Abstract
Background
Sepsis is associated with an elevated risk of late cardiovascular events among hospital survivors.
Methods and Results
We included OptumLabs Data Warehouse patients from 2009 to 2019 who survived a medical/nonsurgical hospitalization lasting at least 2 nights. The association between sepsis during hospitalization, based on explicit and implicit discharge International Classification of Diseases, Ninth Revision (ICD‐9)/Tenth Revision (ICD‐10) diagnosis codes, with subsequent death and rehospitalization was analyzed using Kaplan–Meier survival analysis and multivariable Cox proportional‐hazards models. The study population included 2 258 464 survivors of nonsurgical hospitalization (5 396 051 total patient‐years of follow‐up). A total of 808 673 (35.8%) patients had a sepsis hospitalization, including implicit sepsis only in 448 644, explicit sepsis only in 124 841, and both in 235 188. Patients with sepsis during hospitalization had an elevated risk of all‐cause mortality (adjusted hazard ratio [HR], 1.27 [95% CI, 1.25–1.28]; P<0.001), all‐cause rehospitalization (adjusted HR, 1.38 [95% CI, 1.37–1.39]; P<0.001), and cardiovascular hospitalization (adjusted HR, 1.43 [95% CI, 1.41–1.44]; P<0.001), especially heart failure hospitalization (adjusted HR, 1.51 [95% CI, 1.49–1.53]). Patients with implicit sepsis had higher risk than those with explicit sepsis. A sensitivity analysis using the first hospitalization yielded concordant results for cardiovascular hospitalization (adjusted HR, 1.78 [95% CI, 1.76–1.78]; P<0.001), as did a propensity‐weighted analysis (adjusted HR, 1.52 [95% CI, 1.50–1.54]; P<0.001).
Conclusions
Survivors of sepsis hospitalization are at elevated risk of early and late post‐discharge death as well as cardiovascular and non‐cardiovascular rehospitalization. This hazard spans the spectrum of cardiovascular events and may suggest that sepsis is an important cardiovascular risk factor.
Keywords: heart failure, mortality, myocardial infarction, sepsis
Subject Categories: Heart Failure, Myocardial Infarction, Coronary Artery Disease, Acute Coronary Syndromes, Inflammation
Nonstandard Abbreviation and Acronym
- AKI
acute kidney injury
Clinical Perspective.
What Is New?
In this retrospective cohort analysis of 2.25 million adult hospital survivors, those with sepsis during hospitalization were at higher risk of death, rehospitalization for any cause, and hospitalization for cardiovascular events during follow‐up, even after adjustment for relevant clinical variables or the propensity to have sepsis during hospitalization.
Hospital survivors with sepsis were at higher risk of all major cardiovascular events throughout the duration of follow‐up, including both atherosclerotic and non‐atherosclerotic cardiovascular events, with heart failure hospitalization being both the most common cardiovascular event and the cardiovascular event associated with the greatest attributable hazard from sepsis during hospitalization.
What Are the Clinical Implications?
The excess risk of cardiovascular events in sepsis survivors is substantial enough in magnitude that sepsis should be considered a nontraditional risk factor for cardiovascular events, and further research is needed to determine how best to mitigate this risk in this vulnerable population.
Sepsis is a leading cause of hospitalization, disability, and death worldwide. 1 , 2 Despite improvements in the recognition and management of patients with sepsis, short‐term mortality in this group remains high. 3 , 4 Survivors of hospitalization for sepsis remain at elevated risk of death and other adverse clinical events. 4 , 5 , 6 , 7 The multitude of pathophysiologic mechanisms linking sepsis with adverse outcomes include persistent inflammation with immune dysregulation, oxidative stress, and the consequences of acute organ dysfunction, including acute kidney injury (AKI). 8 , 9 These residual risk pathways remain active after clinical recovery from sepsis, posing the potential for late adverse events to be triggered by a remote episode of sepsis. 8 Sepsis survivors may suffer from neurocognitive impairment, physical weakness, and other functional limitations; additionally, complications directly related to hospitalization and logistical challenges during follow‐up can contribute to health care burden in patients following sepsis. 7
Cardiovascular disease (CVD) is increasingly recognized as both a risk factor for and a potential consequence of sepsis. 10 The role of inflammation and infection as simultaneous drivers of CVD progression and triggers for CVD events has been recognized for many years across a multitude of contexts, including both early events during hospitalization and late postdischarge events. 11 Survivors of sepsis demonstrate an excess risk of mortality and CVD events that may persist for years after hospitalization. 6 , 12 , 13 Complications of sepsis such as AKI have likewise been associated with a higher risk of subsequent CVD events. 14 , 15 , 16 If the elevated risk of CVD events after recovery from sepsis was amenable to preventative therapy, then cardiovascular prevention strategies might be an important component of the care of survivors of sepsis.
Prior studies have observed an association between sepsis and an excess hazard of late postdischarge CVD events; however, a recent meta‐analysis of observational studies found that the quality of evidence supporting this association was low because of methodologic limitations of the published studies. 12 Prior studies used variable definitions for sepsis, often inadequately stratified patients by baseline CVD risk and prevalent CVD, and frequently provided limited detail regarding the type of CVD events that occurred or did not include all major CVD events. 12 Furthermore, the comparison group without sepsis in some prior studies may have included lower‐risk patients, potentially inflating the apparent risk associated with sepsis attributable to higher baseline risk in the sepsis group. Subsequent higher‐quality studies using more robust methods have shown similar associations, but important questions remain. 13 Therefore, we sought to describe the association between sepsis during hospitalization and subsequent death and rehospitalization (including hospitalization for CVD events) among hospital survivors in a large contemporary cohort while accounting for preexisting CVD and acute organ failure during hospitalization. We hypothesized that hospital survivors with sepsis would have a higher risk of subsequent mortality, rehospitalization, and postdischarge CVD events than hospital survivors without sepsis.
METHODS
The authors declare that all supporting data are available within the article and its online supplementary files. This manuscript conforms to the Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines for observational studies. The Mayo Clinic Institutional Review Board determined that this study was exempt from review as it used preexisting, deidentified data.
Study Population
In this retrospective cohort analysis, we used de‐identified administrative claims data from the OptumLabs Data Warehouse, which contains medical and pharmacy claims and enrollment records for commercial and Medicare Advantage insurance enrollees. The database contains longitudinal health information on enrollees, representing a diverse mixture of ages, ethnicities, and geographic regions across the United States. 17 , 18
Our goal was to identify a study population with an increased risk of CVD events to ensure that we did not compare patients with sepsis with high CVD risk with patients without sepsis with low CVD risk. Therefore, we included adult patients (≥18 years) hospitalized for at least 2 nights for nonsurgical encounters (medical disease‐related group) between January 1, 2009, and December 31, 2019. To minimize loss to follow‐up, patients were required to have continuous medical insurance coverage for at least 6 months before the index hospital admission and 30 days after the index hospital discharge. To enrich the study population in patients with or at risk of CVD events, additional inclusion criteria included ≥1 of the following: preexisting CVD diagnosis before hospital admission (Table S1), CVD diagnosis during the index hospitalization, age ≥50 years, age ≥40 years plus ≥1 CVD risk factors (Table S2), or age ≥30 years plus ≥2 CVD risk factors.
Sepsis Definitions
To ensure that our results were not confounded by subsequent sepsis hospitalizations in the nonsepsis group, patients were assigned to mutually exclusive groups on the basis of whether they had a hospitalization with sepsis during the study period. Hospitalizations were categorized as sepsis or nonsepsis on the basis of primary and secondary discharge International Classification of Diseases, Ninth and Tenth Revisions, Clinical Modification (ICD‐9/10‐CM) codes for sepsis or established criteria based on combinations of ICD‐9/10 codes. 1 All patients meeting the criteria for sepsis during any hospitalization within the study period were classified as having sepsis, and all patients who did not meet criteria for sepsis during any hospitalization within the study period were classified as not having sepsis. For patients with multiple hospitalizations during the study period, the first hospitalization for sepsis was used as the index hospitalization (even if there were prior nonsepsis hospitalizations). If there was not a diagnosis of sepsis during a hospitalization, then the first hospitalization was used as the index hospitalization. Sepsis was further classified as explicit, implicit, or both on the basis of ICD‐9/10 diagnosis codes from the index hospitalization. 1 Explicit sepsis was defined as the presence of diagnosis codes for sepsis or septic shock (Table S3). 1 Implicit sepsis was defined using established criteria based on the presence of ≥1 diagnoses for infection combined with ≥1 diagnoses for acute organ dysfunction during the index hospitalization (Table S4). 1 , 19 We performed a sensitivity analysis by reassigning the sepsis and nonsepsis groups on the basis of the discharge diagnoses from the first eligible hospitalization during the study period.
Covariates
Patient demographic characteristics included age (18–54, 55–64, 65–74, 75+ years), sex (female or male), race or ethnicity (Black, White, Asian, Hispanic), region (Midwest, Northeast, South, West), type of health plan (commercial, Medicare Advantage), and year of the index hospitalization. Comorbidities of interest included prior CVD (coronary artery disease, myocardial infarction, prior percutaneous coronary intervention, prior coronary artery bypass grafting or other cardiac surgery, heart failure, cardiomyopathy, atrial fibrillation, supraventricular tachycardia, stroke, ventricular tachycardia, implantable cardioverter‐defibrillator or pacemaker), the number of CVD risk factors (hypertension, hyperlipidemia, diabetes, chronic kidney disease/dialysis, obesity, smoking), and the number of all‐cause hospitalizations in the prior 6 months. Additional covariates included indicators for diagnoses or procedures during the index hospitalization (infection, organ failure, admission to intensive care unit [ICU], use of invasive ventilation, hemodialysis, CVD diagnosis), and index hospitalization length of stay (Table S5).
Outcomes
Patients were followed from index hospital discharge until the end of the study period (December 31, 2020), the end of insurance enrollment, or death, whichever happened first. Mortality was obtained from the Social Security Administration's Death Master File and discharge status information. Outcomes included all‐cause mortality after hospital discharge, all‐cause rehospitalization, and CVD events. A CVD event was defined as the first rehospitalization in which the primary diagnosis was for heart failure, myocardial infarction, angina, atrial fibrillation/flutter, cardiac arrest, coronary disease, valve disease, cardiomyopathy, supraventricular tachycardia, ventricular tachycardia, stroke, or intracranial hemorrhage. Two composite outcomes were also analyzed: (1) all‐cause mortality or all‐cause hospitalization and (2) all‐cause mortality or CVD hospitalization.
Statistical Analysis
Baseline patient characteristics were reported as frequencies (percentages) for categorical data and means (SD) for continuous variables. Unadjusted event rates were reported for each outcome using the number of patients with the given event as numerator and person‐years (time to the first event, death, or end of enrollment) as the denominator. Kaplan–Meier survival analysis was used to estimate postdischarge events rates, with groups compared by log‐rank test. Cox proportional hazards regressions with multivariable adjustment were used to compare nonsepsis to sepsis index hospitalizations for each outcome. Results were presented as hazard ratios (HRs) and 95% CIs. The Fine and Gray method was used to consider death as a competing risk when assessing nonfatal outcomes in the Cox models. The proportional hazard assumptions were tested using Schoenfeld residual plots, and no violations of the assumptions were found. All models included the covariates age, sex, race, region, health plan, index year, history of CVD, number of cardiovascular risk factors, index hospitalization characteristics (infection, organ failure, ICU, ventilator, hemodialysis, CVD diagnosis), and number of prior hospitalizations. Subgroup analyses included stratification by sepsis type, age, sex, race, health plan, pharmacy coverage, prior CVD, number of CVD risk factors, prior hospitalization status, and index hospitalization characteristics (ICU, CVD, organ failure, renal failure, and shock) for each outcome. A propensity score for sepsis was generated using multivariable logistic regression, and the main analysis was repeated using propensity score overlap weighting, with variables included in the propensity score shown in Table S6; all standardized mean differences were < 0.001 after propensity weighting. All analyses were conducted using SAS Enterprise Guide 7.1 (SAS Institute, Cary, NC) and Stata 15.1 (StataCorp, College Station, TX).
RESULTS
Patient Characteristics
We identified 2 258 464 survivors of nonsurgical hospitalizations between 2009 and 2019 (Figure S1), encompassing 5 396 051 total patient‐years of postdischarge follow‐up. The overall mean (SD) age of the cohort was 64.4 (14.6) years. The majority were women (54.4%), White (62.5%), from the South (45.8%), and enrolled in a Medicare Advantage health plan (53.6%).
A total of 808 673 (35.8%) patients had sepsis during at least 1 hospitalization in the study period, including implicit sepsis only in 448 644, explicit sepsis only in 124 841, and both in 235 188 (Figure S1). An ICD‐9/10 code for septic shock was present in 43 188 (12.0%) of patients with explicit sepsis. Patients with sepsis hospitalizations differed substantially from patients hospitalized without sepsis (Table 1), including older age, more Medicare Advantage enrollees, more infection and organ failure, more comorbidities and CVD risk factors, more preexisting and inpatient CVD diagnoses (including all examined CVD diagnoses), greater use of critical care therapies (including the need for ICU care), and a longer hospital length of stay. The most common hospital discharge disease‐related groups in patients without sepsis included cardiovascular/circulatory (19.0%), digestive (15.7%), respiratory (12.8%), nervous system (12.0%), and mental health (5.3%). Additional differences in hospitalization characteristics were observed among patients with sepsis when groups with implicit sepsis, explicit sepsis, and both were compared (Table 2). In general, the group with explicit sepsis had the lowest risk profile, and the group with both implicit and explicit sepsis had the highest risk profile.
Table 1.
Patient Characteristics of Hospitalizations With and Without Sepsis (Any Type)
| No sepsis (N=1 449 821) | Sepsis (N=808 673) | Total (N=2 258 464) | P value | |
|---|---|---|---|---|
| Age, y | <0.0001 | |||
| Mean (SD) | 62.0 (14.8) | 68.7 (13.2) | 64.4 (14.6) | |
| Age group, n (%) | <0.0001 | |||
| 18–54 y | 450 919 (31.1) | 124 249 (15.4) | 575 168 (25.5) | |
| 55–64 y | 347 422 (24.0) | 158 929 (19.7) | 506 351 (22.4) | |
| 65–74 y | 285 200 (19.7) | 194 439 (24.0) | 479 639 (21.2) | |
| 75+ years | 366 280 (25.3) | 331 056 (40.9) | 697 336 (30.9) | |
| Sex, n (%) | <0.0001 | |||
| Female | 789 512 (54.5) | 438 058 (54.2) | 1 227 570 (54.4) | |
| Male | 660 309 (45.5) | 370 615 (45.8) | 1 030 924 (45.6) | |
| Race or ethnicity, n (%) | <0.0001 | |||
| Asian | 29 040 (2.0) | 15 111 (1.9) | 44 151 (2.0) | |
| Black | 175 979 (12.1) | 111 710 (13.8) | 287 689 (12.7) | |
| Hispanic | 104 385 (7.2) | 56 236 (7.0) | 160 621 (7.1) | |
| White | 888 816 (61.3) | 522 351 (64.6) | 1 411 167 (62.5) | |
| Unknown | 251 601 (17.4) | 103 265 (12.8) | 354 866 (15.7) | |
| Region, n (%) | <0.0001 | |||
| Midwest | 404 171 (27.9) | 226 434 (28.0) | 630 605 (27.9) | |
| Northeast | 227 047 (15.7) | 132 902 (16.4) | 359 949 (15.9) | |
| South | 666 092 (45.9) | 368 145 (45.5) | 1 034 237 (45.8) | |
| West | 149 456 (10.3) | 80 006 (9.9) | 229 462 (10.2) | |
| Unknown | 3055 (0.2) | 1186 (0.1) | 4241 (0.2) | |
| Health plan, n (%) | <0.0001 | |||
| Commercial | 789 400 (54.4) | 257 979 (31.9) | 1 047 379 (46.4) | |
| Medicare advantage | 660 421 (45.6) | 550 694 (68.1) | 1 211 115 (53.6) | |
| Index year, n (%) | <0.0001 | |||
| 2009 | 130 672 (9.0) | 42 340 (5.2) | 173 012 (7.7) | |
| 2010 | 118 177 (8.2) | 44 137 (5.5) | 162 314 (7.2) | |
| 2011 | 125 063 (8.6) | 51 960 (6.4) | 177 023 (7.8) | |
| 2012 | 132 417 (9.1) | 58 639 (7.3) | 191 056 (8.5) | |
| 2013 | 131 258 (9.1) | 64 373 (8.0) | 195 631 (8.7) | |
| 2014 | 118 103 (8.1) | 63 891 (7.9) | 181 994 (8.1) | |
| 2015 | 107 710 (7.4) | 61 976 (7.7) | 169 686 (7.5) | |
| 2016 | 119 401 (8.2) | 78 149 (9.7) | 197 550 (8.7) | |
| 2017 | 142 898 (9.9) | 101 541 (12.6) | 244 439 (10.8) | |
| 2018 | 157 028 (10.8) | 116 084 (14.4) | 273 112 (12.1) | |
| 2019 | 167 094 (11.5) | 125 583 (15.5) | 292 677 (13.0) | |
| Prior CVD, n (%) | 642 309 (44.3) | 496 616 (61.4) | 1 138 925 (50.4) | <0.0001 |
| Coronary artery disease | 446 032 (30.8) | 356 481 (44.1) | 802 513 (35.5) | <0.0001 |
| Myocardial infarction | 133 617 (9.2) | 126 051 (15.6) | 259 668 (11.5) | <0.0001 |
| Percutaneous coronary intervention | 103 615 (7.1) | 80 653 (10.0) | 184 268 (8.2) | <0.0001 |
| Coronary artery bypass grafting | 85 075 (5.9) | 73 615 (9.1) | 158 690 (7.0) | <0.0001 |
| Valve procedure | 11 597 (0.8) | 10 459 (1.3) | 22 056 (1.0) | <0.0001 |
| Heart failure | 222 407 (15.3) | 256 655 (31.7) | 479 062 (21.2) | <0.0001 |
| Cardiomyopathy | 100 189 (6.9) | 96 784 (12.0) | 196 973 (8.7) | <0.0001 |
| Atrial fibrillation | 203 769 (14.1) | 189 976 (23.5) | 393 745 (17.4) | <0.0001 |
| Supraventricular tachycardia | 47 782 (3.3) | 37 337 (4.6) | 85 119 (3.8) | <0.0001 |
| Stroke | 133 088 (9.2) | 138 394 (17.1) | 271 482 (12.0) | <0.0001 |
| Ventricular tachycardia | 48 135 (3.3) | 47 666 (5.9) | 95 801 (4.2) | <0.0001 |
| Implanted cardiac device | 69 439 (4.8) | 65 183 (8.1) | 134 622 (6.0) | <0.0001 |
| Number of CVD risk factors | <0.0001 | |||
| 0 | 132 031 (9.1) | 38 526 (4.8) | 170 557 (7.6) | |
| 1 | 236 741 (16.3) | 82 449 (10.2) | 319 190 (14.1) | |
| 2 | 397 542 (27.4) | 170 337 (21.1) | 567 879 (25.1) | |
| 3 | 372 798 (25.7) | 225 234 (27.9) | 598 032 (26.5) | |
| 4 | 222 209 (15.3) | 181 266 (22.4) | 403 475 (17.9) | |
| 5 | 77 777 (5.4) | 91 303 (11.3) | 169 080 (7.5) | |
| 6 | 10 723 (0.7) | 19 558 (2.4) | 30 281 (1.3) | |
| CVD during index hospitalization, n (%) | 651 451 (44.9) | 453 454 (56.1) | 1 104 905 (48.9) | <0.0001 |
| Heart failure | 204 613 (14.1) | 225 294 (27.9) | 429 907 (19.0) | <0.0001 |
| Myocardial infarction | 55 056 (3.8) | 41 637 (5.1) | 96 693 (4.3) | <0.0001 |
| Angina | 37 072 (2.6) | 8435 (1.0) | 45 507 (2.0) | <0.0001 |
| Atrial fibrillation | 207 768 (14.3) | 168 809 (20.9) | 376 577 (16.7) | <0.0001 |
| Arrest | 5626 (0.4) | 6974 (0.9) | 12 600 (0.6) | <0.0001 |
| Coronary artery disease | 267 646 (18.5) | 167 828 (20.8) | 435 474 (19.3) | <0.0001 |
| Valve disease | 176 252 (12.2) | 124 402 (15.4) | 300 654 (13.3) | <0.0001 |
| Cardiomyopathy | 76 338 (5.3) | 54 715 (6.8) | 131 053 (5.8) | <0.0001 |
| Supraventricular tachycardia | 19 657 (1.4) | 15 716 (1.9) | 35 373 (1.6) | <0.0001 |
| Ventricular tachycardia | 27 752 (1.9) | 23 518 (2.9) | 51 270 (2.3) | <0.0001 |
| Stroke | 133 127 (9.2) | 60 554 (7.5) | 193 681 (8.6) | <0.0001 |
| Intracranial bleed | 36 876 (2.5) | 12 669 (1.6) | 49 545 (2.2) | <0.0001 |
| Shock during index hospitalization, n (%) | 7141 (0.5) | 56 228 (7.0) | 63 369 (2.8) | <0.0001 |
| Cardiogenic | 1728 (0.1) | 4616 (0.6) | 6344 (0.3) | <0.0001 |
| Septic | 0 (0.0) | 44 099 (5.5) | 44 099 (1.9) | <0.0001 |
| Infection during index hospitalization | ||||
| Central nervous system | 2621 (0.2) | 3769 (0.5) | 6390 (0.3) | <0.0001 |
| Cardiac/vascular | 9231 (0.6) | 11 786 (1.5) | 21 017 (0.9) | <0.0001 |
| Ear/nose/throat/upper respiratory | 14 039 (1.0) | 15 748 (1.9) | 29 787 (1.3) | <0.0001 |
| Pulmonary | 68 570 (4.7) | 277 549 (34.3) | 346 119 (15.3) | <0.0001 |
| Gastrointestinal | 52 010 (3.6) | 31 670 (3.9) | 83 680 (3.7) | <0.0001 |
| Genitourinary | 81 736 (5.6) | 247 626 (30.6) | 329 362 (14.6) | <0.0001 |
| Skin/soft tissue/bone/joint | 73 925 (5.1) | 82 120 (10.2) | 156 045 (6.9) | <0.0001 |
| Bacterial/fungal | 83 972 (5.8) | 381 206 (47.1) | 465 178 (20.6) | <0.0001 |
| Iatrogenic | 14 444 (1.0) | 21 269 (2.6) | 35 713 (1.6) | <0.0001 |
| Sepsis/bacteremia | 3242 (0.2) | 168 363 (20.8) | 171 605 (7.6) | <0.0001 |
| Index hospitalization, n (%) | ||||
| Organ failure | 337 252 (23.3) | 686 402 (84.9) | 1 023 654 (45.3) | <0.0001 |
| ICU admission | 449 257 (31.0) | 357 378 (44.2) | 806 635 (35.7) | <0.0001 |
| Ventilator | 11 580 (0.8) | 41 662 (5.2) | 53 242 (2.4) | <0.0001 |
| Hemodialysis | 4625 (0.3) | 17 447 (2.2) | 22 072 (1.0) | <0.0001 |
| Length of stay, d | <0.0001 | |||
| Mean (SD) | 4.2 (4.2) | 6.7 (7.5) | 5.1 (5.8) | |
| No. of hospitalizations in prior 6 mo | <0.0001 | |||
| Mean (SD) | 0.1 (0.4) | 0.4 (0.8) | 0.2 (0.6) | |
CVD indicates cardiovascular disease; and ICU, intensive care unit.
Table 2.
Patient Characteristics of Hospitalizations With Sepsis According to the Type of Sepsis
| Both implicit and explicit (N=235 188) | Explicit only (N=124 841) | Implicit only (N=448 644) | Total (N=808 673) | P value | |
|---|---|---|---|---|---|
| Age, y | <0.0001 | ||||
| Mean (SD) | 68.8 (13.2) | 64.0 (14.4) | 69.9 (12.6) | 68.7 (13.2) | |
| Age group, n (%) | <0.0001 | ||||
| 18–54 y | 34 786 (14.8) | 32 687 (26.2) | 56 776 (12.7) | 124 249 (15.4) | |
| 55–64 y | 46 585 (19.8) | 29 249 (23.4) | 83 095 (18.5) | 158 929 (19.7) | |
| 65–74 y | 58 620 (24.9) | 26 736 (21.4) | 109 083 (24.3) | 194 439 (24.0) | |
| 75+ y | 95 197 (40.5) | 36 169 (29.0) | 199 690 (44.5) | 331 056 (40.9) | |
| Sex, n (%) | <0.0001 | ||||
| Female | 117 700 (50.0) | 66 682 (53.4) | 253 676 (56.5) | 438 058 (54.2) | |
| Male | 117 488 (50.0) | 58 159 (46.6) | 194 968 (43.5) | 370 615 (45.8) | |
| Race or ethnicity, n (%) | <0.0001 | ||||
| Asian | 5102 (2.2) | 2806 (2.2) | 7203 (1.6) | 15 111 (1.9) | |
| Black | 31 520 (13.4) | 14 069 (11.3) | 66 121 (14.7) | 111 710 (13.8) | |
| Hispanic | 16 773 (7.1) | 10 560 (8.5) | 28 903 (6.4) | 56 236 (7.0) | |
| White | 151 089 (64.2) | 77 294 (61.9) | 293 968 (65.5) | 522 351 (64.6) | |
| Unknown | 30 704 (13.1) | 20 112 (16.1) | 52 449 (11.7) | 103 265 (12.8) | |
| Region | <0.0001 | ||||
| Midwest | 65 166 (27.7) | 33 670 (27.0) | 127 598 (28.4) | 226 434 (28.0) | |
| Northeast | 39 230 (16.7) | 21 857 (17.5) | 71 815 (16.0) | 132 902 (16.4) | |
| South | 103 667 (44.1) | 54 627 (43.8) | 209 851 (46.8) | 368 145 (45.5) | |
| West | 26 702 (11.4) | 14 451 (11.6) | 38 853 (8.7) | 80 006 (9.9) | |
| Unknown | 423 (0.2) | 236 (0.2) | 527 (0.1) | 1186 (0.1) | |
| Health plan, n (%) | <0.0001 | ||||
| Commercial | 73 111 (31.1) | 59 432 (47.6) | 125 436 (28.0) | 257 979 (31.9) | |
| Medicare advantage | 162 077 (68.9) | 65 409 (52.4) | 323 208 (72.0) | 550 694 (68.1) | |
| Index year, n (%) | <0.0001 | ||||
| 2009 | 8659 (3.7) | 6055 (4.9) | 27 626 (6.2) | 42 340 (5.2) | |
| 2010 | 9074 (3.9) | 6409 (5.1) | 28 654 (6.4) | 44 137 (5.5) | |
| 2011 | 11 630 (4.9) | 7560 (6.1) | 32 770 (7.3) | 51 960 (6.4) | |
| 2012 | 13 601 (5.8) | 8529 (6.8) | 36 509 (8.1) | 58 639 (7.3) | |
| 2013 | 16 087 (6.8) | 9375 (7.5) | 38 911 (8.7) | 64 373 (8.0) | |
| 2014 | 17 523 (7.5) | 10 140 (8.1) | 36 228 (8.1) | 63 891 (7.9) | |
| 2015 | 18 762 (8.0) | 10 420 (8.3) | 32 794 (7.3) | 61 976 (7.7) | |
| 2016 | 24 895 (10.6) | 13 151 (10.5) | 40 103 (8.9) | 78 149 (9.7) | |
| 2017 | 33 288 (14.2) | 15 816 (12.7) | 52 437 (11.7) | 101 541 (12.6) | |
| 2018 | 38 603 (16.4) | 18 195 (14.6) | 59 286 (13.2) | 116 084 (14.4) | |
| 2019 | 43 066 (18.3) | 19 191 (15.4) | 63 326 (14.1) | 125 583 (15.5) | |
| Prior CVD, n (%) | 141 890 (60.3) | 60 720 (48.6) | 294 006 (65.5) | 496 616 (61.4) | <0.0001 |
| Coronary artery disease | 100 358 (42.7) | 42 409 (34.0) | 213 714 (47.6) | 356 481 (44.1) | <0.0001 |
| Myocardial infarction | 35 489 (15.1) | 13 170 (10.5) | 77 392 (17.3) | 126 051 (15.6) | <0.0001 |
| Percutaneous coronary intervention | 22 277 (9.5) | 8911 (7.1) | 49 465 (11.0) | 80 653 (10.0) | <0.0001 |
| Coronary artery bypass grafting | 20 558 (8.7) | 7654 (6.1) | 45 403 (10.1) | 73 615 (9.1) | <0.0001 |
| Valve procedure | 3251 (1.4) | 1338 (1.1) | 5870 (1.3) | 10 459 (1.3) | <0.0001 |
| Heart failure | 70 934 (30.2) | 23 998 (19.2) | 161 723 (36.0) | 256 655 (31.7) | <0.0001 |
| Cardiomyopathy | 26 866 (11.4) | 9081 (7.3) | 60 837 (13.6) | 96 784 (12.0) | <0.0001 |
| Atrial fibrillation | 54 241 (23.1) | 20 462 (16.4) | 115 273 (25.7) | 189 976 (23.5) | <0.0001 |
| Supraventricular tachycardia | 10 647 (4.5) | 4894 (3.9) | 21 796 (4.9) | 37 337 (4.6) | <0.0001 |
| Stroke | 40 243 (17.1) | 15 073 (12.1) | 83 078 (18.5) | 138 394 (17.1) | <0.0001 |
| Ventricular tachycardia | 13 590 (5.8) | 4590 (3.7) | 29 486 (6.6) | 47 666 (5.9) | <0.0001 |
| Implanted device | 17 934 (7.6) | 5946 (4.8) | 41 303 (9.2) | 65 183 (8.1) | <0.0001 |
| Number of cardiovascular risk factors, n (%) | <0.0001 | ||||
| 0 | 11 796 (5.0) | 8455 (6.8) | 18 275 (4.1) | 38 526 (4.8) | |
| 1 | 23 805 (10.1) | 17 328 (13.9) | 41 316 (9.2) | 82 449 (10.2) | |
| 2 | 48 115 (20.5) | 30 498 (24.4) | 91 724 (20.4) | 170 337 (21.1) | |
| 3 | 65 368 (27.8) | 33 735 (27.0) | 126 131 (28.1) | 225 234 (27.9) | |
| 4 | 53 208 (22.6) | 23 873 (19.1) | 104 185 (23.2) | 181 266 (22.4) | |
| 5 | 26 889 (11.4) | 9609 (7.7) | 54 805 (12.2) | 91 303 (11.3) | |
| 6 | 6007 (2.6) | 1343 (1.1) | 12 208 (2.7) | 19 558 (2.4) | |
| CVD during index hospitalization, n (%) | 137 651 (58.5) | 49 267 (39.5) | 266 536 (59.4) | 453 454 (56.1) | <0.0001 |
| Heart failure | 66 993 (28.5) | 15 851 (12.7) | 142 450 (31.8) | 225 294 (27.9) | <0.0001 |
| Myocardial infarction | 17 420 (7.4) | 2812 (2.3) | 21 405 (4.8) | 41 637 (5.1) | <0.0001 |
| Angina | 2020 (0.9) | 997 (0.8) | 5418 (1.2) | 8435 (1.0) | <0.0001 |
| Atrial fibrillation | 52 976 (22.5) | 17 170 (13.8) | 98 663 (22.0) | 168 809 (20.9) | <0.0001 |
| Arrest | 3146 (1.3) | 175 (0.1) | 3653 (0.8) | 6974 (0.9) | <0.0001 |
| Coronary artery disease | 44 354 (18.9) | 18 733 (15.0) | 104 741 (23.3) | 167 828 (20.8) | <0.0001 |
| Valve disease | 40 929 (17.4) | 14 317 (11.5) | 69 156 (15.4) | 124 402 (15.4) | <0.0001 |
| Cardiomyopathy | 16 513 (7.0) | 4037 (3.2) | 34 165 (7.6) | 54 715 (6.8) | <0.0001 |
| Supraventricular tachycardia | 6027 (2.6) | 1862 (1.5) | 7827 (1.7) | 15 716 (1.9) | <0.0001 |
| Ventricular tachycardia | 8741 (3.7) | 1610 (1.3) | 13 167 (2.9) | 23 518 (2.9) | <0.0001 |
| Stroke | 17 790 (7.6) | 5018 (4.0) | 37 746 (8.4) | 60 554 (7.5) | <0.0001 |
| Intracranial bleed | 3246 (1.4) | 819 (0.7) | 8604 (1.9) | 12 669 (1.6) | <0.0001 |
| Shock during index hospitalization, n (%) | 48 182 (20.5) | 509 (0.4) | 7537 (1.7) | 56 228 (7.0) | <0.0001 |
| Cardiogenic | 2762 (1.2) | 31 (0.0) | 1823 (0.4) | 4616 (0.6) | <0.0001 |
| Septic | 42 858 (18.2) | 330 (0.3) | 911 (0.2) | 44 099 (5.5) | <0.0001 |
| Infection during index hospitalization, n (%) | 235 188 (100.0) | 117 667 (94.3) | 448 644 (100.0) | 801 499 (99.1) | <0.0001 |
| Central nervous system | 1199 (0.5) | 551 (0.4) | 2019 (0.5) | 3769 (0.5) | <0.0001 |
| Cardiac/vascular | 2487 (1.1) | 1582 (1.3) | 7717 (1.7) | 11 786 (1.5) | <0.0001 |
| Ear/nose/throat/upper respiratory | 1610 (0.7) | 1879 (1.5) | 12 259 (2.7) | 15 748 (1.9) | <0.0001 |
| Pulmonary | 69 577 (29.6) | 20 889 (16.7) | 187 083 (41.7) | 277 549 (34.3) | <0.0001 |
| Gastrointestinal | 7247 (3.1) | 6853 (5.5) | 17 570 (3.9) | 31 670 (3.9) | <0.0001 |
| Genitourinary | 65 487 (27.8) | 33 508 (26.8) | 148 631 (33.1) | 247 626 (30.6) | <0.0001 |
| Skin/soft tissue/bone/joint | 19 046 (8.1) | 17 619 (14.1) | 45 455 (10.1) | 82 120 (10.2) | <0.0001 |
| Bacterial/fungal | 195 940 (83.3) | 92 910 (74.4) | 92 356 (20.6) | 381 206 (47.1) | <0.0001 |
| Iatrogenic | 8060 (3.4) | 6427 (5.1) | 6782 (1.5) | 21 269 (2.6) | <0.0001 |
| Sepsis/bacteremia | 114 320 (48.6) | 48 276 (38.7) | 5767 (1.3) | 168 363 (20.8) | <0.0001 |
| Index hospitalization, n (%) | |||||
| Organ failure | 235 188 (100.0) | 2570 (2.1) | 448 644 (100.0) | 686 402 (84.9) | <0.0001 |
| ICU admission | 136 785 (58.2) | 34 516 (27.6) | 186 077 (41.5) | 357 378 (44.2) | <0.0001 |
| Ventilator | 22 470 (9.6) | 77 (0.1) | 19 115 (4.3) | 41 662 (5.2) | <0.0001 |
| Hemodialysis | 6548 (2.8) | 78 (0.1) | 10 821 (2.4) | 17 447 (2.2) | <0.0001 |
| Length of stay, d | <0.0001 | ||||
| Mean (SD) | 8.1 (8.3) | 4.9 (4.6) | 6.5 (7.6) | 6.7 (7.5) | |
| No. of hospitalizations in prior 6 mo | <0.0001 | ||||
| Mean (SD) | 0.4 (0.8) | 0.4 (0.8) | 0.4 (0.8) | 0.4 (0.8) | |
CVD indicates cardiovascular disease; and ICU, intensive care unit.
Outcomes
Compared with patients hospitalized without a sepsis diagnosis, patients hospitalized with sepsis had an elevated risk of all examined postdischarge adverse events (Table 3), including all‐cause mortality (adjusted HR, 1.27 [95% CI, 1.25–1.28]; P<0.001; Figure 1), all‐cause rehospitalization (adjusted HR, 1.38 [95% CI, 1.37–1.39]; P<0.001), cardiovascular hospitalization (adjusted HR, 1.43 [95% CI, 1.41–1.44]; P<0.001; Figure 2), and the composite of all‐cause mortality and either all‐cause hospitalization or cardiovascular hospitalization. Event rates were higher in the sepsis group at both early and late time points (Tables S7 through S9). The risk of all postdischarge adverse events was elevated for implicit sepsis, explicit sepsis, and both compared with patients without sepsis, with variability in the magnitude of excess risk based on the sepsis definition (Table 3). The highest crude risk of posthospitalization adverse events was observed in the implicit sepsis group, followed by the group with both implicit and explicit sepsis, followed by the group with explicit sepsis, with the lowest event rate in the group without sepsis (Figures 2 and 3). Survivors of hospitalizations with sepsis were at elevated risk of subsequent hospitalization for each major type of CVD event (coronary, heart failure, stroke, arrhythmia, and shock), hospitalization for infection, and hospitalization for other noncardiovascular/noninfection indications (Table 4). The excess risk associated with sepsis appeared to be highest for hospitalization for heart failure (adjusted HR, 1.51 [95% CI, 1.49–1.53]). Patients with sepsis during hospitalization had an excess risk of rehospitalization for infection (adjusted HR, 1.99 [95% CI, 1.94–2.04]); by definition, subsequent infection hospitalizations in the no‐sepsis group did not meet the criteria for sepsis.
Table 3.
Event Rates (per 100 Person‐Years) and Adjusted Hazard Ratio Values for Mortality and Hospitalization Outcomes Each Sepsis Group to Patients Without Sepsis
| No sepsis (ref) | All sepsis | P value | ||||||
|---|---|---|---|---|---|---|---|---|
| No. with events | Person‐years | Event rate per 100 | No. with events | Person years | Event rate per 100 | Hazard ratio | ||
| (95% CI) | ||||||||
| Outcomes | N=1 449 821 | N=808 673 | ||||||
| Mortality | 167 493 | 3 701 524 | 4.52 | 220 428 | 1 694 527 | 13.01 | 1.27 (1.25–1.28) | <0.001 |
| All‐cause hospitalization* | 604 851 | 2 488 921 | 24.30 | 481 263 | 901 985 | 53.36 | 1.38 (1.37–1.39) | <0.001 |
| CVD hospitalization* | 261 245 | 3 250 160 | 8.04 | 267 829 | 1 314 742 | 20.37 | 1.43 (1.41–1.44) | <0.001 |
| Composite 1† | 661 469 | 2 488 921 | 26.58 | 538 228 | 901 985 | 59.67 | 1.35 (1.34–1.36) | <0.001 |
| Composite 2‡ | 360 403 | 3 250 160 | 11.09 | 377 514 | 1 314 742 | 28.71 | 1.37 (1.35–1.38) | <0.001 |
| No sepsis (ref) | Implicit sepsis only | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcomes | N=1 449 821 | N=448 644 | ||||||
| Mortality | 167 493 | 3 701 524 | 4.52 | 135 616 | 935 100 | 14.50 | 1.23 (1.21–1.25) | <0.001 |
| All‐cause hospitalization* | 604 851 | 2 488 921 | 24.30 | 346 926 | 472 068 | 73.49 | 1.46 (1.44–1.47) | <0.001 |
| CVD hospitalization* | 261 245 | 3 250 160 | 8.04 | 294 021 | 697 599 | 42.15 | 1.54 (1.52–1.56) | <0.001 |
| Composite 1† | 661 469 | 2 488 921 | 26.58 | 314 406 | 427 068 | 73.62 | 1.40 (1.39–1.42) | <0.001 |
| Composite 2‡ | 360 403 | 3 250 160 | 11.09 | 230 492 | 697 599 | 33.04 | 1.43 (1.41–1.44) | <0.001 |
| No sepsis (ref) | Explicit sepsis only | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcomes | N=1 449 821 | N=124 841 | ||||||
| Mortality | 167 493 | 3 701 524 | 4.52 | 20 448 | 294 409 | 6.95 | 1.31 (1.28–1.33) | <0.001 |
| All‐cause hospitalization* | 604 851 | 2 488 921 | 24.30 | 74 236 | 174 319 | 42.59 | 1.33 (1.31–1.34) | <0.001 |
| CVD hospitalization* | 261 245 | 3 250 160 | 8.04 | 50 370 | 248 370 | 20.28 | 1.37 (1.35–1.38) | <0.001 |
| Composite 1† | 661 469 | 2 488 921 | 26.58 | 69 652 | 174 319 | 39.96 | 1.30 (1.29–1.31) | <0.001 |
| Composite 2‡ | 360 403 | 3 250 160 | 11.09 | 39 636 | 248 370 | 15.96 | 1.33 (1.31–1.34) | <0.001 |
| No sepsis (ref) | Both sepsis types | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcomes | N=1 449 821 | N=235 188 | ||||||
| Mortality | 167 493 | 3 701 524 | 4.52 | 64 364 | 465 018 | 13.84 | 1.22 (1.20–1.24) | <0.001 |
| All‐cause hospitalization* | 604 851 | 2 488 921 | 24.30 | 174 031 | 255 598 | 68.09 | 1.32 (1.30–1.33) | <0.001 |
| CVD hospitalization* | 261 245 | 3 250 160 | 8.04 | 142 808 | 368 772 | 38.73 | 1.26 (1.24–1.28) | <0.001 |
| Composite 1† | 661 469 | 2 488 921 | 26.58 | 154 170 | 255 598 | 60.32 | 1.33 (1.31–1.34) | <0.001 |
| Composite 2‡ | 360 403 | 3 250 160 | 11.09 | 107 386 | 368 772 | 29.12 | 1.28 (1.27–1.30) | <0.001 |
CVD indicates cardiovascular disease; and ref, reference group.
Death was considered as a competing risk when examining the risk of all‐cause or CVD rehospitalization.
Composite 1 includes death or all‐cause rehospitalization.
Composite 2 includes death or CVD rehospitalization.
Figure 1. Kaplan–Meier curves demonstrating postdischarge event rates in patients with sepsis (all definitions combined) vs those without sepsis, including all‐cause mortality (top left), all‐cause rehospitalization (top right), the composite of death or all‐cause rehospitalization (bottom left), and the composite of death or cardiovascular hospitalization (bottom right).
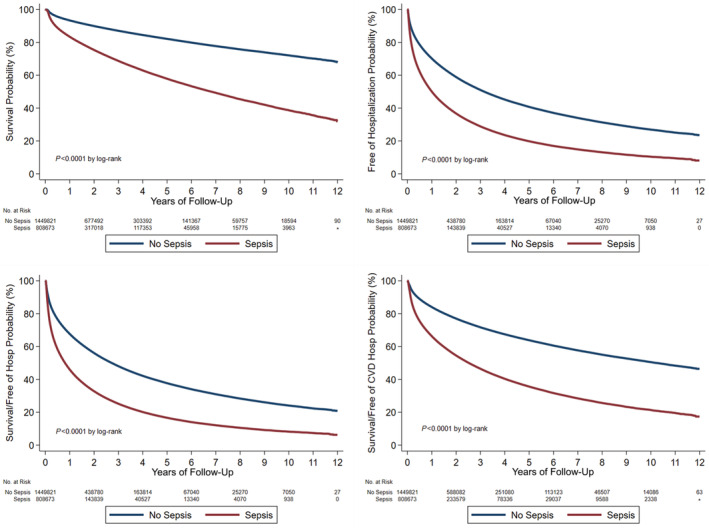
Figure 2. Kaplan–Meier curves demonstrating postdischarge cardiovascular rehospitalization rates in patients with sepsis vs those without sepsis, including all sepsis definitions combined (left) and patients with sepsis separated by the sepsis definition (right).
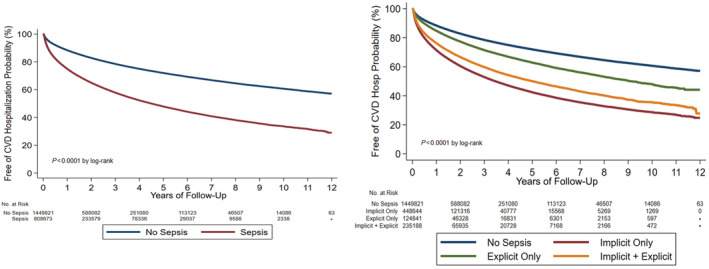
Figure 3. Kaplan–Meier curves demonstrating postdischarge event rates in patients with sepsis (separated by definition) vs those without sepsis, including all‐cause mortality (top left), all‐cause rehospitalization (top right), the composite of death or all‐cause rehospitalization (bottom left) and the composite of death or cardiovascular hospitalization (bottom right).
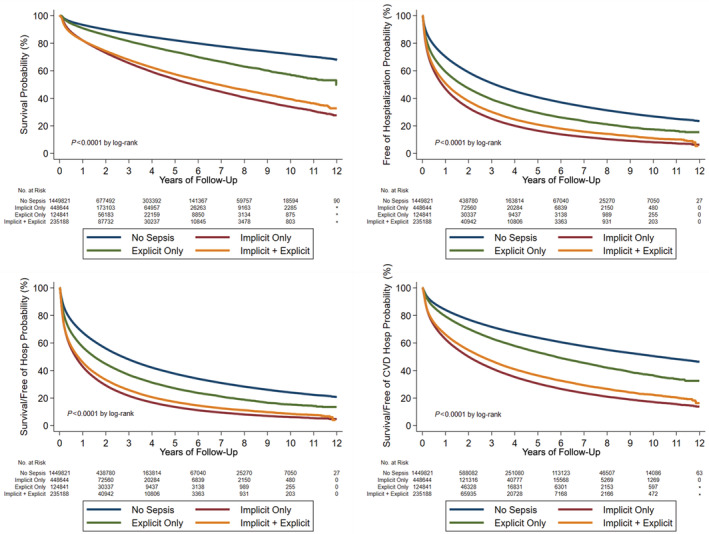
Table 4.
Event Rates (per 100 Person‐Years) and Adjusted Hazard Ratio Values for Individual Hospitalization End Points Comparing the Overall Sepsis Group to Patients Without Sepsis
| No sepsis (ref) | Sepsis | Hazard ratio (95% CI) | P value | |||||
|---|---|---|---|---|---|---|---|---|
| No. with events | Person‐years | Event rate per 100 | No. with events | Person years | Event rate per 100 | |||
| Outcomes | N=1 449 821 | N=808 673 | ||||||
| CVD hospitalization | ||||||||
| Coronary | 88 942 | 139 260 | 63.87 | 84 589 | 109 833 | 77.02 | 1.26 (1.24–1.29) | <0.001 |
| Heart failure | 138 138 | 205 377 | 67.26 | 179 931 | 201 849 | 89.14 | 1.51 (1.49–1.53) | <0.001 |
| Stroke | 71 189 | 114 893 | 61.96 | 64 223 | 88 657 | 72.44 | 1.35 (1.32–1.38) | <0.001 |
| Arrhythmia | 104 505 | 157 507 | 66.35 | 115 046 | 141 100 | 81.54 | 1.45 (1.42–1.47) | <0.001 |
| Shock | 12 125 | 20 674 | 58.65 | 16 968 | 25 335 | 66.97 | 1.38 (1.31–1.45) | <0.001 |
| Infection hospitalization | 32 341 | 51 274 | 63.07 | 66 482 | 78 848 | 84.32 | 1.99 (1.94–2.04) | <0.001 |
| Other non‐CVD/non‐infection hospitalizations | 442 726 | 534 480 | 82.83 | 352 197 | 316 577 | 111.25 | 1.42 (1.40–1.43) | <0.001 |
CVD indicates cardiovascular disease; and ref, reference group.
Subgroup Analyses
The presence of sepsis during hospitalization was associated with an elevated risk of postdischarge all‐cause mortality, all‐cause rehospitalization, and cardiovascular hospitalization in each of the predefined subgroups (Tables S10 through S14). The strength of association between sepsis and outcomes varied across these subgroups, with significant interaction terms observed in most subgroups. For all‐cause postdischarge mortality, the adjusted HR values were higher for patients aged <65 years (particularly those aged <50 years), non‐White race, commercial insurance enrollees, those without CVD (preexisting or diagnosed during hospitalization), those without organ failure (including AKI or shock), those with recent prior hospitalization, and those who did not receive ICU care. The excess risk of all‐cause hospitalization (using death as a competing risk) was consistent in all subgroups, with only modest differences in risk. For cardiovascular hospitalization (using death as a competing risk), the adjusted HR values were higher for patients aged <65 years and commercial insurance enrollees, those without CVD (preexisting or diagnosed during hospitalization), those with recent prior hospitalization, and those with shock or organ failure during hospitalization. Similar patterns were observed across subgroups for the composite outcomes of death and all‐cause or cardiovascular rehospitalization. The risk of postdischarge adverse outcomes varied according to the presence and type of infection diagnosis codes (Table S15).
Sensitivity Analyses
When the main analyses were repeated using propensity score overlap weighting, HR values associated with sepsis were slightly higher for all‐cause mortality (adjusted HR, 1.33 [95% CI, 1.32–1.35]; P<0.001), all‐cause rehospitalization (adjusted HR, 1.47 [95% CI, 1.46–1.48]; P<0.001), and CVD hospitalization (adjusted HR 1.52, 95% CI, 1.50–1.54; P<0.001). Using data from the first hospitalization during the study period, 547 063 patients were classified as sepsis and the remaining 1 659 352 were classified as no sepsis; within the sepsis group, 91 272 were classified as explicit sepsis, 292 748 were classified as implicit sepsis, and 163 043 were classified as both. Patients with sepsis were at elevated risk of postdischarge adverse events (Table 5), with smaller HR values for all‐cause mortality (adjusted HR, 1.10 [95% CI, 1.08–1.11]; P<0.001) and all‐cause rehospitalization (adjusted HR, 1.07 [95% CI, 1.06–1.07]; P<0.001) and a larger HR value for cardiovascular hospitalization (adjusted HR, 1.78 [95% CI, 1.76–1.78]; P<0.001) when compared with the main analysis.
Table 5.
Event Rates (per 100 Patient‐Years) and Adjusted Hazard Ratios for Each of the End Points of Interest in Patients With and Without Sepsis, as a Function of Sepsis Group Using Data From the First Hospitalization During the Study Period (Sensitivity Analysis)
| No sepsis (ref) | Sepsis | Hazard ratio (95% CI) | P Value | |||||
|---|---|---|---|---|---|---|---|---|
| No. with events | Person‐years | Event rate per 100 | No. with events | Person‐years | Event rate per 100 | |||
| Outcomes | N=1 659 352 | N=547 063 | ||||||
| Mortality | 238 248 | 4 390 012 | 5.43 | 131 365 | 1 222 026 | 10.75 | 1.10 (1.08–1.11) | <0.001 |
| All‐cause hospitalization* | 808 926 | 2 676 486 | 30.22 | 302 109 | 699 730 | 43.18 | 1.07 (1.06–1.07) | <0.001 |
| CVD hospitalization* | 261 245 | 3 938 648 | 6.63 | 160 297 | 984 526 | 16.28 | 1.78 (1.76–1.80) | <0.001 |
| Composite 1† | 865 744 | 2 676 486 | 32.35 | 336 890 | 699 730 | 48.15 | 1.07 (1.07–1.08) | <0.001 |
| Composite 2‡ | 431 158 | 3 938 648 | 10.95 | 227 740 | 984 526 | 23.13 | 1.39 (1.38–1.40) | <0.001 |
| No sepsis (ref) | Implicit sepsis only | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcomes | N=1 659 352 | N=292 748 | ||||||
| Mortality | 238 248 | 4 390 012 | 5.43 | 79 501 | 651 202 | 12.21 | 1.13 (1.11–1.14) | <0.001 |
| All‐cause hospitalization* | 808 926 | 2 676 486 | 30.22 | 172 040 | 355 334 | 48.42 | 1.13 (1.12–1.14) | <0.001 |
| CVD hospitalization* | 261 245 | 3 938 648 | 6.63 | 97 308 | 507 145 | 19.19 | 1.96 (1.93–1.99) | <0.001 |
| Composite 1† | 865 744 | 2 676 486 | 32.35 | 191 629 | 355 334 | 53.93 | 1.13 (1.12–1.14) | <0.001 |
| Composite 2‡ | 431 158 | 3 938 648 | 10.95 | 136 038 | 507 145 | 26.82 | 1.48 (1.47–1.50) | <0.001 |
| No sepsis (ref) | Explicit sepsis only | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcomes | N=1 659 352 | N=91 272 | ||||||
| Mortality | 238 248 | 4 390 012 | 5.43 | 12 463 | 225 988 | 5.51 | 1.06 (1.04–1.08) | <0.001 |
| All‐cause hospitalization* | 808 926 | 2 676 486 | 30.22 | 42 919 | 142 887 | 30.04 | 1.00 (0.99–1.01) | 0.469 |
| CVD hospitalization* | 261 245 | 3 938 648 | 6.63 | 18 326 | 195 493 | 9.37 | 1.66 (1.63–1.69) | <0.001 |
| Composite 1† | 865 744 | 2 676 486 | 32.35 | 45 878 | 142 887 | 32.11 | 1.01 (1.00–1.02) | 0.139 |
| Composite 2‡ | 431 158 | 3 938 648 | 10.95 | 24 979 | 195 493 | 12.78 | 1.31 (1.29–1.32) | <0.001 |
| No sepsis (ref) | Both sepsis types | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcomes | N=1 659 352 | N=163 043 | ||||||
| Mortality | 238 248 | 4 390 012 | 5.43 | 39 401 | 344 836 | 11.43 | 1.09 (1.08–1.11) | <0.001 |
| All‐cause hospitalization* | 808 926 | 2 676 486 | 30.22 | 87 105 | 201 509 | 43.23 | 1.03 (1.01–1.04) | <0.001 |
| CVD hospitalization* | 261 245 | 3 938 648 | 6.63 | 44 663 | 281 889 | 15.84 | 1.60 (1.57–1.62) | <0.001 |
| Composite #1† | 865 744 | 2 676 486 | 32.35 | 99 383 | 201 509 | 49.32 | 1.06 (1.05–1.07) | <0.001 |
| Composite #2‡ | 431 158 | 3 938 648 | 10.95 | 66 723 | 281 889 | 23.67 | 1.31 (1.29–1.33) | <0.001 |
CVD indicates cardiovascular disease; and ref, reference group.
Death was considered as a competing risk when examining the risk of all‐cause or CVD rehospitalization.
Composite 1 includes death or all‐cause rehospitalization.
Composite 2 includes death or CVD rehospitalization.
DISCUSSION
Summary of Findings
In this study of >2 million hospitalized nonsurgical patients with or at risk for CVD who survived to discharge, we observed that a high proportion of patients were hospitalized with sepsis on the basis of diagnosis codes. Survivors of sepsis hospitalization were at elevated risk of subsequent death and rehospitalization, with a heightened risk of hospitalizations for CVD events. Patients hospitalized with sepsis were at increased risk of all types of CVD events, including atherosclerotic and nonatherosclerotic events. This excess risk was greatest in magnitude for heart failure hospitalization. The excess risk developed early after hospitalization (within the first 6–12 months) and accumulated over time out to 12 years of follow‐up. An excess risk of adverse events was observed regardless of the sepsis definition (ie, implicit versus explicit) and type of infection. However, the crude event rates were higher among patients with implicit sepsis than those with explicit sepsis, potentially attributable to the uniform presence of organ dysfunction in patients with implicit sepsis. The excess risk of posthospitalization adverse events was consistent across all predefined subgroups, including those with and without CVD or organ failure. The relative hazard posed by sepsis was generally greater among lower‐risk subgroups, such as younger patients, those with commercial insurance, those without CVD, and those without organ failure. When we restricted the analysis to the first hospitalization during the study period, the excess relative hazard of CVD hospitalization associated with sepsis increased, strengthening our main findings. These data highlight the importance of sepsis during hospitalization as a powerful risk factor for subsequent adverse events, including death, reinfection, and major cardiovascular events.
Comparison With Prior Studies
Our results are broadly consistent with prior studies using heterogeneous methodologies that have observed an elevated risk of short‐ and long‐term cardiovascular events and death among survivors of sepsis. 12 A meta‐analysis of 27 observational studies demonstrated an excess risk of myocardial infarction, stroke, and heart failure after hospitalization for sepsis, with pooled adjusted HR values of 1.65 to 1.77 and wide variability across studies. 12 Our study population represents the largest published study of postdischarge CVD outcomes associated with sepsis in medical patients. The only larger study we identified examining postsepsis CVD events specifically examined the short‐term risk of arterial and venous thrombosis associated with postoperative sepsis. 20 Our point estimates for the excess risk of CVD events based on our adjusted HR values are more modest than observed in this meta‐analysis, which may reflect differences in our study population, period of enrollment, sepsis definitions, or CVD end point definitions. Notably, the prior meta‐analysis concluded that many of the included studies were of low quality, and many lacked appropriate nonsepsis controls for comparison, leaving uncertainty about the extent to which differences in baseline characteristics could have biased the findings. 12 A subsequent study published after the meta‐analysis included 250 000 hospital survivors with sepsis from Ontario who were propensity matched to nonsepsis hospital survivors, demonstrating a higher risk of all‐cause mortality and CVD events after sepsis hospitalization. 13 Their reported HR estimates were similar to those we observed using a different cohort and different methodology (HR, 1.26 for all‐cause mortality and 1.30 for major CVD events), and notably these authors observed a higher HR for younger patients as we found. 13 Together with our analysis, this study provides higher‐quality evidence supporting the association between sepsis hospitalization and subsequent CVD events, supporting and expanding on the findings of the recent meta‐analysis. 12 , 13
Unlike prior studies, we specifically enriched our population with higher‐risk medical patients (including older patients with or at risk for CVD) with a minimum 2‐night hospital length of stay to ensure that we did not use low‐risk patients as a comparator group. The high observed event rates in the no‐sepsis group confirm that we achieved this goal and demonstrate that sepsis confers an added hazard even when superimposed on an elevated baseline risk. We included patients with sepsis based on either explicit or implicit criteria, the latter representing a higher‐risk cohort with a greater event rate. As such, we believe that our robust methodology in a broader study population has led to more accurate point estimates as we have adjusted for a more comprehensive number of covariates (particularly those reflecting in‐hospital diagnoses and treatments) and examined several clinically relevant subgroups. As observed in the study from Ontario, the HR estimates for death and CVD hospitalization were higher for several lower‐risk subgroups, including those without prior or current CVD, suggesting that our results might apply equally to populations not enriched in CVD. 13 In addition to a higher risk of subsequent risk of death and hospitalization for CVD events, sepsis survivors were at high risk of reinfection and hospitalization for other causes. 7 This highlights the broad spectrum of elevated risk in this population, warranting close monitoring after hospital discharge.
Sepsis Definition and Subsequent Outcomes
We observed that the association between sepsis and clinical outcomes varied according to the definition of sepsis, which can guide the interpretation of prior studies. Implicit sepsis, defined as the presence of infection and acute organ dysfunction, accounted for most cases of sepsis in our cohort and was associated with a higher risk of adverse postdischarge events, including death and rehospitalization for cardiovascular and noncardiovascular causes. 1 , 19 Explicit sepsis, defined as the presence of specific diagnosis codes for sepsis, was present less often and usually in combination with implicit sepsis. 1 , 21 Contrary to some prior studies, patients with explicit sepsis alone had a lower overall risk profile, with younger age and a lower prevalence of acute organ failure, comorbidities, and the need for critical care therapies. 21 These patients had the lowest risk of subsequent adverse events of all sepsis groups after adjustment, although their risk exceeded that of patients without sepsis. Interestingly, the group with implicit sepsis alone had the highest risk of postdischarge events despite their intermediate severity of illness. This may imply that recognition of sepsis (ie, based on documentation using diagnosis codes) may portend better late outcomes in hospitalized patients by ensuring timely treatment, emphasizing the importance of diagnosing sepsis to ensure optimal patient care. Alternatively, the accuracy of these different sepsis definitions for underlying sepsis could have impacted the association with outcomes simply because of differences in the true prevalence of sepsis in the groups. 1 , 21 , 22 , 23 , 24 The use of ICD codes to identify sepsis cases is challenging because of limited sensitivity and specificity using either explicit or implicit definitions, potentially with underestimation of sepsis prevalence using explicit criteria and overestimation of sepsis prevalence when both criteria are combined. 21 , 22 , 23 , 24 Use of administrative codes to define sepsis will therefore result in some misclassification of the exposure, which is expected to weaken the observed associations. Despite this limitation, the strong associations that we observed using a variety of adjustment methods supports the likelihood that a true association exists.
The requirement for acute organ dysfunction to define implicit sepsis may explain the excess risk of subsequent events among hospital survivors, and subgroup analyses generally demonstrated a weaker association between sepsis and outcomes in patients with organ failure (including AKI or shock). AKI itself is a known predictor of death and CVD events, and our data demonstrate an additive risk of sepsis and AKI for postdischarge events. 14 , 15 , 16 The consistent association between sepsis and adverse outcomes across subgroups with and without various manifestations of critical illness helps to demonstrate the potential hazard from sepsis beyond the known harmful effects of its downstream complications and argues against the presence of critical illness itself as the sole mediator of this association.
Clinical Implications
Patients with sepsis during hospitalization differed substantially from patients without sepsis, with a higher overall risk profile including a greater degree of critical illness and more acute and chronic CVD including prior hospitalizations. However, patients with sepsis remained at higher risk of subsequent adverse outcomes even after adjusting or stratifying for these relevant factors. Prior studies have demonstrated that postdischarge CVD events are associated with higher mortality during follow‐up in survivors of sepsis, suggesting that CVD events likely contribute to the increased late mortality. 25 Our subgroup analyses demonstrated that although the absolute risk of events associated with sepsis was highest in patients with a higher risk profile (including older patients, those with CVD, and those with organ failure), the relative excess hazard was greatest in lower‐risk patients who likely have fewer competing risks. A similar finding was observed in a recent population‐based study, which observed that the hazard associated with sepsis was greater for younger patients who had a lower baseline risk. 13 Therefore, providers need to be cognizant that sepsis during hospitalization can reclassify otherwise low‐risk patients into a group needing diligent follow‐up because of their elevated risk of adverse events. Our analysis adds to the growing evidence base highlighting the multitude of potential complications that survivors of sepsis face after hospital discharge, including death, reinfection, cardiovascular events, and rehospitalization. 12 Survivors of sepsis were at elevated risk of a broad array of CVD events, including atherosclerotic and nonatherosclerotic events, highlighting the multitude of adverse outcomes associated with sepsis and necessitating a comprehensive approach to CVD prevention in this high‐risk group. Among the different CVD events we examined, heart failure was most common, and the adjusted HR value was highest for heart failure, whereas it was highest for myocardial infarction in the prior meta‐analysis. 12 Heart failure, either preexisting or during the index hospitalization, was approximately 2‐fold more common among patients with sepsis, and heart failure is a known risk factor for sepsis. 26 Acute myocardial dysfunction is a well‐known complication of sepsis, and a substantial incidence of new‐onset heart failure has been described in survivors of sepsis. 27 , 28 There are numerous potential explanations for this strong association between sepsis hospitalization and subsequent heart failure, including a variety of pathophysiologic mechanisms by which sepsis‐induced inflammation and neurohormonal activation may aggravate myocardial dysfunction as well as more pragmatic reasons including discontinuation of beneficial heart failure therapies (deprescription) during or after hospitalization. 27 , 28 , 29
Clinicians caring for survivors of sepsis need to be aware of their high risk of both atherosclerotic and nonatherosclerotic (ie, heart failure) CVD events, ensuring close follow‐up and diligent medication optimization. Such an approach may be needed even for patients without preexisting CVD, insofar as sepsis may accelerate underlying CVD progression in at‐risk patients. While the relative excess risk associated with sepsis was generally higher in groups with a lower baseline risk, the absolute excess risk was greater in higher‐risk cohorts, including older patients and those with CVD. This makes it essential to optimize guideline‐directed medical therapies for survivors of sepsis with CVD at hospital discharge and to ensure that medication reconciliation occurs to avoid deprescription. Future studies must determine which, if any, standard CVD prevention medications such as aspirin, statins, renin‐angiotensin‐aldosterone system antagonists, or beta blockers might reduce the risk of subsequent CVD events in sepsis survivors. While prior use of CVD prevention medications such as statins does not appear to influence the short‐term outcomes of patients with sepsis, this does not rule out a potential benefit of these drugs for preventing late atherosclerotic CVD events after discharge. 30 Given the complex residual risk pathways that may drive adverse events among survivors of sepsis (Figure 4), novel mechanistic approaches to CVD prevention in this group may be needed. 9
Figure 4. Conceptual model describing potential pathways by which sepsis during hospitalization may be associated with postdischarge cardiovascular events.

The sepsis syndrome and resultant organ dysfunction can aggravate cardiovascular disease through multiple mechanisms, and this can be exacerbated by purposeful or inadvertent medication deprescription during or after hospitalization. 7 , 9 , 10 , 11 , 12 , 24 , 29
Limitations
As with all observational studies using administrative data, this analysis is limited by the documentation of diagnosis codes to identify diseases of interest. None of these diagnosis codes is completely sensitive or specific, and variability in how these codes were documented across sites over time could have influenced our results. 21 In addition, we used a combination of implicit and explicit sepsis definitions, recognizing that these differ in accuracy and vary in their strength of association with outcomes. 1 , 22 We did not have a gold‐standard sepsis definition for comparison and could not use contemporary clinical sepsis definitions. 31 Because of the timing of our study period, patients with COVID‐19 were not represented in this sample, and the association between sepsis and subsequent CVD outcomes might differ in this group.
This analysis can only demonstrate associations and cannot prove causation, so we could not determine whether sepsis occurs in patients at higher risk of CVD or whether sepsis increases the risk of CVD directly. We were only able to adjust for known confounders available in OptumLabs Data Warehouse, and we cannot exclude residual confounding as a mediator of the observed results. Specifically, we did not have available data on all relevant CVD risk factors, including diet, lifestyle, or family history. We lack sufficient data to provide mechanistic insights about the direction and pathophysiology of the associations between sepsis and cardiovascular disease. We hypothesize that the persistent inflammatory milieu associated with sepsis survivorship may promote the progression of CVD or trigger CVD events, although a multitude of other biological pathways might contribute to the observed associations. 8 , 10 , 11
We specifically focused on medical hospitalizations lasting at least 2 nights to ensure a fair comparator group, precluding us from commenting on patients with surgical hospitalizations or shorter hospitalizations. We included any patient with a sepsis hospitalization in the sepsis group, so our population was enriched in patients with sepsis; therefore, our data cannot be used to determine the prevalence of sepsis hospitalizations directly and our estimates for the excess hazard of subsequent infection hospitalizations are likely exaggerated. OptumLabs Data Warehouse includes only commercial insurance and Medicare Advantage enrollees (who account for approximately one‐third of Medicare enrollees), which could affect the generalizability of our findings. Although this study includes a nationally representative sample of the US population, it does not include Medicaid, Medicare fee‐for‐service, or uninsured populations, who typically have lower income and lower socioeconomic status and may be medically underserved with a worse risk factor profile. While we can identify no specific reason why the direction of the association between sepsis and subsequent outcomes would be divergent in these nonincluded groups (even though nonincluded patients may potentially be at higher risk of CVD and other adverse events), it is likely that the magnitude of this association and overall event rates would differ.
CONCLUSIONS
Sepsis during hospitalization is common in hospital survivors and is associated with a higher risk of subsequent death, rehospitalization, and CVD events. The high global burden of sepsis results in many survivors of sepsis worldwide who will be at elevated risk of CVD complications after hospital discharge. The increased risk for CVD after sepsis hospitalization necessitates diligent follow‐up and optimization of guideline‐directed medical therapies in patients with preexisting CVD. It is imperative to determine the mechanisms underlying this association and whether standard medical therapies for cardiovascular prevention are efficacious for reducing the risk of CVD events associated with sepsis survivorship in the absence of preexisting CVD.
Source of Funding
This study was made possible by indirect funding from the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery and the Mayo Clinic Department of Cardiovascular Medicine. Dr Dunlay is funded by the National Institutes of Health (R01 HL144529). Dr Lawler is supported by a Heart and Stroke Foundation of Canada National New Investigator career award.
Disclosures
None.
Supporting information
Table S1–S15
Figure S1
These data were presented in abstract form at the American College of Cardiology Scientific Sessions, April 2–4, 2022 in Washington, DC.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.027813
For Sources of Funding and Disclosures, see page 16.
See Editorial by Wardi et al.
REFERENCES
- 1. Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the global burden of disease study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, McIntyre L, Ostermann M, Prescott HC, et al. Surviving sepsis campaign: international guidelines for Management of Sepsis and Septic Shock 2021. Crit Care Med. 2021;49:e1063–e1143. doi: 10.1097/CCM.0000000000005337 [DOI] [PubMed] [Google Scholar]
- 3. Prest J, Sathananthan M, Jeganathan N. Current trends in sepsis‐related mortality in the United States. Crit Care Med. 2021;49:1276–1284. doi: 10.1097/CCM.0000000000005017 [DOI] [PubMed] [Google Scholar]
- 4. Meyer N, Harhay MO, Small DS, Prescott HC, Bowles KH, Gaieski DF, Mikkelsen ME. Temporal trends in incidence, sepsis‐related mortality, and hospital‐based acute care after sepsis. Crit Care Med. 2018;46:354–360. doi: 10.1097/CCM.0000000000002872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee JT, Mikkelsen ME, Qi M, Werner RM. Trends in post‐acute care use after admissions for sepsis. Ann Am Thorac Soc. 2020;17:118–121. doi: 10.1513/AnnalsATS.201905-368RL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prescott HC, Osterholzer JJ, Langa KM, Angus DC, Iwashyna TJ. Late mortality after sepsis: propensity matched cohort study. BMJ. 2016;353:i2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prescott HC, Angus DC. Enhancing recovery from sepsis: a review. JAMA. 2018;319:62–75. doi: 10.1001/jama.2017.17687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yende S, Kellum JA, Talisa VB, Peck Palmer OM, Chang CH, Filbin MR, Shapiro NI, Hou PC, Venkat A, LoVecchio F, et al. Long‐term host immune response trajectories among hospitalized patients with sepsis. JAMA Netw Open. 2019;2:e198686. doi: 10.1001/jamanetworkopen.2019.8686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mira JC, Gentile LF, Mathias BJ, Efron PA, Brakenridge SC, Mohr AM, Moore FA, Moldawer LL. Sepsis pathophysiology, chronic critical illness, and persistent inflammation‐immunosuppression and catabolism syndrome. Crit Care Med. 2017;45:253–262. doi: 10.1097/CCM.0000000000002074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Musher DM, Abers MS, Corrales‐Medina VF. Acute infection and myocardial infarction. N Engl J Med. 2019;380:171–176. doi: 10.1056/NEJMra1808137 [DOI] [PubMed] [Google Scholar]
- 11. Lawler PR, Bhatt DL, Godoy LC, Luscher TF, Bonow RO, Verma S, Ridker PM. Targeting cardiovascular inflammation: next steps in clinical translation. Eur Heart J. 2021;42:113–131. doi: 10.1093/eurheartj/ehaa099 [DOI] [PubMed] [Google Scholar]
- 12. Kosyakovsky LB, Angriman F, Katz E, Adhikari NK, Godoy LC, Marshall JC, Ferreyro BL, Lee DS, Rosenson RS, Sattar N, et al. Association between sepsis survivorship and long‐term cardiovascular outcomes in adults: a systematic review and meta‐analysis. Intensive Care Med. 2021;47:931–942. doi: 10.1007/s00134-021-06479-y [DOI] [PubMed] [Google Scholar]
- 13. Angriman F, Rosella LC, Lawler PR, Ko DT, Wunsch H, Scales DC. Sepsis hospitalization and risk of subsequent cardiovascular events in adults: a population‐based matched cohort study. Intensive Care Med. 2022;48:448–457. doi: 10.1007/s00134-022-06634-z [DOI] [PubMed] [Google Scholar]
- 14. Dieter BP, Daratha KB, McPherson SM, Short R, Alicic RZ, Tuttle KR. Association of Acute Kidney Injury with cardiovascular events and death in systolic blood pressure intervention trial. Am J Nephrol. 2019;49:359–367. doi: 10.1159/000499574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Odutayo A, Wong CX, Farkouh M, Altman DG, Hopewell S, Emdin CA, Hunn BH. AKI and long‐term risk for cardiovascular events and mortality. J Am Soc Nephrol. 2017;28:377–387. doi: 10.1681/ASN.2016010105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Omotoso BA, Abdel‐Rahman EM, Xin W, Ma JZ, Scully KW, Arogundade FA, Balogun RA. Acute kidney injury (AKI) outcome, a predictor of long‐term major adverse cardiovascular events (MACE). Clin Nephrol. 2016;85:1–11. doi: 10.5414/CN108671 [DOI] [PubMed] [Google Scholar]
- 17. Wallace PJ, Shah ND, Dennen T, Bleicher PA, Crown WH. Optum labs: building a novel node in the learning health care system. Health Aff (Millwood). 2014;33:1187–1194. doi: 10.1377/hlthaff.2014.0038 [DOI] [PubMed] [Google Scholar]
- 18. Optum Research Data Assets . https://www.optum.com/content/dam/optum/resources/productSheets/5302_Data_Assets_Chart_Sheet_ISPOR.pdf. Accessed October 17, 2020.
- 19. Angus DC, Linde‐Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002 [DOI] [PubMed] [Google Scholar]
- 20. Donze JD, Ridker PM, Finlayson SR, Bates DW. Impact of sepsis on risk of postoperative arterial and venous thromboses: large prospective cohort study. BMJ. 2014;349:g5334. doi: 10.1136/bmj.g5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bouza C, Lopez‐Cuadrado T, Amate‐Blanco JM. Use of explicit ICD9‐CM codes to identify adult severe sepsis: impacts on epidemiological estimates. Crit Care. 2016;20:313. doi: 10.1186/s13054-016-1497-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jolley RJ, Sawka KJ, Yergens DW, Quan H, Jette N, Doig CJ. Validity of administrative data in recording sepsis: a systematic review. Crit Care. 2015;19:139. doi: 10.1186/s13054-015-0847-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iwashyna TJ, Odden A, Rohde J, Bonham C, Kuhn L, Malani P, Chen L, Flanders S. Identifying patients with severe sepsis using administrative claims: patient‐level validation of the angus implementation of the international consensus conference definition of severe sepsis. Med Care. 2014;52:e39–e43. doi: 10.1097/MLR.0b013e318268ac86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Duke GJ, Moran JL, Santamaria JD, Pilcher DV. Sepsis. Lancet. 2020;396:1805. doi: 10.1016/S0140-6736(20)31606-8 [DOI] [PubMed] [Google Scholar]
- 25. Wu MH, Tsou PY, Wang YH, Lee MG, Chao CCT, Lee WC, Lee SH, Hu JR, Wu JY, Chang SS, et al. Impact of post‐sepsis cardiovascular complications on mortality in sepsis survivors: a population‐based study. Crit Care. 2019;23:293. doi: 10.1186/s13054-019-2579-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Walker AMN, Drozd M, Hall M, Patel PA, Paton M, Lowry J, Gierula J, Byrom R, Kearney L, Sapsford RJ, et al. Prevalence and predictors of sepsis death in patients with chronic heart failure and reduced left ventricular ejection fraction. J Am Heart Assoc. 2018;7:e009684. doi: 10.1161/JAHA.118.009684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Walley KR. Sepsis‐induced myocardial dysfunction. Curr Opin Crit Care. 2018;24:292–299. doi: 10.1097/MCC.0000000000000507 [DOI] [PubMed] [Google Scholar]
- 28. Vallabhajosyula S, Jentzer JC, Geske JB, Kumar M, Sakhuja A, Singhal A, Poterucha JT, Kashani K, Murphy JG, Gajic O, et al. New‐onset heart failure and mortality in hospital survivors of sepsis‐related left ventricular dysfunction. Shock. 2018;49:144–149. doi: 10.1097/SHK.0000000000000952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taylor SP, Chou SH, Sierra MF, Shuman TP, McWilliams AD, Taylor BT, Russo M, Evans SL, Rossman W, Murphy S, et al. Association between adherence to recommended care and outcomes for adult survivors of sepsis. Ann Am Thorac Soc. 2020;17:89–97. doi: 10.1513/AnnalsATS.201907-514OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen M, Ji M, Si X. The effects of statin therapy on mortality in patients with sepsis: a meta‐analysis of randomized trials. Medicine (Baltimore). 2018;97:e11578. doi: 10.1097/MD.0000000000011578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Singer M, Deutschman CS, Seymour CW, Shankar‐Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The third international consensus definitions for sepsis and septic shock (Sepsis‐3). JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S15
Figure S1


