference in median scores between arms was 5 points (median, 10.0 UC versus 5.0 SDM; P=0.078, both with and without adjustment for multiple comparisons). The CE was significantly in favor of SDM (U=0.55; P=0.0023 without adjustment, 0.0104 with adjustment). After 1 month, median AF knowledge (median, 6.0 UC versus 7.0 SDM; P=0.0009) as well as its change from baseline was greater in the SDM arm compared with the UC arm (P=0.009).
Table 3.
Primary and Secondary Outcomes
| Month 1 | Month 6 | |||||
|---|---|---|---|---|---|---|
| Usual care (N=506) | Tool intervention (N=495) | P value | Usual care (N=506) | Tool intervention (N=495) | P value | |
| DCS | 0.007* | 0.06 | ||||
| n (%) | 482 (95) | 461 (93) | 455 (90) | 440 (89) | ||
| Median (min, max) | 16.4 (0, 89.1) | 9.4 (0, 81.3) | 10.9 (0, 90.6) | 6.2 (0, 81.3) | ||
| DRS | 0.078† | 0.35 | ||||
| n (%) | 482 (95) | 461 (93) | 454 (90) | 440 (89) | ||
| Median (min, max) | 10.0 (0, 90.0) | 5.0 (0, 80.0) | 5.0 (0, 75.0) | 5.0 (0, 100) | ||
| Weighted DCS and DRS composite score | 0.0009† | 0.043 | ||||
| Preparation for decision making | <0.0001 | <0.0001 | ||||
| n (%) | 482 (95) | 461 (93) | 454 (90) | 440 (89) | ||
| Median (min, max) | 72.5 (0, 100) | 82.5 (0, 100) | 75.0 (0, 100) | 87.5 (0, 100) | ||
| Change of AF knowledge from baseline | 0.009 | 0.007 | ||||
| n (%) | 481 (95) | 461 (93) | 454 (90) | 440 (89) | ||
| Median (min, max) | 0.0 (−4.0, 7.0) | 1.0 (−4.0, 6.0) | 0.0 (−4.0, 7.0) | 1.0 (−4.0, 6.0) | ||
AF indicates atrial fibrillation; DCS, Decision Conflict Scale; and DRS, Decision Regret Scale.
Primary end point.
Key secondary end points: P values were adjustment for 2 end points using the Holm–Bonferroni method.
The treatment effects lessened over time. At 6 months, the difference in medians was 4.7 points for DCS (median, 10.9 UC versus 6.2 SDM; P=0.060) and 0 points for DRS (median, 5.0 UC versus 5.0 SDM; P=0.35). However, the CE was 0.53 with a P value of 0.043. The significant difference between treatment arms for AF knowledge and changes from baseline (median, 6.0 UC versus 7.0 SDM; P=0.007) remained. All P values for 6 month comparisons were not adjusted for multiple comparisons.
Clinical Outcomes
There was no difference in the number of participants with at least 1 clinical outcome (P=0.16) and death (Tables S2 and S3). Similarly, there was no difference in the number of participants experiencing adverse events.
Anticoagulation Decision and Medication Adherence
At 1 and 6 months, there was no significant difference in the anticoagulant decision between the UC and SDM arms (Tables S4 and S5). At 1 and 6 months, there were no significant differences in the number of patient‐reported doses of anticoagulant missed in the past week or past month between the UC and SDM arms (Tables S6 and S7).
Sensitivity Analysis
A missing primary end point was observed for 5.8% of participants (58/1001), and missingness was not at random. The inverse probability weight for missing data correction resulted in a U=0.55 and P=0.012. Two sensitivity analyses also support the significant improvement in DCS 1 month after visit, with P values of 0.018 (imputation by median of opposite arm) and 0.007 (WMW U test with all patients and using ties for all missing observations) (Table S1). We also examined whether treatment behaved differently among prespecified subgroups through testing the interaction between treatment and subgroups. In addition to subgroups according to the 3 stratification variables (sites, prior anticoagulation usage, and high CHA2DS2VASc risk scores), we examined race and ethnicity (Hispanic and Black versus non‐Hispanic and non‐Black) and education (no college versus college and beyond) subgroups. None of the interactions achieved our prespecified significant level. The smallest P value for these interactions was 0.28 (interaction of treatment and site).
DISCUSSION
Main Findings
This multicenter randomized clinical trial demonstrated the beneficial effects of our novel SDM pathway in reducing decisional conflict associated with the decision to use anticoagulation for AF stroke prevention at 1 month, our study's primary end point. The degree of decrease in decisional regret observed in our study is clinically relevant.
The SDM literature highlights potential benefits of computer‐aided patient decision‐making aids that present patients with multiple options and are available simultaneously for real‐time use by clinicians. O'Neill et al searched a total of 666 articles, identifying 7 studies studying 6 different AF stroke prevention decision aids. Of 6 randomized trials, 4 used control arms. These 4 studies with control arms included 3 with usual care. 11 , 12 , 13 They included interventions such as educational booklets alone or booklets accompanied by a worksheet and audiotape, or computerized intervention. None of the published manuscripts provided complete access to these tools.
Our tool consisted of a digital toolkit that the clinician used to calculate personalized stroke risk (Figure 1) as well as a video (Figure 1) that patients viewed before the clinician visit. The digital toolkit included a quiz to assess patients' knowledge with an ability to review the correct answer. It included frequently asked questions that the participants could select. Finally, participants were given a worksheet (Figure 1) to select questions that could be discussed with the clinician. We provide access to the toolkit with the URLs (https://afibguide.com; https://afibguide.com/clinician) and QR codes (Figure 1).
In contrast to our study's findings, a recently published randomized trial of an AF stroke prevention decision aid observed no significant reduction in decisional conflict. This study by Kunneman et al 5 reported DCS scores with a mean of 16.6 (14.4) in the intervention arm and 17.9 (14.9) in the UC arm. The intervention used in this study 11 was an electronic digital tool called the Anticoagulation Choice tool that physicians used during clinician visits. The tool consisted of 2 components: a risk calculator to calculate personalized risks for thromboembolic strokes at 1 and 5 years, and issue cards that supported patient–clinician conversation on patient‐related factors that may affect the choice of agent and the patient's ability to adhere to anticoagulation (eg, diet, recreational activities, and travel). Patients were able to request a printed copy of the tool from their clinician, which they could use later to share with others and to review, confirm, or revisit the decision.
In the Kunneman study, the mean DCS scores in the UC arm was 17.9, higher than observed in our study UC arm, which was 12.5 after the visit, 16.4 at 1 month, and 10.9 at 6 months. A higher score indicates a greater decision conflict, and one might speculate that it would be easier to demonstrate a decrease in decisional conflict in the Kunneman et al study. 5 In this study, only a clinician tool was used. We are unable to ascertain whether our use of low‐health‐literacy patient tools could explain the difference in our study results as compared with study results published by Kunneman et al. 5
We designed our tools with several specific goals in mind. Our video uses a minimal amount of text and is graphically rich to be suitable for patients with a wide range of health literacy. Similarly, our gentle quiz is designed to reinforce key concepts such as the increased risk of stroke in AF. A set of frequently asked questions is designed to address additional questions that the patient may have and to reinforce key messages in the video. The worksheet combined with the clinician tool is designed to enhance communication between the patient and the clinician in discussing the role of anticoagulation for stroke prevention.
Our study only examined a single use of the SDM set of tools. Our study was not powered to examine changes in the DCS score over time, but the positive effect of our 1‐time intervention appears to have lessened over time. We observed that the preparation for decision making was higher in the SDM arm immediately after the visit compared with the UC arm and that this difference was maintained at 1 and 6 months. Indeed, it is expected that a single 1‐time intervention would have a decrease in effect over time. We do not know if repetitive use of our tools would be able to create a more sustained effect. Given that patient‐specific AF risk may change over time, reuse of the toolkit at subsequent visits could be beneficial.
We observed that AF knowledge was greater in the SDM arm compared with the UC arm. Although a study by Aronis et al 8 noted that brief clinic‐based educational interventions rarely result in significant near‐term patient‐centered outcomes (eg, knowledge skills), 30 , 31 , 32 we demonstrated, by contrast, that a brief educational intervention can meaningfully improve AF‐related knowledge not only in the short term (1 month) but also in the medium term (6 months).
Limitations
Our study was not statistically powered to detect differences in cardiovascular outcomes or patient adherence to medications proven to improve outcomes. Similarly, Noseworthy et al 33 reported a clinical trial of an SDM tool in which there was no difference in medication adherence or clinical outcomes as compared with UC. Half of our patients were on anticoagulation before the study, and we do not have an adequate statistical power to examine the effect of the SDM tool on patients with minimal or no prior anticoagulation use. Our study was not powered to determine whether using our SDM tools would result in a change in anticoagulation decision.
We do not know if there is any long‐term (>6 months) impact of our intervention and we do not know if repeating its use will lead to a prolonged effect. Since our intervention uses a patient tool and a clinician tool, we cannot determine whether 1 component had a predominant role in reducing decision conflict.
We anticipated a 5% lost‐to‐follow‐up rate. Despite the pandemic, the study lost‐to‐follow‐up rate remained only 5.8%.
We did not determine the cost of the SDM intervention.
Future research should address these limitations and extend our findings by optimizing the intervention's user interface, identifying facilitators and barriers to SDM toolkit adoption and determining long‐term clinical and quality‐of‐life outcomes.
Implications of Our Study
We have demonstrated the potential impact of a brief educational intervention, enhanced by low‐literacy information technology. We suspect that the intervention's effect may have been facilitated by a low‐literacy approach (minimal text, meaningful images, teach‐back), enhanced by real‐time engagement with a clinician. Use of this toolkit could be considered by clinicians in their decision‐making discussions with patients around anticoagulation for stroke prevention in AF.
Furthermore, this patient and clinician end‐user approach to the design of a low‐health‐literacy digital shared decision‐making tool has potential application to other clinical contexts, particularly technically complex issues where patient preference affects the balance of benefits and harms.
Sources of Funding
The study was conducted at the Joe and Linda Chlapaty Stanford DECIDE Center of the Atrial Fibrillation Strategically Focused Research Network Award from the American Heart Association: 18SFRN34120036.
Disclosures
Dr Russo has disclosed financial relationships with Bayer, BMS‐Pfizer, and Sanofi. Dr Piccini has disclosed financial relationships with Bayer and Boston Scientific. All other authors have no disclosed financial relationships.
Supporting information
Table S1–S7
Acknowledgments
Site Teams:
Cleveland Clinic Foundation: Marilyn Boros, Mina Chung, Jeffery Courson, Michael Crookshanks, Kathleen Davey, Carla Duvall, Diamond Haynes, Carmine Iafelice, Gina Koehler, Milana Leygerman, David Martin, Kenneth Mayuga, Mark Niebauer, Melanie Panko, Veronica Peck, Raquel Rozich, Danielle Schaffer, Lindsey Siemen, Lara Spaller, Jakub Sroubek, Patrick Tchou, Jing Yang.
Cooper University Hospital: Marc Angud, Colleen Brown, Maritza Cotto, Danielle Dondero, Julie Field, Claire Fisher, Matthew Ortman, Andrea Russo, Jennifer Serna, Stephanie Vasey.
East Carolina University: Scarlett Anthony, Joseph Carl Hammerle, Rebecca Harrell, Saleen Khan, Caroline Martin, Aditi Naniwadekar, Rajasekhar Nekkanti, Samuel Sears, Connor Tripp.
Ochsner Medical Center: DeAnna Ames, Michael Bernard, Sharonda Brown, Jennifer Francis, Michael Harrison, Taylor Hart, Elise Hiltbold, Sammy Khatib, Terri Lopez, Hunter McDaniel, Daniel Morin, Glenn Polin, Paul Rogers.
Stanford University/Stanford Health Care: Tina Baykaner, Katie DeSutter, Neisha Fernandes, Gotzone Garay, Karma Lhamo, Amy Lin, Bryant Lin, Chun‐Ying Liu, Ying Lu, Kenneth Mahaffey, Paul Newswanger, Duy Thai Nguyen, Julio Nunes, Nicole Odenwald, Alexander Perino, Idean Pourshams, Marie Preval, Krishna Pundi, Eli Rice, Gerilynn Schott‐Horvick, Rushil Shah, Allysonne Smith, Sadhna Sood, Randall Stafford, Mohan Viswanathan, Paul Wang.
Independent Clinical Monitor
Jonathan Piccini, MD, MHS
Adverse Event Adjudication
Krishna Pundi, MD
This work was presented at AHA Scientific Sessions, November 5–7, 2022, in Chicago, IL.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.028562
For Sources of Funding and Disclosures, see page 11.
References
- 1. Stroke Prevention in Atrial Fibrillation Study . Final results. Circulation. 1991;84:527–539. [DOI] [PubMed] [Google Scholar]
- 2. Warfarin versus aspirin for prevention of thromboembolism in atrial fibrillation: stroke prevention in atrial fibrillation II study. Lancet. 1994;343:687–691. [PubMed] [Google Scholar]
- 3. Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GY. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010;123:638–645.e4. doi: 10.1016/j.amjmed.2009.11.025 [DOI] [PubMed] [Google Scholar]
- 4. Hsu JC, Freeman JV. Underuse of vitamin K antagonist and direct oral anticoagulants for stroke prevention in patients with atrial fibrillation: a contemporary review. Clin Pharmacol Ther. 2018;104:301–310. doi: 10.1002/cpt.1024 [DOI] [PubMed] [Google Scholar]
- 5. Kunneman M, Branda ME, Hargraves IG, Sivly AL, Lee AT, Gorr H, Burnett B, Suzuki T, Jackson EA, Hess E, et al. Assessment of shared decision‐making for stroke prevention in patients with atrial fibrillation: a randomized clinical trial. JAMA Intern Med. 2020;180:1215–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seaburg L, Hess EP, Coylewright M, Ting HH, McLeod CJ, Montori VM. Shared decision making in atrial fibrillation: where we are and where we should be going. Circulation. 2014;129:704–710. doi: 10.1161/CIRCULATIONAHA.113.004498 [DOI] [PubMed] [Google Scholar]
- 7. Devereaux PJ, Anderson DR, Gardner MJ, Putnam W, Flowerdew GJ, Brownell BF, Nagpal S, Cox JL. Differences between perspectives of physicians and patients on anticoagulation in patients with atrial fibrillation: observational study. BMJ. 2001;323:1218–1222. doi: 10.1136/bmj.323.7323.1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aronis KN, Edgar B, Lin W, Martins MAP, Paasche‐Orlow MK, Magnani JW. Health literacy and atrial fibrillation: relevance and future directions for patient‐centered care. Eur Cardiol. 2017. Summer;12:52–57. doi: 10.15420/ecr.2017:2:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mitchell SE, Gardiner PM, Sadikova E, Martin JM, Jack BW, Hibbard JH, Paasche‐Orlow MK. Patient activation and 30‐day post‐discharge hospital utilization. J Gen Intern Med. 2014;29:349–355. doi: 10.1007/s11606-013-2647-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O'Connor AM. User Manual ‐ Decisional Conflict Scale (16 Item Statement Format) [document on the Internet]. Ottawa Hospital Research Institute; © 1993 [updated 2010]:16. Available at: http://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Decisional_Conflict.pdf. [Google Scholar]
- 11. Man‐Son‐Hing LA, O'Connor AM, Biggs J, Drake E, Yetisir E, Hart RG. A patient decision aid regarding antithrombotic therapy for stroke prevention in atrial fibrillation: a randomized controlled trial. JAMA. 1999;282:737–743. doi: 10.1001/jama.282.8.737 [DOI] [PubMed] [Google Scholar]
- 12. Protheroe J, Fahey T, Montgomery AA, Peters TJ. The impact of patients' preferences on the treatment of atrial fibrillation: observational study of patient based decision analysis. BMJ. 2000;320:1380–1384. doi: 10.1136/bmj.320.7246.1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Man‐Son‐Hing M, Gage BF, Montgomery AA, Howitt A, Thomson R, Devereaux PJ, Protheroe J, Fahey T, Armstrong D, Laupacis A. Preference‐based antithrombotic therapy in atrial fibrillation: implications for clinical decision making. Med Decis Making. 2005;25:548–559. doi: 10.1177/0272989X05280558 [DOI] [PubMed] [Google Scholar]
- 14. McAlister FA, Man‐Son‐Hing M, Straus SE, Ghali WA, Anderson D, Majumdar SR, Gibson P, Cox JL, Fradette M, Decision Aid in Atrial Fibrillation (DAAFI) Investigators . Impact of a patient decision aid on care among patients with nonvalvular atrial fibrillation: a cluster randomized trial. CMAJ. 2005;173:496–501. doi: 10.1503/cmaj.050091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holbrook A, Labiris R, Goldsmith CH, Ota K, Harb S, Sebaldt RJ. Influence of decision aids on patient preferences for anticoagulant therapy: a randomized trial. CMAJ. 2007;176:1583–1587. doi: 10.1503/cmaj.060837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thomson RG, Eccles MP, Steen IN, Greenaway J, Stobbart L, Murtagh MJ, May CR. A patient decision aid to support shared decision‐making on anti‐thrombotic treatment of patients with atrial fibrillation: randomized controlled trial. Qual Saf Health Care. 2007;16:216–223. doi: 10.1136/qshc.2006.018481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O'Neill ES, Grande SW, Sherman A, Elwyn G, Coylewright M. Availability of patient decision aids for stroke prevention in atrial fibrillation: a systematic review. Am Heart J. 2017;191:1–11. doi: 10.1016/j.ahj.2017.05.014 Epub 2017 Jun 3 [DOI] [PubMed] [Google Scholar]
- 18. Chung MK, Fagerlin A, Wang PJ, Ajayi TB, Allen LA, Baykaner T, Benjamin EJ, Branda M, Cavanaugh KL, Chen LY, et al. Shared decision making in cardiac electrophysiology procedures and arrhythmia management. Circ Arrhythm Electrophysiol. 2021;14:e007958. doi: 10.1161/CIRCEP.121.007958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–e76. doi: 10.1016/j.jacc.2014.03.022 [DOI] [PubMed] [Google Scholar]
- 20. Rolls CA, Obamiro KO, Chalmers L, Bereznicki LRE. The relationship between knowledge, health literacy, and adherence among patients taking oral anticoagulants for stroke thromboprophylaxis in atrial fibrillation. Cardiovasc Ther. 2017;35. doi: 10.1111/1755-5922.12304 [DOI] [PubMed] [Google Scholar]
- 21. Jankowska‐Polańska B, Katarzyna L, Lidia A, Joanna J, Dudek K, Izabella U. Cognitive function and adherence to anticoagulation treatment in patients with atrial fibrillation. J Geriatr Cardiol. 2016;13:559–565. doi: 10.11909/j.issn.1671-5411.2016.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Deelen BA, van den Bemt PM, Egberts TC, van't Hoff A, Maas HA. Cognitive impairment as determinant for sub‐optimal control of oral anticoagulation treatment in elderly patients with atrial fibrillation. Drugs Aging. 2005;22:353–360. doi: 10.2165/00002512-200522040-00007 [DOI] [PubMed] [Google Scholar]
- 23. Hedberg B, Malm D, Karlsson JE, Årestedt K, Broström A. Factors associated with confidence in decision making and satisfaction with risk communication among patients with atrial fibrillation. Eur J Cardiovasc Nurs. 2017:1474515117741891. doi: 10.1177/1474515117741891 [DOI] [PubMed] [Google Scholar]
- 24. Baykaner T, Pundi K, Lin B, Lu Y, DeSutter K, Lhamo K, Garay G, Nunes JC, Morin DP, Sears SF, et al. The ENHANCE‐AF clinical trial to evaluate an atrial fibrillation shared decision‐making pathway: rationale and study design. Am Heart J. 2022;05(247):68–75. doi: 10.1016/j.ahj.2022.01.013 [DOI] [PubMed] [Google Scholar]
- 25. Brehaut JC, O'Connor AM, Wood TJ, Hack TF, Siminoff L, Gordon E, Feldman‐Stewart D. Validation of a decision regret scale. Med Decis Making. 2003;23:281–292. doi: 10.1177/0272989X03256005 [DOI] [PubMed] [Google Scholar]
- 26. Lu Y, Zhao Q, Zou J, Yan S, Tamaresis JS, Nelson L, Tu XM, Chen J, Tian L. A composite endpoint for treatment benefit according to patient preference. Stat Biopharm Res. 2022;1:15. doi: 10.1080/19466315.2022.2085783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schulman S, Kearon C. Subcommittee on control of anticoagulation of the scientific and standardization Committee of the International Society on thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost. 2005;3:692–694. [DOI] [PubMed] [Google Scholar]
- 28. Bennett C, Graham ID, Kristjansson E, Kearing SA, Clay KF, O'Connor AM. Validation of a preparation for decision making scale. Patient Educ Couns. 2010;78:130–133. doi: 10.1016/j.pec.2009.05.012 [DOI] [PubMed] [Google Scholar]
- 29. Shieh G, Jan SL, Randles RH. On power and sample size determinations for the Wilcoxon–Mann–Whitney test. J Nonparametr Stat. 2006;18:33–43. [Google Scholar]
- 30. Fraenkel L, Street RL Jr, Towle V, O'Leary JR, Iannone L, Van Ness PH, Fried TR. A pilot randomized controlled trial of a decision support tool to improve the quality of communication and decision‐making in individuals with atrial fibrillation. J Am Geriatr Soc. 2012;60:1434–1441. doi: 10.1111/j.1532-5415.2012.04080.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McCabe PJ, Schad S, Hampton A, Holland DE. Knowledge and self management behaviors of patients with recently detected atrial fibrillation. Heart Lung. 2008;37:79–90. doi: 10.1016/j.hrtlng.2007.02.006 [DOI] [PubMed] [Google Scholar]
- 32. Lane DA, Ponsford J, Shelley A, Sirpal A, Lip GY. Patient knowledge and perceptions of atrial fibrillation and anticoagulant therapy: effects of an educational intervention programme. The West Birmingham atrial fibrillation project. Int J Cardiol. 2006;110:354–358. doi: 10.1016/j.ijcard.2005.07.031 [DOI] [PubMed] [Google Scholar]
- 33. Noseworthy PA, Branda ME, Kunneman M, Hargraves IG, Sivly AL, Brito JP, Burnett B, Zeballos‐Palacios C, Linzer M, Suzuki T, et al. Effect of shared decision‐making for stroke prevention on treatment adherence and safety outcomes in patients with atrial fibrillation: a randomized clinical trial. J Am Heart Assoc. 2022;11:e023048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S7


