Abstract
Background
Lectin‐like oxidized low‐density lipoprotein (ox‐LDL) receptor‐1 is a scavenger receptor for oxidized low‐density lipoprotein. In adults, higher soluble lectin‐like ox‐LDL receptor‐1 (sLOX‐1) levels are associated with cardiovascular disease, type 2 diabetes, and obesity, but a similar link in pediatric overweight/obesity remains uncertain.
Methods and Results
Analyses were based on the cross‐sectional HOLBAEK Study, including 4‐ to 19‐year‐olds from an obesity clinic group with body mass index >90th percentile (n=1815) and from a population‐based group (n=2039). Fasting plasma levels of sLOX‐1 and inflammatory markers were quantified, cardiometabolic risk profiles were assessed, and linear and logistic regression analyses were performed. Pubertal/postpubertal children and adolescents from the obesity clinic group exhibited higher sLOX‐1 levels compared with the population (P<0.001). sLOX‐1 positively associated with proinflammatory cytokines, matrix metalloproteinases, body mass index SD score, waist SD score, body fat %, plasma alanine aminotransferase, serum high‐sensitivity C‐reactive protein, plasma low‐density lipoprotein cholesterol, triglycerides, systolic and diastolic blood pressure SD score, and inversely associated with plasma high‐density lipoprotein cholesterol (all P<0.05). sLOX‐1 positively associated with high alanine aminotransferase (odds ratio [OR], 1.16, P=4.1 E‐04), insulin resistance (OR, 1.16, P=8.6 E‐04), dyslipidemia (OR, 1.25, P=1.8 E‐07), and hypertension (OR, 1.12, P=0.02).
Conclusions
sLOX‐1 levels were elevated during and after puberty in children and adolescents with overweight/obesity compared with population‐based peers and associated with inflammatory markers and worsened cardiometabolic risk profiles. sLOX‐1 may serve as an early marker of cardiometabolic risk and inflammation in pediatric overweight/obesity.
Registration
The HOLBAEK Study, formerly known as The Danish Childhood Obesity Biobank, ClinicalTrials.gov identifier number NCT00928473, https://clinicaltrials.gov/ct2/show/NCT00928473 (registered June 2009).
Keywords: adolescent, child, obesity, overweight, soluble lectin‐like oxidized low‐density lipoprotein receptor‐1
Subject Categories: Epidemiology, Obesity, Pediatrics, Risk Factors
Nonstandard Abbreviations and Acronyms
- HOMA‐IR
homeostasis model assessment of insulin resistance
- MMPs
matrix metalloproteinases
- SDS
SD score
- sLOX‐1
soluble lectin‐like oxidized low‐density lipoprotein receptor‐1
Clinical Perspective.
What Is New?
Plasma levels of soluble lectin‐like oxidized low‐density lipoprotein receptor‐1 are higher in pubertal/postpubertal children and adolescents with overweight/obesity compared with population‐based peers.
Higher soluble lectin‐like oxidized low‐density lipoprotein receptor‐1 levels are associated with circulating inflammatory markers and worsened cardiometabolic risk profiles in children and adolescents.
What Are the Clinical Implications?
In adults, plasma concentrations of soluble lectin‐like oxidized low‐density lipoprotein receptor‐1 are a candidate biomarker for stable coronary artery disease, ischemic stroke, and acute coronary syndrome and herein associated with inflammation and cardiometabolic complications in children and adolescents with overweight/obesity.
Obesity during childhood and adolescences increases the likelihood of adiposity tracking into adulthood, along with an increased risk of cardiovascular disease, one of the leading causes of mortality worldwide. 1 Autopsy studies, including the PDAY (Pathobiological Determinants of Atherosclerosis in Youth) study 2 and the Bogalusa Heart Study, 3 demonstrated the presence of atherosclerotic lesions beginning in childhood, accompanied by multiple cardiovascular risk factors, including obesity, hyperglycemia, dyslipidemia, hypertension, and smoking. 4 Cardiovascular events in adulthood are the product of a lifelong pathological process, the foundations of which may begin in childhood and adolescence, and as such, could serve as an optimal window for intervention and prevention. 5
Lectin‐like oxidized low‐density lipoprotein receptor 1 (LOX‐1) has been implicated in the initiation and progression of atherosclerosis. 6 LOX‐1 is a major transmembrane scavenger receptor for oxidized low‐density lipoprotein (ox‐LDL) in endothelial cells, but it is also expressed in macrophages, smooth muscle cells, and fibroblasts. 6 LOX‐1 expression is low under normal conditions but upregulated in response to proatherogenic factors, including ox‐LDL, proinflammatory cytokines, pro‐oxidative, and biomechanical stimuli. 7 Ligand binding to LOX‐1 induces the release of proinflammatory cytokines, matrix metalloproteinases (MMPs), and cell adhesion molecules and activates apoptotic pathways, all of which are crucial in the process of endothelial dysfunction, atherosclerotic plaque development, and vulnerability. 8 Evidence from animal models shows that LOX‐1 gene deficiency reduces atherosclerotic lesions, whereas overexpression exacerbates plaque development and inflammation. 9 Genetic epidemiological studies have shown that single nucleotide polymorphisms in OLR1, which encodes LOX‐1, are associated with atherosclerosis, myocardial infarction, and stable coronary artery disease. 10
A soluble form of LOX‐1 (sLOX‐1) is present in circulation as a result of proteolytic shedding of the receptor's extracellular domain, triggered by factors such as ox‐LDL, CRP (C‐reactive protein), interleukin‐8 (IL‐8), IL‐18, and TNF‐alpha (tumor necrosis factor alpha), mediated by MMPs and ADAMs (a disintegrin and metalloproteinases). 6 Circulating levels of the soluble form are thought to reflect the cellular expression of LOX‐1. 9 Elevated concentrations of sLOX‐1 have been reported in adults with stable coronary artery disease, 11 ischemic stroke, 12 , 13 acute coronary syndrome, 14 , 15 type 2 diabetes (T2D), 16 and obesity. 17 , 18 , 19 Moreover, sLOX‐1 has emerged as a promising biomarker for coronary artery disease, stroke, acute aortic dissection, and acute coronary syndrome. 6 Potentially allowing for disease prediction, classification of disease severity, and monitoring treatment response beyond traditional biomarkers such as hs‐CRP (high‐sensitivity CRP). 13
However, gaps exist in our current understanding of sLOX‐1 in pediatric obesity and its link to inflammation and cardiometabolic risk is limited. 20 , 21 , 22 In the present cross‐sectional study, fasting plasma levels of sLOX‐1 were measured in 3854 Danish children and adolescents from an obesity clinic and from a population‐based control. The aim was to examine whether sLOX‐1 levels associate with markers of inflammation and cardiometabolic risk profiles during childhood and adolescence. We hypothesized that levels of sLOX‐1 would be higher in those from the obesity clinic group compared with population‐based peers and that elevated sLOX‐1 levels would associate with higher levels of proinflammatory cytokines and MMPs, and indicative of worsened cardiometabolic risk profiles, depending on puberty and weight status.
Methods
Study Populations
The data that support the findings of this study are available from the corresponding author upon reasonable request. The present study is based on a subset of the cross‐sectional HOLBAEK Study, previously known as The Danish Childhood Obesity Biobank. 23 , 24 Two groups of children and adolescents were included: (1) an obesity clinic group, the members of which initiated a multidisciplinary childhood obesity management program at Copenhagen University Hospital Holbæk 23 and had a body mass index (BMI) >90th percentile (BMI SD score [SDS] >1.28) according to Danish reference values 25 and (2) a population‐based group, recruited from schools across 11 municipalities in Zealand, Denmark. 24 Both groups were enrolled into The HOLBAEK Study between January 2009 and April 2019.
The exclusion criteria were (1) age at recruitment younger than 4 years or older than 19 years (n=25); (2) race other than White (self‐reported country of origin and ancestry, n=102); (3) diagnosed type 1 diabetes (n=5); (4) diagnosed T2D (n=1); (5) treatment with medications including insulin, liraglutide, metformin, and/or statins (n=4); (6) meeting T2D criteria 26 based on the blood sample taken for this study (fasting plasma glucose >7.0 mmol/L and/or hemoglobin A1c [HbA1c] >48 mmol/mol, n=2); and (7) acute inflammation or infection 27 (serum hs‐CRP >10 mg/L, n=71). Information on ethnicity, diabetes diagnoses, and use of medications were obtained through a questionnaire at inclusion. Following exclusion criteria, 3854 individuals remained with 1815 in the obesity clinic group and 2039 in the population‐based group.
Ethics
According to the Declaration of Helsinki, informed consent was obtained from all participants. Written consent was obtained either from parents/legal guardians of participants younger than 18 years or from the participants when 18 years or older. The study was approved by the Scientific Ethics Committee of Region Zealand, Denmark (protocol no. SJ‐104) and by the Danish Data Protection Agency (REG‐043‐2013).
Anthropometrics
In the obesity clinic group, anthropometrics (height, weight, and waist) were obtained as a part of a clinical examination, whereas the population‐based group was assessed in a mobile laboratory by trained medical professionals, as previously described. 28 BMI SDS were calculated according to a Danish reference 25 and waist SDS was calculated according to age‐ and sex‐specific reference values. 29
Puberty Stage
Tanner stage 30 , 31 was classified by breast development in girls and gonad development in boys, evaluated by a pediatrician in a subset of the obesity clinic group (n=1464) and self‐evaluated using a standard questionnaire with picture pattern recognition in a subset of the population‐based group (n=1456). Self‐assessment has been shown to accurately distinguish between the stages of prepuberty and puberty/postpuberty. 32 Consequently, puberty stage in both groups was defined as prepubertal (Tanner stage 1) versus pubertal/postpubertal (Tanner stage 2–5).
Plasma sLOX‐1 and Inflammatory Markers
Venous blood samples were collected following an overnight fast of at least 8 hours. Proximity extension assay was performed to quantify sLOX‐1 and inflammatory markers using the “Target 96 Cardiovascular II” and “Target 96 Inflammation” panels from Olink Proteomics AB (Uppsala, Sweden) across 2 batches using EDTA plasma stored at −80 °C. Proximity extension assay technology uses nucleic acid labeling of antibodies in combination with quantitative polymerase chain reaction, producing normalized protein expression values as an arbitrary unit on a log2 scale. 33 , 34 Olink batches were bridged and normalized using 16 controls using the “OlinkAnalyze” R package (https://cran.r‐project.org/web/packages/OlinkAnalyze/index.html). Inflammatory markers were selected a priori, based on previously published associations to LOX‐1 (Table S1). Markers were included if >80% of individuals were above the limit of detection. The limit of detection and the percentage of individuals below the limit of detection are listed in Table S1.
Measurement of Cardiometabolic Risk Factors
Whole‐body dual‐energy X‐ray absorptiometry was performed, and total body fat % was quantified in a subset from both the obesity clinic (n=1594) and population‐based (n=267) groups, using a GE Lunar Prodigy (DF+10 031, GE Healthcare, Madison, WI, USA) until October 2009 and thereafter on a GE Lunar iDXA (ME+200 179, GE Healthcare), as previously described. 35
Fasting biochemical measurements described previously by our group include plasma alanine transaminase (ALT), 36 serum hs‐CRP, 37 serum insulin, plasma glucose, whole blood HbA1c, 28 plasma high‐density lipoprotein cholesterol (HDL‐C), plasma LDL cholesterol (LDL‐C), and plasma triglycerides. 38 Homeostasis model assessment of insulin resistance (HOMA‐IR) was calculated as (insulin [mU/L]×glucose [mmol/L])/22.5.
Oscillometric blood pressure (Omron 705IT, Omron Healthcare Co., Ltd., Kyoto, Japan) was measured 3 times on the upper right arm after 5 minutes of rest with subjects in supine position. The mean of the last 2 measurements was calculated and converted to a blood pressure SDS based on age‐, sex‐ and height‐specific reference values from the American Academy of Pediatrics. 39
Defining Cardiometabolic Risk Features
High ALT was defined as fasting plasma ALT concentrations >24.5 U/L in girls and >31.5 U/L in boys, which was determined to be the optimal cutoff for identifying hepatic steatosis by our group (liver fat content of >5% measured by proton magnetic resonance spectroscopy in 458 children and adolescents). 36 IR was defined as HOMA‐IR values above the 90th percentile for age and sex, based on the full population‐based group in the HOLBAEK Study. 28 Hyperglycemia was defined as fasting plasma glucose between 5.6 and 6.9 mmol/L or HbA1c between 39 and 47 mmol/mol, according to the American Diabetes Association guidelines. 26 Dyslipidemia was defined as values above the 95th percentile according to pediatric guidelines, corresponding to total cholesterol >200 mg/dL (5.2 mmol/L), LDL‐C >130 mg/dL (3.4 mmol/L), triglycerides >100 mg/dL (1.1 mmol/L) for 0 to 9 years or >130 mg/dL (1.5 mmol/L) for 10 to 19 years, or HDL‐C <40 mg/dL (1.0 mmol/L). 40 Hypertension was defined as a systolic and/or diastolic blood pressure above the 95th percentile for age, sex, and height based on pediatric guidelines. 39
Statistical Analysis
Statistical analyses were performed in R version 4.2.0. 41 Normality of variable distributions were evaluated. Data were reported as median (interquartile range) for continuous variables and frequencies and percentages for categorical variables. Standardized differences were used to compare the characteristics of the 2 study groups. Wilcoxon rank‐sum tests were applied for continuous variables and χ2‐tests for categorical variables.
Age‐ and sex‐specific percentile curves for sLOX‐1 were calculated using the “Generalized Additive Models for Location, Scale and Shape” R package (https://cran.r‐project.org/web/packages/gamlss/), using Box‐Cox transformation distribution family to account for skewness, with the best fit determined by the Akaike information criterion. 42
For all pooled regression analyses, we constructed models with progressive adjustment. Our first regression model, Model 1, adjusted for age, sex, smoking status, and BMI SDS; except for adiposity traits, which we adjusted for age, sex, and smoking status only. Our second regression model, Model 2, included Model 1 variables plus puberty stage (subset with all available individuals). Nonnormally distributed (right‐skewed) cardiometabolic risk factors were log‐transformed and inflammatory markers were inverse‐normal transformed. For linear regression, estimated β‐effect sizes and 95% CIs were reported as the SD change in inflammatory markers or cardiometabolic risk factors per SD change in sLOX‐1, to facilitate direct comparisons of the strength of associations. For logistic regression, estimated odds ratios (ORs) and 95% CIs were reported for cardiometabolic risk features (0/1) per 1‐SD change in sLOX‐1.
For interaction regression analyses, we estimated whether weight status (overweight/obesity versus normal weight), puberty stage (prepuberty versus puberty/postpuberty), or sex (boys versus girls) modifies the associations between sLOX‐1, inflammatory risk factors, cardiometabolic risk factors or features. For weight status, overweight/obesity was defined as BMI >90th age‐ and sex‐specific Danish percentiles, whereas normal weight was defined as BMI >10th and <90th percentiles. 25 No significant interactions with sex were observed, results not shown.
Statistical significance was set at P<0.05, with no correction for multiple testing.
Results
Characteristics of the Study Groups
Characteristics of the 2 study groups are presented in Table. There were no significant differences in age (P=0.42). There were a higher percentage of boys than girls in the obesity clinic group compared with the population‐based group (P=0.03). A higher percentage of participants from the obesity clinic group were in the prepubertal stage compared with the population‐based group (P<0.001). However, the subset of patients from the obesity clinic with puberty stage data (80.7%) were younger than the subset of participants from the population‐based group with complete assessments (71.4%; P<0.001). A higher percentage of participants from the obesity clinic group were smokers compared with population‐based peers (P<0.001). As expected, the obesity clinic group differed in terms of cardiometabolic risk factors (all P<0.001) including higher BMI SDS, waist SDS, and body fat %, compared with the population‐based group. There were 371 participants (18.2%) with overweight/obesity (BMI>90th percentile) and 119 (5.8%) with underweight (BMI <10th percentile) in the population‐based group. The obesity clinic group exhibited a higher prevalence of cardiometabolic risk features including high ALT (surrogate for hepatic steatosis), IR, hyperglycemia, dyslipidemia, and hypertension (all P<0.001).
Table 1.
Characteristics of Children and Adolescents From the Obesity Clinic and the Population‐Based Groups
| Characteristics | No. | Obesity clinic | No. | Population‐based | Standardized difference |
|---|---|---|---|---|---|
| Age, y | 1815 | 11.8 (9.6 to 14.1) | 2039 | 11.6 (9.0 to 14.4) | 0.00 |
| Sex, male, n (%) | 1815 | 849 (46.8) | 2039 | 881 (43.2) | 0.07 |
| Puberty stage, prepubertal, n (%) | 1464 | 600 (41.0) | 1456 | 497 (34.1) | 0.14 |
| Smoking status, n (%) | 1815 | 2039 | 0.40 | ||
| Nonsmoking | 1096 (60.4) | 1593 (78.1) | |||
| Passive smoking | 664 (36.6) | 425 (20.8) | |||
| Smoking | 55 (3.0) | 21 (1.0) | |||
| Fasting plasma soluble lectin‐like oxidized LDL receptor‐1, normalized protein expression | 1815 | 6.71 (6.43 to 7.00) | 2039 | 6.60 (6.32 to 6.87) | 0.25 |
| Cardiometabolic risk factors | |||||
| Body mass index SDS | 1815 | 2.86 (2.46 to 3.28) | 2039 | 0.26 (−0.43 to 1.01) | 2.97 |
| Waist SDS | 1579 | 2.38 (2.03 to 2.69) | 1885 | −0.01 (−0.67 to 0.67) | 2.86 |
| Body fat, % | 1594 | 43.4 (40.0 to 46.8) | 267 | 26.9 (21.8 to 33.6) | 2.31 |
| Plasma ALT, U/L | 1791 | 23.0 (19.0 to 31.0) | 1999 | 20.0 (16.0 to 23.5) | 0.55 |
| Serum high‐sensitivity C‐reactive protein, mg/L | 956 | 1.17 (0.48 to 2.56) | 629 | 0.40 (0.17 to 0.78) | 0.58 |
| Homeostasis model assessment of insulin resistance, mIU/L | 1773 | 3.69 (2.42 to 5.41) | 2021 | 2.13 (1.48 to 2.99) | 0.66 |
| Whole blood hemoglobin A1c, mmol/mol | 1787 | 33.9 (29.9 to 37.9) | 1997 | 33.5 (30.5 to 36.5) | 0.14 |
| Plasma high‐density lipoprotein cholesterol, mmol/L | 1782 | 1.20 (1.00 to 1.40) | 1993 | 1.50 (1.30 to 1.70) | 0.88 |
| Plasma LDL cholesterol, mmol/L | 1782 | 2.40 (2.00 to 2.90) | 1993 | 2.00 (1.70 to 2.50) | 0.54 |
| Plasma triglycerides, mmol/L | 1782 | 0.90 (0.70 to 1.30) | 1993 | 0.60 (0.50 to 0.80) | 0.84 |
| Systolic BP SDS | 1701 | 0.92 (0.33 to 1.48) | 1771 | 0.64 (0.15 to 1.17) | 0.32 |
| Diastolic BP SDS | 1701 | 0.23 (−0.23 to 0.81) | 1771 | −0.15 (−0.58 to 0.29) | 0.61 |
| Cardiometabolic risk features | |||||
| High ALT, n (%) | 1791 | 599 (33.4) | 1999 | 220 (11.0) | 0.56 |
| Insulin resistance, n (%) | 1741 | 820 (47.1) | 1961 | 191 (9.7) | 0.91 |
| Hyperglycemia, n (%) | 1778 | 311 (17.5) | 1992 | 162 (8.1) | 0.28 |
| Dyslipidemia, n (%) | 1782 | 647 (36.3) | 1993 | 193 (9.7) | 0.67 |
| Hypertension, n (%) | 1701 | 406 (23.9) | 1771 | 179 (10.1) | 0.37 |
Data are shown as medians (interquartile ranges) or frequencies, n (%). Puberty stage is defined as prepubertal (Tanner stage 1) vs pubertal/postpubertal (Tanner stage 2–5). Smoking status is defined as smoking, passive smoking, and nonsmoking. ALT indicates alanine aminotransferase; BP, blood pressure; LDL, low‐density lipoprotein; and SDS, SD score.
Patients in the obesity clinic group had higher fasting plasma sLOX‐1 levels (median 6.71 [interquartile range 6.43, 7.00] normalized protein expression) than participants from the population‐based group (median 6.60 [interquartile range 6.32, 6.87] normalized protein expression; P<0.001) (Table). When stratified by puberty stage, no significant differences in sLOX‐1 levels were observed between groups in prepuberty (P=0.41), whereas a significant difference emerged during puberty/postpuberty (P<0.001). Age‐ and sex‐specific values for sLOX‐1 are shown with 2.5, 50, and 97.5th percentile curves according to study group (Figure S1).
sLOX‐1 Significantly Associates With Inflammatory Markers
There were highly significant positive associations, adjusted for age, sex, smoking status, and BMI SDS, between plasma sLOX‐1 and several plasma cytokines (including CD40 [cluster of differentiation 40], CD40L, IFN‐gamma [interferon‐gamma], IL‐8, IL‐18, latency‐associated peptide TGF‐beta‐1 [transforming growth factor beta‐1], MCP‐1 [monocyte chemotactic protein 1], and TNF), and plasma MMPs (including MMP‐1, MMP‐7, MMP‐10, and MMP‐12). These associations persisted after adjustment for puberty stage. For details, see Figure 1 and Table S2.
Figure 1. Estimated regression β‐effects (95% CIs) for associations of fasting plasma sLOX‐1 (SD‐units) and plasma inflammatory markers (SD‐units) adjusted for age, sex, smoking status, and BMI SD score.
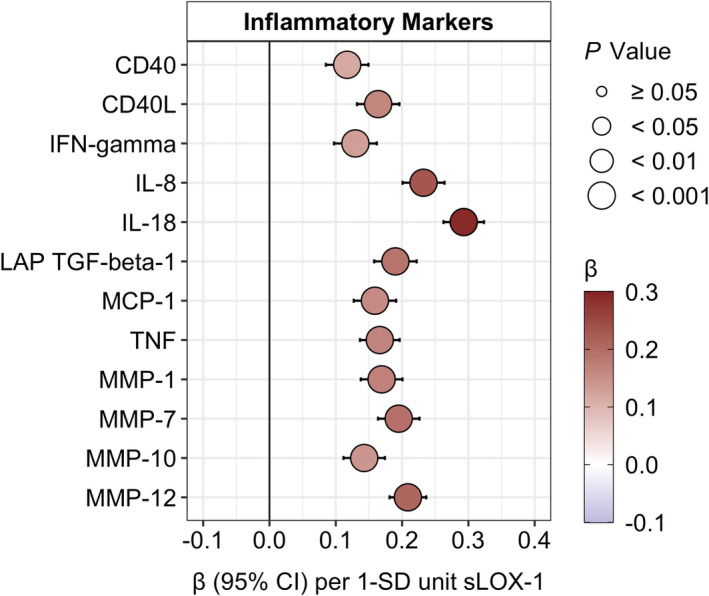
Inflammatory markers (nonnormal distribution) were inverse‐normal transformed. BMI indicates body mass index; CD40, cluster of differentiation 40; CD40L, cluster of differentiation 40 ligand; IFN‐gamma, interferon gamma; IL, interleukin; LAP TGF‐beta‐1, latency‐associated peptide transforming growth factor beta‐1; MCP‐1, monocyte chemotactic protein 1; MMP, matrix metalloproteinase; sLOX‐1, soluble lectin‐like oxidized low‐density lipoprotein; and TNF, tumor necrosis factor.
Regarding weight status, there was a weakly significant interaction (P interaction=0.0499) for MMP‐7, with higher β‐effects with overweight/obesity compared with normal weight (Table S3).
No significant interactions (all P interaction>0.05) between sLOX‐1 and puberty stage were observed for the associations with inflammatory markers (Table S4).
sLOX‐1 Significantly Associates With Cardiometabolic Risk Factors
Fasting plasma sLOX‐1 was positively associated with BMI SDS, waist SDS, and body fat %, adjusted for age, sex, and smoking status. Plasma sLOX‐1 was also positively associated with plasma ALT, serum hs‐CRP, plasma LDL‐C, plasma triglycerides, systolic blood pressure SDS, and diastolic blood pressure SDS, and was negatively associated with plasma HDL‐C, but not with HOMA‐IR or whole blood HbA1c, adjusted for age, sex, smoking status, and BMI SDS. These associations persisted after adjustment for puberty stage, except for a positive association with HOMA‐IR (β=0.04, P=0.009), formerly insignificant. For details, see Figure 2 and Table S5.
Figure 2. Estimated regression β‐effects (95% CIs) for associations of fasting plasma sLOX‐1 (SD‐units) and cardiometabolic risk factors (SD‐units), adjusted for age, sex, smoking status, and BMI SDS (although BMI SDS, waist SDS, and body fat % were not adjusted for BMI SDS).
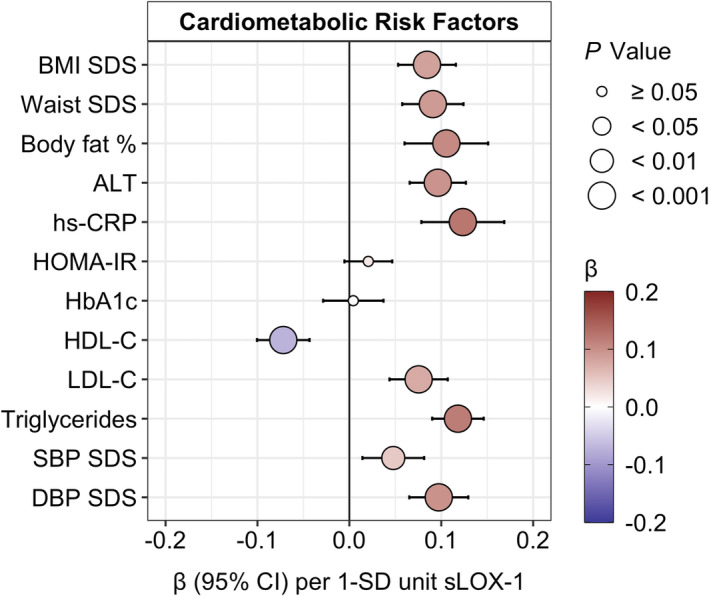
Right‐skewed risk factors were log‐transformed, affecting all but BMI SDS, diastolic blood pressure SDS, and SBP SDS. ALT indicates alanine aminotransferase; BMI, body mass index; DBP, diastolic blood pressure; HbA1c, hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; HOMA‐IR, homeostasis model assessment of insulin resistance; hs‐CRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; SDS, SD score; and sLOX‐1, soluble lectin‐like oxidized low‐density lipoprotein receptor‐1.
There were some significant differences in the magnitude of associations between sLOX‐1 and cardiometabolic risk factors according to weight status (Table S6). There were stronger estimated β‐effects in the children and adolescents with overweight/obesity compared with normal weight individuals for serum hs‐CRP (not significant for Model 2), HOMA‐IR, and plasma LDL‐C (all P interaction<0.05).
Likewise, there were some significant differences in the degree of associations between sLOX‐1 and cardiometabolic risk factors according to puberty stage (Table S7). There were stronger estimated β‐effects in pubertal/postpubertal children and adolescents compared with individuals in prepuberty for BMI SDS, waist SDS, plasma ALT, HOMA‐IR, and plasma triglycerides (all P interaction<0.05).
sLOX‐1 Significantly Associates With Cardiometabolic Risk Features
A 1‐SD increase in sLOX‐1 was associated with a higher prevalence of high ALT (OR, 1.16, P=4.1 E‐04), IR (OR, 1.16, P=8.6 E‐04), dyslipidemia (OR, 1.25, P=1.8 E‐07), and hypertension (OR, 1.12, P=0.02) but not with hyperglycemia (OR, 1.08, P=0.13) adjusted for age, sex, smoking status, and BMI SDS. These associations remained after adjustment for puberty stage. For details, see Figure 3 and Table S8. Larger estimated ORs for the associations between sLOX‐1 with cardiometabolic risk features were observed compared with the same set of associations with hs‐CRP (Table S8).
Figure 3. Estimated ORs (95% CIs) for associations of fasting plasma sLOX‐1 (SD‐units) and cardiometabolic risk features, adjusted for age, sex, smoking status, and BMI SDS.
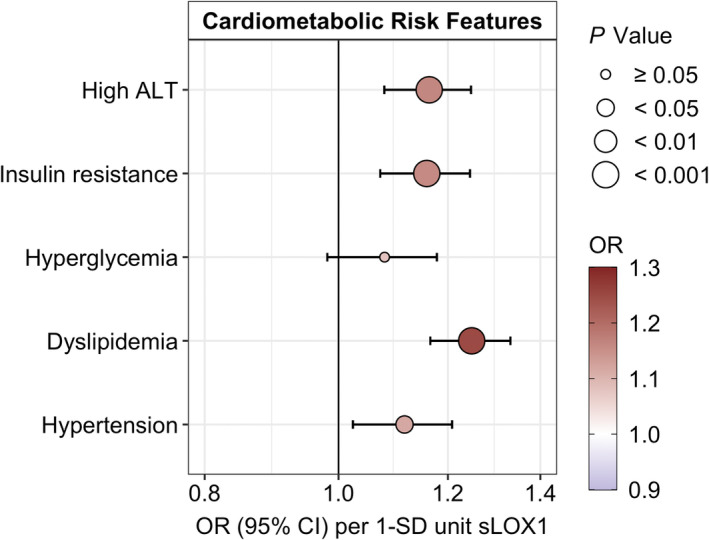
BMI indicates body mass index; high ALT, high alanine transaminase (surrogate measure of hepatic steatosis); OR, odds ratio; SDS, SD score; and sLOX‐1, soluble lectin‐like oxidized low‐density lipoprotein.
There was a general tendency for larger estimated ORs in individuals with overweight/obesity and in pubertal/postpubertal stage. Yet no significant interactions (all P interaction>0.05) were observed for weight status or puberty stage on the associations between sLOX‐1 as an indicator of cardiometabolic risk features (Table S9 and S10).
Discussion
The present study examined sLOX‐1 in children and adolescents with overweight/obesity and population‐based controls. We report 4 main findings: fasting plasma levels of sLOX‐1 are (1) higher in pubertal/postpubertal children and adolescents from an obesity clinic compared with population‐based peers; (2) positively associated with plasma levels of proinflammatory cytokines and MMPs; (3) positively associated with BMI SDS, waist SDS, body fat %, ALT, hs‐CRP, LDL‐C, triglycerides, systolic blood pressure SDS, and diastolic blood pressure SDS and negatively associated with HDL‐C; and (4) associated with increased prevalence of high ALT (a proxy of hepatic steatosis), IR, dyslipidemia, and hypertension but not with hyperglycemia. The positive relationship between sLOX‐1 levels and cardiometabolic risk factors, specifically for HOMA‐IR was strengthened by the presence of both overweight/obesity and initiated in puberty/postpuberty.
Previous pediatric studies have observed elevated ox‐LDL levels in children and adolescents with overweight/obesity compared with normal weight individuals. 20 , 21 Plasma levels of ox‐LDL and LOX‐1 mRNA expression in peripheral blood mononuclear cells were significantly increased in 80 children with Kawasaki disease, to a greater extent in those with coronary artery lesions, compared with 20 febrile and 20 healthy children. 22 Most of the knowledge surrounding the role of sLOX‐1 comes from studies performed in adults. 6 A cross‐sectional study of 51 postmenopausal women demonstrated that plasma sLOX‐1 levels were positively associated with BMI, body fat %, and trunk fat. 18 The present study finds a similar association with adiposity beginning in puberty. Weight reduction resulting from a 12‐week diet and exercise intervention decreased serum sLOX‐1 levels in 32 overweight Japanese middle‐aged men. 17 Future studies should address whether similar reductions in sLOX‐1 levels occur following obesity treatment in the pediatric age range. A longitudinal study including 4703 adults from the Malmö Diet and Cancer cohort demonstrated that high levels of sLOX‐1 were associated with plaque content, ox‐LDL, proinflammatory cytokines, MMPs, and future risk of ischemic stroke. 13 sLOX‐1 levels were also associated with several cardiometabolic risk factors, including age, smoking, diabetes, waist, CRP, HbA1c, HDL‐C, LDL‐C, and triglycerides. 13 This aligns with the current findings where similar associations of sLOX‐1 with proinflammatory cytokines, MMPs, and cardiometabolic risk factors were observed in children and adolescents.
The adipose expandability theory refers to the concept when expansion of subcutaneous adipose tissue storage is exceeded, lipids overflow to visceral and nonadipose tissue, leading to lipotoxicity, resulting in IR and low‐grade inflammation. 43 The presence of obesity in childhood is associated with an increased risk of coronary heart disease in adulthood. 1 The exact mechanisms linking obesity and cardiovascular disease remains unclear; however, expansion of visceral adipose tissue, accompanied by lipotoxicity, and increased systemic inflammation, is thought to play a role in the development of atherosclerosis. 44 LOX‐1 is upregulated by inflammatory cytokines but may also have an inflammatory role itself. 45 Previous studies have shown that LOX‐1 expression is induced in the presence of proatherogenic signals such as ox‐LDL, CRP, IFN‐gamma, IL‐8, and IL‐18, and TNF‐alpha. 6 , 46 , 47 , 48 LOX‐1 also perpetuates inflammatory pathways by triggering CD40/CD40L 49 signaling and secretion of proinflammatory cytokines, such as IL‐6, IL‐8, TGF‐beta‐1, 50 and MCP‐1, 51 as well as modulating MMP activity. 52
There are limitations and strengths in the present study that give perspective. First, this cross‐sectional study may be subject to selection bias with missing Tanner stage data more frequent in older participants from the obesity clinic, who are more likely to be in the pubertal/postpubertal stage. Only a single time point was assessed and therefore future longitudinal studies are warranted. The present study cannot establish causality. Though, a recent study performed in 30 931 adults, demonstrated with intermediate evidence that LOX‐1 was causal in increased waist‐to‐hip ratio and T2D, but not necessarily so for ischemic stroke nor coronary heart disease. 53 Further Mendelian randomization studies are warranted to substantiate these findings. Imaging of the plaque content was not feasible in the present study; therefore, associations between sLOX‐1 and the extent of atherosclerotic lesions cannot be drawn. Lastly, relative concentrations of sLOX‐1 were quantified using proximity extension assay. Therefore, conclusions on the absolute differences between those with overweight/obesity versus the general population cannot be inferred. This study has respective strengths including the relatively large study groups with comprehensive cardiometabolic risk phenotyping.
Conclusions
In conclusion, the current study demonstrates that fasting plasma concentrations of sLOX‐1 were higher in pubertal/postpubertal children and adolescents with overweight/obesity. Levels were associated with circulating inflammatory markers and were indicative of worsened cardiometabolic risk features, beyond traditional markers like hs‐CRP. Inhibitors of LOX‐1 are being tested as therapeutic agents in patients with myocardial infarction and T2D, 54 whereas the soluble form is emerging as a promising biomarker for cardiovascular disease in adults. 6 With the rising prevalence of pediatric obesity and the persistence of adiposity into adulthood accompanied by increased risk for cardiovascular disease, early prevention and intervention strategies are desperately needed. Future studies should validate the clinical utility of sLOX‐1 as a cardiometabolic risk biomarker in adolescents and young adults.
Sources of Funding
This study was supported by The Innovation Fund Denmark (grant number: 0603‐00484B), The Novo Nordisk Foundation (grant number: NNF15OC0016544), The MicrobLiver Challenge (grant number: NNF15OC0016692), Immunometabolism (NNF15SA0018486), MeRIAD (NNF0064142), The Danish Diabetes Academy (grant number: PDMI001‐18 and PDMI002‐18) where funds were contributed by AstraZeneca PLC, and The A.P. Møller Foundation (grant number: 19‐L‐0366). SES is funded by The Novo Nordisk Foundation Copenhagen Bioscience PhD Programme (grant number: NNF18CC0033668). MAVL is funded by The Danish Heart Foundation (grant number: 18‐R125‐A8447). CEF is supported by the BRIDGE – Translational Excellence Programme (grant number: NNF18SA0034956).
Disclosures
Anna E. Jonsson and Mette K. Andersen received funding in the form of salary from the Danish Diabetes Academy contributed by AstraZeneca PLC. Emily L. Ongstad, Ranjitha Gaddipati, Joseph Grimsby, and Christopher J. Rhodes are employed by AstraZeneca PLC and hold shares in the company.
Supporting information
Tables S1–S10
Figure S1
Acknowledgments
The authors would like to acknowledge the participants for their important contribution to the study and the staff at the Department of Biochemistry at Copenhagen University Hospital Holbæk. The authors would also like to thank Birgitte Holløse, Tanja Larsen, Pia Ø. Lind, and Annemette Forman for their technical assistance.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.027042
For Sources of Funding and Disclosures, see page 9.
REFERENCES
- 1. Baker JL, Olsen LW, Sorensen TI. Childhood body‐mass index and the risk of coronary heart disease in adulthood. N Engl J Med. 2007;357:2329–2337. doi: 10.1056/NEJMoa072515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McGill HC Jr, McMahan CA, Malcom GT, Oalmann MC, Strong JP. Effects of serum lipoproteins and smoking on atherosclerosis in young men and women. The PDAY research group. Pathobiological determinants of atherosclerosis in youth. Arterioscler Thromb Vasc Biol. 1997;17:95–106. doi: 10.1161/01.ATV.17.1.95 [DOI] [PubMed] [Google Scholar]
- 3. Berenson GS, Srinivasan SR, Bao W, Newman WP III, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa heart study. N Engl J Med. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302 [DOI] [PubMed] [Google Scholar]
- 4. McGill HC Jr, McMahan CA, Gidding SS. Preventing heart disease in the 21st century: implications of the pathobiological determinants of atherosclerosis in youth (PDAY) study. Circulation. 2008;117:1216–1227. doi: 10.1161/CIRCULATIONAHA.107.717033 [DOI] [PubMed] [Google Scholar]
- 5. McGill HC Jr, McMahan CA, Herderick EE, Malcom GT, Tracy RE, Strong JP. Origin of atherosclerosis in childhood and adolescence. Am J Clin Nutr. 2000;72:1307 S–1315 S. doi: 10.1093/ajcn/72.5.1307s [DOI] [PubMed] [Google Scholar]
- 6. Hofmann A, Brunssen C, Wolk S, Reeps C, Morawietz H. Soluble LOX‐1: a novel biomarker in patients with coronary artery disease, stroke, and acute aortic dissection? J Am Heart Assoc. 2020;9:e013803. doi: 10.1161/JAHA.119.013803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jin P, Cong S. LOX‐1 and atherosclerotic‐related diseases. Clin Chim Acta. 2019;491:24–29. doi: 10.1016/j.cca.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 8. Kattoor AJ, Goel A, Mehta JL. LOX‐1: regulation, signaling and its role in atherosclerosis. Antioxidants (Basel). 2019;8:218. doi: 10.3390/antiox8070218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pirillo A, Norata GD, Catapano AL. LOX‐1, oxldl, and atherosclerosis. Mediators Inflamm. 2013;2013:152786. doi: 10.1155/2013/152786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pothineni NVK, Karathanasis SK, Ding Z, Arulandu A, Varughese KI, Mehta JL. LOX‐1 in atherosclerosis and myocardial ischemia: biology, genetics, and modulation. J Am Coll Cardiol. 2017;69:2759–2768. doi: 10.1016/j.jacc.2017.04.010 [DOI] [PubMed] [Google Scholar]
- 11. Lubrano V, Del Turco S, Nicolini G, Di Cecco P, Basta G. Circulating levels of lectin‐like oxidized low‐density lipoprotein receptor‐1 are associated with inflammatory markers. Lipids. 2008;43:945–950. doi: 10.1007/s11745-008-3227-9 [DOI] [PubMed] [Google Scholar]
- 12. Skarpengland T, Skjelland M, Kong XY, Skagen K, Holm S, Otterdal K, et al. Increased levels of lectin‐like oxidized low‐density lipoprotein receptor‐1 in ischemic stroke and transient ischemic attack. J Am Heart Assoc. 2018;7:e006479. doi: 10.1161/JAHA.117.006479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Markstad H, Edsfeldt A, Yao Mattison I, Bengtsson E, Singh P, Cavalera M, Asciutto G, Björkbacka H, Fredrikson GN, Dias N, et al. High levels of soluble lectinlike oxidized low‐density lipoprotein receptor‐1 are associated with carotid plaque inflammation and increased risk of ischemic stroke. J Am Heart Assoc. 2019;8:e009874. doi: 10.1161/JAHA.118.009874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kobayashi N, Hata N, Kume N, Shinada T, Tomita K, Shirakabe A, Kitamura M, Nozaki A, Inami T, Seino Y, et al. Soluble lectin‐like oxidized ldl receptor‐1 and high‐sensitivity troponin T as diagnostic biomarkers for acute coronary syndrome. Improved values with combination usage in emergency rooms. Circ J. 2011;75:2862–2871. doi: 10.1253/circj.CJ-11-0724 [DOI] [PubMed] [Google Scholar]
- 15. Kraler S, Wenzl FA, Georgiopoulos G, Obeid S, Liberale L, von Eckardstein A, Muller O, Mach F, Räber L, Losdat S, et al. Soluble lectin‐like oxidized low‐density lipoprotein receptor‐1 predicts premature death in acute coronary syndromes. Eur Heart J. 2022;43:1849–1860. doi: 10.1093/eurheartj/ehac143 [DOI] [PubMed] [Google Scholar]
- 16. Tan KC, Shiu SW, Wong Y, Leng L, Bucala R. Soluble lectin‐like oxidized low density lipoprotein receptor‐1 in type 2 diabetes mellitus. J Lipid Res. 2008;49:1438–1444. doi: 10.1194/jlr.M700551-JLR200 [DOI] [PubMed] [Google Scholar]
- 17. Nomata Y, Kume N, Sasai H, Katayama Y, Nakata Y, Okura T, Tanaka K. Weight reduction can decrease circulating soluble lectin‐like oxidized low‐density lipoprotein receptor‐1 levels in overweight middle‐aged men. Metabolism. 2009;58:1209–1214. doi: 10.1016/j.metabol.2009.06.018 [DOI] [PubMed] [Google Scholar]
- 18. Brinkley TE, Kume N, Mitsuoka H, Phares DA, Hagberg JM. Elevated soluble lectin‐like oxidized LDL receptor‐1 (sLOX‐1) levels in obese postmenopausal women. Obesity (Silver Spring). 2008;16:1454–1456. doi: 10.1038/oby.2008.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brinkley TE, Wang X, Kume N, Mitsuoka H, Nicklas BJ. Caloric restriction, aerobic exercise training and soluble lectin‐like oxidized ldl receptor‐1 levels in overweight and obese post‐menopausal women. Int J Obes (Lond). 2011;35:793–799. doi: 10.1038/ijo.2010.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kelly AS, Jacobs DR Jr, Sinaiko AR, Moran A, Steffen LM, Steinberger J. Relation of circulating oxidized LDL to obesity and insulin resistance in children. Pediatr Diabetes. 2010;11:552–555. doi: 10.1111/j.1399-5448.2009.00640.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Norris AL, Steinberger J, Steffen LM, Metzig AM, Schwarzenberg SJ, Kelly AS. Circulating oxidized LDL and inflammation in extreme pediatric obesity. Obesity (Silver Spring). 2011;19:1415–1419. doi: 10.1038/oby.2011.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. He YE, Qiu HX, Wu RZ, Rong X, Xu HT, Xiang RL, Chu MP. Oxidised low‐density lipoprotein and its receptor‐mediated endothelial dysfunction are associated with coronary artery lesions in Kawasaki disease. J Cardiovasc Transl Res. 2020;13:204–214. doi: 10.1007/s12265-019-09908-y [DOI] [PubMed] [Google Scholar]
- 23. Holm JC, Gamborg M, Bille DS, Gr Nb KH, Ward LC, Faerk J. Chronic care treatment of obese children and adolescents. Int J Pediatr Obes. 2011;6:188–196. doi: 10.3109/17477166.2011.575157 [DOI] [PubMed] [Google Scholar]
- 24. Lausten‐Thomsen U, Christiansen M, Fonvig CE, Trier C, Pedersen O, Hansen T, Holm JC. Reference values for serum total adiponectin in healthy non‐obese children and adolescents. Clin Chim Acta. 2015;450:11–14. doi: 10.1016/j.cca.2015.07.012 [DOI] [PubMed] [Google Scholar]
- 25. Nysom K, Molgaard C, Hutchings B, Michaelsen KF. Body mass index of 0 to 45‐y‐old danes: reference values and comparison with published European reference values. Int J Obes Relat Metab Disord. 2001;25:177–184. doi: 10.1038/sj.ijo.0801515 [DOI] [PubMed] [Google Scholar]
- 26. American Diabetes Association . 2. Classification and diagnosis of diabetes: standards of medical care in diabetes‐2021. Diabetes Care. 2021;44:S15–S33. doi: 10.2337/dc21-S002 [DOI] [PubMed] [Google Scholar]
- 27. Schlenz H, Intemann T, Wolters M, Gonzalez‐Gil EM, Nappo A, Fraterman A, et al. C‐reactive protein reference percentiles among pre‐adolescent children in Europe based on the IDEFICS study population. Int J Obes (Lond). 2014;38:S26–S31. doi: 10.1038/ijo.2014.132 [DOI] [PubMed] [Google Scholar]
- 28. Frithioff‐Bojsoe C, Lund MAV, Kloppenborg JT, Nielsen TTH, Fonvig CE, Lausten‐Thomsen U, et al. Glucose metabolism in children and adolescents: population‐based reference values and comparisons to children and adolescents enrolled in obesity treatment. Pediatr Diabetes. 2019;20:538–548. doi: 10.1111/pedi.12859 [DOI] [PubMed] [Google Scholar]
- 29. Sharma AK, Metzger DL, Daymont C, Hadjiyannakis S, Rodd CJ. LMS tables for waist‐circumference and waist‐height ratio Z‐scores in children aged 5‐19 y in NHANES III: association with cardio‐metabolic risks. Pediatr Res. 2015;78:723–729. doi: 10.1038/pr.2015.160 [DOI] [PubMed] [Google Scholar]
- 30. Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rasmussen AR, Wohlfahrt‐Veje C, Tefre de Renzy‐Martin K, Hagen CP, Tinggaard J, Mouritsen A, Mieritz MG, Main KM. Validity of self‐assessment of pubertal maturation. Pediatrics. 2015;135:86–93. doi: 10.1542/peds.2014-0793 [DOI] [PubMed] [Google Scholar]
- 33. Lundberg M, Thorsen SB, Assarsson E, Villablanca A, Tran B, Gee N, et al. Multiplexed homogeneous proximity ligation assays for high‐throughput protein biomarker research in serological material. Mol Cell Proteomics. 2011;10:M110004978. doi: 10.1074/mcp.M110.004978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen Z‐Z, Gerszten RE. Metabolomics and proteomics in type 2 diabetes. Circ Res. 2020;126:1613–1627. doi: 10.1161/CIRCRESAHA.120.315898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nielsen TRH, Fonvig CE, Dahl M, Mollerup PM, Lausten‐Thomsen U, Pedersen O, Hansen T, Holm JC. Childhood obesity treatment; Effects on BMI SDS, body composition, and fasting plasma lipid concentrations. PLoS One. 2018;13:e0190576. doi: 10.1371/journal.pone.0190576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Johansen MJ, Gade J, Stender S, Frithioff‐Bøjsøe C, Lund MAV, Chabanova E, et al. The effect of overweight and obesity on liver biochemical markers in children and adolescents. J Clin Endocrinol Metab. 2020;105:dgz010. doi: 10.1210/clinem/dgz010 [DOI] [PubMed] [Google Scholar]
- 37. Lund MAV, Thostrup AH, Frithioff‐Bojsoe C, Lausten‐Thomsen U, Hedley PL, Pedersen O, et al. Low‐grade inflammation independently associates with cardiometabolic risk in children with overweight/obesity. Nutr Metab Cardiovasc Dis. 2020;30:1544–1553. doi: 10.1016/j.numecd.2020.04.024 [DOI] [PubMed] [Google Scholar]
- 38. Nielsen TRH, Lausten‐Thomsen U, Fonvig CE, Bojsoe C, Pedersen L, Bratholm PS, et al. Dyslipidemia and reference values for fasting plasma lipid concentrations in Danish/North‐European White children and adolescents. BMC Pediatr. 2017;17:116. doi: 10.1186/s12887-017-0868-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Flynn JT, Kaelber DC, Baker‐Smith CM, Blowey D, Carroll AE, Daniels SR, de Ferranti SD, Dionne JM, Falkner B, Flinn SK, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904. doi: 10.1542/peds.2017-1904 [DOI] [PubMed] [Google Scholar]
- 40. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute . Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128:S213–S256. doi: 10.1542/peds.2009-2107C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. R Core Team . R: a Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2022. [Google Scholar]
- 42. Rigby RA, Stasinopoulos DM. Using the Box‐Cox T distribution in GAMLSS to model skewness and kurtosis. Stat Model: Int J. 2016;6:209–229. doi: 10.1191/1471082X06st122oa [DOI] [Google Scholar]
- 43. Virtue S, Vidal‐Puig A. Adipose tissue expandability, lipotoxicity and the metabolic syndrome—an allostatic perspective. Biochim Biophys Acta. 2010;1801:338–349. doi: 10.1016/j.bbalip.2009.12.006 [DOI] [PubMed] [Google Scholar]
- 44. Stefan N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 2020;8:616–627. doi: 10.1016/S2213-8587(20)30110-8 [DOI] [PubMed] [Google Scholar]
- 45. Zeya B, Arjuman A, Chandra NC. Lectin‐like oxidized low‐density lipoprotein (LDL) receptor (LOX‐1): a chameleon receptor for oxidized LDL. Biochemistry. 2016;55:4437–4444. doi: 10.1021/acs.biochem.6b00469 [DOI] [PubMed] [Google Scholar]
- 46. Sagar D, Gaddipati R, Ongstad EL, Bhagroo N, An LL, Wang J, Belkhodja M, Rahman S, Manna Z, Davis MA, et al. LOX‐1: a potential driver of cardiovascular risk in sle patients. PLoS One. 2020;15:e0229184. doi: 10.1371/journal.pone.0229184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Arjuman A, Chandra NC. Differential pro‐inflammatory responses of TNF‐alpha receptors (TNFR1 and TNFR2) on LOX‐1 signalling. Mol Biol Rep. 2015;42:1039–1047. doi: 10.1007/s11033-014-3841-y [DOI] [PubMed] [Google Scholar]
- 48. Mitsuoka H, Kume N, Hayashida K, Inui‐Hayashiada A, Aramaki Y, Toyohara M, Jinnai T, Nishi E, Kita T. Interleukin 18 stimulates release of soluble lectin‐like oxidized LDL receptor‐1 (SLOX‐1). Atherosclerosis. 2009;202:176–182. doi: 10.1016/j.atherosclerosis.2008.04.002 [DOI] [PubMed] [Google Scholar]
- 49. Li D, Liu L, Chen H, Sawamura T, Mehta JL. LOX‐1, an oxidized LDL endothelial receptor, induces CD40/CD40L signaling in human coronary artery endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:816–821. doi: 10.1161/01.ATV.0000066685.13434.FA [DOI] [PubMed] [Google Scholar]
- 50. Hu C, Dandapat A, Sun L, Khan JA, Liu Y, Hermonat PL, Mehta JL. Regulation of TGFBETA1‐mediated collagen formation by LOX‐1: studies based on forced overexpression of TGFBETA1 in wild‐type and LOX‐1 knock‐out mouse cardiac fibroblasts. J Biol Chem. 2008;283:10226–10231. doi: 10.1074/jbc.M708820200 [DOI] [PubMed] [Google Scholar]
- 51. Li D, Mehta JL. Antisense to LOX‐1 inhibits oxidized LDL‐mediated upregulation of monocyte chemoattractant protein‐1 and monocyte adhesion to human coronary artery endothelial cells. Circulation. 2000;101:2889–2895. doi: 10.1161/01.CIR.101.25.2889 [DOI] [PubMed] [Google Scholar]
- 52. Li D, Liu L, Chen H, Sawamura T, Ranganathan S, Mehta JL. Lox‐1 mediates oxidized low‐density lipoprotein‐induced expression of matrix metalloproteinases in human coronary artery endothelial cells. Circulation. 2003;107:612–617. doi: 10.1161/01.CIR.0000047276.52039.FB [DOI] [PubMed] [Google Scholar]
- 53. Folkersen L, Gustafsson S, Wang Q, Hansen DH, Hedman AK, Schork A, Page K, Zhernakova DV, Wu Y, Peters J, et al. Genomic and drug target evaluation of 90 cardiovascular proteins in 30,931 individuals. Nat Metab. 2020;2:1135–1148. doi: 10.1038/s42255-020-00287-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Barreto J, Karathanasis SK, Remaley A, Sposito AC. Role of LOX‐1 (lectin‐like oxidized low‐density lipoprotein receptor 1) as a cardiovascular risk predictor: mechanistic insight and potential clinical use. Arterioscler Thromb Vasc Biol. 2021;41:153–166. doi: 10.1161/ATVBAHA.120.315421 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S10
Figure S1


