Abstract
Background
Hypertrophic cardiomyopathy (HCM) is a genetic disorder affecting not only the myocardium but also the mitral valve (MV) and its apparatus. This study aimed to investigate the prognostic implication of MV disease and its progression in East Asian patients with HCM.
Methods and Results
We assessed MV structure and function on the indexed echocardiogram of 1185 patients with HCM (mean±SD age, 60±14 years; men, 67%) in a longitudinal HCM registry, and 667 patients who performed follow‐up echocardiogram after 3 to 5 years were also analyzed. Progression of mitral regurgitation (MR) was defined as the increase of at least 1 grade. Clinical outcomes were defined as a composite of cardiovascular death, heart failure hospitalization, MV surgery or septal myectomy, and heart transplantation. Most of the entire cohort was nonobstructive type (n=1081 [91.2%]). A total of 278 patients (23.5%) showed at least mild MR on indexed echocardiogram. MR, systolic anterior motion, and mitral annular calcification were more prevalent in patients with obstructive HCM. During 7.0±4.0 years of follow‐up, presence of MR was independently associated with poor clinical outcomes (hazard ratio [HR], 1.60 [95% CI, 1.07–2.40]; P=0.023). On follow‐up echocardiogram, 67 (10.0%) patients showed MR progression, and it was independently associated with poor prognosis (HR, 2.46 [95% CI, 1.29–4.71]; P=0.007).
Conclusions
In East Asian patients with HCM whose major type is nonobstructive, MV disease is common. MR, systolic anterior motion, and mitral annular calcification are more prevalent in patients with obstructive HCM. The presence and progression of MR are associated with a poor prognosis in patients with HCM.
Keywords: hypertrophic cardiomyopathy, mitral regurgitation, mitral valve, prognosis
Subject Categories: Cardiomyopathy, Valvular Heart Disease
Nonstandard Abbreviations and Acronyms
- HCM
hypertrophic cardiomyopathy
- MAC
mitral annular calcification
- MR
mitral regurgitation
- MV
mitral valve
- SAM
systolic anterior motion
Clinical Perspective.
What Is New?
Mitral valve disease in patients with hypertrophic cardiomyopathy is common.
Mitral regurgitation, systolic anterior motion, and mitral annular calcification are more prevalent in patients with obstructive hypertrophic cardiomyopathy.
The presence and progression of mitral regurgitation are associated with a poor prognosis in patients with hypertrophic cardiomyopathy.
What Are the Clinical Implications?
Careful assessment of mitral valve functional abnormalities and detailed evaluation of mitral valve anatomical features are needed to predict subsequent complications in patients with hypertrophic cardiomyopathy.
Hypertrophic cardiomyopathy (HCM) is a genetic disorder characterized by asymmetric left ventricular (LV) hypertrophy and a broad clinical and morphological spectrum. 1 , 2 The prognosis of HCM has recently improved in large part because of the development of interventional and surgical treatments, such as implantable cardioverters‐defibrillators and septal myectomy. 3 , 4 Therefore, the life expectancy of patients with HCM has increased, and risk factors and outcomes of heart failure (HF) in patients with HCM have become important.
Since mitral regurgitation (MR) in patients with HCM first was described in the 1960s, 5 , 6 the diversity of structural and functional mitral valve (MV) alterations in HCM has been elucidated. 7 , 8 , 9 , 10 , 11 The common structural abnormalities of MV, which are observed easily on echocardiogram, include systolic anterior motion (SAM), MV prolapse, and mitral annular calcification (MAC), and these can be accompanied by MR or mitral stenosis (MS). Theoretically, these MV diseases can be a risk factor for HF aggravation through elevation of LV filling pressure in the hypertrophied and stiff myocardium. However, data about the prognostic implication of MV disease and its progression in patients with HCM are scarce. Therefore, in the present study, we sought to investigate the prognostic implication of representative MV disease and functional changes in East Asian patients with HCM in a single‐center longitudinal HCM registry.
METHODS
Anonymized data and materials are available at Yonsei University College of Medicine (Seoul, Korea). In addition, researchers who are interested in accessing the data may request by reasonable contact to Yonsei University College of Medicine.
Study Population
We identified 1193 patients who were diagnosed as having HCM in a single‐center HCM registry between January 2005 and December 2016. The diagnosis of HCM was based on echocardiographic demonstration of a hypertrophied, nondilated LV in the absence of another cardiac or systemic disease that could produce a comparable magnitude of LV hypertrophy. 2 For this study, we excluded patients with previous septal myectomy and prior MV surgery before the indexed echocardiogram. Finally, 1185 patients (mean±SD age, 60±14 years; men, 67%) were included in the analysis. Patients were classified into 2 groups based on presence of LV outflow tract (LVOT) obstruction: obstructive (group 1; n=104) and nonobstructive (group 2; n=1081) HCM. LVOT obstruction was defined as peak pressure gradient of the LVOT ≥30 mm Hg on continuous‐wave Doppler echocardiogram at rest or with physiologic provocation. 2 Patients' clinical data, medications, echocardiographic characteristics, including MV structural and functional abnormalities, and clinical outcomes were reviewed retrospectively. Among the total study population, 667 patients who underwent the follow‐up echocardiogram between 3 and 5 years after the index echocardiogram without any clinical outcomes between the index and follow‐up echocardiogram were additionally collected and analyzed. The study was approved by the Institutional Review Board of Yonsei University Health System (Institutional Review Board number: 4‐2012‐0655) and conducted according to the Declaration of Helsinki. The institutional review board waived the need for the patient's informed consent because of the retrospective nature of this study.
Echocardiography
Two‐dimensional and Doppler measurements were performed using a commercially available ultrasound machine (Vivid E9; GE Health Care, Horten, Norway; Philips iE33; Philips Medical System, Endover, MA, USA) with a 2.5‐ to 3.5‐MHz probe, according to the American Society of Echocardiography guidelines. 12 , 13 LV ejection fraction was measured using the biplane Simpson's method in apical 4‐ and 2‐chamber views. Left atrial (LA) volume index was measured by the biplane method as the end of ventricular systole and indexed to the body surface area. From the mitral inflow velocities, we obtained data on peak velocity of early and late filling and deceleration time of early velocity. Early diastolic tissue Doppler velocities were measured at the septal mitral annulus.
The severity and mechanism of MV dysfunction, including MR and MS, were assessed according to American Society of Echocardiography guidelines. 14 , 15 The degrees of MR and MS, if present, were graded as mild, moderate, or severe using an integrated approach. 14 , 15 Significant valvular dysfunction was defined as at least moderate degree of valvular dysfunction. SAM was evaluated by both the parasternal long‐axis and the apical 3‐chamber views using 2‐dimensional echocardiogram. SAM was defined as the systolic motion of the mitral leaflets into the LVOT, resulting in turbulent flow. 12 MV prolapse was defined as end‐systolic displacement of the MV leaflet at least 2 mm above the plane of the mitral annulus in the parasternal long‐axis view. 16 MAC was defined by an intense echocardiograph‐producing structure at the junction of the atrioventricular groove and both mitral leaflets in the parasternal long or short axis or apical 4‐chamber view. 17 Intrinsic MV disease was defined as a structural problem of the MV leaflet or adjacent structures, represented by MV prolapse or MAC. MR regression/progression was defined as the decreased/increased grade by at least 1 grade on follow‐up echocardiogram. Echocardiographic variables were reviewed by 2 expert cardiologists (DY Kim, J Seo, I Cho, GR Hong, JW Ha, CY Shim) who were blinded to the clinical results.
Clinical Outcomes
Clinical outcomes were defined as a composite of cardiovascular death, HF hospitalization, MV surgery or septal myectomy, and heart transplantation. Surgical treatments, including MV surgery, septal myectomy, and heart transplantation, were decided at the discretion of clinicians. If a patient had at least 2 clinical events, the first event was included for end points.
Statistical Analysis
Continuous variables are presented as mean±SD, and categorical variables are presented as number and percentage for each group. Comparisons of baseline clinical and echocardiographic parameters were analyzed using Student t‐test for continuous variables and χ2 test for categorical variables. Clinical outcomes were constructed using Kaplan‐Meier methods, and comparisons among groups were performed using a log‐rank test. The significance of MV disease or progression of MV disease on clinical outcomes was analyzed with univariate and multivariate Cox proportional hazard regression models. P<0.05 was considered statistically significant. All statistical analyses were performed using SPSS 25.0 software (IBM Corp, Armonk, NY).
RESULTS
Baseline Clinical and Echocardiographic Characteristics
Baseline clinical and echocardiographic characteristics of patients in the 2 groups are shown in Table 1. Most of the entire cohort was nonobstructive type (n=1081 [91.2%]). Compared with obstructive HCM, patients with nonobstructive HCM were older, and more were men. There was no difference in the proportion of comorbidities between the 2 groups, except a history of alcohol septal ablation and implantable cardioverters‐defibrillators. In terms of drug treatment, β‐blockers and calcium channel blockers were prescribed more often in the group with obstructive HCM. Renin‐angiotensin‐aldosterone blockers and diuretics were prescribed more often in the group with nonobstructive HCM, but no statistical differences were seen. As expected, because the classification of both groups was based on echocardiographic findings, patients with obstructive HCM had smaller LV chambers and increased LV wall thickness than those with nonobstructive HCM. In addition, larger mean LA volume index and higher LV ejection fraction, early diastolic mitral inflow velocity, and ratio of early diastolic mitral inflow velocity/early diastolic mitral annular tissue velocity were observed in the group with obstructive HCM compared with the group with nonobstructive HCM.
Table 1.
Baseline Clinical and Echocardiographic Characteristics
| Characteristic | Total (n=1185) | Obstructive HCM (n=104) | Nonobstructive HCM (n=1081) | P value |
|---|---|---|---|---|
| Clinical variables | ||||
| Age, y | 59.5±13.9 | 56.1±15.3 | 59.8±13.7 | 0.009 |
| Male sex, n (%) | 791 (66.8) | 60 (57.7) | 732 (67.7) | 0.038 |
| BMI, kg/m2 | 24.7±3.8 | 24.6±3.4 | 24.8±3.8 | 0.746 |
| Hypertension, n (%) | 606 (51.1) | 53 (51.0) | 553 (51.2) | 0.970 |
| Diabetes, n (%) | 199 (16.8) | 18 (17.3) | 181 (16.7) | 0.883 |
| Dyslipidemia, n (%) | 248 (20.9) | 21 (20.2) | 227 (21.0) | 0.847 |
| CKD, n (%) | 56 (4.7) | 3 (2.9) | 53 (4.9) | 0.354 |
| Atrial fibrillation, n (%) | 258 (21.8) | 18 (17.3) | 240 (22.2) | 0.248 |
| CAD, n (%) | 124 (10.5) | 12 (11.5) | 112 (10.4) | 0.708 |
| Prior alcohol ablation, n (%) | 3 (0.3) | 2 (1.9) | 1 (0.1) | <0.001 |
| Presence of ICD, n (%) | 48 (4.1) | 11 (10.6) | 37 (3.4) | <0.001 |
| Medications, n (%) | ||||
| RAAS blockers | 454 (38.3) | 31 (29.8) | 423 (39.1) | 0.062 |
| β‐Blockers | 562 (47.4) | 65 (62.5) | 497 (46.0) | 0.001 |
| CCBs | 458 (38.6) | 54 (51.9) | 404 (37.4) | 0.004 |
| Diuretics | 233 (19.7) | 13 (12.5) | 220 (20.4) | 0.054 |
| Statin | 304 (25.7) | 27 (26.0) | 277 (25.6) | 0.940 |
| Antiplatelet agents | 466 (39.3) | 35 (33.7) | 431 (39.9) | 0.215 |
| Anticoagulants | 144 (12.2) | 9 (8.7) | 135 (12.5) | 0.253 |
| Echocardiographic variables | ||||
| LVEDD, mm | 46.5±6.8 | 43.9±7.1 | 46.8±6.7 | <0.001 |
| LVESD, mm | 31.6±6.5 | 28.0±5.1 | 31.9±6.5 | <0.001 |
| LV maximal thickness, mm | 18.3±3.7 | 19.9±5.1 | 18.2±3.5 | 0.001 |
| IVS thickness, mm | 14.0±4.3 | 17.5±5.4 | 13.7±4.1 | <0.001 |
| PW thickness, mm | 10.7±2.0 | 11.3±2.6 | 10.6±1.9 | 0.016 |
| LVEF, % | 68.9±8.5 | 73.7±8.2 | 68.5±8.4 | <0.001 |
| LA volume index, mL/m2 | 38.3±20.5 | 42.4±15.4 | 37.8±20.9 | 0.041 |
| E velocity, m/s | 0.63±0.19 | 0.70±0.23 | 0.63±0.19 | 0.003 |
| E/e' | 15.1±6.4 | 18.9±7.6 | 14.7±6.1 | <0.001 |
| RVSP, mm Hg | 28.3±8.2 | 29.1±7.7 | 28.2±8.2 | 0.346 |
BMI indicates body mass index; CAD, coronary artery disease; CCB, calcium channel blocker; CKD, chronic kidney disease; E, mitral inflow early diastolic filling; E/e', ratio of early diastolic mitral inflow velocity/early diastolic mitral annular tissue velocity; HCM, hypertrophic cardiomyopathy; ICD, implantable cardioverter‐defibrillator; IVS, interventricular septum; LA, left atrial; LV, left ventricular; LVEDD, LV end‐diastolic dimension; LVEF, LV ejection fraction; LVESD, LV end‐systolic dimension; PW, posterior wall; RAAS, renin‐angiotensin‐aldosterone system; and RVSP, right ventricular systolic pressure.
MV Functional and Structural Abnormalities
The functional and structural abnormalities of MV are presented in Table 2. Of the total 1185 patients, 278 (23.5%) had at least mild MR. The percentage of patients with MR was high in obstructive HCM, but the overall number of patients was high in nonobstructive HCM because of the higher number of nonobstructive patients in our cohort. A further analysis of 278 patients with MR is described in Table S1. In this result, patients with nonobstructive HCM were older (mean±SD age, 63.7±13.6 versus 57.0±15.8 years; P=0.001) and had more prevalent atrial fibrillation (40.2% versus 23.7%; P=0.020) than those with obstructive HCM. Only 1 patient who had residual LVOT obstruction after alcohol septal ablation showed mild MR. Patients with obstructive HCM with MR had a significantly higher rate of MAC than those with nonobstructive HCM with MR (37.3% versus 16.0%; P<0.001). These findings suggest that the stiffer myocardium and its consequent hemodynamic load in obstructive HCM might have caused structural and functional abnormalities of the MV.
Table 2.
MV Functional and Structural Abnormalities
| Variable | Total (n=1185) | Obstructive HCM (n=104) | Nonobstructive HCM (n=1081) | P value |
|---|---|---|---|---|
| MV function | ||||
| MR, n (%) | 278 (23.5) | 59 (56.7) | 219 (20.3) | <0.001 |
| Mild | 232 (19.6) | 43 (41.3) | 189 (17.5) | |
| Moderate | 35 (3.0) | 13 (12.5) | 22 (2.0) | |
| Severe | 11 (0.9) | 3 (2.9) | 8 (0.7) | |
| MS | 19 (1.6) | 4 (3.8) | 15 (1.4) | 0.057 |
| Mild, n (%) | 19 (1.6) | 4 (3.8) | 15 (1.8) | |
| Moderate, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Severe, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| MDPG, mm Hg | 2.4±1.1 | 2.4±1.3 | 2.3±1.1 | 0.979 |
| MV structure, n (%) | ||||
| SAM | 148 (12.5) | 97 (93.3) | 51 (4.7) | <0.001 |
| MV prolapse | 29 (2.4) | 3 (2.9) | 26 (2.4) | 0.762 |
| Posterior | 8 (0.7) | 2 (1.9) | 6 (0.6) | |
| Anterior | 16 (1.4) | 1 (1.0) | 15 (1.4) | |
| Both | 5 (0.4) | 0 (0.0) | 5 (0.5) | |
| MAC | 154 (13.0) | 29 (27.9) | 125 (11.6) | <0.001 |
| Posterior | 151 (12.7) | 29 (27.9) | 122 (11.3) | |
| Anterior | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Both | 3 (0.3) | 0 (0.0) | 3 (0.3) | |
HCM indicates hypertrophic cardiomyopathy; MAC, mitral annular calcification; MDPG, mean diastolic pressure gradient; MR, mitral regurgitation; MS, mitral stenosis; MV, mitral valve; and SAM, systolic anterior motion.
There was no MS above moderate in either group, and mild MS was rare in 19 (1.6%) patients. The incidence of MS tended to be higher in obstructive HCM, but statistical significance was marginal. In 19 patients with MS, all had the morphological features of MAC, and 1 patient (5.2%) had the combined features of MAC and rheumatic valve disease. On the Doppler assessment of MS, the mean diastolic pressure gradient was 2.4±1.1 mm Hg, and the results showed no differences between the 2 types of HCM. In terms of the degree of MR in all patients with MS, 5 patients (26.3%) had mild MR and 3 patients (15.8%) had significant MR (moderate MR or higher). However, the presence of MR in patients with MS did not show the significant difference of clinical outcomes.
The overall prevalence of SAM, MV prolapse, and MAC was 12.5%, 2.4%, and 13.0%, respectively. MAC was more prevalent in obstructive HCM than in nonobstructive HCM, but there were no significant differences in the prevalence of MV prolapse. The relationship between the structural MV abnormalities and MR is shown in Table S2. The patients with the presence of MAC or MV prolapse revealed a significantly higher incidence of MR and significant MR.
Among 97 patients who had SAM in obstructive HCM, 66 (68.0%) did not have intrinsic MV disease, but 31 (32.0%) had combined intrinsic MV disease. When we analyzed the functional characteristics according to the presence of intrinsic MV disease, patients with combined intrinsic MV disease showed a higher prevalence of either presence of MR (P=0.001) or significant MR (P=0.001) (Table S3).
In 1081 patients with nonobstructive HCM, 30 (2.8%) had significant MR. Among the patients with significant MR, more than half of patients (n=16 [53.3%]) had a degenerative change of MV: 12 patients with leaflet thickening and 4 patients with MAC; 8 patients (26.7%) had MV prolapse, and 4 patients (13.3%) had functional change (3 patients of mitral annular dilation and 1 patient of MV tethering). Two patients (6.7%) had both MAC and MV prolapse.
Cardiovascular Outcomes According to MV Abnormalities in HCM
During 7.0±4.0 years of follow‐up after the indexed echocardiogram, total clinical events occurred in 126 patients (10.6%) (Table 3). There was significantly more MV surgery or septal myectomy events in the group with obstructive HCM than in the group with nonobstructive HCM. However, events of cardiovascular death, HF hospitalization, and heart transplantation were not significantly different between the 2 groups. The causes of cardiovascular death were aggravated HF (n=9), sudden cardiac death (n=9), acute myocardial infarction (n=2), and aortic dissection (n=1), in order of frequency. When the entire cohort was divided by the presence of MR, patients with MR had significantly more events than those without MR, not only in composite clinical outcomes but also in all event categories, except heart transplantation. In addition, clinical events were analyzed according to the presence of MR in each type of HCM. In obstructive HCM, the presence or absence of MR did not make a difference in all clinical events. However, in the group with nonobstructive HCM, patients with the presence of MR showed poorer clinical outcomes, except heart transplantation, compared with those without MR.
Table 3.
Clinical Outcomes
| Outcome | Total (n=1185) | Obstructive (n=104) | Nonobstructive (n=1081) | P value | MR (n=278) | No MR (n=907) | P value |
|---|---|---|---|---|---|---|---|
| Composite clinical outcomes | 126 (10.6) | 24 (23.1) | 102 (9.4) | <0.001 | 60 (21.6) | 66 (7.3) | <0.001 |
| Cardiovascular death | 21 (1.8) | 1 (1.0) | 20 (1.9) | 0.512 | 10 (3.6) | 11 (1.2) | 0.008 |
| HF hospitalization | 90 (7.6) | 7 (6.7) | 83 (7.7) | 0.728 | 38 (13.7) | 52 (5.7) | <0.001 |
| MV surgery/septal myectomy | 23 (1.9) | 19 (18.3) | 4 (0.4) | <0.001 | 17 (6.1) | 6 (0.7) | <0.001 |
| Heart transplantation | 6 (0.5) | 0 (0.0) | 6 (0.6) | 0.446 | 2 (0.7) | 4 (0.4) | 0.567 |
| Obstructive (n=104) | Nonobstructive (n=1081) | |||||
|---|---|---|---|---|---|---|
| MR (n=59) | No MR (n=45) | P value | MR (n=219) | No MR (n=862) | P value | |
| Composite clinical outcomes | 16 (27.1) | 8 (17.8) | 0.263 | 44 (20.1) | 58 (6.7) | <0.001 |
| Cardiovascular death | 1 (1.7) | 0 (0.0) | 0.380 | 9 (4.1) | 11 (1.3) | 0.005 |
| HF hospitalization | 4 (6.8) | 3 (6.7) | 0.982 | 34 (15.5) | 49 (5.7) | <0.001 |
| MV surgery/septal myectomy | 13 (22.0) | 6 (13.0) | 0.255 | 4 (1.8) | 0 (0.0) | <0.001 |
| Heart transplantation | 0 (0.0) | 0 (0.0) | … | 2 (0.9) | 4 (0.5) | 0.424 |
Data are given as number (percentage) of each group. HF indicates heart failure; MR, mitral regurgitation; and MV, mitral valve.
Figures 1 and 2 show event‐free survival based on key patient characteristics. Patients with obstructive HCM had poorer clinical outcomes than those with nonobstructive HCM (log‐rank P<0.001) (Figure 1A). When the patients were divided into 4 groups according to type of HCM and sex, female patients with obstructive HCM had the worst clinical outcomes compared with other groups (log‐rank P<0.001) (Figure 1B). When the patients were divided by presence of MR, those with MR had poorer clinical outcomes than those without MR (Figure 2A). When the patients with HCM were divided into 4 groups according to types of HCM and presence of MR, patients with obstructive HCM and MR had the worst clinical outcomes compared with other groups (log‐rank P<0.001), and the presence of MR was associated with poorer outcomes regardless of HCM type (Figure 2B). When the patients with HCM were divided into 4 groups according to sex and presence of MR, female patients with MR had worse clinical outcomes than other groups (log‐rank P<0.001). Also in this subgroup, the presence of MR was associated with poorer outcomes, regardless of sex (Figure 2C). Table 4 shows the factors associated with clinical outcomes in HCM. In multivariate analysis, female sex (hazard ratio [HR], 2.14 [95% CI, 1.46–3.14]; P<0.001), chronic kidney disease (HR, 1.97 [95% CI, 1.07–3.65]; P=0.031), atrial fibrillation (HR, 2.23 [95% CI, 1.49–3.32]; P<0.001), larger LA volume index (HR, 1.01 [95% CI, 1.01–1.02]; P<0.001), higher ratio of early diastolic mitral inflow velocity/early diastolic mitral annular tissue velocity (HR, 1.05 [95% CI, 1.03–1.08]; P<0.001), presence of MR (HR, 1.60 [95% CI, 1.07–2.40]; P=0.023), and presence of MAC (HR, 1.74 [95% CI, 1.12–2.68]; P=0.013) were significant independent predictors for clinical outcomes.
Figure 1. Kaplan‐Meier analysis of freedom from clinical outcomes, according to type of HCM (A) and sex (B).
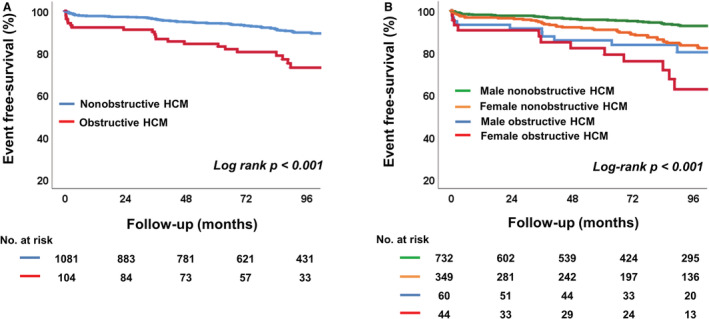
HCM indicates hypertrophic cardiomyopathy.
Figure 2. Kaplan‐Meier analysis of freedom from clinical outcomes.
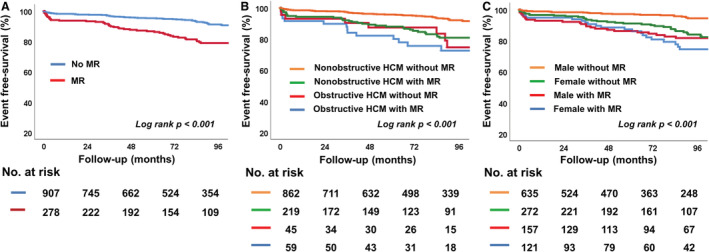
A, Comparison in 2 groups according to presence of MR. B, Comparison in 4 groups classified by type of HCM and presence of MR. C, Comparison in 4 groups classified by sex and presence of MR. HCM indicates hypertrophic cardiomyopathy; and MR, mitral regurgitation.
Table 4.
Cox Regression Analysis for Clinical Outcomes in HCM
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | P value | HR | 95% CI | P value |
| Age | 1.02 | 1.01–1.03 | 0.010 | 0.99 | 0.98–1.01 | 0.391 |
| Female sex | 2.41 | 1.70–3.42 | <0.001 | 2.14 | 1.46–3.14 | <0.001 |
| Hypertension | 1.38 | 0.97–1.97 | 0.074 | 1.25 | 0.84–1.88 | 0.275 |
| Diabetes | 1.47 | 0.96–2.24 | 0.078 | 1.48 | 0.93–2.35 | 0.099 |
| CKD | 3.14 | 1.83–5.39 | <0.001 | 1.97 | 1.07–3.65 | 0.031 |
| Atrial fibrillation | 3.38 | 2.39–4.80 | <0.001 | 2.23 | 1.49–3.32 | <0.001 |
| Obstructive type | 2.59 | 1.66–4.04 | <0.001 | 1.59 | 0.96–2.66 | 0.073 |
| LA volume index | 1.02 | 1.01–1.02 | <0.001 | 1.01 | 1.01–1.02 | <0.001 |
| E/e' | 1.09 | 1.06–1.11 | <0.001 | 1.05 | 1.03–1.08 | <0.001 |
| MR | 3.08 | 2.17–4.38 | <0.001 | 1.60 | 1.07–2.40 | 0.023 |
| MAC | 2.79 | 1.86–4.18 | <0.001 | 1.74 | 1.12–2.68 | 0.013 |
| MV prolapse | 1.93 | 0.85–4.39 | 0.117 | |||
CKD indicates chronic kidney disease; E/e', ratio of early diastolic mitral inflow velocity/early diastolic mitral annular tissue velocity; HCM, hypertrophic cardiomyopathy; HR, hazard ratio; LA, left atrial; MAC, mitral annular calcification; MR, mitral regurgitation; and MV, mitral valve.
Changes of MV Abnormalities and Clinical Outcomes
The changes of echocardiographic variables are shown in Table 5. The mean follow‐up duration between 2 echocardiograms was 4.3±0.6 years. Left chamber size, LV maximal thickness, and LV diastolic parameters were significantly altered, suggesting disease progression in HCM. Although the total incidence of MR had a minimal difference of only 1%, a significant number of patients had MR progression (n=67 [10.0%]) and MR regression (n=53 [7.9%]) on follow‐up echocardiogram. The incidence of MS increased significantly (n=19 [2.8%]) with the increase of MAC (n=52 [7.8%]). The patients with MR progression had poorer clinical outcomes than those without (Figure 3A). When the patients with HCM were divided into 4 groups according to types of HCM and progression of MR, patients with obstructive HCM with MR progression had the worse clinical outcomes (log‐rank P<0.001), and the MR progression was associated with poorer outcomes regardless of HCM type (Figure 3B). In the Cox regression analysis that included the changes of MV abnormalities, including MR and MAC (Table 6), female sex (HR, 2.77 [95% CI, 1.54–4.97]; P<0.001), atrial fibrillation (HR, 2.11 [95% CI, 1.09–4.11]; P=0.028), larger LA volume index (HR, 1.02 [95% CI, 1.00–1.04]; P=0.034), and MR progression (HR, 2.46 [95% CI, 1.29–4.71]; P=0.007), these factors were independently associated with clinical outcomes. Furthermore, on the multiple comparisons in global χ2 test with post hoc analysis by Bonferroni's method (P=0.017), the addition of MR progression over demographic factors (age, sex, hypertension, diabetes, chronic kidney disease, and atrial fibrillation), obstructive type of HCM, and diastolic parameters, such as LA volume index and ratio of early diastolic mitral inflow velocity/early diastolic mitral annular tissue velocity, significantly improved the model's prognostic value for the primary outcomes (P=0.016) (Figure 4).
Table 5.
Baseline and Follow‐Up Echocardiogram in 667 Patients With HCM
| Variable | Baseline | Follow‐up | P value |
|---|---|---|---|
| LVEDD, mm | 47.9±4.7 | 48.4±4.9 | 0.007 |
| LVESD, mm | 30.3±4.4 | 31.0±4.8 | <0.001 |
| LV maximal thickness, mm | 18.4±3.9 | 19.3±4.0 | <0.001 |
| IVS thickness, mm | 14.1±4.4 | 14.5±4.6 | <0.001 |
| PW thickness, mm | 10.7±2.0 | 10.6±1.8 | 0.199 |
| LVEF, % | 69.2±8.1 | 68.1±7.7 | 0.001 |
| LA volume index, mL/m2 | 36.7±14.8 | 43.7±19.6 | <0.001 |
| E velocity, m/s | 0.63±0.2 | 0.66±0.2 | <0.001 |
| E/e' | 14.7±6.0 | 15.6±6.3 | <0.001 |
| RVSP, mm Hg | 28.1±7.7 | 29.3±9.3 | 0.002 |
| MV abnormalities, n (%) | |||
| MR | 150 (22.5) | 157 (23.5) | 0.649 |
| Mild | 128 (19.2) | 129 (19.3) | |
| Moderate | 19 (2.8) | 22 (3.3) | |
| Severe | 3 (0.4) | 6 (0.9) | |
| MR progression | 67 (10.0) | ||
| MR regression | 53 (7.9) | ||
| MS | 10 (1.5) | 29 (4.3) | 0.002 |
| MS progression | 19 (2.8) | ||
| MAC | 91 (13.6) | 132 (19.8) | 0.003 |
| MAC progression | 52 (7.8) | ||
| SAM | 89 (13.3) | 91 (13.6) | 0.893 |
| MV prolapse | 16 (2.4) | 18 (2.7) | 0.728 |
Data are given as mean±SD, unless otherwise indicated. E indicates mitral inflow early diastolic filling; E/e', ratio of early diastolic mitral inflow velocity/early diastolic mitral annular tissue velocity; HCM, hypertrophic cardiomyopathy; IVS, interventricular septum; LA, left atrial; LV, left ventricular; LVEDD, LV end‐diastolic dimension; LVEF, LV ejection fraction; LVESD, LV end‐systolic dimension; MAC, mitral annular calcification; MR, mitral regurgitation; MS, mitral stenosis; MV, mitral valve; PW posterior wall; RVSP, right ventricular systolic pressure; and SAM, systolic anterior motion.
Figure 3. Kaplan‐Meier analysis of freedom from clinical outcomes.
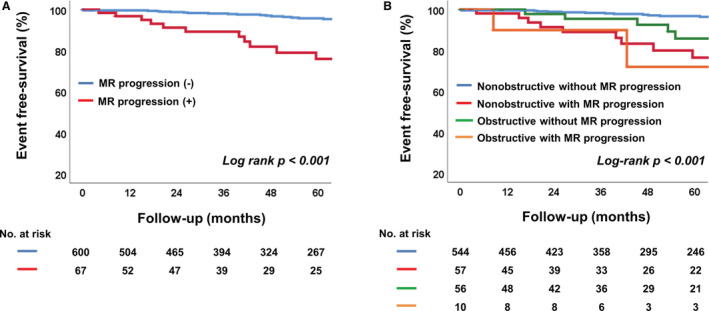
A, Comparison in 2 groups according to the progression of MR. B, Comparison in 4 groups classified by type of hypertrophic cardiomyopathy and the progression of MR. MR indicates mitral regurgitation.
Table 6.
Cox Regression Analysis for Clinical Outcomes in 667 Patients With HCM
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | P value | HR | 95% CI | P value |
| Age | 1.02 | 1.00–1.04 | 0.039 | 1.01 | 0.99–1.03 | 0.492 |
| Female sex | 3.71 | 2.22–6.19 | <0.001 | 2.77 | 1.54–4.97 | 0.001 |
| Hypertension | 1.55 | 0.93–2.58 | 0.095 | 1.01 | 0.56–1.83 | 0.974 |
| Diabetes | 1.94 | 1.05–3.58 | 0.035 | 1.88 | 0.93–3.79 | 0.080 |
| CKD | 3.95 | 1.69–9.24 | 0.002 | 2.66 | 0.95–7.41 | 0.062 |
| Atrial fibrillation | 3.53 | 2.13–5.83 | <0.001 | 2.11 | 1.09–4.11 | 0.028 |
| Obstructive type | 2.04 | 1.10–3.78 | 0.025 | 1.83 | 0.92–3.64 | 0.083 |
| LA volume index | 1.04 | 1.02–1.05 | <0.001 | 1.02 | 1.00–1.04 | 0.034 |
| E/e' | 1.07 | 1.03–1.11 | <0.001 | 1.04 | 1.00–1.07 | 0.069 |
| MR progression | 4.37 | 2.51–7.59 | <0.001 | 2.46 | 1.29–4.71 | 0.007 |
| MAC progression | 3.53 | 1.82–6.83 | <0.001 | 1.64 | 0.79–3.40 | 0.187 |
| MV prolapse | 1.00 | 0.14–7.30 | 0.999 | |||
CKD indicates chronic kidney disease; E/e', ratio of early diastolic mitral inflow velocity/early diastolic mitral annular tissue velocity; HCM, hypertrophic cardiomyopathy; HR, hazard ratio; LA, left atrial; MAC, mitral annular calcification; MR, mitral regurgitation; and MV, mitral valve.
Figure 4. Incremental prognostic value of MR progression over demographic factors, obstructive type of hypertrophic cardiomyopathy, and diastolic parameters, such as LAVI and ratio of E/e' for clinical outcomes.
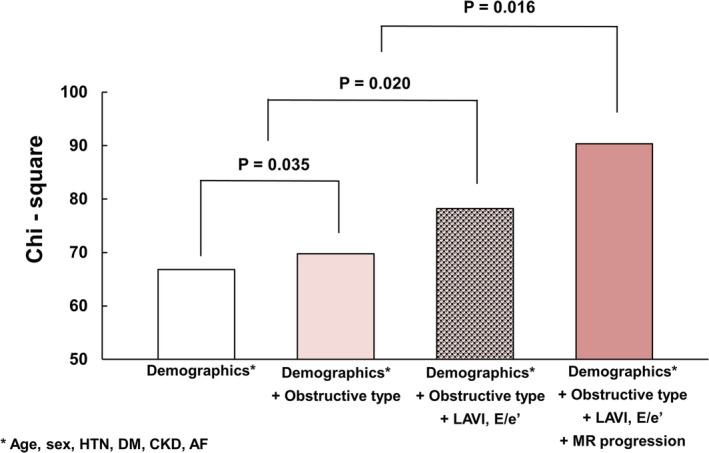
AF indicates atrial fibrillation; CKD, chronic kidney disease; DM, diabetes; E/e', ratio of early diastolic mitral inflow velocity/early diastolic mitral annular tissue velocity; HTN, hypertension; LAVI, left atrial volume index; and MR, mitral regurgitation.
DISCUSSION
This study was conducted in East Asian patients with HCM who were predominantly nonobstructive type, and the principal findings of this study are as follows. First, MV disease was common in patients with HCM, and many types of MV disease were more prevalent in obstructive HCM. Second, the presence of MR and MAC in patients with HCM was a significant contributor to worsening clinical outcomes than other HCM types. Third, progression of MR was an independent prognostic factor of clinical outcomes, along with female sex, atrial fibrillation, and larger LA volume index. These findings stipulate that patients with HCM should be evaluated comprehensively for MV abnormalities, as well as phenotypes associated with the hypertrophied myocardium itself.
Prevalence of MV Disease in HCM
Structural abnormalities of the MV and its apparatus, such as elongated mitral leaflets and displacement and anomalous insertion of papillary muscle, cause SAM or MV prolapse and eventually MR. 6 , 7 , 8 In addition, MAC is often observed in patients with HCM and can be the cause of MV functional abnormalities, such as MS and MR. 17 , 18 , 19 , 20 In particular, several cases of extensive or progressive MAC have been reported in obstructive HCM, perhaps related to elevated peak LV systolic pressure and excess annular tension leading to subsequent annular degeneration. 20 , 21 , 22 In the present study, we investigated the prevalence of SAM, MV prolapse, and MAC as representative MV structural abnormalities that can be diagnosed through transthoracic echocardiogram and evaluated the prevalence and degree of MR and MS as MV functional abnormalities. In this study, the overall prevalence of SAM, MV prolapse, and MAC in collective HCM was 12.5%, 2.4%, and 13.0%, respectively. As expected, SAM and MAC were significantly more common in obstructive HCM. However, there was no difference in the prevalence of MV prolapse between the types of HCM. The reason for the low prevalence of SAM, a hallmark of HCM, was the relatively high number of nonobstructive types in our Korean registry. In East Asia, the proportion of nonobstructive type HCM, including apical type, is higher in the entire HCM registry. 23 Although the proportion of SAM in all subjects was 12.5%, the prevalence of SAM in obstructive HCM was 93.3%.
The prevalence of MAC in the study population was 13.0% in all patients with HCM. In 1990, Fay et al studied echocardiographic characteristics in elderly patients with HCM aged >65 years and found the prevalence of MAC to be 36%. 24 The lower prevalence of MAC in our study compared with the previous one might be explained by a younger population, considering that the occurrence rate of MAC increases according to age and tends to progress over time. That MAC was more common in the obstructive type (27.9%) than in the nonobstructive type (11.6%) is consistent with the mechanism of MAC generation and the results of previous studies.
Relative to SAM and MAC, the prevalence of MV prolapse was far less, at 2.4%. MV prolapse is a common disorder affecting 2% to 3% of the general population and can coexist with HCM. 25 Petrone et al have reported that the prevalence of MV prolapse in 528 patients with HCM was 3%. 9 Our results are consistent with the previous study, and the prevalence of MV prolapse in HCM is similar to that in the general population. In real clinical practice, superimposed MV prolapse with severe MR might be a correctable cause of acute decompensated HF and pulmonary edema in patients with HCM. 25
Some patients had >1 MV abnormality in obstructive HCM, and we found that the patients with both SAM and intrinsic MV disease had more MR and significant MR than those who did not.
In terms of functional abnormalities of MV, the incidence of MR was reported as 23.5% of the total population with HCM. Among them, the obstructive HCM accounted for more than half (56.7%). In our study cohort with East Asian patients, the nonobstructive HCM is predominant. However, in Western patients with HCM, the obstructive type is predominant (about two‐thirds or more), 2 so the incidence of MR in our study population is considered to be lower than that in Western patients with HCM. In patients with MR, atrial fibrillation was more prevalent in the population with nonobstructive HCM. We postulated that the result was influenced by the higher number of aged populations in the group with nonobstructive HCM.
As a result of a comprehensive evaluation of the characteristics of MV disease in our study patients, the prevalence of MV disease was not low in all patients with HCM whose major proportion was nonobstructive, at >90%. Although the prevalence of MV disease was relatively high in patients with obstructive HCM, the proportion of them among entire patients with HCM was low (<10%). In our study of the HCM registry, most of which are nonobstructive types, it has a significant implication that we demonstrated the association between the presence and progression of MR and clinical prognosis using the characteristics of the East Asian population.
Prognostic Implication of MV Dysfunction in HCM
Because patients with HCM have underlying diastolic dysfunction attributable to myocardial fiber disarray and interstitial fibrosis, MR, which is driven by MV functional abnormalities, leads to conveying a volume load to the heart that increases pressure. 25 , 26 With this theoretical background, the various structural abnormalities in HCM and following MV functional abnormality are likely to have clinical implications for the occurrence of HF and cardiovascular outcomes. In this study, patients with MR had poorer outcomes than those without MR. This finding also was found when the patients were divided into obstructive and nonobstructive type HCM and compared. In the subgroup analysis on differences in outcomes of HCM in consideration of sex, 27 the presence of MR had an important prognostic implication in both men and women. Moreover, we found that female patients with HCM with MR had the worst prognosis, whereas male patients with HCM without MR had the best prognosis. Our findings are in line with a recent study that showed the presence of MR in HF with preserved ejection fraction to be more common in women and associated with a poorer prognosis. 28 In addition, the clinical significance of MR was more dominant over the follow‐up period. In this study, the adverse outcomes were predicted more strongly by increased ratio of early diastolic mitral inflow velocity/early diastolic mitral annular tissue velocity (P<0.001) than the presence of MR (P=0.023) in the multivariate analysis of Table 4. However, on follow‐up, MR progression showed the most predictable values (P=0.007) for the poor prognosis among hemodynamic parameters in Table 6. These results suggest that the myocardial disease is the predominant driver of adverse outcomes in this group of patients with HCM at initial presentation. Still, worsening MR plays an important role later in the course.
As described above, most of our cohort has nonobstructive HCM. Although MR in obstructive HCM mainly appears as a posteriorly directed jet by SAM, 25 it is postulated that not only SAM but multifactorial components, such as MAC, MV prolapse, and degeneration of the mitral annulus and leaflet contributed to MR generation, even a mild degree of MR as low severity is associated with structural abnormalities caused by elevated intracardiac pressure of HCM. Therefore, it could be considered that mild MR may forewarn progression and thus poor prognosis of HCM, especially with the nonobstructive type.
The strength of the present study is that it confirmed the clinical implication of MV disease in a large cohort with HCM that was followed up for a median of 7 years. The only study on the prognostic implication of MR in HCM is that by Feneon et al, who reported the clinical outcomes of exercise‐induced MR assessed by exercise stress echocardiography. 26 At a median 29.3 months of follow‐up, 18 of 126 patients with HCM experienced cardiovascular outcomes, and moderate exercise MR along with an LVOT gradient ≥50 mm Hg were associated with poorer outcomes. 26 Although we did not include changes in MR according to exercise, the number of patients was about 10 times higher in our study, and we obtained outcomes during a long‐term follow‐up, which is an advantage. Because MR quantification is challenging during exercise in HCM, and exercise echocardiogram is applied in some patients with HCM, the results of this study using only resting echocardiogram might be more generally applicable in patients with overall HCM. These studies suggest that MR is an important risk factor for poor clinical outcomes in patients with HCM.
Prognostic Implication of MAC in HCM
As mentioned above, the prevalence of MAC is not uncommon at 13%, and multivariable analysis showed the prognostic implication (HR, 1.74 [95% CI, 1.12–2.68]; P=0.013) of MAC. Similar conclusions were reported in other previous HCM studies. Patlolla et al studied the clinical impact of MAC in patients with obstructive HCM who underwent septal myectomy. 29 In this article, of the total of 2113 patients with HCM, MAC was an independent predictor of worse survival rate (HR, 1.46 [95% CI, 1.08–1.97]; P=0.014) after septal myectomy compared with those without MAC. Massera et al also showed the prognostic implication of MAC in a study of a total 304 patients with obstructive HCM. 30 In this study, 141 (46%) patients had MAC, and its offset distance showed a significant association with LVOT obstruction (odds ratio, 1.16 [95% CI, 1.07–1.28]; P=0.001). The patients with MAC underwent septal myectomy to relieve LVOT obstruction, and after the surgery, they showed no deaths over a median follow‐up of 2.7 years. In addition, the higher prevalence of MAC in obstructive HCM is also associated with a poor prognosis of MAC. The intraventricular high pressure of LV observed in obstructive HCM contributes to the formation of calcification of the annulus. Also, conversely, MAC itself induces anterior displacement of the mitral leaflet, causing LVOT obstruction. These phenomena also support the poor prognosis of MAC.
Limitations
This study has several limitations. First, this study was a single‐center retrospective study, and there was some possibility of selection bias. Second, there are some cases where it is a challenge to quantify MR in patients with HCM, particularly when there is LVOT obstruction, and not all the patients had provocation tests for diagnosis of HCM, which could make the underdiagnosis of obstructive HCM. Third, subjects with HCM were recruited only from a tertiary hospital, and we excluded patients who had already undergone septal myectomy or MV surgery, a factor that might be a selection bias. However, we included >1000 patients with HCM over 10 years, and this might compensate for the possibility of selection bias.
CONCLUSIONS
MV disease is common in East Asian patients with HCM whose major type is nonobstructive, and MR, SAM, and MAC are more prevalent in patients with obstructive HCM. The presence and progression of MR is a prognostic factor in the occurrence of poorer clinical outcomes; therefore, careful assessment of MV functional abnormalities and detailed evaluation of MV anatomical features are needed to predict subsequent complications in patients with HCM.
Sources of Funding
This study was supported by a faculty research grant of Yonsei University College of Medicine (6‐2021‐0096).
Disclosures
None.
Supporting information
Table S1–S3
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.024792
For Sources of Funding and Disclosures, see page 11.
REFERENCES
- 1. Maron BJ, Ommen SR, Semsarian C, Spirito P, Olivotto I, Maron MS. Hypertrophic cardiomyopathy: present and future, with translation into contemporary cardiovascular medicine. J Am Coll Cardiol. 2014;64:83–99. doi: 10.1016/j.jacc.2014.05.003 [DOI] [PubMed] [Google Scholar]
- 2. Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P, Evanovich LL, Hung J, Joglar JA, Kantor P, et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy. Circulation. 2020;142:e558–e631. doi: 10.1161/CIR.0000000000000937 [DOI] [PubMed] [Google Scholar]
- 3. Maron BJ, Spirito P, Shen WK, Haas TS, Formisano F, Link MS, Epstein AE, Almquist AK, Daubert JP, Lawrenz T, et al. Implantable cardioverter‐defibirllators and prevention of sudden cardiac death in hypertrophic cardiomyopathy. JAMA. 2007;298:405–412. doi: 10.1001/jama.298.4.405 [DOI] [PubMed] [Google Scholar]
- 4. Ommen SR, Maron BJ, Olivotto I, Maron MS, Cecchi F, Betocchi S, Gersh BJ, Ackerman MJ, McCully RB, Dearani JA, et al. Long‐term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;46:470–476. doi: 10.1016/j.jacc.2005.02.090 [DOI] [PubMed] [Google Scholar]
- 5. Wigle ED, Trimble AS, Adelman AG, Bigelow WG. Surgery in muscular subaortic stenosis. Prog Cardiovasc Dis. 1968;11:83–112. doi: 10.1016/0033-0620(68)90021-2 [DOI] [PubMed] [Google Scholar]
- 6. Simon AL, Ross J, Gault JH. Angiographic anatomy of the left ventricle and mitral valve in idiopathic hypertrophic subaortic stenosis. Circulation. 1967;38:852–867. doi: 10.1161/01.CIR.36.6.852 [DOI] [PubMed] [Google Scholar]
- 7. Klues HG, Maron BJ, Dollar AL, Roberts WC. Diversity of structural mitral valve alterations in hypertrophic cardiomyopathy. Circulation. 1992;85:1651–1660. doi: 10.1161/01.CIR.85.5.1651 [DOI] [PubMed] [Google Scholar]
- 8. Grigg LE, Wigle ED, Williams WG, Daniel LB, Rakowski H. Transesophageal doppler echocardiography in obstructive hypertrophic cardiomyopathy: clarification of pathophysiology and importance in intraoperative decision making. J Am Coll Cardiol. 1992;20:42–52. doi: 10.1016/0735-1097(92)90135-A [DOI] [PubMed] [Google Scholar]
- 9. Petrone RK, Klues HG, Panza JA, Peterson EE, Maron BJ. Coexistence of mitral valve prolapse in a consecutive group of 528 patients with hypertrophic cardiomyopathy assessed with echocardiography. J Am Coll Cardiol. 1992;20:242–247. doi: 10.1016/0735-1097(92)90137-C [DOI] [PubMed] [Google Scholar]
- 10. Maron MS, Olivotto J, Harrigan C, Appelbaum E, Gibson CM, Lesser JR, Haas TS, Udelson JE, Manning WJ, Maron BJ. Mitral valve abnormalities identified by cardiovascular magnetic resonance represent a primary phenotype expression of hypertrophic cardiomyopathy. Circulation. 2011;124:40–47. doi: 10.1161/CIRCULATIONAHA.110.985812 [DOI] [PubMed] [Google Scholar]
- 11. Sherrid MV, Balaram S, Kim B, Axel L, Swistel DG. The mitral valve in obstructive hypertrophic cardiomyopathy a test in context. J Am Coll Cardiol. 2016;67:1846–1858. doi: 10.1016/j.jacc.2016.01.071 [DOI] [PubMed] [Google Scholar]
- 12. Nagueh SF, Bierig SM, Budoff MJ, Desai M, Dilsizian V, Eidem B, Goldstein SA, Hung J, Maron MS, Ommen SR, et al. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with hypertrophic cardiomyopathy: endorsed by the American Society of Nuclear Cardiology, Society for Cardiovascular Magnetic Resonance, and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr. 2011;24:473–498. doi: 10.1016/j.echo.2011.03.006 [DOI] [PubMed] [Google Scholar]
- 13. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–271. doi: 10.1093/ehjci/jev014 [DOI] [PubMed] [Google Scholar]
- 14. Zoghbi WA, Adams D, Bonow RO, Sarano ME, Foster E, Grayburn PA, Hahn RT, Han Y, Hung J, Lang RM, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30:303–371. doi: 10.1016/j.echo.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 15. Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Lung B, Otto CM, Pellikka PA, Quiñones M. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22:1–23; quiz 101–2, 1. doi: 10.1016/j.echo.2008.11.029 [DOI] [PubMed] [Google Scholar]
- 16. Levine RA, Stathogiannis E, Newell JB, Harrigan P, Weyman AE. Reconsideration of echocardiographic standards of mitral valve prolapse: Lack of association between leaflet displacement isolated to the apical four chamber view and independent echocardiographic evidence of abnormality. J Am Coll Cardiol. 1998;11:1010–1019. doi: 10.1016/S0735-1097(98)90059-6 [DOI] [PubMed] [Google Scholar]
- 17. Abramowitz Y, Jilaihawi H, Chakravarty T, Mack MJ, Makkar RR. Mitral annulus calcification. J Am Coll Cardiol. 2015;66:1934–1941. doi: 10.1016/j.jacc.2015.08.872 [DOI] [PubMed] [Google Scholar]
- 18. Bertrand PB, Churchill TW, Yucel E, Namasivayam M, Bernard S, Nagata Y, He W, Andrews CT, Picard MH, Weyman AE, et al. Prognostic importance of the transmitral pressure gradient in mitral annular calcification with associated with mitral valve dysfunction. Eur Heart J. 2020;41:4321–4328. doi: 10.1093/eurheartj/ehaa819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Movva R, Murthy K, Romero‐Corral A, Rammohan HR, Fumo P, Pressman GS. Calcification of the mitral valve and annulus: systemic evaluation of effects on valve anaotmy and function. J Am Soc Echocardiogr. 2013;26:1135–1142. doi: 10.1016/j.echo.2013.06.014 [DOI] [PubMed] [Google Scholar]
- 20. Ullah W, Haas D. Caseous mitral valve calcificaiton and concurrent hypertropic obstructive cardiomyopathy: a rare cause of stroke. Int J Cardiol Heart Vasc. 2020;30:100647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Puri P, Sarma R, Ostrzega EI, Varadarajan P, Pai RG. Massive posterior mitral annular calcification causing dynamic left ventricular outflow tract obstruction: mechanism and management implications. J Am Soc Echocardiogr. 2005;18(1106):e3–e5. [DOI] [PubMed] [Google Scholar]
- 22. Kim D, Shim CY, Hong GR, Jeong H, Ha JW. Morphological and functional characteristics of mitral annular calcification and their relationship to stroke. PLoS One. 2020;15:e0227753. doi: 10.1371/journal.pone.0227753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moon J, Shim CY, Ha JW, Cho IJ, Kang MK, Yang W, Jang Y, Chung N, Cho S. Clinical and echocardiographic predictors of outcomes in patients with apical hypertrophic cardiomyopathy. Am J Cardiol. 2011;108:1614–1619. doi: 10.1016/j.amjcard.2011.07.024 [DOI] [PubMed] [Google Scholar]
- 24. Fay WP, Taliercio CP, Ilstrup DM, Tajik AJ, Gersh BJ. Natural history of hypertrophic cardiomyopathy in the elderly. J Am Coll Cardiol. 1990;16:821–826. doi: 10.1016/S0735-1097(10)80328-6 [DOI] [PubMed] [Google Scholar]
- 25. Kuperstein R, Klempfner R, Ofek E, Maor E, Freimark D, Sternik L, Goldenberg I, Raanani E, Arad M. De novo mitral regurgitation as a cause of heart failure exacerbation in patients with hypertrophic cardiomyopathy. Int J Cardiol. 2018;252:122–127. doi: 10.1016/j.ijcard.2017.11.060 [DOI] [PubMed] [Google Scholar]
- 26. Feneon D, Schnell F, Galli E, Bernard A, Mabo P, Daubert J, Leclercq C, Carre F, Donal E. Impact of exercise‐induced mitral regurgitation on hypertrophic cardiomyopathy outcomes. Eur Heart J Cardiovasc Imaging. 2016;17:1110–1117. doi: 10.1093/ehjci/jev242 [DOI] [PubMed] [Google Scholar]
- 27. Olivotto I, Maron MS, Adabag AS, Casey SA, Vargiu D, Link MS, Udelson JE, Cecchi F, Maron BJ. Gender‐related differences in the clinical presentation and outcome of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;46:480–487. doi: 10.1016/j.jacc.2005.04.043 [DOI] [PubMed] [Google Scholar]
- 28. Arora S, Sivaraj K, Hendrickson M, Chang PP, Weickert T, Qamar A, Vaduganathan M, Caughey MC, Pandey A, Cavender MA, et al. Prevalence and prognostic significance of mitral regurgitation in acute decompensated heart failure: the ARIC study. JACC Hear Fail. 2021;9:179–189. doi: 10.1016/j.jchf.2020.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Patlolla SH, Schaff HV, Nishimura RA, Geske JB, Lahr BD, Lee AT, Eleid MF, Ommen SR, Dearani JA. Mitral annular calcification in obstructive hypertrophic cardiomyopathy: prevalence and outcomes. Ann Thorac Surg. 2022;114:1–9. doi: 10.1016/j.athoracsur.2021.09.077 [DOI] [PubMed] [Google Scholar]
- 30. Massera D, Xia Y, Li B, Riedy K, Swistel DG, Sherrid MV. Mitral annular calcification in hypertrophic cardiomyopathy. Int J Cardiol. 2022;349:83–89. doi: 10.1016/j.ijcard.2021.11.058 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S3


