Abstract
Background
We aimed to determine the effect of integrating Atrial Fibrillation Better Care pathway compliance in relation to achievement of systolic blood pressure (SBP) targets and good control of time in therapeutic range (TTR) on clinical outcomes in patients with atrial fibrillation.
Methods and Results
We prospectively enrolled patients with nonvalvular atrial fibrillation from 27 hospitals in Thailand. All clinical outcomes were recorded. Main outcomes were the composite of all‐cause death or ischemic stroke/systemic embolism (SSE), as well as secondary outcomes of all‐cause death, SSE, major bleeding, intracranial hemorrhage, and heart failure. An SBP of 120 to 140 mm Hg was considered good blood pressure control. Target TTR was a TTR ≥65%. A total of 3405 patients were studied (mean age 67.8 years, 41.8% female). Full ABC pathway compliance was evident in 42.7%. For blood pressure control, 41.9% had SBP within target, whereas 35.9% of those on warfarin had TTR within target. The incidence rates of all‐cause death/SSE, all‐cause death, SSE, major bleeding, intracranial hemorrhage, and heart failure were 5.29, 4.21, 1.51, 2.25, 0.78, and 2.84 per 100 person‐years respectively. Adjusted hazard ratios and 95% CI of Atrial Fibrillation Better Care pathway compliance for all‐cause death/SSE, all‐cause death, and heart failure were 0.76 (0.62–0.94), 0.79 (0.62–0.99), and 0.69 (0.51–0.94), respectively, compared with noncompliance. Patients with Atrial Fibrillation Better Care compliance and SBP within target had a better outcome or TTR within target had better outcomes.
Conclusions
In COOL‐AF (Cohort of Antithrombotic Use and Optimal International Normalized Ratio Level in Patients With Non‐Valvular Atrial Fibrillation in Thailand), a multicenter nationwide prospective cohort of patients with atrial fibrillation, achieving SBP within target and TTR ≥ 65% has added value to Atrial Fibrillation Better Care pathway compliance in the reduction of adverse clinical outcomes in patients with atrial fibrillation.
Keywords: ABC pathway, death, ischemic stroke, major bleeding, systolic blood pressure, time in therapeutic range
Subject Categories: Atrial Fibrillation
Nonstandard Abbreviations and Acronyms
- ABC
Atrial Fibrillation Better Care
- COOL‐AF
Cohort of Antithrombotic Use and Optimal International Normalized Ratio Level in Patients With Non‐Valvular Atrial Fibrillation in Thailand
- ICH
intracranial hemorrhage
- OAC
oral anticoagulant
- SSE
ischemic stroke/systemic embolism
- TTR
time in therapeutic range
Clinical Perspective.
What Is New?
Patients with atrial fibrillation had a high rate of adverse clinical outcomes including death, ischemic stroke/systemic embolism, major bleeding, and heart failure.
Achieving systolic blood pressure within target and time in therapeutic range of international normalized ratio has an incremental value to Atrial Fibrillation Better Care pathway compliance in the reduction of adverse clinical outcomes in patients with atrial fibrillation.
What Are the Clinical Implications?
Treatment of patients with atrial fibrillation should not focus only on stroke prevention but should provide a holistic approach including symptom management and treatment of comorbidities.
Treatment of comorbidities in patients with atrial fibrillation should not focus only on giving the medications but also on achieving the target of treatments.
Nonvalvular atrial fibrillation (AF) is one of the leading causes of cardiovascular morbidity and mortality, 1 , 2 as well as hospitalizations and increasing health care costs. 3 A majority of patients with AF are at risk of ischemic stroke and, therefore, need oral anticoagulant (OAC) for stroke prevention. 4 Although OAC reduces stroke and all‐cause mortality in AF, a residual risk of death and other cardiovascular complications remains. 5 A significant proportion of patients with AF have comorbidities such as hypertension, and much more focus has been directed to a more holistic or integrated care approach to AF management, based on the Atrial Fibrillation Better Care (ABC) pathway. 6 Compliance with such an approach is associated with improved outcomes overall 7 , 8 , 9 , 10 , 11 and hence, adopted by guidelines. 12 , 13
Specific factors that have a strong impact on clinical outcomes include optimal blood pressure level 14 , 15 and time in therapeutic range (TTR) for those who are on warfarin. 16 , 17 Of note, the majority of OAC use in Thailand (and many other developing countries) is warfarin owing to the low cost. Indeed, good TTR control was associated with a reduced risk of ischemic stroke, major bleeding, and death. 16 , 18 , 19 Also, the optimal systolic blood pressure (SBP) should be 120 to 140 mm Hg. 14 , 15 , 20
We therefore hypothesized that the clinical outcomes of patients who comply with the ABC pathway would be better if blood pressure and TTR were optimized, compared with those who did not. The aim of this study was to determine the incremental effect of integrating ABC pathway compliance in relation to achievement of blood pressure targets and good TTR on clinical outcomes in a prospective cohort of patients with AF on all‐cause death, ischemic stroke/systemic embolism (SSE), major bleeding, intracranial hemorrhage (ICH), and heart failure (HF) during the mean follow‐up of 32 months.
METHODS
The data set that was used to support the results and conclusion of this study is included within the article. The additional data are available from the corresponding author upon reasonable request.
Study Population
The COOL‐AF (Cohort of Antithrombotic Use and Optimal International Normalized Ratio [INR] Level in Patients With NonValvular Atrial Fibrillation in Thailand) registry is a prospective multicenter nationwide registry of patients with nonvalvular AF that was conducted in 27 centers in Thailand. Patients with nonvalvular AF aged at least 18 years were enrolled. ECG confirmation of AF is required. Patients with the following conditions were excluded: (1) mechanical heart valve, (2) rheumatic mitral valve disease, (3) AF from transient conditions, (4) life expectancy less than 3 years, (4) pregnancy, (5) hematologic disease that increased risk of bleeding, (6) unable to have the follow‐up visit, and (7) refuse participation. Primary objective of the COOL‐AF registry is to determine antithrombotic pattern and to identify optimal INR for Thai population and clinical outcomes. This study was approved by the institutional review board of participating hospitals. Each patient gave informed consent. The study was conducted in accordance with the principles set forth in the Declaration of Helsinki and the International Conference on Harmonization for Good Clinical Practice Guidelines.
Study Protocol
Investigators were instructed to enroll consecutive cases. Investigators reviewed medical records and interviewed patients to acquire the baseline information. The data were recorded in the case record form and entered in the web‐based system. Once the required information was recorded, the data were transferred to the central data management site where the data were validated to ensure the correctness. Site monitoring was performed for all participating hospitals to check the data quality with the source document and confirm the good clinical practice compliance of the study teams. Follow‐up data at 6, 12, 18, 24, 30, and 36 months after the initial visit were recorded.
Data Collection
Baseline information was (1) demographic data, (2) weight and height, (3) vital signs, (4) symptoms and physical examination, (5) types and duration of AF, (6) laboratory data including INR, (7) medical history, (8) investigations such as echocardiogram, and (9) medications. Each component of the CHA2DS2VASc score was scored and recorded, as follows: C=congestive HF (1 point); H=hypertension (1 point); A=age >75 years (2 points); D=diabetes (1 point); S=stroke (2 points); V=vascular disease (1 point); A=age 65–74 (1 point); and Sc=female sex category (1 point). Each component of the HAS‐BLED score was scored and recorded, as follows: uncontrolled Hypertension, Abnormal renal, or liver function; history of Stroke; history of Bleeding; Labile INR; Elderly (age above 65 years); and Drugs or alcohol (1 point each). ABC pathway data were collected according to the original description, 6 as described in Figure 1; A for Avoid stroke is defined by the appropriate use of OAC, B for Better symptom management is defined by the European Heart Rhythm Association symptom scale score of no more than 2, and C for Cardiovascular and comorbidity management is defined as the appropriate use of medications for hypertension, coronary artery disease, ischemic stroke, HF, and diabetes.
Figure 1. Flow diagram of study population.
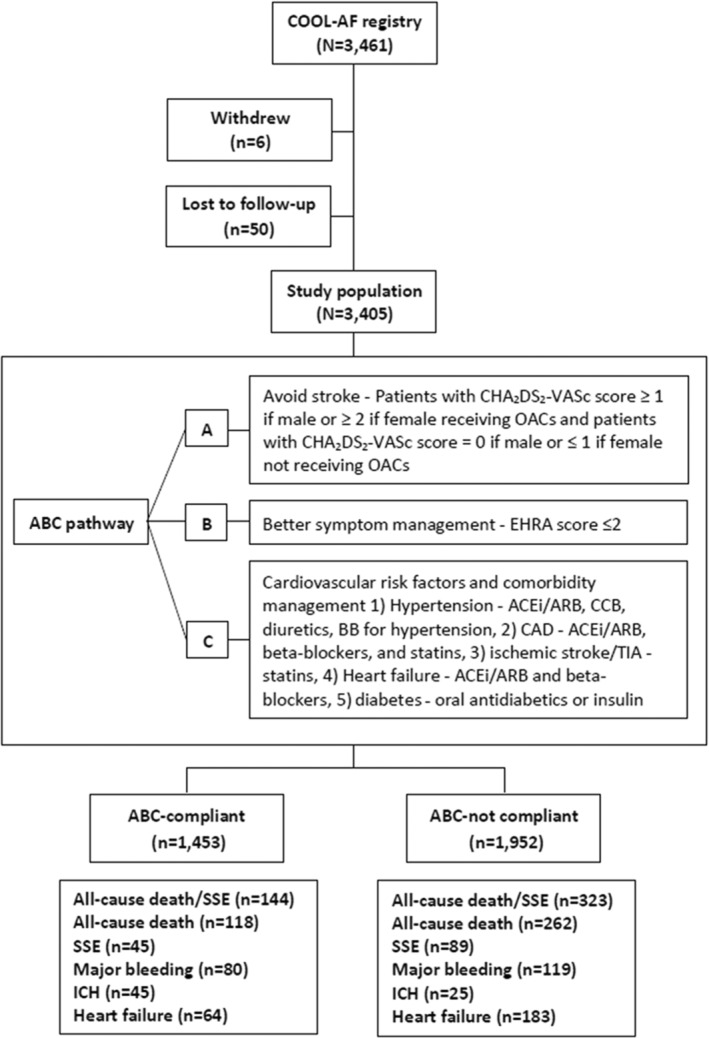
ABC indicates Atrial Fibrillation Better Care; ACEi/ARB, angiotensin‐converting enzyme inhibitors/angiotensin II receptor antagonists; BB, beta blocker; CAD, coronary artery disease; CCB, calcium channel blocker; COOL‐AF, Cohort of Antithrombotic Use and Optimal International Normalized Ratio Level in Patients With Non‐Valvular Atrial Fibrillation in Thailand; EHRA, European Heart Rhythm Association; ICH, intracranial hemorrhage; OAC, oral anticoagulant; SSE, ischemic stroke/systemic embolism; and TIA, transient ischemic attack.
Data collected during the follow‐up visits included outcome data. In patients who used warfarin, TTR was calculated using the method proposed by Rosendaal et al. 21 In brief, we collected INR data and the date of INR test of every visit. From the 2 adjacent INR results, we estimated the percentage of time between the 2 adjacent visits when the INR was within range of 2 to 3. We then calculated the days within range of the 2 INR intervals from the percentage of time within range divided by number of days between the 2 INR tests. We then made a sum of days within range of every interval between the 2 INR tests. TTR was calculated from the overall days within range divided by the total number of days started from the first INR test to the last INR test.
Outcomes
Main outcomes were the composite of all‐cause death or SSE, as well as secondary outcomes of all‐cause death, SSE, major bleeding, ICH, and HF. Ischemic stroke or transient ischemic attack was defined as acute onset of focal neurological deficit lasting more than 24 hours for ischemic stroke and <24 hours for transient ischemic attack. Whether positive or negative, imaging data from computerized tomography brain scan or magnetic resonance imaging were required to be uploaded into the web‐based system. Systemic embolism was defined by both clinical and objective evidence of sudden loss of end‐organ perfusion, and major bleeding was defined by International Society of Thrombosis and Haemostasis criteria. 22 ICH was defined as the bleeding within the skull, which could be intracerebral bleeds, subarachnoid bleeds, subdural bleeds, and epidural bleeds. Our ICH definition does not include microbleeds and secondary hemorrhagic transformation. 23 Secondary hemorrhagic transformation was excluded from the clinical and imaging information, that is, evidence of mottled hemorrhage within the larger area of brain infarction. HF event was defined as a hospital admission or a presentation of the patient for an urgent, unscheduled clinic/office/emergency department visit, with a primary diagnosis of HF, whereby the patient exhibits new or worsening symptoms of HF on presentation, has objective evidence of new or worsening HF, and receives initiation or intensification of treatment specifically for HF. 24
Statistical Analysis
The primary analysis was to analyze the difference in rate of clinical outcomes between patients with and without ABC pathway compliance. ABC compliance is defined according to the original description 6 as shown in Figure 1. ABC compliance was defined as compliance in all 3 components of ABC pathway. The primary analysis is to compare outcomes between patients with ABC compliance and noncompliance. Descriptive data were displayed as mean and SD for normal distribution variables and median and interquartile range for nonparametric data. Categorical variables were shown as frequency and percentages. Comparisons of continuous data were made by the independent samples t‐test. Incidence rates of each clinical outcome were calculated and reported as rate per 100 person‐years, which was calculated from the number of each event in the interested group divided by the denominator derived from the sum of follow‐up duration (in years) of that group divided by 100. Chi‐square test or Fisher exact test was used for comparison of categorical data. Nonparametric statistics were used as needed. Multivariable Cox‐proportional hazard model was used to determine the predictive values for clinical outcomes using backward elimination with P value <0.05 as the stopping criteria. Hazard ratio (HR) and 95% CI were reported for the unadjusted and adjusted model with the use of age, sex, diabetes, dyslipidemia, hypertension, chronic kidney disease, history of HF, history of coronary artery disease, and history of ischemic stroke/transient ischemic attack as covariates. Patients were grouped according to ABC compliance status for the analysis. We used an SBP target of 120 to 140 mm Hg (at least 120 and <140 mm Hg), which has been reported by our group to have the best mortality outcome. 14 Target TTR was defined as ≥65%. 16
Sensitivity analysis was performed by treating ABC compliance as 3 groups: 0‐ to 1‐factor, 2‐factor, and 3‐factor ABC compliance. Table demonstrated the details of ABC and the definition of compliance to A, B, or C. We defined 0‐ to 1‐factor compliance as a group with 0 or 1 item of A, B, or C compliance according to the definition shown in Figure 1. Two‐ or 3‐factor compliance was defined as 2 or 3 items of ABC are compliant respectively. All statistics were performed using the SPSS statistical software version 18.0 (SPSS, Inc., Chicago, IL, USA) and STATA Statistical Software Release 17 (StataCorp LLC, College Station, TX, USA). A P value <0.05 was considered statistically significant.
Table .
Baseline Characteristics of Study Population
| Characteristics | All (N=3405) | ABC‐compliant (n=1453) | ABC‐not compliant (n=1952) | P value |
|---|---|---|---|---|
| Age, y | 67.8±11.3 | 66.8±11.0 | 68.6±11.4 | <0.001† |
| Female sex | 1424 (41.8%) | 576 (39.6%) | 848 (43.4%) | 0.026* |
| Body mass index, kg/m2 | 25.2±4.7 | 25.3±4.8 | 25.1±4.7 | 0.113 |
| Systolic BP, mm Hg | 128.5±18.4 | 128.2±18.1 | 128.7±18.6 | 0.456 |
| Diastolic BP, mm Hg | 75.2±12.7 | 75.6±12.4 | 75.0±12.8 | 0.157 |
| Pulse, bpm | 77.4±16.2 | 77.1±15.8 | 77.6±16.5 | 0.367 |
| Time after diagnosis of AF, y | 3.4±4.3 | 3.3±4.2 | 3.5±4.4 | 0.177 |
| Atrial fibrillation | 0.212 | |||
| Paroxysmal | 1148 (33.7%) | 492 (33.9%) | 656 (33.6%) | |
| Persistent | 645 (18.9%) | 256 (17.6%) | 389 (19.9%) | |
| Permanent | 1612 (47.3%) | 705 (48.5%) | 907 (46.5%) | |
| Symptomatic AF | 2620 (76.9%) | 1107 (76.2%) | 1513 (77.5%) | 0.365 |
| History of heart failure | 913 (26.8%) | 193 (13.3%) | 720 (36.9%) | <0.001* |
| History of coronary artery disease | 547 (16.1%) | 177 (12.2%) | 370 (19.0%) | <0.001* |
| Cardiac implantable electronic device | 341 (10.0%) | 142 (9.8%) | 199 (10.2%) | 0.685 |
| History of ischemic stroke/transient ischemic attack | 592 (17.4%) | 229 (15.8%) | 363 (18.6%) | 0.031* |
| Diabetes | 839 (24.6%) | 309 (21.3%) | 530 (27.2%) | <0.001* |
| Hypertension | 2330 (68.4%) | 982 (67.6%) | 1348 (69.1%) | 0.360 |
| Smoking | 678 (19.9%) | 270 (18.6%) | 408 (20.9%) | 0.094 |
| Dyslipidemia | 1917 (56.3%) | 762 (52.4%) | 1155 (59.2%) | <0.001* |
| Renal replacement therapy | 40 (1.2%) | 6 (0.4%) | 34 (1.7%) | <0.001* |
| Dementia | 29 (0.9%) | 5 (0.3%) | 24 (1.2%) | 0.005* |
| Systemic embolism | 25 (0.7%) | 9 (0.6%) | 16 (0.8%) | 0.498 |
| History of peripheral vascular disease | 44 (1.3%) | 11 (0.8%) | 33 (1.7%) | 0.017* |
| History of carotid occlusive disease | 8 (0.2%) | 4 (0.3%) | 4 (0.2%) | 0.730 |
| History of stent use | 253 (7.4%) | 95 (6.5%) | 158 (8.1%) | 0.087 |
| History of coronary artery bypass graft | 65 (1.9%) | 16 (1.1%) | 49 (2.5%) | 0.003* |
| History of alcohol abuse, | 140 (4.1%) | 52 (3.6%) | 88 (4.5%) | 0.177 |
| History of bleeding | 324 (9.5%) | 118 (8.1%) | 206 (10.6%) | 0.017* |
| Chronic kidney disease | 1756 (51.6%) | 676 (46.5%) | 1080 (55.3%) | <0.001* |
| Anemia | 1293 (38.0%) | 447 (30.8%) | 846 (43.3%) | <0.001* |
| Left ventricular ejection fraction | 60.2±13.7 | 60.4±13.2 | 59.5±14.1 | 0.305 |
| CHA2DS2‐VASc score* | 0.001* | |||
| Low risk | 287 (8.4%) | 151 (10.4%) | 136 (7.0%) | |
| Intermediate risk | 548 (16.1%) | 243 (16.7%) | 305 (15.6%) | |
| High risk | 2570 (75.5%) | 1059 (72.9%) | 1511 (77.4%) | |
| HAS‐BLED score | <0.001* | |||
| 0 | 490 (14.4%) | 256 (17.6%) | 234 (12.0%) | |
| 1–2 | 1255 (36.9%) | 620 (42.7%) | 635 (32.5%) | |
| ≥3 | 1660 (48.8%) | 577 (39.7%) | 1083 (55.5%) | |
| Antiplatelet | 892 (26.2%) | 217 (14.9%) | 675 (24.6%) | <0.001* |
| Anticoagulant | 2568 (75.4%) | 1302 (89.6%) | 1266 (64.9%) | <0.001* |
| Warfarin | 2340 (68.7%) | 1172 (80.7%) | 1168 (59.8%) | <0.001* |
| Non‐vitamin K antagonist anticoagulants | 228 (6.7%) | 130 (8.9%) | 98 (5.0%) | <0.001* |
| Beta blocker | 2477 (72.7%) | 1123 (77.3%) | 1354 (69.4%) | <0.001* |
| Calcium channel blocker | 935 (27.5%) | 440 (30.3%) | 495 (25.4%) | 0.001* |
| Digitalis | 539 (15.8%) | 209 (14.4%) | 330 (16.9%) | 0.046* |
| Mineralocorticoid receptor antagonists | 280 (8.2%) | 117 (8.1%) | 163 (8.4%) | 0.754 |
| Statin | 2014 (59.1%) | 972 (66.9%) | 1042 (53.4%) | <0.001* |
| Angiotensin‐converting enzyme inhibitors/angiotensin II receptor antagonists | 1557 (45.7%) | 771 (53.1%) | 786 (40.3%) | <0.001* |
| Nonsteroidal anti‐inflammatory drugs /cyclooxygenase‐2 inhibitor | 83 (2.4%) | 29 (2.0%) | 54 (2.8%) | 0.149 |
ABC indicates Atrial Fibrillation Better Care; AF, atrial fibrillation; and BP, blood pressure.
Low risk=CHA2DS2‐VASc score=0 in men or 1 in women, intermediate risk=CHA2DS2‐VASc score=1 in men or 2 in women, high risk=CHA2DS2‐VASc score >1 in men or >2 in women.
Statistical significance (P<0.05).
RESULTS
Study Population
We studied a total of 3405 patients (mean age 67.8±11.3 years; 1424 [41.8%] were female). Proportions of the study population that were compliant to A, B, and C criteria were 2561 (77.9%), 2608 (76.6%), and 2345 (68.9%). Overall, 1453 (42.7%) were compliant to all components of the ABC pathway. Baseline characteristics of patients with and without ABC compliance are shown in Table. Patients with ABC compliance had a younger age, less frequently female. History of HF, coronary artery disease, ischemic stroke/transient ischemic attack, diabetes, dyslipidemia, chronic kidney disease/renal replacement therapy, dementia, peripheral arterial disease, coronary artery bypass grafting, history of bleeding, and anemia were less in the ABC‐compliant group. Mean CHA2DS2‐VASc and HAS‐BLED scores were lower in the ABC‐compliant group. OACs and many cardiovascular medications were used more and antiplatelets were used less in the ABC‐compliant group.
Clinical Outcomes
Average follow‐up duration was 31.8±8.7 months or 9026.7 person‐years. According to the study protocol, the follow‐up data were entered in the web‐based system every 6 months at months 6, 12, 18, 24, 30, and 36. The average follow‐up duration was calculated from the mean of follow‐up duration of each patient. Because there were some patients with loss to follow‐up or who withdrew consent and some patients who died during the follow‐up, the average follow‐up time was 31.8±8.7 months. The incidence rates of all‐cause death/SSE, all‐cause death, SSE, major bleeding, ICH, and HF were 5.29 (4.82–5.79), 4.21 (3.80–4.65), 1.51 (1.26–1.78), 2.25 (1.95–2.59), 0.78 (0.61–0.98), and 2.84 (2.49–3.21) per 100 person‐years respectively. Flow diagram of study population and clinical outcomes is shown in Figure 1. Tables S1–S4 show the proportions of patients with A, B, and C compliance.
Table S2 demonstrates incidence rate of each clinical outcome in patients who were ABC‐compliant and in patients who were compliant in each of the A, B, and C components. Overall, compliance with the ABC pathway was associated with a significant reduction in the occurrence of clinical outcomes. Comparisons of incidence rate of clinical outcomes in patients with and without ABC compliance are shown in Figures S1–S4. The ABC‐compliant group had a lower incidence rates of all‐cause death/SSE (3.77 versus 6.45 per 100 person‐years, P<0.001), all‐cause death (3.03 versus 5.11 per 100 person‐years, P<0.001), SSE (1.17 versus 1.76 per 100 person‐years, P=0.001), major bleeding (2.10 versus 2.36 per 100 person‐years, P=0.210), ICH (0.64 versus 0.88 per 100 person‐years, P=0.106), and HF (1.67 versus 3.75 per 100 person‐years, P<0.001), when compared with those without ABC compliance.
Univariable and Multivariable Analysis
Univariable and multivariable Cox proportional hazard model was performed. Figure 2 shows forest plot of unadjusted (blue color) and adjusted (red color) HRs and 95% CIs of all clinical outcomes in the ABC‐compliant and noncompliant groups. The ABC‐compliant group had a reduction in all‐cause death/SSE (adjusted HR, 0.76 [95% CI, 0.62–0.94]), all‐cause death (adjusted HR, 0.79 [95% CI, 0.62–0.99]), and HF outcomes (adjusted HR, 0.69 [95% CI, 0.51–0.94]), both for unadjusted and adjusted analysis. For SSE outcome, the ABC‐compliant group had a better outcome on univariable analysis but was nonsignificant with wide 95% CIs on multivariable analysis.
Figure 2. Forest plot of unadjusted and adjusted hazard ratio of ABC‐compliant status and clinical outcome.
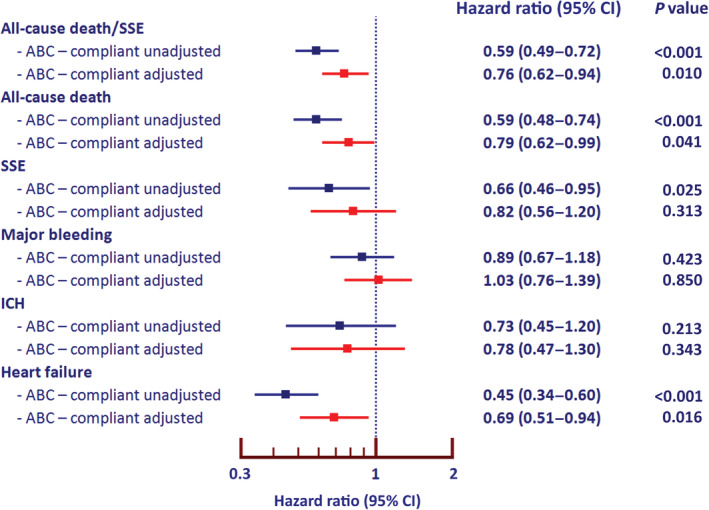
ABC indicates Atrial Fibrillation Better Care; ICH, intracranial hemorrhage; and SSE, ischemic stroke/systemic embolism.
ABC Pathway Compliance in Combination With Achievement of SBP Targets
SBP target was achieved in 1427 (41.9%) patients. Table S3 demonstrates baseline characteristics between patients with SBP at target and not at target. The data of ABC pathway compliance and achievement of SBP target were analyzed by classifying patients into 4 groups: (1) ABC noncompliant and SBP not at target, n=1142 (33.5%); (2) ABC noncompliant and SBP at target, n=810 (23.8%); (3) ABC‐compliant and SBP not at target, n=836 (24.6%); and (4) ABC‐compliant and SBP at target, 617 (18.1%). Figure 3 showed forest plots of unadjusted and adjusted HRs and 95% CIs of all clinical outcomes using the patients who were ABC noncompliant and SBP not at target as the reference group. Patients with ABC compliance and SBP at target were the group with the best outcomes for all‐cause death/SSE (adjusted HR, 0.71 [95% CI, 0.53–0.96]), all‐cause death (adjusted HR, 0.68 [95% CI, 0.48–0.96]), and HF (adjusted HR, 0.60 [95% CI, 0.39–0.94]).
Figure 3. Forest plot of ABC‐compliant status and achievement of systolic blood pressure target and clinical outcomes.
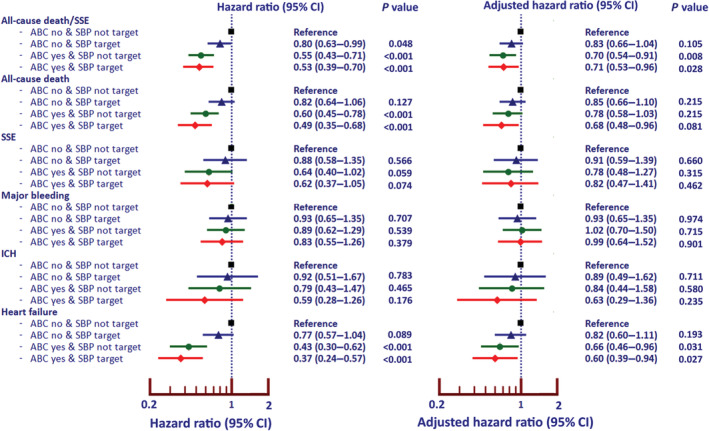
ABC indicates Atrial Fibrillation Better Care; ICH, intracranial hemorrhage; and SSE, ischemic stroke/systemic embolism.
ABC Pathway Compliance in Combination With Achievement of TTR Target
Warfarin was used in 2340 patients (68.7%). Among patients who were on warfarin, the number of INRs allow TTR to be calculated in 2233 patients (65.6% of all patients). A target TTR of ≥65% was achieved in 801 (35.9%). Table S4 demonstrates baseline characteristics between patients with TTR < and ≥65%. The data of ABC pathway and achievement of TTR target were analyzed by classifying patients into 4 groups: (1) ABC noncompliant and TTR <65%, n=759 (22.3%); (2) ABC noncompliant and TTR ≥65%, n=385 (11.3%); (3) ABC‐compliant and TTR <65%, n=735 (21.6%); and (4) ABC‐compliant and TTR ≥65%, n=416 (12.2%). Figure 4 shows the forest plot of unadjusted and adjusted HRs and 95% CIs of all clinical outcomes using patients who were ABC noncompliant and TTR not at target as reference group. Patients with ABC compliance and TTR at target were the group with the best outcomes for all‐cause death/SSE (adjusted HR, 0.48 [95% CI, 0.32–0.73]), all‐cause death (adjusted HR, 0.44 [95% CI, 0.27–0.71]), major bleeding (adjusted HR, 0.52 [95% CI, 0.30–0.89]), ICH (adjusted HR, 0.22 [95% CI, 0.08–0.63]), and HF (adjusted HR, 0.43 [95% CI, 0.24–0.76]).
Figure 4. Forest plot of ABC‐compliant status and achievement of target level of time in therapeutic range (TTR) and clinical outcomes.
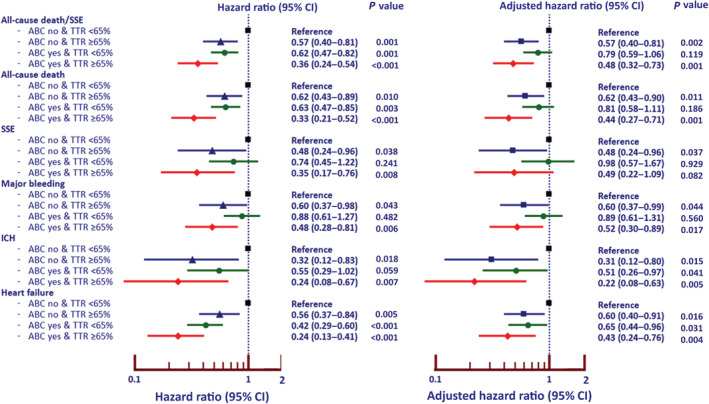
ABC indicates Atrial Fibrillation Better Care; ICH, intracranial hemorrhage; and SSE, ischemic stroke/systemic embolism.
Sensitivity Analysis
Sensitivity analysis was performed by classifying ABC pathway compliance into 3 groups: 0 to 1 factor compliant, 2 factors compliant, and 3 factors compliant. The risks of all‐cause death/SSE, all‐cause death, SSE, and HF increased in patients with poor ABC compliance compared with those with better ABC compliance. Figures S2 and S3 show bar graphs of the incidence rates of clinical outcomes and forest plot of outcomes according to the 3 groups of ABC compliance. The results indicated that the magnitude of reduction of clinical outcomes mainly depended on the levels of ABC pathway achievement that each patient had.
DISCUSSION
The principal findings from this study are that patients with AF who had ABC pathway compliance had a better clinical outcome compared with those who were noncompliant, and the more components of ABC were achieved, the better the clinical outcomes. Second, ABC compliance has added value on top of SBP target achievement for the reduction of adverse outcomes. Third, for patients with warfarin, ABC compliance and good TTR control complement each other for better cardiovascular outcomes in patients with AF.
The ABC pathway was been introduced to provide holistic or integrated care management in patients with AF. 6 The majority of patients with AF need OAC to prevent ischemic stroke, whereas patient‐centered, symptom‐directed decisions on rate or rhythm control are part of the management approach. Although rate control should be considered for all patients with AF, a rhythm control strategy is appropriate especially in those newly diagnosed, paroxysmal, and symptomatic. 25 Most patients with AF also had multiple comorbidities such as diabetes, chronic kidney disease, and hypertension, and the ABC pathway focuses on the appropriate management of all such comorbidities and lifestyle changes. ABC pathway has been one of the recommended strategies for AF management in recent guidelines. 12 , 13 , 26
Many studies have shown the advantages of compliance with integrated ABC pathway care in AF management, 7 , 8 , 9 , 10 including studies from Asian 7 , 8 and Western populations. 9 , 10 The results of these studies demonstrated a consistent benefit of using an integrated care ABC pathway in the improvement of clinical outcomes. A systematic review and meta‐analysis of benefit of ABC pathway showed that ABC pathway compliance was associated with a reduction in all‐cause death, cardiovascular death, ischemic stroke, and major bleeding of 58%, 63%, 45%, and 31%, respectively. 11 The results of the present study from a prospectively collected nationwide cohort demonstrated that using the ABC pathway was associated with a reduction in all‐cause death/SSE, all‐cause death, SSE, and HF. Multivariable analysis showed that benefit of ABC remained significant for all‐cause death/SSE, all‐cause death, and HF, although there was no statistically significant effect on major bleeding.
Additional novelty of our study includes showing the benefit of ABC pathway on top of the achievement of optimal SBP target and TTR target (for those who were on warfarin) in patients with AF. Previous studies have shown the advantage of SBP control in patients with AF, 15 , 27 although not in combination with ABC pathway compliance. Data from the COOL‐AF study previously demonstrated a benefit of SBP control on clinical outcome of patients with AF, 14 with a J‐curve on mortality but no J‐curve effect on ischemic stroke and major bleeding, and suggested an optimal SBP target of 120 to 140 mm Hg. Of note, the nature of these prior studies was different: one study was clinical trial data, 15 one study was national health insurance data, 27 and one study was a prospective cohort. 14 previous study that also demonstrated a J‐curve effect on clinical outcome. 15 We demonstrate an additional benefit of ABC on top of SBP target achievement on reducing all‐cause death/SSE, all‐cause death, and HF.
Previous studies have shown that achievement of good TTR target was associated with better outcomes in patients with AF. 17 , 19 The results of our previous report demonstrated a benefit of good TTR control in the reduction of death, ischemic stroke, and major bleeding. 16 This is especially important in an Asian population because the rate of major bleeding and ICH is generally higher than in a Western population. 28 , 29 , 30 However, only 36% of patients had a TTR ≥65%. 16 The result of this study demonstrated that among patients with AF who were on warfarin, the integration of ABC pathway with good TTR would have additional advantages on the protection of patients from adverse clinical outcomes.
Compliance with the ABC pathway was defined according to the original study. 6 Most of the recommendations in the ABC pathway were based on whether the patients received appropriate treatment for stroke prevention, symptoms, and comorbidities. ABC‐guided management, 7 , 8 , 9 , 10 attainment of good SBP control, 14 , 15 , 27 and achievement of a high TTR 16 , 17 , 19 have been demonstrated to have an impact on clinical outcomes. The results of our study indicated that we should not focus only on giving the medications but also on achieving the target of treatments.
Limitations
There were some limitations of this study. First, this is a multicenter study based on 27 hospitals, most of which were large. We did not have data indicating whether care of patients with AF was similar or different between large hospitals versus small hospitals. Second, despite 75.4% of our study population using OAC, the majority of them use warfarin, and only 8.9% of OAC were non‐vitamin K antagonist OACs. The choice of OAC use may affect the clinical outcome. Lastly, the lack of detailed information about ABC compliance during follow‐up is also a study limitation.
CONCLUSIONS
In this multicenter nationwide prospective cohort of patients with AF, achieving SBP within target and TTR ≥ 65% has added value to ABC pathway compliance in the reduction of adverse clinical outcomes in patients with AF.
Sources of Funding
This study was funded by a grant from the Heart Association of Thailand under the Royal Patronage of H.M. the King. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
Disclosures
G.Y.H.L. is a consultant and speaker for BMS/Pfizer, Boehringer Ingelheim, and Daiichi‐Sankyo. No fees are directly received personally. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S4
Figures S1–S3
Acknowledgments
The authors gratefully acknowledge Ahthit Yindeengam and Poom Sairat for data management. Author Contributions: All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.028463
For Sources of Funding and Disclosures, see page 10.
References
- 1. Tanaka Y, Shah NS, Passman R, Greenland P, Lloyd‐Jones DM, Khan SS. Trends in cardiovascular mortality related to atrial fibrillation in the United States, 2011 to 2018. J Am Heart Assoc. 2021;10:e020163. doi: 10.1161/JAHA.120.020163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xia Z, Dang W, Jiang Y, Liu S, Yue L, Jia F, Sun Q, Shi L, Sun J, Li J, et al. Association between atrial fibrillation and the risk of cardiovascular mortality among elderly adults with ischemic stroke in Northeast China: a community‐based prospective study. Front Aging Neurosci. 2022;14:836425. doi: 10.3389/fnagi.2022.836425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burdett P, Lip GYH. Atrial fibrillation in the United Kingdom: predicting costs of an emerging epidemic recognising and forecasting the cost drivers of atrial fibrillation‐related costs. Eur Heart J Qual Care Clin Outcomes. 2022;8:187–194. doi: 10.1093/ehjqcco/qcaa093 [DOI] [PubMed] [Google Scholar]
- 4. Camm AJ, Accetta G, Ambrosio G, Atar D, Bassand JP, Berge E, Cools F, Fitzmaurice DA, Goldhaber SZ, Goto S, et al. Evolving antithrombotic treatment patterns for patients with newly diagnosed atrial fibrillation. Heart. 2017;103:307–314. doi: 10.1136/heartjnl-2016-309832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pokorney SD, Piccini JP, Stevens SR, Patel MR, Pieper KS, Halperin JL, Breithardt G, Singer DE, Hankey GJ, Hacke W, et al. Cause of death and predictors of all‐cause mortality in anticoagulated patients with nonvalvular atrial fibrillation: data from Rocket AF. J Am Heart Assoc. 2016;5:e002197. doi: 10.1161/JAHA.115.002197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lip GYH. The abc pathway: an integrated approach to improve af management. Nat Rev Cardiol. 2017;14:627–628. doi: 10.1038/nrcardio.2017.153 [DOI] [PubMed] [Google Scholar]
- 7. Yoon M, Yang PS, Jang E, Yu HT, Kim TH, Uhm JS, Kim JY, Sung JH, Pak HN, Lee MH, et al. Improved population‐based clinical outcomes of patients with atrial fibrillation by compliance with the simple ABC (Atrial Fibrillation Better Care) pathway for integrated care management: a nationwide cohort study. Thromb Haemost. 2019;119:1695–1703. doi: 10.1055/s-0039-1693516 [DOI] [PubMed] [Google Scholar]
- 8. Guo Y, Lane DA, Wang L, Zhang H, Wang H, Zhang W, Wen J, Xing Y, Wu F, Xia Y, et al. Mobile health technology to improve care for patients with atrial fibrillation. J Am Coll Cardiol. 2020;75:1523–1534. doi: 10.1016/j.jacc.2020.01.052 [DOI] [PubMed] [Google Scholar]
- 9. Proietti M, Lip GYH, Laroche C, Fauchier L, Marin F, Nabauer M, Potpara T, Dan GA, Kalarus Z, Tavazzi L, et al. Relation of outcomes to ABC (atrial fibrillation better care) pathway adherent care in European patients with atrial fibrillation: an analysis from the ESC‐EHRA EORP atrial fibrillation general long‐term (AFGEN LT) registry. Europace. 2021;23:174–183. doi: 10.1093/europace/euaa274 [DOI] [PubMed] [Google Scholar]
- 10. Pastori D, Menichelli D, Violi F, Pignatelli P, Gregory YH; ATHERO‐AF study group . The Atrial Fibrillation Better Care (ABC) pathway and cardiac complications in atrial fibrillation: a potential sex‐based difference. The Athero‐AF study. Eur J Intern Med. 2021;85:80–85. doi: 10.1016/j.ejim.2020.12.011 [DOI] [PubMed] [Google Scholar]
- 11. Romiti GF, Pastori D, Rivera‐Caravaca JM, Ding WY, Gue YX, Menichelli D, Gumprecht J, Koziel M, Yang PS, Guo Y, et al. Adherence to the ‘Atrial Fibrillation Better Care’ pathway in patients with atrial fibrillation: impact on clinical outcomes—a systematic review and meta‐analysis of 285,000 patients. Thromb Haemost. 2022;122:406–414. doi: 10.1055/a-1515-9630 [DOI] [PubMed] [Google Scholar]
- 12. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom‐Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2020;42:373–498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 13. Chao TF, Joung B, Takahashi Y, Lim TW, Choi EK, Chan YH, Guo Y, Sriratanasathavorn C, Oh S, Okumura K, et al. 2021 focused update consensus guidelines of the Asia Pacific Heart Rhythm Society on stroke prevention in atrial fibrillation: executive summary. Thromb Haemost. 2022;122:20–47. doi: 10.1055/s-0041-1739411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krittayaphong R, Pumprueg S, Ratanasumawong K, Sairat P, Lip GYH, COOL‐AF Investigators. Average systolic blood pressure and clinical outcomes in patients with atrial fibrillation: prospective data from COOL‐AF registry. Clin Interv Aging. 2021;16:1835–1846. doi: 10.2147/CIA.S335321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bohm M, Brueckmann M, Eikelboom JW, Ezekowitz M, Frassdorf M, Hijazi Z, Hohnloser SH, Mahfoud F, Schmieder RE, Schumacher H, et al. Cardiovascular outcomes, bleeding risk, and achieved blood pressure in patients on long‐term anticoagulation with the thrombin antagonist dabigatran or warfarin: data from the RE‐LY trial. Eur Heart J. 2020;41:2848–2859. doi: 10.1093/eurheartj/ehaa247 [DOI] [PubMed] [Google Scholar]
- 16. Krittayaphong R, Chantrarat T, Rojjarekampai R, Jittham P, Sairat P, Lip GYH. Poor time in therapeutic range control is associated with adverse clinical outcomes in patients with non‐valvular atrial fibrillation: a report from the nationwide COOL‐AF registry. J Clin Med. 2020;9:1698. doi: 10.3390/jcm9061698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haas S, Ten Cate H, Accetta G, Angchaisuksiri P, Bassand JP, Camm AJ, Corbalan R, Darius H, Fitzmaurice DA, Goldhaber SZ, et al. Quality of vitamin K antagonist control and 1‐year outcomes in patients with atrial fibrillation: a global perspective from the Garfield‐AF registry. PLoS One. 2016;11:e0164076. doi: 10.1371/journal.pone.0164076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chao TF, Guo Y. Should we adopt a standard international normalized ratio range of 2.0 to 3.0 for Asian patients with atrial fibrillation? An appeal for evidence‐based management, not eminence‐based recommendations. Thromb Haemost. 2020;120:366–368. doi: 10.1055/s-0040-1702230 [DOI] [PubMed] [Google Scholar]
- 19. Pandey AK, Xu K, Zhang L, Gupta S, Eikelboom J, Cook O, McIntyre WF, Lopes RD, Crowther M, Belley‐Cote EP, et al. Lower versus standard INR targets in atrial fibrillation: a systematic review and meta‐analysis of randomized controlled trials. Thromb Haemost. 2020;120:484–494. doi: 10.1055/s-0039-3401823 [DOI] [PubMed] [Google Scholar]
- 20. Kim D, Yang PS, Kim TH, Jang E, Shin H, Kim HY, Yu HT, Uhm JS, Kim JY, Pak HN, et al. Ideal blood pressure in patients with atrial fibrillation. J Am Coll Cardiol. 2018;72:1233–1245. doi: 10.1016/j.jacc.2018.05.076 [DOI] [PubMed] [Google Scholar]
- 21. Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69:236–239. doi: 10.1055/s-0038-1651587 [DOI] [PubMed] [Google Scholar]
- 22. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x [DOI] [PubMed] [Google Scholar]
- 23. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449 [DOI] [PubMed] [Google Scholar]
- 24. Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, Fonarow GC, Jacobs JP, Jaff MR, Lichtman JH, et al. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). Circulation. 2015;132:302–361. doi: 10.1161/CIR.0000000000000156 [DOI] [PubMed] [Google Scholar]
- 25. Piccini JP, Fauchier L. Rhythm control in atrial fibrillation. Lancet. 2016;388:829–840. doi: 10.1016/S0140-6736(16)31277-6 [DOI] [PubMed] [Google Scholar]
- 26. Lip GYH, Banerjee A, Boriani G, Chiang CE, Fargo R, Freedman B, Lane DA, Ruff CT, Turakhia M, Werring D, et al. Antithrombotic therapy for atrial fibrillation: chest guideline and expert panel report. Chest. 2018;154:1121–1201. doi: 10.1016/j.chest.2018.07.040 [DOI] [PubMed] [Google Scholar]
- 27. Kim TH, Yang PS, Yu HT, Jang E, Shin H, Kim HY, Uhm JS, Kim JY, Sung JH, Pak HN, et al. Effect of hypertension duration and blood pressure level on ischaemic stroke risk in atrial fibrillation: nationwide data covering the entire Korean population. Eur Heart J. 2019;40:809–819. doi: 10.1093/eurheartj/ehy877 [DOI] [PubMed] [Google Scholar]
- 28. Lip GY, Wang KL, Chiang CE. Non‐vitamin K antagonist oral anticoagulants (NOACs) for stroke prevention in Asian patients with atrial fibrillation: time for a reappraisal. Int J Cardiol. 2015;180:246–254. doi: 10.1016/j.ijcard.2014.11.182 [DOI] [PubMed] [Google Scholar]
- 29. Gorog DA, Gue YX, Chao TF, Fauchier L, Ferreiro JL, Huber K, Konstantinidis SV, Lane DA, Marin F, Oldgren J, et al. Assessment and mitigation of bleeding risk in atrial fibrillation and venous thromboembolism: executive summary of a European and Asia‐Pacific expert consensus paper. Thromb Haemost. 2022;122:1625–1652. doi: 10.1055/s-0042-1750385 [DOI] [PubMed] [Google Scholar]
- 30. Kim HK, Tantry US, Smith SC Jr, Jeong MH, Park SJ, Kim MH, Lim DS, Shin ES, Park DW, Huo Y, et al. The East Asian paradox: an updated position statement on the challenges to the current antithrombotic strategy in patients with cardiovascular disease. Thromb Haemost. 2021;121:422–432. doi: 10.1055/s-0040-1718729 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4
Figures S1–S3


