Abstract
Background
The role of uric acid is gaining increasing importance in the evaluation of cardiovascular disease, but its relationship with atrial fibrillation (AF) is unclear. This study aims to investigate the association between uric acid levels and risk of new‐onset AF.
Methods and Results
A total of 339 604 individuals 30 to 60 years of age and free from cardiovascular disease at baseline (1985–1996) in the Swedish AMORIS (Apolipoprotein‐Mortality Risk) cohort were followed until December 31, 2019 for incident AF. Cox regression models were used to examine the association between uric acid and AF, adjusting for potential confounders and stratifying by incident cardiovascular disease. Over a mean follow‐up of 25.9 years, 46 516 incident AF cases occurred. Compared with the lowest uric acid quartile, each of the upper 3 quartiles were associated with an increased risk of AF in a dose–response manner. Adjusted hazard ratios were 1.09 (95% CI, 1.06–1.12) for second quartile, 1.19 (95% CI, 1.16–1.23) for third quartile, and 1.45 (95% CI, 1.41–1.49) for fourth quartile. The association was similar among individuals with and without incident hypertension, diabetes, heart failure, or coronary heart disease. The dose–response pattern was further supported in a subsample of individuals with repeated measurements of uric acid.
Conclusions
Elevated uric acid was associated with an increased risk of AF, not only among people with cardiovascular disease and cardiovascular risk factors but also among those without. Future investigations are needed to examine whether lowering uric acid is relevant for AF prevention.
Keywords: atrial fibrillation, biomarkers, cardiovascular disease, cohort studies, uric acid
Subject Categories: Atrial Fibrillation, Epidemiology, Risk Factors
Nonstandard Abbreviation and Acronym
- AMORIS
Apolipoprotein‐Mortality Risk
Clinical Perspective.
What Is New?
In this large population‐based study of 339 604 individuals, elevated uric acid in midlife is associated with an increased risk of atrial fibrillation later in life, not only among individuals with cardiovascular diseases and cardiovascular risk factors but also among those without.
What Are the Clinical Implications?
Elevated uric acid may not only operate through cardiovascular pathways to increase the risk of atrial fibrillation, it may also have a direct influence on the development of atrial fibrillation via other mechanisms.
Future investigations are needed to examine whether lowering uric acid is relevant for the prevention of atrial fibrillation in the general population settings.
Atrial fibrillation (AF) is the most common cardiac arrhythmia in old age, with an increasing incidence and prevalence worldwide. 1 , 2 Many studies have been conducted aiming at stroke prevention in patients with AF, whereas less is known about primary prevention of AF in the general population. Mounting evidence has shown that conventional cardiovascular risk factors (older age, male sex, smoking, obesity, hypertension, hyperlipidemia, and diabetes) are unable to fully explain the development of AF. 3 , 4 , 5
Uric acid is the final product of purine metabolism, catalyzed by xanthine oxidase. Its role in cardiovascular disease (CVD) and cardiometabolic disorders is gaining increasing importance, and elevated uric acid is associated with a higher risk of hypertension, diabetes, and coronary heart disease (CHD) in large studies. 6 , 7 Recent data, such as the Atherosclerosis Risk in Communities study and the Norwegian Tromsø Study, showed that high uric acid levels may also elevate the risk of AF. 8 , 9 Uric acid is highly related to inflammation and oxidative stress, which play a central role in the cause of AF. 6 , 10 However, robust evidence on the association between uric acid and AF risk is still scarce, especially among younger and healthier individuals. Most past studies were cross‐sectional or conducted in selected populations. It is also not known whether increased uric acid contributes to a higher risk of AF independently of established AF risk factors (eg, CVD and cardiovascular risk factors), or if it is just a marker for overall cardiometabolic burden. In addition, most previous studies have been limited by one‐time biomarker measurement, 11 and the reported associations might therefore be over‐ or underestimated because of changes in uric acid levels over time. Because the pathogenesis of AF is still not entirely clear, elucidating the association between uric acid and AF using a large prospective cohort may help to gain a better understanding of AF risk factors and thus lend support to better preventive strategies.
In this study, we aimed to investigate the association between uric acid levels in midlife (30–60 years of age) and the risk of AF later in life, using data from 339 604 healthy individuals in the Swedish AMORIS (Apolipoprotein‐Mortality Risk) cohort. Uric acid was measured at baseline, as well as repeatedly over a 5‐year period. The large sample size also allowed stratified analysis to examine whether the association differs by co‐occurring CVD and cardiovascular risk factors.
METHODS
Data Availability Statement
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the Department of Environmental Medicine, Karolinska Institutet (contact information available at https://ki.se/en/imm/amoris).
Study Population
The large population‐based AMORIS cohort was designed to study the associations between metabolism and inflammation biomarkers and chronic diseases. 12 The cohort included in total 812 073 Swedish individuals with enrollment and blood measurements from 1985 to 1996. These individuals had their blood tests taken either as part of a health assessment conducted at their workplaces (screening), or from regular blood examinations within primary or occupational health care. All laboratory tests were conducted on fresh blood samples by the Central Automation Laboratory, Stockholm, Sweden. Several clinically commonly assessed biomarkers (eg, total cholesterol, triglycerides, glucose, and serum creatinine) were included in a standard analysis package offered by the Central Automation Laboratory, and these biomarkers were available for a large proportion of all participants. 12 Uric acid is one of the biomarkers in the package, and 531 276 cohort members (65.4%) had at least 1 uric acid measurement.
Participants were followed up until December 31, 2019 with regard to vital status and hospital diagnoses through linkage with multiple national registers (eg, National Patient Register, Cause of Death Register, Total Population Register), using a unique Swedish personal identification number for each individual. All participants were followed from the date of their first laboratory test until the date of AF diagnosis, date of death, emigration, or end of follow‐up (December 31, 2019), whichever came first.
Figure 1 shows the flowcharts of the study population. A total of 350 384 individuals, whose uric acid level was measured at least once and who were 30 to 60 years of age at the first measurement, were included. Of these, 32.6% came from occupational health screening, 41.4% came from primary or occupational health care, and the rest underwent health examinations for undocumented reasons. We then excluded 1068 participants with a history of AF at baseline. We further excluded 7709 individuals with preexisting heart failure (HF), CHD, stroke or transient ischemic attack, hypertension, and diabetes; 72 individuals who died on the sampling date; 952 individuals who immigrated to Sweden; and 979 individuals who were lost to follow‐up because they lacked registration in the Total Population Register during the follow‐up, which means that any migration out of the country and vital status was unknown. Eventually, data from 339 604 individuals were analyzed for association between baseline uric acid and incident AF.
Figure 1. Flowchart of the study population.
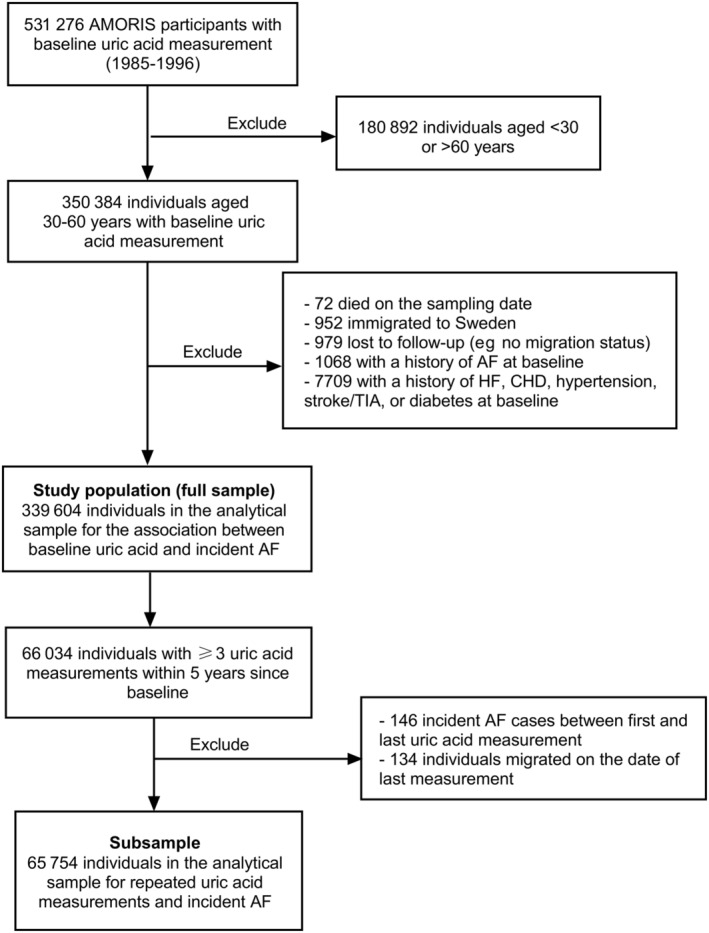
AF indicates atrial fibrillation; AMORIS, Apolipoprotein‐Related Mortality Risk; CHD, coronary heart disease; HF, heart failure; and TIA, transient ischemic attack.
To reduce within‐person variations in uric acid levels over time, we examined all individuals with at least 3 uric acid measurements within 5 years since baseline, for a total of 66 034 individuals (Figure 1). Of these, we further excluded 146 incident AF cases that occurred between the first and the last uric acid measurement and 134 individuals migrating on the date of last measurement. A total of 65 754 individuals were eventually included in this subsample.
This study complies with the Declaration of Helsinki and has been approved by the regional ethical committee at Karolinska Institutet, Stockholm, Sweden (reference number 2018/2401–31). The ethical board waived the need for informed consent because of the large number of participants in the cohort and because half of them had already died at the time of linkage of the cohort to registers after ethical vetting.
Assessment of Serum Uric Acid and Other Biomarkers
Serum uric acid (micromoles per liter) was measured by the enzymatic uricase method. Variation for uric acid determinations were <2.8% at 164 μmol/L (2.76 mg/dL), 2.3% at 470 μmol/L (7.90 mg/dL), and 1.8% at 624 μmol/L (10.49 mg/dL). Covariates included sex (men/women), age (years), blood glucose (millimoles per liter), total cholesterol (millimoles per liter), triglycerides (millimoles per liter), and estimated glomerular filtration rate (eGFR; milliliters per minute per 1.73 m2) at baseline.
Identification of AF and Other Conditions
Individuals developing AF during the follow‐up were identified in the Swedish National Patient Register and the Cause of Death Register. The Swedish National Patient Register contains information on hospital discharge records from inpatient care regionally since 1964 and nationally since 1987, and data on specialized outpatient care were available nationally since 2001. Information retrieved from this register includes the dates and discharge diagnoses of each hospital visit, and all discharge diagnoses were coded according to the International Classification of Diseases, Eighth Revision, Ninth Revision, and Tenth Revision (ICD‐8, ICD‐9, ICD‐10). A previous study showed that the sensitivity and specificity of AF diagnosis in the Swedish Patient Register were 80% and 93%, respectively, between 2001 and 2011. 13 The National Cause of Death Register is a complete register of all deaths in Sweden since 1952, with ICD codes of underlying and contributing causes of deaths. In this study, incident AF was identified as the first diagnosis appearing in either the National Patient Register or the Cause of Death Register (ICD‐8: 427.90 and 427.92; ICD‐9: 427.3; ICD‐10: I48).
Among those who developed incident AF, we ascertained incident diabetes (ICD‐8: 250; ICD‐9: 250, 251.D; ICD‐10: E10, E11, E13, E14), hypertension (ICD‐8: 400–404; ICD‐9: 401–405; ICD‐10: I10, I13, I15), HF (ICD‐8: 427.0, 427.1; ICD‐9: 402, 404, 425, 428; ICD‐10: I110, I130, I132, I27, I280, I42, I43, I515, I517, I528), or CHD (ICD‐8: 410–414; ICD‐9: 410–414; ICD‐10: I20‐25) cases only if they occurred before, on the same date, or within 6 months after AF diagnosis. Among those who did not develop incident AF, incident diabetes, hypertension, HF, or CHD cases that occurred before death, migration, or the end of follow‐up were ascertained.
Statistical Analysis
Cox proportional hazards models were used to examine the association between baseline uric acid and the risk of incident AF, with attained age as the time scale. Concentrations of uric acid were first dichotomized, and the cutoff value for hyperuricemia was >420 μmol/L in men and >340 μmol/L in women. 14 We also categorized uric acid into quartiles (separately in men and women), with the first quartile as the reference group (≤282 μmol/L for men and ≤205 μmol/L for women). The second quartile of uric acid corresponds to 283 to 319 μmol/L for men and 206 to 236 μmol/L for women, the third quartile corresponds to 320 to 361 μmol/L for men and 237 to 273 μmol/L for women, and the fourth quartile corresponds to ≥362 μmol/L for men and ≥274 μmol/L for women. All models were adjusted for age, sex, blood glucose, total cholesterol, triglycerides, and eGFR at the time of uric acid measurement. Stratified analyses were also performed to examine whether the association between uric acid and incident AF differs between people with and without incident diabetes, hypertension, HF, or CHD.
To reduce within‐person variations in uric acid assessments over time, a 5‐year cumulative average of uric acid was analyzed in relation to AF among a subsample of individuals with repeated measures of uric acid. Because uric acid is reported to be highly correlated over time within individuals, 15 taking the cumulative average provides a more precise estimate of uric acid levels over the 5‐year period. We then used the average to define hyperuricemia and to create quartiles in the same way as described in the baseline analysis. The start of follow‐up in this subsample is the day after the last uric acid measurement, and Cox regression models were applied to assess the association between cumulative average uric acid and incident AF, adjusting for age, sex, blood glucose, total cholesterol, triglycerides, and eGFR at baseline.
To assess whether the associations could be confounded by body mass index (BMI), we performed sensitivity analyses in a subgroup of 56 851 participants for whom BMI data were available on the same date or within 5 years before blood measurement. We then further adjusted all models for BMI. We also excluded people with gout diagnosis (ICD‐8, ICD‐9: 274, ICD‐10: M10) before baseline or during follow‐up, as a sensitivity analysis.
All statistical analyses were conducted using Stata 16.1 (StataCorp, College Station, TX). Effect estimates were presented as adjusted hazard ratio (HR) and 95% CI.
RESULTS
A total of 339 604 individuals were followed for a mean follow‐up of 25.9 years (SD, 7.9 years), and 46 516 incident AF cases (13.7%) were identified. Among those who developed incident AF, 32 287 individuals (69.4%) also developed incident hypertension, diabetes, HF, or CHD. Among those who did not develop incident AF, 121 397 (41.4%) individuals had incident hypertension, diabetes, HF, or CHD. The distribution of participant characteristics across uric acid quartiles is shown in the Table. Individuals with higher uric acid levels at baseline were more likely to have lower eGFR; higher total cholesterol, glucose, and triglycerides; and more frequently experienced incident cardiovascular events during the follow‐up.
Table 1.
Baseline Characteristics of the Study Population by Uric Acid Quartiles
| Characteristics | Total sample, n=339 604 | Uric acid quartiles | |||
|---|---|---|---|---|---|
| 1, n=86 738 | 2, n=83 987 | 3, n=84 617 | 4, n=84 262 | ||
| Women, n (%) | 154 047 (45.4) | 39 557 (45.6) | 37 871 (45.1) | 38 687 (45.7) | 37 932 (45.0) |
| Age, y, mean (SD) | 44.6 (8.0) | 43.2 (7.7) | 43.5 (7.9) | 44.2 (8.0) | 45.5 (8.2) |
| Uric acid, μmol/L, mean (SD) | 287.2 (72.6) | 218.6 (43.2) | 265.3 (41.1) | 300.2 (44.1) | 366.5 (62.5) |
| Total cholesterol, mmol/L, mean (SD) | 5.6 (1.1) | 5.4 (1.0) | 5.5 (1.0) | 5.7 (1.1) | 5.9 (1.2) |
| Glucose, mmol/L, mean (SD) | 4.9 (1.0) | 4.9 (1.3) | 4.9 (0.9) | 4.9 (0.9) | 5.1 (1.0) |
| Triglycerides, mmol/L, mean (SD) | 1.3 (1.0) | 1.1 (0.7) | 1.2 (0.8) | 1.3 (0.9) | 1.7 (1.4) |
| eGFR, mL/min per 1.73 m2, mean (SD) | 90.7 (13.7) | 94.8 (12.9) | 91.8 (13.1) | 89.6 (13.4) | 86.4 (14.2) |
| Incident AF, n (%) | 46 516 (13.7) | 10 033 (11.6) | 10 629 (12.7) | 11 817 (14.0) | 14 037 (16.7) |
| Incident cardiovascular conditions, n (%) | 153 684 (45.3) | 32 308 (37.3) | 34 835 (41.5) | 39 425 (46.6) | 47 116 (55.9) |
| Diabetes, n (%) | 41 804 (12.3) | 6403 (7.4) | 7783 (9.3) | 10 357 (12.2) | 17 261 (20.5) |
| Hypertension, n (%) | 120 712 (35.6) | 24 813 (28.6) | 27 308 (32.5) | 31 189 (36.9) | 37 402 (44.4) |
| Coronary heart disease, n (%) | 52 582 (15.5) | 11 054 (12.7) | 11 838 (14.1) | 13 295 (15.7) | 16 395 (19.5) |
| Heart failure, n (%) | 28 919 (8.5) | 5657 (6.5) | 6059 (7.2) | 7286 (8.6) | 9917 (11.8) |
| Body mass index,* kg/m2 | 24.4 (3.7) | 23.1 (3.1) | 23.8 (3.3) | 24.6 (3.6) | 26.3 (4.3) |
AF indicates atrial fibrillation; and eGRF, estimated glomerular filtration rate.
Data on body mass index were available in a subsample of 56 851 individuals.
Figure 2 shows the incidence rate and HR for AF by uric acid quartiles at baseline, shown in the total sample and stratified by incident hypertension, diabetes, HF, or CHD. All HRs were adjusted for age, sex, total cholesterol, glucose, eGFR, and triglycerides at the time of uric acid measurement. In the total sample, the upper 3 uric acid quartiles were associated with an increased risk of AF in a dose–response manner (second quartile: HR, 1.09 [95% CI, 1.06–1.12]; third quartile: HR, 1.19 [95% CI, 1.16–1.23]; fourth quartile: HR, 1.45 [95% CI, 1.41–1.49]), compared with the first. The HRs did not differ substantially between people who developed incident hypertension, diabetes, HF, or CHD and those who did not. However, with regard to absolute risk, the incidence rate of AF was substantially higher among people with incident hypertension, diabetes, HF, or CHD than those without. When uric acid was analyzed as a dichotomous variable, hyperuricemia at baseline was associated with an increased risk of AF (HR, 1.48 [95% CI, 1.43–1.53]) in the multivariate model (Table S1). Excluding individuals with gout diagnosis at baseline or during the follow‐up did not alter the results (HR, 1.08 [95% CI, 1.05–1.11] for the second uric acid quartile, 1.20 [95% CI, 1.16–1.23] for the third quartile, and 1.48 [95% CI, 1.43–1.52] for the fourth quartile, as compared with the first quartile). Furthermore, restricting the study sample to participants coming only from health screenings (n=111 573) yielded similar results as the main analyses (HR, 1.05 [95% CI, 1.00–1.11] for the second uric acid quartile, 1.18 [95% CI, 1.13–1.24] for the third quartile, and 1.43 [95% CI, 1.36–1.51] for the fourth quartile, as compared with the first quartile).
Figure 2. Hazard ratios and incidence rate (95% CI) of atrial fibrillation associated with quartiles of baseline uric acid in the total sample and stratified by incident hypertension, diabetes, heart failure, or coronary heart disease during follow‐up.
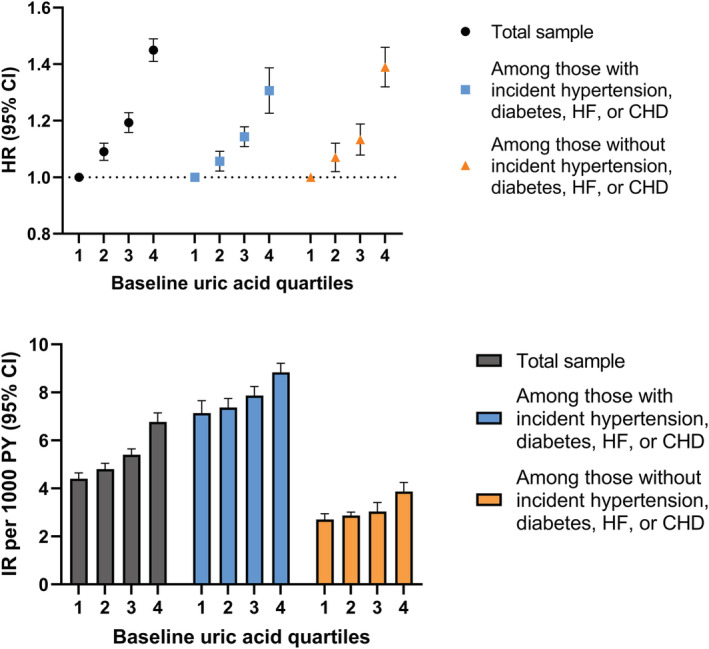
All hazard ratios were adjusted for age, sex, total cholesterol, glucose, estimated glomerular filtration rate, and triglycerides at the time of uric acid measurement. CHD indicates coronary heart disease; HF, heart failure; HR, hazard ratio; IR, incidence rate; and PY, person‐years.
To reduce within‐person variations in uric acid levels over time, a subsample of 65 754 individuals with ≥3 uric acid measurements within 5 years since baseline were included. The mean number of repeated uric acid measurements per person was 4.1 (SD, 1.7). A total of 51.0% had 3 uric acid measurements, 23.2% had 4, 13.1% had 5, and 13.6% had ≥6 (Figure S1). Figure 3 shows the HRs for AF by quartiles of cumulative average uric acid. The results are shown in the total sample as well as stratified by incident hypertension, diabetes, HF, or CHD. Similar to the results on baseline uric acid, the upper 3 quartiles of cumulative average uric acid were associated with a higher risk of AF in a dose–response manner (second quartile: HR, 1.18 [95% CI, 1.11–1.25]; third quartile: HR, 1.35 [95% CI, 1.27–1.44]; fourth quartile: HR, 1.68 [95% CI, 1.26–1.80]), compared with the first quartile. Similar associations were observed in the stratified analyses, meaning that the relative association between uric acid and AF was present both among individuals with and without incident hypertension, diabetes, HF, or CHD.
Figure 3. Hazard ratios (95% CI) of atrial fibrillation by quartiles of cumulative average uric acid in the total sample and stratified by incident hypertension, diabetes, heart failure, or coronary heart disease during follow‐up.
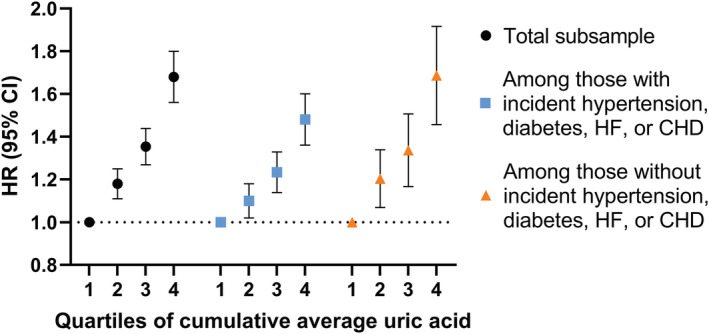
All hazard ratios were adjusted for age, sex, total cholesterol, glucose, estimated glomerular filtration rate, and triglycerides at the time of first uric acid measurement. CHD indicates coronary heart disease; HF, heart failure; and HR, hazard ratio.
Results of the sensitivity analyses showed that further adjusting for BMI attenuated the associations, but a higher risk of AF associated with hyperuricemia (HR, 1.24 [95% CI, 1.14–1.37]) or upper uric acid quartiles (third quartile: HR, 1.09 [95% CI, 1.02–1.17]; fourth quartile: HR, 1.21 [95% CI, 1.12–1.30]) still remained (Table S2).
DISCUSSION
Most previous studies investigating the association between uric acid and AF are of cross‐sectional nature. Only a few longitudinal studies exist, 8 , 9 , 11 , 16 , 17 and they mostly include older adults with preexisting cardiovascular conditions. This makes it difficult to disentangle the causal directions, as well as to know if the association differs by coexisting CVD and cardiovascular risk factors. In this large population‐based cohort study, the first and by far the largest to elucidate the association between uric acid and risk of AF in a younger population free of cardiovascular conditions at baseline, we found that increased uric acid in middle adulthood (30–60 years of age) was a significant risk factor for new‐onset AF. We also found that the association between uric acid and AF was similar among individuals who developed CVD during follow‐up and among those who did not. We additionally adjusted for a wide range of metabolic biomarkers that may correlate with uric acid and AF (eg, glucose, total cholesterol, eGFR, triglycerides) to minimize residual confounding. Taken together, this suggests that uric acid may not only be a reflection of cardiometabolic burden that further leads to AF, but it can also contribute to the onset of AF in a pathway other than prior onset of cardiovascular conditions. This hypothesis is strengthened by the dose–response relationship between long‐term elevation of uric acid and risk of AF.
Cumulating evidence supports serum uric acid as a marker for CVD and a measure of cardiovascular risk factor load. 6 , 7 In large population‐based studies, high uric acid levels were associated with various cardiovascular end points, including stroke, CHD, and HF. 18 , 19 , 20 , 21 Evidence from randomized controlled trials and animal experiments suggests that uric acid could be an independent causal factor for hypertension, 22 , 23 , 24 which is a known risk factor for AF. Although the mechanisms are not entirely clear, it has been suggested that inhibition of endothelial nitric oxide, microvascular renal lesions, and vascular inflammation could play a central role in the relationship between high uric acid and hypertension and other cardiovascular outcomes. 6 , 18 In the current study, it is likely that uric acid increases the risk of cardiovascular risk factors and CVD, which in turn elevates the risk of AF later in life, as shown in our stratified analyses.
In the current study, we also found a dose–response relationship between uric acid and AF in people without incident CVD and cardiovascular risk factors. The effect estimate for upper quartiles of uric acid in these individuals is even larger than those with CVD predisposed to AF (HR, 1.39 versus 1.23). It is, however, worth noting that the absolute risk of AF (ie, incidence rate) is still considerably higher among those with incident CVD than those without. Nevertheless, all these findings could suggest a potential direct association between uric acid and the onset of AF, independent of cardiovascular risk factors and co‐occurring CVD. The mechanism behind this association remains elusive. Uric acid is known to promote inflammation via the activation of proinflammatory cytokines, and inflammation has been suggested to be essential in the pathophysiological mechanisms of AF. 10 , 25 However, it is still debated whether inflammation is a cause or a consequence of AF. 26 Many studies have suggested that inflammation contributes to at least some type of AF, and inflammatory biomarkers, such as C‐reactive protein, were shown to be associated with an increased risk of AF in population‐based settings. 27 , 28 Moreover, uric acid can promote oxidative stress by reducing the production of nitric oxide in arterial endothelial cells and inhibiting vasodilation, which may be involved in the pathogenesis of AF. 25 , 29 Uric acid also has a direct effect on vascular smooth muscle cells proliferation by stimulating the vascular renin‐angiotensin system, 29 , 30 which may further promote arterial stiffness and increases the risk of AF development. 31 These potential mechanisms are supported by experimental studies where high uric acid levels were shown to directly promote atrial remodeling, ionic channel remodeling, and large left atrial size, leading to a higher risk of AF. 25 , 30
Our study is one of the first with repeated measures of uric acid. Uric acid levels are subject to change over time because of lifestyle, diet, or medication use. 32 Moreover, the risk could be different among individuals who have a 1‐point elevation of uric acid, from those with a longer duration of elevated uric acid. Only 1 study has examined repeated measurements of uric acid in relation to incident AF, using data from 107 360 individuals followed up for a mean of 6.7 years. 11 However, this study included all uric acid measurements from baseline to the end of follow‐up, which increases the risk of reverse causality (ie, a change to higher uric acid level could be a result of AF pathology). In our study, we allowed for a 5‐year exposure window to assess the average uric acid level, and all incident AF cases that occurred before the last uric acid measurement were excluded. This method reduces the risk that long‐term uric acid elevation could be a consequence of AF and lends weight to the inference on a temporal relationship between high uric acid and higher AF risk.
Our study has several strengths, including repeated measures of uric acid using a well‐documented method in the same clinical laboratory, a long follow‐up using high‐quality Swedish registers, a large enough sample to stratify by incident CVD and cardiovascular risk factors, and adjustments of several important metabolic biomarkers. However, there are also limitations. Medication data were not available at the time of uric acid measurement in this study. Uric acid levels can be influenced by medications such as diuretics. However, participants were relatively young, and individuals with a history of cardiovascular morbidities were excluded; therefore, it is unlikely that many were on cardiovascular medications at the time of enrollment. Moreover, other urate‐lowering medications, such as allopurinol, which is commonly used to prevent or treat gout, can also influence uric acid levels. It is possible that normal uric acid at baseline for some individuals is a result of prior treatment, or some with high levels at baseline can start treatment during follow‐up. However, in our sensitivity analyses, excluding people with a gout diagnosis did not alter the results. Furthermore, we could not consider lifestyle factors (eg, physical activity, diet, and alcohol consumption) because of lack of data, although uric acid levels can be reflective, at least partly, of lifestyle and dietary patterns. Moreover, it is likely that individuals with high uric acid at baseline received medical advice on lifestyle changes during the follow‐up, such as reducing the consumption of alcohol and purine‐rich food, 33 , 34 which may in turn lower the risk of developing AF. Therefore, the observed association between high uric acid and higher AF risk in this study may be underestimated. Of all lifestyle‐related factors, BMI is most strongly associated with risk of AF and thus could most likely have acted as a confounder. Therefore, in the sensitivity analyses, we additionally adjusted for BMI, and the association between high uric acid levels and higher risk of AF still remained. This suggests that the observed association cannot be fully explained by BMI. Nevertheless, residual confounding is still possible.
Taken together, our study shows that elevated uric acid levels in middle adulthood are associated with a higher risk of AF later in life. High uric acid not only operates through CVD and cardiovascular risk factors to increase the risk of AF, it may also have a direct influence on the development of AF via other mechanisms. Future studies are needed to further elucidate the mechanism by which uric acid may increase the risk of AF and in controlled randomized studies examine whether lowering uric acid levels may prevent the development of AF in a general population setting.
Sources of Funding
This study was supported by the Swedish Research Council (grant number 2020–01938), Loo and Hans Osterman Foundation for Medical Research (grant number 2021–00702), and Karolinska Institutet Research Foundation (grant number 2020–01569).
Disclosures
None.
Supporting information
Table S1–S2
Figure S1
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.027089
For Sources of Funding and Disclosures, see page 8.
References
- 1. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH, Zheng ZJ, et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton‐Cheh C, Lubitz SA, Magnani JW, Ellinor PT, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham heart study: a cohort study. Lancet. 2015;386:154–162. doi: 10.1016/S0140-6736(14)61774-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allan V, Honarbakhsh S, Casas JP, Wallace J, Hunter R, Schilling R, Perel P, Morley K, Banerjee A, Hemingway H. Are cardiovascular risk factors also associated with the incidence of atrial fibrillation?: a systematic review and field synopsis of 23 factors in 32 population‐based cohorts of 20 million participants. Thromb Haemost. 2017;117:837–850. doi: 10.1160/TH16-11-0825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D'Agostino RB, Newton‐Cheh C, Yamamoto JF, Magnani JW, Tadros TM, et al. Development of a risk score for atrial fibrillation (Framingham heart study): a community‐based cohort study. Lancet. 2009;373:739–745. doi: 10.1016/S0140-6736(09)60443-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ, MacLehose R, Konety S, Alonso A. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the atherosclerosis risk in communities (ARIC) study. Circulation. 2011;123:1501–1508. doi: 10.1161/CIRCULATIONAHA.110.009035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee SJ, Oh BK, Sung K‐C. Uric acid and cardiometabolic diseases. Clin Hypertens. 2020;26:13. doi: 10.1186/s40885-020-00146-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feig DI, Kang D‐H, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–1821. doi: 10.1056/NEJMra0800885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tamariz L, Agarwal S, Soliman EZ, Chamberlain AM, Prineas R, Folsom AR, Ambrose M, Alonso A. Association of serum uric acid with incident atrial fibrillation (from the atherosclerosis risk in communities [ARIC] study). Am J Cardiol. 2011;108:1272–1276. doi: 10.1016/j.amjcard.2011.06.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nyrnes A, Toft I, Njølstad I, Mathiesen EB, Wilsgaard T, Hansen JB, Løchen ML. Uric acid is associated with future atrial fibrillation: an 11‐year follow‐up of 6308 men and women—the Tromso study. Europace. 2014;16:320–326. doi: 10.1093/europace/eut260 [DOI] [PubMed] [Google Scholar]
- 10. Aviles RJ, Martin DO, Apperson‐Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. doi: 10.1161/01.CIR.0000103131.70301.4F [DOI] [PubMed] [Google Scholar]
- 11. Li S, Cheng J, Cui L, Gurol ME, Bhatt DL, Fonarow GC, Benjamin EJ, Xing A, Xia Y, Wu S, et al. Cohort study of repeated measurements of serum urate and risk of incident atrial fibrillation. J Am Heart Assoc. 2019;8:e012020. doi: 10.1161/JAHA.119.012020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Walldius G, Malmström H, Jungner I, de Faire U, Lambe M, Van Hemelrijck M, Hammar N. Cohort profile: the AMORIS cohort. Int J Epidemiol. 2017;46:1103–1103. doi: 10.1093/ije/dyw333 [DOI] [PubMed] [Google Scholar]
- 13. Baturova MA, Lindgren A, Carlson J, Shubik YV, Olsson SB, Platonov PG. Atrial fibrillation in patients with ischaemic stroke in the Swedish national patient registers: how much do we miss? Europace. 2014;16:1714–1719. doi: 10.1093/europace/euu165 [DOI] [PubMed] [Google Scholar]
- 14. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and nutrition examination survey 2007‐2008. Arthritis Rheum. 2011;63:3136–3141. doi: 10.1002/art.30520 [DOI] [PubMed] [Google Scholar]
- 15. Wang H, Jacobs DR, Gaffo AL, Gross MD, Goff DC, Carr JJ. Longitudinal association between serum urate and subclinical atherosclerosis: the coronary artery risk development in young adults (CARDIA) study. J Intern Med. 2013;274:594–609. doi: 10.1111/joim.12120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kawasoe S, Kubozono T, Yoshifuku S, Ojima S, Miyata M, Miyahara H, Maenohara S, Ohishi M. Uric acid level and new‐onset atrial fibrillation in the Japanese general population ‐ longitudinal study. Circ J. 2018;83:156–163. doi: 10.1253/circj.CJ-18-0508 [DOI] [PubMed] [Google Scholar]
- 17. Kwon CH, Lee SH, Lee JY, Ryu S, Sung KC. Uric acid and risk of atrial fibrillation in the Korean general population. Circ J. 2018;82:2728–2735. doi: 10.1253/circj.CJ-18-0748 [DOI] [PubMed] [Google Scholar]
- 18. Kanbay M, Segal M, Afsar B, Kang DH, Rodriguez‐Iturbe B, Johnson RJ. The role of uric acid in the pathogenesis of human cardiovascular disease. Heart. 2013;99:759–766. doi: 10.1136/heartjnl-2012-302535 [DOI] [PubMed] [Google Scholar]
- 19. Niskanen LK, Laaksonen DE, Nyyssönen K, Alfthan G, Lakka HM, Lakka TA, Salonen JT. Uric acid level as a risk factor for cardiovascular and all‐cause mortality in middle‐aged men: a prospective cohort study. Arch Intern Med. 2004;164:1546–1551. doi: 10.1001/archinte.164.14.1546 [DOI] [PubMed] [Google Scholar]
- 20. Huang H, Huang B, Li Y, Huang Y, Li J, Yao H, Jing X, Chen J, Wang J. Uric acid and risk of heart failure: a systematic review and meta‐analysis. Eur J Heart Fail. 2014;16:15–24. doi: 10.1093/eurjhf/hft132 [DOI] [PubMed] [Google Scholar]
- 21. Zhong C, Zhong X, Xu T, Xu T, Zhang Y. Sex‐specific relationship between serum uric acid and risk of stroke: a dose‐response meta‐analysis of prospective studies. J Am Heart Assoc. 2017;6:e005042. doi: 10.1161/JAHA.116.005042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuwabara M, Hisatome I, Niwa K, Hara S, Roncal‐Jimenez CA, Bjornstad P, Nakagawa T, Andres‐Hernando A, Sato Y, Jensen T, et al. Uric acid is a strong risk marker for developing hypertension from prehypertension: a 5‐year Japanese cohort study. Hypertension. 2018;71:78–86. doi: 10.1161/HYPERTENSIONAHA.117.10370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Forman JP, Scheven L, De Jong PE, Bakker SJL, Curhan GC, Gansevoort RT. Association between sodium intake and change in uric acid, urine albumin excretion, and the risk of developing hypertension. Circulation. 2012;125:3108–3116. doi: 10.1161/CIRCULATIONAHA.112.096115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, Lan HY, Kivlighn S, Johnson RJ. Elevated uric acid increases blood pressure in the rat by a novel crystal‐independent mechanism. Hypertension. 2001;38:1101–1106. doi: 10.1161/hy1101.092839 [DOI] [PubMed] [Google Scholar]
- 25. Deng Y, Liu F, Yang X, Xia Y. The key role of uric acid in oxidative stress, inflammation, fibrosis, apoptosis, and immunity in the pathogenesis of atrial fibrillation. Front Cardiovasc Med. 2021;8:641136. doi: 10.3389/fcvm.2021.641136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guo Y, Lip GYH, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012;60:2263–2270. doi: 10.1016/j.jacc.2012.04.063 [DOI] [PubMed] [Google Scholar]
- 27. Peña JM, MacFadyen J, Glynn RJ, Ridker PM. High‐sensitivity C‐reactive protein, statin therapy, and risks of atrial fibrillation: an exploratory analysis of the JUPITER trial. Eur Heart J. 2012;33:531–537. doi: 10.1093/eurheartj/ehr460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Conen D, Ridker PM, Everett BM, Tedrow UB, Rose L, Cook NR, Buring JE, Albert CM. A multimarker approach to assess the influence of inflammation on the incidence of atrial fibrillation in women. Eur Heart J. 2010;31:1730–1736. doi: 10.1093/eurheartj/ehq146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Corry DB, Eslami P, Yamamoto K, Nyby MD, Makino H, Tuck ML. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin‐angiotensin system. J Hypertens. 2008;26:269–275. doi: 10.1097/HJH.0b013e3282f240bf [DOI] [PubMed] [Google Scholar]
- 30. Kang DH, Han L, Ouyang X, Kahn AM, Kanellis J, Li P, Feng L, Nakagawa T, Watanabe S, Hosoyamada M, et al. Uric acid causes vascular smooth muscle cell proliferation by entering cells via a functional urate transporter. Am J Nephrol. 2005;25:425–433. doi: 10.1159/000087713 [DOI] [PubMed] [Google Scholar]
- 31. Shaikh AY, Wang N, Yin X, Larson MG, Vasan RS, Hamburg NM, Magnani JW, Ellinor PT, Lubitz SA, Mitchell GF, et al. Relations of arterial stiffness and brachial flow‐mediated dilation with new‐onset atrial fibrillation. Hypertension. 2016;68:590–596. doi: 10.1161/HYPERTENSIONAHA.116.07650 [DOI] [PubMed] [Google Scholar]
- 32. Choi HK, Liu S, Curhan G. Intake of purine‐rich foods, protein, and dairy products and relationship to serum levels of uric acid: the third national health and nutrition examination survey. Arthritis Rheum. 2005;52:283–289. doi: 10.1002/art.20761 [DOI] [PubMed] [Google Scholar]
- 33. De Oliveira EP, Burini RC. High plasma uric acid concentration: causes and consequences. Diabetol Metab Syndr. 2012;4:4. doi: 10.1186/1758-5996-4-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Choi HK. A prescription for lifestyle change in patients with hyperuricemia and gout. Curr Opin Rheumatol. 2010;22:165–172. doi: 10.1097/BOR.0b013e328335ef38 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S2
Figure S1
Data Availability Statement
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the Department of Environmental Medicine, Karolinska Institutet (contact information available at https://ki.se/en/imm/amoris).


