Abstract
Three-dimensional (3D) dried blood spheroids formed on hydrophobic paper is a new microsampling platform that can stabilize labile molecules in whole blood stored in ambient air at room temperature. In this study, we define the ideal conditions for preparing the dried blood spheroids. The physical morphology of 3D dried blood spheroids is found to be largely impacted by unregulated relative humidity of the surrounding environment. A solution of KOH placed in an enclosed chamber offers a facile way to control humidity. We also report a general polymer coating strategy that serves to stabilize dried biofluids when prepared under ordinary ambient conditions without regulated humidity. Dried blood spheroids coated in xanthan gum polymer exhibited enhanced chemical and physical stability. The same xanthan gum polymer provided chemical stability for 2D dried blood spots when compared with the conventional non-coated samples. We have expanded the application of xanthan gum to less viscous biofluids such as urine to induce an artificial protective barrier that also provides enhanced stability for labile performance enhancing drugs stored at room temperature. The impact of polymer coating on direct biofluid analysis via paper spray mass spectrometry was determined by comparing relative ionization efficiency, percent difference of ionization efficiency, and matrix effects of performance enhancing drugs that were spiked in undiluted raw urine. Acceptable analytical performance was recorded for all three criteria, including high ionization efficiencies that ranged from 77 to 93% in the presence of the xanthan gum polymer.
Graphical Abstract
INTRODUCTION
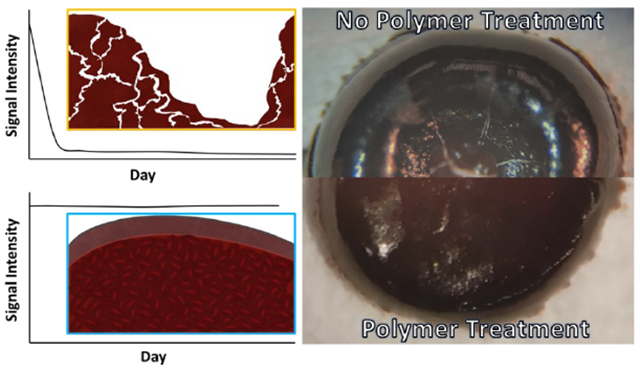
Recently, we introduced the three-dimensional (3D) dried blood spheroid microsampling platform that provides dry-state room temperature storage conditions for labile biological (e.g., enzymes)1 and organic compounds.1–3 It is the only microsampling method that is capable of preserving red blood cells in the dry state by limiting hemolysis when the blood sample is stored at room temperature.2,4–7 This feature is important because it has implications in diagnosing certain diseases like malaria using stored dry blood samples via microscopy which is based on the examination of infected red blood cells. The enhanced stability observed in 3D dried blood spheroids is attributed to the critical radius of insulation mechanism, enabled by a surface thin film, which preserves the bulk interior of the spheroid. The objective of the current work is to carefully delineate sample preparation conditions that facilitate stable formation of blood spheroids on hydrophobic paper. We take insights learned to establish artificial barriers, based on xanthan gum polymer, for less viscous biofluids like urine that lack endogenous biopolymers/bioparticles to form 3D structures on their own when dried in paper. Important experiments were also designed to quantify the effect of the artificial barrier on direct mass spectrometry (MS) analysis of the dried biofluid samples. Therefore, the novelty of the current study includes (1) the development of two complementary field-ready methods to stabilize dried biofluids, (2) the discovery of water-soluble xanthan gum as a matrix for protecting biofluids (blood and urine) at room temperature, and (3) the establishment of quantitative parameters to evaluate the effect of xanthan gum matrix on direct MS analysis.
The use of paper as a sampling medium in the form of 2D dried blood spot (DBS) offers ease of transportation (e.g., non-biohazardous dry-state samples), universal availability, and is cost effective.8 In addition to these advantages, the surface of paper is highly customizable and can be modified through physical and chemical methods to fine-tune the surface parameters.9 However, 2D-DBS suffer from some disadvantages, with the most notable being their cold storage10 requirements and the hematocrit effect,11 which stems from non-uniform distribution of analytes within the core of the paper substrate. By making the surface of paper hydrophobic through gas-phase silanization, we observed the blood samples to bead up as a spherical drop,1,12 which is subsequently adsorbed onto the paper as a 3D blood spheroid. This adsorptive mechanism of microsampling is different from all other cellulose-based blood collection methods, which use an absorptive mechanism to sample blood. While the highly porous nature of hydrophilic paper typically used to create 2D-DBS via absorption is known to denature enzymes, the same porous property predisposes analytes in the blood to oxidative stress, causing labile organic compounds and red blood cells to degrade quickly when stored in ambient air.
The protective mechanism of the 3D dried blood spheroids platform has been characterized recently to involve the formation of a surface barrier/thin film from lysed red blood cells present at the air-blood interface of the spheroid.2 Scanning electron microscopy (SEM) images showed the presence of tightly-packed self-assembled red blood cells, which subsequently led to the formation of the semi-impenetrable passivation thin film at the surface of the spheroid. It is conceivable that instabilities in the formation of this surface film can lead to unwanted analyte degradation. For example, the formation of a diffused or cracked surface thin film can allow air into the interior of the dried blood spheroid, which can cause rapid chemical decomposition. Indeed, aspects of our experiments have shown that placement of 2D DBS samples in vacuum desiccator under air-tight conditions prevented labile analytes (e.g., cocaine and benzoylecgonine) from degradation.1 Rapid degradation occurred when the same samples were left in the open air suggesting oxidative stress from atmospheric air is a major contributor (among other factors) to analyte degradation. We confirmed this through the detection of molecular oxygen adducts of cocaine in dried blood stored in open air.
Therefore, it was essential for us to study the optimal drying conditions to enable the reproducible formation of morphologically stable dried blood spheroids. We present two straightforward approaches to prevent unwanted physical morphological changes during blood spheroid preparation: regulation of humidity and treatment with exogenous polymer solution. More importantly, we take insights learned to create 3D structures from non-viscous biofluids like urine which would otherwise dry as a 2D film that is susceptible to rapid degradation, whether prepared in hydrophilic or hydrophobic paper substrates. The chemical changes in the stored biofluids were monitored through direct paper spray mass spectrometry (MS). In the current study, we evaluated the impact of polymer coating on the direct analysis step in terms of ionization efficiency and matrix effects during ambient ionization processes.
EXPERIMENTAL SECTION
Chemicals and Reagents.
Standard solutions of cocaine (1.0 mg/mL) and cocaine-d3 (100 μg/mL) were obtained from Cerilliant (Round Rock, TX). All solvents (Methanol (99.9%, HPLC grade), Ethyl Acetate (≥ 99.5 %), Acetonitrile (HPLC plus grade)), standard solutions (1.0 mg/mL) of clenbuterol hydrochloride (≥ 95%), clenbuterol-d9 hydrochloride solution, trenbolone solution, nandrolone solution, Glutaraldehyde solution (Grade 1, 25% in water), methyltrichlorosilane (≥ 99.9%) and xanthan gum from Xanthomonas campestris were obtained from Sigma-Aldrich (St. Louis, MO). 18.2 MΩ water was obtained from a Milli-Q water purification system (Millipore, Billerica, MA, USA). Human blood from a healthy single donor was collected in K2 EDTA-capped blood collection tubes and lithium heparin collection tubes from Medline (Northfield, IL) with IRB exemption. All experiments were performed in accordance with the guidelines of the Office of Responsible Research Practices (ORRP) and approved by the ethics committee at The Ohio State University. Informed consents were obtained from human participants of this study. Human urine from the same single donor was collected in 50 mL Falchion tubes (Sarstedt inc., Newton, NC). 1X Phosphate Buffered Saline Tablets and phosphate buffer solution (1.0 M, pH 7.4) were purchased from AMRESCO (Solon, Ohio). Whatman chromatography filter paper (grade 1) was purchased from Whatman (Little Chalfont, England).
Paper Spray Mass Spectrometry.
Mass spectra were recorded using Thermo Scientific LTQ Finnegan linear ion trap mass spectrometer (San Jose, CA). MS parameters used were as follows: 200 °C capillary temperature, three microscans, and 60% Slens voltage. Ethyl acetate was used as spray solvent to avoid solubilization of the water-soluble protecting xanthan gum polymer as well as sample matrix. Spray voltage was 5 kV unless otherwise specified. The paper triangles were held in line with the inlet at a tip-to-inlet distance of 5 mm. Thermo Fisher Scientific Xcalibur 2.2 SP1 software was applied for MS data collecting and processing. Tandem MS (MS/MS) with collision-induced dissociation (CID) was utilized for analyte identification. For the CID experiments, 1.5 Th (mass/charge units) for the isolation window and 18-43% collision energy (manufacturer’s unit) were chosen.
Hydrophobic Paper Preparation.
Precut filter paper was treated in a vacuum desiccator with 0.5 mL of methyltrichlorosilane. Typically, 0.5 mL of the silanization reagent was used for precut paper triangles derived from 4–5 sheets of filter paper. Treatment time was kept at 60 min. A detailed description of this treatment process and time optimization have been described in previous works.12,13 Untreated paper was not subject to this reaction. Paper triangles were 9.5 mm base × 16.6 mm height. Paper squares used in SEM were 10 mm base × 10 mm height. Samples were pipetted onto the paper surface and allowed to dry overnight unless otherwise stated.
Sample Preparation for Stability Studies.
Standard stock solutions of 10 μg/mL cocaine, clenbuterol, and trenbolone were made via serial dilutions and spiked into biofluids to final concentrations of 5 (cocaine) and 2 μg/mL (clenbuterol and trenbolone), respectively. Similarly, internal standard stock solutions were made and spiked separately into spray solvent at a final concentration of 20 ng/mL (clenbuterol-d9 hydrochloride solution), 500 ng/mL (nandrolone solution), and 1 μg/mL (cocaine-d3). Aliquots of spiked sample were then spotted onto paper substrates to final absolute concentrations of 20 ng cocaine/sample spot, 20 ng clenbuterol/sample spot, and 50 ng trenbolone/sample spot. The spotted samples were then stored under ambient conditions to dry for at least 24 h prior to analysis by mass spectrometry. Samples analyzed on day 0 were dried for at least 4 h and analyzed within the same day after the sample has completely dried. Samples to be coated with polymer solution were first air dried for 2 h. A solution of ~5 mg/mL xanthan gum polymer was then used to coat the sample, which were re-dried for at least 2 h prior to analysis. All samples were analyzed using paper spray tandem mass spectrometry by monitoring the following signal transitions: MS/MS transition m/z 304 → 182 for cocaine relative to internal standard transition, cocaine-d3, m/z 307 → 185; MS/MS transition m/z 277 → 259 → 203 for clenbuterol relative to internal standard transition, clenbuterol-d9, m/z 286 → 268 → 204; and MS/MS transition m/z 271 → 227 for trenbolone relative to internal standard transition, nandrolone, m/z 275 → 145.
Sample Preparation for Matrix Effects.
Stock solutions of clenbuterol and trenbolone were first made via serial dilutions to a final concentration of 100 μg/mL. Next, clenbuterol and trenbolone were spiked into pure solution and urine to final concentrations of 2 and 10 μg/mL, respectively. Clenbuterol (8 ng/sample) and trenbolone (40 ng/sample) were spotted onto hydrophilic and hydrophobic paper triangles from both pure solution and urine stock solutions. A subset of each of these samples were then coated with ~5 mg/mL solution of xanthan gum polymer. The signals of clenbuterol and trenbolone were monitored relative to their internal standard spiked separately in spray solvent in MS/MS mode. Samples were air dried for a minimum of 2 h (4 h if polymer coating) and stored at −20 °C until analysis.
Sample Preparation for Relative Ionization Efficiency.
Stock solutions of clenbuterol and trenbolone were first made via serial dilutions to a final concentration of 10 μg/mL. Next, various solutions containing different ratios of the performance enhancing drugs and internal standards (A:IS) were created by mixing the corresponding analyte and internal standard to final concentration ratios of 2:3, 1:1, 3:2, 7:3, and 4:1 in pure solution. The smallest concentration for clenbuterol was 1.6 ng per sample spot while the smallest concentration for trenbolone was 4 ng per sample spot. Then, aliquots of these prepared samples were spotted onto hydrophilic and hydrophobic paper triangles. The samples were air dried for a minimum of 2 h (4 h if polymer coating) and stored at −20 °C until analysis. The samples were extracted with ethyl acetate, 0.1% formic acid and the signal for clenbuterol and trenbolone were monitored relative to their internal standards in MS/MS mode.
RESULTS AND DISCUSSION
Controlling Humidity During the Preparation of Dried Blood Spheroids.
Droplets of whole blood on hydrophobic paper substrate have previously been shown to dry in a series of stages, where the first stage involves the evaporation of water from the droplet of blood.2 The rate of evaporation, which is influenced by ambient relative humidity (RH), affects the resulting morphology and drying time of the spheroid.14 When a blood spheroid dries too quickly, the resulting 3D dried blood spheroid can crack, exposing the interior bulk to environmental stressors.15 To determine the impact of RH on the drying of droplets of whole blood on hydrophobic paper, wet spheroids were stored under various regulated and non-regulated humidity systems and the resulting drying times and morphologies were monitored. Chemical changes induced in the samples were also monitored by paper spray mass spectrometry.
A low (estimated ~55%) and high (estimated ≥ 85%) percent RH were selected for experiments in which we regulated the spheroid drying process. Two additional conditions were employed where RH was not controlled. Here, the wet spheroids were either dried with exposure to open air (in open pipette tip box) or without exposure to open air with samples placed in a closed pipette tip box. Details outlining the preparation of these humidity chambers can be found in the Supporting Information. Under lower humidity conditions, the spheroids dried in ≤ 2 h exhibiting severe cracking and/or cratering as shown in Figure 1A, i. On the contrary, the spheroids dried within 48 h under higher humidity conditions but did not exhibit any cracking or cratering, forming a fully intact 3D dried blood spheroid (Figure 1A, ii). A control sample set was created (without control of RH) and was left to dry under ambient conditions (open box, exposure to surrounding environment). The control sample set dried in about 2 h with cracking and/or cratering (Figure 1B, i). A secondary control was created (without control of RH) and was left to dry under ambient conditions (closed box, no exposure to open air). In this case, the spheroids dried in approximately 2 h with more optimal morphology (Figure 1B, ii) analogous to those dried under high RH. The cracking and cratering can be attributed to buckling instabilities that occur due to a more rapid rate of evaporation, which is a known drying phenomenon of sessile droplets of blood that have not wet the surface to a greater extent.16
Figure 1.

3D dried blood spheroids (10 μL) air-dried under (Ai, ii) controlled relative humidity conditions and (Bi, ii) ambient conditions (e.g., uncontrolled relative humidity). (Ai) 3D dried blood spheroids dried under a low relative humidity of approximately 55%. Cracks and cratering were observed although drying time was reduced to about 1 h. (Aii) 3D dried blood spheroids dried under a high relative humidity of approximately 85%. Little to no cracks are observed for these samples at the cost of longer drying time, which took > 48 h. (Bi) 3D dried blood spheroids dried under ambient storage conditions with the storage box open, exposing the samples to ambient relative humidity. The drying time is approximately 2 h and cracking and cratering are observed. (Bii) 3D dried blood spheroids dried under ambient storage conditions with the storage box closed without exposure to open air for drying. These samples dried in approximately 2 h.
Figure 2.
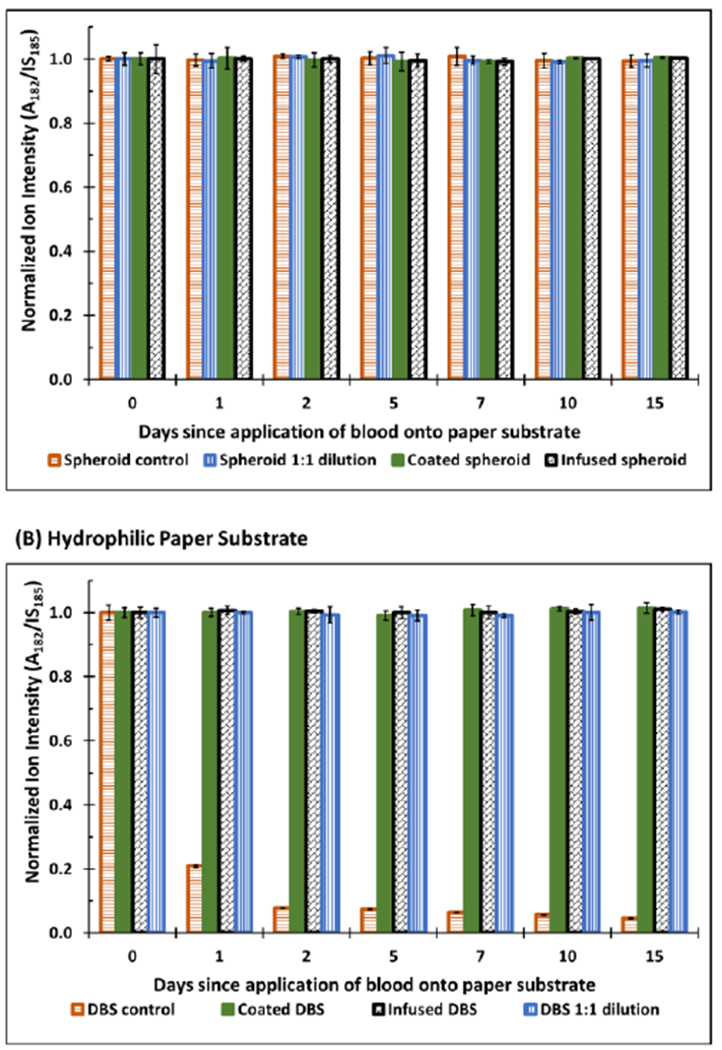
Data showing the stability of cocaine spiked in whole blood (10 μL) on (A) hydrophobic and (B) hydrophilic paper substrates with or without xanthan gum treatment. Different methods of application of polymer were studied to determine if the treatment step affects the resulting enhanced stability. Samples were either diluted 1:1 with exogenous polymer xanthan gum solution (1:1 dilution), coated with exogenous xanthan gum solution after air-drying (coated), or infused with xanthan gum immediately after being deposited onto paper substrates (infused). Corresponding information pertaining to %RSD (<10%) may be found in the Supplementary Information (Table S1)
As demonstrated through various storage conditions, ideal ambient drying conditions are not always feasible, especially in the case of field collection or in an environment where the humidity cannot be regulated. Samples stored under non-regulated drying conditions (e.g., no control of relative humidity and closed box with no exposure to surrounding air) can still yield spheroids that are cracked if the RH is low in the surrounding storage environment. It is important to note that while chemical stability of molecules depend on the presence of a thin film, the morphological structure of the spheroid itself also contributes to this factor. That is, structural artifacts in the spheroid sample (e.g., cracks) can result in decreased stability of molecules stored in the dried blood spheroid. Example is shown in Figure S1 where the induction of cracks in spheroids resulted in rapid analyte degradation although the surface thin film was present. The use of a KOH humidifying solution in a simple pipette box can serve to provide a convenient way to prepare dried blood spheroids in the open field during sample collection to reduce the probability of the forming structural artifacts in the dried blood spheroid samples.
Polymer-Fortified Dried Blood Spheroids.
Aside from humidity control, we further explored the use of exogenous polymer to control cracking and cratering during spheroid preparation. In this case, a suitable polymer should be highly soluble in water to enable effective mixing with biofluids, present minimal chemical and physical interference during analyte extraction/analysis, as well as having physical rigidity to form 3D scaffolding after drying. After initial investigation, we identified xanthan gum as an excellent polymer for stabilization of labile compounds in biofluids. Our preliminary experiments involved the testing of two other polymers: polyvinylpyrrolidine (PVP) and chitosan. PVP and chitosan were not suitable polymers for this platform because of several factors. First, PVP and chitosan exhibited partial or no dissolution in water. Second, they exhibited less physical rigidity where biofluids fortified with PVP and chitosan easily removed from the surface of paper. Thus, PVP and chitosan were not structurally viable for transportation and storage in paper envelope. Importantly, PVP and chitosan were unable to provide enhanced stability for cocaine in blood (data not shown), when stored under room temperature storage conditions.
The process for polymer coating is described in the Supporting Information (Figures S2 and S3). When xanthan gum solution was added on top of the air-dried samples, the dried samples were re-dissolved with concomitant mixing, forming a new sample spot with a relatively larger surface area. This process was characterized using methylene blue dye as a model system (Figure S4). The resultant thin film of polymer encases the sample and provides an artificial protective layer that protects the inner bulk from environmental stressors. To investigate the efficacy of this artificial protective layer, we used MS to monitor the stability of cocaine in 3D dried blood spheroids and 2D-DBS. The results from these experiments are summarized in Figure 2 where a stable cocaine signal was obtained for all samples prepared on hydrophobic paper (Figure 2A) and polymer-coated samples on hydrophilic paper (Figure 2B). On the contrary, the signal for cocaine in non-coated 2D-DBS samples prepared on hydrophilic paper degraded quickly (within two days) after room temperature storage (brown trace in Figure 2B). Notice how cocaine stability is maintained upon application of the xanthan gum polymer solution on the same samples prepared in hydrophilic paper. From these results, it was evident that the polymer coating had provided an artificial protective barrier similar to the naturally occurring barrier observed in 3D dried blood spheroids that helps reduce the rate at which the sample degrades. We expect a similar mechanism of critical radius of insulation to provide this enhanced stabilization, where the polymer coating limits the influx ambient air into the interior of the dried samples. As will be discussed later, the presence of the xanthan gum does present some matrix effects during paper spray, so the lower signal detected here for the bare paper substrate cannot be attributed to inefficient extraction at longer storage periods. The xanthan gum polymer coating is less susceptible to humidity changes as shown in the enhanced stability observed when sample is stored under unregulated ambient conditions.
Investigating the Mode of Applying Polymer Solution on Spheroids.
Overall, we investigated three methods of polymer application. We sought to determine if the mode of polymer solution application has any influence on the effectiveness of the resulting artificial protective barrier. The application methods investigated include (1) coating of a completely air-dried sample with polymer solution (Figure S5), (2) infusion of polymer into a freshly spotted blood sample (1:1 %v/v, sample volume/polymer solution, Figure S6)), and (3) dilution of whole blood with polymer solution (1:1 %v/v, sample volume/polymer solution, Figure S7) followed by spotting of sample onto paper substrate and subsequent air-drying prior to analysis. The samples were subsequently characterized using SEM. Sample preparation steps for these experiments are detailed in the Supporting Information.
While several notable differences in physical morphology of the blood samples were observed from the SEM images, each method conferred enhanced stability to cocaine prepared in 2D-DBS samples. Polymer coating of a completely air-dried sample leads to a sample spot with a greater surface area when compared to the non-coated sample (Figure S5Ai and Aii). The 3D blood spheroid samples were found to maintain their natural protective barrier while also having an artificial barrier on the exterior surface (Figure S5C). Infusion of polymer solution into freshly deposited 3D blood spheroids resulted in an inhomogeneous mixture of blood and polymer, with localized dense patches of polymer solution (Figure S6Dii). The polymer coating helps maintain red blood cell morphology on the exterior surface, which normally undergo hemolysis after 80 min of air drying (Figure S6Di).2 This is important because it shows that the presence of the polymer has no detrimental effect of the chemical composition of the spheroid, including limited lysing of red blood cells.
Fresh DBS samples that were infused with polymer solution resulted in localized regions in which the polymer sample was deposited (Figure S6C). The reduced rate of wetting is likely due to reduced flow of blood at the center of the wet blood spot in addition to the highly viscous nature of the polymer solution. Interestingly, intact red blood cells were observed within the pores of the paper substrate for 2D DBS samples after 2 h of storage. This makes sense since the blood was not allowed to dry before depositing the polymer solution. Note: for traditional 2D DBS samples (without coating), red blood cells lyse within 10 min after drying in ambient air.2 Dilution of whole blood with polymer followed by sample deposition onto hydrophilic paper substrate resulted in a more homogenous mixture of blood and polymer when compared to the infused samples, where the polymer solution was drop-cast onto blood already deposited in the paper. The exterior surface of the resultant 3D scaffold of the dried blood prepared by sample dilution with polymer exhibited an artificial polymer barrier that protects red blood cells that are observable near the surface (Figure S7D). We observed a thin film barrier of approximately 1.3 μm that coated the surface to offer protection of sample beneath (Figure S8A). Here too, intact red blood cells are observed after 2 h of drying under room temperature conditions (Figure S8B).
The main differences in sample preparation of polymer-treated samples were total drying times required for the 2D and 3D samples, prepared on hydrophilic and hydrophobic paper substrates, respectively. Generally, the 2D coated samples dried faster than the polymer-coated 3D samples. This implies, ideally, different methods of applying the polymer solution must be used for 2D versus 3D samples. Intrinsically, the coating method has a longer total drying time of 4 h while the dilution and infusion methods only required 2 h due to the rewetting processes. Overall, the coating of dried blood with polymer solution represented the most ideal method for stabilizing the 3D dried spheroids. This method does not rely on a specific volume of polymer solution and instead only requires that the sample is fully coated. In addition to the polymer treatment method, it is important to use extraction solvent that can penetrate the polymer-coated samples, while also avoiding the re-dissolution of polymer. In this work, ethyl acetate was selected as an extraction/spray solvent since it is immiscible with water and can penetrate approximately 34% into the core of a spheroid.1 The induced artificial barrier was found to provide enhanced stability to samples stored under room temperature. From experimental data collected, the methods of infusion and prior sample dilution are ideal for preserving red blood cells in 2D-DBS while also enhancing chemical stability of analytes that rapidly degrade under room temperature storage. While the method of drop-casting polymer solution onto a previously dried 2D blood sample provides enhanced chemical stability, it does not provide physical protection to red blood cells. Therefore, microsampling platforms that utilize the absorption mechanism (i.e., 2D samples on hydrophilic paper substrate) would benefit more from the method of dilution and infusion of polymer onto freshly prepared blood spots.
Polymer-Fortified Dried Urine Spots.
Using insights gained from these studies, the polymer coating methodology was extended to dried urine spots (DUS) to facilitate storage under room temperature conditions to alleviate cold storage requirements. The storage and transportation of urine specimens is a major concern in drug testing. With increasing interest in self-reported drug use, it becomes necessary to collect samples away from laboratory settings. For remote areas lacking cold storage conditions, DUS prepared on paper substrates can serve as a viable method compared with liquid samples. That is, although dried urine samples have higher stability than solution-phase samples, the DUS still require cold temperatures for long-term storage.17,18 An opportunity to provide long-term storage at room temperature for DUS is important because urine is most often the preferred sample for drug testing due to ease of collection and the fact that the concentrations of drugs and metabolites tend to be higher than in blood, which in turn can provide lower detection limits.19
Urine collection on filter paper is limited by two main factors: the first involves stability – the method is generally considered viable when the DUS samples are kept frozen or refrigerated;20 (2) the second challenge involves the elution of analyte from the hydrophilic paper substrate – this typically requires excessive dilution, something that necessitates the use of highly sensitive analytical methods for detection.21 Analytical challenges associated with dilution can be reduced by employing paper spray (PS) MS, which allows direct analyte detection from the dried matrix spot without the need for prior extraction steps.
We have previously attempted to stabilize urine samples spiked with performance enhancing drug stored in the dry state and stored on hydrophobic paper at room temperature.13 For all analytes screened in the previous work, rapid degradation was observed within the first five days of room temperature storage except for hydrochlorothiazide.13 This is not surprising because although urine forms a spherical drop when applied on a hydrophobic paper substrate, it dries as a 2D thin film due to the absence of biomacromolecules such as red blood cells or any other component that would form a 3D structure. Data collected in this study using polymer-coated dried blood spheroids and DBS samples have provided critical insight that led us to apply the polymer coating to DUS to induce an artificial barrier to provide enhanced protection at room temperature.
As a novel paper-based method for urine sampling, the xanthan gum-based dried matrix spot not only stabilizes labile compounds in urine, but also enables direct analysis of the stored samples without any major pre-treatment steps. For the DUS experiments in this work, the drying time was kept at a consistent 2 hours since the molecules are stable for > 4 h, but it should be noted that DUS and DBS typically dry in < 0.5 h. These samples are generally dried overnight at room temperature storage before subject to sample clean-up and analysis. The stability of two selected performance enhancing drugs, trenbolone and clenbuterol, were investigated for polymer coated and non-coated DUS on hydrophilic paper substrates. Figures 3A and 3B show the positive-ion mode paper spray mass spectra for 20 ng and 50 ng of clenbuterol and trenbolone, respectively, analyzed directly from DUS samples that had been coated with xanthan polymer. As can be observed, the pseudo-molecular ions [M+H]+ were detected at m/z 277 and 271, respectively, for clenbuterol and trenbolone. It should be noted that analysis of undiluted raw urine is not a trivial task and often requires prior analyte extraction steps.22 The complexity of the sample increased when xanthan gum was added, as shown in the background noise detected in the upper mass range of the spectrum (Figure 3) The corresponding mass spectra for the analysis of clenbuterol and trenbolone spiked in urine without xanthan gum treatment are provided in Figure S9.
Figure 3.
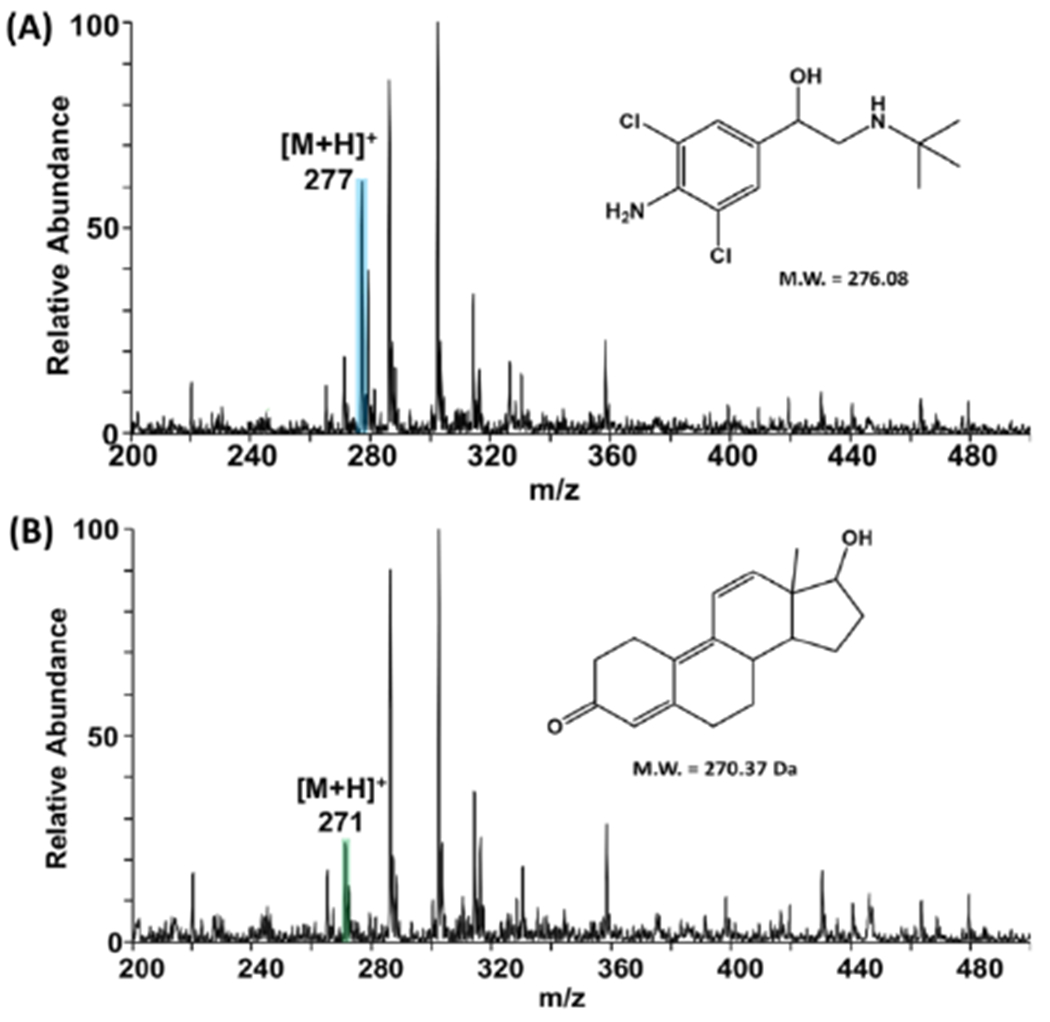
Typical positive-ion mode PS MS full mass spectra obtained for (A) clenbuterol and (B) trenbolone spiked in urine spotted on hydrophilic paper coated with xanthan gum polymer. Corresponding MS/MS spectra for clenbuterol and trenbolone are provided in Supporting Information (Figure S9).
The stability of clenbuterol and trenbolone in the DUS samples was subsequently evaluated, with and without polymer coating, over a two-week period by monitoring tandem MS signal for each analyte (see Figure S10A and B for clenbuterol and Figure S10C for trenbolone). All DUS samples containing xanthan gum coating exhibited greater stability than those samples without polymer coating. Clenbuterol present in DUS without xanthan coating rapidly degraded completely within the first two days of storage at room temperature (Figure 4A). Trenbolone on the other hand degraded slowly in DUS prepared without xanthan coating; even here, the signal reduced to approximately 30% after 7 days of storage (Figure 4B). Storage of non-coated DUS at room temperature for more than 10 days resulted in complete degradation of trenbolone. This effect was reversed when the same DUS samples were stored under similar conditions in the presence of xanthan polymer where only a small reduction in trenbolone signal was detected after day 8. These results confirm the protective power of artificial surface layers.
Figure 4.
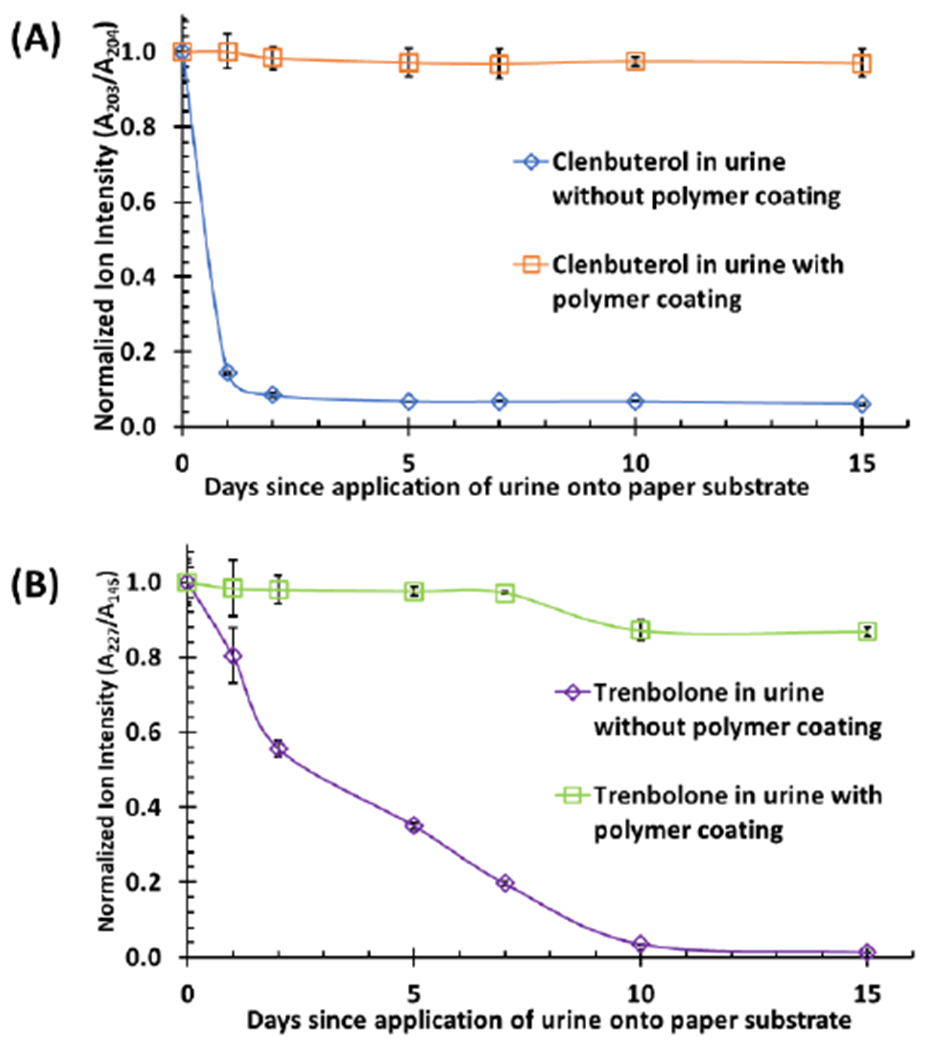
(A) Stability of dried urine spot containing 20 ng clenbuterol stored on hydrophilic paper, with and without xanthan gum polymer coating. The spray solvent (ethyl acetate with 0.1% formic acid) used for online extraction and paper spray contained 20 ng/mL clenbuterol-d9 internal standard (IS, transition, m/z 286 → 268 → 204). (B) Stability of dried urine spot containing 50 ng trenbolone stored on hydrophilic paper, with and without polymer coating. 1 μg/mL nandrolone (transition: m/z 275 → 145) in ethyl acetate with 0.1% formic acid was used as an internal standard (IS). All signal (A/IS) for each storage time was normalized to A/IS of day zero (0). Corresponding information pertaining to %RSD (<10%) may be found in the Supplementary Information (Table S1).
Investigation of the Effects of Xanthan Gum on Direct MS Analysis.
This work reports the first use of xanthan gum polymer as an additive in paper spray, so it was important to investigate any possible matrix effects this polymer might introduce during the analysis step. As a simpler matrix, DUS presented a more suitable system for this purpose than dried blood.
Overall, we compared three related parameters: relative ionization efficiency (obtained via a calibration curve), percent difference (via comparison of slopes from ionization efficiency curves), and matrix effects (by comparing absolute analyte signal in urine versus pure solutions). Relative ionization efficiency was determined through a calibration curve comparing different ratios of analyte and internal standard mixed together. The resulting slope of each plot (Figure S11) was used to determine the relative ionization efficiency,23,24 as summarized in Table 1. This data describes the ease of ionizing the analyte with respect to the internal standard from each paper substrate (hydrophobic versus hydrophilic paper). It is important to note that it is hard to separate the ionization efficiency and recovery processes from online extraction of paper-based platforms.25 Although the two processes are indiscernible in this experiment, this relationship provides an indirect way to estimate the specific effect the xanthan gum polymer has on the extraction and analysis steps. To determine the impact of polymer on the ionization efficiency/recovery, we compared data derived from DUS prepared in the absence of xanthan gum versus dried matrix spot that consisted of both urine and xanthan gum polymer in the dry sample spot. The percent difference between these two groups of urine samples provided insight on interferences exerted by xanthan gum in paper spray.
Table 1.
Relative ionization efficiencies as derived from comparison between samples coated with xanthan gum polymer and those without the polymer stored on hydrophobic and hydrophilic paper substrates.
| Analyte* | Relative Ionization Efficiency (%) (Slope from A/IS versus concentration curve) ‡ |
|||
|---|---|---|---|---|
| DUS on Hydrophobic Paper | DUS on Hydrophilic Paper | |||
| Without Xanthan |
With Xanthan |
Without Xanthan |
With Xanthan |
|
| Clenbuterol | 92.07 ± 1.26 | 82.41 ± 1.53 | 70.70 ± 0.41 | 58.10 ± 0.72 |
| Trenbolone | 93.34 ± 1.38 | 76.98 ± 1.58 | 88.00 ± 0.37 | 70.36 ± 1.26 |
Clenbuterol-d9 used as internal standard for clenbuterol, and nandrolone as internal standard for trenbolone.
See Figure S11 for raw data concerning analyte (A)/IS versus concentration plots.
The ionization efficiency of the analyte is equal to that of the internal standard if the slope of the A/IS signal versus concentration curve is equal to unity (1) (i.e., direct correlation between changes in analyte concentration and internal standard concentrations). We observed slopes of 0.92 and 0.93 for clenbuterol/clenbuterol-d9 and trenbolone/nandrolone analyte-internal standard pairs, respectively, when the DUS samples were prepared on hydrophobic paper substrate in the absence of xanthan gum polymer. These results suggest that there likely are discrepancies in extraction and/or analysis steps. In the presence of xanthan gum matrix, the slopes determined for the same analyte/internal standard pairs reduced to 0.82 and 0.77, respectively (Table 1). These represent a percent difference of 10% and 18% for clenbuterol and trenbolone analytes, respectively. Clearly this effect represents a hindrance in extraction and/or transportation posed by the presence of the xanthan gum polymer. The larger percent difference observed for trenbolone may reflect the significant structural difference when compared with the selected internal standard, nandrolone, which subsequently results in marked difference in their interaction with the xanthan gum polymer. Interestingly, significant deviation of slopes from unity was observed for DUS samples prepared on hydrophilic paper substrates. In the absence of xanthan gum polymer, recorded slopes were 0.71 and 0.88 for clenbuterol/clenbuterol-d9 and trenbolone/nandrolone pairs, respectively, when the DUS samples were prepared on hydrophilic paper substrate.
Since the aqueous urine sample completely wets the hydrophilic paper, these results suggest that interactions between the analytes in sample and paper substrate (e.g., paper treatment agents such as sizing agents, paper acidity or presence of metals) can negatively impact the analysis. These effects can significantly be reduced by preventing the urine sample from wetting the paper and absorbing into the core of the paper. The slopes recorded for hydrophilic paper further decreased to 0.58 and 0.70, respectively, for clenbuterol and trenbolone when DUS is coated with xanthan polymer. Here too, we calculated percent differences of 18% and 20%; these values compare well with those derived from hydrophobic paper substrates (Table S2). Since it is often challenging to differentiate ionization and recovery processes, the good agreements obtained here support the interpretation that the percent difference parameter can be used to estimate the effect of the xanthan gum matrix on ion yield during paper spray.
To further validate this explanation, we investigated matrix effects that might come from the urine sample itself due to the presence of endogenous interfering compounds. Here, we compared the absolute analyte signal from DUS samples to analyte signal derived from dried analyte spots prepared from pure solutions. The results of this experiment are summarized in Table S3, which suggest that in terms of absolute analyte signal, the urine sample does not present major matrix effects when allowed to dry on hydrophobic paper substrates. That is, we recorded ratios of ion signal from urine samples (Iurine) to signal from pure samples (Ipure) to be greater than 80% (i.e., Iurine/Ipure × 100) for samples prepared on hydrophobic paper. On the contrary, 55% was calculated for samples prepared on hydrophilic paper. Again, this confirms that the filter paper substrate presents a major challenge (e.g., interference between analyte in sample and paper substrate) in paper spray. The use of the hydrophobic paper not only provides an efficient means to prepare 3D biofluid scaffolds to preserve analyte integrity in the dry state but also as a sensitive method for direct MS analysis of the stored samples without significant dilution.12 Further development of this field-amenable paper-based platform will combine the fundamental insights from this study with our recently described embossment strategy26 to expand applications to clinical samples. For example, on-going studies involve the analysis of collected samples for the detection of malaria metabolites.
CONCLUSION
In summary, we have delineated experimental conditions that lead to the stable formation of 3D dried blood spheroids without morphological changes such as cracks and cratering during the drying of whole blood in ambient air. In the absence of regulated storage conditions, such cracks/cratering were found to cause rapid degradation of labile cocaine which is due to the influx of atmospheric air into the interior of the dried blood. The use of off-the-shelf KOH solutions allowed effective control of humidity that were found to be mainly responsible for the physical changes in the dried blood spheroids. The chemical stability observed for species present inside the 3D dried blood spheroid is attributed to the critical radius of insulation, only possible when a semi-impenetrable thin film is formed at the surface of the spheroid.
Guided by insight, we identified a water-soluble xanthan gum polymer that offered a facile mechanism to form stable spheroids irrespective of the relative humidity of the surrounding environment where the blood sample has been processed. The xanthan gum polymer preserved not only the stability of chemical species in the blood sample, but also stabilized red blood cells in the dry state and at room temperature. This result is comparable to the natural protective barriers that form in 3D dried blood spheroids on hydrophobic paper substrate. Stabilization of the 3D biofluid scaffold is important in preventing exposure of the interior bulk to environmental stressors that may initiate degradation of sample. The efficacy of artificially induced protective barriers from polymer coating was investigated for both whole blood and non-viscous raw urine samples. For blood samples, we studied the stability of both the traditional 2D dried blood spots prepared in hydrophilic paper substrate and 3D dried blood spheroids prepared in hydrophobic paper. In both cases, the xanthan gum polymer-coating method enabled enhanced stability of cocaine, clenbuterol, and trenbolone. This is contrary to non-coated 2D dried blood spot samples, which degraded rapidly within 2 days.
With raw urine representing a less complex biological matrix than whole blood, it enabled us to readily quantify the effect of the presence of xanthan gum on direct paper spray MS analyses of the dried biofluid samples. This was evaluated through three important experiments: 1) the examination of relative ionization efficiency, which was achieved through careful calibration, 2) a novel percent difference parameter that compared the slopes from ionization efficiency curves; this parameter was important to quantify the specific effect of the polymer matrix, and 3) validation experiment that independently assessed matrix effects via the comparison of absolute analyte signal in urine that derived from pure solutions.
Supplementary Material
ACKNOWLEDGMENT
This research was supported by National Institute of Health (Grant Number R01-AI-143809), Sloan Foundation (Grant Number FG-2020-12734), and National Science Foundation (Award Number CHE-1900271). Electron microscopy was performed at the Center for Electron Microscopy and Analysis (CEMAS).
Footnotes
Supporting Information
Experimental details are provided in the Supporting Information that includes hydrophobic paper preparation, sample preparation for stability studies, matrix effects and relative ionization efficiency of performance enhancing drugs in DUS, procedure for spheroid fixation in glutaraldehyde solution, SEM parameters, humidity chamber preparation, tandem mass spectra, and additional discussion on application of polymer methodologies.
The authors declare no competing financial interest.
REFERENCES
- (1).Damon DE; Yin M; Allen DM; Maher YS; Tanny CJ; Oyola-Reynoso S; Smith BL; Maher S; Thuo MM; Badu-Tawiah AK Dried Blood Spheroids for Dry-State Room Temperature Stabilization of Microliter Blood Samples. Anal. Chem 2018, 90 (15), 9353–9358. [DOI] [PubMed] [Google Scholar]
- (2).Frey BS; Damon DE; Allen DM; Baker J; Asamoah S; Badu-Tawiah AK Protective Mechanism of Dried Blood Spheroids: Stabilization of Labile Analytes in Whole Blood, Plasma, and Serum. Analyst 2021, 146, 6780–6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Swiner DJ; Jackson S; Durisek GR; Walsh BK; Kouatli Y; Badu-Tawiah AK Microsampling with Cotton Thread: Storage and Ultra-Sensitive Analysis by Thread Spray Mass Spectrometry. Analytica Chimica Acta 2019, 1082, 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Field JW Blood Examination and Prognosis in Acute Falciparum Malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 1949, 43 (1), 33–48. [DOI] [PubMed] [Google Scholar]
- (5).Shute PG; Maryon M A Satisfactory Technique for Storing and Staining Malaria and Other Parasites in Old Blood Films. Transactions of the Royal Society of Tropical Medicine and Hygiene 1960, 54 (5), 415–418. [Google Scholar]
- (6).Singh J; Ray A; Nair C A Preliminary Note on the Preservation of Unstained Blood Smears. Indian Journal of Malariology 1949, 3 (4), 327–329. [Google Scholar]
- (7).Field JW The Microscopic Diagnosis of Human Malaria, Part I. Study No. 23 from the Institute for Medical Research, Federation of Malaya, 1948. [Google Scholar]
- (8).Akyazi T ; Basabe-Desmonts L; Benito-Lopez F Review on Microfluidic Paper-Based Analytical Devices towards Commercialisation. Analytica Chimica Acta 2018, 1001, 1–17. [DOI] [PubMed] [Google Scholar]
- (9).Frey BS; Damon DE; Badu-Tawiah AK Emerging Trends in Paper Spray Mass Spectrometry: Microsampling, Storage, Direct Analysis, and Applications. Mass Spec Rev 2020, 39(4), 336–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Batterman S; Chernyak S Performance and Storage Integrity of Dried Blood Spots for PCB, BFR and Pesticide Measurements. Science of The Total Environment 2014, 494–495, 252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Delahaye L; Veenhof H; Koch BCP Alternative Sampling Devices to Collect Dried Blood Microsamples: State-of-the-Art. Ther Drug Monit 2021, 43 (3), 12. [DOI] [PubMed] [Google Scholar]
- (12).Damon DE; Davis KM; Moreira CR; Capone P; Cruttenden R; Badu-Tawiah AK Direct Biofluid Analysis Using Hydrophobic Paper Spray Mass Spectrometry. Anal. Chem 2016, 88 (3), 1878–1884. [DOI] [PubMed] [Google Scholar]
- (13).Rossini EL; Kulyk DS; Ansu-Gyeabourh E; Sahraeian T; Pezza HR; Badu-Tawiah AK Direct Analysis of Doping Agents in Raw Urine Using Hydrophobic Paper Spray Mass Spectrometry. J. Am. Soc. Mass Spectrom 2020, 31 (6), 1212–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Bou Zeid W; Brutin D Influence of Relative Humidity on Spreading, Pattern Formation and Adhesion of a Drying Drop of Whole Blood. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2013, 430, 1–7. [Google Scholar]
- (15).Bou Zeid W; Vicente J; Brutin D Influence of Evaporation Rate on Cracks’ Formation of a Drying Drop of Whole Blood. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2013, 432, 139–146. [Google Scholar]
- (16).Sobac B; Brutin D Desiccation of a Sessile Drop of Blood: Cracks, Folds Formation and Delamination. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2014, 448, 34–44. [Google Scholar]
- (17).Palmer EA; Cooper HJ; Dunn WB Investigation of the 12-Month Stability of Dried Blood and Urine Spots Applying Untargeted UHPLC-MS Metabolomic Assays. Anal. Chem 2019, 91 (22), 14306–14313. [DOI] [PubMed] [Google Scholar]
- (18).Breier AC; Cé J; Coelho JC Correlation of the Levels of Glycosaminoglycans between Urine and Dried Urine in Filter Paper Samples and Their Stability over Time under Different Storage Temperatures. Clinica Chimica Acta 2014, 433, 49–53. [DOI] [PubMed] [Google Scholar]
- (19).Tests for Drugs of Abuse. The Medical Letter on Drugs and Therapeutics 2002, 44 (1137), 71–73. [PubMed] [Google Scholar]
- (20).DuBey IS; Caplan YH The Storage of Forensic Urine Drug Specimens as Dry Stains: Recovery and Stability. Journal of Forensic Sciences 1996, 41 (5), 845–850. [PubMed] [Google Scholar]
- (21).Meany DL; Clarke W Opiate DAU Screening Using Dried Urine Specimens. Clinica Chimica Acta 2009, 401 (1–2), 188–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Huang G; Chen H; Zhang X; Cooks RG; Ouyang Z Rapid Screening of Anabolic Steroids in Urine by Reactive Desorption Electrospray Ionization. Anal. Chem 2007, 79 (21), 8327–8332. [DOI] [PubMed] [Google Scholar]
- (23).Zhou W; Yang S; Wang PG Matrix Effects and Application of Matrix Effect Factor. Bioanalysis 2017, 9 (23), 1839–1844. [DOI] [PubMed] [Google Scholar]
- (24).Chen S; Wan Q; Badu-Tawiah AK Picomole-Scale Real-Time Photoreaction Screening: Discovery of the Visible-Light-Promoted Dehydrogenation of Tetrahydroquinolines under Ambient Conditions. Angewandte Chemie International Edition 2016, 55 (32), 9345–9349. [DOI] [PubMed] [Google Scholar]
- (25).Vega C; Spence C; Zhang C; Bills BJ; Manicke NE Ionization Suppression and Recovery in Direct Biofluid Analysis Using Paper Spray Mass Spectrometry. Journal of The American Society for Mass Spectrometry 2016, 27 (4), 726–734. [DOI] [PubMed] [Google Scholar]
- (26).Frey BS; Heiss DR; Badu-Tawiah AK Embossed Paper Platform for Whole Blood Collection, Room Temperature Storage, and Direct Analysis by Pinhole Paper Spray Mass Spectrometry. Anal. Chem 2022, 94 (10), 4417–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


