Abstract
Although alcohol expectancies and subjective response are independent predictors of drinking, social–cognitive theory suggests that expectancies may distort one’s subjective response, creating discrepancies between expected and actual alcohol effects. A recent cross-sectional study found that unmet expectancies (using difference scores) were associated with heavier drinking. However, cross-sectional data cannot establish temporal precedence, and using difference scores ignores important conditional main effects. As such, the current study sought to evaluate how expectancy-subjective response discrepancies predict future drinking using prospective data and an interaction approach. Participants (N = 258) were randomly assigned to consume alcohol (target BAC = .08%) within a placebo-controlled alcohol administration session. Alcohol expectancies and subjective response were assessed across the full valence by arousal affective space using parallel measures. Results indicated a significant high arousal positive (HIGH+) interaction, such that, as HIGH+ expectancies increased, individuals at low and mean levels of HIGH+ subjective response drank more heavily 12 months later. There was also a significant high arousal negative (HIGH−) interaction with a similar pattern of moderated effects. No interactions were found for low arousal effects. These results indicate that individuals with unmet HIGH+ and HIGH− expectancies drink more heavily 12 months later, controlling for prior drinking. This suggests that clinicians may consider recommending specific interventions (e.g., expectancy challenges vs. pharmacotherapy) based upon an individual’s levels of expectancies and subjective response to optimize intervention efficacy.
Keywords: alcohol expectancies, subjective response, heavy drinking, social learning
Heavy drinking is a major public health concern, as it is the third leading cause of preventable death in the United States and is associated with negative outcomes such as risky sexual behavior, drunk driving, physical injuries, and alcohol use disorders (AUDs; e.g., Chassin, Pitts, & Prost, 2002; Gmel, Kuntsche, & Rehm, 2011; Perkins, 2002; Rehm, Shield, Joharchi, & Shuper, 2012; Wechsler, Davenport, Dowdall, Moeykens, & Castillo, 1994, 1995). Thus, it is important to understand prospective risk factors to target via prevention and early intervention efforts. Both expecting positive alcohol effects, referred to as alcohol expectancies (e.g., I will become sociable, fun) and pharmacological responses to alcohol (e.g., increased stimulant/dampened sedative subjective response) are prospective predictors of heavy drinking (e.g., Jones, Corbin, & Fromme, 2001; King, de Wit, McNamara, & Cao, 2011, King, Hasin, O’Connor, McNamara, & Cao, 2016; Wiers, van Woerden, Smulders, & de Jong, 2002). However, the two are primarily examined separately, ignoring potentially additive or interactive effects. This is an important limitation as correlations between expectancies and their subjective response counterparts are modest (Fridberg et al., 2017; Morean, Corbin, & Treat, 2015), and expectancies, when modeled alone, may provide a distorted, overestimation of an individual’s true subjective response when drinking. The few studies that have investigated the implications of such discrepancies are cross-sectional and/or have statistical limitations. Thus, the current study tested whether expectancies, subjective response, and their interaction prospectively predicted future drinking, using novel measures of expectancies and subjective response in the context of an alcohol administration study.
Alcohol Expectancies
Alcohol expectancies are typically conceptualized within a social learning perspective (Bandura, 1977; Rotter, Chance, & Phares, 1972), suggesting constant, interactive relations among cognitions, behaviors, and environments. This creates a reciprocal determinism, such that both person- (cognitions and behaviors) and environment-level influences affect one another (Bandura, 1977) and predict behavior. Expectancies serve a cognitive function in social learning, as they represent conditioned responses about how alcohol will affect an individual based on direct and indirect experience. Both direct exposure to alcohol (Aas, Leigh, Anderssen, & Jakobsen, 1998; Dunn & Goldman, 1996; Sher, Wood, Wood, & Raskin, 1996; Smith, Goldman, Greenbaum, & Christiansen, 1995) and indirect exposure to alcohol cues/content via media messages (Austin & Knaus, 2000; Austin, Pinkleton, & Fujioka, 2000; Dal Cin et al., 2009), peers (e.g., Boyd, Sceeles, Tapert, Brown, & Nagel, 2018; Martino, Collins, Ellickson, Schell, & McCaffrey, 2006), and family (Brown, Creamer, & Stetson, 1987; Colder, Chassin, Stice, & Curran, 1997; Waddell, Blake, Sternberg, Ruof, & Chassin, 2020) lead to the formation of and changes in expectancies. Thus, early alcohol expectancies are formed from observing alcohol use through socializing agents such as parents, peers, and the media, whereas once alcohol use initiates, personal drinking appears to be the strongest driver of expectancy change. Several studies suggest reciprocal effects of alcohol use and expectancies, such that as alcohol use increases, so do positive expectancies, particularly in adolescence (Aas et al., 1998; Smith et al., 1995), and college age (Sher et al., 1996; Walther, Pedersen, Gnagy, Pelham, & Molina, 2019).
Across ages, positive expectancies (e.g., sociability, liquid courage) are consistently associated with frequent and heavier drinking, growth in drinking, and alcohol-related problems (e.g., Fromme & D’Amico, 2000; Ham & Hope, 2003; Jones et al., 2001; Lee, Oei, & Greeley, 1999; Leigh & Stacy, 2004; Mann, Chassin, & Sher, 1987; Turrisi, Wiersma, & Hughes, 2000), whereas negative expectancies are inconsistently linked to use and/or problems (e.g., Collins, Koutsky, Morsheimer, & MacLean, 2001; Jones et al., 2001; Mann et al., 1987; McMahon, Jones, & O’Donnell, 1994; Southwick, Steele, Marlatt, & Linde, 1981). In addition to differences in relations based on the valence of expectancies (positive vs. negative), recent studies suggest that level of arousal (low vs. high) may also be critical. Morean, Corbin, and Treat (2012) found that high arousal positive (HIGH+) expectancies (e.g., sociable, talkative) were positively associated with all indices of heavy drinking and alcohol problems, and that HIGH− expectancies (e.g., aggressive, rude) were positively associated with binge drinking and alcohol problems. In contrast, Morean and colleagues (2012) found that low arousal positive (e.g., relaxed, calm) and low arousal negative expectancies (e.g., woozy, wobbly) were associated with less drinking and problems, largely replicating the results of a study by Rather, Goldman, Roehrich, and Brannick, 1992. Follow-up studies replicated these findings in high school students (Morean, Zellers, Tamler, & Krishnan-Sarin, 2016)., emphasizing the importance of arousal in assessing expectancies and their relations with drinking outcomes.
Subjective Response
In-the-moment subjective response also serves as a risk factor for alcohol use and problems. Subjective response was first conceptualized as acute changes in mood and the amount of alcohol needed to achieve intoxication (Judd, Hubbard, Janowsky, Huey, & Atewall, 1977; Mayfield, 1968; Schuckit, 1980). However, subsequent research has identified specific domains of subjective response, namely stimulation and sedation (Martin, Earleywine, Musty, Perrine, & Swift, 1993). Theoretical models focused on subjective intoxication versus stimulation/sedation differ in their predictions of how subjective response relates to drinking outcomes.
The low-level of response model (e.g., Schuckit, 1984; Schuckit, 2009) posits that individuals at highest risk for AUD (i.e., those with a family history of AUD) feel less acute alcohol effects, leading to heavier drinking, higher tolerance, and risk for AUD (Schuckit, 2009). This model has received support from several studies across 25 years of follow-up (Schuckit, 1994; Schuckit, 1998; Schuckit & Smith, 1996; Schuckit & Smith, 2000; Schuckit, Smith, Anderson, & Brown, 2004; Trim, Schuckit, & Smith, 2009). In contrast, the differentiator model (DM; Newlin & Thomson, 1990) posits that those at highest risk for future drinking and alcohol problems may be more sensitive to stimulant subjective alcohol effects (on the ascending limb), and less sensitive to sedating subjective effects (on the descending limb). The DM has also received robust support across studies (Corbin, Gearhardt, & Fromme, 2008; Erblich, Earleywine, Erblich, & Bovbjerg, 2003; Holdstock, King, & de Wit, 2000; King et al., 2011; King, McNamara, Hasn, & Cao, 2014; King et al., 2016; King, Houle, de Wit, Holdstock, & Schuster, 2002; Martin et al., 1993; Morean & Corbin, 2010; Quinn & Fromme, 2011; Thomas, Drobes, Voronin, & Anton, 2004).
Although the DM differentiates subjective alcohol effects by levels of arousal, the items are confounded by valence such that the most commonly used items in DM studies primarily measure HIGH+ (stimulant) and low arousal negative (sedative) subjective responses. However, more recent work has demonstrated that HIGH− subjective response is related to typical drinking quantity, binge drinking, and alcohol problems, whereas HIGH+ subjective response is related to drinking and driving (Morean, Corbin, & Treat, 2013). Low arousal positive and negative subjective response, much like expectancies, were found to be associated with less drinking and problems.
Expectancy–Subjective Response Discrepancies
As noted previously, alcohol expectancies and subjective response are seldom modeled together in single studies. According to reciprocal determinism (Bandura, 2004, 2012), expectancies and subjective response should become highly correlated with drinking experience, as particular expectancies strengthen after feeling particular subjective effects when drinking. However, the relationship between the two appears to be more complex. Bandura’s (2012) social cognitive theory posits that cognitions about an event/behavior can provide an inaccurate portrayal of reality, such that alcohol expectancies may misrepresent one’s actual subjective experiences during alcohol consumption. Three studies to date have provided support for this notion. Fromme and Dunn (1992) found that participants expected more positive and negative alcohol effects than they acutely experienced, Wall, Thrussell, and Lalonde (2003) found that participants expected more risk/aggression than they acutely experienced, and Fridberg, Rueger, Smith, and King (2017) found that stimulant and sedative expectancies accounted for only 40% of the variance in their subjective response counterparts.
Although prior studies have detected discrepancies between expectancies and subjective response, only one study has tested how discrepancies may drive further drinking behavior. Morean and colleagues (2015) found that overestimating HIGH− effects was associated with greater binge drinking and alcohol problems, whereas overestimating HIGH+ effects was associated with more drinking and driving. Overestimating low arousal negative effects was related to less binge drinking and alcohol problems, whereas overestimating low arousal positive effects was only related to less drinking and driving (Morean et al., 2015).
The finding that overestimating HIGH− effects lead to more binge drinking and problems is consistent with operant conditioning (Skinner, 1963). That is, expecting undesirable effects but not actually feeling them may be experienced as avoiding the aversive, punishing outcomes thought to be associated with alcohol use. In contrast, the theoretical underpinnings of the relation between discrepancies for HIGH+ effects and drinking and driving are less apparent. Morean et al. (2012) proposed that overestimating HIGH+ effects may be associated with less perceived impairment, which could lead to drinking and driving. Alternatively, this pattern of findings could be explained by goal-directed behavior, where an individual is motivated to achieve the expected effects that he or she fails to receive. In fact, the lack of actual HIGH+ effects may be experienced as a thwarting of goals (Gollwitzer & Oettingen, 2011), leading to a strengthening of goal-directed behavior (e.g., further consumption and risky behavior).
The Morean et al. (2012) study was important in being the first to test effects of discrepancies between expectancies and subjective response. However, the cross-sectional design limits the ability to understand temporal relations among expectancies, subjective response, and drinking outcomes. In addition to the potential for spurious correlations and truncating the variance of the construct of interest, discrepancy scores do not provide a good measure of similarity and can have limited interpretability, primarily due to confounding the discrepancy with the constituent main effect correlations and variances (e.g., Griffin, Murray, & Gonzalez, 1999; Peter, Churchill, & Brown, 1993). Particularly of interest to the current research questions, the use of discrepancy scores fails to consider main effects, and thus ignore participants’ absolute levels of expectancies and subjective response.
Including both main effects and the interaction in the model could delineate for whom particular interventions might be most effective. For example, if an individual has high expectancies and mean level subjective response (e.g., expectancies 8/10; subjective response 5/10), they may benefit most from an expectancy challenge (Darkes & Goldman, 1993), which seeks to correct strong and highly distorted expectancies (i.e., align them with one’s subjective response) and provide psychoeducation about expectancies as a risk factor for drinking. However, an individual with mean level expectancies and low subjective response (e.g., expectancies 5/10; subjective response 2/10, respectively) may benefit less from the same expectancy challenge intervention; without main effects in the model, these two individuals would be considered identical with respect to their expectancy-subjective response discrepancy. Similarly, an individual who has high expectancies and subjective response (e.g., expectancies 7/10; subjective response 8/10, respectively; no/small discrepancy) may benefit most from a preventative intervention seeking to reduce pharmacological effects of alcohol (e.g., naltrexone; O’Malley et al., 2015), whereas an individual with a similar discrepancy but low levels of expectancies and subjective response (e.g., expectancies = 3/10; subjective response = 4/10, respectively) might derive less benefit from this type of intervention. Thus, it is critical to understand discrepancies between expectancies and subjective response in the context of an individual’s overall levels of each.
Proposed Study and Hypotheses
The current study sought to address important gaps in the literature by investigating how alcohol expectancies, subjective response, and their interaction influence an individual’s typical drinking quantity 1 year later, using data from a placebo-controlled alcohol administration session with longitudinal follow up. Given the focus on interactions between expectancies and subjective response to alcohol, only data from participants who received alcohol were included. In line with the pursuit of goal-directed behavior (Ajzen & Madden, 1986), we hypothesized that participants who expect high but feel low levels of HIGH+ effects would be more likely to continue drinking in an effort to chase after expected positive effects. We also anticipated that those who expect high but feel low levels of HIGH− effects would continue drinking due to an absence of negative (punishing) effects, in line with operant conditioning (Skinner, 1963). This hypothesis is also supported by cross-sectional results from Morean et al. (2015). We considered the effects of low arousal positive and negative effects exploratory given inconsistencies between theory and the limited prior empirical findings.
Method
Participants
Participants were recruited via flyers posted around a southwestern university, the surrounding community, and through online Listervs (e.g., Craigslist) and advertisements (e.g., Facebook). After an initial phone screen, 547 participants came into the lab for a baseline assessment (hereafter referred to as T1), and 448 met eligibility for the alcohol administration session. Participants were deemed eligible if they endorsed binge drinking (≥4 drinks for women, ≥5 for men) at least once in the last month. Exclusion criteria included last-month alcohol dependence, a last-month depressive or anxiety disorder, serious mental illness or medical conditions, use of psychotropic or pain medicine, negative reactions to alcohol, daily or near daily marijuana use, past treatment seeking for alcohol problems, and pregnancy/nursing. A total of 270 (of 448) participants were in the alcohol condition. Given the goals of the current study, participants in the placebo condition were excluded from analyses. This was done because (1) discrepancies for placebo subjective response do not represent real-world drinking experience (i.e., one does not drink to consume alcohol but actually consume placebo in the real world); (2) examining discrepancies between expectancies and placebo response taps into discrepancies between explicit and implicit expectancies, which is an interesting but fundamentally different question; and (3) expectancies were assessed for anticipated effects after consuming four to five drinks, rather than for drinking alcohol in general, which may get at more placebo-like drinking responses.
In addition, 12 participants in the alcohol condition were excluded because they reported a peak BAC under .06%. These participants were removed to ensure that (1) BAC levels were high enough to capture pharmacological alcohol effects (vs. expectancies) and (2) each participant in the sample had a comparable BAC on the ascending and descending limbs of the blood alcohol curve. Participants were 21 to 25 years old, 42.6% female, mostly college students (79%), and represented the general racial (66.1% Caucasian) and ethnic (26.1% Hispanic/Latinx) composition of the community (see Table 1 for descriptive statistics). A total of 232 (89%) participants reported provided follow-up data (hereafter referred to as T2). Participants who did not report follow-up data 6 or 12 months later had significantly higher levels of HIGH+ subjective response, t(45.92) 2.28, p = .03, and were more likely to be female, t(34.27) −.3.05, p < .01, but did not differ on other variables used in the study.
Table 1.
Demographic
| Variable | N | M (SD) |
|---|---|---|
|
| ||
| Age | 258 | 22.25 (1.23) |
|
| ||
| College student (Yes/No) | 258 | |
| Yes | 204 (79%) | |
| No | 54 (21%) | |
| Sex | 258 | |
| Male | 148 (57.4%) | |
| Female | 110(42.6%) | |
| Race | 258 | |
| White/Caucasian | 170 (65.9%) | |
| Black/African American | 20 (7.8%) | |
| Asian | 28 (10.9%) | |
| American Indian/Native | 4(1.6%) | |
| Other | 35 (13.6%) | |
| Missing | 1 (.4%) | |
| Ethnicity | 258 | |
| Hispanic/Latinx | 65 (25.2) | |
| Non-Hispanic/Latinx | 184 (71.3%) | |
| Missing | 9 (3.0%) | |
Note. N = 258.
Procedure
All procedures were approved by the Arizona State University Institutional Review Board. After completing online or telephone screening, eligible participants came to the laboratory for a T1 structured clinical interview (Grant et al., 2003) and a battery of questionnaires. If participants met inclusion criteria, they were scheduled to attend an alcohol administration session on a weekday between 4 p.m. and 6 p.m. Participants were asked not to consume alcohol 24 hr prior to coming into the lab and not to consume any food or caffeine 4 hr before the session. Before arriving at the alcohol administration session, participants were randomly assigned to one of four conditions crossing physical (bar vs. lab) and social (solitary vs. group) contexts. Within each context, participants were randomized to an alcohol or placebo condition. To adequately power within-alcohol-condition analyses examining effects of subjective alcohol response on later drinking outcomes, randomization was done at a 6:4 (alcohol:placebo) ratio.
Upon arrival for the alcohol administration session, research assistants verified the participant’s age and reviewed the consent document completed during the first session. T1 BAC was tested to ensure a .00% BAC, and female participants were administered a pregnancy test and confirmed a negative result prior to participating. Research assistants then used computer algorithms (Curtin & Fairchild, 2003) to prepare drinks with fixed amounts of vodka, cranberry juice, lemon–lime soda, and lime juice to dose each participant to a .08% BAC. Alcohol dose was individualized per participant sex and weight and then combined with a mixer at a 3:1 (mixer:vodka) ratio.
Research assistants (who were blind to beverage condition) served participants three drinks and told them that they had 6 min to consume each drink, with a 1-min break after each 6-min period. After drink consumption, BAC readings were taken every 10 min. BAC readings were taken using handheld breath alcohol testers (Alco-Sensor, Intoximeters, St. Louis, MO), which demonstrate high reliability and correspondence with other BAC assessments (Gibb, Yee, Johnston, Martin, & Nowak, 1984). Once a BAC of .06% was reached, the ascending limb assessments began. Descending limb assessments were given when BAC measurements closely matched the ascending limb BAC. After completing all assessments, participants were held in the lab until their BAC fell below a .03%, and were then debriefed, paid, and provided transportation home. Participants were contacted 6 and 12 months later and asked to provide web-based assessments of drinking. Participants were paid $12/hr for the T1 session/alcohol administration, and $5 per session (two sessions) if they attended their originally scheduled sessions. Participants were paid $20 at the 6-month and $30 at the 12-month follow-up for participation.
Measures
Demographics.
Age and sex were assessed at T1.
Alcohol use.
T1 and follow-up alcohol use data was assessed via the timeline followback (TLFB; Sobell & Sobell, 1992). Participants reported on the frequency, quantity, and amount of time spent drinking for each of the last 30 days. The T1 TLFB was administered in person by a research assistant, and the follow-up TLFBs were completed via a web-based calendar. In both cases, standard drink charts were provided to ensure participants used the same definition of a standard drink. The TLFB shows strong validity in younger adult and alcohol-dependent populations and is positively correlated with other measures of heavy drinking (Carey, Carey, Maisto, & Henson, 2004; Sobell & Sobell, 1992). Drinking quantity (i.e., drinks per drinking day) was calculated as the total number of drinks over the past 30 days divided by the total number of drinking days. TLFB scores at 6- and 12-month follow ups and were averaged across the two to obtain a more reliable measure of drinking quantity across the 12-month period.
In addition to the TLFB, the Daily Drinking Questionnaire (DDQ; Collins, Parks, & Marlatt, 1985), was administered to participants at the 12-month follow-up, measuring participants’ drinking quantity during a typical week over the last year. The DDQ (over the last 30 days) is highly correlated with other measures of drinking quantity such as the Drinking Practices Questionnaire (Collins et al., 1985). In the current sample, the DDQ was highly correlated with the combined TLFB score across 6- and 12-months (r = .67, p < .001).
Alcohol expectancies.
Alcohol expectancies were measured with the Anticipated Effects of Alcohol Scale (AEAS; Morean et al., 2012). The AEAS includes 22-items that assess HIGH+, HIGH−, low arousal positive, and low arousal negative expectancies. The AEAS assesses the extent to which individuals believe they will feel specific effects “immediately after” and “90 min after” drinking four to five drinks (depending on sex, respectively) in a 2-hr period on a scale of 0 (not at all) to 10 (extremely). Alcohol administration paradigms have shown that, when dosed to a .08% BAC, BAC measurements will typically reach a BAC of .06% “immediately after” or shortly after consumption, and descend back to .06% about “90 min later” (e.g., Morean et al., 2013). We used the “immediately after” and “90 min after” assessments to correspond to ascending and descending limb measurements of subjective response, respectively. Only items that were also on the parallel subjective response scale (see below and Table 1 in the online supplemental material) were included to allow for direct comparisons between expectancies and subjective response. HIGH+ (i.e., lively, talkative, funny, fun), HIGH− (i.e., aggressive, demanding, rude), Low Arousal Positive (i.e., mellow, calm, relaxed), and Low Arousal Negative (i.e., dizzy, wobbly, calm) Expectancy subscales had adequate internal consistency (α = .73–.95).
Subjective response.
Subjective response was assessed with the Subjective Effects of Alcohol Scale (SEAS; Morean et al., 2013) The SEAS matches the format of the AEAS, breaking subjective response into the four quadrants of affective space. The SEAS assesses the extent to which alcohol produces feelings at the “present time,” and participants completed the SEAS on the ascending and descending BAC limbs. Only matched items on the AEAS were used (see online supplemental material Table 1). The matched items on the SEAS had good internal consistency for all subscales (α = .79–94) across both limbs.
Power Analysis
Power analysis was conducted using G*Power 3 (Faul, Erdfelder, Lang, & Buchner, 2007). For the focal test of a two-way interaction between expectancies and subjective response in a multiple regression including all covariates, the sample size was adequately powered (>.80) to detect a small to medium effect size across models (f2 = .038).
Data Analytic Plan
Before conducting primary analyses, all variable distributions were examined for outliers. Outliers were winsorized by replacing any value more than three standard deviations from the mean with a value of one higher than the highest value within the distribution (Tabachnick, Fidell, & Ullman, 2007). Maximum likelihood with robust standard errors (MLR) estimation was used in all analyses. All continuous variables were mean-centered in analyses to reduce nonessential multicollinearity (Cohen, Cohen, West, & Aiken, 2003). Zero-order correlations, variance inflation factors (VIF), and tolerance were examined for all variables to investigate multicollinearity.
The primary analyses used multiple regression via MPlus Version 7.4 (Muthén & Muthén, 2017). Full information maximum likelihood (FIML) was used to handle missing data (Schafer & Graham, 2002), and all exogenous variables were allowed to freely covary. We estimated a series of models in which follow-up drinks per drinking day was regressed on expectancies, subjective response, and their interaction within each quadrant of affective space (analyzed separately). All analyses included T1 drinks per drinking day, age, sex, physical context, and social context as covariates. The context variables were dummy coded. Sex was also dummy coded, and age was entered as a continuous covariate.
In line with the DM, we tested models for high arousal effects using scores on the ascending limb, and low arousal effects using scores on the descending limb of the BAC. Significant interactions were probed by estimating simple regression equations at one standard deviation above the mean, at the mean, and one standard deviation below the mean of the moderator (i.e., subjective response; Aiken, West, & Reno, 1991). For models where one standard deviation below the mean fell outside of the distribution of scores (i.e., HIGH−), we examined appropriate data points to estimate similar distances above and below the mean. Separate models were run without the interaction term to report main effects. Unstandardized effects are shown in the text and standardized effects are in tables.
Results
Descriptive Statistics, Multicollinearity, and Bivariate Correlations
There were outliers for T1 and T2 drinking quantity (i.e., less than 2% of cases; two cases at T1, four cases at T2) and each were given a score of one higher than the highest score within the distribution (Tabachnick et al., 2007). With the censoring of these outliers, distributions were within reasonable limits of normality (skewness = ~1.2, kurtosis ~1.8). Correlations between matched domains of expectancies and subjective response were moderate in magnitude. No correlation was greater than .39 (r = .21 –.39, p < .001), and no variable had a variance inflation factor greater than 2 or tolerance less than 0.5.
Bivariate correlations are shown in Table 2. T1 and T2 drinking were correlated with male sex (r = −.20, −.23, respectively, p < .01) and with weaker low arousal negative expectancies (r = −.16, −.21, respectively, p < .05). T1 drinking was correlated with weaker low arousal negative subjective response (r = −.12, p < .05) and stronger HIGH+ subjective response (r = .12, p < .05).
Table 2.
Means, Standard Deviations, and Bivariate Correlations Across Study Variables
| Variable | M (SD) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| 1. T1 Drinking | 4.62 (2.24) | — | .54** | −.28** | − .04 | .06 | −.06 | .02 | .04 | − .01 | −.16** | .12* | .09 | .08 | −.12* |
| 2. T2 Drinking | 4.30 (2.01) | — | −.23** | −.09 | .11 | .05 | .08 | − .03 | .11† | −.21** | .01 | − .02 | .01 | −.11† | |
| 3. Sex | 42.6% Female | — | − .08 | .04 | −.02 | .03 | −.09 | − .16* | .10 | .20** | .17** | − .07 | −.12 | ||
| 4. Age | 22.25 (1.23) | — | − .06 | .01 | .03 | .11† | .12† | .09 | −.03 | .16* | − .04 | −.09 | |||
| 5. Physical context | 50% Bar | — | .02 | −.11 | .02 | −.13* | − .07 | −.06 | − .07 | −.13 | −.05 | ||||
| 6. Social context | 52% Social | — | − .01 | .10 | − .04 | .15* | .21** | .09 | .08 | .08 | |||||
| 7. AEAS HIGH+ | 7.41 (1.51) | — | − .01 | .28** | .10 | .26** | .10 | .28** | .01 | ||||||
| 8. AEAS HIGH− | 1.70 (1.72) | — | −.13* | .30** | .12 | .39** | − .06 | .11† | |||||||
| 9. AEAS LOW+ | 5.83 (2.02) | — | .04 | .13* | .04 | .36** | .02 | ||||||||
| 10. AEAS LOW− | 2.64 (2.22) | — | <.01 | .12† | −.05 | .21** | |||||||||
| 11. SEAS HIGH+ | 6.27 (2.19) | — | .16* | .31** | .22** | ||||||||||
| 12. SEAS HIGH− | .64(1.19) | — | .04 | .25** | |||||||||||
| 13. SEAS LOW+ | 6.32(2.11) | — | .10 | ||||||||||||
| 14. SEAS LOW− | .95 (1.47) | — | |||||||||||||
Note. AEAS = expectancies; HIGH+ = high arousal positive; HIGH− = high arousal negative; LOW+ = low arousal positive; LOW− = low arousal negative; SEAS = subjective response.
p < .10.
p < .05.
p < .01.
HIGH+ Effects
In the HIGH+ model (see Table 3), sex and T1 drinking were significant predictors of T2 drinking, with men (b = −.49, p = .04) and heavier drinkers at T1 (b = .47, p < .001) drinking more at T2. Stronger HIGH+ expectancies were marginally associated with greater T2 drinking quantity (b = .12, p = .07), whereas HIGH+ subjective response was inversely (albeit marginally) associated with T2 drinking quantity (b = −.09, p < .09).
Table 3.
Parameter Estimates for Primary Models Across Affective Space
| High arousal positive |
High arousal negative |
|||||||||||||||
| Main effects model |
Interaction model |
Main effects model |
Interaction model |
|||||||||||||
| Variables | B | SE | 95% CI | P | B | SE | 95% CI | P | B | SE | 95% CI | P | B | SE | 95% CI | P |
|
| ||||||||||||||||
| Covariate | ||||||||||||||||
| Age | −.09 | .05 | [−.18, .01] | .06 | −.08 | .05 | [−.17, .02] | .11 | −.08 | .05 | [−.17, .02] | .11 | −.08 | .05 | [−.17, .02] | .12 |
| Sex | −.12 | .06 | [−.24, .01] | .04 | −.13 | .06 | [−.25, .02] | .03 | −.11 | .06 | [−.23, .01] | .07 | −.12 | .06 | [−.23, .01] | .07 |
| Social context | .03 | .05 | [−.08, .13] | .59 | .02 | .05 | [−.08, .13] | .69 | .02 | .05 | [−.08, .13] | .65 | .01 | .05 | [−.09, .12] | .80 |
| Physical context | .05 | .05 | [−.06, .16] | .33 | .06 | .06 | [−.05, .17] | .28 | .07 | .05 | [−.04, .18] | .20 | .08 | .05 | [−.03, .18] | .16 |
| T1 drinking (TLFB) Principal predictor | .52 | .06 | [.40, .64] | <.001 | .52 | .06 | [.40, .64] | < .001 | .52 | .06 | [.40, .64] | < .001 | .52 | .06 | [.41, .64] | < .001 |
| Principal predictor | ||||||||||||||||
| Expectancies | .09 | .05 | [−.01, .20] | <.07 | .09 | .05 | 001[−.001, .18] | <.06 | −.03 | .06 | [−.14, .08] | .56 | .01 | .06 | [−.11, .12] | .95 |
| Subjective response Interaction | −.09 | .05 | [−.20, .01] | <.09 | −.11 | .06 | [−.21, .000] | <.06 | −.02 | .06 | [−.14, .09] | .69 | −.01 | .06 | [−.12, .13] | .82 |
| Expectancies × Subjective Response | −.12 | .06 | [−.23, −.01] | .009 | −.12 | .06 | [−.23, −.01] | .04 | ||||||||
|
| ||||||||||||||||
| Low arousal positive |
Low arousal negative |
|||||||||||||||
| Main effects model |
Interaction model |
Main effects model |
Interaction model |
|||||||||||||
| B | SE | 95% CI | P | B | SE | 95% CI | P | B | SE | 95% CI | P | B | SE | 95% CI | P | |
| Covariate | ||||||||||||||||
| Age | −.10 | .05 | [−.19, −.01] | .04 | −.10 | .05 | [−.19, −.01] | .04 | −.08 | .05 | [−.17, .02] | .12 | −.08 | .05 | [−.18, .02] | .12 |
| Sex | −.09 | .06 | [−.21, .04] | .16 | −.09 | .06 | [−.21, .03] | .16 | −.11 | .06 | [−.23, .01] | .07 | −.11 | .06 | [−.23, .01] | .07 |
| Social context | .03 | .05 | [−.08, .13] | .64 | .03 | .05 | [−.08, .13] | .64 | .02 | .05 | [−.09, .12] | .74 | .02 | .06 | [−.09, .12] | .78 |
| Physical context | .06 | .06 | [−.05, .17] | .28 | .06 | .06 | [−.05, .17] | .28 | .06 | .05 | [−.05, .16] | .29 | .06 | .05 | [−.05, .16] | .29 |
| T1 drinking (TLFB) Principal predictor | .53 | .06 | [.41, .65] | < .001 | .53 | .06 | [.41, .65] | < .001 | .50 | .06 | [.40, .62] | < .001 | .50 | .06 | [.38, .62] | < .001 |
| Principal predictor | ||||||||||||||||
| Expectancies | .12 | .06 | [.01, .24] | .04 | .12 | .06 | [.01, .24] | .04 | −.09 | .05 | [−.20, .01] | <.08 | −.09 | .05 | [−.20, .01] | <.08 |
| Subjective response Interaction | −.09 | .06 | [−.21, .03] | .12 | −.09 | .06 | [−.21, .03] | .13 | −.04 | .05 | [−.13, .05] | .36 | −.04 | .05 | [−.13, .05] | .40 |
| Expectancies × Subjective Response | .01 | .06 | [−.11, .12] | .94 | −.01 | .05 | [−.11, .05] | .82 | ||||||||
Note. All model parameters are standardized. Paths in boldface type are significant at p < .05. T1 = baseline; TLFB = timeline followback.
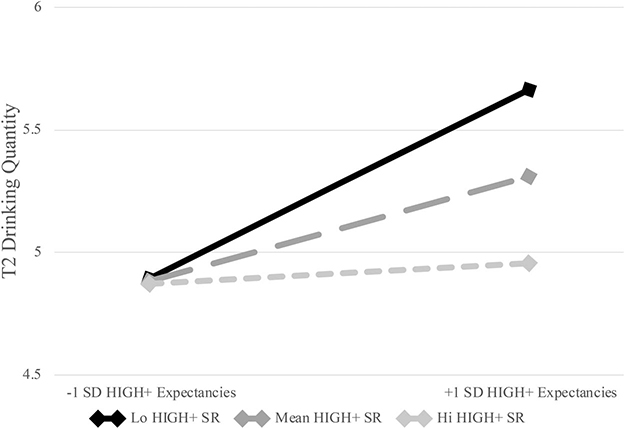
The interaction between HIGH+ expectancies and subjective response was robustly significant (b = −.05, p = .009). Post hoc probing found significant simple slopes for HIGH+ expectancies at the mean (b = .12, p = .04) and 1 SD below the mean (b = .22, p = .001) of HIGH+ subjective response, but not at 1 SD above the mean (b = .01, p = .93; see Figure 1). Regions of significance testing suggested that the slope for HIGH+ expectancies became significant below a value (centered) of .07 (regions of significance = .07, 9.15; see Figure 2). Thus, for individuals at mean and low levels of HIGH+ subjective response, as expectancies increased, T2 drinking quantity significantly increased (see Figures 1 and 2).
Figure 1.
High arousal positive (HIGH) expectancies by subjective response interaction. Simple slopes were significant for mean and low levels of high arousal positive subjective response.
Figure 2.
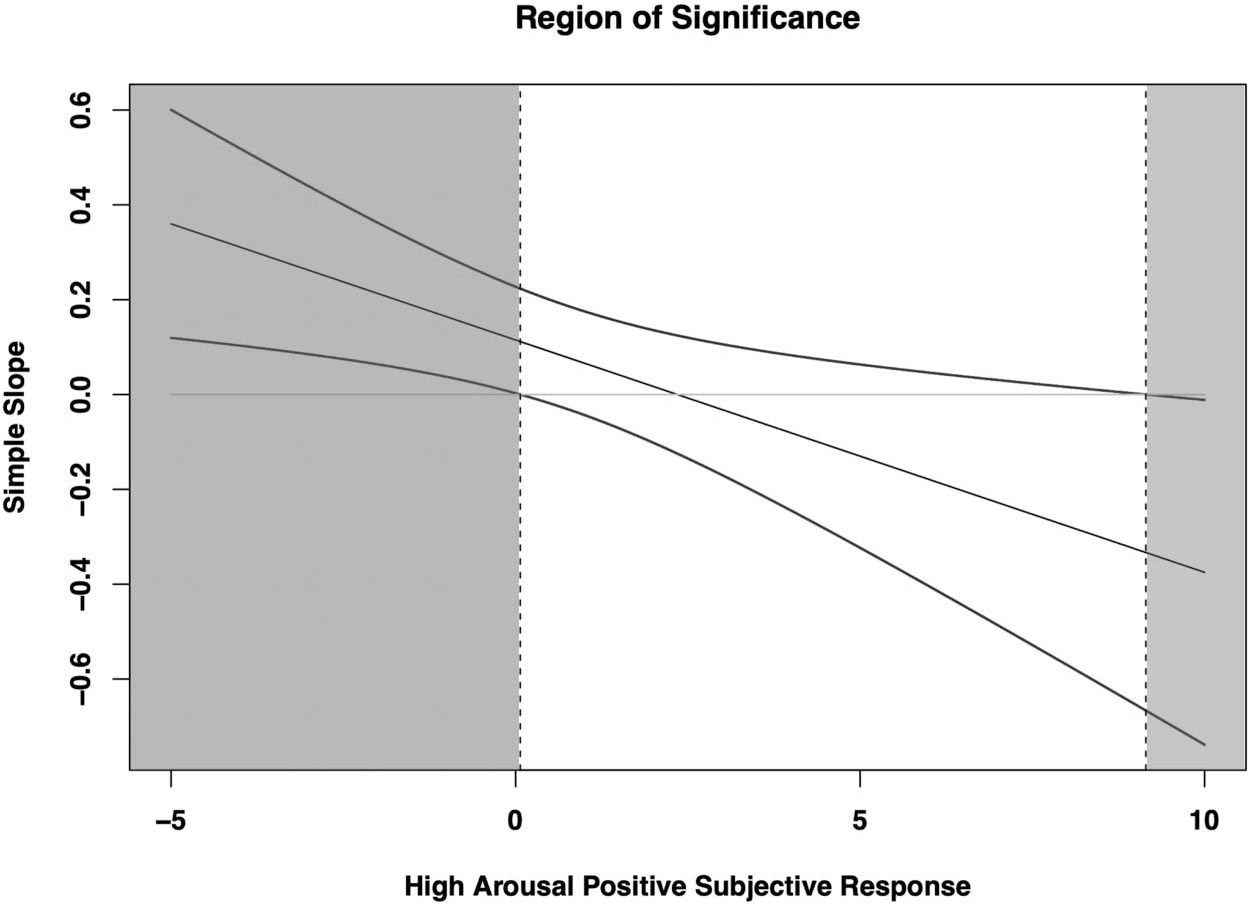
High arousal positive expectancies by subjective response region of significance. Model estimates were mean-centered, and thus a value of zero is equal to mean levels of high arousal positive subjective response.
HIGH− Effects
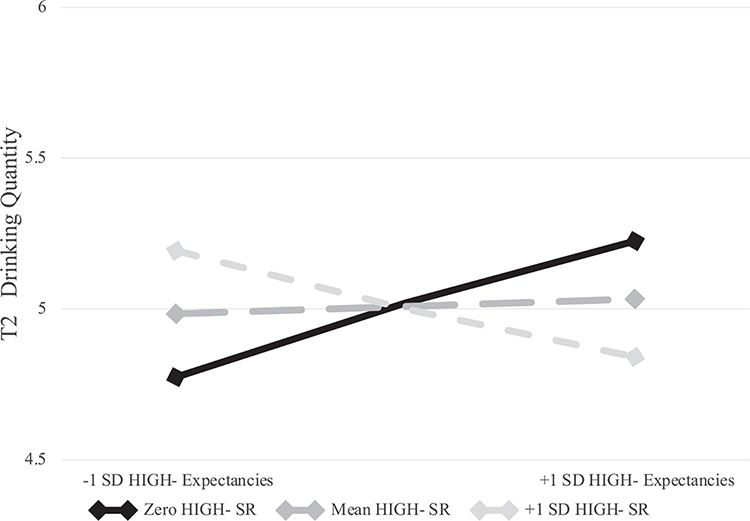
Before fitting the HIGH− model, HIGH− subjective response was log transformed. This was done due to substantial positive skew and kurtosis (2.9 and 9.3, respectively) and substantial non-normality of residuals as shown via P-P plots. In the HIGH− model (see Table 3), the only significant covariate was T1 drinking quantity (b = .47, p < .001), such that heavier drinkers at T1 drank more at T2. Neither HIGH− subjective response nor expectancies predicted T2 drinking quantity. However, the interaction was statistically significant (b = −.12, p = .04).
To probe the HIGH− interaction, we estimated simple slopes for HIGH− expectancies at high levels of HIGH− subjective response (one standard deviation above the mean), mean subjective response, and low subjective response (subjective response = 0). Because of the low number of individuals who endorsed HIGH− effects, one standard deviation below the mean was outside the range of the data. Thus, we used the value of zero which was roughly one standard deviation below the mean (SD = .86). The simple slopes for expectancies at low (b = .11, p = .30), mean (b = .01, p = .94), and high (b = −.12, p = .07) levels of HIGH− subjective response were all nonsignificant, though the latter effect approached significance. Importantly, the overall interaction implied that there was statistically significant variation across these simple slopes: graphic depiction of the interaction (see Figure 3) suggests that, as subjective response increased, effects of expectancies on T2 drinking went from moderately positive to slightly negative.
Figure 3.
High arousal negative (HIGH−) expectancies by subjective response interaction. No simple slopes were statistically significant.
Low Arousal Positive Effects
In the low arousal positive model (see Table 3), T1 drinking quantity (b = .47, p < .001) and age (b = −.16, p = .04) were associated with T2 drinking quantity, such that heavier drinkers and younger participants at T1 drank more at T2. Low arousal positive expectancies (b = .12, p < .05), but not subjective response, were associated with T2 drinking, such that stronger low arousal positive expectancies were associated with heavier T2 drinking. No significant interaction emerged between low arousal positive expectancies and subjective response (b = .01, p = .94).
Low Arousal Negative Effects
In the low arousal negative model (see Table 3), T1 drinking quantity was associated with T2 drinking quantity (b = .45, p < .001), such that heavier drinkers at T1 drank more at T2. T2 drinking was not significantly associated with either low arousal negative expectancies or subjective response. No significant interaction emerged between low arousal negative expectancies and subjective response (b = −.01, p = .82).
Sensitivity Analyses
Because the TLFB did not capture drinking across the full year between T1 and T2, we conducted a sensitivity analysis using scores on the Daily Drinking Questionnaire (DDQ; Collins et al., 1985), which measured participants typical drinking quantity “during a typical week over the past year” at T2. All models were reestimated with this score as the outcome. The interaction between HIGH+ expectancies and subjective response remained significant (b = −.09, p < .02), whereas the interactions for HIGH− (b = −.06, p = .42), low arousal negative (b = .02, p = .76), and low arousal positive effects (b = .01, p = .65) were all nonsignificant. For a full list of all parameters see Supplementary Table 2.
Discussion
The current study sought to test the prospective effects of discrepancies between alcohol expectancies and subjective response using longitudinal data. We tested the unique effects of expectancies and subjective response in addition to their interaction, parsing out shared variance within an additive risk framework. Whereas the one prior study of discrepancies between expectancies and subjective response examined difference scores for expectancies and subjective response (Morean et al., 2015), this is the first study to use a moderation approach. In addition, this also the first study to examine discrepancies between expectancies and subjective response as prospective predictors of later drinking, controlling for T1 drinking. This is critical for establishing temporal precedence of effects.
The first hypothesis was that stronger HIGH+ expectancies would be prospectively associated with greater drinking among those with weaker (but not stronger) levels of HIGH+ subjective response. Results supported this hypothesis, showing that for those at low and average levels of HIGH+ subjective response, stronger expectancies were associated with heavier drinking. As depicted in Figure 1, individuals with low subjective response and high expectancies were the heaviest drinkers at follow-up. These findings fit within the theory of goal-directed behavior. Individuals who expect higher levels of HIGH+ effects, but experience mean or low levels of these effects in-the-moment, may be more motivated to continue drinking, as their expectations likely guide them to anticipate that drinking will cause such effects. In other words, an individual with unmet expectancies may attempt to chase appetitive HIGH+ effects by continuing to drink, whereas an individual whose expectancies are met by their subjective response may be satisfied and not feel the need to continue drinking.
The second hypothesis was that, similar to HIGH+ expectancies, stronger HIGH− expectancies would be associated with heavier drinking among those with weaker (but not stronger) HIGH− subjective response. The results provided some support for this hypothesis. Figure 3 shows that stronger HIGH− expectancies were positively related to drinking at low levels of HIGH− subjective response. Thus, it is possible that individuals who expect but do not feel HIGH− effects may continue drinking due to the absence of expected aversive effects. However, given that this effect was not replicated with the DDQ data, future research is needed to determine the reliability of this finding.
Although exploratory, we tested the same framework for low arousal positive and negative effects. Neither interaction was significant. Particularly for the low arousal positive interaction, it may be that high arousal effects are more vivid and provide a larger difference from expectancies than low arousal effects. Mean levels of low arousal positive (expectancies = 5.83; subjective response = 6.32) and HIGH+ effects (expectancies = 7.41, subjective response = 6.27) support this notion, with a larger difference for HIGH+ effects.
These findings, particularly those involving HIGH+ effects, have potentially important treatment implications. As stated earlier, two potential intervention efforts to lower heavy drinking are pharmacotherapy to reduce subjective response (e.g., naltrexone) and expectancy challenge to realign one’s expectancies with their actual subjective response. Thus, delineating the interplay between specific cognitive and subjective individual risk factors may help clinicians to tailor interventions to different phenotypic expressions, rather than using blanket-level interventions for all heavy drinkers. Because those who expected but did not acutely feel HIGH+ effects drank more heavily (than those with weaker expectancies), an expectancy challenge may be the most effective intervention for these individuals. Presumably, if expectancies were reduced, drinking would also decrease, as has been shown in expectancy challenge efficacy studies (e.g., Scott-Sheldon, Terry, Carey, Garey, & Carey, 2012). Although future research is needed to determine if individuals with larger discrepancies benefit more from expectancy challenges, prior research highlights the value of tailoring prevention efforts to unique characteristics of individuals. For example, personality-centered interventions (e.g., Conrod et al., 2000; Conrod, Castellanos, & Mackie, 2008) match brief interventions to individuals based upon their personality profiles, and show promising results. Moreover, given that highly impulsive individuals have higher positive expectancies (e.g., Corbin, Iwamoto, & Fromme, 2011; Settles, Cyders, & Smith, 2010), combining both personality- and expectancy-centered interventions may be particularly efficacious for those with high expectancies and mean/low subjective response.
In contrast, because expectancy challenges seek to align expectancies with one’s actual subjective response, individuals with elevated levels of HIGH+ subjective response may benefit less from this approach. Considering these individuals have comparable expectancies and subjective response (i.e., both high), an intervention seeking to align the two may provide little benefit, as there is little realigning needed. Instead, these individuals may benefit most from pharmacotherapy (e.g., naltrexone), which seeks to blunt the positive, reinforcing effects of alcohol. Naltrexone is thought to be most effective in reducing drinking for those with high reward and low relief subjective response phenotypes (e.g., Mann et al., 2018; Witkiewitz, Roos, Mann, & Kranzler, 2019), and thus may be particularly suited to those with high levels of HIGH+ (i.e., rewarding) subjective response. In addition, because expectancies are partially formed and adjusted from direct experiences with alcohol (e.g., Smith et al., 1995; Sher et al., 1996), reductions in HIGH+ subjective response could provide a dual effect on both expectancies and drinking. Although these hypotheses need empirical investigation, extant research suggests that pharmacotherapy is effective in reducing heavy drinking across studies, with a move toward making pharmacotherapies such as naltrexone more accessible as a low-burden, preventative tool in nontreatment seeking heavy drinkers (e.g., O’Malley et al., 2015; Epler, Sher, Loomis, & O’Malley, 2009). Thus, future research should test these hypothesized effects in naltrexone studies.
Although expectancy-subjective response interactions were the main focus of the current study, the current approach allowed us to test how each confers risk for drinking above and beyond the other. Considering that subjective response is a combination of both expected and pharmacological effects, controlling for expectancies provides a better estimate of the unique impact of subjective response. Of particular interest, HIGH+ subjective response was not related to later alcohol consumption above and beyond expectancies. This was surprising given support for relations between stimulant (HIGH+) subjective response and later drinking outcomes in prior research (e.g., King et al., 2011). However, it is worth noting that HIGH+ subjective response was significantly correlated with concurrent drinking. This suggests that, in the current sample, subjective response may be a stronger concurrent rather than prospective correlate of drinking.
A main effect of expectancies but not subjective response also emerged for low-arousal positive effects. Moreover, controlling for low arousal positive subjective response strengthened the effect of expectancies on drinking behavior; this was also replicated in the DDQ analyses. Low arousal positive expectancies were positively but not significantly correlated with later drinking behavior in the bivariate correlations but became a significant predictor of later drinking in the multivariate regression model. This suggests that subjective alcohol effects may suppress the link between low arousal positive expectancies and drinking behavior. A prior study found that low arousal positive subjective response did not differ between participants receiving alcohol versus placebo (e.g., Corbin, Scott, Boyd, Menary, & Enders, 2015), suggesting that the relative lack of low arousal positive effects under alcohol may serve to reduce the impact of strong low arousal positive expectancies. These findings suggest that challenging inaccurate HIGH+ expectancies, while also bringing awareness to social and contextual factors that may lead to low arousal positive expectancies, may be efficacious in efforts to reduce drinking.
Although the presence of expectancy main effects and absence of subjective response main effects is intriguing, it is possible that this pattern of results was due to the lack of control for placebo response. Due to the study question at hand, we excluded placebo participants to allow firm assertions about alcohol-induced subjective response. Thus, prior studies examining subjective response and later drinking have tested differences across alcohol versus placebo to account for the implicit expectancy effects of being in a drinking environment and believing that one has consumed alcohol. However, the current study tested explicit expectancies as reported by participants. These two conceptualizations of expectancy effects address different fundamental questions and could lead to different results. Future studies are needed to directly examine this possibility.
Although the current study advanced previous research by using prospective data and an interaction approach, findings must be interpreted considering limitations. First, the current study included social drinkers between the ages of 21 to 25, and excluded participants who either did not endorse at least occasional binge drinking or had a diagnosable AUD. As such, the sample was relatively homogeneous with respect to typical drinking patterns, which could limit variability in drinking and generalizability beyond the current sample. Second, the current study relied on self-report drinking, expectancies, and subjective response rather than objective measurements. Although previous research has suggested that self-report alcohol use does not differ from collateral reports (Babor, Steinberg, Anton, & Del Boca, 2000; LaForge et al., 2005), future research could use Ecological Momentary Assessment coupled with biosensor data. Third, the analyses were based on data from a larger study focused on contextual influences on subjective response. As such, participants were randomly assigned to four contexts (solitary lab, solitary bar, group lab, group bar), which could have impacted the pattern of results. Although the analyses controlled for effects of context, there is reason to believe that contextual effects could affect a variety of subjective response patterns (Corbin et al., 2015) in ways that may not be adequately addressed by covarying context main effects. Although outside the scope of the present study, future research should replicate the present findings within a single context and/or investigate the moderating role of contextual/environmental factors.
In addition, although participants were asked to refrain from use of drugs before the alcohol administration session, we did not drug test participants before alcohol administration. Therefore, there is no way to know if participants had recently used illicit drugs which may affect subjective response to alcohol (e.g., Piasecki et al., 2011; Sokolovsky, Gunn, Micalizzi, White, & Jackson, 2020). Last, participants who did not report follow-up data had higher levels of HIGH+ subjective response, suggesting that those at greatest risk may have been least likely to complete all assessments. This limits the ability to generalize the findings beyond the sample who completed the follow-up assessments.
Despite these limitations, this is the first study to our knowledge that has used prospective data and an interaction framework to investigate the combined effects of expectancies and subjective response in predicting future drinking. In addition, we are aware of only one other study (Morean et al., 2015) that has examined the effects of discrepancies between expectancies and subjective response for the full range of alcohol effects. Our analyses suggest that those who had stronger HIGH+ expectancies but had weaker subjective response to alcohol drank the most heavily 12 months later. In addition, the current study found some evidence that those who expected but did not feel HIGH− effects drank more than those who both expected and experienced strong HIGH− effects. These findings suggest that those with unrealistic HIGH+ expectancies may benefit most from an expectancy challenge and provide some evidence that interventions should consider including negative expectancies/subjective response in heavy drinking prevention programs. Future research and replication are needed to confirm this pattern of findings, and fully understand underlying mechanisms and treatment implications. Future research should look at differences across sex and if the pattern of findings extends to negative alcohol consequences/harms.
Supplementary Material
Public Health Significance.
Using an interaction approach and longitudinal data, the current study found that individuals who had stronger high arousal positive expectancies coupled with mean/low levels of high arousal positive subjective response drank more heavily across a 12-month period. Findings suggest that tailoring interventions based on the interplay between an individual’s expectancies and subjective response might lead to stronger intervention efficacy.
Acknowledgments
This study was supported by Grant R01 AA021148 from the National Institute on Alcohol Abuse and Alcoholism to William R. Corbin. Portions of the findings were submitted to a symposium held at the 2020, 43rd Annual Research Society on Alcoholism Meeting in San Diego, California.
Footnotes
Supplemental materials: http://dx.doi.org/10.1037/pha0000430.supp
References
- Aas HN, Leigh BC, Anderssen N, & Jakobsen R (1998). Two-year longitudinal study of alcohol expectancies and drinking among Norwegian adolescents. Addiction, 93, 373–384. 10.1046/j.1360-0443.1998.9333736.x [DOI] [PubMed] [Google Scholar]
- Aiken LS, West SG, & Reno RR (1991). Multiple regression: Testing and interpreting interactions. Atlanta, GA: SAGE. [Google Scholar]
- Ajzen I, & Madden TJ (1986). Prediction of goal-directed behavior: Attitudes, intentions, and perceived behavioral control. Journal of Experimental Social Psychology, 22, 453–474. 10.1016/0022-1031(86)90045-4 [DOI] [Google Scholar]
- Austin EW, & Knaus C (2000). Predicting the potential for risky behavior among those ‘too young’ to drink as the result of appealing advertising. Journal of Health Communication, 5, 13–27. 10.1080/108107300126722 [DOI] [PubMed] [Google Scholar]
- Austin EW, Pinkleton BE, & Fujioka Y (2000). The role of interpretation processes and parental discussion in the media’s effects on adolescents’ use of alcohol. Pediatrics, 105, 343–349. 10.1542/peds.105.2.343 [DOI] [PubMed] [Google Scholar]
- Babor TF, Steinberg K, Anton RAY, & Del Boca F (2000). Talk is cheap: Measuring drinking outcomes in clinical trials. Journal of Studies on Alcohol, 61, 55–63. 10.15288/jsa.2000.61.55 [DOI] [PubMed] [Google Scholar]
- Bandura A (1977). Self-efficacy: Toward a unifying theory of behavioral change. Psychological Review, 84, 191–215. 10.1037/0033-295X.84.2.191 [DOI] [PubMed] [Google Scholar]
- Bandura A (2004). Model of causality in social learning theory. In Freeman A, Mahoney MJ, Devito P, & Martin D(Eds.), Cognition and psychotherapy (2nd ed., pp. 25–44). New York, NY: Springer. [Google Scholar]
- Bandura A (2012). Social cognitive theory. In Lange PAM, Kruglanski AW, & Higgens ET (Eds.), Handbook of theories of social psychology (Vol. 1, pp. 349–374). Thousand Oaks, CA: SAGE. 10.4135/9781446249215.n18 [DOI] [Google Scholar]
- Boyd SJ, Sceeles EM, Tapert SF, Brown SA, & Nagel BJ (2018). Reciprocal relations between positive alcohol expectancies and peer use on adolescent drinking: An accelerated autoregressive cross-lagged model using the NCANDA sample. Psychology of Addictive Behaviors, 32, 517–527. 10.1037/adb0000371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Creamer VA, & Stetson BA (1987). Adolescent alcohol expectancies in relation to personal and parental drinking patterns. Journal of Abnormal Psychology, 96, 17–121. 10.1037/0021-843X.96.2.117 [DOI] [PubMed] [Google Scholar]
- Carey KB, Carey MP, Maisto SA, & Henson JM (2004). Temporal stability of the timeline followback interview for alcohol and drug use with psychiatric outpatients. Journal of Studies on Alcohol, 65, 774–781. 10.15288/jsa.2004.65.774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassin L, Pitts SC, & Prost J (2002). Binge drinking trajectories from adolescence to emerging adulthood in a high-risk sample: Predictors and substance abuse outcomes. Journal of Consulting and Clinical Psychology, 70, 67–78. 10.1037/0022-006X.70.1.67 [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, & Aiken LS (2003). Applied multiple correlation/regression analysis for the social sciences. Hillsdale, NJ: Erlbaum. [Google Scholar]
- Colder CR, Chassin L, Stice EM, & Curran PJ (1997). Alcohol expectancies as potential mediators of parent alcoholism effects on the development of adolescent heavy drinking. Journal of Research on Adolescence, 7, 349–374. 10.1207/s15327795jra0704_1 [DOI] [Google Scholar]
- Collins RL, Koutsky JR, Morsheimer ET, & MacLean MG (2001). Binge drinking among underage college students: A test of a restraint-based conceptualization of risk for alcohol abuse. Psychology of Addictive Behaviors, 15, 333–340. 10.1037/0893-164X.15.4.333 [DOI] [PubMed] [Google Scholar]
- Collins RL, Parks GA, & Marlatt GA (1985). Social determinants of alcohol consumption: The effects of social interaction and model status on the self-administration of alcohol. Journal of Consulting and Clinical Psychology, 53, 189–200. 10.1037/0022-006X.53.2.189 [DOI] [PubMed] [Google Scholar]
- Conrod PJ, Castellanos N, & Mackie C (2008). Personality-targeted interventions delay the growth of adolescent drinking and binge drinking. Journal of Child Psychology and Psychiatry, 49, 181–190. [DOI] [PubMed] [Google Scholar]
- Conrod PJ, Stewart SH, Pihl RO, Côté S, Fontaine V, & Dongier M (2000). Efficacy of brief coping skills interventions that match different personality profiles of female substance abusers. Psychology of Addictive Behaviors, 14, 231. [DOI] [PubMed] [Google Scholar]
- Corbin WR, Gearhardt A, & Fromme K (2008). Stimulant alcohol effects prime within session drinking behavior. Psychopharmacology, 197, 327–337. 10.1007/s00213-007-1039-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin WR, Iwamoto DK, & Fromme K (2011). A comprehensive longitudinal test of the acquired preparedness model for alcohol use and related problems. Journal of Studies on Alcohol and Drugs, 72, 602–610. 10.15288/jsad.2011.72.602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin WR, Scott C, Boyd SJ, Menary KR, & Enders CK (2015). Contextual influences on subjective and behavioral responses to alcohol. Experimental and Clinical Psychopharmacology, 23, 59–70. 10.1037/a0038760 [DOI] [PubMed] [Google Scholar]
- Curtin JJ, & Fairchild BA (2003). Alcohol and cognitive control: Implications for regulation of behavior during response conflict. Journal of Abnormal Psychology, 112, 424–436. 10.1037/0021-843X.112.3.424 [DOI] [PubMed] [Google Scholar]
- Dal Cin S, Worth KA, Gerrard M, Gibbons FX, Stoolmiller M, Wills TA, & Sargent JD (2009). Watching and drinking: Expectancies, prototypes, and friends’ alcohol use mediate the effect of exposure to alcohol use in movies and adolescent drinking. Health Psychology, 28, 473–483. 10.1037/a0014777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darkes J, & Goldman MS (1993). Expectancy challenge and drinking reduction: Experimental evidence for a meditational process. Journal of Consulting and Clinical Psychology, 61, 344–353. 10.1037/0022-006X.61.2.344 [DOI] [PubMed] [Google Scholar]
- Dunn ME, & Goldman MS (1996). Empirical modeling of an alcohol expectancy memory network in elementary school children as a function of grade. Experimental and Clinical Psychopharmacology, 4, 209–217. 10.1037/1064-1297.4.2.209 [DOI] [Google Scholar]
- Epler AJ, Sher KJ, Loomis TB, & O’Malley SS (2009). College student receptiveness to various alcohol treatment options. Journal of American College Health, 58, 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erblich J, Earleywine M, Erblich B, & Bovbjerg DH (2003). Biphasic stimulant and sedative effects of ethanol: Are children of alcoholics really different? Addictive Behaviors, 28, 1129–1139. 10.1016/S0306-4603(02)00221-6 [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, & Buchner A (2007). G Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39, 175–191. 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- Fridberg DJ, Rueger SY, Smith P, & King AC (2017). Association of anticipated and laboratory-derived alcohol stimulation, sedation, and reward. Alcoholism, Clinical and Experimental Research, 41, 1361–1369. 10.1111/acer.13415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme K, & D’Amico EJ (2000). Measuring adolescent alcohol outcome expectancies. Psychology of Addictive Behaviors, 14, 206–212. 10.1037/0893-164X.14.2.206 [DOI] [PubMed] [Google Scholar]
- Fromme K, & Dunn ME (1992). Alcohol expectancies, social and environmental cues as determinants of drinking and perceived reinforcement. Addictive Behaviors, 17, 167–177. 10.1016/0306-4603(92)90021-M [DOI] [PubMed] [Google Scholar]
- Gibb KA, Yee AS, Johnston CC, Martin SD, & Nowak RM (1984). Accuracy and usefulness of a breath alcohol analyzer. Annals of Emergency Medicine, 13, 516–520. 10.1016/S0196-0644(84)80517-X [DOI] [PubMed] [Google Scholar]
- Gmel G, Kuntsche E, & Rehm J (2011). Risky single-occasion drinking: Bingeing is not bingeing. Addiction, 106, 1037–1045. 10.1111/j.1360-0443.2010.03167.x [DOI] [PubMed] [Google Scholar]
- Gollwitzer PM, & Oettingen G (2011). Planning promotes goal striving. In Vohs KD & Baumeister RF (Eds.), Handbook of self-regulation: Research, theory, and applications (pp. 162–185). New York, NY: Guilford Press. [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou PS, Kay W, & Pickering R (2003). The Alcohol Use Disorder and Associated Disabilities Interview Schedule-IV (AUDADIS-IV): Reliability of alcohol consumption, tobacco use, family history of depression and psychiatric diagnostic modules in a general population sample. Drug and Alcohol Dependence, 71, 7–16. 10.1016/S0376-8716(03)00070-X [DOI] [PubMed] [Google Scholar]
- Griffin D, Murray S, & Gonzalez R (1999). Difference score correlations in relationship research: A conceptual primer. Personal Relationships, 6, 505–518. 10.1111/j.1475-6811.1999.tb00206.x [DOI] [Google Scholar]
- Ham LS, & Hope DA (2003). College students and problematic drinking: A review of the literature. Clinical Psychology Review, 23, 719–759. 10.1016/S0272-7358(03)00071-0 [DOI] [PubMed] [Google Scholar]
- Holdstock L, King AC, & de Wit H (2000). Subjective and objective responses to ethanol in moderate/heavy and light social drinkers. Alcoholism, Clinical and Experimental Research, 24, 789–794. 10.1111/j.1530-0277.2000.tb02057.x [DOI] [PubMed] [Google Scholar]
- Jones BT, Corbin W, & Fromme K (2001). A review of expectancy theory and alcohol consumption. Addiction, 96, 57–72. 10.1046/j.1360-0443.2001.961575.x [DOI] [PubMed] [Google Scholar]
- Judd LL, Hubbard RB, Janowsky DS, Huey LY, & Atewall PA (1977). The effect of lithium carbonate on affect, mood, and personality of normal subjects. Archives of General Psychiatry, 34, 346–351. 10.1001/archpsyc.1977.01770150104012 [DOI] [PubMed] [Google Scholar]
- King AC, de Wit H, McNamara PJ, & Cao D (2011). Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Archives of General Psychiatry, 68, 389–399. 10.1001/archgenpsychiatry.2011.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Hasin D, O’Connor SJ, McNamara PJ, & Cao D (2016). A prospective 5-year re-examination of alcohol response in heavy drinkers progressing in alcohol use disorder. Biological Psychiatry, 79, 489–498. 10.1016/j.biopsych.2015.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Houle T, de Wit H, Holdstock L, & Schuster A (2002). Biphasic alcohol response differs in heavy versus light drinkers. Alcoholism, Clinical and Experimental Research, 26, 827–835. 10.1111/j.1530-0277.2002.tb02611.x [DOI] [PubMed] [Google Scholar]
- King AC, McNamara PJ, Hasin DS, & Cao D (2014). Alcohol challenge responses predict future alcohol use disorder symptoms: A 6-year prospective study. Biological Psychiatry, 75, 798–806. 10.1016/j.biopsych.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laforge RG, Borsari B, & Baer JS (2005). The utility of collateral informant assessment in college alcohol research: Results from a longitudinal prevention trial. Journal of Studies on Alcohol, 66, 479–487. 10.15288/jsa.2005.66.479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NK, Oei TPS, & Greeley JD (1999). The interaction of alcohol expectancies and drinking refusal self-efficacy in high- and low-risk drinkers. Addiction Research, 7, 91–102. 10.3109/16066359909004377 [DOI] [Google Scholar]
- Leigh BC, & Stacy AW (2004). Alcohol expectancies and drinking in different age groups. Addiction, 99, 215–227. 10.1111/j.1360-0443.2003.00641.x [DOI] [PubMed] [Google Scholar]
- Mann K, Roos CR, Hoffmann S, Nakovics H, Lemenager T, Heinz A, & Witkiewitz K (2018). Precision medicine in alcohol dependence: A controlled trial testing pharmacotherapy response among reward and relief drinking phenotypes. Neuropsychopharmacology, 43, 891–899. 10.1038/npp.2017.282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann LM, Chassin L, & Sher KJ (1987). Alcohol expectancies and the risk for alcoholism. Journal of Consulting and Clinical Psychology, 55, 411–417. 10.1037/0022-006X.55.3.411 [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, & Swift RM (1993). Development and validation of the Biphasic Alcohol Effects Scale. Alcoholism, Clinical and Experimental Research, 17, 140–146. 10.1111/j.1530-0277.1993.tb00739.x [DOI] [PubMed] [Google Scholar]
- Martino SC, Collins RL, Ellickson PL, Schell TL, & McCaffrey D (2006). Socio-environmental influences on adolescents’ alcohol outcome expectancies: A prospective analysis. Addiction, 101, 971–983. 10.1111/j.1360-0443.2006.01445.x [DOI] [PubMed] [Google Scholar]
- Mayfield DG (1968). Psychopharmacology of alcohol: Affective change with intoxication, drinking behavior, and affective state. Journal of Nervous and Mental Disease, 146, 314–321. 10.1097/00005053-196804000-00006 [DOI] [PubMed] [Google Scholar]
- McMahon J, Jones BT, & O’Donnell P (1994). Comparing positive and negative alcohol expectancies in male and female social drinkers. Addiction Research, 1, 349–365. 10.3109/16066359409005202 [DOI] [Google Scholar]
- Morean ME, & Corbin WR (2010). Subjective response to alcohol: A critical review of the literature. Alcoholism, Clinical and Experimental Research, 34, 385–395. 10.1111/j.1530-0277.2009.01103.x [DOI] [PubMed] [Google Scholar]
- Morean ME, Corbin WR, & Treat TA (2012). The Anticipated Effects of Alcohol Scale: Development and psychometric evaluation of a novel assessment tool for measuring alcohol expectancies. Psychological Assessment, 24, 1008–1023. 10.1037/a0028982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, Corbin WR, & Treat TA (2013). The Subjective Effects of Alcohol Scale: Development and psychometric evaluation of a novel assessment tool for measuring subjective response to alcohol. Psychological Assessment, 25, 780–795. 10.1037/a0032542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, Corbin WR, & Treat TA (2015). Evaluating the accuracy of alcohol expectancies relative to subjective response to alcohol. Addictive Behaviors, 51, 197–203. 10.1016/j.addbeh.2015.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, Zellers S, Tamler M, & Krishnan-Sarin S (2016). Psychometric validation of measures of alcohol expectancies, retrospective subjective response, and positive drinking consequences for use with adolescents. Addictive Behaviors, 58, 182–187. 10.1016/j.addbeh.2016.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (2017). Mplus user’s guide (1998–2017). Los Angeles, CA: Author. [Google Scholar]
- Newlin DB, & Thomson JB (1990). Alcohol challenge with sons of alcoholics: A critical review and analysis. Psychological Bulletin, 108, 383–402. 10.1037/0033-2909.108.3.383 [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Corbin WR, Leeman RF, DeMartini KS, Fucito LM, Ikomi J, . . . Gueorguieva R. (2015). Reduction of alcohol drinking in young adults by naltrexone: A double-blind, placebo-controlled, randomized clinical trial of efficacy and safety. The Journal of Clinical Psychiatry, 76, e207–e213. 10.4088/JCP.13m08934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins HW (2002). Surveying the damage: A review of research on consequences of alcohol misuse in college populations. Journal of Studies on Alcohol Supplement, 14, 91–100. 10.15288/jsas.2002.s14.91 [DOI] [PubMed] [Google Scholar]
- Peter JP, Churchill GA Jr., & Brown TJ (1993). Caution in the use of difference scores in consumer research. The Journal of Consumer Research, 19, 655–662. 10.1086/209329 [DOI] [Google Scholar]
- Piasecki TM, Jahng S, Wood PK, Robertson BM, Epler AJ, Cronk NJ, . . . Sher KJ. (2011). The subjective effects of alcohol–tobacco co-use: An ecological momentary assessment investigation. Journal of Abnormal Psychology, 120, 557–571. 10.1037/a0023033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn PD, & Fromme K (2011). Subjective response to alcohol challenge: A quantitative review. Alcoholism, Clinical and Experimental Research, 35, 1759–1770. 10.1111/j.1530-0277.2011.01521.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rather BC, Goldman MS, Roehrich L, & Brannick M (1992). Empirical modeling of an alcohol expectancy memory network using multidimensional scaling. Journal of Abnormal Psychology, 101, 174–183. 10.1037/0021-843X.101.1.174 [DOI] [PubMed] [Google Scholar]
- Rehm J, Shield KD, Joharchi N, & Shuper PA (2012). Alcohol consumption and the intention to engage in unprotected sex: Systematic review and meta-analysis of experimental studies. Addiction, 107, 51–59. 10.1111/j.1360-0443.2011.03621.x [DOI] [PubMed] [Google Scholar]
- Rotter JB, Chance JE, & Phares EJ (1972). Applications of a social learning theory of personality. New York, NY: Holt, Rinehart & Winston. [Google Scholar]
- Schafer JL, & Graham JW (2002). Missing data: Our view of the state of the art. Psychological Methods, 7, 147–177. 10.1037/1082-989X.7.2.147 [DOI] [PubMed] [Google Scholar]
- Schuckit MA (1980). Self-rating of alcohol intoxication by young men with and without family histories of alcoholism. Journal of Studies on Alcohol, 41, 242–249. 10.15288/jsa.1980.41.242 [DOI] [PubMed] [Google Scholar]
- Schuckit MA (1984). Subjective responses to alcohol in sons of alcoholics and control subjects. Archives of General Psychiatry, 41, 879–884. 10.1001/archpsyc.1984.01790200061008 [DOI] [PubMed] [Google Scholar]
- Schuckit MA (1994). Low level of response to alcohol as a predictor of future alcoholism. The American Journal of Psychiatry, 151, 184–189. 10.1176/ajp.151.2.184 [DOI] [PubMed] [Google Scholar]
- Schuckit MA (1998). Biological, psychological and environmental predictors of the alcoholism risk: A longitudinal study. Journal of Studies on Alcohol, 59, 485–494. 10.15288/jsa.1998.59.485 [DOI] [PubMed] [Google Scholar]
- Schuckit MA (2009). Alcohol-use disorders. Lancet, 373, 492–501. 10.1016/S0140-6736(09)60009-X [DOI] [PubMed] [Google Scholar]
- Schuckit MA, & Smith TL (1996). An 8-year follow-up of 450 sons of alcoholic and control subjects. Archives of General Psychiatry, 53, 202–210. 10.1001/archpsyc.1996.01830030020005 [DOI] [PubMed] [Google Scholar]
- Schuckit MA, & Smith TL (2000). The relationships of a family history of alcohol dependence, a low level of response to alcohol and six domains of life functioning to the development of alcohol use disorders. Journal of Studies on Alcohol, 61, 827–835. 10.15288/jsa.2000.61.827 [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Anderson KG, & Brown SA (2004). Testing the level of response to alcohol: Social information processing model of alcoholism risk—A 20-year prospective study. Alcoholism, Clinical and Experimental Research, 28, 1881–1889. 10.1097/01.ALC.0000148111.43332.A5 [DOI] [PubMed] [Google Scholar]
- Scott-Sheldon LA, Terry DL, Carey KB, Garey L, & Carey MP (2012). Efficacy of expectancy challenge interventions to reduce college student drinking: A meta-analytic review. Psychology of Addictive Behaviors, 26, 393–405. 10.1037/a0027565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settles RF, Cyders M, & Smith GT (2010). Longitudinal validation of the acquired preparedness model of drinking risk. Psychology of Addictive Behaviors, 24, 198–207. 10.1037/a0017631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher KJ, Wood MD, Wood PK, & Raskin G (1996). Alcohol outcome expectancies and alcohol use: A latent variable cross-lagged panel study. Journal of Abnormal Psychology, 105, 561–574. 10.1037/0021-843X.105.4.561 [DOI] [PubMed] [Google Scholar]
- Skinner BF (1963). Operant behavior. American Psychologist, 18, 503–515. 10.1037/h0045185 [DOI] [Google Scholar]
- Smith GT, Goldman MS, Greenbaum PE, & Christiansen BA (1995). The expectancy for social facilitation from drinking: The divergent paths of high-expectancy and low-expectancy adolescents. Journal of Abnormal Psychology, 104, 32–40. 10.1037/0021-843X.104.1.32 [DOI] [PubMed] [Google Scholar]
- Sobell LC, & Sobell MB (1992). Timeline follow-back. In Litten R & Allen J (Eds.), Measuring alcohol consumption (pp. 41–72). Totowa, NJ: Humana Press. 10.1007/978-1-4612-0357-5_3 [DOI] [Google Scholar]
- Sokolovsky AW, Gunn RL, Micalizzi L, White HR, & Jackson KM (2020). Alcohol and marijuana co-use: Consequences, subjective intoxication, and the operationalization of simultaneous use. Drug and Alcohol Dependence, 212, 107986. 10.1016/j.drugalcdep.2020.107986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick L, Steele C, Marlatt GA, & Linde UM (1981). Alcoholrelated expectancies: Defined by phase of intoxication and drinking experience. Journal of Consulting and Clinical Psychology, 49, 713–721. 10.1037/0022-006X.49.5.713 [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS, & Ullman JB (2007). Using multivariate statistics (Vol. 5). Boston, MA: Pearson. [Google Scholar]
- Thomas SE, Drobes DJ, Voronin K, & Anton RF (2004). Following alcohol consumption, non-treatment-seeking alcoholics report greater stimulation but similar sedation compared with social drinkers. Journal of Studies on Alcohol, 65, 330–335. 10.15288/jsa.2004.65.330 [DOI] [PubMed] [Google Scholar]
- Trim RS, Schuckit MA, & Smith TL (2009). The relationships of the level of response to alcohol and characteristics to alcohol use disorders across adulthood: A discrete-time survival analysis. Alcoholism, Clinical and Experimental Research, 9, 1562–1570. 10.1111/j.1530-0277.2009.00984.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrisi R, Wiersma KA, & Hughes KK (2000). Binge-drinkingrelated consequences in college students: Role of drinking beliefs and mother –teen communications. Psychology of Addictive Behaviors, 14, 342–355. 10.1037/0893-164X.14.4.342 [DOI] [PubMed] [Google Scholar]
- Waddell JT, Blake AJ, Sternberg A, Ruof A, & Chassin L (2020). The effects of observable parent alcohol consequences and parent alcohol disorder on adolescent alcohol expectancies. Alcoholism, Clinical and Experimental Research, 44, 973–982. 10.1111/acer.14298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall AM, Thrussell C, & Lalonde RN (2003). Do alcohol expectancies become intoxicated outcomes? A test of social-learning theory in a naturalistic bar setting. Addictive Behaviors, 28, 1271–1283. 10.1016/S0306-4603(02)00253-8 [DOI] [PubMed] [Google Scholar]
- Walther CAP, Pedersen SL, Gnagy E, Pelham WE, & Molina BSG (2019). Specificity of expectancies prospectively predicting alcohol and marijuana use in adulthood in the Pittsburgh ADHD longitudinal study. Psychology of Addictive Behaviors, 33, 117–127. 10.1037/adb0000439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler H, Davenport A, Dowdall G, Moeykens B, & Castillo S (1994). Health and behavioral consequences of binge drinking in college: A national survey of students at 140 campuses. Journal of the American Medical Association, 272, 1672–1677. 10.1001/jama.1994.03520210056032 [DOI] [PubMed] [Google Scholar]
- Wechsler H, Dowdall GW, Davenport A, & Castillo S (1995). Correlates of college student binge drinking. American Journal of Public Health, 85, 921–926. 10.2105/AJPH.85.7.921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiers RW, van Woerden NV, Smulders FTY, & de Jong PT (2002). Implicit and explicit alcohol-related cognitions in heavy and light drinkers. Journal of Abnormal Psychology, 111, 648–658. 10.1037/0021-843X.111.4.648 [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Roos CR, Mann K, & Kranzler HR (2019). Advancing precision medicine for alcohol use disorder: Replication and extension of reward drinking as a predictor of naltrexone response. Alcoholism, Clinical and Experimental Research, 43, 2395–2405. 10.1111/acer.14183 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.