Abstract
Background
Understanding current trends in cholesterol screening, lipid levels, and lipid management therapies may inform health policy and practice.
Methods and Results
In 50 928 US adult National Health and Nutrition Examination Survey (NHANES) participants, trends were assessed in cholesterol screening, mean levels of total cholesterol, triglycerides, low‐density‐lipoprotein cholesterol, and lipid‐lowering medication use from 1999 through 2018. Point estimates were also calculated using the 2017 to March 2020 prepandemic data set. The age‐ and sex‐adjusted proportion of having cholesterol screened within 5 years increased from 63.2% (95% CI, 60.0–66.3) in 1999 to 2000 to 72.5% (95% CI, 69.5–75.3) in 2017 to 2018 (P<0.001 for linear trend). Mean total cholesterol decreased from 203.3 mg/dL (95% CI, 201.0–205.7) in 1999 to 2000 to 188.4 mg/dL in 2017 to 2018 (95% CI, 185.4–191.5) (P<0.001 for nonlinear trend). The mean triglyceride level decreased from 121.3 mg/dL (95% CI, 116.4–126.4) in 1999 to 2000 to 91.4 mg/dL (95% CI, 88.4–94.6) in 2017 to 2018 (P<0.001 for nonlinear trend). Low‐density lipoprotein cholesterol decreased from 127.9 mg/dL (95% CI, 125.3–130.5) in 1999 to 2000 to 111.7 mg/dL (95% CI, 109.0–114.4) in 2017 to 2018 (P<0.001 for nonlinear trend). Among statin‐eligible US adults, the proportion of statin use increased from 14.9% (95% CI, 12.2–17.9) in 1999 to 2000 to 27.8% (95% CI, 23.0–33.2) in 2017 to 2018 (P<0.001 for nonlinear trend). Statin use increased in adults with diabetes aged 40 to 75 years from 21.4% in 1999 to 2000 to 51.9% in 2017 to 2018 (P<0.001 for overall linear trend). Statin use plateaued in all other groups. The proportions of using ezetimibe and proprotein convertase subtilisin/kexin type 9 inhibitors were 3.7% (95% CI, 1.3–9.8) and 0.03% (95% CI, 0.01–0.15) in 2017 to March 2020, respectively.
Conclusions
From 1999 through 2018, cholesterol screening increased while mean total cholesterol, triglycerides, and low‐density lipoprotein cholesterol levels decreased, with a modest increase in statin use and low uptake of nonstatin therapy in the US population.
Keywords: epidemiology, lipids, preventive cardiology, statins
Subject Categories: Epidemiology, Risk Factors
Nonstandard Abbreviations and Acronyms
- NHANES
National Health and Nutrition Examination Survey
- PCSK9
proprotein convertase subtilisin/kexin type 9
- TC
total cholesterol
JEL
EpidemiologyRisk Factors
Clinical Perspective.
What Is New?
Using the most recent data from the National Health and Nutrition Examination Survey, we updated trends in cholesterol screening, lipid levels, and lipid‐lowering medication use in the US population from 1999 to 2018, with point estimates for 2017 to March 2020.
Interim data show continued improvement in cholesterol screening, with a respective decrease in mean total cholesterol, triglycerides, and low‐density lipoprotein cholesterol levels, and a modest increase in statin use but low uptake of nonstatin therapy.
Only the subgroup of statin‐eligible patients with diabetes aged 40 to 75 years showed an increase in statin use after 2015 to 2016, and 60.4% of patients with established atherosclerotic cardiovascular disease were not prescribed a statin in 2017 to March 2020.
What Are the Clinical Implications?
There is a need to intensify national efforts to improve the utilization of evidence‐based lipid‐lowering therapies at the systems, clinician, and patient levels, with opportunities to revisit risk discussions at multiple timepoints in a patient's care.
A key population of eligible patients with a concerning trend in statin use in recent years includes patients with established atherosclerotic cardiovascular disease.
Despite advances in antilipid therapy with the advent of proprotein convertase subtilisin/kexin type 9 inhibitors and ezetimibe in recent years, there is room for improvement to increase adoption of these nonstatin therapies.
Cardiovascular disease remains the leading cause of death in the United States. 1 , 2 Serum cholesterol and its lipoprotein carriers are key risk factors in the development of atherosclerotic cardiovascular disease (ASCVD). 3 Low‐density lipoprotein cholesterol (LDL‐C) is the target of global prevention guidelines, as randomized controlled trials have shown that ASCVD risk can be reduced by screening and interventions to reduce LDL‐C. Triglycerides and high‐density lipoprotein cholesterol (HDL‐C) are also important biomarkers of cardiovascular disease risk. 4 , 5 , 6
Previous literature has examined cholesterol screening, treatment, total cholesterol (TC), triglycerides, LDL‐C, and HDL‐C levels, but trends have not been updated with the most recent survey data. 2 , 3 , 7 , 8 , 9 Therefore, using nearly 2 decades of data (1999–2018) from the National Health and Nutrition Examination Survey (NHANES), we aimed to evaluate and update the trends in cholesterol screening, lipid levels, and lipid‐lowering medication use in the US population.
METHODS
Data Availability
The authors declare that all supporting data are available within the article and its online supplementary files.
Study Population
The NHANES is a cross‐sectional survey that uses a stratified and multistage probability‐cluster sampling scheme to assess the health and nutritional status of the US noninstitutionalized, civilian population. 10 In 55 081 participants aged ≥20 years from NHANES 1999 to 2018, we excluded those who did not have laboratory samples collected (n=2683), were pregnant at the time of examination (n=1469), or who were missing data for all variables of our interest (n=1), leaving a final sample size of 50 928 for the primary trends analysis. The study protocols were approved by the institutional review board of the National Center for Health Statistics, and all study participants provided written informed consent.
Variables
NHANES participants reported age, sex (male or female), race and ethnicity (non‐Hispanic Asian, Hispanic, non‐Hispanic Black, and non‐Hispanic White), education (<9th grade, 9–11th grade, high school graduate, some college, or college graduate), income (<100%, 100%–299%, 300%–499%, or ≥500% of the federal poverty level), and health insurance type (private, government‐based, and uninsured). Representative information for non‐Hispanic Asian Americans was available in NHANES since the 2011 to 2012 cycle. 11 History of ASCVD was defined as self‐report of any coronary heart disease, myocardial infarction, angina, or stroke. 12 Diagnosis of diabetes was defined by a self‐reported history of diabetes, fasting glucose ≥126 mg/dL, or hemoglobin A1c ≥6.5%. The 10‐year ASCVD risk was calculated using the pooled cohort equations for participants without a self‐reported history of ASCVD. 12 , 13
Outcomes
Outcomes regarding cholesterol screening were derived from the questionnaire questions “Ever had blood cholesterol checked” and “When was blood cholesterol last checked.” Four measures of lipid levels were assessed: TC, triglycerides, LDL‐C, and HDL‐C. TC was measured in a series of enzymatic reactions. 14 Triglycerides were measured enzymatically using a series of coupled reactions in which triglycerides were hydrolyzed to produce glycerol. 15 HDL‐C was measured by the heparin‐manganese precipitation method technique. 16 LDL‐C was calculated from measured values of TC, triglycerides, and HDL‐C according to the Hopkins/Martin equation. 17 Lipid‐lowering medication and drug classes (eg, statins, fibric acid derivatives, ezetimibe, bile acid sequestrants, and PCSK9 [proprotein convertase subtilisin/kexin type 9] inhibitors) were defined using the Multum Lexicon standardized drug code or therapeutic classification scheme. 18
Statistical Analysis
All statistical analyses followed the recommended analytic guidelines and accounted for the complex NHANES sampling design, oversampling, and survey nonresponse. 19 Examination weights were used for the analysis of cholesterol screening, total cholesterol, HDL‐C, and medication use. Fasting weights were used in the analysis of triglycerides and LDL‐C, which were collected as fasting samples. When combining survey cycles, we followed analytic recommendations set by NHANES and constructed weights using 4‐year weights for 1999 to 2002 and 2‐year weights for 2002 to 2018. 20 Baseline characteristics were compared in 2‐year survey intervals from 1999 through 2018.
Outcome measures were age‐ and sex‐adjusted using the direct method to the 2000 US Census projected population by 6 age‐sex groups (men and women of 20–39, 40–59 years, and >60 years). 21 For cholesterol screening, we assessed the proportions of having cholesterol screened ever or within 5 years. For lipid levels, we calculated the arithmetic means of TC, LDL‐C, and HDL‐C; geometric means were presented for triglycerides as the distribution was heavily skewed. We also assessed the prevalence of high LDL‐C (defined as ≥130 mg/dL without a history of ASCVD or ≥70 mg/dL with prior ASCVD). For medication use, we investigated the proportions by drug classes or combinations (eg, statin or statin plus another nonstatin therapy) among the overall statin‐eligible population defined by the 2018 American College of Cardiology/American Heart Association (ACC/AHA) guideline criteria. 9 This population included individuals with a history of ASCVD, LDL‐C ≥190 mg/dL, diabetes aged 40 to 75 years, or 10‐year ASCVD risk ≥7.5% aged 40 to 75 years and LDL‐C 70 to 189 mg/dL. We also assessed statin use by these statin‐eligibility subgroups.
Trends analyses were conducted using the NHANES 1999 to 2018 data. We used the midpoint of each 2‐year survey time as a continuous variable to test for linear trends using linear or logistic regression models. If the overall model fit improved after adding a quadratic term of survey time, we then modeled the trends using piecewise spline models with 1 inflection point to facilitate clinical interpretations of nonlinear trends (Data S1). We conducted exploratory analyses to examine homogeneity of trends by race and ethnicity using an interaction term of time with subgroups in the regression models.
To provide the most updated estimates on key outcomes, we also conducted a cross‐sectional analysis in NHANES participants using the combined data from 2017 to March 2020, up to the point when the NHANES program was suspended because of the coronavirus disease 2019 pandemic. 22 Among 9232 participants aged ≥20 years from NHANES 2017 to March 2020, we excluded those who did not have laboratory samples collected (n=688) or were pregnant at the time of examination (n=87), leaving a sample size of 8457 for the cross‐sectional analysis. However, the 2017 to March 2020 data set was not included in the trends analysis, as recommended by the analytic guidelines. 22
Participants with missing data on respective outcomes were excluded for the primary analysis, with missingness of 3.0% for cholesterol screening, 6.2% for total cholesterol and HDL, 6.8% for triglycerides and LDL cholesterol, and 0.1% for medication use. Per recommendations, 23 we conducted sensitivity analyses to account for the missingness of lipid levels using multiple imputation via multivariate normal distribution with 5 imputed data sets (more details in Data S1). All analyses were performed using Stata version 15.1 (StataCorp LLC) and R software version 3.6.3 (R Foundation for Statistical Computing), with a 2‐sided P value <0.05 considered statistically significant.
RESULTS
NHANES 1999 to 2018
The mean age of the study participants steadily increased from 46.4 years in 1999 to 2000 to 48.5 years in 2017 to 2018 (Table 1). The proportion of women was stable from 1999 through 2018, at ≈51%. The proportions of non‐Hispanic Asian participants ranged from 5.3% to 6.1%, Hispanic from 11.7% to 16.5%, non‐Hispanic Black from 11.2% to 12.1%, and non‐Hispanic White from 65.4% to 76.3% across all survey cycles. The proportions of individuals with less than a high school education declined from 24.9% in 1999 to 2000 to 11.2% in 2017 to 2018. The proportions of persons with a family income below the poverty threshold varied from 10.8% to 17.7% and those who did not have health insurance from 13.5% to 20.8% across all cycles.
Table 1.
Weighted Characteristics of Study Participants, NHANES 1999 to 2018
| Characteristics | 1999 to 2000 | 2001 to 2002 | 2003 to 2004 | 2005 to 2006 | 2007 to 2008 | 2009 to 2010 | 2011 to 2012 | 2013 to 2014 | 2015 to 2016 | 2017 to 2018 |
|---|---|---|---|---|---|---|---|---|---|---|
| No. of participants (unweighted) | 4186 | 4731 | 4523 | 4448 | 5650 | 5991 | 5262 | 5523 | 5404 | 5210 |
| Age, mean (SE), y | 46.4 (0.4) | 46.5 (0.5) | 46.6 (0.5) | 47.0 (0.7) | 47.0 (0.4) | 47.2 (0.5) | 47.6 (0.8) | 47.7 (0.4) | 48.2 (0.6) | 48.5 (0.5) |
| Women, n (%) | 2112 (51.1) | 2345 (51.3) | 2248 (51.3) | 2164 (50.9) | 2853 (51.4) | 3062 (51.2) | 2642 (51.5) | 2854 (51.4) | 2780 (51.3) | 2669 (51.3) |
| Race and ethnicity, n (%)* | n=4058 | n=4585 | n=4331 | n=4275 | n=5420 | n=5661 | n=5113 | n=5361 | n=5210 | n=4945 |
| Non‐Hispanic Asian | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 745 (5.3) | 630 (5.5) | 640 (6.0) | 748 (6.1) |
| Hispanic | 1384 (15.8) | 1185 (13.2) | 1031 (11.9) | 992 (11.7) | 1588 (13.9) | 1692 (14.5) | 1050 (14.6) | 1230 (15.1) | 1651 (15.7) | 1179 (16.5) |
| Non‐Hispanic Black | 820 (11.3) | 927 (11.0) | 899 (11.8) | 1045 (12.1) | 1171 (11.9) | 1082 (12.2) | 1387 (11.7) | 1127 (11.6) | 1145 (11.8) | 1225 (12.0) |
| Non‐Hispanic White | 1854 (73.0) | 2473 (75.8) | 2401 (76.3) | 2238 (76.2) | 2661 (74.2) | 2887 (73.3) | 1931 (68.3) | 2374 (67.8) | 1774 (66.5) | 1793 (65.4) |
| Education, n (%) | n=4172 | n=4723 | n=4514 | n=4441 | n=5644 | n=5977 | n=5258 | n=5518 | n=5401 | n=5198 |
| Less than 9th grade | 820 (8.0) | 687 (6.9) | 675 (6.9) | 561 (6.6) | 758 (6.9) | 744 (6.4) | 512 (5.9) | 436 (4.6) | 651 (6.1) | 450 (3.8) |
| 9th to 11th grade | 834 (16.9) | 768 (12.6) | 670 (11.6) | 673 (11.1) | 993 (13.7) | 965 (12.6) | 743 (11.0) | 753 (10.7) | 634 (8.5) | 590 (7.4) |
| High school graduate | 945 (26.2) | 1109 (25.3) | 1150 (27.3) | 1070 (25.2) | 1399 (25.5) | 1376 (22.9) | 1106 (20.3) | 1245 (21.8) | 1171 (20.7) | 1240 (27.2) |
| Some college or Associate degree | 927 (27.0) | 1225 (29.4) | 1222 (31.2) | 1267 (31.4) | 1443 (28.8) | 1676 (30.4) | 1575 (32.0) | 1699 (32.7) | 1599 (32.5) | 1669 (30.7) |
| College graduate or higher | 646 (21.9) | 934 (25.8) | 797 (23.0) | 870 (25.8) | 1051 (25.2) | 1216 (27.7) | 1322 (30.9) | 1385 (30.2) | 1346 (32.2) | 1249 (30.8) |
| Family income‐to‐poverty ratio, n (%) | n=3586 | n=4392 | n=4259 | n=4237 | n=5126 | n=5410 | n=4814 | n=5098 | n=4845 | n=4522 |
| <100% | 724 (15.4) | 757 (13.6) | 775 (12.7) | 705 (10.8) | 1056 (14.1) | 1208 (14.6) | 1226 (17.7) | 1138 (15.7) | 1087 (14.3) | 826 (12.5) |
| 100% to 299% | 1547 (38.0) | 1819 (35.1) | 1901 (38.4) | 1752 (35.7) | 2268 (36.9) | 2282 (35.6) | 1898 (35.3) | 2010 (35.9) | 2148 (37.2) | 2039 (36.2) |
| 300% to 499% | 719 (23.9) | 937 (25.5) | 892 (26.8) | 966 (27.5) | 914 (22.7) | 1023 (24.3) | 868 (22.5) | 1002 (22.5) | 821 (21.4) | 862 (24.4) |
| ≥500% | 596 (22.8) | 879 (25.8) | 691 (22.1) | 814 (26.0) | 888 (26.3) | 897 (25.5) | 822 (24.4) | 948 (25.9) | 789 (27.0) | 795 (27.0) |
| Type of health insurance, n (%)† | n=4090 | n=4626 | n=4461 | n=4430 | n=5635 | n=5971 | n=5235 | n=5493 | n=5330 | n=5134 |
| Private | 1918 (58.5) | 2233 (59.4) | 1833 (54.9) | 1935 (54.8) | 2300 (55.0) | 2332 (52.4) | 2112 (50.5) | 2340 (51.5) | 2102 (49.9) | 1847 (45.5) |
| Government | 1310 (22.9) | 1517 (23.8) | 1748 (27.0) | 1504 (26.3) | 1997 (25.5) | 2114 (26.8) | 1871 (29.5) | 1987 (30.4) | 2295 (36.6) | 2505 (40.6) |
| Uninsured | 862 (18.6) | 876 (16.8) | 880 (18.2) | 991 (19.0) | 1338 (19.5) | 1525 (20.8) | 1252 (20.1) | 1166 (18.1) | 933 (13.5) | 782 (13.9) |
| History of atherosclerotic cardiovascular disease, n (%)‡ | 437 (8.0) | 491 (7.8) | 577 (9.0) | 483 (8.2) | 602 (7.8) | 596 (7.5) | 489 (7.7) | 513 (7.9) | 537 (8.0) | 590 (9.0) |
NHANES indicates National Health and Nutrition Examination Survey; and SE, standard error.
Non‐Hispanic Asian was included in the demographic survey questionnaire starting in NHANES 2011–2012.
Government insurance included Medicare, Medi‐Gap, Medicaid, State Children's Health Insurance Program, military health care, Indian Health Service, and other government insurance.
Atherosclerotic cardiovascular disease was defined as a self‐reported history of coronary heart disease, heart attack, stroke, or angina.
Cholesterol Screening
Age‐ and sex‐adjusted proportions of ever cholesterol screening increased from 70.3% (95% CI, 67.1–73.3) in 1999 to 2000 to 74.3% (95% CI, 72.5–76.0) in 2011 to 2012, then increased at a faster rate to 81.1% (95% CI, 78.8–83.2) in 2017 to 2018 in the overall population (P=0.009 for overall nonlinear trend) (Figure 1). Differences in slopes between 1999 to 2012 and 2013 to 2018 time periods were significant (P<0.001). Proportions of cholesterol screening within 5 years were slightly lower than those of ever screened, but the trends were largely consistent. Overall, the proportion of having cholesterol screened within 5 years increased from 63.2% (95% CI, 60.0–66.3) in 1999 to 2000 to 72.5% (95% CI, 69.5–75.3) in 2017 to 2018 (P<0.001 for overall linear trend).
Figure 1. Age‐/sex‐adjusted trends in cholesterol screening in US adults, NHANES 1999 to 2018.
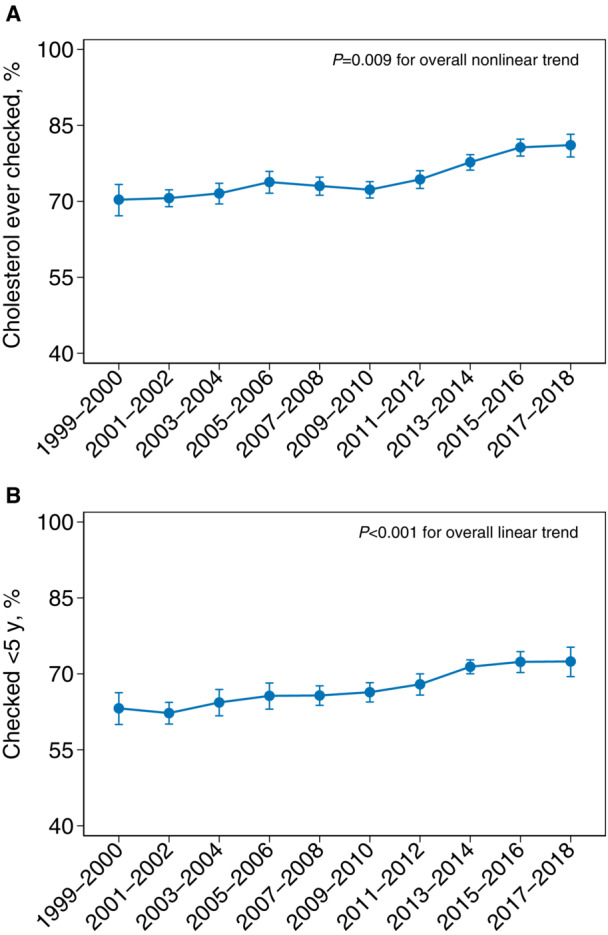
A,Cholesterol ever screened. B, Cholesterol screened within 5 years. Error bars indicate 95% CIs. NHANES indicates National Health and Nutrition Examination Survey.
Lipid Levels
Age‐ and sex‐adjusted mean TC decreased from 203.3 mg/dL (95% CI, 201.0–205.7) in 1999 to 2000 to 188.4 mg/dL (95% CI, 185.4–191.5) in 2017 to 2018 (P<0.001 for overall linear trend) in the overall US population (Figure 2). Mean triglycerides decreased from 121.3 mg/dL (95% CI, 116.4, 126.4) in 1999 to 2000 to 111.4 mg/dL (95% CI, 107.5–115.5) in 2007 to 2008, then continued to decrease to 91.4 mg/dL (95% CI, 88.4–94.6) in 2017 to 2018 (P<0.001 for overall nonlinear trend). Differences in slopes for mean triglycerides between the 1999 to 2008 and 2009 to 2018 time periods were significant (P<0.001). Mean LDL‐C levels in the overall population decreased drastically from 127.9 mg/dL (95% CI, 125.3–130.5) in 1999 to 2000 to 118.9 mg/dL (95% CI, 116.8–121.0) in 2003 to 2004, then continued to decline to 111.7 mg/dL (95% CI, 109.0–114.4) in 2017 to 2018 (P<0.001 for overall nonlinear trend). Mean HDL‐C increased from 1999 through 2006, and then fluctuated around 54 mg/dL after 2005 to 2006 (P<0.001 for overall nonlinear trend). Estimates of mean lipid levels after multiple imputations were similar to results from the primary analysis (Figure S1 and Table S1).
Figure 2. Age‐sex‐adjusted trends in lipid levels in US adults, National Health and Nutrition Examination Survey (NHANES) 1999 to 2018.
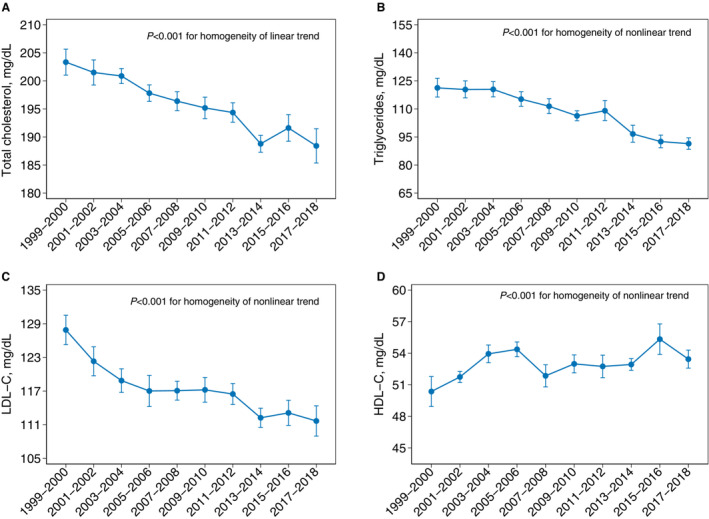
A,Mean total cholesterol. B, Mean triglycerides. C, Mean LDL‐C. D, HDL‐C. Screened within 5 years. Error bars indicate 95% CIs. HDL‐C indicates high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; and NHANES, National Health and Nutrition Examination Survey.
During the same period, the prevalence of LDL‐C ≥130 mg/dL among US adults without ASCVD decreased from 44.0% (95% CI, 41.5–46.4) in 1999 to 2000 to 26.4% (95% CI, 24.1–28.7) in 2017 to 2018 (P<0.001 for overall linear trend) (Figure S2). The prevalence of LDL‐C ≥70 mg/dL among US adults with ASCVD showed a nonlinear declining trend, with 95.8% (95% CI 72.2, 99.5) in 1999 to 2000 and 76.3% (95% CI, 64.7–84.9) in 2017 to 2018 (P=0.017 for overall nonlinear trend).
Lipid‐Lowering Medication
Among US adults eligible for statin treatment by the 2018 ACC/AHA guideline criteria, the age‐ and sex‐adjusted proportion of statin use increased from 14.9% (95% CI, 12.2–17.9) in 1999 to 2000 to 24.6% (95% CI, 20.4–29.3) in 2007 to 2008 and continued to improve, yet at a slower rate, to 27.8% (95% CI, 23.0–33.2) in 2017 to 2018 (P<0.001 for overall nonlinear trend) (Figure 3). The slopes between 1999 to 2008 and 2009 to 2018 periods were significantly different (P<0.001). When stratified by statin‐eligibility subgroups, the adjusted proportion of statin use increased from 23.0% (95% CI, 18.9–28.8) in 1999 to 2000 to 37.0% (95% CI, 30.2–44.2) in 2011 to 2012, and then did not increase afterwards in US adults with a history of ASCVD (P=0.005 for nonlinear trend). Among US adults aged 40 to 75 years with 10‐year ASCVD risk ≥7.5% and LDL‐C 70 to 189 mg/dL, statin use increased from 6.8% (95% CI, 3.9–11.6) in 1999 to 2000 to 31.1% (95% CI, 21.3–42.9) in 2013 to 2014 and declined to 19.1% (95% CI, 14.8–24.2) in 2017 to 2018 (P=0.03 for nonlinear trend). On the contrary, among US adults with diabetes aged 40 to 75 years, the proportion of statin use increased steadily from 21.4% (95% CI, 13.2–32.7) in 1999 to 2000 to 51.9% (95% CI, 41.2–62.5) in 2017 to 2018 (P<0.001 for overall linear trend).
Figure 3. Age‐sex‐adjusted trends in statin use in US adults by statin‐eligible groups, NHANES 1999 to 2018.
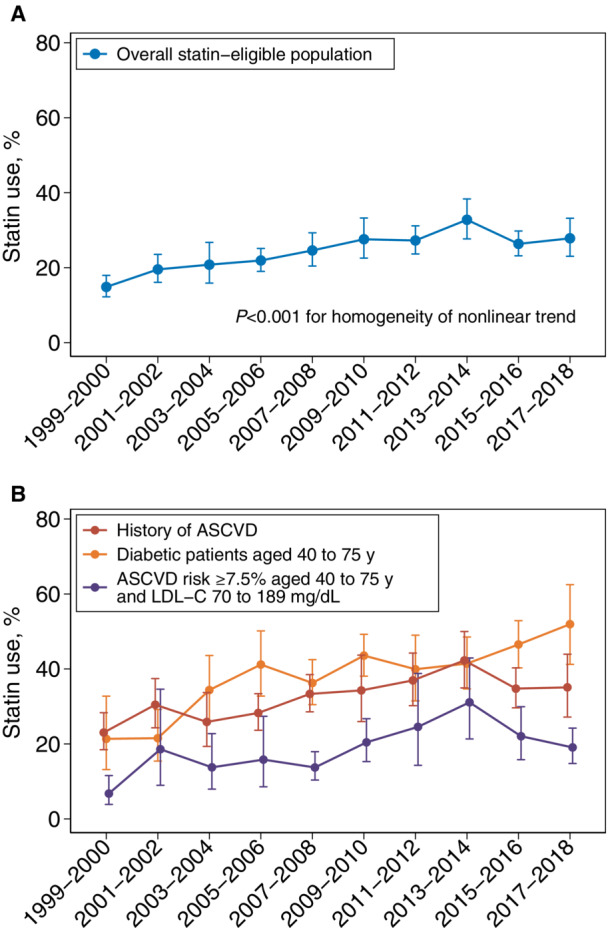
A,Overall. B, By statin‐eligible groups. ASCVD was defined as a self‐reported history of coronary heart disease, heart attack, stroke, or angina. Error bars indicate 95% CIs. ASCVD indicates atherosclerotic cardiovascular disease; and NHANES, National Health and Nutrition Examination Survey.
Age‐ and sex‐adjusted proportions of statin plus another lipid‐lowering medication remained mostly <5% from 1999 through 2018 (Figure S3). Use of ezetimibe declined after 2007 to 2008, while use of fibric acid agents and bile acid sequestrants remained consistently low (Figure S4). Age‐ and sex‐adjusted proportion of inhibitor use in statin‐eligible US adults was 0.02% (95% CI, 0.01–0.16) in 2017 to 2018.
Exploratory Analyses by Race and Ethnicity
There were significant differences in trends by race and ethnicity with respect to proportions of both cholesterol screening measures and in mean TC and LDL‐C levels (all P<0.01 for homogeneity of trends) (Figures S5 and S6). The age‐ and sex‐adjusted proportions of cholesterol ever screened and cholesterol screening within 5 years were consistently and significantly lower in Hispanic participants compared with White participants (P<0.001 for group differences across all survey cycles) (Table S2). Mean triglyceride levels in Black individuals and HDL‐C levels in Hispanic individuals were consistently lower (P<0.05 for group differences across all survey cycles), when compared with White individuals (Table S3).
NHANES 2017 to March 2020 Prepandemic
In the 2017 to March 2020 prepandemic population, age‐ and sex‐adjusted proportions of ever cholesterol screening and cholesterol screening within 5 years among US adults were 81.3% (95% CI, 79.5–83.0) and 72.6% (95% CI, 70.5–74.6), respectively (Table 2). Mean levels of TC, triglycerides, LDL‐C, and HDL‐C were 186.6 mg/dL (95% CI, 184.3–189.0), 90.6 mg/dL (95% CI, 87.9–93.3), 110.5 mg/dL (95% CI, 108.2–112.9), and 53.5 mg/dL (95% CI, 52.8–54.2), respectively. Among US adults eligible for statins, the adjusted proportions were 32.1% (95% CI, 24.0–41.6) in statin use, 4.0% (95% CI, 1.3–11.1) in statin use plus another nonstatin therapy, 3.7% (95% CI, 1.3–9.8) in ezetimibe use, and 0.03% (95% CI, 0.01–0.15) for PCSK9 inhibitors.
Table 2.
Age‐/Sex‐Adjusted Point Estimates (95% CIs) in Cholesterol Screening, Lipid Levels, and Lipid‐Lowering Medication Use
| Outcomes | NHANES 2017 to 2018 | NHANES 2017 to March 2020 prepandemic |
|---|---|---|
| Proportions of cholesterol screening, % | ||
| Ever screened | 81.1 (78.8–83.2) | 81.3 (79.5–83.0) |
| Screened within 5 y | 72.5 (69.5–75.3) | 72.6 (70.5–74.6) |
| Mean lipid levels, mg/dL | ||
| Total cholesterol | 188.4 (185.4–191.5) | 186.6 (184.3–189.0) |
| Triglycerides | 91.4 (88.4–94.6) | 90.6 (87.9–93.3) |
| LDL‐C | 111.7 (109.0–114.4) | 110.5 (108.2–112.9) |
| HDL‐C | 53.4 (52.6–54.3) | 53.5 (52.8–54.2) |
| Proportions of lipid‐lowering medication use (among statin‐eligible US adults), % | ||
| Statin only | 27.8 (23.0–33.2) | 32.1 (24.0–41.6) |
| Stain plus another lipid‐lowering medication | 1.5 (1.0–2.4) | 4.0 (1.3–11.1) |
| Ezetimibe | 1.5 (0.8–2.7) | 3.7 (1.3–9.8) |
| Fibric acid agents | 1.6 (0.9–2.9) | 2.3 (1.3–3.8) |
| Bile acid sequestrants | 0.5 (0.2–1.3) | 0.4 (0.2–1.2) |
| PCSK9 inhibitors | 0.02 (0.01–0.16) | 0.03 (0.01–0.15) |
HDL‐C indicates high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; NHANES, National Health and Nutrition Examination Survey; and PCSK9, proprotein convertase subtilisin/kexin type 9.
DISCUSSION
This analysis of a nationally representative sample of >50 000 patients comprehensively examines and updates trends in lipid levels and lipid‐lowering medication use over the past 2 decades. Our study found a continuation of favorable temporal trends 3 , 7 , 8 in serum cholesterol screening, mean levels of TC, triglycerides, LDL‐C, and HDL‐C, and lipid‐lowering medication use among US adults aged ≥20 years from 1999 through 2018. The proportions of ever cholesterol screening and cholesterol screening within 5 years increased over the past 2 decades, to 81.1% and 72.5%, respectively. Mean population TC declined to 188.4 mg/dL in 2017 to 2018 from 203.3 mg/dL in 1999 to 2000. Mean triglyceride and LDL‐C levels also declined to 91.4 mg/dL and 111.7 mg/dL, respectively, in 2017 to 2018, while mean HDL‐C fluctuated around 54 mg/dL after a mild increase during 1999 to 2004.
Any trend comparison between the 2017 to March 2020 data and data from the previous 2‐year NHANES cycles should be interpreted with caution, and the Centers for Disease Control and Prevention (CDC) recommend considering the historical context of trends when doing so. 24 With that caveat, the point estimates derived from 2017 to March 2020 appear to show marginally higher percentages of cholesterol screening and lipid‐lowering medication use and lower lipid levels, with 32.1% of eligible patients prescribed a statin and 4% prescribed a statin in addition to another lipid‐lowering medication in 2017 to March 2020. In part, these results may be a consequence of an improvement in screening, clinician‐patient risk discussion, and prescription of statin and nonstatin therapies over time. It is also possible that the 2017 to March 2020 point estimates reflect some uptake of the 2018 AHA/ACC Guideline on the Management of Blood Cholesterol. 6 , 25 However, the incomplete data collected during the 2019 to 2020 cycle were not nationally representative and consequently combined with the 2017 to 2018 NHANES cycle, which would not reflect the AHA/ACC guidelines as they were released late in 2018. Thus, despite efforts made to adjust and weight the 2017 to 2020 data in order to make it nationally representative, potential unequal rates of dissemination and uptake by geographical location make it challenging to ascertain the extent to which guideline uptake was reflected or assess for potential improvement over time. Regardless, with less than a third of eligible patients prescribed a statin in 2017 to March 2020, there remains significant room for improvement.
Lipid management is an important priority for the CDC. However, of the 3 Healthy People 2020 goals released by the CDC that target hyperlipidemia, only 1 was achieved. Specifically, only the Healthy People 2020 goal of reducing the proportion of adults with TC ≥240 mg/dL to <13.5% was achieved; this proportion was 9.7% in 2017 to March 2020, based on our analysis. Moreover, in the most recent NHANES survey cycle (2017 to March 2020), ≈26% of US adults without ASCVD had levels of LDL‐C ≥130 mg/dL, and 82% of US adults with ASCVD had LDL‐C ≥70 mg/dL. 6 It is challenging to assess rates of control given that the most recent guidelines focus on treating risk rather than targeting specific lipid levels. However, previously reported barriers to the utilization of evidence‐based therapies might play a role in our findings. 26 , 27 , 28 , 29 These include clinical inertia, lack of robust systems to systematically identify eligible patients, clinician lack of confidence navigating perceived statin intolerance, and patient fear of side effects or discontinuation because of perceived side effects. The literature also highlights gaps in patient knowledge, presenting an opportunity for increased patient education and shared decision‐making. 30 Given that cardiovascular risk reduction is proportional to LDL‐C reduction and that aggressive lowering of LDL‐C further improves cardiovascular outcomes, our findings highlight the need to intensify national efforts to improve guideline‐concordant therapy with multipronged interventions at the systems, clinician, and patient levels. 31
Our work aligns with and extends previously described trends in statin use. 8 , 29 , 32 In particular, an evaluation of NHANES 2005 to 2016 showed that overall statin use remained unchanged between 2013 to 2014 and 2015 to 2016. With an additional 2 years of data, our study shows a continuation of this plateau. The temporal trends in statin use by statin‐eligible groups before 2015 to 2016 were similar between our results and the ones from Patel et al. 8 However, in terms of proportion estimates, our numbers were ≈10% lower than their study, likely attributable to variations in the definitions of ASCVD as well as the inclusion of LDL‐C ≥190 mg/dL and exclusion of LDL‐C conditions for participants with diabetes in our study. Notably, we found that of all the subgroups of statin‐eligible adults, statin use only continued to increase after 2015 to 2016 in the group of patients with diabetes aged 40 to 75 years. In US adults with ASCVD risk ≥7.5% aged 40 to 75 years and LDL‐C 70 to 189 mg/dL, statin use declined after 2013 to 2014. Increased recognition of coronary artery calcification scoring and identification of risk enhancers for risk stratification for patients with ASCVD risk 7.5% to 20% may have contributed to the trend in patients with ASCVD risk ≥7.5%. 33 Our work is also consistent with and extends contemporary work showing low use of statin therapy in patients with established ASCVD. 29 We observed that the age‐/sex‐adjusted proportion of statin use was 35.1% in 2017 to 2018 and 39.6% in 2017 to March 2020 among US adults with ASCVD, which is lower than the corresponding value in the study by Nelson et al (50.1%). However, our sample includes patients who were uninsured and, thus, it is understandable that our patient population would have a greater gap compared with a patient population of only privately insured patients. Regardless, it is concerning that such a large proportion of patients with ASCVD (60.4% in 2017 to March 2020) were not taking a statin. Contemporary literature suggests that at least some of this is caused by nonadherence, underscoring the value of revisiting risk discussion at multiple timepoints in a patient's care continuum. 25 This could be complemented by smart decision‐support tools for clinicians that clearly describe indications at the point‐of‐care, search tools that allow clinicians to look up recommendations to answer practical questions, particularly around side effects, and empowering nonphysician members of the team to be involved in revisiting statin initiation. 29 , 34
There are a few limitations worth noting. First, given our cross‐sectional study design, we were unable to provide any definitive explanation for causes behind the observed trends. Future studies may explore plausible causes and suggest any clinical actions to correct or mitigate suboptimal cholesterol trends. Second, we could not assess longitudinal changes in outcomes of interest at an individual level, and NHANES does not provide data on duration of medication use. Thus, our cohort included patients who may have been prescribed a statin that were subsequently discontinued over time. Understanding what proportion of eligible patients was prescribed a statin that was then discontinued, and reasons for doing so, should be a high priority to address statin underuse particularly in high‐risk populations such as patients with established ASCVD. On a similar note, given the cross‐sectional study design, we were also unable to determine whether the trends we observed have directly led to deleterious clinical outcomes; nevertheless, the relationship of LDL‐C and ASCVD outcomes is well‐established. Third, because of the impact of the coronavirus pandemic, we were unable to include data past 2018 in the trends analysis and were thus unable to fully capture the impact on lipid management by the release of 2018 ACC/AHA guidelines. 33 Fourth, even though NHANES is a representative sample of the US noninstitutionalized population, our findings may not be generalizable to other populations. Fifth, similar to prior literature, 35 we used the Pooled Cohort Equations to estimate 10‐year ASCVD risk in Hispanic and Asian individuals, which has not been validated. Sixth, although we have incorporated survey weights to account for participant nonresponse, 6.2% to 6.8% of missing lipid values could have affected our estimates. Nevertheless, our sensitivity analysis using multiple imputation yielded similar results compared with the primary analysis. Last, data are only available up to March 2020, and the coronavirus pandemic likely had a major impact on all assessed outcomes; this must be taken into account when extending implications for cardiovascular prevention to the postpandemic era.
In a nationally representative sample of US adults aged ≥20 years, cholesterol screening increased while mean TC, triglyceride, and LDL‐C levels decreased from 1999 to 2018. However, we found room for improvement with respect to LDL‐C levels in patients with ASCVD and statin use in statin‐eligible US adults. Use of nonstatin lipid‐lowering therapies such as ezetimibe and PCSK9 inhibitors also remains low. Multifaceted strategies to optimize utilization of lipid‐lowering therapies are needed.
Sources of Funding
None.
Disclosures
Dr Martin is a founder of and holds equity in Corrie Health; has received material support from Apple and iHealth; has received funding from the Maryland Innovation Initiative, Wallace H. Coulter Translational Research Partnership, Louis B. Thalheimer Fund, the Johns Hopkins Individualized Health Initiative, the American Heart Association (20SFRN35380046, 20SFRN35490003, COVID19‐811000, #878924, and #882415), the Patient‐Centered Outcomes Research Institute (ME‐2019C1‐15 328), the National Institutes of Health (P01 HL108800 and R01AG071032), the David and June Trone Family Foundation, the Pollin Digital Innovation Fund, the PJ Schafer Cardiovascular Research Fund, Sandra and Larry Small, CASCADE FH, Google, and Amgen; has received personal fees for serving on scientific advisory boards for Amgen, AstraZeneca, Dalcor, Esperion, Kaneka, Novartis, Novo Nordisk, Sanofi, and 89bio; and is a coinventor on a system for low‐density lipoprotein cholesterol estimation, for which Johns Hopkins has abandoned the patent application to make the system available without intellectual property restrictions. The remaining authors have no disclosures to report.
Supporting information
Data S1
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.028205
Y. Gao and L. M. Shah contributed equally.
This work was presented as an oral moderated poster presentation at the American College of Cardiology Scientific Sessions, April 2‐4, 2022.
For Sources of Funding and Disclosures, see page 9.
References
- 1. Murphy SL, Kochanek KD, Xu J, Arias E. Mortality in the United States, 2020. NCHS Data Brief. 2021;427:1–8. doi: 10.15620/cdc:112079 [DOI] [PubMed] [Google Scholar]
- 2. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore‐Mensah Y, et al. Heart disease and stroke statistics‐2022 update: a report from the American Heart Association. Circulation. 2022;145(8):e153–e639. doi: 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 3. Rosinger A, Carroll MD, Lacher D, Ogden C. Trends in total cholesterol, triglycerides, and low‐density lipoprotein in US adults, 1999‐2014. JAMA Cardiol. 2017;2:339–341. doi: 10.1001/jamacardio.2016.4396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris‐Etherton PM, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2292–2333. doi: 10.1161/CIR.0b013e3182160726 [DOI] [PubMed] [Google Scholar]
- 5. Voight BF, Peloso GM, Orho‐Melander M, Frikke‐Schmidt R, Barbalic M, Jensen MK, Hindy G, Holm H, Ding EL, Johnson T, et al. Plasma hdl cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380:572–580. doi: 10.1016/S0140-6736(12)60312-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella‐Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APHA/ASPC/NLA/PCNa guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;139:e1082–e1143. doi: 10.1161/CIR.0000000000000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. He J, Zhu Z, Bundy JD, Dorans KS, Chen J, Hamm LL. Trends in cardiovascular risk factors in us adults by race and ethnicity and socioeconomic status, 1999‐2018. JAMA. 2021;326:1286–1298. doi: 10.1001/jama.2021.15187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Patel N, Bhargava A, Kalra R, Parcha V, Arora G, Muntner P, Arora P. Trends in lipid, lipoproteins, and statin use among U.S. adults: impact of 2013 cholesterol guidelines. J Am Coll Cardiol. 2019;74:2525–2528. doi: 10.1016/j.jacc.2019.09.026 [DOI] [PubMed] [Google Scholar]
- 9. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129:S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a [DOI] [PubMed] [Google Scholar]
- 10. Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National health and nutrition examination survey: plan and operations, 1999‐2010. Vital and Health Statistics. Series 1; Washington, DC: National Center for Health Statistics; 2013:1–37. [PubMed] [Google Scholar]
- 11. Paulose‐Ram R, Burt V, Broitman L, Ahluwalia N. Overview of asian american data collection, release, and analysis: national health and nutrition examination survey 2011‐2018. Am J Public Health. 2017;107:916–921. doi: 10.2105/AJPH.2017.303815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fan W, Philip S, Toth PP, Granowitz C, Nathan DW. Estimated ascvd risk according to statin use in us adults with borderline triglycerides: results from national health and nutrition examination survey (nhanes) 2007‐2014. Am J Prev Cardiol. 2020;3:100087. doi: 10.1016/j.ajpc.2020.100087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goff DC Jr, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- 14. National Health and Nutrition Examination Survey . 2017–2018 data documentation, codebook, and frequencies (cholesterol). February 2020. Accessed November 2022. https://wwwn.cdc.gov/Nchs/Nhanes/2017‐2018/TCHOL_J.htm
- 15. National Health and Nutrition Examination Survey . Laboratory procedure manual (triglycerides). December 2020. Accessed November 2022. https://wwwn.cdc.gov/nchs/data/nhanes/2017‐2018/labmethods/TRIGLY‐J‐MET‐508.pdf
- 16. National Health and Nutrition Examination Survey . 2001–2002 data documentation, codebook, and frequencies (cholesterol). April 2010. Accessed November 2022. https://wwwn.cdc.gov/Nchs/Nhanes/2001‐2002/L13_B.htm
- 17. Martin SS, Blaha MJ, Elshazly MB, Toth PP, Kwiterovich PO, Blumenthal RS, Jones SR. Comparison of a novel method vs the friedewald equation for estimating low‐density lipoprotein cholesterol levels from the standard lipid profile. JAMA. 2013;310:2061–2068. doi: 10.1001/jama.2013.280532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Health and Nutrition Examination Survey . 1988–2020 data documentation, codebook, and frequencies (prescription medications). September 2021. Accessed November 2022. https://wwwn.cdc.gov/nchs/nhanes/1999‐2000/RXQ_DRUG.htm
- 19. Johnson CL, Paulose‐Ram R, Ogden CL, Carroll MD, Kruszon‐Moran D, Dohrmann SM, Curtin LR. National health and nutrition examination survey: analytic guidelines, 1999‐2010. Vital and Health Statistics. Series 2; Washington, DC: National Center for Health Statistics; 2013:1–24. [PubMed] [Google Scholar]
- 20. National Health and Nutrition Examination Survey . Module 3: weighting.
- 21. Ingram DD, Malec DJ, Makuc DM, Kruszon‐Moran D, Gindi RM, Albert M, Beresovsky V, Hamilton BE, Holmes J, Schiller J, et al. National center for health statistics guidelines for analysis of trends. Vital and Health Statistics. Series 2; Washington, DC: National Center for Health Statistics; 2018:1–71. [PubMed] [Google Scholar]
- 22. National Center for Health Statistics . Nhanes analytic guidance and brief overview for the 2017‐march 2020 pre‐pandemic data files. 2021.
- 23. Jakobsen JC, Gluud C, Wetterslev J, Winkel P. When and how should multiple imputation be used for handling missing data in randomised clinical trials ‐ a practical guide with flowcharts. BMC Med Res Methodol. 2017;17:162. doi: 10.1186/s12874-017-0442-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Akinbami LJ, Chen TC, Davy O, Ogden CL, Fink S, Clark J, Riddles MK, Mohadjer LK. National health and nutrition examination survey, 2017‐march 2020 prepandemic file: sample design, estimation, and analytic guidelines. Vital and Health Statistics. Series 1; Washington, DC: National Center for Health Statistics; 2022:1–36. [PubMed] [Google Scholar]
- 25. Martin SS, Sperling LS, Blaha MJ, Wilson PW, Gluckman TJ, Blumenthal RS, Stone NJ. Clinician‐patient risk discussion for atherosclerotic cardiovascular disease prevention: importance to implementation of the 2013 acc/aha guidelines. J Am Coll Cardiol. 2015;65:1361–1368. doi: 10.1016/j.jacc.2015.01.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Campbell DJ, Lee‐Krueger RC, McBrien K, Anderson T, Quan H, Leung AA, Chen G, Lu M, Naugler C, Butalia S. Strategies for enhancing the initiation of cholesterol lowering medication among patients at high cardiovascular disease risk: a qualitative descriptive exploration of patient and general practitioners' perspectives on a facilitated relay intervention in Alberta, Canada. BMJ Open. 2020;10:e038469. doi: 10.1136/bmjopen-2020-038469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Virani SS, Ballantyne CM, Petersen LA. Guideline‐concordant statin therapy use in secondary prevention: should the medical community wait for divine intervention? J Am Coll Cardiol. 2022;79:1814–1817. doi: 10.1016/j.jacc.2022.02.042 [DOI] [PubMed] [Google Scholar]
- 28. Bradley CK, Wang TY, Li S, Robinson JG, Roger VL, Goldberg AC, Virani SS, Louie MJ, Lee LV, Peterson ED, et al. Patient‐reported reasons for declining or discontinuing statin therapy: insights from the palm registry. J Am Heart Assoc. 2019;8:e011765. doi: 10.1161/JAHA.118.011765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nelson AJ, Haynes K, Shambhu S, Eapen Z, Cziraky MJ, Nanna MG, Calvert SB, Gallagher K, Pagidipati NJ, Granger CB. High‐intensity statin use among patients with atherosclerosis in the U.S. J Am Coll Cardiol. 2022;79:1802–1813. doi: 10.1016/j.jacc.2022.02.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nelson AJ, O'Brien EC, Kaltenbach LA, Green JB, Lopes RD, Morse CG, Al‐Khalidi HR, Aroda VR, Cavender MA, Gaynor T, et al. Use of lipid‐, blood pressure‐, and glucose‐lowering pharmacotherapy in patients with type 2 diabetes and atherosclerotic cardiovascular disease. JAMA Netw Open. 2022;5:e2148030. doi: 10.1001/jamanetworkopen.2021.48030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chamberlain AM, Gong Y, Shaw KM, Bian J, Song WL, Linton MF, Fonseca V, Price‐Haywood E, Guhl E, King JB, et al. Pcsk9 inhibitor use in the real world: data from the national patient‐centered research network. J Am Heart Assoc. 2019;8:e011246. doi: 10.1161/JAHA.118.011246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wong ND, Young D, Zhao Y, Nguyen H, Caballes J, Khan I, Sanchez RJ. Prevalence of the American College of Cardiology/American Heart Association statin eligibility groups, statin use, and low‐density lipoprotein cholesterol control in us adults using the national health and nutrition examination survey 2011‐2012. J Clin Lipidol. 2016;10:1109–1118. doi: 10.1016/j.jacl.2016.06.011 [DOI] [PubMed] [Google Scholar]
- 33. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella‐Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APHA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;139:e1046–e1081. [DOI] [PubMed] [Google Scholar]
- 34. Adusumalli S, Westover JE, Jacoby DS, Small DS, VanZandbergen C, Chen J, Cavella AM, Pepe R, Rareshide CAL, Snider CK, et al. Effect of passive choice and active choice interventions in the electronic health record to cardiologists on statin prescribing: a cluster randomized clinical trial. JAMA Cardiol. 2021;6:40–48. doi: 10.1001/jamacardio.2020.4730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rodriguez F, Chung S, Blum MR, Coulet A, Basu S, Palaniappan LP. Atherosclerotic cardiovascular disease risk prediction in disaggregated asian and hispanic subgroups using electronic health records. J Am Heart Assoc. 2019;8:e011874. doi: 10.1161/JAHA.118.011874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wells C. Approaches to Imputing Missing Data in Complex Survey Data. July 2018. Accessed December 2022. https://www.stata.com/meeting/canada18/slides/canada18_Wells.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Data Availability Statement
The authors declare that all supporting data are available within the article and its online supplementary files.


