Abstract
Burkholderia thailandensis, an opportunistic pathogen found in the environment, is a bacterium closely related to B. pseudomallei, the cause of melioidosis. Human B. thailandensis infections are uncommon. We isolated B. thailandensis from water in Texas and Puerto Rico and soil in Mississippi in the United States, demonstrating a potential public health risk.
Keywords: Burkholderia, Burkholderia thailandensis, Burkholderia pseudomallei, environmental pathogens, bacteria, water, waterborne infections, genomic islands, local adaptation, Western Hemisphere, Puerto Rico, Mississippi, Arkansas, Louisiana, Oklahoma, Texas, United States
Burkholderia thailandensis, a gram-negative bacterium found in the environment, poses a public health threat both because of its ability to cause infections as an opportunistic pathogen and potential misidentification as the more pathogenic B. pseudomallei, its closest phylogenetic relative (1–4). B. pseudomallei, designated a Select Agent by the US Federal Select Agents Program and the causative pathogen of melioidosis, and B. thailandensis are found in the environment in some tropical regions, including Southeast Asia and northern Australia. B. thailandensis, a Biosafety Level 2 organism not classified as a Select Agent (3), has fewer safety restrictions than B. pseudomallei, and because it can be handled outside of Biosafety Level 3 laboratories, it is used by researchers as a surrogate in some experiments (5). In laboratory analyses, B. thailandensis is challenging to distinguish from B. pseudomallei because of their similar biochemical phenotypes, the only difference being that B. thailandensis can assimilate L-arabinose (1,3). B. thailandensis was described after researchers observed reduced virulence in an environmental isolate thought to be B. pseudomallei. Subsequent 16S rRNA gene analysis revealed a novel Burkholderia species named B. thailandensis after the geographic origin of the type strain (3).
Human B. thailandensis infections are uncommon (1,4), especially in the Western Hemisphere. Three previous clinical cases in that region have been reported, all from the southern United States: Louisiana in 1997, Texas in 2003 (1), and Arkansas in 2017 (4). Environmental sampling related to the 2003 case in Texas and previous environmental sampling for B. pseudomallei complex members did not recover B. thailandensis (6). B. thailandensis has been described primarily from the environment in Southeast Asia and Australia (3,7) and, recently, Africa (8). Occurrence of B. thailandensis in the environment in the Western Hemisphere remains poorly understood. We used a systematic approach to detect and isolate B. thailandensis from soil and water samples collected in Texas in November 2019 and November 2020 (9) and Puerto Rico during December 2018–March 2020.
The Study
We collected 2,540 environmental samples, 370 (280 soil, 80 water, 10 environmental water tank scrapes) from Texas and 2,170 (1,650 soil, 520 water) from throughout Puerto Rico. From the collected samples, we detected B. thailandensis DNA in 10 complex broth samples, 4 from Texas and 6 from Puerto Rico (Appendix). Culturing (10) yielded B. thailandensis isolates from 5 samples, 1 from Texas and 4 from Puerto Rico. In addition, we isolated B. thailandensis from a soil sample collected in Mississippi in July 2022 during a melioidosis cluster investigation (11). Further, in 2021, we identified B. thailandensis infection in a 4th case-patient in the United States (Oklahoma) (Table). The patient was suspected to have aspirated water after a motor vehicle rollover into water; he died because of multiple complications (Appendix).
Table. Genomes from global isolates used to generate whole-genome phylogeny in study of Burkholderia thailandensis from the environment in the United States*.
| Isolate | Alternative ID | Country (state/territory) | Sample type (source) | Year | MLST | GenBank accession no. |
|---|---|---|---|---|---|---|
| Bt10009† | 165–01_P1_S7 | USA (TX) | Environmental (water) | 2019 | 1758 | JALGJD00000000 |
| Bt10013† | 203–09_P1_S27 | USA (PR) | Environmental (water) | 2020 | 1772 | JALGJC00000000 |
| Bt9795† | 61_10_S54_S1 copy3 | USA (PR) | Environmental (water) | 2018 | 1772 | WCIR00000000 |
| Bt9920† | 89–06_P1_S1 | USA (PR) | Environmental (water) | 2018 | 1772 | WCIQ00000000 |
| Bt9942† | 91–08_P2_S1 | USA (PR) | Environmental (water) | 2018 | 1772 | WCIP00000000 |
| BtMS2022a† | USA (MS) | Environmental (soil) | 2022 | 2019 | SRR22548212 | |
| BtOK2021a† | USA (OK) | Clinical (human) | 2021 | 1772 | SRR22548210 | |
| 2.1 | Vietnam | Environmental (soil) | 2017 | 696 | GCA_002803565.1 | |
| 82172 | 34; 2002721621 | France | Veterinary (horse) | 1982 | 73 | GCA_001555485.1 |
| Bt4 | 49639 | Australia | Environmental | Unknown | 699 | GCA_000170395.1 |
| BtAR2017 | USA (AR) | Clinical (human) | 2017 | 101 | GCA_004684955.1 | |
| E1 | Papua New Guinea | Environmental | 1995 | 669 | GCA_001524325.1 | |
| E254 | Thailand | Environmental (soil) | 1992 | 345 | GCA_000765375.1 | |
| E264 | ATCC 700388 | Thailand | Environmental (soil) | 1994 | 80 | GCA_003568605.1 |
| E444 | E0444 | Thailand | Environmental (soil) | 2002 | 79 | GCA_000567945.1 |
| E555 | Cambodia | Environmental | 2005 | 696 | GCF_000179515.1 | |
| H0587 | 2002721121 | USA (LA) | Clinical (human) | 1997 | 101 | GCA_000567905.1 |
| MSMB59 | Australia | Environmental (soil) | 2006 | 669 | GCA_001718595.1 | |
| MSMB60 | Australia | Environmental (soil) | 2006 | 669 | GCA_001524345.1 | |
| Phuket 4W-1 | Thailand | Environmental (water) | 1965 | 80 | GCA_000877335.1 | |
| TXDOH | CDC3015869; 2003015869 | USA (TX) | Clinical (human) | 2003 | 101 | GCA_002888425.1 |
| USAMRU Malaysia no. 20 | 2002721744 | Malaysia | Unknown | Unknown | 80 | GCA_000706745.1 |
*Phylogeny shown in Figure. MLST, multilocus sequence type; USAMRU, US Army Medical Research Unit; TXDOH, Texas Department of Health. †Isolated in this study.
We used whole-genome analysis of those 7 isolates (National Center for Biotechnology Information BioProject nos. PRJNA575701, PRJNA818328, PRJNA908850) to place them within a larger phylogeographic context, including other B. thailandensis isolates from the United States and other global locations (Table; Figure). Environmental B. thailandensis isolates from Texas and Mississippi grouped in the same clade with clinical isolates from Texas and Louisiana and 2 environmental isolates from Asia. The 2021 clinical isolate from Oklahoma was most closely related to the isolate from the 2003 clinical case in Texas. Environmental isolates from Texas and Mississippi differed by more (4,639 single-nucleotide polymorphisms [SNPs]) than environmental isolates from Thailand and Australia (2,671 SNPs); B. pseudomallei isolates found in Australia and Asia are more diverse than isolates in the Americas (10). We observed little diversity among the 4 B. thailandensis isolates from Puerto Rico; total diversity was 62 SNPs, and distance between any 2 isolates was 28–36 SNPs.
Figure.
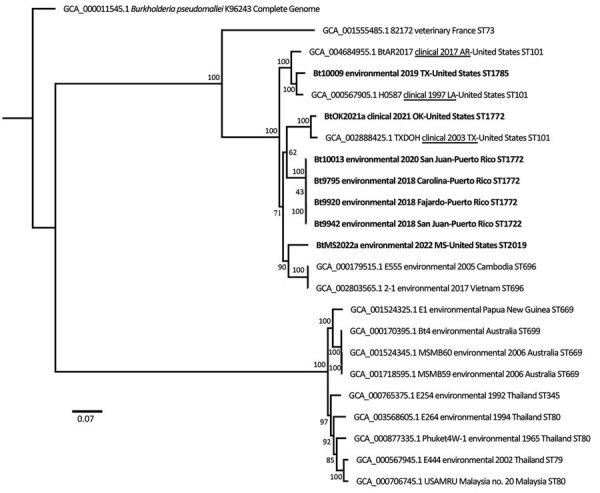
Whole-genome maximum-likelihood phylogeny of global isolates in study of Burkholderia thailandensis from the environment in the United States (Table). Tree was constructed with 1,000 bootstrap replicates and rooted with B. pseudomallei. Bold indicates B. thailandensis genomes generated from isolates collected in this study; other B. thailandensis from the Western Hemisphere have epidemiologic information underlined. Scale bar indicates 3,000 SNPs.
Among the isolates identified in our study, in silico multilocus sequence type analysis (https://pubmlst.org/organisms/burkholderia-pseudomallei) revealed novel ace allele 106 in the 4 isolates from Puerto Rico and the clinical isolate from Oklahoma, assigning all 5 to novel sequence type (ST) 1772. Novel gltB allele 175 was identified in the isolate from Texas, which was assigned to novel ST1785. The isolate from Mississippi, which had a unique combination of alleles, was assigned to novel ST2019.
Conclusions
Our study confirms B. thailandensis endemicity in the environment in the United States, albeit of rare occurrence and low abundance, requiring extensive sampling to detect; we found B. thailandensis at only 3.7% of collection sites in Puerto Rico and 8% in Texas. However, the pathogen could be present in other unsampled areas in the southern United States and Puerto Rico. Substantial culturing was required to isolate bacteria from PCR-positive samples, suggesting low abundance or its presence being outcompeted by other bacteria. B. thailandensis abundance might vary seasonally or on the basis of precipitation levels.
We detected B. thailandensis in Texas and Puerto Rico only from water samples, although they comprised only 24% of total (water and soil) samples collected at positive sites; all soil samples were negative for B. thailandensis. In contrast, in Thailand, B. thailandensis is most commonly isolated from soil (12). All 4 clinical cases from the United States were associated with traumatic injuries (1,4), 3 involving water (1), demonstrating the public health risk for disease from traumatic injuries related to contaminated water. This risk is especially relevant in Puerto Rico where B. thailandensis was detected within neighborhoods of the largest city, San Juan. Puerto Rico and the southeastern United States are prone to hurricane-induced flooding, which could increase the risk for infection by both B. thailandensis and B. pseudomallei (13).
Although samples were collected from 3 municipalities in northeastern Puerto Rico during a 1-year period, we found little phylogenetic diversity among the isolates, suggesting B. thailandensis may be widespread but rare in the environment in Puerto Rico and the result of a single introduction, as previously suggested for B. pseudomallei in Puerto Rico (10). We found evidence of possible local adaptation in Puerto Rico, which supports this hypothesis. We identified 113 genes unique to B. thailandensis isolates from Puerto Rico (Appendix), many of them potentially colocated in genomic islands, a pattern similar to one previously observed among B. pseudomallei isolates from Puerto Rico (10). Of note, 2 genes common to all B. thailandensis from Puerto Rico were present in some B. pseudomallei isolates from Puerto Rico but absent from all other global B. pseudomallei genomes (Appendix). In contrast, thousands of SNPs were found among B. thailandensis strains in the continental United States (Arkansas, Louisiana, Mississippi, Oklahoma, and 2003 clinical and 2019 environmental isolates from Texas). This finding suggests a long-term but cryptic presence of B. thailandensis in the southern United States, perhaps in water. It is unknown how long B. thailandensis can persist in water, but B. pseudomallei can survive in water for >16 years without nutrients (14).
Our study provides valuable information regarding B. thailandensis occurrence and the potential of water to serve as a reservoir and source of infection for this opportunistic pathogen in the southern United States and Puerto Rico, especially following flooding events. Because likely autochthonous melioidosis cases also have been reported from Texas (15), Puerto Rico (10), and Mississippi (11), clinicians should be aware of the potential of misidentifying B. thailandensis as B. pseudomallei because of their morphologic and biochemical similarities.
Additional information about Burkholderia thailandensis found in the environment in the United States.
Acknowledgments
We thank the Mississippi State Health Department, the Oklahoma Department of Health, animal health technicians from the US Department of Agriculture Animal and Plant Health Inspection Service, Veterinary Services in Puerto Rico, and undergraduate researchers at Northern Arizona University.
Funding for this project was provided by the Centers for Disease Control and Prevention through award nos. 75D30118C00594 and 75D30119P05696.
Biography
Dr. Hall is a research scientist at the Pathogen and Microbiome Institute in Flagstaff, Arizona, USA, and has conducted research on Burkholderia pseudomallei for 10 years. She has conducted environmental surveys for B. pseudomallei and other Burkholderia spp. in the United States in Arizona, Louisiana, Florida, Puerto Rico, Texas, and the US Virgin Islands, and in Australia.
Footnotes
Suggested citation for this article: Hall CM, Stone NE, Martz M, Hutton SM, Santana-Propper E, Versluis L, et al. Burkholderia thailandensis isolated from the environment, United States. Emerg Infect Dis. 2023 Mar [date cited]. https://doi.org/10.3201/eid2903.221245
References
- 1.Glass MB, Gee JE, Steigerwalt AG, Cavuoti D, Barton T, Hardy RD, et al. Pneumonia and septicemia caused by Burkholderia thailandensis in the United States. J Clin Microbiol. 2006;44:4601–4. 10.1128/JCM.01585-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu B, Tong X, He H, Yang Y, Chen H, Yang X, et al. Misidentification of Burkholderia pseudomallei, China. Emerg Infect Dis. 2021;27:964–6. 10.3201/eid2703.191769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brett PJ, DeShazer D, Woods DE. Burkholderia thailandensis sp. nov., a Burkholderia pseudomallei-like species. Int J Syst Bacteriol. 1998;48:317–20. 10.1099/00207713-48-1-317 [DOI] [PubMed] [Google Scholar]
- 4.Gee JE, Elrod MG, Gulvik CA, Haselow DT, Waters C, Liu L, et al. Burkholderia thailandensis isolated from infected wound, Arkansas, USA. Emerg Infect Dis. 2018;24:2091–4. 10.3201/eid2411.180821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morici LA, Heang J, Tate T, Didier PJ, Roy CJ. Differential susceptibility of inbred mouse strains to Burkholderia thailandensis aerosol infection. Microb Pathog. 2010;48:9–17. 10.1016/j.micpath.2009.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall CM, Busch JD, Shippy K, Allender CJ, Kaestli M, Mayo M, et al. Diverse Burkholderia species isolated from soils in the southern United States with no evidence of B. pseudomallei. PLoS One. 2015;10:e0143254. 10.1371/journal.pone.0143254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ginther JL, Mayo M, Warrington SD, Kaestli M, Mullins T, Wagner DM, et al. Identification of Burkholderia pseudomallei near-neighbor species in the Northern Territory of Australia. PLoS Negl Trop Dis. 2015;9:e0003892. 10.1371/journal.pntd.0003892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birnie E, van ’t Hof S, Bijnsdorp A, Mansaray Y, Huizenga E, van der Ende A, et al. Identification of Burkholderia thailandensis with novel genotypes in the soil of central Sierra Leone. PLoS Negl Trop Dis. 2019;13:e0007402. 10.1371/journal.pntd.0007402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall CM, Romero-Alvarez D, Martz M, Santana-Propper E, Versluis L, Jiménez L, et al. Low risk of acquiring melioidosis from the environment in the continental United States. PLoS One. 2022;17:e0270997. 10.1371/journal.pone.0270997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall CM, Jaramillo S, Jimenez R, Stone NE, Centner H, Busch JD, et al. Burkholderia pseudomallei, the causative agent of melioidosis, is rare but ecologically established and widely dispersed in the environment in Puerto Rico. PLoS Negl Trop Dis. 2019;13:e0007727. 10.1371/journal.pntd.0007727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Melioidosis locally endemic in areas of the Mississippi Gulf Coast after Burkholderia pseudomallei isolated in soil and water and linked to two cases—Mississippi, 2020. and 2022 [cited 2022 Jul 27]. https://emergency.cdc.gov/han/2022/han00470.asp
- 12.Hantrakun V, Thaipadungpanit J, Rongkard P, Srilohasin P, Amornchai P, Langla S, et al. Presence of B. thailandensis and B. thailandensis expressing B. pseudomallei-like capsular polysaccharide in Thailand, and their associations with serological response to B. pseudomallei. Plos Neglect Trop D. 2018;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodgetts K, Kleinecke M, Woerle C, Kaestli M, Budd R, Webb JR, et al. Melioidosis in the remote Katherine region of northern Australia. PLoS Negl Trop Dis. 2022;16:e0010486. 10.1371/journal.pntd.0010486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pumpuang A, Chantratita N, Wikraiphat C, Saiprom N, Day NPJ, Peacock SJ, et al. Survival of Burkholderia pseudomallei in distilled water for 16 years. Trans R Soc Trop Med Hyg. 2011;105:598–600. 10.1016/j.trstmh.2011.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cossaboom CM, Marinova-Petkova A, Strysko J, Rodriguez G, Maness T, Ocampo J, et al. Melioidosis in a resident of Texas with no recent travel history, United States. Emerg Infect Dis. 2020;26:1295–9. 10.3201/eid2606.190975 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional information about Burkholderia thailandensis found in the environment in the United States.


