Abstract
Background
Heart failure (HF) has been increasing in prevalence, and a need exists for biomarkers with improved predictive and prognostic ability. GDF‐15 (growth differentiation factor‐15) is a novel biomarker associated with HF mortality, but no serial studies of GDF‐15 have been conducted. This study aimed to investigate the association between GDF‐15 levels over time and the occurrence of ventricular arrhythmias, HF hospitalizations, and all‐cause mortality.
Methods and Results
We used a retrospective case–control design to analyze 148 patients with ischemic and nonischemic cardiomyopathies and primary prevention implantable cardioverter‐defibrillator (ICD) from the PROSe‐ICD (Prospective Observational Study of the ICD in Sudden Cardiac Death Prevention) cohort. Patients had blood drawn every 6 months and after each appropriate ICD therapy and were followed for a median follow‐up of 4.6 years, between 2005 to 2019. We compared serum GDF‐15 levels within ±90 days of an event among those with a ventricular tachycardia/fibrillation event requiring ICD therapies and those hospitalized for decompensated HF. A comparator/control group comprised patients with GDF‐15 levels available during 2‐year follow‐up periods without events. Median follow‐up was 4.6 years in the 148 patients studied (mean age 58±12, 27% women). The HF cohort had greater median GDF‐15 values within 90 days (1797 pg/mL) and 30 days (2039 pg/mL) compared with the control group (1062 pg/mL, both P<0.0001). No difference was found between the ventricular tachycardia/fibrillation subgroup within 90 days (1173 pg/mL, P=0.60) or 30 days (1173 pg/mL, P=0.78) and the control group. GDF‐15 was also significantly predictive of mortality (hazard ratio, 3.17 [95% CI, 2.33–4.30]).
Conclusions
GDF‐15 levels are associated with HF hospitalization and mortality but not ventricular arrhythmic events.
Keywords: arrhythmias, biomarkers, heart failure, mortality
Subject Categories: Biomarkers, Heart Failure, Arrhythmias
Clinical Perspective.
What Is New?
GDF‐15 (growth differentiation factor‐15) is a novel biomarker that was found to predict mortality and decompensated heart failure events requiring admission with intravenous diuretics but was not predictive of ventricular arrhythmias.
What Are the Clinical Implications?
Patients with high‐risk heart failure may benefit from improved prognostication and goal‐directed medical therapy titration using serial GDF‐15 measurements when compared with existing biomarkers such as pro‐brain natriuretic peptide.
Nonstandard Abbreviations and Acronyms
- ATP
antitachycardia pacing
- GDF‐15
growth differentiation factor‐15
- hsCRP
high‐sensitivity C‐reactive protein
- PROSe‐ICD
Prospective Observational Study of the ICD in Sudden Death Prevention
- VT/VF
ventricular tachycardia/fibrillation
GDF‐15 (growth differentiation factor‐15) is part of the transforming growth factor‐β family of cytokines that is secreted at low levels by most cells. Its increased expression can be induced by various cellular stressors, including hypoxia, inflammation, or oxidative stress. 1 , 2 GDF‐15 is reported to be a useful prognostic biomarker for mortality in patients with heart failure (HF) 3 , 4 , 5 or myocardial infarction 6 , 7 as well as for thrombosis among patients with atrial fibrillation. 8 , 9 , 10 However, little information is available about the use of GDF‐15 as a biomarker for sudden arrhythmic death independent of HF exacerbations. Additionally, there have been no studies evaluating longitudinal variations in GDF‐15 values to determine if serial GDF‐15 levels are a better predictor of future clinical events than single baseline values.
We conducted this retrospective case–control study to assess changes in GDF‐15 levels in patients from PROSe‐ICD (Prospective Observational Study of the ICD in Sudden Cardiac Death Prevention), which enrolled and followed patients with left ventricular ejection fraction ≤35% who received guideline‐directed primary prevention defibrillators. We compared GDF‐15 levels among 3 groups: patients with hospital admissions for acute decompensated HF or with episodes of ventricular tachycardia/fibrillation (VT/VF) requiring implantable cardioverter defibrillator (ICD) therapies and a control group with neither event. We also performed a secondary analysis to assess the relationship between serial GDF‐15 levels and mortality.
METHODS
The data, analytic methods, and study materials that support the findings of this study are available from the corresponding author upon reasonable request. PROSe‐ICD (Prospective Observational Study of the ICD in Sudden Death Prevention) is a multicenter prospective cohort study that enrolled 1189 patients from 5 sites with ongoing follow‐up. 11 The study population consists of primary prevention ICD recipients between ages 18 and 80 years with ischemic or nonischemic cardiomyopathy with an ejection fraction ≤35% on optimal pharmacotherapy. 11 Full trial design, blood draw, and processing procedures have been published previously. 11 Briefly, patients in this cohort were evaluated every 6 months after ICD implantation and soon after any ICD shock with blood work performed at these study visits. All ICD shocks were reviewed and electrograms adjudicated by at least 2 electrophysiologists; a third reviewer resolved disagreements. An appropriate ICD shock was defined as that delivered for VT/VF above the rate cutoff for the device. Antitachycardia pacing (ATP) delivered for an appropriate VT/VF event also qualified as VT/VF events. We included ATP events along with ICD shocks, given previously published data showing similar outcomes with either ATP or shock events. 12 HF hospitalizations were determined from medical record review and required intravenous diuretic therapy. GDF‐15 was quantified using the R&D Human GDF‐15 Quantikine ELISA Kit (R&D Systems, Minneapolis, MN) and reported as pg/mL. Assay performance in the laboratory yielded a limit of detection of 2 pg/mL, an interassay coefficient of variance of 3.2%, and an intra‐assay coefficient of variance of 2.5%.
We used a retrospective case–control study design using available frozen blood samples from 148 patients (637 samples) to identify 2 groups of patients with events: (1) those with an appropriate ICD therapy, including appropriate shocks or ATP (VT/VF cohort, n=39); and (2) those with a hospital admission for HF (HF cohort, n=36). Control samples were matched based on ejection fraction, which led to a higher rate of men in both cohorts. Twelve patients had VT/VF and hospital admissions for HF and are included in both subgroups. Importantly, of these 12 patients, none had a VT/VF and HF event concurrently (within 90 days of each other). We compared GDF‐15 levels between those with blood samples within 90 days before and after (1) an appropriate ICD therapy (VT/VF 90‐day cohort, n=33) and (2) a hospital admission for HF (HF 90‐day cohort, n=32). The samples were chosen after a chart review was performed and dates obtained for HF admission or VT/VF events, as occurring before or following the event (Figure S1–S9). A comparison control group of 85 patients, defined as patients having no HF and/or VT/VF events, was identified. Control patients with follow‐up duration of <2 years and/or recovered left ventricular ejection fraction (>40%) were excluded. Subgroup analysis was further performed among patients with blood specimens within 30 days of the VT/VF event (n=26) or HF event (n=21). Secondary analysis was performed to assess the relationship between serial GDF‐15 levels before death and time to death in the full patient cohort (n=148). As a sensitivity analysis to examine the effects of potential bias in our Cox proportional hazards regression model, we duplicated each observation in the control group and reran the analysis to examine changes.
Categorical data are presented as frequencies and percentages, and continuous data are presented as means and SD or medians with 25th and 75th percentiles, as appropriate for the distribution. Chi‐squared or Fisher exact tests were used to compare categorical variables between groups, and Student t or Wilcoxon rank‐sum tests were used to compare continuous variables between groups. GDF‐15 was log‐transformed for all adjusted analyses. Mixed models were used to assess differences in log‐GDF‐15 values between the control group and each of the cohorts (HF, VT/VF) separately, accounting for repeated measures of GDF‐15 within a patient; models were run with and without adjustment for factors with complete data that were statistically significantly associated with cohort in bivariate analysis or were considered to be potential confounders. Survival data were displayed in Kaplan–Meier survival curves and compared using the log‐rank test, using the GDF‐15 value most recently assessed before the time of event, dichotomized at a threshold of ≥3000 pg/mL versus <3000 pg/mL as used by Lourenco et al. 13 Cox proportional hazards regression models were used to evaluate risk factors for mortality; factors with complete data that were statistically significantly associated with mortality in bivariate analysis or considered to be potential confounders were included in these models. Date of implant or date of first GDF‐15 sample was used as time zero. GDF‐15 was log‐transformed and treated as a time‐dependent covariate. Sensitivity analyses were run with only the Johns Hopkins site included (n=138). SAS v9.4 (Cary, NC) was used for all analyses, with P values <0.05 considered to indicate statistical significance. This study was approved by the institutional review boards of all participating sites and all patients signed informed consent for blood draws and clinical data collection.
RESULTS
Baseline characteristics of the study cohort are shown by event group in Table 1. Patients were well matched with regards to baseline characteristics in the HF and VT/VF subgroups, with the notable exception of higher female prevalence in the HF cohort (44%) when compared with the control population (22%). Additionally, the HF and VT/VF subgroups each had higher hsCRP (high‐sensitivity C‐reactive protein) levels than the control group, and the HF subgroup was also found to have higher interleukin‐6 levels compared with controls. Among the 148 patients, there were 637 blood samples drawn over an average follow‐up period of 4.6 years in this study. The control cohort (n=85) was followed for a median of 5.7 years (25th percentile, 75th percentile 1, 8.1). The HF cohort had a significantly shorter median follow‐up time with a time to event of 1.1 years (25th percentile, 75th percentile 0.36, 4.9; P<0.01), but the VT/VF cohort follow‐up time was not significantly different (median 3.9 years, 25th percentile, 75th percentile 1.1, 7.2; P=0.12). The distribution and timing of the samples are shown in Figure 1. The control cohort (343 samples, 85 patients) had a median GDF‐15 value of 1062 pg/mL (25th percentile, 75th percentile 737, 1740), which was not statistically different from the VT/VF subgroup at 30 days before or after an arrhythmic event (32 samples, 26 patients, median 1173 pg/mL, 25th percentile, 75th percentile 812, 1548) or 90 days (44 samples, 33 patients, median 1173 pg/mL, 25th percentile, 75th percentile 814, 1766) (Figure 2, Table 2). In contrast, the HF cohort had significantly elevated GDF‐15 values at 30 days before or after an HF admission (29 samples, 21 patients, median 2039 pg/mL, 25th percentile, 75th percentile 1313, 5318) and 90 days (47 samples, 32 patients, median 1797 pg/mL, 25th percentile, 75th percentile 1313, 5014) compared with the control group (Figure 2, Table 2). Notably, 12 patients had both HF and VT/VF events and were included in both subgroups, although no HF and VT/VF events in these patients occurred concurrently (within 90 days of the other), and they had similar outcomes to the HF and VT cohorts, respectively. For the 12 patients who had both VT/VF and HF, median GDF‐15 values were not higher compared with the HF group alone (1227 pg/mL, 25th percentile, 75th percentile 803, 1688). Atrial fibrillation was associated with significantly increased GDF‐15 values in all subgroups (Table S1–S9). Both ischemic and nonischemic cardiomyopathy subtypes showed no statistically significant difference between subgroups when adjusted for age at implantation, sex, race, hsCRP, and interleukin‐6 in control, HF, and VT/VF cohorts (Table S2). In the VT/VF subgroup, there was no significant difference between median (25%, 75%), GDF‐15 levels between patients who received ICD shocks (1029 [716–1735] pg/mL) versus those who only received ATP treatment (893 [674–1389] pg/mL) (P=0.21). GDF‐15 was positively correlated with other serum biomarkers, including hsIL‐6, hsCRP, interleukin‐10, tumor necrosis factor‐alpha, and pro‐brain natriuretic peptide (Table S3). We were able to add each biomarker one at a time to our model with GDF‐15 as a time‐dependent covariate. As a result, we found that GDF‐15 remains the strongest marker even after the addition of any of these biomarkers (Tables S4–S9).
Table 1.
Baseline Demographics of Overall Study Population, Control Cohort, HF Cohort, and VT/VF Cohort With Respect to Race, Age, Sex, and Various Cardiac Risk Factors
| Demographics | Overall (n=148) | Controls (n=85) | CHF (n=36) | VT/VF (n=39) | P value (HF vs controls) | P value (VT/VF vs controls) |
|---|---|---|---|---|---|---|
| Mean (SD) or Median (25th percentile, 75th percentile) | Mean (SD) or Median (25th percentile, 75th percentile) | Mean (SD) or Median (25th percentile, 75th percentile) | Mean (SD) or Median (25th percentile, 75th percentile) | |||
| Age at device implantation, y | 58.4 (12.5) | 59.0 (13.0) | 56.5 (10.7) | 56.5 (12.4) | 0.14 | 0.16 |
| Body mass index, kg/m2 | 29.4 (5.77) | 28.8 (6.08) | 30.8 (5.96) | 29.8 (4.31) | 0.06 | 0.14 |
| Creatinine, mg/dL | 1.1 (0.37) | 1.1 (0.4) | 1.16 (0.38) | 1.03 (0.22) | 0.24 | 0.88 |
| Glucose, mg/dL | 112 (33.7) | 109 (23.5) | 120 (51.4) | 116 (48.6) | 0.66 | 0.47 |
| hsIL‐6 | 1.99 (1.15, 3.93) | 1.67 (0.92, 3.01) | 2.5 (1.69, 6.4)* | 2.17 (1.28, 4.35) | 0.0274* | 0.09 |
| hsCRP | 3.83 (1.59, 9.51) | 2.6 (1.25, 7.04) | 6.99 (2.65, 13.2)* | 4.96 (2.69, 10.3)* | 0.0185* | 0.0152* |
| Il‐10 | 1.43 (0.9, 2.64) | 1.4 (0.86, 2.96) | 1.56 (0.96, 2.23) | 1.24 (0.77, 2.64) | 0.93 | 0.80 |
| TNF‐ɑ | 2937 (2236, 4376) | 2979 (2236, 3902) | 2770 (2155, 4450) | 2780 (2095, 3545) | 0.99 | 0.37 |
| Potassium, mg/dL | 4.19 (0.39) | 4.16 (0.41) | 4.24 (0.4) | 4.23 (0.3) | 0.29 | 0.25 |
| Pro‐BNP | 2.74 (1.91, 3.9) | 2.41 (1.76, 3.39) | 3.11 (2.24, 4.23) | 2.89 (2, 3.9) | 0.052 | 0.18 |
| Sodium, mg/dL | 139 (2.94) | 139 (2.76) | 138 (3.48) | 138 (2.86) | 0.14 | 0.20 |
| Troponin I | 0.01 (0, 0.04) | 0 (0, 0.02) | 0.02 (0, 0.05) | 0.01 (0, 0.05) | 0.12 | 0.18 |
| Ejection fraction, % | 20.6 (7.08) | 20.3 (7) | 18.3 (6.65) | 22.3 (7.55) | 0.13 | 0.16 |
| eGFR CKD‐EPI, mL/min/1.73 m2 | 77.9 (24.4) | 78.7 (24.3) | 73.2 (26.0) | 81.3 (21.1) | 0.17 | 0.90 |
| Supine right SBP (mm HG) | 120 (19.9) | 121 (18.5) | 119 (23.0) | 119 (19.1) | 0.45 | 0.51 |
| Supine right DBP (mm HG) | 72.9 (11.1) | 73.6 (10.9) | 71.5 (11.6) | 72.4 (10.3) | 0.26 | 0.60 |
| Descriptive characteristics | n (%) | n (%) | n (%) | n (%) | ||
|---|---|---|---|---|---|---|
| Female sex | 40 (27.0) | 19 (22.4) | 16 (44.4)* | 9 (23.1) | 0.0143* | 0.93 |
| Black race | 44 (29.7) | 23 (27.1) | 16 (44.4) | 9 (23.1) | 0.15 | 0.78 |
| White race | 102 (68.9) | 61 (71.8) | 20 (55.6) | 29 (74.4) | ||
| Never smoker | 50 (33.8) | 32 (37.7) | 12 (33.3) | 10 (25.6) | 0.88 | 0.29 |
| Recent smoker | 24 (16.2) | 12 (14.1) | 6 (16.7) | 9 (23.1) | ||
| Biventricular device | 4 (2.7) | 1 (1.2) | 1 (2.8) | 2 (5.1) | 0.41 | 0.38 |
| Dual chamber device | 20 (13.5) | 8 (9.4) | 7 (19.4) | 6 (15.4) | ||
| Dual/Biventricular device | 37 (25) | 23 (27.1) | 9 (25) | 8 (20.5) | ||
| Single chamber device | 87 (58.8) | 53 (62.4) | 19 (52.8) | 23 (59) | ||
| History of atrial fibrillation | 33 (22.3) | 19 (22.4) | 7 (19.4) | 9 (23.1) | 0.72 | 0.93 |
| Hyperlipidemia | 56 (37.8) | 32 (37.7) | 17 (47.2) | 11 (28.2) | 0.33 | 0.31 |
| Diabetes | 42 (28.4) | 23 (27.1) | 14 (38.9) | 8 (20.5) | 0.20 | 0.43 |
| Hypertension | 79 (53.4) | 51 (60) | 21 (58.3) | 11 (28.2)* | 0.86 | 0.0010* |
| Ischemic cardiomyopathy | 71 (48) | 42 (49.4) | 14 (38.9) | 20 (51.3) | 0.29 | 0.85 |
| Nonischemic cardiomyopathy | 83 (56.1) | 47 (55.3) | 24 (66.7) | 21 (53.9) | 0.25 | 0.88 |
| Medication use | ||||||
| ACE inhibitor | 103 (69.6) | 59 (69.4) | 27 (75) | 26 (66.7) | 0.54 | 0.76 |
| Beta‐blocker | 132 (89.2) | 77 (90.6) | 33 (91.7) | 33 (84.6) | 0.85 | 0.33 |
| Calcium channel blocker | 12 (8.1) | 9 (10.6) | 0* | 3 (7.7) | 0.0424* | 0.61 |
| ARB | 33 (22.3) | 22 (25.9) | 6 (16.7) | 8 (20.5) | 0.27 | 0.52 |
| Aldosterone antagonist | 30 (20.3) | 14 (16.5) | 10 (27.8) | 10 (25.6) | 0.15 | 0.23 |
| Digoxin | 34 (23) | 19 (22.4) | 9 (25) | 9 (23.1) | 0.75 | 0.93 |
| Loop diuretic | 101 (68.2) | 53 (62.4) | 33 (91.7)* | 25 (64.1) | 0.0011* | 0.85 |
| Thiazide diuretic | 12 (8.1) | 7 (8.2) | 3 (8.3) | 3 (7.7) | 0.99 | 0.92 |
| Statin | 97 (65.5) | 57 (67.1) | 20 (55.6) | 26 (66.7) | 0.23 | 0.97 |
| Clopidogrel | 21 (14.2) | 9 (10.6) | 6 (16.7) | 6 (15.4) | 0.35 | 0.45 |
| Warfarin | 52 (35.1) | 26 (30.6) | 14 (38.9) | 18 (46.2) | 0.38 | 0.09 |
| Aspirin | 103 (69.6) | 59 (69.4) | 26 (72.2) | 24 (61.5) | 0.76 | 0.39 |
| SSRI | 11 (7.4) | 7 (8.2) | 4 (11.1) | 1 (2.6) | 0.62 | 0.23 |
| Any antidepressant | 23 (15.5) | 11 (12.9) | 9 (25) | 5 (12.8) | 0.10 | 0.99 |
| Site of follow‐up | ||||||
| JHU | 138 (93.2) | 75 (88.2) | 36 (100)* | 39 (100)* | 0.0317* | 0.0255* |
P values for heart failure and ventricular tachycardia/fibrillation cohorts, versus control population, are shown. *Significant differences (defined as P value <0.05). Notably, 12 patients had both heart failure and ventricular tachycardia/fibrillation events and were included in both subgroups.
ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; CHF, congestive heart failure; eGFR, estimated glomerular filtration rate, using CKD‐EPI equation; hsCRP, high‐sensitivity C‐reactive protein; hsIL‐6, high‐sensitivity Interleukin‐6; JHU, Johns Hopkins University; SSRI, selective serotonin reuptake inhibitor; and TNF‐ɑ, tumor necrosis factor alpha.
Figure 1. GDF‐15 (growth differentiation factor‐15) values (pg/mL) with respect to time to decompensated heart failure admission (blue dots) or ventricular tachycardia/fibrillation (red crosses) event.
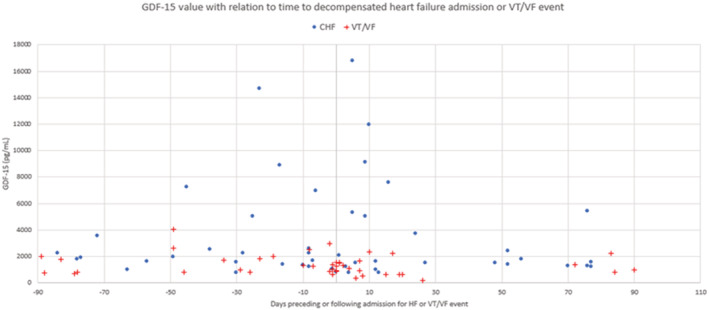
Negative numbers indicate days preceding heart failure admission date or ventricular tachycardia/fibrillation event, whereas positive numbers indicate days after the heart failure admission date or ventricular tachycardia/fibrillation event. CHF indicates congestive heart failure; GDF‐15, growth differentiation factor‐15; and VT/VF, ventricular tachycardia/fibrillation.
Figure 2. GDF‐15 (growth differentiation factor‐15) values (pg/mL) for heart failure and ventricular tachycardia/fibrillation subgroups at 90 days and 30 days before and after an event, as well as for the control group.
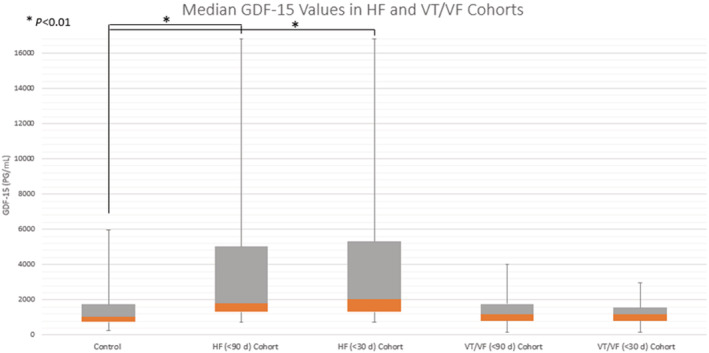
Median GDF‐15 values are shown, with the positive interquartile range shown in the gray box and negative interquartile range in orange. Maximum and minimum values for each group are represented by the whiskers. *P<0.01 from adjusted mixed model. GDF‐15 indicates growth differentiation factor‐15; HF, heart failure; and VT/VF, ventricular tachycardia/fibrillation.
Table 2.
Median GDF‐15 Values (in pg/mL) for the HF and VT/VF Cohorts
| Group | Samples, n | Patients, n | GDF‐15 Median (25th percentile, 75th percentile) | Unadjusted P value | Adjusted P value |
|---|---|---|---|---|---|
| HF (<90 d) cohort | 47 | 32 | 1797 (1313, 5014) | <0.0001 | <0.0001 |
| HF (<30 d) cohort | 29 | 21 | 2039 (1313, 5318) | <0.0001 | <0.0001 |
| VT/VF (<90 d) cohort | 44 | 33 | 1173 (814, 1766) | 0.57 | 0.48 |
| VT/VF (<30 d) cohort | 32 | 26 | 1173 (812, 1548) | 0.28 | 0.66 |
| Control | 343 | 85 | 1062 (737, 1740) | N/A | N/A |
The control group includes 343 samples in patients who did not experience a decompensated heart failure or ventricular tachycardia/fibrillation event within a 30‐ or 90‐day period. Adjusted P values were adjusted for age at implantation, sex, race, atrial fibrillation, high‐sensitivity C‐reactive protein, and interleukin‐6 in the heart failure cohorts, and age at implantation, sex, race, atrial fibrillation, and high‐sensitivity C‐reactive protein levels in the ventricular tachycardia/fibrillation cohorts.
GDF‐15 indicates growth differentiation factor 15; HF, heart failure; and VT/VF, ventricular tachycardia/fibrillation.
Additionally, GDF‐15 was found to be associated with a significantly greater mortality, in both unadjusted and adjusted Cox proportional hazards modeling with log‐transformed serial levels (unadjusted hazard ratio, 2.99 [95% CI, 2.28–3.93]; adjusted hazard ratio, 3.17 [95% CI, 2.33–4.30]), and with Kaplan–Meier analysis using a cutoff GDF‐15 value of ≥3000 pg/mL, compared with patients with lower values, over 7.5 years of follow‐up (Figure 3). Sensitivity analyses found no change in results when limited to the Johns Hopkins site or with duplication of the control observations.
Figure 3. Kaplan–Meier curve for patient mortality stratified by GDF‐15 level.
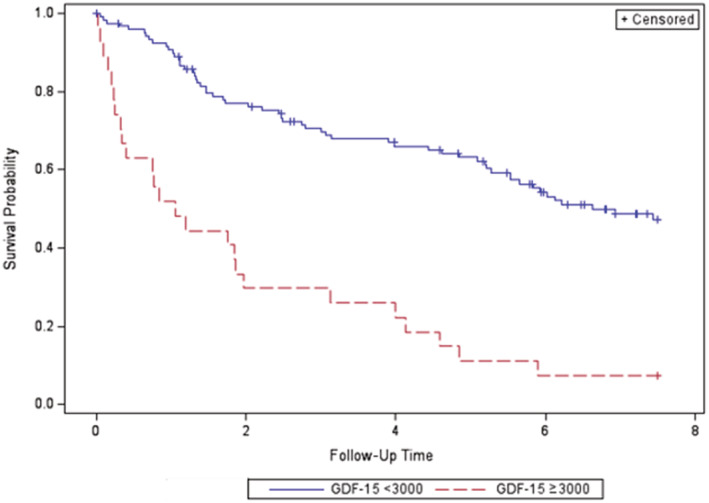
Values <3000 pg/mL are shown in blue, and values ≥3000 pg/mL are shown in red. Data were censored after 7.5 years of follow‐up (P<0.0001). GDF‐15 indicates growth differentiation factor‐15.
DISCUSSION
To our knowledge, the current study is the first to assess serial changes in GDF‐15 levels and expands our knowledge by evaluating not only the relationship to HF events and mortality but also to adjudicated ventricular arrhythmias in a cardiomyopathy cohort at high‐risk of sudden cardiac death. We found significantly higher GDF‐15 levels as early as 90 days preceding a decompensated HF hospitalization compared with controls. Increasing GDF‐15 levels were temporally related to increased risk for all‐cause mortality, analyzed as serial measures or using a threshold of ≥3000 pg/mL. GDF‐15 levels, however, were not associated with risk of subsequent VT/VF, and levels among those patients with VT/VF did not differ from that of controls.
The prognostic value of GDF‐15 has been compared previously to multiple other biomarkers, including NT‐proBNP (N‐terminal pro‐brain natriuretic peptide), hsCRP, and high‐sensitivity troponin T. 6 , 14 , 15 , 16 , 17 Lok et al conducted an analysis of the prognostic value of GDF‐15 versus NT‐proBNP, hsCRP, high‐sensitivity troponin T, and galectin‐3 and found a calculated area under the curve for all‐cause mortality of 0.63 for NT‐proBNP as compared with 0.78 for GDF‐15, making the latter a more powerful predictor of mortality for patients with HF than NT‐proBNP. 15 Similar results have been found for the predictive value of GDF‐15 for patients with myocardial infarction, 6 although no significant difference has been found in patients with HF and a preserved ejection fraction. 15 Interestingly, the elevated hsCRP and interleukin‐6 values in the HF and VT/VF cohorts may help illustrate the higher inflammatory and oxidative stress burden in patients with HF, especially around the time of an exacerbation, and may provide mechanistic insights into GDF‐15 elevation.
A recent study by Lourenco et al examined GDF‐15 values in patients with acute HF and showed a significantly higher 1‐year risk of death; hazard ratio, 2.59; 95% CI, 1.41–4.76, with cutoff values of ≥3000 pg/mL. 13 Our study adds to the predictive value of GDF‐15 by showing its association not only with mortality but also HF decompensation in patients with HF with a reduced ejection fraction. In addition, it helps to illustrate the relatively normal median baseline GDF‐15 values around 1200 pg/mL of patients with HF with a reduced ejection fraction when outside of a 3‐month window for a decompensated HF event. In contrast to a prior study, 7 GDF‐15 was not elevated in patients with ventricular arrhythmias in our cohort, but significant differences exist between the 2 studies. The prior study comprised patients dying suddenly after acute myocardial infarction, whereas our cohort comprised those with chronic cardiomyopathy. Most ventricular arrhythmias in patients with chronic HF occur as a result of scar‐mediated reentry pathways, rather than ischemic events, which could further contribute to the lack of association of GDF‐15 with ventricular arrhythmias in our study. Additionally, in our study, VT/VF events occurred largely independent of HF exacerbations, while the prior study did not separate HF exacerbations and sudden cardiac death events. Thus, many arrhythmic events may have occurred in the setting of worsening HF exacerbations in the prior study.
GDF‐15, outside of reproductive organs, has been shown to have low levels of expression at baseline but can be induced by cellular stressors, including inflammation, myocardial ischemia, and cancer. 18 GDF‐15 has been described as a cardiokine (analogous to an adipokine), whose expression increases during cellular stress and functions as a mitochondrial regulator, potentially with a role in energy homeostasis. 18 , 19 Mechanistically, the hypoxic stress associated with decompensated HF, along with mitochondrial dysfunction, could explain the GDF‐15 elevation in these cohorts.
Our study has several limitations. First, the study design is prone to selection bias, especially because the number of GDF‐15 samples per patient was proportional to the duration of follow‐up. Additionally, the small number of patients in whom HF or VT/VF events occurred and in whom blood specimens were available within 90 days (33 and 32 patients, respectively) results in a small effective sample size for which an event occurred and may not be representative of a larger population. GDF‐15 values may be elevated in a variety of different conditions, including poor physical activity, mitochondrial disease, diabetes, kidney disease, or cancer, and while we controlled for diabetes and renal insufficiency, these other noncardiac causes of GDF‐15 elevation were not evaluated in this study and leave the potential for confounding attributable to noncardiac elevations of GDF‐15. 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 Studies have also found differences in GDF‐15 levels in male versus female neonates and in pregnant females. While further studies are needed for elucidating sexual differences in GDF‐15 expression, a female predominance in our HF group may have theoretically skewed our results. 29 , 30
Nonetheless, this study has several important clinical implications. First, it helps to illustrate the predictive value of GDF‐15 with respect to mortality and decompensated HF admissions. Second, this study shows that GDF‐15 does not predict future VT/VF events. Third, elevated GDF‐15 values may identify a subset of patients at high risk of mortality who may benefit from close clinical follow‐up.
Given the significant elevation of GDF‐15 values around decompensated HF events, this biomarker may become helpful clinically for the management of HF, although larger prospective studies are required to determine patient subgroups who would derive the most benefit from monitoring and whether intervening early could reduce hospitalizations and improve outcome. We did not find any significant relationship between ventricular arrhythmic events and GDF‐15 values.
Sources of Funding
This work was supported by the National Institutes of Health (R01HL132181 to G.F.T. and K.C.W.) and by a Research Grant from the Lovin’ Every Day Foundation (to A.S.B.).
Disclosures
None.
Supporting information
Tables S1–S9
Figure S1
Acknowledgments
We thank and acknowledge the patients for their participation. We also acknowledge the support given by the departments of Internal Medicine and Cardiology at Johns Hopkins and Virginia‐Tech Carilion for their support of this research.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.026003
For Sources of Funding and Disclosures, see page 8.
References
- 1. Wollert K, Kempf T, Wallentin L. Growth differentiation factor 15 as a biomarker in cardiovascular disease. Clin Chem. 2017;63:140–151. doi: 10.1373/clinchem.2016.255174 [DOI] [PubMed] [Google Scholar]
- 2. Xu X, Li Z, Gao W. Growth differentiation factor 15 in cardiovascular diseases: from bench to bedside. Biomarkers. 2011;16:466–475. doi: 10.3109/1354750X.2011.580006 [DOI] [PubMed] [Google Scholar]
- 3. Kempf T, Haehling S, Peter T, Allhoff T, Cicoira M, Doehner W, Ponikowski P, Filippatos G, Rozentryt P, Drexler H, et al. Prognostic utility of growth differentiation factor‐15 in patients with chronic heart failure. JACC. 2007;50:1054–1060. doi: 10.1016/j.jacc.2007.04.091 [DOI] [PubMed] [Google Scholar]
- 4. George M, Jena A, Srivatsan V, Muthukumar R, Dhandapani VE. GDF 15‐a novel biomarker in the offing for heart failure. Curr Cardiol Rev. 2016;12:37–46. doi: 10.2174/1573403X12666160111125304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sharma A, Stevens S, Lucas J, Fiuzat M, Adams K, Whellan D, Donahue M, Kitzman D, Pina I, Zannad F, et al. Utility of growth differentiation factor‐15, a marker of oxidative stress and inflammation, in chronic heart failure: insights from the HF‐ACTION study. JACC Heart Fail. 2017;5:724–734. doi: 10.1016/j.jchf.2017.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khan S, Ng K, Dhillon O, Kelly D, Quinn P, Squire I, Davies J, Ng L. Growth differentiation factor‐15 as a prognostic marker in patients with acute myocardial infarction. Eur Heart J. 2009;30:1057–1065. doi: 10.1093/eurheartj/ehn600 [DOI] [PubMed] [Google Scholar]
- 7. Andersson J, Fall T, Delicano R, Wennberg P, Jansson J. GDF‐15 is associated with sudden cardiac death due to incident myocardial infarction. Resuscitation. 2020;152:165–169. doi: 10.1016/j.resuscitation.2020.05.001 [DOI] [PubMed] [Google Scholar]
- 8. Wallentin L, Hijazi Z, Andersson U, Alexander J, Caterina R, Hanna M, Horowitz J, Hylek E, Lopes R, Asberg S, et al. Growth differentiation factor 15, a marker of oxidative stress and inflammation, for risk assessment in patients with atrial fibrillation. Circulation. 2014;130:1847–1858. doi: 10.1161/CIRCULATIONAHA.114.011204 [DOI] [PubMed] [Google Scholar]
- 9. Santema B, Chan M, Tromp J, Dokter M, Wal H, Emmens J, Takens J, Samani N, Ng L, Lang C, et al. The influence of atrial fibrillation on the levels of NT‐proBNP versus GDF‐15 in patients with heart failure. Clin Res Cardiol. 2020;109:331–338. doi: 10.1007/s00392-019-01513-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marín F, Roldán V. GDF‐15 and risk stratification in atrial fibrillation. Nat Rev Cardiol. 2015;12:8–9. doi: 10.1038/nrcardio.2014.190 [DOI] [PubMed] [Google Scholar]
- 11. Cheng A, Dalal D, Butcher B, Norgard S, Zhang Y, Dickfeld T, Eldadah Z, Ellenbogen K, Guallar E, Tomaselli G. Prospective observational study of implantable cardioverter‐defibrillators in primary prevention of sudden cardiac death: study design and cohort description. J Am Heart Assoc. 2013;2:e000083. doi: 10.1161/JAHA.112.000083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moss A, Schuger C, Beck C, Brown M, Cannom D, Daubert J, Estes M, Greenberg H, Hall J, Huang D, et al. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med. 2012;367:2275–2283. doi: 10.1056/NEJMoa1211107 [DOI] [PubMed] [Google Scholar]
- 13. Lourenco P, Cunha FM, Ferreira‐Coimbra J, Barroso I, Guimaraes J, Bettencourt P. Dynamics of growth differentiation factor 15 in acute heart failure. ESC Heart Fail. 2021;8:2527–2534. doi: 10.1002/ehf2.13377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lok DJ, Klip IT, Lok SI, Porte P, Badings E, Winjngaarden J, Voors A, Boer R, Veldhuisen D, Meer P. Incremental prognostic power of novel biomarkers (growth‐differentiation factor‐15, high‐sensitivity C‐reactive protein, galectin‐3, and high‐sensitivity troponin‐T) in patients with advanced chronic heart failure. Am J Cardiol. 2013;112:831–837. doi: 10.1016/j.amjcard.2013.05.013 [DOI] [PubMed] [Google Scholar]
- 15. Santhanakrishnan R, Chong JP, Ng TP, Ling L, Sim D, Leong K, Yeo P, Ong H, Jaufeerally F, Wong R, et al. Growth differentiation factor 15, ST2, high‐sensitivity troponin T, and N‐terminal pro brain natriuretic peptide in heart failure with preserved vs. reduced ejection fraction. Eur J Heart Fail. 2012;14:1338–1347. doi: 10.1093/eurjhf/hfs130 [DOI] [PubMed] [Google Scholar]
- 16. Wang TJ, Wollert KC, Larson MG, Coglianese E, McCabe E, Cheng S, Ho J, Fradley M, Ghorbani A, Xanthakis V, et al. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham heart study. Circulation. 2012;126:1596–1604. doi: 10.1161/CIRCULATIONAHA.112.129437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xanthakis V, Larson MG, Wollert KC, Aragam J, Cheng S, Ho J, Coglianese E, Levy D, Colucci W, Felker G, et al. Association of novel biomarkers of cardiovascular stress with left ventricular hypertrophy and dysfunction: implications for screening. J Am Heart Assoc. 2013;2:e000399. doi: 10.1161/JAHA.113.000399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wischhusen J, Melero I, Fridman WH. Growth/differentiation factor‐15 (GDF‐15): from biomarker to novel targetable immune checkpoint. Front Immunol. 2020;11:951. doi: 10.3389/fimmu.2020.00951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rochette L, Zeller M, Cottin Y, Vergely C. Insights into mechanisms of GDF15 and receptor GFRAL: therapeutic targets. Trends Endocrinol Metab. 2020;31:939–951. doi: 10.1016/j.tem.2020.10.004 [DOI] [PubMed] [Google Scholar]
- 20. Conte M, Martucci M, Mosconi G, Chiariello A, Cappucilli C, Totti V, Santoro A, Franceschi C, Salvioli S. GDF15 plasma level is inversely associated with level of physical activity and correlates with markers of inflammation and muscle weakness. Front Immunol. 2020;11:915. doi: 10.3389/fimmu.2020.00915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vocka M, Langer D, Fryba V, Petrtyl J, Hanus T, Kalousova M, Zima T, Petruzelka L. Growth/differentiation factor 15 (GDF‐15) as new potential serum marker in patients with metastatic colorectal cancer. Cancer Biomark. 2018;21:869–874. doi: 10.3233/CBM-170792 [DOI] [PubMed] [Google Scholar]
- 22. Bao X, Borné Y, Muhammad IF, Nilsson J, Lind L, Melander O, Niu K, Orho M, Engstrom G. Growth differentiation factor 15 is positively associated with incidence of diabetes mellitus: the Malmö diet and cancer‐cardiovascular cohort. Diabetologia. 2019;62:78–86. doi: 10.1007/s00125-018-4751-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nair V, Cohen C, Smith M, Bellovich K, Bhat Z, Bobadilla M, Brosius F, Boer I, Essioux L, Formentini I, et al. Growth differentiation factor–15 and risk of CKD progression. J Am Soc Nephrol. 2017;28:2233–2240. doi: 10.1681/ASN.2016080919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Molfino A, Amabile M, Imbimbo G, Rizzo V, Pediconi F, Catalano C, Emiliani A, Belli R, Ramaccini C, Parisi C, et al. Association between growth differentiation factor‐15 (GDF‐15) serum levels, anorexia and low muscle mass among cancer patients. Cancers. 2021;13:1–10. doi: 10.3390/cancers13010099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luan H, Wang A, Hilliard B, Carvalho F, Rosen C, Ahasic A, Herzog E, Kang I, Pisani M, Yu S, et al. GDF15 is an inflammation‐induced central mediator of tissue tolerance. Cell. 2019;178:1231–1244. doi: 10.1016/j.cell.2019.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yatsuga S, Fujita Y, Ishii A, Fukumoto Y, Arahata H, Kakuma T, Kojima T, Ito M, Tanaka M, Saiki R, et al. Growth differentiation factor 15 as a useful biomarker for mitochondrial disorders. Ann Neurol. 2015;78:814–823. doi: 10.1002/ana.24506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Montero R, Yubero D, Villarroya J, Henares D, Jou C, Rodriguez M, Ramos F, Nascimento A, Ortez C, Campistol J, et al. GDF‐15 is elevated in children with mitochondrial diseases and is induced by mitochondrial dysfunction. PLoS One. 2016;11:e0148709. doi: 10.1371/journal.pone.0148709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Formichi P, Cardone N, Taglia I, Cardaiola E, Salvatore S, Gerfo A, Simoncini C, Montano V, Siliciano G, Mancuso M, et al. Fibroblast growth factor 21 and growth differentiation factor 15 are sensitive biomarkers of mitochondrial diseases due to mitochondrial transfer‐RNA mutations and mitochondrial DNA deletions. Neurol Sci. 2020;41:3653–3662. doi: 10.1007/s10072-020-04422-5 [DOI] [PubMed] [Google Scholar]
- 29. Zhang Y, Jiang W, Wang L, Lingappan K. Sex‐specific differences in the modulation of growth differentiation factor 15 (GDF15) by hyperoxia in vivo and in vitro: role of Hif‐1α. Toxicol Appl Pharmacol. 2017;332:8–14. doi: 10.1016/j.taap.2017.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Andersson‐Hall U, Svedin P, Mallard C, Blennow K, Zetterberg H, Holmang A. Growth differentiation factor 15 increases in both cerebrospinal fluid and serum during pregnancy. PLoS One. 2021;16:e0248980. doi: 10.1371/journal.pone.0248980 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S9
Figure S1


