Abstract
Microbial proline 4-hydroxylases, which hydroxylate free l-proline to trans-4-hydroxy-l-proline, were screened in order to establish an industrial system for biotransformation of l-proline to trans-4-hydroxy-l-proline. Enzyme activities were detected in eight strains, including strains of Dactylosporangium spp. and Amycolatopsis spp. The Dactylosporangium sp. strain RH1 enzyme was partially purified 3,300-fold and was estimated to be a monomer polypeptide with an apparent molecular mass of 31 kDa by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Degenerate primers based on the N-terminal amino acid sequence of the 31-kDa polypeptide were synthesized in order to amplify the corresponding 71-bp DNA fragment. A 5.5-kbp DNA fragment was isolated by using the 71-bp fragment labeled with digoxigenin as a probe for a genomic library of Dactylosporangium sp. strain RH1 constructed in Escherichia coli. One of the open reading frames found in the cloned DNA, which encoded a 272-amino-acid polypeptide (molecular mass, 29,715 daltons), was thought to be a proline 4-hydroxylase gene. The gene was expressed in E. coli as a fused protein with the N-terminal 34 amino acids of the β-galactosidase α-fragment. The E. coli recombinant exhibited proline 4-hydroxylase activity that was 13.6-fold higher than the activity in the original strain, Dactylosporangium sp. strain RH1. No homology was detected with other 2-oxoglutarate-dependent dioxygenases when databases were searched; however, the histidine motif conserved in 2-oxoglutarate-dependent dioxygenases was found in the gene.
trans-4-Hydroxy-l-proline is a chiral synthon that is useful for chemical synthesis of pharmaceuticals (26). This compound is manufactured industrially by acid hydrolysis of mammalian collagen, and no biocatalytic method for production of hydroxyprolines has been described.
Hydroxyprolines have been found in certain proteins, such as collagen (14), and in some peptide antibiotics, such as actinomycin (12) and etamycin (29). In mammalian systems, l-proline is hydroxylated to trans-4-hydroxy-l-proline by procollagen-proline dioxygenase (prolyl hydroxylase) (EC 1.14.11.2). This enzyme belongs to the family of 2-oxoglutarate-dependent dioxygenases, which require 2-oxoglutarate and dioxygen as cosubstrates and ferrous ion as a cofactor (2). It accepts only peptidyl proline as a substrate, and free l-proline is not accepted. In contrast, proline 4-hydroxylase, which hydroxylates free l-proline to free trans-4-hydroxy-l-proline, has been found in a microbial system. However, the enzyme activity was found only during biosynthesis of etamycin in Streptomyces griseoviridus P-8648 (1, 15, 24). The proline 4-hydroxylase gene has never been cloned.
To determine the system which makes trans-4-hydroxy-l-proline, we began by studying the enzymatic conversion of l-proline, which is industrially produced by fermentation, to trans-4-hydroxy-l-proline through the action of proline 4-hydroxylase. Since the previously reported activity was so weak that the specific activity of the purified enzyme preparation was 907 pmol/min per mg of protein (15), extensive screening of microbial proline 4-hydroxylases was necessary to obtain an enzyme with high activity. Previously, we described a sensitive and efficient screening system for the enzyme (20). Here, the results of proline 4-hydroxylase screening, partial purification, cloning, and expression of the proline 4-hydroxylase gene in Escherichia coli are reported.
MATERIALS AND METHODS
Chemicals.
Most chemicals were purchased from Nacalai Tesque (Kyoto, Japan); l-proline was obtained from Kyowa Hakko Kogyo Co., Ltd. (Tokyo, Japan).
Culture medium and cultural conditions.
HT medium and SR3 medium have been described previously (20). Df1 medium consisted of 50 g of soluble starch per liter, 15 g of soy bean meal per liter, 0.5 g of KH2PO4 per liter, 0.5 g of MgSO4 · 7H2O per liter, and 5 g of calcium carbonate per liter, and the pH was adjusted to 7.0. All cultures were grown at 28°C. HT medium was used to isolate microorganisms during the screening study. The microorganisms were aerobically cultured with shaking for 2 days in 10 ml of SR3 medium. Then 1 ml of each culture was inoculated into 10 ml of Df1 medium and cultured for 2 days with shaking. Cells were collected by centrifugation and used for the whole-cell reaction study. Df1 medium containing 1 g of l-Pro per liter was used for a large-scale culture of Dactylosporangium sp. strain RH1. RH1 cells were grown in a 30-liter fermentor containing 18 liters of the medium for 3 days with agitation (385 rpm) and aeration (1 liter/liter/min). The cells were harvested by centrifugation and washed once with a cold 0.85% (wt/vol) NaCl solution.
Taxonomic characterization of the strains.
The cultural characteristics and morphology of strains RH1, RH2, RH3, RH4, and RH5 were determined by the methods of the International Streptomyces Project (27). The sugars in whole-cell hydrolysates, menaquinones, phospholipids, and mycolic acids were analyzed as described previously (4, 18, 19, 34).
Analytical methods.
Hydroxyprolines and proline were detected by postcolumn derivatization with 7-chloro-4-nitrobenzo-2-oxa-1,3-diazole chloride (NBD) (25), as well as precolumn derivatization (16).
Protein concentrations were determined with a Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, Calif.), as well as by determining the approximate absorbance at 280 nm. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed with 12.5% (wt/vol) polyacrylamide slab gels by using a Tris-glycine buffer system (13). Proteins were detected with a Quick-CBB kit (Wako Pure Chemical Industries, Osaka, Japan).
The N-terminal amino acid sequence of a 31-kDa polypeptide was determined by performing automated Edman degradation with a model PPSQ-10 protein sequencer (Shimadzu Seisakusho, Kyoto, Japan). The internal amino acid sequences of a 31-kDa polypeptide were determined by using proteolytic peptides digested with lysyl endopeptidase (Wako Pure Chemical Industries).
Enzyme assay.
Cellular proline 4-hydroxylase activities were measured by the whole-cell reaction procedure. Whole-cell reactions were performed as described previously (20). Cell-free enzyme activities were assayed as follows. Each reaction mixture (250 μl) contained 80 mM 2-(N-morpholino)ethanesulfonic acid (MES) buffer (pH 6.5), 4 mM l-proline, 8 mM 2-oxoglutarate, 2 mM ferrous sulfate, 4 mM l-ascorbic acid, and an enzyme preparation. The reaction mixtures were incubated at 35°C for 10 min, and then the reactions were terminated. The amount of trans-4-hydroxy-l-proline in each mixture was determined. For the assay in which recombinant E. coli was used, the reaction mixture contained 240 mM MES (pH 6.5), 12 mM l-proline, 24 mM 2-oxoglutarate, 8 mM ascorbic acid, 4 mM FeSO4, and cell extract. One unit of enzyme activity was defined as the amount of enzyme that hydroxylated 1 nmol of l-proline to trans-4-hydroxy-l-proline per 1 min.
Enzyme purification.
Buffer A [50 mM N-tris-(hydroxymethyl)methyl-3-aminopropane sulfonic acid (TAPS) (pH 9.0) containing 2 mM dithiothreitol, 0.2 mM EDTA, and 20% (vol/vol) glycerol] and buffer B (50 mM TAPS [pH 8.0] containing 2 mM dithiothreitol, 0.1% [wt/vol] Tween 20, and 20% [vol/vol] glycerol) were used for enzyme purification. All procedures were performed at 0 to 5°C. RH1 cells suspended in buffer A were disrupted with a Dyno-mill (Willy A Bachofen Maschinenfabrik, Basel, Switzerland) and centrifuged (8,000 × g, 10 min). The supernatant was applied to a STREAMLINE DEAE column (Pharmacia), which was eluted with 0.2 M NaCl in buffer A. Then the pooled active fraction was applied to a DEAE-Sepharose column (Pharmacia), which was eluted with a linear 0 to 1 M NaCl gradient in buffer A. The pooled active fraction was applied to a Butyl Sepharose 4 Fast Flow column (Pharmacia) equilibrated with 3 M NaCl in buffer A. The column was washed stepwise with 3, 2, and 1 M NaCl in buffer A and then with buffer A. The pooled active fraction was applied to a HiTrap Phenyl Sepharose HP column (Pharmacia) equilibrated with 3 M NaCl in buffer A. The active fractions were eluted with 2 M NaCl in buffer A. Tween 20 was added to the active solution at a concentration of 0.1% (wt/vol). Then the solution was desalted with a PD-10 column (Pharmacia) and was applied to a Reactive Red 120-agarose column (type 3000-CL; Sigma Chemical Co., St. Louis, Mo.) equilibrated with buffer A containing 0.1% (wt/vol) Tween 20. Active fractions were eluted with a linear 0 to 1.5 M NaCl gradient in the same buffer. After it was desalted with a PD-10 column, the active solution was applied to a Reactive Blue 72-agarose column (Sigma) directly connected to a Resource Q column. The active fractions were eluted from the Resource Q column with a linear 0 to 0.2 M NaCl gradient in buffer B and used for characterization of the enzyme.
Cloning and nucleotide sequencing of the proline 4-hydroxylase gene.
Degenerate oligoprimers F1 (5′-ATGCT[C/G]AC[C/G]CC[A/C/G/T]AC[A/C/G/T]GA) and R1 (5′-GG[C/G]CC[C/G]AG[A/C/G/T]CC[A/G]TC[C/T]TC) corresponding to the N-terminal amino acid sequence of the 31-kDa polypeptide were synthesized and used for PCR with genomic DNA of Dactylosporangium sp. strain RH1 prepared by the method of Hopwood et al. (10). The amplified fragments were cloned into the SmaI site of pUC18. The cloned 71-bp fragment was labeled with digoxigenin (DIG) by performing a PCR with a PCR DIG labeling kit (Boehringer, Mannheim, Germany). Approximately 5.5-kb DNA fragments that hybridized to the DIG-labeled 71-bp fragment were recovered from XhoI-digested RH1 genomic DNA and ligated with XhoI-digested λ ZAPII vector DNA (Stratagene). The ligated DNA was packaged in vitro with Gigapack II Gold packaging extract (Stratagene). The library was screened by performing plaque hybridization with the DIG-labeled probe and a DIG detection kit (Boehringer). Plasmid pRH71 was excised in vivo from a positive phage by using E. coli SOLR (Stratagene) as the host.
A 2.7-kb portion of the inserted 5.5-kb fragment of pRH71 was sequenced by the chain termination method by using a Taq DyeDeoxy terminator cycle sequencing kit (Perkin-Elmer, Norwalk, Conn.).
Construction of expression plasmid for the proline 4-hydroxylase gene.
Plasmid pRH71 was digested with SacI and blunted with a DNA blunting kit (Takara). A 2.4-kb fragment was recovered, digested with XbaI, and then ligated to EcoRV-XbaI-digested pBluescript II KS(+), which resulted in pES1-23a. E. coli DH1 was used as the host for expression of the proline 4-hydroxylase gene.
RESULTS
Screening for microbial proline 4-hydroxylases.
Screening for proline 4-hydroxylases was done by the whole-cell reaction method as described previously (20). The screening procedure was focused mainly on actinomycetes grown on HT medium to which hydroxyproline-containing actinomycin I or proline analogs were sometimes added. More than 3,000 microbial strains isolated from soil and etamycin-producing strains, including S. griseoviridus P-8648 (24), were examined. Five strains, RH1, RH2, RH3, RH4, and RH5, were selected as proline 4-hydroxylase producers (Table 1). Strains RH1, RH3, and RH4 were identified as Dactylosporangium strains (7, 32) based on the following taxonomic characteristics: branched substrate mycelia on which claviform sporangia containing one row of two to four spores were observed; meso-diaminopimelic acid was present in the cell walls; whole-cell hydrolysates contained arabinose and xylose as diagnostic sugars; and the major isoprenoid quinones were MK-9(H6) and MK-9(H8). Strains RH2 and RH5 were identified as Amycolatopsis strains (7, 9). In these strains, branched substrate and aerial mycelia were observed, but spores, sporangia, synnemata, and other morphological characteristics were not; meso-diaminopimelic acid was present in the cell walls; whole-cell hydrolysates contained arabinose and galactose; the major isoprenoid quinone was MK-9(H4); phosphatidylethanolamine was present and no mycolic acids were present.
TABLE 1.
Results of screening for microbial proline 4-hydroxylases
| Strain | Proline 4-hydroxylase activity
|
Etamycin production | |
|---|---|---|---|
| Cellular activity (U/g of cells) | Sp act (U/mg of protein) | ||
| S. griseoviridus P-8648 | 22.0 | 1.63 | + |
| S. griseoviridus JCM 4250 | 20.0 | 0.86 | + |
| S. daghestanicus JCM 4365 | 9.0 | 0.14 | + |
| Dactylosporangium sp. strain RH1 | 28.0 | 2.31 | − |
| Dactylosporangium sp. strain RH3 | 21.5 | 0.22 | − |
| Dactylosporangium sp. strain RH4 | 4.0 | NDa | − |
| Amycolatopsis sp. strain RH2 | 6.9 | ND | − |
| Amycolatopsis sp. strain RH5 | 3.0 | ND | − |
ND, not determined.
As a result of the screening analysis, Dactylosporangium sp. strain RH1 was chosen for further study since it exhibited the highest level of proline 4-hydroxylase activity.
Partial purification of proline 4-hydroxylase.
The proline 4-hydroxylase of Dactylosporangium sp. strain RH1 was partially purified (Table 2). Although the proline hydroxylase activity was enriched 3,300-fold, two major bands, one at 80 kDa and one at 31 kDa, were identified in an SDS-PAGE analysis of the purified enzyme preparation. The 31-kDa polypeptide band on SDS-PAGE gels was thought to correspond to proline 4-hydroxylase based on the results of two different gel filtration chromatography analyses performed with YMC-Pack Diol-2000 and TSKgel G-3000. The N-terminal amino acid sequence and two internal amino acid sequences of the 31-kDa polypeptide were determined to be MLTPTELKQY(R)EAGYLLIEDGLGP, XXNRTDNALPAQAAPRP, and AARDAT (where X is an amino acid that was not identified and parentheses indicate an amino acid whose identity was not certain).
TABLE 2.
Purification of l-proline 4-hydroxylase from Dactylosporangium sp. strain RH1
| Stepa | Total activity (U) | Total protein (mg) | Sp act (U/mg) | Purifi-cation (fold) | Yield (%) |
|---|---|---|---|---|---|
| Cell extract | 3,590 | 5,700 | 0.631 | 1.00 | 100 |
| STREAMLINE (DEAE) | 2,880 | 2,550 | 1.13 | 1.79 | 80.2 |
| DEAE-Sepharose FF | 1,690 | 324 | 5.22 | 8.29 | 47.0 |
| Butyl Sepharose 4 FF | 1,690 | 44.1 | 38.3 | 60.8 | 47.0 |
| Phenyl Sepharose 6 FF | 781 | 4.50 | 174 | 275 | 21.7 |
| Reactive Red 120 | 931 | 1.11 | 836 | 1,330 | 26.9 |
| Reactive Blue 72-Resource Q | 454 | 0.20 | 2,080 | 3,300 | 12.6 |
The enzyme purification, enzyme assay, and protein determination procedures were carried out as described in the text.
Cloning and expression of the proline 4-hydroxylase gene in E. coli.
Plasmid pRH71, which contained the 5.5-kb XhoI fragment of RH1 genomic DNA, was isolated by the plaque hybridization method performed with the DIG-labeled 71-bp probe corresponding to the N-terminal amino acid sequence of the 31-kDa polypeptide. Sequencing of a 2.7-kb portion of the 5.5-kb fragment revealed that there were four open reading frames (ORFs) in the fragment, although ORF 3 was truncated and ORF 0 was not completely sequenced. ORF 1 is an 816-bp ORF that encodes a 272-amino-acid polypeptide (molecular mass, 29,715 daltons) containing amino acid sequences identical to the N-terminal and internal amino acid sequences of the 31-kDa polypeptide. The initiation codon, ATG, is preceded by a probable ribosome-binding site, AGGAG. The G+C content of ORF 1 is 74%, and 98% of the codons used in ORF 1 have G or C at the third position. A high G+C content and a codon usage bias are characteristics of genes from actinomycetes (33).
Expression plasmid pES1-23a, in which ORF 1 was expressed under the lac promoter as a fused protein consisting of the N-terminal 34 amino acids of the β-galactosidase α-fragment followed by 265 amino acids (amino acids 8 to 272) of ORF 1, was constructed (Fig. 1). Proline 4-hydroxylase activity was detected in E. coli DH1 containing pES1-23a, and this activity was 13.6-fold higher than the activity in Dactylosporangium sp. strain RH1, indicating that ORF 1 encodes a proline 4-hydroxylase. The GenBank accession number of the proline 4-hydroxylase structural gene is D78338.
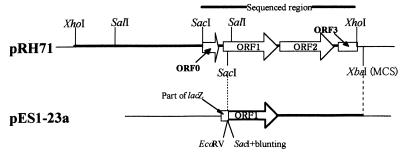
FIG. 1.
Physical structures of plasmids. The inserted DNA fragments are indicated by lines. A 5.5-kb XhoI fragment of pRH71 is indicated by a heavy line. The region sequenced is indicated. The predicted ORFs are shown as open arrows and an open box, which indicates a 3′-truncated gene. It is thought that in pES1-23a ORF 1 is expressed as a lacZ-fused protein (open box plus an open arrow under the lac promoter). The positions of ORF 2 and ORF 3 in pES1-23a are not shown.
Comparison with other 2-oxoglutarate-dependent dioxygenases.
The search for sequences homologous to the deduced amino acid sequences of ORF 0 to ORF 3 with the Genetics Computer Group package program (Genetics Computer Group, Madison, Wis.) revealed no significant similarities (i.e., the levels of identity were less than 40%) to the genes in the GenBank database, including the genes for prolyl 4-hydroxylase and other 2-oxoglutarate-dependent dioxygenases. We also detected no homology with proline 3-hydroxylase of Streptomyces sp. strain TH1 (21). However, the His-1 motif HXD (X indicates any amino acid), which is conserved in the 2-oxoglutarate-dependent dioxygenase family (23), seemed to be conserved in proline 4-hydroxylase at amino acids 109 to 111 (Table 3). The His-2 motif was not assigned to any part of the amino acid sequence of proline 4-hydroxylase (27, 31).
TABLE 3.
Comparison of amino acid sequences of the His-1 motif
| Enzymea | His-1 motif | Reference |
|---|---|---|
| Deacetoxycepharosporin C synthase | PHYDLSTIT | 28 |
| Isopenicillin synthase | WHEDVSLIT | 3 |
| Lysyl hydroxylase | PHHDASTFT | 22 |
| Prolyl 4-hydroxylase α-subunit | PHFDFARKD | 8 |
| Ethylene-forming enzyme | AHTDYGLL | 6 |
| Plant TOME8 | QHTDIGFVT | 5 |
| Hyoscyamine 6β-hydroxylase | GHYDGNLIT | 17 |
| Proline 3-hydroxylase | PHRDFVELD | 21 |
| Proline 4-hydroxylase | WHQDYIFWA | This study |
Data are shown for deacetoxycepharosporin C synthase from Cepharosporium acremonium, isopenicillin N synthase from Penicillium chrysogenum, lysyl hydroxylase from chicks, prolyl 4-hydroxylase α-subunit from humans, ethylene-forming enzyme from Pseudomonas syringae pv. phaseolicola PK2, plant TOME8 from tomato, hyoscyamine 6β-hydroxylase from Hyoscyamus niger, proline 3-hydroxylase from Streptomyces sp. strain TH1, and proline 4-hydroxylase from Dactylosporangium sp. strain RH1. Completely conserved histidine (H) and aspartic acid (D) residues are indicated by boldface type.
DISCUSSION
Hydroxyproline has been found in prokaryotic cells primarily in peptide antibiotics, such as actinomycin (12), etamycin (29), and telomycin (11); the only exception to this is a hydroxyproline-rich protein that has been found in Staphylococcus aureus (30). Proline 4-hydroxylase activity was found only during biosynthesis of etamycin in S. griseoviridus P-8648 (1, 15, 24). In the screening study reported here, five actinomycete strains were found to produce proline 4-hydroxylases, as were three etamycin-producing Streptomyces strains. These five actinomycete strains produced no etamycin as far as we could determine (Table 1). During the screening study we also found that Streptomyces and Bacillus strains produced proline 3-hydroxylases as well as telomycin-producing Streptomyces strains produce these enzymes (20). Our preliminary results suggested that the strains identified in this screening study produced peptidelike compounds containing hydroxyprolines other than etamycin and telomycin (20). Since hydroxyproline isomers, such as cis-4-hydroxy-d-proline, cis-4-hydroxy-l-proline, and trans-3-hydroxy-l-proline, are known to exist in nature along with trans-4-hydroxy-l-proline and cis-3-hydroxy-l-proline (14), there may be other proline hydroxylases with different regio- and stereospecificities.
Recently, Lawrence et al. described the purification and characterization of l-proline 4-hydroxylase from etamycin-producing S. griseoviridus P-8648 (15). The characteristics of proline 4-hydroxylase from S. griseoviridus P-8648 that were reported resembled those of the proline 4-hydroxylase described here with regard to the requirements for the reaction (i.e., Km values for l-proline and 2-oxoglutarate, which are substrates of the reaction and substrate specificity). However, the two enzymes were quite different in the following ways. First, Lawrence et al. reported that l-ascorbate inhibited 4-hydroxylation and proposed that l-ascorbate competed with 2-oxoglutarate for the same binding site on 4-hydroxylase. However, excess l-ascorbate never inhibited the 4-hydroxylase activity of Dactylosporangium sp. strain RH1. Second, the specific activity of partially purified 4-hydroxylase from Dactylosporangium sp. strain RH1 (2,080 nmol/min per mg of protein) was much higher than the reported activity of the purified 4-hydroxylase from S. griseoviridus P-8648 (907 pmol/min per mg of protein). This must be an advantage for industrial applications.
The proline 4-hydroxylase described here is classified as a 2-oxoglutarate-dependent dioxygenase because it requires 2-oxoglutarate and ferrous ion for a reaction. The deduced amino acid sequence of the enzyme has the same His motif that 2-oxoglutarate-dependent dioxygenase has (23). The HXD amino acid sequence in the His-1 motif is strongly conserved in the 2-oxoglutarate-dependent dioxygenase family, including proline 4-hydroxylase and proline 3-hydroxylase, as well as in isopenicillin N synthase, which is not a 2-oxoglutarate-dependent dioxygenase but requires ferrous ion and dioxygen. The histidine and aspartic acid residues in the His-1 motif and the histidine residue in the His-2 motif were found to take part in holding a ferrous ion in the enzyme by crystallographic studies of isopenicillin N synthase (27) and deacetoxycepharosporin C synthase (31). The histidine and aspartic acid residues are also conserved in both proline 4-hydroxylase and proline 3-hydroxylase (Table 3).
The proline 4-hydroxylase described here catalyzed highly regio- and stereospecific hydroxylation of l-proline. This rigidity of the reaction specificity makes the enzyme useful as a biocatalyst for the production of trans-4-hydroxy-l-proline from l-proline, which is industrially produced by fermentation. The enzyme also should be of some utility for preparing hydroxyproline isomers and some analogs. Cloning and expression of the proline 4-hydroxylase gene should lead to an effective method for producing trans-4-hydroxy-l-proline industrially.
ACKNOWLEDGMENTS
We thank Ruriko Nishimura for capable technical assistance throughout the experiments. We also thank Keiichi Yano and Kazuhisa Uchida for N-terminal sequencing of the enzyme and technical advice.
REFERENCES
- 1.Baldwin J E, Field R A, Lawrence C C, Merritt K D, Schofield C J. Substrate specificity of proline 4-hydroxylase: chemical and enzymatic synthesis of 2S,3R,4S-epoxyproline. Tetrahedron Lett. 1993;34:7489–7492. [Google Scholar]
- 2.Cardinale G J, Udenfriend S. Prolyl hydroxylase. Adv Enzymol. 1974;41:245–300. doi: 10.1002/9780470122860.ch6. [DOI] [PubMed] [Google Scholar]
- 3.Carr L G, Skatrud P L, Scheetz II M E, Queener S W, Ingolia T D. Cloning and expression of the isopenicillin N synthetase gene from Penicillium chrysogenum. Gene. 1986;48:257–266. doi: 10.1016/0378-1119(86)90084-3. [DOI] [PubMed] [Google Scholar]
- 4.Collins M D, Pirouz T, Goodfellow M, Minnekin D E. Distribution of menaquinones in actinomycetes and corynebacteria. J Gen Microbiol. 1977;100:221–230. doi: 10.1099/00221287-100-2-221. [DOI] [PubMed] [Google Scholar]
- 5.Deikman J, Fischer R L. Interaction of a DNA binding factor with the 5′-flanking region of an ethylene-responsive fruit ripening gene from tomato. EMBO J. 1988;7:3315–3320. doi: 10.1002/j.1460-2075.1988.tb03202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukuda H, Ogawa T, Ishihara K, Fujii T, Nagahama K, Omata T, Inoue Y, Tanase S, Morino Y. Molecular cloning in Escherichia coli, expression, and nucleotide sequence of the gene for the ethylene-forming enzyme of Pseudomonas syringae pv. phaseolicola PK2. Biochem Biophys Res Commun. 1992;188:826–832. doi: 10.1016/0006-291x(92)91131-9. [DOI] [PubMed] [Google Scholar]
- 7.Hasegawa T, Takizawa M, Tanida S. A rapid analysis for chemical grouping of aerobic actinomycetes. J Gen Appl Microbiol. 1983;29:319. [Google Scholar]
- 8.Helaakoski T, Vuori K, Myllyla R, Kivirikko K I, Pihlajaniemi T. Molecular cloning of the α-subunit of human prolyl 4-hydroxylase: the complete cDNA-derived amino acid sequence and evidence for alternative splicing of RNA transcripts. Proc Natl Acad Sci USA. 1989;86:4392–4396. doi: 10.1073/pnas.86.12.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holt J G, Krieg N R, Sneath P H A, Staley J T, Williams S T. Bergey’s manual of determinative bacteriology. 9th ed. Baltimore, Md: William & Wilkins Co.; 1994. pp. 625–650. [Google Scholar]
- 10.Hopwood D A, Bibb M J, Chater K F, Kieser T, Bruton C J, Kieser H M, Lydiate D J, Smith C P, Ward J M, Schrempf H. Genetic manipulation of Streptomyces, a laboratory manual. Norwich, United Kingdom: The John Innes Foundation; 1985. [Google Scholar]
- 11.Irreverre F, Morita K, Ishii S, Witkop B. Occurrence of cis- and trans-3-hydroxy-l-proline in acid hydrolysate of telomycin. Biochem Biophys Res Commun. 1962;9:69–71. doi: 10.1016/0006-291x(62)90089-x. [DOI] [PubMed] [Google Scholar]
- 12.Katz E, Prockop D J, Udenfriend S. Precursors of the hydroxyproline and ketoproline in actinomycin. J Biol Chem. 1962;237:1585–1588. [PubMed] [Google Scholar]
- 13.King J, Laemmli U K. Polypeptides of the tail fibres of bacteriophage T4. J Mol Biol. 1971;62:465–477. doi: 10.1016/0022-2836(71)90148-3. [DOI] [PubMed] [Google Scholar]
- 14.Kuttan R, Radhakrishnan A N. Biochemistry of the hydroxyprolines. Adv Enzymol. 1973;37:273–347. doi: 10.1002/9780470122822.ch5. [DOI] [PubMed] [Google Scholar]
- 15.Lawrence C C, Sobey W J, Field R A, Baldwin J E, Schofield C J. Purification and initial characterization of proline 4-hydroxylase from Streptomyces griseoviridus P8648: a 2-oxoacid, ferrous-dependent dioxygenase involved in etamycin biosynthesis. Biochem J. 1996;313:185–191. doi: 10.1042/bj3130185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindbald W J, Diegelmann R F. Quantitation of hydroxyproline isomers in acid hydrolysates by high-performance liquid chromatography. Anal Biochem. 1984;138:390–395. doi: 10.1016/0003-2697(84)90827-3. [DOI] [PubMed] [Google Scholar]
- 17.Matsuda J, Okabe S, Hashimoto T, Yamada Y. Molecular cloning of hyoscyamine 6 β-hydroxylase, a 2-oxoglutarate-dependent dioxygenase, from cultured roots of Hyoscyamus niger. J Biol Chem. 1991;266:9460–9464. [PubMed] [Google Scholar]
- 18.Minnikin D E, Alshamaony L, Goodfellow M. Differentiation of Mycobacterium, Nocardia, and related taxa by thin-layer chromatographic analysis of whole-organism methanolysates. J Gen Microbiol. 1975;88:200–204. doi: 10.1099/00221287-88-1-200. [DOI] [PubMed] [Google Scholar]
- 19.Minnikin D E, O’Donnell A G, Goodfellow M, Alderson G, Schaal A, Parlett J H. An integrated procedure for extraction of bacterial isoprenoid quinones and polar lipids. J Microbiol Methods. 1984;2:233–241. [Google Scholar]
- 20.Mori H, Shibasaki T, Uozaki Y, Ochiai K, Ozaki A. Detection of novel proline 3-hydroxylase activities in Streptomyces and Bacillus spp. by regio- and stereospecific hydroxylation of l-proline. Appl Environ Microbiol. 1996;62:1903–1907. doi: 10.1128/aem.62.6.1903-1907.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mori H, Shibasaki T, Yano K, Ozaki A. Purification and cloning of a proline 3-hydroxylase, a novel enzyme which hydroxylates free l-proline to cis-3-hydroxy-l-proline. J Bacteriol. 1997;179:5677–5683. doi: 10.1128/jb.179.18.5677-5683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myllyla R, Pihlajaniemi T, Pajunen L, Turpeenniemi Hujanen T, Kivirikko K I. Molecular cloning of chick lysyl hydroxylase. Little homology in primary structure to the two types of subunit of prolyl 4-hydroxylase. J Biol Chem. 1991;266:2805–2810. [PubMed] [Google Scholar]
- 23.Myllyla R, Gunzler V, Kivirikko K I, Kaska D D. Modification of vertebrate and algal prolyl 4-hydroxylases and vertebrate lysyl hydroxylase by diethyl pyrocarbonate. Evidence for histidine residues in the catalytic site of 2-oxoglutarate-coupled dioxygenases. Biochem J. 1992;286:923–927. doi: 10.1042/bj2860923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onishi M, Okumura Y, Okamoto R, Ishikura T. Proline hydroxylation by cell free extract of a streptomycete. Biochem Biophys Res Commun. 1984;120:45–51. doi: 10.1016/0006-291x(84)91411-6. [DOI] [PubMed] [Google Scholar]
- 25.Ozaki A, Shibasaki T, Mori H. Specific proline and hydroxyproline detection method by post-column derivatization for high-performance liquid chromatography. Biosci Biotechnol Biochem. 1995;59:1764–1765. [Google Scholar]
- 26.Remuzon P. trans-4-Hydroxy-l-proline, a novel and versatile chiral starting block. Tetrahedron. 1996;52:13803–13835. [Google Scholar]
- 27.Roach P L, Clifton I J, Fulop V, Harlos K, Barton G J, Hajdu J, Andersson I, Schofield C J, Baldwin J E. Crystal structure of isopenicillin N synthase is the first from a new structural family of enzymes. Nature. 1995;375:700–704. doi: 10.1038/375700a0. [DOI] [PubMed] [Google Scholar]
- 28.Samson S M, Dotzlaf J E, Slisz M L, Becker G W, Frank R M V, Veal L E, Yeh W, Miller J R, Queener S W, Ingolia T D. Cloning and expression of the fungal expandase/hydroxylase gene involved in cepharosporin biosynthesis. Bio/Technology. 1987;5:1207–1214. [Google Scholar]
- 29.Sheehan J C, Zachau H G, Lawson The structure of etamycin. J Am Chem Soc. 1958;80:3349–3355. [Google Scholar]
- 30.Usui Y, Yoshida K, San Clemente C L. Hydroxyproline-rich protein in the capsule of a strain of Staphylococcus aureus. Can J Microbiol. 1981;27:955–958. doi: 10.1139/m81-150. [DOI] [PubMed] [Google Scholar]
- 31.Valegard K, van Scheltinga A C, Lloyd M D, Hara T, Ramaswamy S, Perrakis A, Thompson A, Lee H J, Baldwin J E, Schofield C J, Hajdu J, Andersson I. Structure of a cephalosporin synthase. Nature. 1998;394:805–809. doi: 10.1038/29575. [DOI] [PubMed] [Google Scholar]
- 32.Vobis G. Genus Dactylosporangium Thiemann, Pagani and Beretta 1967a, 43AL. In: Williams S T, Sharpe M E, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 4. Baltimore, Md: Williams & Wilkins Co.; 1989. pp. 2437–2442. [Google Scholar]
- 33.Wright F, Bibb M J. Codon usage in the G+C-rich Streptomyces genome. Gene. 1992;113:55–65. doi: 10.1016/0378-1119(92)90669-g. [DOI] [PubMed] [Google Scholar]
- 34.Yokota A, Hasegawa T. The analysis of madurose, an actinomycete whole-cell sugar, by HPLC after anzymatic treatment. J Gen Appl Microbiol. 1988;34:445–449. [Google Scholar]