Abstract
Detailed studies of the Gram-negative model bacterium, Escherichia coli, have demonstrated that post-transcriptional events exert important and possibly greater control over gene regulation than transcription initiation or effective translation. Thus, over past 30 years, considerable effort has been invested in understanding the pathways of mRNA turnover in E. coli. Although it is assumed that most of the ribonucleases and accessory proteins involved in mRNA decay have been identified, our understanding of the regulation of mRNA decay is still incomplete. Furthermore, the vast majority of the studies on mRNA decay have been conducted on exponentially growing cells. Thus, the mechanism of mRNA decay as currently outlined may not accurately reflect what happens when cells find themselves under a variety of stress conditions, such as, nutrient starvation, changes in pH and temperature, as well as a host of others. While the cellular machinery for degradation is relatively constant over a wide range of conditions, intracellular levels of specific ribonucleases can vary depending on the growth conditions. Substrate competition will also modulate ribonucleolytic activity. Post-transcriptional modifications of transcripts by polyadenylating enzymes may favor a specific ribonuclease activity. Interactions with small regulatory RNAs and RNA binding proteins add additional complexities to mRNA functionality and stability. Since many of the ribonucleases are found at the inner membrane, the physical location of a transcript may help determine its half-life. Here we discuss the properties and role of the enzymes involved in mRNA decay as well as the multiple factors that may affect mRNA decay under various in vivo conditions.
Keywords: RNase E, RNase P, RNase III, polyadenylation, sRNAs, exonucleases
Introduction
One of the most important aspects of gene regulation is the ability of the regulatory mechanisms to rapidly modulate messenger RNA (mRNA) stability, thus allowing an organism to adapt to changes in its environment. Although David Apirion (Apirion 1973) first proposed a model for mRNA decay in 1973, at that time very little was known about the enzymes that actually were responsible for the degradation of mRNAs. In fact, it was generally assumed that mRNA decay was strictly a salvage pathway. Accordingly, there was little inherent interest in studying the mechanism of mRNA decay and its possible role(s) in gene regulation. When coupled with the fact that for many years working with RNA was considered to be technically challenging and existing procedures were somewhat primitive, the study of mRNA decay played second fiddle to the analysis of transcription initiation, elongation and termination. In addition, some of the first ribonucleases discovered, such as RNase III (Robertson et al. 1967) and RNase E (Apirion and Lasser 1978) were initially thought to be only involved in rRNA processing (Dunn and Studier 1973; Ghora and Apirion 1978; Misra and Apirion 1979).
About the time RNase E was first identified, Ono and Kuwano (Kuwano et al. 1977; Ono and Kuwano 1979; Ono and Kuwano 1980) described a conditional mutant (called ams-1) of E. coli that led to an increase in the chemical half-life of total RNA. It was subsequently shown that the ams-1 and rne-3071 mutations were alleles of the same gene, which is now called rne (Mudd et al. 1990; Babitzke and Kushner 1991). As will be discussed later, RNase E has been shown to play major roles in both mRNA decay and tRNA processing (Li and Deutscher 2002; Ow and Kushner 2002; Bernstein et al. 2004; Stead et al. 2011; Clarke et al. 2014).
An important step forward in understanding the pathways of mRNA decay was the observation that inactivation of RNase II (encoded by rnb) and polynucleotide phosphorylase (PNPase, encoded by pnp), two 3’ → 5’ exonucleases, led to synthetic lethality and the accumulation of partially degraded mRNA fragments (Donovan and Kushner 1986). Subsequently, the ability to visualize discreet mRNA breakdown products in a triple mutant (ams-1 pnp-7 rnb-500) using polyacrylamide northern blots (Arraiano et al. 1988) also provided an important connection between the ribonucleases and their substrates.
Over the next 33 years, numerous additional ribonucleases, accessory proteins and small regulatory RNAs (sRNAs) have been identified and characterized (Cao and Sarkar 1992; Carpousis et al. 1994; Ghosh and Deutscher 1999; Gottesman 2004; Perwez and Kushner 2006; Deana et al. 2008). The development of powerful new techniques, which include macroarrays, microarrays, high density tiling arrays and RNA-seq analysis has led to the identification of ribonuclease targets at the genome-wide level. Experiments using these procedures have shown that depending on the growth conditions each of the ribonucleases may target specific mRNA(s) for decay (Ow et al. 2000; Mohanty and Kushner 2003; Ow et al. 2003; Mohanty and Kushner 2006; Perwez and Kushner 2006; Stead et al. 2011; Pobre and Arraiano 2015).
The discovery of polyadenylation of mRNAs in E. coli, generally targeting them for more rapid decay (O’Hara et al. 1995; Mohanty and Kushner 1999), added another level of complexity to the study of mRNA decay. The deletion of the structural gene (pcnB) for poly(A) polymerase I (PAP I) leads to the stabilization of a significant number of mRNAs, as will be discussed in more detail later. Subsequently, it was shown that Rho-independent transcription terminators serve as polyadenylation signals in E. coli (Mohanty et al. 2004; Mohanty and Kushner 2006). It is worth noting that PAP I is only found in a limited number of Gram-negative bacteria (Raynal and Carpousis 1999) and there is little evidence to suggest that it is found in any Gram-positive bacteria.
In this review we will first describe the known mechanisms of mRNA decay primarily in Gram-negative bacteria, by outlining the functions of the various ribonucleases and other accessory proteins. We will also discuss the significant roles that sRNAs play in both regulating gene expression and mRNA decay. Finally, we will address the known and hypothesized factors that help regulate mRNA decay. Table 1 provides a listing of all the currently known ribonucleases and proteins that may play a role in either the decay of mRNAs or the regulation of their decay in E. coli.
Table 1.
Ribonucleases and accessory proteins involved in mRNA decay in Escherichia coli.
| RNases/Proteins | Gene | Activity | Known functions |
|---|---|---|---|
| RNase E | rne | Endoribonuclease | mRNA decay, tRNA processing, tRNA maturation, and degradation of rRNA, degradation of sRNAs |
| RNase G | rng | Endoribonuclease | mRNA decay, rRNA processing |
| RNase III | rnc | Endoribonuclease | mRNA decay, rRNA processing, sRNA degradation |
| RNase P | rnpA, rnpB | Endoribonuclease | Separation of pre-tRNAs from polycistronic transcripts, tRNA 5’ end maturation, mRNA decay* |
| YbeY | ybeY | Endoribonuclease | rRNA maturation |
| RNase I | rna | Endoribonuclease | Specific function unknown, Located in the periplasmic space |
| RNase HI | rnhA | Endoribonuclease | DNA replication |
| RNase HII | rnhB | Endoribonuclease | DNA replication |
| RNase Z/RNase BN | rbn | Endoribonuclease/3’ → 5’ exoribonuclease | mRNA decay, sRNA degradation, tRNA maturation |
| PNPase | pnp | 3’ → 5’ exoribonuclease | mRNA decay, rRNA degradation, sRNA degradation; Biosynthetic activity adds polynucleotide tails to mRNA decay intermediates |
| RNase II | rnb | 3’ → 5’ exoribonuclease | mRNA decay |
| RNase R | rnr | 3’ → 5’ exoribonuclease | mRNA decay, rRNA maturation and degradation |
| RNase PH | rph | 3’ → 5’ exoribonuclease | tRNA maturation |
| RNase T | rnt | 3’ → 5’ exoribonuclease | tRNA and rRNA maturation |
| RNase D | rnd | 3’ → 5’ exoribonuclease | tRNA maturation |
| oligoribonuclease | orn | 3’ → 5’ exoribonuclease | mRNA decay (oligoribonucleotides 2–5 nt in length) |
| RNase AM | trpH | 5’ → 3’ exoribonuclease | rRNA maturation |
| Poly(A) polymerase (PAP I) | pcnB | Polyadenylation | Poly(A) tails provides unstructured binding sites for 3’ → 5’ exonucleases. Promotes degradation of full-length transcripts and decay intermediates. |
| Hfq | hfq | RNA binding protein | Modulation of poly(A) addition, promotes mRNA/sRNA interaction |
| ProQ | proQ | RNA binding protein | RNA/RNA interaction, inhibition of exonucleolytic decay |
| CsrA | csrA | RNA binding protein | mRNA stability |
Based on RNA-seq analysis of the rnpA49 transcriptome (Mohanty and Kushner, unpublished results).
The process of mRNA decay in bacteria has previously been reviewed by several authors (Mohanty and Kushner 2016; Bechhofer and Deutscher 2019). Readers are encouraged to consult these recent reviews for more information on Gram-positive bacteria.
Participants in mRNA decay
All mRNA decay in E. coli is accomplished by the combined actions of endoribonucleases and exoribonucleases (Table 1). There are at least eight endoribonucleases (RNases E, G, P, III, YbeY, I, HI and HII) and seven exoribonucleases [polynucleotide phosphorylase (PNPase), RNase II, RNase R, RNase PH, RNase T, RNase D and oligoribonuclease) that have been identified in E. coli (Table 1). However, only five endoribonucleases (RNases E, G, P, III, and Z) and four exoribonucleases (PNPase, RNase II, RNase R and oligoribonuclease) are known to be significant players in mRNA decay and will be discussed in more detail here. In addition, RNase BN, initially identified as a 3’ → 5’ exonuclease, has also been shown to have endonuclease activity (called RNase Z) (Perwez and Kushner 2006; Dutta et al. 2012). While all the exonucleases mentioned above degrade RNA in the 3’ → 5’ direction, the recently identified RNase AM, is the only E. coli exoribonuclease, which degrades RNA in the 5’ → 3’. This enzyme seems to be only required for 5’-end maturation of rRNAs (Jain 2020). The rest of the RNases that are primarily involved in metabolic processes other than mRNA decay have been reviewed recently (Mohanty and Kushner 2018; Bechhofer and Deutscher 2019) and will not be discussed here. The consensus is that most if not all mRNA decay begins with endonucleolytic cleavages generating decay intermediates followed by further shortening of the decay intermediates by 3’ → 5’ exonucleases to short oligomers (2–5 nucleotides), which are converted to mononucleotides by oligoribonuclease (Figure 1).
Figure 1.
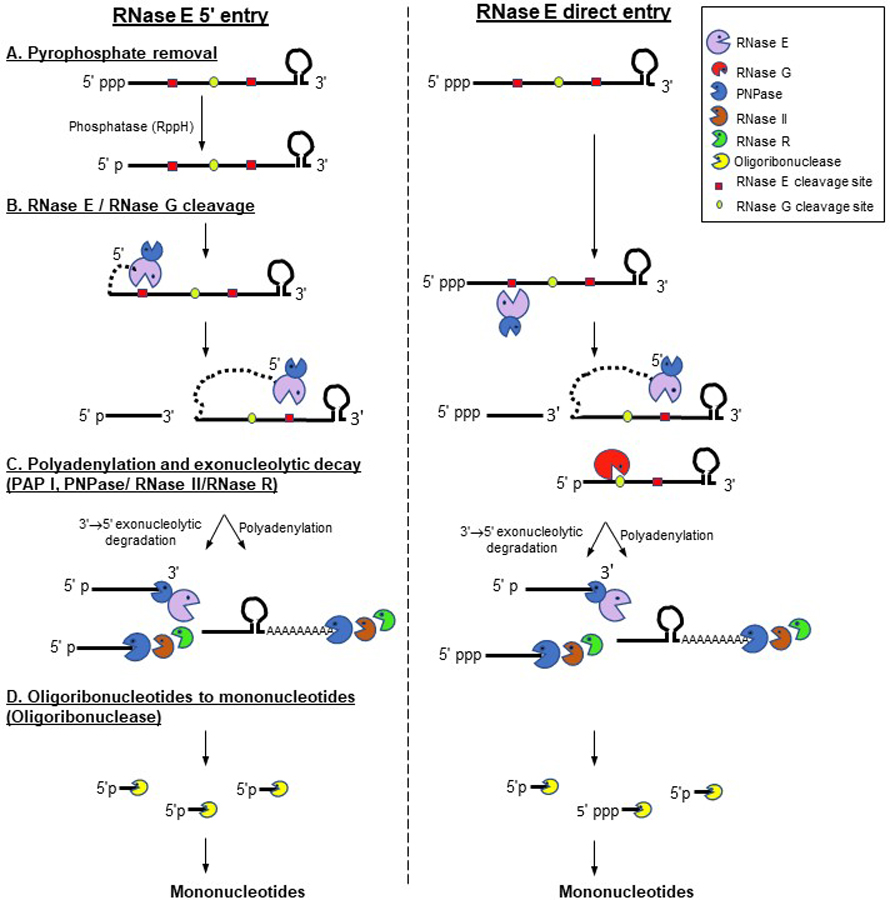
Schematic view of mRNA decay initiated by RNase E-based degradosome. In this diagram a single mRNA with a 5’ triphosphate and a 3’ Rho-independent transcription terminator is shown as substrate. Only polynucleotide phosphorylase (PNPase) is shown associated with RNase E whereas other components of the degradosome (RhlB RNA helicase and enolase) have been omitted for the sake of simplicity. RNase E can initiate mRNA decay from the 5’ end following dephosphorylation of the triphosphate (Step A) of the primary transcript by RppH to a monophosphate (left hand side of figure). Alternatively, RNase E can bypass the 5’ triphosphate using its direct entry mechanism (right hand side of the figure). After the initial RNase E endonucleolytic cleavage (Step B), which generates decay intermediates with 5’ monophosphate ends, RNase E can proceed in the 5’ → 3’ direction generating additional large oligonucleotides. RNase G can also initiate the decay of a 5’ monophophorylated mRNA or use RNase E cleaved decay intermediates as substrates. The endonucleolytically generated oligonucleotides are degraded exonucleolytically by PNPase, RNase II and in some cases RNase R (Step C). PNPase and RNase II can be inhibited by secondary structures (Spickler and Mackie 2000) including the Rho-independent terminator sequence, where the addition of poly(A) tails by poly(A) polymerase provides single-stranded binding sites for the 3’ → 5’ exonucleases. All of the three 3’ → 5’ exonucleases disassociate leaving short oligonucleotides (2–5 nt in length), which are converted to mononucleotides by oligoribonuclease (Step D). This figure is available in color online.
Endoribonucleases
RNase E.
RNase E is involved in many aspects of post-transcriptional RNA metabolism which include mRNA decay, the processing and maturation of 5S (Apirion and Lasser 1978) and 16S rRNAs (Li et al. 1999), degradation of rRNAs (Sulthana et al. 2016), maturation of tmRNA (Lin-Chao et al. 1999), the processing of polycistronic and monocistronic tRNA transcripts (Li and Deutscher 2002; Ow and Kushner 2002), the processing of the M1 RNA (Lundberg and Altman 1995; Sim et al. 2002) and the cleavage of many non-coding regulatory RNAs including sRNAs (Lin-Chao and Cohen 1991; Briani et al. 2002; Moll et al. 2003). Using a high-density tiling array, the enzyme has been shown to be involved in the initiation of decay of approximately 60% of the E. coli transcriptome (Stead et al. 2011).
RNase E levels are autoregulated through its interaction with a secondary structure present in the 5’ untranslated region (UTR) of the rne transcript (Jain and Belasco 1995; Schuck et al. 2009). It is a large protein of over 1000 amino acids and is normally present in the cell as part of a multiprotein complex called the degradosome that also contains PNPase, the RhlB RNA helicase and the glycolytic enzyme enolase (Carpousis et al. 1994; Py et al. 1996a). The N terminal region (1–529 amino acids) of the RNase E protein contains the catalytic domain and the C terminal region (530–1061 amino acids) encompasses the binding sites for the other proteins associated with the degradosome. It has also been shown that the RNA binding protein, Hfq can compete for the RhlB RNA helicase binding site (Ikeda et al. 2011). An eleven amino acid domain in the N terminal region of the protein facilitates its association with the inner membrane of the cell (Khemici et al. 2008). Recent data indicate that the degradosome rapidly diffuses along the inner membrane and forms short-lived foci (Strahl et al. 2015; Muthunayake et al. 2020).
RNase E has been shown to be a 5’-end dependent endonuclease that is inhibited by 5’ triphosphates (Mackie 1998; Tock et al. 2000). Although conversion of a primary transcript with 5’ triphosphate to 5’ monophosphate is perceived as the rate-limiting step of RNase E cleavage (Deana et al, 2008), the enzyme can overcome this requirement by cleaving both mRNAs and tRNAs using a direct entry mechanism (Baker and Mackie 2003; Kime et al. 2010; Garrey and Mackie 2011; Clarke et al. 2014; Kime et al. 2014) (Figure 1). Past studies have shown that the enzyme cleaves single-stranded RNA, preferably in the A/U rich sequences (Ehretsmann et al. 1992; McDowall et al. 1994; McDowall et al. 1995). More recent work using RNA-seq analysis of the E. coli transcriptome has mapped large numbers of in vivo RNase E cleavage sites, which suggest a minimal 5 nt cleavage site of “RN↓WUU (R as G/A, W as A/U and N as any nucleotide) with a strong preference for uridine at the +2 position (Chao et al. 2017). RNase E cleavages generate RNA fragments with 5’-phosphate and 3’-hydroxyl termini. Although the enzyme is essential for cell viability, the exact reason is yet to be unequivocally determined. Reports have been published suggesting that the initiation of processing of polycistronic tRNA precursors serves as the essential function of RNase E (Li and Deutscher 2002; Ow and Kushner 2002; Perwez et al. 2008). However, it has also been argued that the selective mRNA turnover is the essential function of the enzyme (Hammarlof et al. 2015).
RNase G.
This enzyme is an ortholog of RNase E, but lacks the degradosome scaffold region (Wachi et al. 1997; Li et al. 1999; Wachi et al. 1999). The protein has 35% identity and 50% similarity with the RNase E N-terminal catalytic domain (McDowall and Cohen 1996). Interestingly, many Gram-negative bacteria have either an RNase G or RNase E homolog unlike E. coli which has both. The RNase G level inn the cell is only ~3% of that of RNase E level (Lee et al. 2002). Both enzymes share several common properties, such as being inhibited by the presence of a 5’ triphosphate, preferring single-stranded AU-rich sequences as substrates, and producing cleavage products with 5’-phosphate and 3’-hydroxyl termini (Tock et al. 2000). However, while RNase E is essential for cell viability, the loss of RNase G has no significant effect on the bacterial growth rate. Nonetheless, inactivating both enzymes has a significant impact on the processing and/or degradation of certain substrates, suggesting some overlapping substrate specificity (Ow et al. 2003). The initial report that overexpression of RNase G partially complemented rne-1 temperature sensitive mutant (Lee et al. 2002) has not been confirmed. In fact, RNase G cannot complement an RNase E deletion mutant under normal physiological conditions unless it has been mutated (Chung et al. 2010).
RNase G participates in mRNA decay to a very limited extent (Umitsuki et al. 2001; Wachi et al. 2001; Kaga et al. 2002; Ow et al. 2003). Only 18 mRNAs were found to have increased abundance in the absence of RNase G in a microarray study (Lee et al. 2002). While the enzyme is not able to carry out processing of polycistronic tRNA transcripts (Ow et al. 2003), it has been shown to remove the Rho-independent transcription terminator associated with the secG leuU transcript (Mohanty & Kushner, 2008). In addition, it is required for the maturation of 5’ terminus of 16S rRNA (Li et al. 1999; Wachi et al. 1999).
RNase P.
Like RNase E, RNase P is essential for cell viability but unlike RNase E, it is present in almost all bacterial species. In addition, it is unusual in that it is a ribozyme that contains a catalytic RNA subunit (M1 RNA, encoded by rnpB) and a protein subunit (C5, encoded by rnpA) (Altman 1989). While the M1 RNA has some catalytic activity both in vitro and in vivo (Guerrier-Takada et al. 1983), the C5 protein is required for full RNase P activity in vivo (Altman 1990). It is the only enzyme responsible for the 5’ end maturation of all the E. coli tRNAs (Altman 1989). RNase P is also required for the separation of at least 25 pre-tRNAs from the polycistronic tRNA transcripts (Mohanty and Kushner 2007; Mohanty and Kushner 2008; Agrawal et al. 2014; Mohanty et al. 2020). For many years, tRNA 5’ end maturation was considered to be the essential function of RNase P, since it was believed to be a prerequisite for tRNA aminoacylation at the 3’ end. However, recent studies have shown that while 5’ immature tRNAs can be aminoacylated, at least seven primary polycistronic tRNA transcripts, containing 25 tRNAs will not be processed in the absence of RNase P (Mohanty et al, 2020). Thus, it is now argued that the essential function of RNase P is its role in generating pre-tRNAs from polycistronic operons (Mohanty et al. 2020).
The involvement of RNase P in the processing and maturation of 4.5S RNA (Peck-Miller and Altman 1991), tmRNA and C4 antisense RNAs of bacteriophage P1 and P7 (Komine et al. 1994) has also been demonstrated. The role of RNase P in mRNA decay and processing was believed to be minimal based on limited analysis of specific mRNAs and microarray studies (Alifano et al. 1994; Li and Altman 2003; Li et al. 2003). However, a recent transcriptome-wide analysis using RNA-seq of the temperature sensitive rnpA49 mutant has shown that the steady-state levels of ∼44% of all the mRNAs transcribed in exponentially growing E. coli cells were increased upon the inactivation of the enzyme, suggesting it might also be a major player in mRNA decay, (Mohanty and Kushner, manuscript in preparation).
While RNase P cleavage products contain a 5’-phosphate and 3’-hydroxyl termini, the mechanism of its catalytic activity on various substrates is still not completely understood. Based on the assumption that pre-tRNAs were its only substrates, extensive studies involving biochemical, phylogenetic and crystal structure of a RNase P-pre-tRNA complex were conducted to understand how the RNA and protein component of RNase P cooperate in tRNA maturation. Based on these studies, the consensus agreement is that the enzyme recognizes the three-dimensional folded structure of a pre-tRNA to accurately carry out catalysis (Suwa et al. 2009; Reiter et al. 2010; Wu et al. 2011). However, since RNase P is now known to cleave substrates larger than individual pre-tRNAs, “an one size fit all” model for RNase P catalysis may not be applicable. In fact, a recent in vitro study has shown that the enzyme utilizes a distributive mechanism involving dissociation between cleavages of a dicistronic tRNA substrate where the length and structure of the 5’ leader and 3’ trailer sequences can modulate the enzyme activity (Zhao and Harris 2019). Furthermore, RNase P processing proceeds in the 3’ → 5’ direction, both in vivo and in vitro, based on the analysis of polycistronic tRNA substrates (Agrawal et al. 2014; Zhao and Harris 2019) as opposed to RNase E which generally proceeds in the 5’ → 3’ direction (Richards and Belasco 2019).
RNase III.
RNase III is unique in E. coli as it is the only ribonuclease that is specific for double-stranded RNA. It was first identified based on its ability to process rRNA operons by cleaving the double-stranded stem regions formed by the pre-16S and pre-23S rRNA species (King et al. 1984). However, very little 30S rRNA accumulates in the absence of the enzyme suggesting an alternate processing pathway (G. Chaudhury and S. Kushner, unpublished results), which results in incompletely processed, but functional 23S rRNA species and fully matured 16S rRNA species (King et al. 1984). Thus, the enzyme is not essential in E. coli, although a significant growth defect is observed in its absence (G. Chaudhury and S. Kushner, unpublished results).
RNase III also was shown to affect the stability of a limited number of mRNAs (Regnier and Grunberg-Manago 1989; Opdyke et al. 2011; Lim et al. 2012). A recent genome-wide study using a high-density tiling array has suggested that RNase III may be involved in the stability of ∼12% of the E. coli transcriptome (Stead et al. 2011). In addition, the ability of the enzyme to degrade specific sRNAs also indirectly affects mRNA decay (Viegas et al. 2011).
Unlike RNase E, RNase III does not have a strongly conserved preferred cleavage site. Instead, it relies on a contextual structure to identify its substrate, an approach similar to that utilized by RNase P. Specifically, RNase III recognizes the elements of a dsRNA structure of about 22 base pairs for cleavage. The dsRNA structure does not have to be perfectly complementary and can be formed either by intramolecular base pairing or from two separate RNA strands (Robertson 1982; Court 1993; Pertzev and Nicholson 2006), with cleavage occurring in one or both RNA strands (Nicholson 1999). An in vitro study has suggested that the ability of the enzyme to bind and cleave is determined in part by the sequence content of two discrete double-helical elements of the dsRNA (Pertzev and Nicholson 2006). The cleavage products contain 5’-phosphate and 3’-hydroxyl termini.
RNase Z.
This enzyme belongs to a superfamily of endoribonucleases, which cleave pre-tRNAs lacking an encoded CCA immediately after the discriminator nucleotides (Pellegrini et al. 2003), generating a substrate that can be used for post-transcriptional addition of the CCA trinucleotide by tRNA nucleotidyltransferase (Schiffer et al. 2002; Vogel et al. 2005). This enzyme is essential in the organisms where large numbers of their tRNAs do not have the CCA encoded in the tRNA genes, such as eukaryotes and Gram-positive bacteria. In contrast, with all of the 86 tRNA genes in E. coli having the CCA trinucleotide encoded (Blattner et al. 1997), it is not clear why the bacterium might have an RNase Z homolog. Unknowingly, the E. coli RNase Z homolog (originally called elaC) had been identified years earlier as a 3’ → 5’ exonuclease and called RNase BN (Ezraty et al. 2005). Subsequently, it was discovered that both enzymes are encoded by the same structural gene rbn (Dutta and Deutscher 2009) and that the enzyme can function both as an endo- and exonuclease in vivo (Dutta et al. 2012).
A rbn deletion mutant has no growth defect. As an endonuclease, RNase Z prefers double-stranded RNA over single-stranded RNA as substrates and produces cleavage products as small as four nucleotides (Dutta and Deutscher 2009). In the absence of more preferred exoribonucleases, (i.e.,RNase II, RNase D, RNase T and RNase PH), E. coli RNase Z has been shown to play a backup role in the 3’ end maturation of the pre-tRNAs (Asha et al. 1983; Kelly and Deutscher 1992).
Although the E. coli RNase Z does not play a significant role in tRNA processing, it has been shown to play some role in mRNA decay (Perwez and Kushner 2006). Over 150 mRNAs were shown to have increased abundance of more than two-fold in the rnz/rbn deletion mutant compared to the wild-type strain in a genome-wide analysis using macroarray. However, many of these mRNAs are also the substrates for RNase E and thus their half-lives increased dramatically in the RNase E RNase Z double mutant, which suggested a backup role of RNase Z in mRNA decay (Perwez and Kushner 2006).
The level of the rbn mRNA is subject to growth phase-dependent regulation, which is directly related to the abundance of certain sRNAs (Chen et al. 2016). It has been shown that the rbn message undergoes RNase E cleavage resulting in decreased levels of RNase Z in stationary phase. However, the rbn message is protected from RNase E cleavage by the GvcB sRNA and the RNA binding protein Hfq resulting in increased RNase Z levels during exponential growth (Chen et al. 2019). The increased endonucleolytic activity of RNase Z during exponential growth of the cell is believed to be responsible for regulating the level of 6S RNA, a global transcription regulator, which is now considered to be the most likely primary function of the enzyme.
Exoribonucleases
PNPase (polynucleotide phosphorylase).
This enzyme is an unusual 3’ → 5’ exonuclease encoded by pnp, which utilizes a phosphorolytic mechanism to degrade unstructured single-stranded RNA substrates in the presence of inorganic phosphate, generating nucleoside diphosphates (Godefroy-Colburn and Grunberg-Manago 1972). The enzyme has an equilibrium constant of 1 so that if the pool of nucleoside diphosphates builds up, the enzyme can synthesize RNA in an untemplated reaction. In fact, the enzyme was originally discovered by Ochoa who thought that his group had discovered RNA polymerase (Grunberg-Manago et al. 1955; Grunberg-Manago et al. 1956). Since the inorganic phosphate concentration in E. coli is over 10 mM (Shulman et al. 1979), it was long assumed that the enzyme worked exclusively as a ribonuclease. However, Mohanty and Kushner (Mohanty and Kushner 2000) demonstrated that in vivo the enzyme works both degradatively and biosynthetically. In its in vivo biosynthetic mode, it generates polynucleotide tails on mRNA breakdown products (Mohanty and Kushner 2000; Mohanty et al. 2004).
While the enzyme is not essential for cell viability, a pnp deletion mutant grows slower at 37°C (Mohanty and Kushner 2003) and effectively stops growing at temperatures below 20°C (Hossain et al. 2016). PNPase RNase II and PNPase RNase R double mutants are synthetic lethals. Large mRNA and rRNA fragments accumulate in both temperature sensitive mutants at the nonpermissive temperature, which suggests that PNPase has functional overlap with both RNase II and RNase R (Donovan and Kushner 1986; Cheng et al. 1998).
In addition, PNPase has been shown to be associated with other proteins forming various complexes, which play important roles in mRNA decay. The best studied among these is the RNase E-based multienzyme degradosome complex, which also contains the RNA helicase RhlB and enolase (Py et al. 1996b). In addition, PNPase is also found associated with the polyadenylation complex containing PAP I and the RNA binding protein Hfq (Mohanty et al. 2004) as well as forming an independent complex with the RNA helicase RhlB (Lin and Lin-Chao 2005). However, the importance of these latter two PNPase complexes in vivo is not fully understood.
The enzyme needs at least a 6–9 nucleotide single-stranded region to bind to initiate the degradative reaction and dissociates from substrates of 3–5 nucleotides. It is strongly inhibited by secondary structures (Spickler and Mackie 2000). Thus, addition of poly(A) tails by PAP I to decay intermediates containing secondary structures facilitates their faster degradation. The accumulation of endonucleolytically generated cleavage products in pnp mutants (Donovan and Kushner 1986; Hajnsdorf et al. 1996; Spickler and Mackie 2000) has strongly suggested that the enzyme uses decay intermediates as its primary substrates. However, the effect of the enzyme on the stability of the full-length mRNAs has also been reported (Hajnsdorf et al. 1994). In addition, genome-wide analyses of a pnp deletion transcriptome showed that PNPase participates in the degradation of a limited number of mRNAs (Mohanty and Kushner 2003; Pobre and Arraiano 2015).
RNase II.
This enzyme, encoded by the rnb gene, was one of the first ribonucleases of E. coli to be identified (Singer and Tolbert 1965; Nossal and Singer 1968). It belongs to RNR family of enzymes and degrades RNA using a hydrolytic mechanism. It is a highly processive 3’ → 5’ exonuclease accounting for 95–98% of the exonuclease activity in E. coli cell extracts (Cheng et al. 1998). The enzyme needs a minimum of 9–10 nts of single-stranded RNA to bind (Cheng and Deutscher 2002). RNA substrates less than 10 nts in length lead to distributive mode of action for the enzyme (Zuo et al. 2006). RNase II is very sensitive to secondary structures similar to PNPase (Spickler and Mackie 2000). Thus addition of poly(A) tails stimulates its activity (Marujo et al. 2000; Mohanty and Kushner 2000). While deletion of the structural gene has no effect on growth and cell viability, a pnp rnb double mutant is a synthetic lethal accumulating mRNA fragments (Donovan and Kushner 1986). However, an RNase II single mutant becomes inviable during an extended period in stationary phase or amino acid starvation. This lethality is suppressed in the absence of RNase PH, suggesting the regulation of RNase PH activity by RNase II during stationary phase (Sulthana et al. 2017).
Genome-wide analyses has shown that the enzyme has a limited effect on the decay of full-length mRNAs (Mohanty and Kushner 2003; Pobre and Arraiano 2015). However, the enzyme plays a significant role in stabilizing ~31% of mRNAs in E. coli, which includes almost all of the rRNA protein genes (Mohanty and Kushner 2003). This result may be related to its ability to efficiently remove poly(A) tails from many substrates (Marujo et al. 2000; Mohanty and Kushner 2000), generating substrates that are not suitable for other exoribonucleases. RNase II has also been shown to be required for RelE-independent A-site mRNA cleavage during ribosome pausing where the enzyme degrades the sequences downstream of the A-site (Garza-Sanchez et al. 2009).
RNase R.
This ribonuclease, encoded by rnr (initially called vacB) gene, is a homolog of RNase II and is of particular interest because, unlike RNase II and PNPase, it is not inhibited by secondary structures (Cheng and Deutscher 2002). RNase R has an intrinsic ATP-dependent RNA helicase activity, which is required for its proper functioning in RNA metabolism (Hossain et al. 2016). It requires a single-stranded region of at least 10 nucleotides for optimal substrate binding (Vincent and Deutscher 2006). Although a rnr deleted strain has no significant growth defects above 30°C, it grows much slower below 25°C (Hossain et al. 2016). The absence of both RNase R and PNPase leads to a significant accumulation of 16S and 23S rRNA breakdown products and a defect in ribosome assembly resulting in inviability (Cheng and Deutscher 2003).
RNase R is probably a minor player in mRNA decay, since RNA-seq analysis of an Δrnr mutant showed increased steady-state levels of only ∼6% of the genomic mRNAs (Pobre and Arraiano 2015). In fact, the most important function of RNase R is believed to be in degrading mRNA breakdown products with extensive secondary structures, such as REP elements (Cheng and Deutscher 2005).
Oligoribonuclease.
Oligoribonuclease, encoded by the orn gene, is highly specific for short oligonucleotides of 2–5 nt in length. While PNPase, RNase II and RNase R all effectively degrade large RNA molecules, none of them can completely process their substrates to mononucleotides and leave short oligonucleotides (2–4 nt), which accumulate in the absence of oligoribonuclease (Ghosh and Deutscher 1999). Although some experimental results suggest that the enzyme is essential for cell viability (Ghosh and Deutscher 1999), it has not been unequivocally proven.
RNase AM.
With the identification of a protein associated with the trpH gene in E. coli that could hydrolyze RNA molecules in the 5’ → 3’ direction (Ghodge and Raushel 2015), the long held thinking that there were no 5’ → 3’ exonucleases in E. coli and probably other Gram-negative bacteria has been disproved. The apparent absence of such exonucleases was quite surprising, since they play an important role in mRNA decay in both eukaryotes (Jinek et al. 2011) and Gram-positive bacteria (Mathy et al. 2007). This protein, which is now designated as RNase AM, was recently shown to be responsible for removing the extra nucleotides from the 5’ termini of the pre-5S, pre-23S and pre-16S rRNAs to generate their respective mature 5’ ends (Jain 2020). However, RNase AM does not substitute for RNase P in the processing of the immature 5’ ends of pre-tRNAs (Mohanty and Kushner, unpublished results). It is thus not clear how many substrates RNase AM has in the bacterium and whether it plays any role in mRNA decay.
mRNA decay pathways
With the existence of such a large number of both endo- and exoribonucleases, it is not surprising that each ribonuclease has a subset of preferred substrates (Mohanty and Kushner 2018). The recent genome-wide analyses of various ribonuclease mutants suggest that endoribonucleases affect the abundances of many more transcripts compared to exoribonucleases (Mohanty and Kushner 2003; Bernstein et al. 2004; Stead et al. 2011; Pobre and Arraiano 2015).
In vivo, most of the mRNA decay is initiated by the RNase E-based degradosome (Figure 1). This multiprotein mRNA degrading machine is directly associated with the inner membrane of the bacterium (Khemici et al. 2008; Strahl et al. 2015). RNase E can either bind to the 5’ terminus of an mRNA with a 5’ monophosphate or enter directly downstream of the 5’ terminus (Baker and Mackie 2003; Clarke et al. 2014). Subsequently, it can move in the 5’ → 3’ direction looking for downstream cleavage sites unless it is blocked by secondary structures (Richards and Belasco 2019). RNase G, an RNase E ortholog, can also carry out the endonucleolytic cleavages generating the large oligonucleotides, which are further degraded by various 3’ → 5’ exonucleases including PNPase, which is part of the degradosome complex. The RhlB RNA helicase, which is also part of the degradosome complex, is also believed to facilitate PNPase degradation by unwinding any secondary structures within the mRNA decay intermediates. The final step in the pathway involves oligoribonuclease degrading the very short oligoribonucleotides (2–5 nt) left over from the activity of PNPase, RNase II and/or RNase R (Figure 1).
Furthermore, recent data suggest that poly(A) tails added after the Rho-independent transcription terminators of polycistronic transcripts induce RNase E-mediated decay of such transcripts (Mildenhall et al. 2016). Since PAP I is also believed to be located at the inner membrane (Carabetta et al. 2010), such a result is not particularly surprising. Contrary to the initial assumption that there was a considerable excess of RNase E relative to PAP I, mass spectrometric analysis indicates that there are between 438–477 RNase E molecules per cell (Ishihama et al. 2008; Wisniewski and Rakus 2014). Considering that RNase E exists in vivo as a tetramer (Callaghan et al. 2003), this would indicate that there are only ∼110–120 degradosomes per cell, which is not significantly greater than the estimates of PAP I molecules (50–110) (Mohanty et al. 2004; Ishihama et al. 2008; Wisniewski and Rakus 2014).
The contributions of RNase G and RNase III to the initiation of mRNA decay are probably not very significant. Furthermore, there is currently no evidence that RNase G is associated with inner membrane. However, being an ortholog of RNase E and also a 5’ end-dependent ribonuclease (Tock et al. 2000), it is reasonable to assume that RNase G also decays mRNAs in the 5’ → 3’ direction. In contrast, the majority of RNase III is believed to be associated with inner membrane (Miczak et al. 1991), although this work has never been confirmed. The enzyme cleaves an RNA-RNA double-stranded stem of a minimum of 22 nt (Pertzev and Nicholson 2006). These structures are significantly longer than the double-stranded stems of Rho-independent transcription terminators. E. coli RNase III does cleave the secondary structure of its own mRNA (rnc) in the 5’ untranslated region causing the rapid degradation of the mRNA (Matsunaga et al. 1996). A recent study that identified genome-wide RNase III cleavage sites in E. coli observed that the enzyme regulates mRNA decay in several ways (Gordon et al. 2017). The authors demonstrated that RNase III cleavages led to either destabilization or stabilization of the transcripts and degradation of the transcripts encoding leader peptides involved in transcriptional attenuation. Furthermore, RNase III has been shown to cleave the MicA sRNA and its target ompA mRNA in Salmonella typhimurium, which is analogous to a eukaryotic RNAi system (Viegas et al. 2011).
It should be pointed out that some mRNAs are not substrates for any of the endoribonucleases and appear to be decayed entirely by the cell’s 3’ → 5’ exonucleases (Mohanty and Kushner 2003; Pobre and Arraiano 2015), although these mRNAs represent only a small fraction of the cell’s transcriptome. However, mRNA fragments arising from cleavages by any of the five endonucleases become substrates primarily for PNPase. RNase II may also be involved in some cases (Hajnsdorf et al, 1994; Haugel-Neilsen et al, 1996), since the rnb pnp double mutant is inviable and mRNA fragments accumulate at the nonpermissive temperature (Donovan and Kushner 1986). RNase R, the 3’ → 5’ exonuclease that is not inhibited by secondary structures is primarily employed in the degradation of rRNAs derived from nonfunctional ribosomes (Basturea et al. 2011). It is not clear at this time why the loss of PNPase and RNase II leads to synthetic lethality, while an RNase II RNase R double mutant does not show any significant phenotypic effect.
While RNase E has been shown to be responsible for the initiation of ~60% of the mRNAs decay in E. coli (Stead et al. 2011), RNase G, RNase III, PNPase, RNase II and RNase R collectively accounts for only~15% of the mRNAs (Lee et al. 2002; Mohanty and Kushner 2003; Stead et al. 2011; Pobre and Arraiano 2015). Thus, additional ribonuclease(s) may be involved in initiating the decay of ~25% of the mRNAs. In fact, an RNA-seq analysis suggests that RNase P affects the steady-state level of ~44% of the mRNAs and the majority of these are not substrates of RNase E (Mohanty and Kushner, manuscript in preparation). At the present time, nothing is known about the hypothesized mechanism of RNase P mediated mRNA decay pathway. While RNase P activity and the C5 protein component of holoenzyme have been shown to be associated with inner membrane, the M1 RNA component is cytosolic (Miczak et al. 1991). Both in vivo and in vitro experiments examining the processing of polycistronic tRNA transcripts suggest that RNase P acts in the 3’ → 5’ direction and can remove Rho-independent transcription terminators (Agrawal et al. 2014; Zhao and Harris 2019). It is thus not unreasonable to think that the enzyme will act in a similar fashion on its mRNA targets.
Regulation of mRNA stability
The steady-state level of a particular mRNA is the outcome of its rate of decay (half-life) versus its rate of transcription, both of which are most likely controlled by some or all of the factors that are discussed below (Figure 2).
Figure 2.
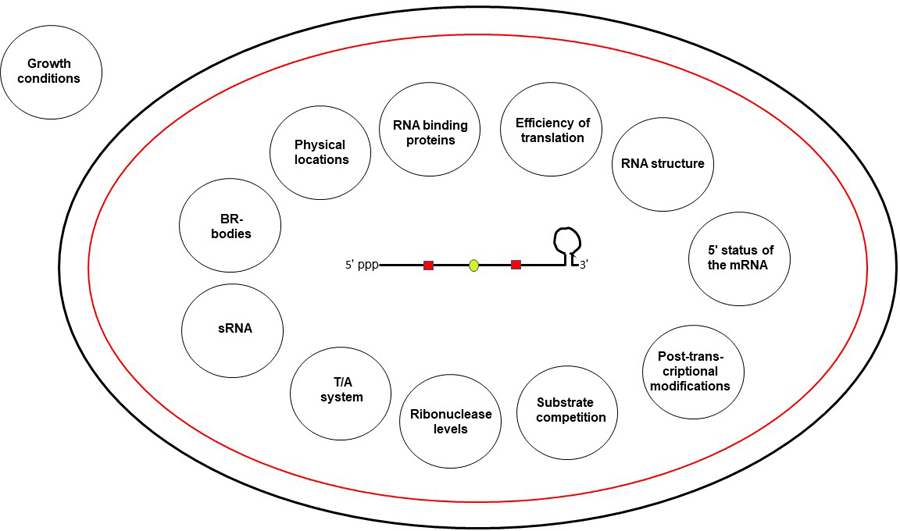
A schematic presentation of all the factors regulating mRNA stability in E. coli. A single mRNA molecule (as described in the legend to Figure 1) is shown at the center of a bacterium represented by a black outer membrane and a red inner membrane. The conditions under which each of the factor (shown inside the circle) affects the mRNA stability are described in the text. This figure is available in color online.
Growth conditions.
The half-lives of mRNAs can change significantly depending on the growth conditions, which helps the bacterium rapidly adjust to the available nutrient pools as well as any other change in its environment. The analysis of mRNA turnover and its regulation has generally focused on exponentially growing cells. Under these conditions the cell is not under nutrient limitations or any particular kind of stress. In these circumstances decay of most mRNAs is most likely regulated by their proximity to the inner membrane where the RNase E-based degradosomes and the polyadenylation complexes are located (Figure 3).
Figure 3.

Schematic view of the locations of the major enzymes involved in mRNA decay in E. coli. The outer membrane of the bacterium is shown in black, while the inner membrane is shown in red. The nucleoid, which normally occupies almost 60% of the internal cell volume, has been shrunk to provide more space for the enzymes and mRNA molecules (squiggly black lines). Once transcribed, some of the mRNA molecules have to find their way (short arrow) to the inner membrane (mechanism unknown) for their decay, since most of the ribonucleases including the primary ribonuclease RNase E are believed to be associated with the inner membrane. The RNase E-based degradosome is shown as a single large oval for the sake of simplicity. Its main components are shown outside of the cell. The Hfq protein is associated with some degradosomes. Poly(A) polymerase (PAP I) is also shown near the inner membrane. For the sake of simplicity, it is not depicted as part of the polyadenylation complex that also contains PNPase and Hfq (Mohanty et al. 2004). RNase III is shown attached to mRNAs that contain long stem loop structures near the inner membrane, although the exact location of this enzyme is not well established. RNase P activity is believed to be associated with inner membrane (Miczak et al. 1991). The figure is not drawn to scale. This figure is available in color online.
Once a cell enters stationary phase as a result of nutrient limitation, mRNA decay is governed by a very different set of rules. A recent microarray analysis of the E. coli transcriptome during conditions that followed the transition from glucose supported growth to acetate utilization to carbon starvation showed that most of the regulation in gene expression was actually driven by changes in transcriptional regulation (Morin et al. 2020). Thus, as the cells switch from exponential growth into stationary phase, the half-lives of a significant number of transcripts increased, while the total number of transcripts decreased. A further increase in half-lives was observed when the cells started to consume acetate and subsequently entered starvation. Thus, the half-lives which ranged between ∼1 – 8 minutes in exponentially growing cells increased to ∼3– 19 minutes under starvation conditions.
Furthermore, bacteria in nature encounter a variety of situations beyond nutrient starvation that are far from ideal. Some of these may include cold shock, heat shock, deficiencies in iron or nitrogen, osmotic shock, and pH shock to name just a few. Many of these changes lead to the induction of the stringent response, which results in a rapid cessation of rRNA synthesis and a significant reduction in the number of functional ribosomes. The concomitant reduction in protein synthesis leads to greater spacing of ribosomes on mRNAs making them more susceptible to attack by the various endonucleases, particularly by RNase E. At the same time, the transcription of many sRNAs is increased leading to the more rapid turnover of a large number of targeted mRNAs. In addition, some of the toxin/antitoxin systems will be activated releasing a series of endoribonucleases that are not active in exponentially growing cells. These ribonucleases target mRNAs at locations distinct from those enzymes that perform the bulk of mRNA turnover in exponentially growing cells.
Almost all stress conditions lead to increased sRNAs levels (Gottesman and Storz 2011). Many of the sRNAs have multiple mRNA targets. The higher levels of sRNAs drive increased interactions with target mRNAs, usually resulting in their more rapid turnover. These sRNA/mRNA interactions arising from the result of cell stress provide an important mechanism for the cell to rapidly downshift its biosynthetic apparatus to promote survival under difficult conditions. However, the impact of sRNAs is more specific than the dramatic reduction in functional ribosomes arising from the activation of the stringent response. Finally, it should be pointed out that very few experiments have actually been carried to measure mRNA half-lives under various stress conditions and to determine what fraction of the transcriptome is affected by a particular stress condition.
Interactions with small regulatory RNAs (sRNAs).
A large number of mRNAs have their half-lives modulated in part by whether they are being targeted by a sRNA. It is currently thought that there are 80–100 sRNAs in E. coli ranging in length from 60–300 nt, which can either be cis- or trans-acting. cis acting sRNAs are encoded on the strand complementary to their target mRNA, while trans-acting sRNAs can act at a distance and in many cases have multiple targets (Storz et al. 2005; Waters and Storz 2009; Storz et al. 2011). In general, sRNAs are not expressed constitutively. Rather their synthesis is induced under a variety of stress conditions such as nutrient limitation, iron or nitrogen starvation, osmotic stress or changes in pH (Gottesman and Storz 2011). The RNA binding protein Hfq (see below) is required for the majority of the trans-acting sRNAs to efficiently interact with their target mRNAs (Vogel and Luisi 2011). In fact, with the development of the RIL-seq technique (RNA interaction by ligation and sequencing) Melamed et al. (Melamed et al. 2016) identified ∼2800 Hfq-bound RNA pairs, strongly indicating that sRNA/mRNA interactions play a major role in post-transcriptional gene regulation.
Many sRNAs have multiple targets and usually function by binding their target mRNAs at or near the ribosome binding site promoting the degradation of the mRNA (Caron et al. 2010; Gottesman and Storz 2011). RNase E is usually the endoribonuclease that initiates the decay involving sRNA/mRNA interaction (Caron et al. 2010; Gottesman and Storz 2011), but RNase III can also be involved (Lalaouna et al. 2013). It has been shown that PNPase is involved in the degradation of sRNAs that are not bound to target mRNAs (De Lay and Gottesman 2011; Andrade et al. 2012; Bandyra et al. 2016). In a limited number of cases, interaction of a sRNA with mRNA increases its translatability, usually by unmasking the ribosome binding site (Gottesman and Storz 2011). In these cases, the half-life of the target mRNA will increase, since the mRNAs that are being actively translated are protected from endonucleolytic cleavages in part by bound ribosomes.
Toxin/Antitoxin systems.
E. coli has been shown to have at least 33 toxin/antitoxin (TA) systems that play significant roles under various stress conditions by helping the cell rapidly adjust to changes in its environment. Some are induced by amino acid starvation and carbon starvation, while others respond to the DNA damage (Christensen-Dalsgaard et al. 2010). In most of these systems the toxin proteins are endoribonucleases [these enzymes have been called mRNA interferases (Inouye 2006)] and their most common targets are mRNAs at specific nucleotide sequences as the mRNA are being translated (Gerdes and Maisonneuve 2012). However, these enzymes can be either ribosome-dependent or -independent and represent a powerful broad-spectrum mechanism for rapidly destroying large numbers of functional mRNAs, which bypasses the normal mRNA decay pathways. For example, the MazF enzyme, which recognizes the sequence ACA (Zhang et al. 2003), only cleaves mRNAs containing this sequence. These ribonucleases are not active during exponential growth because they form a stable complex with their cognate antitoxins (Gerdes and Maisonneuve 2012). The free antitoxins are, however, highly unstable if not complexed with the toxins and thus need to be continuously synthesized to inhibit toxin activity (Yamaguchi and Inouye 2009).
Interestingly, E. coli appears to harbor an RNA repair system to recover from the stress-induced RNA damage. RtcA RNA cyclase, which converts RNA 3’-phosphate ends to 2’,3’-cyclic phosphate and RtcB ligase that joins 2’,3’-cyclic phosphate and 5’-OH RNA ends to form a 3’–5’ phosphodiester splice junction are encoded in rtcBA operon and predicted to carry out the healing and sealing functions respectively (Tanaka and Shuman 2011). In fact, the cleavage at the 3’-ends of 16S rRNA by MazF during amino acid starvation producing specialized ribosomes have been shown to be re-ligated by RtcB ligase both in vitro and in vivo (Temmel et al. 2017). However, a recent genome-wide transcriptome analysis demonstrating that MazF does not produce a large pool of specialized ribosomes has contradicted the previous observation (Culviner and Laub 2018). Instead, the MazF cleavages inhibit the bacterial growth by disrupting ribosome biogenesis.
Alterations in intracellular ribonuclease levels.
The intracellular levels of various ribonucleases can vary depending on the growth conditions. For example, the levels of RNase E, PNPase and RNase III, the major ribonucleases that participate in mRNA decay are known to be controlled by a variety of mechanisms including autoregulation (Bardwell et al. 1989; Matsunaga et al. 1996; Jarrige et al. 2001; Carzaniga et al. 2009; Schuck et al. 2009; Briani et al. 2016). In addition, the trans-acting proteins RraA, RraB (regulator of ribonuclease activity) (Gao et al. 2006) and ribosomal protein L4 (Singh et al. 2009) also downregulate RNase E activity by binding to C terminus, leading to the stabilization of several RNase E-dependent mRNAs (Lee et al. 2003; Gorna et al. 2010). RraA binds to a specific segment in the C terminal segment of the protein and interferes with the activity of the RhlB RNA helicase and, indirectly PNPase (Gorna et al. 2010). The protein can also interfere with the activities of other DEAD box RNA helicases (Gorna et al. 2010).
An adaptor protein, RapZ, has been shown to be required in order for RNase E to cleave the sRNA GlmZ. This sRNA activates translation of the glmS mRNA, which leads to increased synthesis of glucosamine-6-phosphate, a key metabolite required for cell envelope biosynthesis. When there are high levels of glucosamine-6-phosphate in the cell, GlmZ is inactivated by RNase E cleavage, which is strictly dependent on RapZ (Gopel et al. 2013). At low intracellular levels of glucosamine-6-phosphate there is increased synthesis of a second sRNA (GlmY), whose function is to sequester the RapZ protein, leading to increased levels of GlmZ, thereby stimulating the translation of the glmS mRNA.
The levels of RNase E and PNPase have also been shown to increase under conditions that stimulate higher decay activity, such as increased polyadenylation of transcripts (Mohanty and Kushner 2002). Stress conditions, such as cold shock induces increased PNPase levels by interfering with its autoregulation (Beran and Simons 2001; Mathy et al. 2001). Interestingly, the level of RNase II and PNPase negatively regulate each other’s synthesis (Zilhao et al. 1996). Thus, the absence of RNase II leads to increased PNPase levels and overproduction of RNase II decreases PNPase levels. Similarly, the absence of PNPase increases RNase II levels and overproduction of PNPase decreases RNase II levels. It has also been shown that RNase II levels change in different media, a phenomenon that is regulated by gmr (gene modulating RNase II). In fact, a three-fold increase in RNase II levels was observed in a gmr deletion strain (Cairrao et al. 2001). RNase II levels have also shown to be regulated by the acetylation of the residue Lys501 (Song et al. 2016).
RNase R levels have been shown to increase under stress conditions that include starvation, cold shock and stationary phase (Cairrao et al. 2003). The increase in RNase R levels during stationary phase results in the destabilization of the full-length ompA mRNA (Andrade et al. 2006). Furthermore, not all of the RNase R in exponentially growing cell is free, which is believed to be deleterious and can cause severe RNA degradation. Rather, ~80% of the RNase R in a growing cell is bound to ribosomes (Liang and Deutscher 2013).
With regard to actual enzyme levels within the cell, there appears to be considerable variation between how many molecules are present in exponential growing cells versus stationary phase cultures for many of the ribonucleases. For example, based on two studies that employed mass spectrometry to determine protein levels (Ishihama et al. 2008; Wisniewski and Rakus 2014), the number of PNPase molecules was determined to be 5,700 in exponentially growing cells, but only 911 in stationary phase cells. However, there was significantly less variation for the endoribonucleases, RNase III (383 in exponential phase vs. 294 in stationary phase) and RNase E (444 in exponential phase vs. 438 in stationary phase). In fact, RNase E appears to be in excess in the cell in either growth condition, since the enzyme levels have to be significantly reduced before any phenotypic effect is observed (Jain and Belasco 1995; Ow et al. 2002). One interesting observation from these two studies is that PNPase appears to be the most abundant ribonuclease in cell under all growth conditions.
Substrate competition.
What has not always been appreciated is the fact that for a particular endoribonuclease, which can cleave multiple substrates, there will always be an issue of substrate competition. Thus, another very important regulatory element of mRNA decay is the competition for mRNA substrates by the ribonucleases based on their binding affinities. As recently pointed out, even though RNase E is involved in the decay of a large number of mRNA substrates, its binding affinity will vary among the various species (Etienne et al. 2020). Since there is an excess of mRNA substrates relative to the number of ribonuclease proteins, mRNAs that have a higher binding affinity for a particular RNase will be decayed preferentially over those mRNAs that bind with lower affinities. Thus, binding affinities will play a significant role in regulating the decay of particular mRNAs independent of structural features at either terminus or proximity to the inner membrane or physical location on the nucleoid.
Post-transcriptional modifications.
The addition of untemplated nucleotides to the 3’-ends of transcripts is one of the common post-transcriptional modifications that can affect their stability. In eukaryotes every mRNA is post-transcriptionally modified with either a poly(A) or a poly(U) tail, as in the case for histone mRNAs (Mullen and Marzluff 2008). In E. coli and some other Gram-negative bacteria, mRNAs can be post-transcriptionally modified by either PAP I or PNPase. Although PAP I was first discovered in 1962 (August et al. 1962), its structural gene (pcnB) was not identified for another 30 years (Cao and Sarkar 1992). Subsequently, it was shown that the addition of poly(A) tails serves as a targeting signal for rapid mRNA decay (O’Hara et al. 1995; Mohanty and Kushner 1999). A genome-wide analysis suggests that transcripts from 90% of the expressed genes in E. coli are polyadenylated to some extent, but the specific percentage of polyadenylated transcript for each of the genes can vary significantly (Mohanty and Kushner 2006). Several studies have shown that less than 2% of the any particular mRNA substrate is actually polyadenylated at any given time in E. coli. (Cao and Sarkar 1992; Mohanty and Kushner 1999; Mohanty and Kushner 2006)
Surprisingly, while PAPI can add poly(A) tails onto almost any RNA molecule in vitro (Yehudai-Resheff and Schuster 2000), but in vivo many decay and processing intermediates are selectively subject to polyadenylation, particularly the ones with strong secondary structures (Spickler and Mackie 2000). The unstructured poly(A) tails provide strong binding sites for almost all the 3’ → 5’ exoribonucleases (Hajnsdorf et al. 1994; Spickler and Mackie 2000; Cheng and Deutscher 2005; Andrade et al. 2009). While the mechanism of substrate selection by PAP I is yet to be understood, a polyadenylation complex comprising PAP I, PNPase and the RNA binding protein Hfq has been shown to target transcripts with Rho-independent transcription terminators suggesting such terminators serve as polyadenylation signals (Mohanty et al. 2004; Mohanty and Kushner 2006). Recent work indicates that a Rho-independent transcription terminator at the 3’ terminus of a polycistronic mRNA also plays a role in the polyadenylation of transcripts from ORFs within the operon (Mildenhall et al. 2016). It is not entirely clear how PNPase and Hfq function in the polyadenylation complex. However, in the absence of the either protein, PAP I mediated polyadenylation after Rho-independent transcription terminators is significantly altered (Mohanty et al. 2004; Mildenhall et al. 2016).
PAP I is a member of a large superfamily that includes tRNA nucleotidyltransferases. In fact, a number of potential poly(A) polymerases in Gram-positive bacteria have turned out to be tRNA nucleotidyltransferases. Although PAP I plays a significant role in the regulation of mRNA decay in E. coli (Mohanty and Kushner 2010a), the enzyme is only found in a limited number of Gram-negative bacteria (Raynal and Carpousis 1999). One possible explanation for the limited presence of PAP I enzymes may be related to its toxicity (Mohanty & Kushner, 1999), which involves polyadenylation of 79/86 of the E. coli pre-tRNAs (Mohanty et al. 2012). Thus, PAP I is under tight regulation in E. coli and deregulation of PAP I leads to the inhibition of protein synthesis and cell death (Mohanty and Kushner 2013).
E. coli and the vast majority of other prokaryotes contain PNPase, an enzyme that can carry out both 3’ → 5’ exonucleolytic degradation of single-stranded RNA, as well as synthesizing polynucleotide tails in the presence of high concentrations of nucleoside diphosphates (Grunberg-Manago 1963). Since the degradative activity of PNPase requires inorganic phosphate and its concentration in E. coli is ∼13 mM (Shulman et al. 1979), it was assumed for many years that the enzyme only worked degradatively in vivo. Instead, it turns out that the enzyme can function both degradatively and biosynthetically in the bacterium (Mohanty and Kushner 2000). However, unlike PAP I added poly(A) tails, PNPase synthesized tails occur almost exclusively on mRNA decay fragments or very near the 5’ terminus of an mRNA (Mohanty et al. 2004). These polynucleotide tails can be very long (> 50 nt), but they do not have any significant secondary structure (Mohanty and Kushner 2010a). Unlike what has been observed with poly(A) tails, there is no direct evidence at the present time that polynucleotide tails play any role in regulating mRNA decay. In fact, they probably arise from the fact that PNPase has an equilibrium constant of one so that the forward and reverse activities of the enzyme are controlled by the availability of either inorganic phosphate or ribonucleoside diphosphates. Thus, it is possible that this property of PNPase helps the enzyme to be more efficient, particularly on substrates that are either shorter in length or contain significant secondary structures (Mohanty et al. 2004; Mohanty and Kushner 2010a).
5’-status of the mRNA.
Unlike eukaryotes where mRNAs are protected from degradation by a methylated guanosine (7mG) cap at the 5’-end, E. coli mRNAs appear to be protected from degradation by a host of different chemical moieties at the 5’ end (Vasilyev et al. 2019), which can profoundly influence their half-lives. Many primary transcripts with a 5’ triphosphate are protected from the RNase E and RNase G endonucleolytic cleavages due to these enzymes’ strong preference for an unpaired 5’ end with a single phosphate (Mackie 1998; Tock et al. 2000). Thus, conversion of a triphosphate 5’ end to monophosphate 5’ end may play a key role in mRNA stability. Accordingly, initially it was believed that RNA pyrophosphohydrolase (RppH encoded by the rppH gene) plays the key role by converting a triphosphate RNA to a 5’-monophosphate RNA, which would trigger RNase E mediated decay (Deana et al. 2008). More recently it has been shown that the preferred substrate for the RppH protein is actually a diphosphate (Luciano et al. 2017; Luciano et al. 2018). Furthermore, it has been demonstrated that the RppH enzyme has preferred nucleotides downstream of the triphosphate (Foley et al. 2015). Thus, it was not surprising that the impact of this enzyme is limited, since the steady-state levels of only a small number of mRNAs were changed in a mutant lacking the enzyme (Deana et al. 2008).
A subset of regulatory small RNAs (sRNA) contain a 5’-nicotinamide adenine dinucleotide (NAD) cap (Cahova et al. 2015). While the mechanism of NAD-cap biosynthesis is still unknown, it strongly stabilizes the RNA from degradation in vitro by RNase E (Bandyra et al. 2012) and processing by RppH (Deana et al. 2008). However, NudC, another member of the Nudix family of phosphohydrolases, is highly effective in decapping the NAD-cap, but has no effect on 5’ triphosphorylated RNA (Cahova et al. 2015). Interestingly, although the percentage of NAD-capped sRNAs increased two-fold in a nudC deletion mutant, no significant change in the in vivo half-lives of sRNAs was observed (Cahova et al. 2015), indicating the presence of either additional decapping enzyme(s) or an alternative decay pathway. Furthermore, some sRNAs have been reported to contain coenzyme A (CoA) at their 5’ ends, although its significance has yet to be determined (Kowtoniuk et al. 2009).
RNA structure.
RNA structure, particularly secondary or tertiary structures at either or both the 5’ and 3’ termini of an mRNA can have a significant impact on its half-life. The presence of secondary structures in the 5’ untranslated region (UTR) significantly stabilizes the downstream sequences, presumably by blocking RNase E from initiating mRNA decay (Emory and Belasco 1990; Emory et al. 1992; Jain and Belasco 1995; Bricker and Belasco 1999). Similarly, secondary structures at the 3’ end of an mRNA significantly inhibit both the major exonucleases, PNPase and RNase II (Spickler and Mackie 2000). Thus, mRNAs terminated with a Rho-independent transcription terminator (almost half of the E. coli transcripts) (Lesnik et al. 2001) or a Rho-dependent transcription terminator will be differentially susceptible to these exonucleases. Generally, mRNAs with a Rho-independent transcription terminator are not good substrates for either of these 3’ → 5’ exonucleases with a few limited exceptions. For example, the Rho-independent transcription terminator associated with the primary leuX tRNA transcript is removed by PNPase without the need of RhlB helicase activity (Mohanty and Kushner 2010b).
Although RNase R is not inhibited by the secondary structures, it needs a 10–12 nt single-stranded region to bind to a substrate (Cheng and Deutscher 2005). Since the single-stranded extensions beyond the stems associated with Rho-independent transcription terminators are generally only 1–5 nt in length, unless there is a post-transcriptional addition of a poly(A) tail (see above), RNase R will not play any significant role in degrading a primary mRNA transcript. In fact, the role of RNase R in mRNA decay is probably minimal (Pobre and Arraiano 2015). mRNAs that are terminated in a Rho-dependent fashion are potential substrates for all three 3’ → 5’ exonucleases since this type of termination event does not generate secondary structures.
In addition to Rho-independent transcription terminators, E. coli is also known to contain a significant number of repetitive extragenic palindromes (REP elements) (Higgins et al. 1988) that form large stem-loops in the spacer regions of some polycistronic messages. These are structural barriers that protect the 5’ proximal sequences from the action of PNPase and RNase II (Newbury et al. 1987a; Newbury et al. 1987b). Both PNPase and the RhlB RNA helicase along with PAP I are required to degrade the REP sequences (Khemici and Carpousis 2004).
The five DEAD-box RNA helicases in E. coli encoded by the rhlB, rhlE, dbpA, srmB, and deaD (csdA) genes (Jagessar and Jain 2010) have been shown to play different roles in RNA metabolism. An interaction of the DbpA helicase with helix 92 of the 23S ribosomal RNA promotes its ATPase and helicase activities (Diges and Uhlenbeck 2001). The SrmB and DeaD (CsdA) helicases have been implicated in ribosome biogenesis (Charollais et al. 2003; Charollais et al. 2004). However, the CsdA RNA helicase, which is also a cold shock protein, has been shown to be associated with RNase E in a so-called cold shock degradosome (Prud’homme-Genereux et al. 2004). While the role of the RhlE helicase in E. coli is not clear, but it is associated with the cold shock degradosome in Caulobacter crescentus, another Gram-negative bacterium (Aguirre et al. 2017).
In terms of mRNA decay, the most relevant RNA helicase is RhlB, since it is an integral part of the RNase E-based degradosome (Coburn et al. 1999; Khemici et al. 2004). It has been shown that the RhlB RNA helicase is necessary for the RNase E-based degradosome to degrade ribosome free mRNA molecules (Khemici et al. 2005). Microarray analysis of a rhlB mutant indicated that the decay of specific polycistronic mRNA operons was significantly affected in the absence of the helicase, although the total number of affected genes was relatively small (Bernstein et al. 2004).
Efficiency of translation.
The efficiency of mRNA translation plays a significant role in regulating the decay of individual mRNAs. Specifically, a high density of ribosomes on a mRNA will preclude endonucleolytic access to cleavage sites (Braun et al. 1998). Furthermore, AU sequences within the 5’ untranslated region of an mRNA enhances its translation and stability (Komarova et al. 2005). In addition, it has been shown that poorly translated mRNAs that arise from noncanonical ribosome binding sites (RBS) are turned over more rapidly than those containing an RBS that is closer to the canonical sequence (Richards et al. 2012). Reduction in translation efficiency resulting from the presence of rare codons in the early regions of the mRNA can in some circumstances also lead to more rapid decay of the transcript.
RNA binding proteins (Chaperones)
Hfq.
Hfq is a small RNA binding protein that was discovered based on its role in the replication of the bacteriophage Qβ (Franze de Fernandez et al. 1968). The protein has two binding surfaces, one that prefers A/U rich regions in mRNA molecules and the other that interacts with small regulatory RNAs (Schumacher et al. 2002; Link et al. 2009). Since the Hfq protein is required to assist the interactions of most trans-acting sRNAs with their target mRNAs, its role in mRNA decay is most likely indirect (Gottesman et al. 2001). However, Hfq has also been reported to be associated with the RNase E based degradosome (Morita and Aiba 2011). Thus, it may have a more direct role in mRNA decay, which has yet to be demonstrated.
ProQ.
ProQ is a recently identified RNA chaperone that belongs to the FinO family of RNA binding proteins (Olejniczak and Storz 2017). There are a number of features that make ProQ an very interesting RNA chaperone. Besides having multiple target sites in both E. coli and Salmonella enterica (Holmqvist et al. 2018), the protein appears to bind at the 3’ termini of mRNAs, protecting them from 3’ → 5’ exonucleolytic decay. However, recent work on RNA-RNA interactomes has shown that the ProQ network is smaller than that observed for Hfq, where about one third of the RNA-RNA interactions were common between the two proteins (Melamed et al. 2020).
CsrA.
The CsrA protein in E. coli typically binds to an mRNA either promoting or repressing translation. The small ∼7 kDa protein that forms a homodimer (Gutierrez et al. 2005) has been shown to bind to an AUGGA sequence that is localized in the loop of a stem-loop (Dubey et al. 2005). The binding of CsrA can either promote mRNA decay by inhibiting translation or stabilize a transcript by increasing its translatability. The protein can also directly affect mRNA stability by either blocking or promoting access to ribonucleases (Yakhnin et al. 2013). The action of CsrA is controlled in part by action of two small RNAs, CsrB and CsrC, which bind with high affinity to the CsrA protein (Romeo et al. 2013). Compared to the Hfq and ProQ RNA binding proteins, CsrA has a more limited number of target mRNAs (Quendera et al. 2020).
Physical location.
The cytoplasm of E. coli is very crowded with the nucleoid occupying up to 60% of the bacterium’s volume. In cells that are actively growing the actual volume of the nucleoid is probably larger when considering simultaneous DNA replication and transcription. It is thus likely that many mRNAs will be transcribed in close proximity to the inner membrane. Given that a number of the enzymes involved in mRNA decay are associated with the inner member [(RNase E-based degradosome, RNase P, RNase III, PAP I, (Miczak et al. 1991; Diestra et al. 2009; Carabetta et al. 2010; Lu and Taghbalout 2013)] and the interaction between the degradosome and polyadenylation after Rho-independent transcription terminators (Mildenhall et al. 2016), it seems likely that a significant amount of mRNA decay takes place at the inner membrane (Figure 3).
However, not all mRNAs will be transcribed in close proximity to the inner membrane. Given that the compaction of the bacterial genome is not random, a significant number of mRNAs will be transcribed at internal locations. In fact, a study of RNA dynamics has revealed RNA foci at various locations around the nucleoid (Golding and Cox 2004). Thus, the half-lives of mRNAs not transcribed close to the inner membrane might be expected to longer, either because they need to diffuse to the inner membrane or because of reduced accessibility of a particular ribonuclease.
As was pointed out in an earlier review on the subject of the regulation of mRNA decay (Mohanty and Kushner 2016), there are considerable differences in the half-lives of a variety of mRNAs that are very similar in size and method of transcription termination. For example, the half-lives of the lpp, rpsT, cspE, and rpoS transcripts have half-lives that vary from 2.1 min to more than 10 min (O’Hara et al. 1995; Mohanty and Kushner 1999; Ow et al. 2003; Perwez and Kushner 2006). Although a longer half-life could result from it not being a substrate for RNase E, it could also be a function of the cellular location of the particular gene. It should be noted that at this point in time the role of physical location of a mRNA affecting its half-life is purely speculative as no experiments have yet been carried out to see if changing the location of a particular gene on the E. coli genome results in a significant change in its half-life.
BR-bodies.
It has recently been shown that there is an association of RNAs and proteins into membrane-less organelles similar to the P bodies found in eukaryotic cells (Muthunayake et al. 2020). What is interesting about the BR-bodies is that they preferentially form with untranslated mRNAs. In fact, they seem to specifically exclude mRNAs associated with ribosomes (Muthunayake et al. 2020). Some sRNAs and antisense RNAs are also found in the BR-bodies. Thus, it may be that the primary function of BR-bodies is to sequester and degrade RNAs that are no longer functional avoiding the decay of functional mRNAs.
Overview
RNA decay versus processing
Initially it was assumed that all post-transcriptional RNA metabolism was part of a general salvage pathway designed for the primary purpose of recycling nucleotides. Subsequently, it was recognized that not all RNase activity involved degradation, but rather was required to process precursor RNA molecules into their functionally active mature forms. The two most striking examples of this type of reaction are the processing and maturation of tRNAs and rRNAs. For example, RNase P participates in both separation of pre-tRNAs from polycistronic operons and tRNA 5’-end maturation. RNase T, RNase PH, RNase D and RNase BN are involved in trimming pre-tRNA 3’-ends for 3’-end maturation. RNase III is involved in separation of rRNA precursors by cleaving within the double-stranded regions of the primary 30S rRNA transcript. More recent studies have shown RNase E and RNase III are required for maturation of many sRNAs.
Although many of the RNases involved in post-transcriptional RNA processing also participate in mRNA decay, the actual details associated with this process are much more complicated than previously thought. Furthermore, the regulation of the process is far less well understood. What seems clear is that much depends on the actual state of the cell at any particular time. If the cell is in a nutrient rich environment and is growing exponentially, the fate of a particular mRNA is going to be controlled by a combination of structural considerations (i.e. the presence of secondary and/or tertiary regions at either its 5’ or 3’ end) and how rapidly it is being translated along with its physical location within the cell. If a mRNA is located close to the cell’s inner membrane, it will be an inviting target for degradation by the degradosome. Since the bulk of the sRNAs are not present at significant levels during exponential growth, sRNA mediated regulation is not going to have a major impact on mRNA decay. What will be important is the combination of ribonuclease abundance and the binding affinities of individual mRNAs for the various ribonucleases. These circumstances result in setting up a competition among mRNAs for the relatively limited number of ribonuclease molecules. In addition, since mRNA half-lives have been shown to dependent in part on growth rate, overall mRNAs will decay more rapidly when the cell is in exponential phase of growth.
With regard to nutrient starvation and any other type of stress, the regulation of mRNA decay is considerably more complex. Because in addition to all the factors that play a role during exponential growth, there is an overlay of the changes in ribonuclease levels under a particular cellular condition, such as the stringent response, induction of sRNAs and the activation of toxin/antitoxin systems. For example, it has been shown that RNase R can be highly unstable depending on both growth and stress conditions (Chen and Deutscher 2010). PNPase levels change over 6-fold between exponential growing cells and stationary phase cells (Ishihama et al. 2008; Wisniewski and Rakus 2014). In the case of the stringent response, rRNA synthesis is rapidly reduced leading to a reduction in both ribosomes and functional tRNAs. Reduced ribosome levels will lead to decreased spacing on mRNAs being translated making them more accessible to decay by BR bodies.
In the case of sRNAs, their impact on mRNA decay under stress conditions is more selective in that each sRNA, while it may have more than one target, generally will only affect specific pathways that are directly related to the particular stress event. Iron starvation will elicit the synthesis of a different set of sRNAs than those that respond to nitrogen starvation.
Future Directions
The consensus view of mRNA decay in E. coli at the present time is that RNase E is the primary enzyme responsible for the initiation of decay for the vast majority of mRNAs under most circumstances. RNase III, G, Z and the primary 3’ → 5’ exonucleases (PNPase, RNase II and RNase R) play secondary roles. However, RNase P is not thought to be significantly involved in mRNA decay. Although some people think that RNase E is responsible for the initiation of all mRNA decay, the recent transcriptome analyses shows that RNase E only affects the steady-state levels of ~60% of all E. coli mRNAs in exponentially growing cells (Stead et al. 2011). The same study also found that RNase III is responsible for changes in ∼12% of the transcripts. That leaves possibly between 30–40% of the transcripts unaccounted for. Is there a yet to be discovered endoribonuclease or can either RNase G, RNase Z or RNase P or some combination of them initiate mRNA decay on those transcripts that are not substrates for either RNase E or RNase III?
In fact, new results obtained from an RNA-seq analysis of a temperature sensitive RNase P mutant (rnpA49) may provide the answer (Mohanty and Kushner, manuscript in preparation). Specifically, inactivation of RNase P leads to changes in the steady-state levels of ∼44% of the mRNAs transcribed in exponentially growing cells. Furthermore, the mRNAs affected by inactivation of RNase P do not appear to be substrates for RNase E and RNase E targeted mRNAs are not substrates for RNase P. Thus, it is possible that the bulk of initiation of mRNA decay in E. coli will be accounted for by these two enzymes along with RNase III. All of the other endonucleases probably play secondary roles. Clearly, more work is needed to confirm the RNase P RNA-seq data.
Furthermore, the role of CRISPR (clustered regularly interspaced short palindromic repeats) Cas (CRISPR associated) system, a small RNA based adaptive immune system to combat viral infections, in prokaryotic RNA metabolism is yet to be uncovered. Cas proteins encode nucleases which target the DNA or RNA of the viruses with the help of crRNA (CRISPR RNA). E. coli K12 (MG1655) contains two CRISPR regions (I and II) where CRISPR I cluster is associated with eight cas genes and no separate Cas genes are found near CRSPR II. Recently, the promoters to the Cas genes near CRISPR I have been characterized (Pul et al. 2010).
It should be noted that increased or decreased levels of a particular mRNA observed in either microarray or RNA-seq experiments may arise from secondary effects associated with the stabilization or destabilization of a particular sRNA or as yet some unidentified factor. For example, stabilization of a transacting sRNA in an RNase E mutant may lead to a reduced steady-state level of one or more of its target mRNA(s), even though the mRNA is normally not a substrate for the enzyme. Since there is evidence RNase E, RNase P and RNase III can be involved in the degradation of a variety of sRNAs, it is likely that some of the changes in transcriptome-wide mRNA steady-state levels are the result of changes in sRNA levels.
Finally, it is worth pointing out that prior to microarrays and RNA-seq, mRNA half-lives were determined using northern blot analysis. With this approach it was possible to visualize the loss of the full-length transcript, which would accurately reflect its true chemical half-life. However, this process is not efficient when it comes to transcriptome-wide determination of mRNA half-lives. In contrast, while microarrays and RNA-seq studies can easily handle transcriptome-wide determinations, these techniques do not discriminate between full-length transcripts and decay intermediates. Thus, there is an ongoing debate between accuracy and determination of genome-wide measurements using these techniques.
Footnotes
Declaration of interest
This work was supported in part by a grant (GM08554) from the National Institutes of General Medical Sciences to S.R.K.
References
- Agrawal A, Mohanty BK, Kushner SR. 2014. Processing of the seven valine tRNAs in Escherichia coli involves novel features of RNase P. Nucleic Acids Res 42: 11166–11179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre AA, Vicente AM, Hardwick SW, Alvelos DM, Mazzon RR, Luisi BF, Marques MV. 2017. Association of the cold shock DEAD-Box RNA helicase RhlE to the RNA degradosome in Caulobacter crescentus. J Bacteriol 199: UNSPe00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alifano P, Rivellini F, Piscitelli C, Arraiano CM, Bruni CB, Carlomagno MS. 1994. Ribonuclease E provides substrates for ribonuclease P-dependent processing of a polycistronic mRNA. Gen Develop 8: 3021–3031. [DOI] [PubMed] [Google Scholar]
- Altman S 1989. Ribonuclease P: an enzyme with a catalytic RNA subunit. Adv Enzymol Relat Areas Mol Biol 62: 1–36. [DOI] [PubMed] [Google Scholar]
- Altman S. 1990. Nobel lecture. Enzymatic cleavage of RNA by RNA. Biosci Rep 10: 317–337. [DOI] [PubMed] [Google Scholar]
- Andrade JM, Cairrao F, Arraiano CM. 2006. RNase R affects gene expression in stationary phase: regulation of ompA. Mol Microbiol 60: 219–228. [DOI] [PubMed] [Google Scholar]
- Andrade JM, Pobre V, Matos AM, Arraiano CM. 2012. The crucial role of PNPase in the degradation of small RNAs that are not associated with Hfq. RNA 18: 844–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade JM, Pobre V, Silva IJ, Domingues S, Arraiano CM. 2009. The role of 3’−5’ exoribonucleases in RNA degradation. Prog Mol Biol Transl Sci 85: 187–229. [DOI] [PubMed] [Google Scholar]
- Apirion D 1973. Degradation of RNA in Escherichia coli: A hypothesis. Mol Gen Genet 122: 313–322. [DOI] [PubMed] [Google Scholar]
- Apirion D, Lasser AB. 1978. A conditional lethal mutant of Escherichia coli which affects the processing of ribosomal RNA. J Biol Chem 253: 1738–1742. [PubMed] [Google Scholar]
- Arraiano CM, Yancey SD, Kushner SR. 1988. Stabilization of discrete mRNA breakdown products in ams pnp rnb multiple mutants of Escherichia coli K-12. J Bacteriol 170: 4625–4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asha PK, Blouin RT, Zaniewski R, Deutscher MP. 1983. Ribonuclease BN: Identification and partial characterization of a new tRNA processing enzyme. Proc Natl Acad Sci U S A 80: 3301–3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- August J, Ortiz PJ, Hurwitz J. 1962. Ribonucleic acid-dependent ribonucleotide incorporation. I. Purification and properties of the enzyme. J Biol Chem 237: 3786–3793. [PubMed] [Google Scholar]
- Babitzke P, Kushner SR. 1991. The Ams (altered mRNA stability) protein and ribonuclease E are encoded by the same structural gene of Escherichia coli. Proc Natl Acad Sci U S A 88: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KE, Mackie GA. 2003. Ectopic RNase E sites promote bypass of 5’-end-dependent mRNA decay in Escherichia coli. Mol Microbiol 47: 75–88. [DOI] [PubMed] [Google Scholar]
- Bandyra KJ, Said N, Pfeiffer V, Gorna MW, Vogel J, Luisi BF. 2012. The seed region of a small RNA drives the controlled destruction of the target mRNA by the endoribonuclease RNase E. Mol Cell 47: 943–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyra KJ, Sinha D, Syrjanen J, Luisi BF, De Lay NR. 2016. The ribonuclease polynucleotide phosphorylase can interact with small regulatory RNAs in both protective and degradative modes. RNA 22: 360–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell JCA, Regnier P, Chen S-M, Nakamura Y, Grunberg-Manago M, Court DL. 1989. Autoregulation of RNase III operon by mRNA processing. EMBO J 8: 3401–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basturea GN, Zundel MA, Deutscher MP. 2011. Degradation of ribosomal RNA during starvation: comparison to quality control during steady-state growth and a role for RNase PH. RNA 17: 338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechhofer DH, Deutscher MP. 2019. Bacterial ribonucleases and their roles in RNA metabolism. Crit Rev Biochem Mol Biol 54: 242–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran RK, Simons RW. 2001. Cold-temperature induction of Escherichia coli polynucleotide phosphorylase by reversal of its autoregulation. Mol Microbiol 39: 112–125. [DOI] [PubMed] [Google Scholar]
- Bernstein JA, Lin P-H, Cohen SN, Lin-Chao S. 2004. Global analysis of Escherichia coli RNA degradosome function using DNA microarrays. Proc Natl Acad Sci U S A 101: 2758–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner FR, Plunkett III G, Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF et al. 1997. The complete sequence of Escherichia coli K-12. Science 277: 1453–1474. [DOI] [PubMed] [Google Scholar]
- Braun F, Le Derout J, Régnier P. 1998. Ribosomes inhibit an RNase E cleavage which induces the decay of the rpsO mRNA of Escherichia coli. EMBO J 17: 4790–4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briani F, Carzaniga T, Deho G. 2016. Regulation and functions of bacterial PNPase. Wiley Interdiscip Rev RNA 7: 241–258. [DOI] [PubMed] [Google Scholar]
- Briani F, Del Vecchio E, Domenico M, Hajnsdorf E, Regnier P, Ghisotti D, Deho G. 2002. RNase E and polyadenyl polymerase I are involved in maturation of CI RNA, the P4 phage immunity factor. J Mol Biol 318: 321–331. [DOI] [PubMed] [Google Scholar]
- Bricker AL, Belasco JG. 1999. Importance of a 5’ stem-loop for longevity of papA mRNA in Escherichia coli. J Bacteriol 181: 3587–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahova H, Winz ML, Hofer K, Nubel G, Jaschke A. 2015. NAD captureSeq indicates NAD as a bacterial cap for a subset of regulatory RNAs. Nature 519: 374–377. [DOI] [PubMed] [Google Scholar]
- Cairrao F, Chora A, Zilhao R, Carpousis AJ, Arraiano CM. 2001. RNase II levels change according to the growth conditions: characterization of gmr, a new Escherichia coli gene involved in the modulation of RNase II. Mol Microbiol 39: 1550–1561. [DOI] [PubMed] [Google Scholar]
- Cairrao F, Cruz A, Mori H, Arraiano CM. 2003. Cold shock induction of RNase R and its role in the maturation of the quality control mediator SsrA/tmRNA. Mol Microbiol 50: 1349–1360. [DOI] [PubMed] [Google Scholar]
- Callaghan AJ, Grossman JG, Redko YU, Ilag LL, Moncrieffe MC, Symmons MF, Robinson CV, McDowall KJ, Luisi BF. 2003. Quaternary structure and catalytic activity of the Escherichia coli ribonuclease E amino-terminal catalytic domain. Biochemistry 42: 12848–13855. [DOI] [PubMed] [Google Scholar]
- Cao G-J, Sarkar N 1992. Identification of the gene for an Escherichia coli poly(A) polymerase. Proc Natl Acad Sci U S A 89: 10380–10384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabetta VJ, Silhavy TJ, Cristea IM. 2010. The response regulator SprE (RssB) is required for maintaining poly(A) polymerase I-degradosome association during stationary phase. J Bacteriol 192: 3713–3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron MP, Lafontaine DA, Masse E. 2010. Small RNA-mediated regulation at the level of transcript stability. RNA Biol 7: 140–144. [DOI] [PubMed] [Google Scholar]
- Carpousis AJ, Van Houwe G, Ehretsmann C, Krisch HM. 1994. Copurification of E. coli RNAase E and PNPase: evidence for a specific association between two enzymes important in RNA processing and degradation. Cell 76: 889–900. [DOI] [PubMed] [Google Scholar]
- Carzaniga T, Briani F, Zangrossi S, Merlino G, Marchi P, Deho G. 2009. Autogenous regulation of Escherichia coli polynucleotide phosphorylase expression revisited. J Bacteriol 191: 1738–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Y, Li L, Girodat D, Forstner KU, Said N, Corcoran C, Smiga M, Papenfort K, Reinhardt R, Wieden HJ et al. 2017. In vivo cleavage map illuminates the central role of RNase E in coding and non-coding RNA pathways. Mol Cell 65: 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charollais J, Dreyfus M, Iost I. 2004. CsdA, a cold-shock RNA helicase from Escherichia coli, is involved in the biogenesis of 50S ribosomal subunit. Nucleic Acids Res 32: 2751–2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charollais J, Pflieger D, Vinh J, Dreyfus M, Iost I. 2003. The DEAD-box RNA helicase SrmB is involved in the assembly of 50S ribosomal subunits in Escherichia coli. Mol Microbiol 48: 1253–1265. [DOI] [PubMed] [Google Scholar]
- Chen C, Deutscher MP. 2010. RNase R is a highly unstable protein regulated by growth phase and stress. RNA 16: 667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Dutta T, Deutscher MP. 2016. Growth Phase-dependent Variation of RNase BN/Z Affects Small RNAs: Regulation of 6S RNA. J Biol Chem 291: 26435–26442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Previero A, Deutscher MP. 2019. A novel mechanism of ribonuclease regulation: GcvB and Hfq stabilize the mRNA that encodes RNase BN/Z during exponential phase. J Biol Chem 294: 19997–20008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z-F, Deutscher MP. 2005. An important role for RNase R in mRNA decay. Mol Cell 17: 313–318. [DOI] [PubMed] [Google Scholar]
- Cheng ZF, Deutscher MP. 2002. Purification and characterization of the Escherichia coli exoribonuclease RNase R. Comparison with RNase II. J Biol Chem 277: 21624–21649. [DOI] [PubMed] [Google Scholar]
- Cheng ZF. 2003. Quality control of ribosomal RNA mediated by polynucleotide phosphorylase and RNase R. Proc Natl Acad Sci U S A 100: 6388–6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng ZF, Zuo Y, Li Z, Rudd KE, Deutscher MP. 1998. The vacB gene required for virulence in Shigella flexneri and Escherichia coli encodes the exoribonuclease RNase R. J Biol Chem 273: 14077–14080. [DOI] [PubMed] [Google Scholar]
- Christensen-Dalsgaard M, Jorgensen MG, Gerdes K. 2010. Three new RelE-homologous mRNA interferases of Escherichia coli differentially induced by environmental stresses. Mol Microbiol 75: 333–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung D-H, Min Z, Wang B-C, Kushner SR. 2010. Single amino acid changes in the predicted RNase H domain of E. coli RNase G lead to the complementation of RNase E mutants. RNA 16: 1371–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JE, Kime L, Romero AD, McDowall KJ. 2014. Direct entry by RNase E is a major pathway for the degradation and processing of RNA in Escherichia coli. Nucleic Acids Res 42: 11733–11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn GA, Miao X, Briant DJ, Mackie GA. 1999. Reconstitution of a minimal RNA degradosome demonstrates functional coordination between a 3’ exonuclease and a DEAD-box RNA helicase. Genes Dev 13: 2594–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court D 1993. RNA processing and degradation by RNase III. in Control of messenger RNA stability (eds. Belasco J, Brawerman G), pp. 71–117. Academic Press, New York. [Google Scholar]
- Culviner PH, Laub MT. 2018. Global Analysis of the E. coli Toxin MazF Reveals Widespread Cleavage of mRNA and the Inhibition of rRNA Maturation and Ribosome Biogenesis. Mol Cell 70: 868–880 e810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lay N, Gottesman S. 2011. Role of polynucleotide phosphorylase in sRNA function in Escherichia coli. RNA 17: 1172–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deana A, Celesnik H, Belasco JG. 2008. The bacterial enzyme RppH triggers messenger RNA degradation by 5’ pyrophosphate removal. Nature 451: 355–358. [DOI] [PubMed] [Google Scholar]
- Diestra E, Cayrol B, Arluison V, Risco C. 2009. Cellular electron microscopy imaging reveals the localization of the Hfq protein close to the bacterial membrane. PLoS One 4: e8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diges CM, Uhlenbeck OC. 2001. Escherichia coli DbpA is an RNA helicase that requires hairpin 92 of 23S rRNA. EMBO J 20: 5503–5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan WP, Kushner SR. 1986. Polynucleotide phosphorylase and ribonuclease II are required for cell viability and mRNA turnover in Escherichia coli K-12. Proc Natl Acad Sci U S A 83: 120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey AK, Baker CS, Romeo T, Babitzke P. 2005. RNA sequence and secondary structure participate in high-affinity CsrA-RNA interaction. RNA 11: 1579–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn JJ, Studier FW. 1973. T7 early RNAs and Escherichia coli ribosomal RNAs are cut from large precursor RNAs in vivo by ribonuclease III. Proc Natl Acad Sci USA 70: 3296–3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta T, Deutscher MP. 2009. Catalytic properties of RNase BN/RNase Z from Escherichia coli: RNase BN is both an exo- and endoribonuclease. J Biol Chem 284: 15425–15431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta T, Malhotra A, Deutscher MP. 2012. Exoribonuclease and endoribonuclease activities of RNase BN/RNase Z both function in vivo. J Biol Chem 287: 35747–35755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehretsmann CP, Carpousis AJ, Krisch HM. 1992. Specificity of Escherichia coli endoribonuclease RNase E: in vivo and in vitro analysis of mutants in a bacteriophage T4 mRNA processing site. Genes Dev 6: 149–159. [DOI] [PubMed] [Google Scholar]
- Emory SA, Belasco JG. 1990. The ompA 5’ untranslated RNA segment functions in Escherichia coli as a growth-rate-regulated mRNA stabilizer whose activity is unrelated to translational efficiency. J Bacteriol 172: 4472–4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emory SA, Bouvet P, Belasco JG. 1992. A 5’-terminal stem-loop structure can stabilize mRNA in Escherichia coli. Genes Dev 6: 135–148. [DOI] [PubMed] [Google Scholar]
- Etienne TA, Cocaign-Bousquet M, Ropers D. 2020. Competitive effects in bacterial mRNA decay. J Theor Biol: 110333. [DOI] [PubMed] [Google Scholar]
- Ezraty B, Dahlgren B, Deutscher MP. 2005. The RNase Z homologue encoded by Escherichia coli elaC gene is RNase BN. J Biol Chem 280: 16542–16545. [DOI] [PubMed] [Google Scholar]
- Foley PL, Hsieh PK, Luciano DJ, Belasco JG. 2015. Specificity and Evolutionary Conservation of the Escherichia coli RNA Pyrophosphohydrolase RppH. J Biol Chem: 10.1074/jbc.M1114.634659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franze de Fernandez MT, Eoyang L, August TL. 1968. Factor fraction required for the synthesis of bacteriophage Qβ-RNA. Nature 219: 588–590. [DOI] [PubMed] [Google Scholar]
- Gao JJ, Lee K, Zhao M, Qiu J, Zhan XM, Saxena A, Moore CJ, Cohen SN, Georgiou G. 2006. Differential modulation of E. coli mRNA abundance by inhibitory proteins that alter the composition of the degradosome. Mol Microbiol 61: 394–406. [DOI] [PubMed] [Google Scholar]
- Garrey SM, Mackie GA. 2011. Roles of the 5’-phosphate sensor domain in RNase E. Mol Microbiol 80: 1613–1624. [DOI] [PubMed] [Google Scholar]
- Garza-Sanchez F, Shoji S, Fredrick K, Hayes CS. 2009. RNase II is important for A-site mRNA cleavage during ribosome pausing. Mol Microbiol 73: 882–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes K, Maisonneuve E. 2012. Bacterial persistence and toxin-antitoxin loci. Annu Rev Microbiol 66: 103–123. [DOI] [PubMed] [Google Scholar]
- Ghodge SV, Raushel FM. 2015. Discovery of a previously unrecognized ribonuclease from Escherichia coli that hydrolyzes 5’-Phosphorylated fragments of RNA. Biochemistry 54: 2911–2918. [DOI] [PubMed] [Google Scholar]
- Ghora BK, Apirion D. 1978. Structural analysis and in vitro processing to p5 rRNA of a 9S RNA molecule isolated from an rne mutant of E. coli. Cell 15: 1055–1066. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Deutscher MP. 1999. Oligoribonuclease is an essential component of the mRNA decay pathway. Proc Natl Acad Sci USA 96: 4372–4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godefroy-Colburn T, Grunberg-Manago M. 1972. Polynucleotide phosphorylase. in The Enzymes (ed. Boyer PD), pp. 533–574. Academic Press, New York. [Google Scholar]
- Golding I, Cox EC. 2004. RNA dynamics in live Escherichia coli cells. Proc Natl Acad Sci USA 101: 11310–11315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopel Y, Papenfort K, Reichenbach B, Vogel J, Gorke B. 2013. Targeted decay of a regulatory small RNA by an adaptor protein for RNase E and counteraction by an anti-adaptor RNA. Genes Dev 27: 552–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon GC, Cameron JC, Pfleger BF. 2017. RNA sequencing identifies new RNase III cleavage sites in Escherichia coli and reveals increased regulation of mRNA. MBio 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorna MW, Pietras Z, Tsai YC, Callaghan AJ, Hernandez H, Robinson CV, Luisi BF. 2010. The regulatory protein RraA modulates RNA-binding and helicase activities of the E. coli RNA degradosome. RNA 16: 553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S 2004. The small RNA regulators of Escherichia coli: Roles and mechanisms. Ann Rev Microbiology 58: 303–328. [DOI] [PubMed] [Google Scholar]
- Gottesman S, Storz G. 2011. Bacterial small RNA regulators: versatile roles and rapidly evolving variations. Cold Spring Harb Perspect Biol 3: 10.1101/cshperspect.a003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S, Storz G, Rosenow C, Majdalani N, Repoila F, Wassarman KM. 2001. Small RNA regulators of translation: mechanisms of action and approaches for identifying new small RNAs. Cold Spring Harb Symp Quant Biol 66: 353–362. [DOI] [PubMed] [Google Scholar]
- Grunberg-Manago M 1963. Polynucleotide phosphorylase. Prog Nucl Acids Res 1: 93–133. [Google Scholar]
- Grunberg-Manago M, Ortiz PJ, Ochoa S. 1955. Enzymatic synthesis of nucleic acidlike polynucleotides. Science 122: 907–910. [DOI] [PubMed] [Google Scholar]
- Grunberg-Manago M. 1956. Enzymic synthesis of polynucleotides: 1. Polynucleotide phosphorylase of Azotobacter vinelandiia. Biochim Biophys Acta 20: 269–285. [DOI] [PubMed] [Google Scholar]
- Guerrier-Takada C, Gardiner K, Marsh T, Pace N, Altman S. 1983. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell 35: 849–857. [DOI] [PubMed] [Google Scholar]
- Gutierrez P, Li Y, Osborne MJ, Pomerantseva E, Liu Q, Gehring K. 2005. Solution structure of the carbon storage regulator protein CsrA from Escherichia coli. J Bacteriol 187: 3496–3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnsdorf E, Braun F, Haugel-Nielsen J, Le Derout J, Régnier P. 1996. Multiple degradation pathways of the rpsO mRNA of Escherichia coli. RNase E interacts with the 5’ and 3’ extremities of the primary transcript. Biochimie 78: 416–424. [DOI] [PubMed] [Google Scholar]
- Hajnsdorf E, Steier O, Coscoy L, Teysset L, Régnier P. 1994. Roles of RNase E, RNase II and PNPase in the degradation of the rpsO transcripts of Escherichia coli: stabilizing function of RNase II and evidence for efficient degradation in an ams pnp rnb mutant. EMBO J 13: 3368–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlof DL, Bergman JM, Garmendia E, Hughes D. 2015. Turnover of mRNAs is one of the essential functions of RNase E. Mol Microbiol 98: 34–45. [DOI] [PubMed] [Google Scholar]
- Higgins CF, McLaren RS, Newbury SF. 1988. Repetitive extragenic palindromic sequences, mRNA stability and gene expression: Evolution by gene conversion?-A review. Gene 72: 3–14. [DOI] [PubMed] [Google Scholar]
- Holmqvist E, Li L, Bischler T, Barquist L, Vogel J. 2018. Global Maps of ProQ Binding In Vivo Reveal Target Recognition via RNA Structure and Stability Control at mRNA 3’ Ends. Mol Cell 70: 971–982 e976. [DOI] [PubMed] [Google Scholar]
- Hossain ST, Malhotra A, Deutscher MP. 2016. How RNase R degrades structured RNA: Role of the helicase activity and the S1 domain. J Biol Chem 291: 7877–7887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Yagi M, Morita T, Aiba H. 2011. Hfq binding at RhlB-recognition region of RNase E is crucial for the rapid degradation of target mRNAs mediated by sRNAs in Escherichia coli. Mol Microbiol 79: 419–432. [DOI] [PubMed] [Google Scholar]
- Inouye M 2006. The discovery of mRNA interferases: implication in bacterial physiology and application to biotechnology. J Cell Physiol 209: 670–676. [DOI] [PubMed] [Google Scholar]
- Ishihama Y, Schmidt T, Rappsilber J, Mann M, Hartl FU, Kerner MJ, Frishman D. 2008. Protein abundance profiling of the Escherichia coli cytosol. BMC Genomics 9: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagessar KL, Jain C. 2010. Functional and molecular analysis of Escherichia coli strains lacking multiple DEAD-box helicases. RNA 16: 1386–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain C 2020. RNase AM, a 5’ to 3’ exonuclease, matures the 5’ end of all three ribosomal RNAs in E. coli. Nucleic Acids Res 48: 5616–5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain C, Belasco JG. 1995. RNase E autoregulates its synthesis by controlling the degradation rate of its own mRNA in Escherichia coli: unusual sensitivity of the rne transcript to RNase E activity. Genes Dev 9: 84–96. [DOI] [PubMed] [Google Scholar]
- Jarrige A-C, Mathy N, Portier C. 2001. PNPase autocontrols its expression by degrading a double-stranded structure in the pnp mRNA leader. EMBO J 20: 6845–6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M, Coyle SM, Doudna JA. 2011. Coupled 5’ nucleotide recognition and processivity in Xrn1-mediated mRNA decay. Mol Cell 41: 600–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaga N, Umitsuki G, Nagai K, Wachi M. 2002. RNase G-dependent degradation of the eno mRNA encoding a glycolysis enzyme enolase in Escherichia coli. Biosci Biotechn and Biochem 66: 2216–2220. [DOI] [PubMed] [Google Scholar]
- Kelly KO, Deutscher MP. 1992. Characterization of Escherichia coli RNase PH. J Biol Chem 267: 17153–17158. [PubMed] [Google Scholar]
- Khemici V, Carpousis AJ. 2004. The RNA degradosome and poly(A) polymerase of Escherichia coli are required in vivo for the degradation of small mRNA decay intermediates containing REP-stabilizers. Mol Microbiol 51: 777–790. [DOI] [PubMed] [Google Scholar]
- Khemici V, Poljak L, Luisi BF, Carpousis AJ. 2008. The RNase E of Escherichia coli is a membrane-binding protein. Mol Microbiol 70: 799–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khemici V, Poljak L, Toesca I, Carpousis AJ. 2005. Evidence in vivo that the DEAD-box helicase RhlB facilitates the degradation of ribosome-free mRNA by RNase E. Proc Natl Acad Sci USA 102: 6913–6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khemici V, Toesca I, Poljak L, Vanzo NF, Carpousis AJ. 2004. The RNase E of Escherichia coli has at least two binding sites for DEAD-box RNA helicases: functional replacement of RhlB by RhlE. Mol Microbiol 54: 1422–1430. [DOI] [PubMed] [Google Scholar]
- Kime L, Clarke JE, Romero AD, Grasby JA, McDowall KJ. 2014. Adjacent single-stranded regions mediate processing of tRNA precursors by RNase E direct entry. Nucleic Acids Res 42: 4577–4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kime L, Jourdan SS, Stead JA, Hidalgo-Sastre A, McDowall KJ. 2010. Rapid cleavage of RNA by RNase E in the absence of 5’ monophosphate stimulation. Mol Microbiol 76: 590–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King TC, Sirdeshmukh R, Schlessinger D. 1984. RNase III cleavage is obligate for maturation but not for function of Escherichia coli pre-23S rRNA. Proc Natl Acad Sci USA 81: 185–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarova AV, Tchufistova LS, Dreyfus M, Boni IV. 2005. AU-rich sequences within 5’ untranslated leaders enhance translation and stabilize mRNA in Escherichia coli. J Bacteriology 187: 1344–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komine Y, Kitabatake M, Yokigawa T, Nishikawa K, Inokuchi H. 1994. A tRNA-like structure is present in 10Sa RNA, a small stable RNA from Escherichia coli. Proc Natl Acad Sci USA 91: 9223–9227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowtoniuk WE, Shen Y, Heemstra JM, Agarwal I, Liu DR. 2009. A chemical screen for biological small molecule-RNA conjugates reveals CoA-linked RNA. Proc Natl Acad Sci USA 106: 7768–7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwano M, Ono M, Endo H, Hori K, Nakamura K, Hirota Y, Ohnishi Y. 1977. Gene affecting longevity of messenger RNA: a mutant of Escherichia coli with altered mRNA stability. Mol Gen Genet 154: 279–285. [DOI] [PubMed] [Google Scholar]
- Lalaouna D, Simoneau-Roy M, Lafontaine D, Masse E. 2013. Regulatory RNAs and target mRNA decay in prokaryotes. Biochim Biophys Acta 1829: 742–747. [DOI] [PubMed] [Google Scholar]
- Lee K, Bernstein JA, Cohen SN. 2002. RNase G complementation of rne null mutation identified functional interrelationships with RNase E in Escherichia coli. Mol Microbiology 43: 1445–1456. [DOI] [PubMed] [Google Scholar]
- Lee K, Zhan X, Gao J, Feng Y, Meganathan R, Cohen SN, Georgiou G. 2003. RraA: a protein inhibitor of RNase E activity that globally modulates RNA abundance in E. coli. Cell 114: 623–634. [PubMed] [Google Scholar]
- Lesnik EA, Sampath R, Levene HB, Henderson TJ, McNeil JA, Ecker DJ. 2001. Prediction of Rho-independent transcriptional terminators in Escherichia coli. Nucleic Acids Res 29: 3583–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Altman S. 2003. A specific endoribonuclease, RNase P, affects gene expression of polycistronic operon mRNAs. Proc Natl Acad Sci USA 100: 13213–13218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Cole K, Altman S. 2003. The effect of a single, temperature-sensitive mutation on global gene expression in Escherichia coli. RNA 9: 518–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Deutscher MP. 2002. RNase E plays an essential role in the maturation of Escherichia coli tRNA precursors. RNA 8: 97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Pandit S, Deutscher MP. 1999. RNase G (CafA protein) and RNase E are both required for the 5’ maturation of 16S ribosomal RNA. EMBO J 18: 2878–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W, Deutscher MP. 2013. Ribosomes regulate the stability and action of the exoribonuclease RNase R. J Biol Chem 288: 34791–34798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim B, Sim SH, Sim M, Kim K, Jeon CO, Lee Y, Ha NC, Lee K. 2012. RNase III controls the degradation of corA mRNA in Escherichia coli. J Bacteriol 194: 2214–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin-Chao S, Cohen S. 1991. The rate of processing and degradation of antisense RNA I regulates the replication of ColE1-type plasmids in vivo. Cell 65: 1233–1242. [DOI] [PubMed] [Google Scholar]
- Lin-Chao S, Wei C-L, Lin Y-T. 1999. RNase E is required for the maturation of ssrA and normal ssrA RNA peptide-tagging activity. Proc Natl Acad Sci USA 96: 12406–12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P-H, Lin-Chao S 2005. RhlB helicase rather than enolase is the Β-subunit of the Escherichia coli polynucleotide phosphorylase (PNPase)-exoribonucleolytic complex. Proc Natl Acad Sci USA 102: 16590–16595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link TM, Valentin-Hansen P, Brennan RG. 2009. Structure of Escherichia coli Hfq bound to polyriboadenylate RNA. Proc Natl Acad Sci USA 106: 19292–19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F, Taghbalout A. 2013. Membrane association via an amino-terminal amphipathic helix is required for the cellular organization and function of RNase II. J Biol Chem 288: 7241–7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano DJ, Vasilyev N, Richards J, Serganov A, Belasco JG. 2017. A novel RNA phosphorylation state enables 5’ end-dependent degradation in Escherichia coli. Mol Cell 67: 44–54 e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano DJ. 2018. Importance of a diphosphorylated intermediate for RppH-dependent RNA degradation. RNA Biol 15: 703–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg U, Altman S. 1995. Processing of the precursor to the catalytic RNA subunit of RNase P from Escherichia coli. RNA 1: 327–334. [PMC free article] [PubMed] [Google Scholar]
- Mackie GA. 1998. Ribonuclease E is a 5’-end-dependent endonuclease. Nature 395: 720–723. [DOI] [PubMed] [Google Scholar]
- Marujo PE, Hajnsdorf E, Le Derout J, Andrade R, Arraiano CM, Regnier P. 2000. RNase II removes the oligo(A) tails that destabilize the rpsO mRNA of Escherichia coli. RNA 6: 1185–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathy N, Benard L, Pellegrini O, Daou R, Wen T, Condon C. 2007. 5’-to-3’ exoribonuclease activity in Bacteria: Role of RNase J1 in rRNA maturation and 5’ stability of mRNA. Cell 129: 681–692. [DOI] [PubMed] [Google Scholar]
- Mathy N, Jarrige A-C, Le Meur MR, Portier C. 2001. Increased expression of Escherichia coli polynucleotide phosphorylase at low temperatures is linked to a decrease in the efficiency of autocontrol. J Bacteriol 183: 3848–3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga J, Simons EL, Simons RW. 1996. RNase III autoregulation: Structure and function of rncO, the posttranscriptional “operator”. RNA 2: 1228–1240. [PMC free article] [PubMed] [Google Scholar]
- McDowall KJ, Cohen SN. 1996. The N-terminal domain of the rne gene product has RNase E activity and is non-overlapping with the arginine-rich RNA-binding motif. J Mol Biol 255: 349–355. [DOI] [PubMed] [Google Scholar]
- McDowall KJ, Kaberdin VR, Wu S-W, Cohen SN, Lin-Chao S. 1995. Site-specific RNase E cleavage of oligonucleotides and inhibition by stem-loops. Nature 374: 287–290. [DOI] [PubMed] [Google Scholar]
- McDowall KJ, Lin-Chao S, Cohen SN. 1994. A + U content rather than a particular nucleotide order determines the specificity of RNase E cleavage. J Biol Chem 269: 10790–10796. [PubMed] [Google Scholar]
- Melamed S, Adams PP, Zhang A, Zhang H, Storz G. 2020. RNA-RNA interactomes of ProQ and Hfq reveal overlapping and competing roles. Mol Cell 77: 411–425 e417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed S, Peer A, Faigenbaum-Romm R, Gatt YE, Reiss N, Bar A, Altuvia Y, Argaman L, Margalit H. 2016. Global Mapping of Small RNA-Target Interactions in Bacteria. Mol Cell 63: 884–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczak A, Srivastava RAK, Apirion D. 1991. Location of the RNA-processing enzymes RNase III, RNase E, and RNase P. Mol Microbiol 5: 1801–1810. [DOI] [PubMed] [Google Scholar]
- Mildenhall KB, Wiese N, Chung D, Maples VF, Mohanty BK, Kushner SR. 2016. RNase E-based degradosome modulates polyadenylation of mRNAs after Rho-independent transcription terminators in Escherichia coli. Mol Microbiol 101: 645–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra TK, Apirion D. 1979. RNase E, an RNA processing enzyme from Escherichia coli. J Biol Chem 254: 11154–11159. [PubMed] [Google Scholar]
- Mohanty BK, Agrawal A, Kushner SR. 2020. Generation of pre-tRNAs from polycistronic operons is the essential function of RNase P in Escherichia coli. Nucleic Acids Res 48: 2564–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty BK, Kushner SR. 1999. Analysis of the function of Escherichia coli poly(A) polymerase I in RNA metabolism. Mol Microbiol 34: 1094–1108. [DOI] [PubMed] [Google Scholar]
- Mohanty BK. 2000. Polynucleotide phosphorylase functions both as a 3’ – 5’ exonuclease and a poly(A) polymerase in Escherichia coli. Proc Natl Acad Sci USA 97: 11966–11971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty BK. 2002. Polyadenylation of Escherichia coli transcripts plays an integral role in regulating intracellular levels of polynucleotide phosphorylase and RNase E. Mol Microbiol 45: 1315–1324. [DOI] [PubMed] [Google Scholar]
- Mohanty BK. 2003. Genomic analysis in Escherichia coli demonstrates differential roles for polynucleotide phosphorylase and RNase II in mRNA abundance and decay. Mol Microbiol 50: 645–658. [DOI] [PubMed] [Google Scholar]
- Mohanty BK. 2006. The majority of Escherichia coli mRNAs undergo post-transcriptional modification in exponentially growing cells. Nucleic Acids Res 34: 5695–5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty BK. 2007. Ribonuclease P processes polycistronic tRNA transcripts in Escherichia coli independent of ribonuclease E. Nucleic Acids Res 35: 7614–7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty BK. 2008. Rho-independent transcription terminators inhibit RNase P processing of the secG leuU and metT tRNA polycistronic transcripts in Escherichia coli. Nucleic Acids Res 36: 364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty BK, Kushner SR. 2010a. Bacterial/archaeal/organellar polyadenylation. WIREs RNA 2: ,256–276. [Google Scholar]
- Mohanty BK, Kushner SR. 2010b. Processing of the Escherichia coli leuX tRNA transcript, encoding tRNAleu5, requires either the 3’−5’ exoribonuclease polynucleotide phosphorylase or RNase P to remove the Rho-independent transcription terminator. Nucleic Acids Res 38: 597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty BK. 2013. Deregulation of poly(A) polymerase I in Escherichia coli inhibits protein synthesis and leads to cell death. Nucleic Acids Res 41: 1757–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty BK. 2016. Regulation of mRNA decay in bacteria. Ann Rev Microbiol 70: 25–44. [DOI] [PubMed] [Google Scholar]
- Mohanty BK, Kushner SR. 2018. Enzymes involved in post-transcriptional RNA metabolism in Gram-negative bacteria. Microbiology Spectrum 6: RWR-0011–2017. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty BK, Maples VF, Kushner SR. 2004. The Sm-like protein Hfq regulates polyadenylation dependent mRNA decay in Escherichia coli. Mol Microbiol 54: 905–920. [DOI] [PubMed] [Google Scholar]
- Mohanty BK. 2012. Polyadenylation helps regulate functional tRNA levels in Escherichia coli. Nucleic Acids Res 40: 4589–4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll I, Afonyuskhin T, Vytvytska O, Kaberdin VR, Blasi U. 2003. Coincident Hfq binding and RNase E cleavage sites on mRNA and small regulator RNAs. RNA 9: 1308–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin M, Enjalbert B, Ropers D, Girbal L, Cocaign-Bousquet M. 2020. Genomewide Stabilization of mRNA during a “Feast-to-Famine” Growth Transition in Escherichia coli. mSphere 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T, Aiba H. 2011. RNase E action at a distance: degradation of target mRNAs mediated by an Hfq-binding small RNA in bacteria. Genes Dev 25: 294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd EA, Krisch HM, Higgins CF. 1990. RNase E, an endoribonuclease, has a general role in the chemical decay of Escherichia coli mRNA: evidence that rne and ams are the same genetic locus. Mol Microbiol 4: 2127–2135. [DOI] [PubMed] [Google Scholar]
- Mullen TE, Marzluff WF. 2008. Degradation of histone mRNA requires oligouridylation followed by decapping and simultaneous degradation of the mRNA both 5’ to 3’ and 3’ to 5’. Genes Dev 22: 50–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthunayake NS, Tomares DT, Childers WS, Schrader JM. 2020. Phase-separated bacterial ribonucleoprotein bodies organize mRNA decay. Wiley Interdiscip Rev RNA: e1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbury SF, Smith NH, Higgins CF. 1987a. Differential mRNA stability controls relative gene expression within a polycistronic operon. Cell 51: 1131–1143. [DOI] [PubMed] [Google Scholar]
- Newbury SF, Smith NH, Robinson EC, Hiles ID, Higgins CF. 1987b. Stabilization of translationally active mRNA by prokaryotic REP sequences. Cell 48: 297–310. [DOI] [PubMed] [Google Scholar]
- Nicholson A 1999. Function, mechanism and regulation of bacterial ribonucleases. FEMS Microbiology Reviews 23: 371–390. [DOI] [PubMed] [Google Scholar]
- Nossal NG, Singer MF. 1968. The processive degradation of individual polynucleotide chains. J Biol Chem 243: 913–922. [PubMed] [Google Scholar]
- O’Hara EB, Chekanova JA, Ingle CA, Kushner ZR, Peters E, Kushner SR. 1995. Polyadenylylation helps regulate mRNA decay in Escherichia coli. Proc Natl Acad Sci U S A 92: 1807–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olejniczak M, Storz G. 2017. ProQ/FinO-domain proteins: another ubiquitous family of RNA matchmakers? Mol Microbiol 104: 905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M, Kuwano M. 1979. A conditional lethal mutation in an Escherichia coli strain with a longer chemical lifetime of mRNA. J Mol Biol 129: 343–357. [DOI] [PubMed] [Google Scholar]
- Ono M. 1980. Chromosomal location of a gene for chemical longevity of messenger ribonucleic acid in a temperature-sensitive mutant of Escherichia coli. J Bacteriol 142: 325–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opdyke JA, Fozo EM, Hemm MR, Storz G. 2011. RNase III participates in GadY-dependent cleavage of the gadX-gadW mRNA. J Mol Biol 406: 29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ow MC, Kushner SR. 2002. Initiation of tRNA maturation by RNase E is essential for cell viability in E. coli. Genes Dev 16: 1102–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ow MC, Liu Q, Kushner SR. 2000. Analysis of mRNA decay and rRNA processing in Escherichia coli in the absence of RNase E-based degradosome assembly. Mol Microbiol 38: 854–866. [DOI] [PubMed] [Google Scholar]
- Ow MC, Liu Q, Mohanty BK, Andrew ME, Maples VF, Kushner SR. 2002. RNase E levels in Escherichia coli are controlled by a complex regulatory system that involves transcription of the rne gene from three promoters. Mol Microbiol 43: 159–171. [DOI] [PubMed] [Google Scholar]
- Ow MC, Perwez T, Kushner SR. 2003. RNase G of Escherichia coli exhibits only limited functional overlap with its essential homologue, RNase E. Mol Microbiol 49: 607–622. [DOI] [PubMed] [Google Scholar]
- Peck-Miller KA, Altman S. 1991. Kinetics of the processing of the precursor to 4.5 S RNA, a naturally occurring substrate for RNase P from Escherichia coli. J Mol Biol 221: 1–5. [DOI] [PubMed] [Google Scholar]
- Pellegrini O, Nezzar J, Marchfelder A, Putzer H, Condon C. 2003. Endonucleolytic processing of CCA-less tRNA precursors by RNase E in Bacillus subtilis. EMBO J 22: 4534–4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertzev AV, Nicholson AW. 2006. Characteization of RNA sequence determinants and antideterminants of processing reactivity for a minimal substrate of Escherichia coli ribonuclease III. Nucleic Acids Res 34: 3708–3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perwez T, Hami D, Maples VF, Min Z, Wang BC, Kushner SR. 2008. Intragenic suppressors of temperature-sensitive rne mutations lead to the dissociation of RNase E activity on mRNA and tRNA substrates in Escherichia coli. Nucleic Acids Res 36: 5306–5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perwez T, Kushner SR. 2006. RNase Z in Escherichia coli plays a significant role in mRNA decay. Mol Microbiol 60: 723–737. [DOI] [PubMed] [Google Scholar]
- Pobre V, Arraiano CM. 2015. Next generation sequencing analysis reveals that the ribonucleases RNase II, RNase R and PNPase affect bacterial motility and biofilm formation in E. coli. BMC Genomics 16: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prud’homme-Genereux A, Beran RK, Iost I, Ramey CS, Mackie GA, Simons RW. 2004. Physical and functional interactions among RNase E, polynucleotide phosphorylase and the cold-shock protein, CsdA: evidence for a ‘cold shock degradosome’. Mol Microbiol 54: 1409–1421. [DOI] [PubMed] [Google Scholar]
- Pul U, Wurm R, Arslan Z, Geissen R, Hofmann N, Wagner R. 2010. Identification and characterization of E. coli CRISPR-cas promoters and their silencing by H-NS. Mol Microbiol 75: 1495–1512. [DOI] [PubMed] [Google Scholar]
- Py B, Higgins CF, Krisch HM, Carpousis AJ. 1996a. A DEAD-box RNA helicase in the Escherichia coli RNA degradosome. Nature 381: 169–172. [DOI] [PubMed] [Google Scholar]
- Py B, Higgins CF, Krisch HM, Carpousis AJ. 1996b. A DEAD-box RNA helicase in the Escherichia coli RNA degradosome. Nature 381: 169–172. [DOI] [PubMed] [Google Scholar]
- Quendera AP, Seixas AF, Dos Santos RF, Santos I, Silva JPN, Arraiano CM, Andrade JM. 2020. RNA-Binding Proteins Driving the Regulatory Activity of Small Non-coding RNAs in Bacteria. Front Mol Biosci 7: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynal LC, Carpousis AJ. 1999. Poly(A) polymerase I of Escherichia coli: characterization of the catalytic domain, an RNA binding site and regions for the interaction with proteins involved in mRNA degradation. Mol Microbiol 32: 765–775. [DOI] [PubMed] [Google Scholar]
- Regnier P, Grunberg-Manago M. 1989. Cleavage by RNase III in the transcripts of the metY-nus-infB operon of Escherichia coli releases the tRNA and initiates the decay of the downstream mRNA. J Mol Biol 210: 293–302. [DOI] [PubMed] [Google Scholar]
- Reiter NJ, Osterman A, Torres-Larios A, Swinger KK, Pan T, Mondragon A. 2010. Structure of a bacterial ribonuclease P holoenzyme in complex with tRNA. Nature 468: 784–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J, Belasco JG. 2019. Obstacles to scanning by RNase E govern bacterial mRNA lifetimes by hindering access to distal cleavage sites. Mol Cell 74: 284–295 e285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J, Luciano DJ, Belasco JG. 2012. Influence of translation on RppH-dependent mRNA degradation in Escherichia coli. Mol Microbiol 86: 1063–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson HD. 1982. Escherichia coli ribonuclease III cleavage sites. Cell 30: 669–672. [DOI] [PubMed] [Google Scholar]
- Robertson HD, Webster RE, Zinder ND. 1967. A nuclease specific for double-stranded RNA. Virology 12: 718–719. [DOI] [PubMed] [Google Scholar]
- Romeo T, Vakulskas CA, Babitzke P. 2013. Post-transcriptional regulation on a global scale: form and function of Csr/Rsm systems. Environ Microbiol 15: 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer S, Rosch S, Marchfelder A. 2002. Assigning a function to a conserved group of proteins: the tRNA 3’ processing enzymes. EMBO J 21: 2769–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck A, Diwa A, Belasco JG. 2009. RNase E autoregulates its synthesis in Escherichia coli by binding directly to a stem-loop in the rne 5’ untranslated region. Mol Microbiol 72: 470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher MA, Pearson RF, Moller T, Valentin-Hansen P, Brennan RG. 2002. Structures of the pleiotropic translational regulator Hfq and an Hfq-RNA complex: a bacterial Sm-like protein. EMBO J 21: 3546–3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman RG, Brown TR, Ugurbil K, Ogawa S, Cohen SM, den Hollander JA. 1979. Cellular applications of 31P and 13C nuclear magnetic resonance. Science 205: 160–166. [DOI] [PubMed] [Google Scholar]
- Sim S, Kim KS, Lee Y. 2002. 3’-end processing of precursor M1 RNA by the N-terminal half of RNase E. FEBS Lett 529: 225–231. [DOI] [PubMed] [Google Scholar]
- Singer MF, Tolbert G. 1965. Purification and properties of a potassium-activated phosphodiesterase (RNase II) from Escherichia coli. Biochemistry 4: 1319–1330. [DOI] [PubMed] [Google Scholar]
- Singh D, Chang SJ, Lin PH, Averina OV, Kaberdin VR, Lin-Chao S. 2009. Regulation of ribonuclease E activity by the L4 ribosomal protein of Escherichia coli. Proc Natl Acad Sci USA 106: 864–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Wang G, Malhotra A, Deutscher MP, Liang W. 2016. Reversible acetylation on Lys501 regulates the activity of RNase II. Nucleic Acids Res 44: 1979–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spickler C, Mackie GA. 2000. Action of RNase II and polynucleotide phosphorylase against RNAs containing stem-loops of defined structure. J Bacteriol 182: 2422–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead MB, Marshburn S, Mohanty BK, Mitra J, Pena Castillo L, Ray D, van Bakel H, Hughes TR, Kushner SR. 2011. Analysis of Escherichia coli RNase E and RNase III activity in vivo using tiling microarrays. Nucleic Acids Res 39: 3188–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz G, Altuvia S, Wassarman KM. 2005. An abundance of RNA regulators. Annu Rev Biochem 74: 199–217. [DOI] [PubMed] [Google Scholar]
- Storz G, Vogel J, Wassarman KM. 2011. Regulation by small RNAs in bacteria: expanding frontiers. Mol Cell 43: 880–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahl H, Turlan C, Khalid S, Bond PJ, Kebalo JM, Peyron P, Poljak L, Bouvier M, Hamoen L, Luisi BF et al. 2015. Membrane recognition and dynamics of the RNA degradosome. PLoS Genet 11: e1004961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulthana S, Basturea GN, Deutscher MP. 2016. Elucidation of pathways of ribosomal RNA degradation: an essential role for RNase E. RNA 22: 1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulthana S, Quesada E, Deutscher MP. 2017. RNase II regulates RNase PH and is essential for cell survival during starvation and stationary phase. RNA 23: 1456–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwa S, Nagai Y, Fujimoto A, Kikuchi Y, Tanaka T. 2009. Analysis on substrate specificity of Escherichia coli ribonuclease P using shape variants of pre-tRNA: proposal of subsites model for substrate shape recognition. J Biochem 145: 151–160. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Shuman S. 2011. RtcB is the RNA ligase component of an Escherichia coli RNA repair operon. J Biol Chem 286: 7727–7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temmel H, Muller C, Sauert M, Vesper O, Reiss A, Popow J, Martinez J, Moll I. 2017. The RNA ligase RtcB reverses MazF-induced ribosome heterogeneity in Escherichia coli. Nucleic Acids Res 45: 4708–4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tock MR, Walsh AP, Carroll G, McDowall KJ. 2000. The CafA protein required for the 5’-maturation of 16 S rRNA is a 5’-end-dependent ribonuclease that has context-dependent broad sequence specificity. J Biol Chem 275: 8726–8732. [DOI] [PubMed] [Google Scholar]
- Umitsuki G, Wachi M, Takada A, Hikichi T, Nagia K. 2001. Involvement of RNase G in in vivo mRNA metabolism in Escherichia coli. Genes Cells 6: 403–410. [DOI] [PubMed] [Google Scholar]
- Vasilyev N, Gao A, Serganov A. 2019. Noncanonical features and modifications on the 5’-end of bacterial sRNAs and mRNAs. Wiley Interdiscip Rev RNA 10: e1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viegas SC, Silva IJ, Saramago M, Domingues S, Arraiano CM. 2011. Regulation of the small regulatory RNA MicA by ribonuclease III: a target-dependent pathway. Nucleic Acids Res 39: 2918–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent HA, Deutscher MP. 2006. Substrate recognition and catalysis by the exoribonuclease RNase R. J Biol Chem 281: 29769–29775. [DOI] [PubMed] [Google Scholar]
- Vogel A, Schilling O, Spath B, Marchfelder A. 2005. The tRNase Z family of proteins: physiological functions, substrate specificity and structural properties. BiolChem 386: 1253–1264. [DOI] [PubMed] [Google Scholar]
- Vogel J, Luisi BF. 2011. Hfq and its constellation of RNA. Nat Rev Microbiol 9: 578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachi M, Naoko K, Umitsuki G, Clarke DP, Nagai K. 2001. A novel RNase G mutant that is defective in degradation of adhE mRNA but proficient in the processing of 16S rRNA precursor. Biochem Biophys Res Comm 289: 1301–1306. [DOI] [PubMed] [Google Scholar]
- Wachi M, Takada A, Nagai K. 1999. Overproduction of the outer-membrane proteins FepA and Fhu responsible for iron transport in Escherichia coli hfq::cat mutant. Biochem Biophys Res Comm 264: 525–529. [DOI] [PubMed] [Google Scholar]
- Wachi M, Umitsuki G, Nagai K. 1997. Functional relationship between Escherichia coli RNase E and the CafA protein. Mol Gen Genet 253: 515–519. [DOI] [PubMed] [Google Scholar]
- Waters LS, Storz G. 2009. Regulatory RNAs in bacteria. Cell 136: 615–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski JR, Rakus D. 2014. Quantitative analysis of the Escherichia coli proteome. Data Brief 1: 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Chen Y, Lindell M, Mao G, Kirsebom LA. 2011. Functional coupling between a distal interaction and the cleavage site in bacterial RNase-P-RNA-mediated cleavage. J Mol Biol 411: 384–396. [DOI] [PubMed] [Google Scholar]
- Yakhnin AV, Baker CS, Vakulskas CA, Yakhnin H, Berezin I, Romeo T, Babitzke P. 2013. CsrA activates flhDC expression by protecting flhDC mRNA from RNase E-mediated cleavage. Mol Microbiol 87: 851–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Inouye M. 2009. mRNA interferases, sequence-specific endoribonucleases from the toxin-antitoxin systems. Prog Mol Biol Transl Sci 85: 467–500. [DOI] [PubMed] [Google Scholar]
- Yehudai-Resheff S, Schuster G. 2000. Characterization of the E.coli poly(A) polymerase: nucleotide specificity, RNA-binding affinities and RNA structure dependence. Nucleic Acids Res 28: 1139–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang J, Hoeflich KP, Ikura M, Qing G, Inouye M. 2003. MazF cleaves cellular mRNAs specifically at ACA to block protein synthesis in Escherichia coli. Mol Cell 12: 913–923. [DOI] [PubMed] [Google Scholar]
- Zhao J, Harris ME. 2019. Distributive enzyme binding controlled by local RNA context results in 3’ to 5’ directional processing of dicistronic tRNA precursors by Escherichia coli ribonuclease P. Nucleic Acids Res 47: 1451–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilhao R, Cairrao R, Régnier P, Arraiano CM. 1996. PNPase modulates RNase II expression in Escherichia coli: Implications for mRNA decay and cell metabolism. Mol Microbiol 20: 1033–1042. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Vincent HA, Zhang J, Wang Y, Deutscher MP, Malhotra A. 2006. Structural basis for processivity and single-strand specificity of RNase II. Mol Cell 24: 149–156. [DOI] [PubMed] [Google Scholar]


