Abstract
Background
Prior research suggests an association between clinical outcomes in heart failure (HF) and social determinants of health (SDoH). Because providers should identify and address SDoH in care delivery, we evaluated how SDoH have been defined, measured, and evaluated in studies that examine HF outcomes.
Methods and Results
Following Preferred Reporting Items for Systematic Reviews and Meta‐Analysis guidelines, databases were searched for observational or interventional studies published between 2009 and 2021 that assessed the influence of SDoH on outcomes. Selected articles were assessed for quality using a validated rating scheme. We identified 1373 unique articles for screening; 104 were selected for full‐text review, and 59 met the inclusion criteria, including retrospective and prospective cohort, cross‐sectional, and intervention studies. The majority examined readmissions and hospitalizations (k=33), mortality or survival (k=29), and success of medical devices and transplantation (k=8). SDoH examined most commonly included race, ethnicity, age, sex, socioeconomic status, and education or health literacy. Studies used a range of 1 to 9 SDoH as primary independent variables and 0 to 7 SDoH as controls. Multiple data sources were employed and frequently were electronic medical records linked with national surveys and disease registries. The effects of SDoH on HF outcomes were inconsistent because of the heterogeneity of data sources and SDoH constructs.
Conclusions
Our systematic review reveals shortcomings in measurement and deployment of SDoH variables in HF care. Validated measures need to be prospectively and intentionally collected to facilitate appropriate analysis, reporting, and replication of data across studies and inform the design of appropriate, evidence‐based interventions that can ameliorate significant HF morbidity and societal costs.
Keywords: heart failure, social determinants of health, systematic review
Subject Categories: Heart Failure, Lifestyle, Race and Ethnicity, Risk Factors, Aging
Nonstandard Abbreviations and Acronyms
- EMR
electronic medical record
- SDoH
social determinants of health
Clinical Perspective.
What Is New?
The association between social determinants of health (SDoH) and heart failure outcomes has been extensively studied, yet the findings from this systematic review suggest that substantial gaps exist in our understanding of the ways in which SDoH influence heart failure outcomes.
The wide variability of SDoH constructs and proxy measures used across studies may account for the differences in reported results.
What Are the Clinical Implications?
The use of validated SDoH measures that are standardized across studies is necessary to inform the design of evidence‐based interventions that support patient‐centered management of heart failure.
Future research should focus on prospectively and intentionally collecting SDoH data in a way that facilitates appropriate analysis, reporting, and replication of data across heart failure studies.
Heart failure (HF) is a growing public health epidemic that is expected to affect >8 million Americans aged ≥18 years and cost the US health system nearly $69.7 billion by 2030. 1 , 2 Although the major clinical risk factors for HF are well known, 3 , 4 the prevalence of these factors vary based on social determinants of health (SDoH). SDoH, defined as the conditions in which people are born, live, learn, work, play, worship, and age, are generally grouped into the following 5 domains: economic stability, education access and quality, healthcare access and quality, neighborhood and built environment, and social and community context. 5 For example, economic instability (eg, poverty, unemployment) is associated with reduced ability to afford the resources needed to manage HF, such as healthy foods, stable housing, and working utilities. Poverty is also associated with increased toxic stress and related adverse health effects (eg, high blood pressure, inflammation). 5 Poor education is associated with limited health literacy and numeracy, skills needed to self‐manage HF. 5 Lack of access to high‐quality health care is associated with limited receipt of timely preventive care and treatment for chronic illness. 5 Neighborhoods with unsafe air or water or limited access to parks and sidewalks are not only associated with greater exposure to health and safety risks but also reduced opportunities for physical activity. 5 Social and community context, characteristics of the relationships among people and the settings in which they interact, are associated with mental health outcomes, health behaviors, morbidity, and mortality. Supportive social networks, for example, are associated with reduced depression and anxiety, improved ability to cope with health issues, transportation and caregiving support, and overall better health. Social cohesion and civic engagement are associated with reducing the negative health impacts of structural racism, discrimination, and implicit bias (eg, stress, depression). 5 These upstream SDoH are factors, in addition to traditional physiological characteristics, that are associated with the risk that a person will be adversely affected by a wide range of downstream health outcomes. 6 , 7 Prior research suggests that SDoH may contribute ≈30% to 55% to health outcomes (World Health Organization, 2021) 8 and be a direct cause of acute exacerbations of chronic diseases by influencing lifestyle, behavior, stress, and environmental exposures. 9 The American Heart Association has highlighted the importance of focusing on individual‐ and neighborhood‐level SDoH in facilitating self‐care and navigation across the healthcare system and in developing interventions to improve the health outcomes of patients with HF. 10
During the past decade, US policy makers have accelerated attempts to address adverse HF outcomes, such as mortality, readmissions, and higher costs, through reimbursement models that link Medicare and Medicaid payments to outcomes (eg, Hospital Readmissions Reduction Program, Accountable Care Organizations). In response to this pressure, providers are increasingly exploring models of care that address both clinical risk factors and SDoH for patients with HF. Impediments to such efforts include challenges in measuring and evaluating the influence of SDoH in HF care and, subsequently, developing risk‐stratification models that address or control for the influence of SDoH on HF outcomes. Such information can be used in US health policy and healthcare decision making to improve risk adjustment in value‐based care models and develop and evaluate evidence‐based interventions that improve HF care.
Therefore, although a top‐level summary of SDoH data was performed in the American Heart Association's Scientific Statement, we conducted a systematic and critical review using Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) guidelines and recent findings relevant to US settings. The objective of this study was to summarize how SDoH have been included, defined, measured, and evaluated in studies that examine outcomes in HF. Our specific aims were to (1) characterize the constructs of SDoH included in research that examines HF outcomes and (2) summarize the current state of evidence about the influence of SDoH on HF outcomes.
METHODS
As a systematic review based on data from published studies, this work does not require approval from an ethical standards committee. The authors declare that all supporting data are available in the article (and its online supplementary files). The protocol for this review is consistent with the PRISMA 11 and the PRISMA Equity 2012 extension 12 statements. Inclusion and exclusion criteria, information sources, and data collection procedures were defined a priori.
Search Strategy and Study Selection
A research librarian searched PubMed, Cumulative Index to Nursing and Allied Health Literature, Scopus, ABI/INFORM, and Web of Science for articles that met the inclusion criteria and were published from January 2009 through March 2021. The start date was selected to align with the beginning of national policy discussions about healthcare reform and the Affordable Care Act. In general, the following words and related Medical Subject Headings terms were used to search relevant databases: “heart failure,” “hospitalization,” “hospital readmission,” “emergency service,” “mortality,” “complications,” “predict,” and “factor.” The full search strategy for each database is detailed in Table S1. The search retrieved observational and interventional studies that focused on patients with HF and had the primary or secondary objective of examining or addressing SDoH risk factors related to adverse HF outcomes. The reference lists of relevant systematic reviews and gray literature were searched manually for additional articles.
To be eligible for inclusion, studies must have been available as a full‐text article, written in English, and published in a peer‐reviewed journal. In addition, the studies must have been original investigations, reported quantitative data, and featured documentation and discussion of the influence of SDoH on HF outcomes. Results could be reported at the patient, hospital, or community level. We excluded studies in which SDoH were used only to adjust for potentially confounding effects in statistical models, with no discussion of their association with HF outcomes. We limited our review to studies conducted in US populations considering important differences between the United States and other countries in healthcare and social welfare policies and infrastructures. Reviews, editorials, and opinion articles were also excluded.
A total of 2 reviewers independently screened all titles and abstracts for eligibility based on the inclusion and exclusion criteria. Following this procedure, the full texts of articles eligible for further review were examined in detail. The reviewers were not blinded to the authors, journals, or funding sources. Disagreements regarding eligibility were resolved by consensus or intervention of a third team member.
Data Extraction and Quality Assessment
Data from included studies were abstracted by at least 2 reviewers, independently and in duplicate, using a standardized electronic form developed for this review. The abstracted data included study type (observational: retrospective, prospective, cross‐sectional, or intervention), geographic location, population (sample size and unique characteristics), data sources, measured outcomes, SDoH predictors, intervention characteristics (if applicable), and results. Study authors were not contacted for additional data. Results are reported for adjusted models only. For cases in which multiple cohorts were studied (eg, acute myocardial infarction or community‐acquired pneumonia in addition to HF), we limited the reporting of results to the HF cohort only. CIs are reported at α=0.05 unless otherwise indicated. Results were not analyzed across studies because of the heterogeneity of the interventions, study designs, and outcomes.
A total of 2 reviewers independently assessed study quality using a modified Oxford Centre for Evidence‐Based Medicine rating scheme and evaluated for interrater comparability. Using this methodology, studies were rated 1 (highest quality) to 5 (lowest quality). Studies receiving the highest rating were properly powered and conducted randomized controlled trials, well‐designed controlled trials without randomization or prospective comparative cohort trials were rated 2, case control and retrospective cohort studies were rated 3, case series and cross‐sectional studies were rated 4, and opinion and case reports (rating 5) were not included in this review. Disagreements were resolved by consensus.
RESULTS
Study Characteristics
The search identified 1373 unique records for title/abstract screen; 104 were selected for full‐text review. The PRISMA study flow diagram summarizing the article screening process is depicted in Figure S1. In total, 59 articles met the criteria for inclusion (Table S2). 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 The studies were published from 2009 to 2021 and included observation periods that spanned from 1987 to 2018.
The study characteristics are summarized in Table 1. These included retrospective cohort (k=30), prospective cohort (k=19), cross‐sectional (k=5), and intervention (k=5) studies. A total of 24 studies evaluated nationally representative samples of patients with HF, whereas others were conducted in regional or state urban/suburban (k=25), urban/suburban and rural (k=8), or rural only (k=2) hospitals or clinics. The studies used multiple data sources, including electronic medical records (EMRs; k=25), national surveys (k=16), research studies (k=14), Medicare claims (k=13), patient questionnaires (k=10), disease registries (k=8), state or national death indices (k=6), and health exchanges or administrative data (k=6). Only 1 study analyzed state‐level data, 55 whereas all other studies analyzed patient‐level data (k=58). The primary outcomes measured were mortality or survival (k=29), readmissions (k=33), and medical device and transplantation use and complications (k=8). Other use outcomes were measured less frequently (eg, hospital length of stay, emergency department visits, postdischarge follow‐up visits).
Table 1.
Characteristics of Included Studies (k=59)
| Characteristic | No. (%)* | Studies |
|---|---|---|
| Study type | ||
| Retrospective cohort | 30 (51) | 13, 17, 18, 19, 21, 22, 25, 26, 27, 28, 29, 30, 38, 40, 41, 43, 46, 47, 49, 52, 55, 56, 57, 64, 65, 66, 70, 71 |
| Prospective cohort | 19 (32) | 14, 15, 20, 23, 31, 32, 33, 39, 42, 44, 48, 50, 51, 53, 58, 59, 63, 67, 68, 69 |
| Cross‐sectional | 5 (8) | 34, 36, 54, 61, 62 |
| Intervention | 5 (8) | 16, 24, 35, 36, 37, 45, 60 |
| Study setting | ||
| National | 24 (41) | 18, 21, 22, 23, 26, 27, 30, 34, 38, 40, 41, 47, 48, 49, 53, 55, 56, 59, 61, 62, 63, 66, 70, 71 |
| Regional or state | ||
| Urban | 25 (42) | 13, 15, 16, 17, 20, 24, 25, 28, 31, 32, 33, 35, 36, 37, 42, 43, 45, 46, 51, 52, 54, 57, 58, 60, 65 |
| Urban/rural | 8 (14) | 14, 29, 39, 44, 50, 64, 67, 69 |
| Rural | 2 (3) | 19, 68 |
| Level of analysis | ||
| Patient | 58 (98) | 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71 |
| State | 1 (2) | 55 |
| Data source† | ||
| Electronic medical record | 25 (42) | 13, 15, 16, 17, 19, 24, 25, 28, 33, 39, 40, 43, 45, 46, 51, 52, 53, 54, 57, 58, 59, 60, 62, 65, 69 |
| National surveys | 16 (27) | 13, 14, 26, 27, 29, 32, 47, 50, 55, 56, 59, 61, 62, 70, 71 |
| Study data | 14 (24) | 14, 16, 18, 31, 32, 35, 36, 39, 42, 46, 53, 59, 67, 68 |
| Medicare claims | 13 (22) | 22, 23, 26, 27, 38, 39, 41, 47, 55, 56, 59, 62, 63 |
| Patient questionnaires | 10 (17) | 15, 20, 24, 33, 35, 37, 42, 44, 51, 58 |
| Disease registries | 8 (14) | 13, 21, 27, 30, 41, 48, 63, 64 |
| Data exchange and/or administrative data | 6 (10) | 42, 44, 49, 50, 66, 69 |
| Death index | 6 (10) | 28, 34, 40, 43, 57, 58 |
| Other data† | 7 (12) | 39, 42, 53, 54, 55, 56, 69 |
| Outcomes analyzed | ||
| Mortality or survival | 29 (49) |
30 d 15 , 26 , 27 , 28 , 40 , 52 , 56 , 57 , 62 , 63 60 d 57 1 y 63 Other 21 , 30 , 32 , 34 , 35 , 39 , 42 , 44 , 46 , 51 , 57 , 58 , 62 , 64 , 65 , 70 |
| Readmissions | 33 (56) | 13, 15, 16, 17, 19, 20, 21, 22, 23, 24, 26, 27, 28, 32, 33, 35, 38, 40, 43, 45, 46, 47, 49, 50, 52, 55, 56, 57, 60, 61, 63, 65, 66 |
| Medical device or transplantation, including complications | 8 (14) | 13, 21, 29, 30, 31, 48, 61, 70 |
| Other‡ | 26 (44) | 13, 40, 63, 64, 70, 71 |
Percentages may not equal 100% because of rounding.
Percentages may not equal 100% because of rounding or inclusion of >1 data source. Other data sources include patient and family interviews (k=5), Kaiser Family Foundation reports (k=1), Dartmouth Atlas (k=1), content analysis of obituaries (k=1), and medication event monitoring system (k=1).
Other use included postdischarge follow‐up (k=5), 22 , 24 , 41 , 64 , 66 length of stay (k=5), 13 , 40 , 63 , 64 , 70 hospitalizations (k=5), 18 , 44 , 68 , 71 emergency department visits (k=4), 20 , 22 , 44 , 46 , 53 financial penalties or costs (k=2), self‐care (k=2), evidence‐based care processes (k=2), and hospice (k=1).
Social Determinants of Health
The frequencies of SDoH examined as primary and control factors in the studies are illustrated in the Figure and referenced in Table S3. On average, the 59 studies examined 3.5 SDoH as primary regressors of interest (SD, 2.1; range, 1–9) and controlled for 2.5 sociodemographic factors (SD, 2.1; range, 0–7). The SDoH most commonly examined were age (k=55), race and ethnicity (k=55), and sex (k=54). A majority of studies examined multiple socioeconomic status (SES) constructs as primary contributors to HF outcomes, including insurance status (k=27), income or wealth (k=14), education or health literacy (k=13), employment (k=2), urban/rural residence (k=9), social instability (k=3), and composite measures of individual‐ or neighborhood‐level SES (k=12). For example, studies measured social (in)stability by calculating the number of home address or zip code changes by an individual or in a neighborhood and measured neighborhood SES using the Area Deprivation Index or median household income. Social support was measured directly through self‐report (k=8) and via proxy: marital status (k=11), living status (k=6), and children (k=1). The cultural constructs of language (k=3) or spirituality (k=3) were also examined. In total, 19 studies used a validated scale to measure specific SDoH domains (Table S4).
Figure . Summary of SDoH analyzed in the studies (k=59).
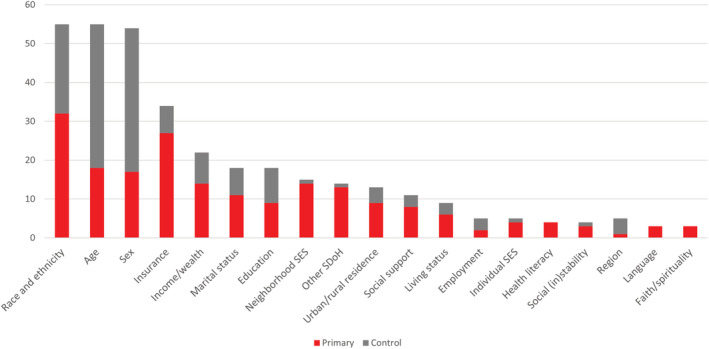
Other SDoH: access to care (k=3), veteran priority group (k=2), other psychosocial risk (k=2), cumulative SDoH burden (k=2), children (k=1), SHS exposure (k=1), hospice (k=1), public health infrastructure (k=1), and self‐efficacy (k=2). SDoH indicates social determinants of health; SES, socioeconomic status; and SHS, second hand smoke.
Association Between SDoH and HF Outcomes
Mortality or Survival
The included studies measured mortality rate as the percentage of people in the observation group who died of HF during a specific period of time (eg, 30 days, 60 days, 1 year); the survival rate measured the percentage of people in the group who were still alive for a specific period of time after they were diagnosed with or started treatment for HF. The 29 studies that examined mortality or survival are presented in Table 2. In these studies, Black race (compared with White race) was associated with both decreased 26 , 27 , 28 , 63 and increased 52 , 59 , 70 mortality or survival; similarly, Hispanic ethnicity (compared with non‐Hispanic White ethnicity) was associated with decreased 27 , 63 and increased mortality risk. 52 , 59 , 70 Older age (compared with younger age groups) was associated with increased HF mortality risk in several studies, 42 , 43 , 46 , 51 whereas younger (compared with older) age was associated with increased in‐hospital mortality risk in a study examining cardiogenic shock unrelated to acute coronary syndrome. 70 In 1 study, no significant association between age and mortality was found. 28 In 2 studies, female sex (compared with male sex) was associated with increased 70 and decreased 43 mortality risk; in 2 other studies, no significant association was found. 28 , 44 Although 1 study found that having an educational attainment of high school or less (compared with at least some college) and lower (compared with higher) health literacy were associated with increased mortality risk, 46 education was not significantly associated with mortality risk in 4 studies. 13 , 27 , 56 , 59 Primary language spoken at home was not significantly associated with mortality risk. 28 No significant association between insurance status and HF mortality risk was observed, 13 , 28 , 32 , 42 , 64 with the exception of 1 study, where lack of insurance was associated with decreased mortality risk. 42 Marital status was an inconsistent influence: being married was associated with increased 58 or decreased mortality risk 43 ; in 2 studies, 13 , 65 no significant association between marital status and mortality risk was found. Faith/spirituality was associated with decreased mortality risk, 51 whereas a lack of faith identification was associated with increased mortality risk. 40 In 3 studies, no significant association between neighborhood SES and mortality was observed 28 , 40 , 42 ; in 1 study, it was associated with increased HF mortality risk. 38 Compared with limited access, greater access to cardiology services was also associated with decreased HF mortality risk. 57
Table 2.
Effect of Social Determinants of Health on Mortality or Survival, k=29 Studies
| Social determinants of health | Increased risk | Decreased risk | No statistically significant association found |
|---|---|---|---|
| Black race | 52, 59, 70 | 26, 27, 28, 63 | … |
| Hispanic ethnicity | 52, 59, 70 | 27, 63 | … |
| Age |
Younger 70 |
… | 28 |
| Sex | 70 | 43 | 28, 44 |
| Education | Low 46 | … | 13, 27, 56, 59 |
| Language | … | … | 28 |
| Insurance | … | Lack 42 | 13, 28, 32, 64 |
| Married marital status | 58 | 43 | 13, 65 |
| Faith/spirituality | Lack 40 | 51 | … |
| Neighborhood socioeconomic status | 38 | … | 28, 40, 42 |
| Access to cardiology | … | 57 | … |
Readmissions
The studies examined hospital readmissions as an unplanned episode in which a patient who had been discharged from a hospital is admitted again in a certain period of time (eg, 30, 60, 90 days) after the index admission for HF. The 33 studies that examined readmissions are presented in Table 3. Factors associated with increased readmissions included Black race 22 , 23 , 26 , 49 , 52 , 63 and Hispanic ethnicity 22 , 63 (compared with other or unknown 23 or Asian, Hawaiian or Pacific Islander, Native American, other, or mixed 56 race and ethnicity), Medicare 15 , 22 , 52 and Medicaid 32 (compared with private insurance), male sex (compared with female sex), 15 older age (compared with younger age), 23 unmarried (compared with married), 15 or disabled (compared with those without a disability). 17 , 38 Additional factors included poverty 38 or lower neighborhood‐level income (compared with higher income), 32 low neighborhood SES (compared with medium and high income), 38 , 50 higher (compared with lower) proportion of neighborhood‐level white collar workers, 27 social (housing) instability (compared with higher stability), 15 , 38 education (high school or less compared with more than high school), 27 and low (compared with high) social support. 21 Factors associated with decreased readmissions included several from the preceding list, including Black race 23 or Non‐White race (Asian, Hawaiian or Pacific Islander, Native American, other, or mixed) race (compared with White race) 56 and older age (compared with younger age). 28 , 49 Others included private (compared with public) 22 , 49 , 61 or no insurance (compared with private or public insurance), 61 female sex (compared with male sex), 23 , 49 married (compared with unmarried), independently living with family (compared with living alone or in a nursing facility), 43 and having children (compared with no children). 47 Educational attainment of a bachelor's degree or higher (compared with high school or less), 56 higher (compared with lower) neighborhood‐level income, 47 , 56 and access to cardiology 28 , 57 , 60 or navigation services (compared with limited or no access to these services) were also associated with decreased readmissions. 16 , 24 , 45 In several studies, some of these same factors, as well as others, were not associated with readmissions: individual‐level 17 , 19 , 27 , 28 , 47 , 57 or state‐level race and ethnicity 55 ; insurance status, 13 , 17 including Medicaid 22 , 47 ; sex 17 , 19 , 22 , 55 , 61 ; age 17 , 19 , 22 , 43 , 61 ; marital status 13 , 17 , 19 , 43 , 47 , 65 ; living status 19 , 47 ; individual‐level 13 and neighborhood‐level income 26 , 27 , 56 ; neighborhood‐level SES 15 , 40 ; neighborhood‐level home value 27 ; urban or rural residence 13 , 28 , 40 ; education 13 ; language 19 ; faith/spirituality 40 ; and low health literacy. 46
Table 3.
Effect of Social Determinants of Health on Readmissions, k=33 Studies
| Social determinants of health | Increased risk | Decreased risk | No statistically significant association found |
|---|---|---|---|
| Black race | 22, 23, 26, 49, 52, 63 |
Black race 23 Non‐White race (Asian, Hawaiian or Pacific Islander, Native American, other, or mixed) 56 |
… |
| Hispanic ethnicity | 22, 63 | … |
Individual 17 , 19 , 27 , 28 , 47 , 57 State level 55 |
| Insurance |
Medicaid 32 |
No 61 |
|
| Sex | Male 15 | Female 23 , 49 | 17, 19, 22, 55, 61 |
| Older age | 23 | 28, 49 | 17, 19, 22, 43, 61 |
| Marital status | Unmarried 15 | Married 43 | 13, 17, 19, 43, 47, 65 |
| Disabled | 17,38 | … | … |
| Poverty | 38 | … | … |
| Neighborhood income | Low 32 | High 47 , 56 | 26, 27, 56 |
| Neighborhood socioeconomic status | 38, 50 | … | 15, 40 |
| Neighborhood‐level white collar workers | 27 | … | … |
| Social instability | 15, 38 | … | … |
| Education | High school or less 27 | Bachelor's or higher 56 | 13 |
| Social support | 21 | … | … |
| Children | … | 47 | … |
| Access to cardiology | … | 16, 24, 45 | … |
| Living status | … | … | 19, 47 |
| Individual income | … | … | 13 |
| Neighborhood‐level home value | … | … | 27 |
| Urban or rural | … | … | 13, 28, 40 |
| Language | … | … | Individual 19 |
| Faith/spirituality | … | … | 40 |
| Health literacy | … | … | 46 |
| State‐level language | 55 | … | … |
| State‐level median household income | 55 | … | … |
In 1 state‐level study, states with greater proportions of residents speaking a primary language other than English at home (compared with speaking English) were associated with decreased probability of ranking “worse” on readmissions compared with other states. States with higher median incomes were associated with increased probability to be “worse” than the US national rate. 55
Medical Devices or Transplantation
A total of 8 studies examined implantation of manufactured medical devices, including a continuous flow left ventricular assist device as bridge‐to‐transplant or destination therapy for patients with HF, implantable cardioverter‐defibrillator implantation, mechanical circulatory support devices, or heart transplantation (Table 4). Some studies also examined complications related to these procedures, such as device‐related infection, gastrointestinal bleeding, device thrombosis, stroke, or any cause rehospitalization. In these studies, younger age (compared with older age), 70 male sex (compared with female sex), 30 , 48 , 70 and state Medicaid expansion (compared with nonexpansion) 29 were associated with increased probability of device implantation or transplantation. Black race (compared with White race), 30 , 48 older age (compared with younger age), 48 female sex (compared with male sex), 61 and being uninsured (compared with public or private insurance) 48 were associated with decreased probability of device implantation or transplantation. In 6 studies, race and ethnicity, 29 , 31 , 70 age, 61 education, 13 , 30 , 31 income, 13 , 31 insurance status, 13 , 30 , 31 , 61 urban/rural residence, 13 and neighborhood‐level SES 30 were not significantly associated with medical device implantation or transplantation.
Table 4.
Effect of Social Determinants of Health on Medical Devices or Transplantation, k=8 Studies
| Social determinants of health | Increased risk | Decreased risk | No statistically significant association found |
|---|---|---|---|
| Age | Younger 70 | Older 48 | 61 |
| Sex | Male 30 , 48 , 70 | Female 61 | … |
| State Medicaid expansion | 29 | … | … |
| Black race | … | 30, 48 | … |
| Uninsured | … | 48 | 13, 30, 31, 61 |
| Race and ethnicity | … | … | 29, 31, 70 |
| Education | … | 13, 30, 31 | |
| Income | … | … | 13, 31 |
| Urban/rural | … | … | 13 |
| Neighborhood‐level socioeconomic status | … | … | 30 |
Other Outcomes
Other outcomes examined with similar variability in the findings included hospital length of stay 13 , 40 , 63 , 64 , 70 ; hospitalizations other than readmissions, such as index hospitalizations for HF (eg, hospitalization for which the International Classification of Diseases Ninth Revision [ICD‐9] clinical modification discharge code for HF was used as the first listed discharge code) 18 , 44 , 68 , 71 ; emergency department visits not resulting in hospitalization 20 , 22 , 44 , 46 , 53 ; and postdischarge planned follow‐up visits, such as primary care visits in a certain time frame. 22 , 24 , 41 , 64 , 66
Quality of Evidence
Of the 59 studies, 1 was rated as 1 (randomized clinical trial), 22 were rated 2 (well‐designed controlled trials without randomization, prospective comparative cohort trials), 30 were rated 3 (case‐control studies, retrospective cohort studies), and 6 were rated 4 (case series with or without intervention, cross‐sectional studies).
DISCUSSION
This systematic review of HF literature suggests that substantial gaps exist in our understanding of the ways in which SDoH are measured and the relationship of SDoH to HF outcomes. Of 59 studies meeting criteria, including data derived from retrospective and prospective cohorts, cross‐sectional designs, and interventions, the majority examined mortality/survival and hospital readmissions. Studies used a wide range of SDoH as primary independent variables and as controls, with data derived from multiple sources, often EMRs linked with national surveys, disease registries, or data from large national studies. SDoH most commonly evaluated included race and ethnicity, age, and sex, whereas socioeconomic status, social support, educational status, and health literacy were assessed less frequently. However, we found that studies investigating the influence of SDoH on HF outcomes produced disparate results. Consistent with a systematic review of articles published between 1981 and 2008, 72 this finding highlights the need for more targeted research to clarify the impact of SDoH on HF, particularly in light of the rapid evolution of SDoH data collection and use during the past 25 years. Indeed, the heterogeneity of study designs, target populations, SDoH constructs, covariates used to adjust the statistical models, and HF outcomes in published studies are underlying reasons for differences in the reported results. However, recent imperatives, such as the proliferation of value‐based payment models and Medicare readmissions penalties, drive the need to better understand and address SDoH in conjunction with clinical risk factors for patients with HF and other chronic diseases.
Specifically, many of the measured SDoH lacked construct validity. For example, in assessing social support and its relationship to HF readmissions, proxy EMR variables were used, including marital status and whether the patient had a child. The degree to which these proxies have been validated is not clear. Few studies used accepted measures of social support or social isolation. Because variables listed as SDoH may be poor proxies for SDoH constructs, they may not be well suited for understanding potentially policy‐responsive effects of SDoH on HF outcomes. For example, race and ethnicity were the most commonly included SDoH. Although these variables are easy to measure and routinely included in EMRs and disease registries, other variables potentially more relevant to poor health outcomes exist (eg, racism, discrimination, provider uncertainty, implicit bias) and can be measured using validated instruments and approaches (eg, Everyday Discrimination Scale, Experiences of Discrimination Measure, Patient Experience Measures from the Consumer Assessment of Healthcare Providers & Systems Clinician & Group Survey). Our results are also consistent with recent studies that call for greater clarity in conceptualizing and more specificity in measuring SDoH to support improved clinical and shared decision making and patient self‐care. 10 , 73 , 74 Moreover, our findings regarding the inconsistent definitions and lack of construct validity in SDoH measures help to explain, in part, the differences in results across studies that investigate the effect of SDoH on HF mortality and readmissions. 75 , 76 The use of standardized SDoH measures and covariates across studies could help to produce more consistent results and enable researchers to conduct robust meta‐analyses.
The heterogeneity of data sources and SDoH constructs employed across studies may also explain the lack of consistent results. Most studies were secondary, retrospective analyses of EMRs, which may have data quality issues and, more important, were not designed to specifically address SDoH. Furthermore, most studies focus on readmissions and mortality, which are publicly reported measures, rather than on the relationship between SDoH, processes of care (eg, length of stay, follow‐up visits), and patients' self‐reported outcomes. Indeed, researchers and policy makers use what is available, and the focus on collecting and documenting SDoH in EMRs and other data repositories is relatively new. However, some social constructs (such as language and spirituality) may be important but difficult to assess and include in studies. Conversely, larger‐scale community factors (eg, county characteristics) are measured in more standardized ways and are publicly available.
Another potentially important finding is that significant gaps remain in our understanding of how various pathways, and interactions among various SDoH in those pathways, may explain differences in results regarding specific SDoH across studies. Although some studies focused on a target population or reported stratified results based on a specific SDoH (eg, race and ethnicity), none of the studies explored interactions between SDOH domains to examine the potential role of intersectionality on HF outcomes. For example, race by sex or race by income are potentially important interactions that warrant investigation. These overlapping socioeconomic identities may combine to exacerbate or ameliorate discrimination or privilege. Such knowledge would help to disentangle the degree to which specific SDoH mediate or moderate outcomes or whether the cumulative or intersectional impact of SDoH explain greater, lesser, or no effect of SDoH on HF outcomes.
Our systematic review has limitations that might limit generalizability. We purposefully did not include studies outside the United States. Although these studies may give additional insight, differences in lifestyles, behaviors, social structure, and healthcare delivery systems limit their applicability to the US setting. Furthermore, in the United States, there has been increased attention paid to SDoH in large part because of the recognition of health inequities in the American healthcare system. Our search may have excluded studies that do not identify variables as SDoH. Despite our efforts to identify a comprehensive set of articles, it is possible that our search missed relevant studies that fit our inclusion criteria. We attempted to minimize this possibility by reviewing reference lists of included articles and related systematic reviews. We did not contact the study authors, so we may have missed some information. Publication and potential researcher bias in establishing study inclusion criteria may also have been present in our study.
In conclusion, a critical and systematic assessment of studies reporting on the influence of SDoH on HF outcomes reveals significant shortcomings in the measurement and deployment of SDoH variables. Although the problem may be generic and not specific to HF, additional investigations may be warranted (eg, in diabetes care) to identify standardized measures that can be applied across conditions and studies.
Given the limitations of EMRs for measuring SDoH, which may have constrained studies in the past, significant redesign may be required not only in its structure but also in the facilitation of data collection by providers. Ultimately, validated SDoH measures need to be prospectively and intentionally collected in a way that is analyzable, reportable, and actionable in health policy and healthcare decision making. A key challenge will be to figure out how SDoH modules in EMRs can be individualized by disease state and can be incorporated in a way that will not disturb current workflows. We suggest that 1 way to move forward will be to launch a series of demonstration projects using a relatively parsimonious list of key variables that are selected based on expert consensus opinion. These variables should go beyond the demographic variables commonly used to adjust for confounding effects in statistical models (eg, age, sex, race and ethnicity) to include variables, and interactions between variables, for which the demographic variables may serve as proxies. We also recommend that researchers clearly define SDoH variables and provide a rationale for their use, particularly in cases when proxies for SDoH are included. We argue that having common definitions and an understanding of SDoH will reduce the variability in the findings of future studies. The data derived from such projects will facilitate the design of appropriate risk adjustment models and interventions that can effectively influence outcomes, thereby supporting the dissemination and implementation of evidence‐based care and providing additional meaning to the concept of patient‐centered management of HF.
Sources of Funding
None.
Disclosures
None.
Supporting information
Tables S1–S4
Figure S1
Acknowledgments
Enard and Hauptman had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Enard and Hauptman contributed to the concept and design. Enard, Coleman, Yakubu, Butcher, Tao, and Hauptman contributed to the acquisition, analysis, or interpretation of the data. Enard, Coleman, Yakubu, and Hauptman drafted the manuscript. Enard, Coleman, Yakubu, Tao, and Hauptman critically revised the manuscript for important intellectual content.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.026590
This work was presented at the American Heart Association's Quality of Care & Outcomes Research Scientific Sessions, May 14–15, 2021.
For Sources of Funding and Disclosures, see page 9.
References
- 1. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart disease and stroke statistics‐2021 update: a report from the American Heart Association. Circulation. 2018;143:e254–e743. doi: 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 2. Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roger VL. Epidemiology of heart failure. Circ Res. 2013;113:646–659. doi: 10.1161/CIRCRESAHA.113.300268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. He J, Zhu Z, Bundy JD, Dorans KS, Chen J, Hamm LL. Trends in cardiovascular risk factors in US adults by race and ethnicity and socioeconomic status, 1999‐2018. JAMA. 2021;326:1286–1298. doi: 10.1001/jama.2021.15187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. U.S. Department of Health and Human Services Office of Disease Prevention and Health Promotion . Healthy People 2030. https://health.gov/healthypeople/objectives‐and‐data/social‐determinants‐health. Accessed March 30, 2021. [PubMed]
- 6. Centers for Disease Control and Prevention . Social Determinants of Health: Know What Affects Health. https://www.cdc.gov/socialdeterminants/index.html. Accessed March 30, 2021.
- 7. Enard KR, Hauptman PJ. Heart failure, shared decision‐making, and social determinants of health: an upstream perspective. JAMA Cardiol. 2019;4:609–610. doi: 10.1001/jamacardio.2019.1763 [DOI] [PubMed] [Google Scholar]
- 8. World Health Organization . Social Determinants of Health. https://www.who.int/health‐topics/social‐determinants‐of‐health#tab=tab_1. Accessed October 06, 2012.
- 9. Cockerham WC, Hamby BW, Oates GR. The social determinants of chronic disease. Am J Prev Med. 2017;52:S5–S12. doi: 10.1016/j.amepre.2016.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. White‐Williams C, Rossi LP, Bittner VA, Driscoll A, Durant RW, Granger BB, Graven LJ, Kitko L, Newlin K, Shirey M. Addressing social determinants of health in the care of patients with heart failure: a scientific statement from the American Heart Association. Circulation. 2020;141:e841–e863. doi: 10.1161/CIR.0000000000000767 [DOI] [PubMed] [Google Scholar]
- 11. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Welch V, Petticrew M, Tugwell P, Moher D, O'Neill J, Waters E, White H. Prisma‐equity 2012 extension: reporting guidelines for systematic reviews with a focus on health equity. PLoS Med. 2012;9:e1001333. doi: 10.1371/journal.pmed.1001333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ahmed M, Magar S, Jeng E, Arnaoutakis G, Beaver T, Vilaro J, Klodell C, Aranda J. Effects of socioeconomic status on clinical outcomes with ventricular assist devices. Clin Cardiol. 2018;41:1463–1467. doi: 10.1002/clc.23070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Akwo E, Kabagambe E, Wang T, Harrell F, Blot W, Mumma M, Gupta D, Lipworth L. Heart failure incidence and mortality in the southern community cohort study. Circ Heart Fail. 2017;10:e003553. doi: 10.1161/CIRCHEARTFAILURE.116.003553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Amarasingham R, Moore BJ, Tabak YP, Drazner MH, Clark CA, Zhang S, Reed WG, Swanson TS, Ma Y, Halm EA. An automated model to identify heart failure patients at risk for 30‐day readmission or death using electronic medical record data. Med Care. 2010;48:981–988. doi: 10.1097/MLR.0b013e3181ef60d9 [DOI] [PubMed] [Google Scholar]
- 16. Asthana V, Sundararajan M, Ackah RL, Karun V, Misra A, Pritchett A, Bugga P, Siler‐Fisher A, Peacock WF. Heart failure education in the emergency department markedly reduces readmissions in un‐ and under‐insured patients. Am J Emerg Med. 2018;36:2166–2171. doi: 10.1016/j.ajem.2018.03.057 [DOI] [PubMed] [Google Scholar]
- 17. Bradford C, Shah BM, Shane P, Wachi N, Sahota K. Patient and clinical characteristics that heighten risk for heart failure readmission. Res Social Adm Pharm. 2017;13:1070–1081. doi: 10.1016/j.sapharm.2016.11.002 [DOI] [PubMed] [Google Scholar]
- 18. Breathett K, Kohler LN, Eaton CB, Franceschini N, Garcia L, Klein L, Martin LW, Ochs‐Balcom HM, Shadyab AH, Cené CW. When the at‐risk do not develop heart failure: understanding positive deviance among post‐menopausal African American and Hispanic women. J Card Fail. 2021;27:217–223. doi: 10.1016/j.cardfail.2020.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carlson B, Hoyt H, Gillespie K, Kunath J, Lewis D, Bratzke LC. Predictors of heart failure readmission in a high‐risk primarily Hispanic population in a rural setting. J Cardiovasc Nurs. 2019;34:267–274. doi: 10.1097/JCN.0000000000000567 [DOI] [PubMed] [Google Scholar]
- 20. Cox SR, Liebl MG, McComb MN, Chau JQ, Wilson AA, Achi M, Garey KW, Wallace D. Association between health literacy and 30‐day healthcare use after hospital discharge in the heart failure population. Res Social Adm Pharm. 2017;13:754–758. doi: 10.1016/j.sapharm.2016.09.003 [DOI] [PubMed] [Google Scholar]
- 21. DeFilippis EM, Breathett K, Donald EM, Nakagawa S, Takeda K, Takayama H, Truby LK, Sayer G, Colombo PC, Yuzefpolskaya M, et al. Psychosocial risk and its association with outcomes in continuous‐flow left ventricular assist device patients. Circ Heart Fail. 2020;13:e006910. doi: 10.1161/CIRCHEARTFAILURE.120.006910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. DeLia D, Tong J, Gaboda D, Casalino LP. Post‐discharge follow‐up visits and hospital utilization by Medicare patients, 2007‐2010. Medicare Medicaid Res Rev. 2014;4:E1–E19. doi: 10.5600/mmrr.004.02.a01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dharmarajan K, Hsieh AF, Lin Z, Bueno H, Ross JS, Horwitz LI, Barreto‐Filho JA, Kim N, Bernheim SM, Suter LG, et al. Diagnoses and timing of 30‐day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355–363. doi: 10.1001/jama.2012.216476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Di Palo KE, Patel K, Assafin M, Piña IL. Implementation of a patient navigator program to reduce 30‐day heart failure readmission rate. Prog Cardiovasc Dis. 2017;60:259–266. doi: 10.1016/j.pcad.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 25. Distelhorst K, Claussen R, Dion K, Bena JF, Morrison SL, Walker D, Tai HL, Albert NM. Factors associated with adherence to 14‐day office appointments after heart failure discharge. J Card Fail. 2018;24:407–411. doi: 10.1016/j.cardfail.2018.04.006 [DOI] [PubMed] [Google Scholar]
- 26. Downing NS, Wang C, Gupta A, Wang Y, Nuti SV, Ross JS, Bernheim SM, Lin Z, Normand ST, Krumholz HM. Association of racial and socioeconomic disparities with outcomes among patients hospitalized with acute myocardial infarction, heart failure, and pneumonia: an analysis of within‐ and between‐hospital variation. JAMA Netw Open. 2018;1:e182044. doi: 10.1001/jamanetworkopen.2018.2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eapen ZJ, McCoy LA, Fonarow GC, Yancy CW, Miranda ML, Peterson ED, Califf RM, Hernandez AF. Utility of socioeconomic status in predicting 30‐day outcomes after heart failure hospitalization. Circ Heart Fail. 2015;8:473–480. doi: 10.1161/CIRCHEARTFAILURE.114.001879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eberly LA, Richterman A, Beckett AG, Wispelwey B, Marsh RH, Cleveland Manchanda EC, Chang CY, Glynn RJ, Brooks KC, Boxer R, et al. Identification of racial inequities in access to specialized inpatient heart failure care at an academic medical center. Circ Heart Fail. 2019;12:e006214. doi: 10.1161/CIRCHEARTFAILURE.119.006214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ehsan A, Zeymo A, McDermott J, Shara NM, Sellke FW, Yousefzai R, Al‐Refaie WB. Utilization of left ventricular assist devices in vulnerable adults across medicaid expansion. J Surg Res. 2019;243:503–508. doi: 10.1016/j.jss.2019.05.015 [DOI] [PubMed] [Google Scholar]
- 30. Emani S, Tumin D, Foraker RE, Hayes D Jr, Smith SA. Impact of insurance status on heart transplant wait‐list mortality for patients with left ventricular assist devices. Clin Transplant. 2017;31. doi: 10.1111/ctr.12875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Flint K, Chaussee EL, Henderson K, Breathett K, Khazanie P, Thompson JS, McIlvennan CK, Larue SJ, Matlock DD, Allen LA. Social determinants of health and rates of implantation for patients considering destination therapy left ventricular assist device. J Card Fail. 2021;27:497–500. doi: 10.1016/j.cardfail.2020.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Foraker RE, Rose KM, Suchindran CM, Chang PP, McNeill AM, Rosamond WD. Socioeconomic status, medicaid coverage, clinical comorbidity, and rehospitalization or death after an incident heart failure hospitalization: atherosclerosis risk in communities cohort (1987 to 2004). Circ Heart Fail. 2011;4:308–316. doi: 10.1161/CIRCHEARTFAILURE.110.959031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gilotra NA, Shpigel A, Okwuosa IS, Tamrat R, Flowers D, Russell SD. Patients commonly believe their heart failure hospitalizations are preventable and identify worsening heart failure, nonadherence, and a knowledge gap as reasons for admission. J Card Fail. 2017;23:252–256. doi: 10.1016/j.cardfail.2016.09.024 [DOI] [PubMed] [Google Scholar]
- 34. He X, Zhao J, He J, Dong Y, Liu C. Association of household secondhand smoke exposure and mortality risk in patients with heart failure. BMC Cardiovasc Disord. 2019;19:280. doi: 10.1186/s12872-019-1269-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heisler M, Halasyamani L, Cowen ME, Davis MD, Resnicow K, Strawderman RL, Choi H, Mase R, Piette JD. Randomized controlled effectiveness trial of reciprocal peer support in heart failure. Circ Heart Fail. 2013;6:246–253. doi: 10.1161/CIRCHEARTFAILURE.112.000147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Irani E, Moore SE, Hickman RL, Dolansky MA, Josephson RA, Hughes JW. The contribution of living arrangements, social support, and self‐efficacy to self‐management behaviors among individuals with heart failure: a path analysis. J Cardiovasc Nurs. 2019;34:319–326. doi: 10.1097/JCN.0000000000000581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Johansson M, Athilingam P. Structured telephone support intervention: improved heart failure outcomes. JMIR Aging. 2020;3:e13513. doi: 10.2196/13513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Joynt Maddox KE, Reidhead M, Hu J, Kind AJH, Zaslavsky AM, Nagasako EM, Nerenz DR. Adjusting for social risk factors impacts performance and penalties in the hospital readmissions reduction program. Health Serv Res. 2019;54:327–336. doi: 10.1111/1475-6773.13133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kaiser P, Allen N, Delaney JAC, Hirsch CH, Carnethon M, Arnold AM, Odden MC. The association of prediagnosis social support with survival after heart failure in the cardiovascular health study. Ann Epidemiol. 2020;42:73–77. doi: 10.1016/j.annepidem.2019.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Knighton AJ, Savitz LA, Benuzillo J, VanDerslice JA. It takes a village: exploring the impact of social determinants on delivery system outcomes for heart failure patients. Healthc (Amst). 2018;6:112–116. doi: 10.1016/j.hjdsi.2017.06.001 [DOI] [PubMed] [Google Scholar]
- 41. Kociol RD, Greiner MA, Fonarow GC, Hammill BG, Heidenreich PA, Yancy CW, Peterson ED, Curtis LH, Hernandez AF. Associations of patient demographic characteristics and regional physician density with early physician follow‐up among Medicare beneficiaries hospitalized with heart failure. Am J Cardiol. 2011;108:985–991. doi: 10.1016/j.amjcard.2011.05.032 [DOI] [PubMed] [Google Scholar]
- 42. Kostelanetz S, Di Gravio C, Schildcrout JS, Roumie CL, Conway D, Kripalani S. Should we implement geographic or patient‐reported social determinants of health measures in cardiovascular patients. Ethn Dis. 2021;31:9–22. doi: 10.18865/ed.31.1.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lu MLR, Davila CD, Shah M, Wheeler DS, Ziccardi MR, Banerji S, Figueredo VM. Marital status and living condition as predictors of mortality and readmissions among African Americans with heart failure. Int J Cardiol. 2016;222:313–318. doi: 10.1016/j.ijcard.2016.07.185 [DOI] [PubMed] [Google Scholar]
- 44. Manemann SM, Chamberlain AM, Roger VL, Griffin JM, Boyd CM, Cudjoe TKM, Jensen D, Weston SA, Fabbri M, Jiang R, et al. Perceived social isolation and outcomes in patients with heart failure. J Am Heart Assoc. 2018;7:e008069. doi: 10.1161/JAHA.117.008069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McKinley D, Moye‐Dickerson P, Davis S, Akil A. Impact of a pharmacist‐led intervention on 30‐day readmission and assessment of factors predictive of readmission in African American men with heart failure. Am J Mens Health. 2019;13:1557988318814295. doi: 10.1177/1557988318814295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McNaughton CD, Cawthon C, Kripalani S, Liu D, Storrow AB, Roumie CL. Health literacy and mortality: a cohort study of patients hospitalized for acute heart failure. J Am Heart Assoc. 2015;4:e000682. doi: 10.1161/JAHA.115.000682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Meddings J, Reichert H, Smith SN, Iwashyna TJ, Langa KM, Hofer TP, McMahon LF Jr. The impact of disability and social determinants of health on condition‐specific readmissions beyond Medicare risk adjustments: a cohort study. J Gen Intern Med. 2017;32:71–80. doi: 10.1007/s11606-016-3869-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mehra MR, Yancy CW, Albert NM, Curtis AB, Stough WG, Gheorghiade M, Heywood JT, McBride ML, O'Connor CM, Reynolds D, et al. Evidence of clinical practice heterogeneity in the use of implantable cardioverter‐defibrillators in heart failure and post‐myocardial infarction left ventricular dysfunction: findings from IMPROVE HF. Heart Rhythm. 2009;6:1727–1734. doi: 10.1016/j.hrthm.2009.08.022 [DOI] [PubMed] [Google Scholar]
- 49. Mirkin KA, Enomoto LM, Caputo GM, Hollenbeak CS. Risk factors for 30‐day readmission in patients with congestive heart failure. Heart Lung. 2017;46:357–362. doi: 10.1016/j.hrtlng.2017.06.005 [DOI] [PubMed] [Google Scholar]
- 50. Nagasako EM, Reidhead M, Waterman B, Dunagan WC. Adding socioeconomic data to hospital readmissions calculations may produce more useful results. Health Aff (Millwood). 2014;33:786–791. doi: 10.1377/hlthaff.2013.1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Park CL, Aldwin CM, Soyoung C, George L, Suresh DP, Bliss D. Spiritual peace predicts 5‐year mortality in congestive heart failure patients. Health Psychol. 2016;35:203–210. doi: 10.1037/hea0000271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Patel SA, Krasnow M, Long K, Shirey T, Dickert N, Morris AA. Excess 30‐day heart failure readmissions and mortality in Black patients increases with neighborhood deprivation. Circ Heart Fail. 2020;13:e007947. doi: 10.1161/CIRCHEARTFAILURE.120.007947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pinheiro LC, Reshetnyak E, Sterling MR, Levitan EB, Safford MM, Goyal P. Multiple vulnerabilities to health disparities and incident heart failure hospitalization in the REGARDS study. Circ Cardiovasc Qual Outcomes. 2020;13:e006438. doi: 10.1161/CIRCOUTCOMES.119.006438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Russell D, Baik D, Jordan L, Dooley F, Hummel SL, Prigerson HG, Bowles KH, Creber RM. Factors associated with live discharge of heart failure patients from hospice: a multimethod study. JACC Heart Fail. 2019;7:550–557. doi: 10.1016/j.jchf.2019.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schmeida M, Savrin RA. Heart failure rehospitalization of the Medicare FFS patient: a state‐level analysis exploring 30‐day readmission factors. Prof Case Manag. 2012;17:155–161. doi: 10.1097/NCM.0b013e31824c5fca [DOI] [PubMed] [Google Scholar]
- 56. Schopfer DW, Whooley MA, Stamos TD. Hospital compliance with performance measures and 30‐day outcomes in patients with heart failure. Am Heart J. 2012;164:80–86. doi: 10.1016/j.ahj.2012.04.017 [DOI] [PubMed] [Google Scholar]
- 57. Selim AM, Mazurek JA, Iqbal M, Wang D, Negassa A, Zolty R. Mortality and readmission rates in patients hospitalized for acute decompensated heart failure: a comparison between cardiology and general‐medicine service outcomes in an underserved population. Clin Cardiol. 2015;38:131–138. doi: 10.1002/clc.22372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shen BJ, Xu Y, Eisenberg S. Psychosocial and physiological predictors of mortality in patients of heart failure: independent effects of marital status and C‐reactive protein. Int J Behav Med. 2017;24:83–91. doi: 10.1007/s12529-016-9579-2 [DOI] [PubMed] [Google Scholar]
- 59. Sterling MR, Ringel JB, Pinheiro LC, Safford MM, Levitan EB, Phillips E, Brown TM, Goyal P. Social determinants of health and 90‐day mortality after hospitalization for heart failure in the REGARDS study. J Am Heart Assoc. 2020;9:e014836. doi: 10.1161/JAHA.119.014836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tabit CE, Coplan MJ, Spencer KT, Alcain CF, Spiegel T, Vohra AS, Adelman D, Liao JK, Sanghani RM. Cardiology consultation in the emergency department reduces re‐hospitalizations for low‐socioeconomic patients with acute decompensated heart failure. Am J Med. 2017;130:1112.e1117–1112.e1131. doi: 10.1016/j.amjmed.2017.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tripathi B, Arora S, Kumar V, Thakur K, Lahewala S, Patel N, Dave M, Shah M, Savani S, Sharma P, et al. Hospital complications and causes of 90‐day readmissions after implantation of left ventricular assist devices. Am J Cardiol. 2018;122:420–430. doi: 10.1016/j.amjcard.2018.04.015 [DOI] [PubMed] [Google Scholar]
- 62. Trivedi AN, Jiang L, Silva G, Wu WC, Mor V, Fine MJ, Kressin NR, Gutman R. Evaluation of changes in Veterans Affairs medical centers' mortality rates after risk adjustment for socioeconomic status. JAMA Netw Open. 2020;3:e2024345. doi: 10.1001/jamanetworkopen.2020.24345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Vivo RP, Krim SR, Liang L, Neely M, Hernandez AF, Eapen ZJ, Peterson ED, Bhatt DL, Heidenreich PA, Yancy CW, et al. Short‐ and long‐term rehospitalization and mortality for heart failure in 4 racial/ethnic populations. J Am Heart Assoc. 2014;3:e001134. doi: 10.1161/JAHA.114.001134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wadhera RK, Joynt Maddox KE, Fonarow GC, Zhao X, Heidenreich PA, DeVore AD, Matsouaka RA, Hernandez AF, Yancy CW, Bhatt DL. Association of the Affordable Care Act's Medicaid expansion with care quality and outcomes for low‐income patients hospitalized with heart failure. Circ Cardiovasc Qual Outcomes. 2018;11:e004729. doi: 10.1161/CIRCOUTCOMES.118.004729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Watkins T, Mansi M, Thompson J, Mansi I, Parish R. Effect of marital status on clinical outcome of heart failure. J Investig Med. 2013;61:835–841. doi: 10.2310/JIM.0b013e31828c823e [DOI] [PubMed] [Google Scholar]
- 66. Wray CM, Vali M, Byers A, Keyhani S. Examining the association of social determinants of health with missed clinic visits in patients with heart failure in the Veterans Health Administration. J Gen Intern Med. 2020;35:1591–1592. doi: 10.1007/s11606-019-05507-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wu JR, Lennie TA, Dekker RL, Biddle MJ, Moser DK. Medication adherence, depressive symptoms, and cardiac event‐free survival in patients with heart failure. J Card Fail. 2013;19:317–324. doi: 10.1016/j.cardfail.2013.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wu JR, Moser DK, DeWalt DA, Rayens MK, Dracup K. Health literacy mediates the relationship between age and health outcomes in patients with heart failure. Circ Heart Fail. 2016;9:e002250. doi: 10.1161/CIRCHEARTFAILURE.115.002250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wu JR, Moser DK, Rayens MK, De Jong MJ, Chung ML, Riegel B, Lennie TA. Rurality and event‐free survival in patients with heart failure. Heart Lung. 2010;39:512–520. doi: 10.1016/j.hrtlng.2009.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yandrapalli S, Sanaani A, Harikrishnan P, Aronow WS, Frishman WH, Lanier GM, Ahmed A, Fonarow GC. Cardiogenic shock during heart failure hospitalizations: age‐, sex‐, and race‐stratified trends in incidence and outcomes. Am Heart J. 2019;213:18–29. doi: 10.1016/j.ahj.2019.03.015 [DOI] [PubMed] [Google Scholar]
- 71. Ziaeian B, Kominski GF, Ong MK, Mays VM, Brook RH, Fonarow GC. National differences in trends for heart failure hospitalizations by sex and race/ethnicity. Circ Cardiovasc Qual Outcomes. 2017;10:e003552. doi: 10.1161/CIRCOUTCOMES.116.003552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Calvillo‐King L, Arnold D, Eubank KJ, Lo M, Yunyongying P, Stieglitz H, Halm EA. Impact of social factors on risk of readmission or mortality in pneumonia and heart failure: systematic review. J Gen Intern Med. 2013;28:269–282. doi: 10.1007/s11606-012-2235-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Biermann O, Mwoka M, Ettman CK, Abdalla SM, Shawky S, Ambuko J, Pearson M, Zeinali Z, Galea S, Mberu B, et al. Data, social determinants, and better decision‐making for health: the 3‐D commission. J Urban Health. 2021;98:4–14. doi: 10.1007/s11524-021-00556-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Elias RR, Jutte DP, Moore A. Exploring consensus across sectors for measuring the social determinants of health. SSM Popul Health. 2019;7:100395. doi: 10.1016/j.ssmph.2019.100395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Baker MC, Alberti PM, Tsao TY, Fluegge K, Howland RE, Haberman M. Social determinants matter for hospital readmission policy: insights from New York City. Health Aff (Millwood). 2021;40:645–654. doi: 10.1377/hlthaff.2020.01742 [DOI] [PubMed] [Google Scholar]
- 76. Gupta A, Allen LA, Bhatt DL, Cox M, DeVore AD, Heidenreich PA, Hernandez AF, Peterson ED, Matsouaka RA, Yancy CW, et al. Association of the hospital readmissions reduction program implementation with readmission and mortality outcomes in heart failure. JAMA Cardiol. 2018;3:44–53. doi: 10.1001/jamacardio.2017.4265 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4
Figure S1


