Abstract
Background
Both BNT162b2 (Pfizer–BioNTech) and ChAdOx1 nCoV-19 (Oxford–AstraZeneca) vaccines have shown high efficacy against COVID-19 in randomized controlled trials. However, their comparative effectiveness against COVID-19 is unclear in the real world. We evaluated the comparative effectiveness of the BNT162b2 and ChAdOx1 nCoV-19 vaccines against COVID-19 in the UK general population.
Methods
We emulated a target trial using IQVIA Medical Research Database (IMRD), an electronic primary care database from the UK (2021). We included 1,311,075 participants, consisting of 637,549 men and 673,526 women age≥18 years, who received vaccination with BNT162b2 or ChAdOx1 nCoV-19 between January 1 and August 31, 2021. The outcomes consisted of confirmed diagnosis of SARS-CoV-2 infection, hospitalisation for COVID-19 and death from COVID-19 in the IMRD. We performed a cox-proportional hazard model to compare the risk of each outcome variable between the two vaccines adjusting for potential confounders with time-stratified overlap weighting of propensity score (PS).
Results
During a mean of 6.7 months of follow-up, 20,070 confirmed SARS-CoV-2 infection occurred in individuals who received BNT162b2 vaccine (PS weighted incidence rate: 3.65 per 1000 person-months), and 31,611 SARS-CoV-2 infection occurred in those who received ChAdOx1 nCoV-19 vaccine (PS weighted incidence rate: 5.25 per 1000 person-months). The time-stratified PS weighted rate difference of SARS-CoV-2 infection for BNT162b2 group vs. ChAdOx1 nCoV-19 group was -1.60 per 1000 person-months (95% confidence interval [CI]: -1.76 to -1.43 per 1000 person-months), and the hazard ratio was 0.69 (95% CI: 0.68 to 0.71). The results were similar across the stratum of sex, age (<65 and ≥65 years), and study periods (i.e., alpha-variant predominance period and delta-variant predominance period). The PS weighted incidence of hospitalisation for COVID-19 was also lower in the BNT162b2 vaccine group than that in the ChAdOx1 vaccine group (RD: -0.09, 95%CI: -0.13 to -0.05 per 1000 person-months; HR: 0.65, 95%CI: 0.57 to 0.74). No significant difference in the risk of death from COVID-19 was observed between the two comparison groups.
Conclusions
In this population-based study, the BNT162b2 vaccine appears to be more efficacious than the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2 infection and hospitalisation for COVID-19 but not death from COVID-19.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-023-02795-w.
Keywords: COVID-19, Vaccine, BNT162b2, ChAdOx1 nCoV-19
Background
Covid-19 vaccination is critical for controlling the COVID-19 pandemic. The United Kingdom (UK) implemented a COVID-19 vaccination program after the emergency use approval of the Pfizer-BioNTech messenger RNA (mRNA) vaccine, BNT162b2, in December 2020 [1]. Later, the vaccination program was expanded to include the Oxford-AstraZeneca adenovirus (AdV) vector vaccine, ChAdOx1 nCoV-19 [2] (hereafter referred to ChAdOx1), and Moderna mRNA vaccine mRNA-1273 [3]. To date, the majority have received two doses of either BNT162b2 or ChAdOx1 vaccines in the UK [4, 5]. To date, the percentage of fully vaccinated population remains low (<20%) in most countries of Africa and several countries of West Asia [6].
Previous studies reported that the AdV vector vaccine might induce higher levels of specific T cells, whereas the mRNA vaccine might induce higher antibody titers [7–9]. Results from the phase III clinical trials showed 95% efficacy for BNT162b2 [10] and 70% efficacy for ChAdOx1 [11] against COVID-19 after two vaccine doses. However, the results from the observational studies were inconsistent when the effectiveness of these two vaccines was compared with non-vaccination. Some cohort studies [12–15] and a test negative case-control study [16] showed higher effectiveness against COVID-19 for the BNT162b2 vaccine than the ChAdOx1 vaccine when compared with unvaccinated individuals, whereas other cohort studies [4, 17–19] and test negative case-control study [20] found no apparent difference in the effectiveness of these two vaccines against COVID-19 compared with unvaccinated individuals. To date, there is a paucity of evidence of head-to-head comparisons of these two vaccines, leaving knowledge gaps regarding which vaccine is more effective against COVID-19.
Therefore, we emulated a target trial to evaluate the comparative effectiveness of the BNT162b2 vaccine vs. the ChAdOx1 vaccine against COVID-19 using data from a UK primary care database.
Methods
Data sources
We used data from the IQVIA Medical Research Database (IMRD) (previously called The Health Improvement Network (THIN)), an electronic health records database of general practitioner (GP) based healthcare covering 839 practices and 19 million individuals in the UK. The IMRD was established in 2003 as a collaboration between the company owning Vision (In Practice Systems) and the CSD Medical Research Group (now Quintiles IMS). The database contains computerised information on socio-demographics, anthropometric characteristics, lifestyle factors, and details from visits to GPs (i.e., prescriptions, diagnosis, diagnoses and interventions from specialist referrals, hospital admissions, and results of laboratory tests). The READ classification system is used to code specific diagnoses [21], whereas a dictionary based on the Multilex classification system is used to code drugs [22]. The validity of the IMRD for use in clinical and epidemiological research studies has been demonstrated in a previous study [23]. The scientific review committee for the IMRD database and the institutional review board at Xiangya Hospital approved this study, with a waiver of informed consent. This study followed the recommendations of the STROBE initiative for reporting observational studies in epidemiology [24].
Study design and cohort definition
We emulated a target trial to compare the risk of SARS-CoV-2 infection and its sequelae, i.e., hospitalisation and death in individuals who received the BNT162b2 vaccine with those who received the ChAdOx1 vaccine. We included individuals between 18 to 89 years of age, who received their first covid vaccination with either of these two vaccines from January 1, 2021, to August 31, 2021, and had at least two years of continuous enrolment with a general practice prior to entering the study. The details of vaccination records were based on the READ code in IMRD (Additional file 1: Table S1) [22]. The date of the first dose of either the BNT162b2 vaccine or the ChAdOx1 vaccine was assigned as the index date. Individuals were excluded if they had received a SARS-CoV-2 infection diagnosis or a different COVID-19 vaccine (i.e., mRNA-1273 [Moderna] and Ad26.CoV2.S [Janssen/Johnson &Johnson]) prior to the index date.
Emulation of the target trial
We divided the baseline study period into weekly time blocks. Eligible individuals were allocated into these blocks according to their index dates. In each weekly time block we calculated the propensity score (PS) and adopted an overlap weighting approach to balance baseline characteristics (Fig. 1) [25, 26]. Specifically, the PS for the BNT162b2 vaccine was calculated in each weekly time block using a logistic regression model that included potential confounders. Individuals receiving the BNT162b2 vaccine were weighted by the probability of not receiving the BNT162b2 vaccine, i.e., 1-PS, and individuals receiving the ChAdOx1 vaccine were weighted by the probability of receiving the BNT162b2 vaccine, i.e., PS. Overlap weights were bounded and smoothly reduced the influence of individuals at the tails of the PS distribution without making any exclusions.
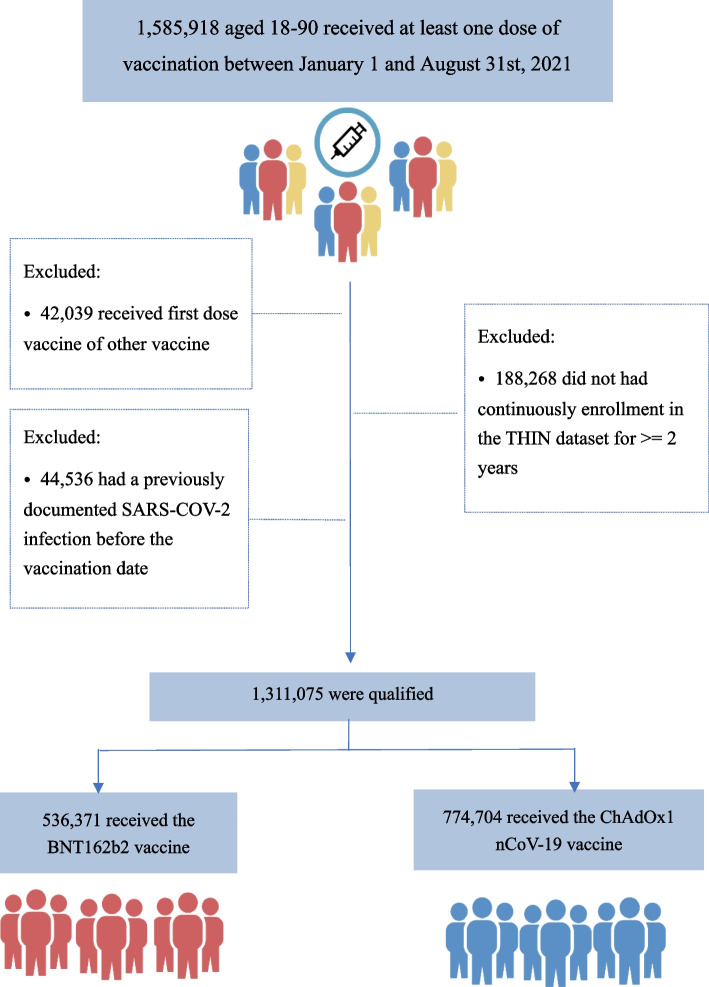
Fig. 1.
Selection process of participants for the emulation of a target trial evaluating the comparative effectiveness of the BNT162b2 and ChAdOx1 nCoV-19 vaccines
Assessment of outcome
The primary outcome was a confirmed diagnosis of SARS-CoV-2 infection, and the secondary outcomes were hospitalisation for COVID-19 and death from COVID-19. A confirmed diagnosis of SARS-CoV-2 infection was based on READ codes recommended in national guidelines (Additional file 1: Table S1) [27, 28]. According to National Health Service Guidance and Standard Operating Procedures for Primary Care, and the UK Faculty of Clinical Informatics guidelines, confirmed SARS-CoV-2 infection codes represent a positive RT-PCR test [29]. Hospitalisation for COVID-19 was defined as a hospitalisation record in the IMRD [30] within 30 days after documentation of SARS-CoV-2 infection, and death from COVID-19 was defined as a death within 30 days after documentation of SARS-CoV-2 infection.
Assessment of negative control outcome
We used two negative control outcomes (i.e., SARS-CoV-2 infection occurred over the 14 days after receiving the first dose of the COVID-19 vaccine and death from cardiovascular disease [CVD] defined as a death within 30 days before or after documentation of myocardial infarction, stroke or heart failure) to evaluate for potential residual confounding [31]. No difference in the risk of the outcomes between two comparison groups should be observed unless there were residual confounders (e.g., health status or healthcare-seeking behaviour).
Assessment covariates
The variables in the PS model consisted of sociodemographic factors (i.e., age, sex, Townsend Deprivation Index), geographic location, race, body mass index (BMI), lifestyle factors (i.e., alcohol use and smoking status), influenza vaccination history during the past one year before the index date, comorbidities prior to the index date (i.e., hypertension, diabetes, chronic kidney disease, pneumonia or infection, chronic obstructive pulmonary disease, influenza, cancer, venous thrombosis, atrial fibrillation, ischemic heart disease, congestive heart failure, stroke, trauma, fracture, liver disease, fall, dementia, and depression), medication use (antidiabetic, antihypertensive, statin, diuretics, glucocorticoids, non-steroidal anti-inflammatory drugs, opioids, proton-pump inhibitors, biologic disease modifying antirheumatic drugs) and healthcare utilization during the past one year immediately before the index date. A directed acyclic graph of the comparative effectiveness of BNT162b2 and ChAdOx1 nCoV-19 vaccines against COVID-19 and potential confounders was shown in Additional file 1: Figure S1.
Statistical analysis
The baseline characteristics of individuals who received the BNT162b2 vaccine were compared with individuals who received the ChAdOx1 vaccine using standardized difference. For the primary outcome, person-months of follow-up for each individual were calculated as the amount of time from the index date to the first of the following events: confirmed diagnoses of SARS-CoV-2 infection, death, disenrollment from a GP practice participating in IMRD, 10 months follow-up, receiving a different COVID-19 vaccine of the second dose, or the end of the study (October 31, 2021). We calculated the rate of SARS-CoV-2 infection and plotted cumulative incidence curves of SARS-CoV-2 infection for individuals who received the BNT162b2 vaccine and individuals who received the ChAdOx1 vaccine, respectively. We estimated the absolute rate difference (RD) of SARS-CoV-2 infection between the two comparison groups. We obtained the hazard ratio (HR) of SARS-CoV-2 infection for the individuals who received the BNT162b2 vaccine vs. those who received the ChAdOx1 vaccine using Cox proportional hazard model accounting for competing event (i.e., death). We tested the proportional hazard assumption by plotting the cumulative incidence curve of each outcome. We then conducted a weighted cox regression to obtain a non-proportional HR if the proportional hazard assumption was violated [32]. We repeated the analysis to evaluate the comparative effectiveness of the two vaccines for secondary outcomes.
We performed four sensitivity analyses to assess the robustness of the study findings. First, we conducted subgroup analyses to assess the comparative effectiveness of these two vaccines according to sex (male vs. female), age (≥65 vs. <65 years), and study period (i.e., SARS-CoV-2 alpha-variant predominance period from January 1 to May 16, 2021 vs. delta-variant predominance period from May 17 to October 31, 2021 [13]). Second, to evaluate the potential residual confounding effect, we examined the relation of the BNT162b2 vaccine vs. the ChAdOx1 vaccine to the risk of SARS-CoV-2 infection in the first 14 days after the first dose of vaccination and death from CVD, respectively. Third, we performed the analysis by including the participants who had SARS-COV-2 infection prior to the index date. In this analysis, we added the history of previous SARS-COV-2 infection when calculating the PS. Fourth, we assessed the risk of SARS-CoV-2 infection among participants who received two doses of vaccines and one dose of vaccine separately.
All P values were 2-sided and P<0.05 was considered significant for all tests. All statistical analyses were performed with SAS software, version 9.4 (SAS Institute, Cary, North Carolina, USA) and R Studio, version 1.1,456 (R Foundation, Vienna, Austria).
Results
Among 1,585,918 individuals aged 18-90 years who received at least one dose of vaccination between January 1 and August 31, 2021, in the IMRD, 536,371 individuals received the BNT162b2 vaccine and 774,704 received the ChAdOx1 nCoV-19 vaccine. The baseline characteristics of the study population are shown in Table 1. More than 90% of individuals in each group received a second dose of the same vaccine. Before PS overlap weighting, individuals who received the BNT162b2 vaccine were younger, had a lower BMI, a lower percentage of influenza vaccination, comorbidities, and medication use. After PS overlap weighting, all measured baseline characteristics were well-balanced between the two comparison groups (all standardized difference < 0.02).
Table 1.
Baseline characteristics of individuals newly received the BNT162b2 and the ChAdOx1 vaccine
| Before propensity-score overlap weighting | After propensity-score overlap weighting | |||||
|---|---|---|---|---|---|---|
| BNT162b2 | ChAdOx1 | Standardized difference |
BNT162b2 | ChAdOx1 | Standardized difference |
|
| Number | 536,371 | 774,704 | 163,716 | 163,716 | ||
| Demographics | ||||||
| Age, mean (SD), y | 46.37 (19.13) | 57.82 (14.82) | 0.669 | 58.32 (15.77) | 58.32 (16.75) | <0.001 |
| Race (%) | 0.075 | <0.001 | ||||
| White | 42.7 | 46 | 45 | 45 | ||
| Other | 3.4 | 2.6 | 2.8 | 2.8 | ||
| Missing | 53.9 | 51.3 | 52.2 | 52.2 | ||
| Socioeconomic deprivation index score (%)a | 0.052 | <0.001 | ||||
| 1 | 15.5 | 15.8 | 16.7 | 16.7 | ||
| 2 | 18.5 | 19.7 | 19.8 | 19.8 | ||
| 3 | 19.7 | 20.1 | 19.8 | 19.8 | ||
| 4 | 18.6 | 18.6 | 17.7 | 17.7 | ||
| 5 | 15.5 | 14.9 | 14.2 | 14.2 | ||
| Missing | 12.2 | 10.9 | 11.9 | 11.9 | ||
| Female (%) | 52.9 | 50.3 | 0.052 | 52.2 | 52.2 | <0.001 |
| BMI, mean (SD), kg/m2 | 27.44 (5.87) | 28.45 (6.30) | 0.165 | 27.98 (5.80) | 28.07 (6.00) | 0.015 |
| Region | 0.105 | <0.001 | ||||
| England | 14.8 | 13.6 | 15.1 | 15.1 | ||
| Northern Ireland | 15.3 | 12.3 | 17.6 | 17.6 | ||
| Scotland | 39.1 | 42.8 | 38.1 | 38.1 | ||
| Wales | 30.8 | 31.4 | 29.2 | 29.2 | ||
| Influenza vaccination (%)b | 31.8 | 45 | 0.272 | 49.1 | 49.1 | <0.001 |
| Lifestyle factors | ||||||
| Drinking (%) | 0.037 | <0.001 | ||||
| None | 19.1 | 18.1 | 18.2 | 18.2 | ||
| Past | 2.9 | 3.4 | 3.5 | 3.5 | ||
| Current | 77.9 | 78.5 | 78.3 | 78.3 | ||
| Smoking (%) | 0.134 | <0.001 | ||||
| None | 62 | 55.8 | 56.9 | 56.9 | ||
| Past | 21.7 | 26.8 | 27.5 | 27.5 | ||
| Current | 16.2 | 17.4 | 15.6 | 15.6 | ||
| Comorbidity (%) | ||||||
| Hypertension | 18.2 | 28.7 | 0.249 | 30.5 | 30.5 | <0.001 |
| Diabetes | 8.9 | 14 | 0.162 | 14.1 | 14.1 | <0.001 |
| Chronic kidney disease | 3.0 | 5.0 | 0.102 | 5.5 | 5.5 | <0.001 |
| Pneumonia or infection | 5.4 | 6.8 | 0.060 | 6.7 | 6.7 | <0.001 |
| Chronic obstructive pulmonary disease | 2.3 | 4.4 | 0.112 | 4.6 | 4.6 | <0.001 |
| Influenza | 3.1 | 3.6 | 0.030 | 3.6 | 3.6 | <0.001 |
| Cancer | 6.3 | 9.2 | 0.106 | 10.4 | 10.4 | <0.001 |
| Venous thrombosis | 1.5 | 2.6 | 0.079 | 2.5 | 2.5 | <0.001 |
| Atrial fibrillation | 2.2 | 3.8 | 0.095 | 4.1 | 4.1 | <0.001 |
| Ischemic heart disease | 4 | 6.7 | 0.120 | 7.3 | 7.3 | <0.001 |
| Myocardial infarction | 1.8 | 3.2 | 0.088 | 3.3 | 3.3 | <0.001 |
| Congestive heart failure | 1.1 | 2.0 | 0.075 | 2.0 | 2.0 | <0.001 |
| Stroke | 1.4 | 2.6 | 0.085 | 2.6 | 2.6 | <0.001 |
| Trauma | 0.9 | 1.1 | 0.025 | 1.0 | 1.0 | <0.001 |
| Fracture | 31.2 | 33.3 | 0.044 | 32.6 | 32.6 | <0.001 |
| Liver disease | 2.2 | 3.4 | 0.075 | 3.4 | 3.4 | <0.001 |
| Fall | 5.8 | 7.8 | 0.079 | 8.0 | 8.0 | <0.001 |
| Dementia | 0.6 | 1.1 | 0.053 | 1.1 | 1.1 | <0.001 |
| Depression | 12.4 | 15.2 | 0.083 | 14.4 | 14.4 | <0.001 |
| Medication (%)b | ||||||
| Antihypertensive | 22.1 | 33 | 0.247 | 34.8 | 34.8 | <0.001 |
| Antidiabetic | 4.5 | 7.9 | 0.142 | 7.6 | 7.6 | <0.001 |
| Statin | 15 | 23.2 | 0.212 | 25.9 | 25.9 | <0.001 |
| Loop diuretics | 2.0 | 3.9 | 0.117 | 3.8 | 3.8 | <0.001 |
| Thiazide diuretics | 3.6 | 5.5 | 0.093 | 6.2 | 6.2 | <0.001 |
| Glucocorticoids | 3.3 | 5 | 0.084 | 5.2 | 5.2 | <0.001 |
| NSAIDs | 16.1 | 21.4 | 0.136 | 21.2 | 21.2 | <0.001 |
| Opioids | 5.0 | 8.1 | 0.127 | 7.6 | 7.6 | <0.001 |
| PPIs | 20.1 | 27.9 | 0.182 | 28.5 | 28.5 | <0.001 |
| DMARDs | 1.1 | 1.7 | 0.051 | 1.9 | 1.9 | <0.001 |
| Healthcare utilization, mean (SD)b | ||||||
| Hospitalizationsb | 0.19 (0.70) | 0.24 (0.86) | 0.057 | 0.24 (0.82) | 0.24 (0.80) | <0.001 |
| General practice visitsb | 1.69 (3.13) | 2.13 (3.95) | 0.123 | 2.13 (3.87) | 2.13 (3.53) | <0.001 |
| Specialist referralsb | 0.21 (0.62) | 0.23 (0.66) | 0.026 | 0.24 (0.67) | 0.24 (0.69) | <0.001 |
BMI Body mass index, n number, y years, SD Standard deviation, NSAIDs Non-steroidal anti-inflammatory drugs, PPIs Proton-pump inhibitors, DMARDs Biologic disease modifying antirheumatic drugs
aThe Socio-Economic Deprivation Index was measured by the Townsend Deprivation Index, which was grouped into quintiles from 1 (least deprived) to 5 (most deprived)
bFrequency during the past year
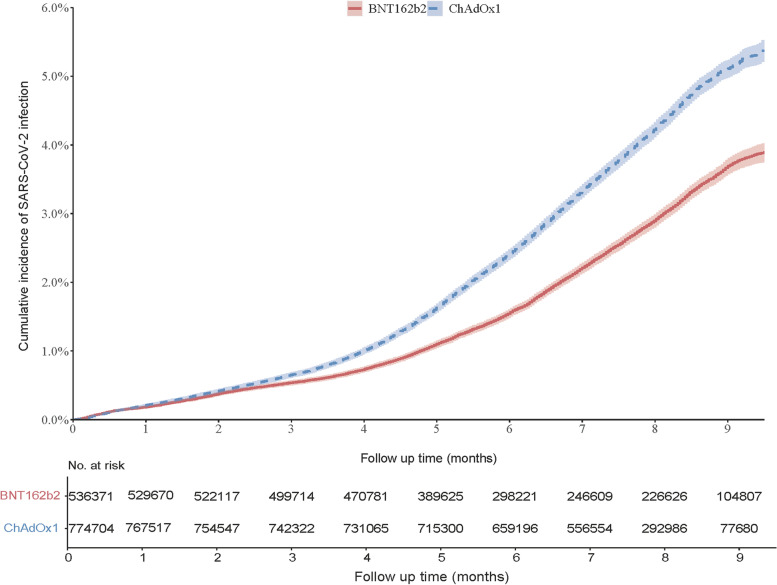
During a mean of 6.7 months of follow-up, 20,070 confirmed SARS-CoV-2 infection cases occurred in the BNT162b2 vaccine group, and 31,611 cases occurred in the ChAdOx1 nCoV-19 vaccine group. The weighted incidence of COVID-19 was lower in the BNT162b2 vaccine group (3.65 per 1000 person-months) than that in the ChAdOx1 vaccine group (5.25 per 1000 person-months) (Fig. 2 and Table 2). Compared with those who received the ChAdOx1 vaccine, the RD of SARS-CoV-2 infection among individuals who received the BNT162b2 vaccine was -1.60 (95% confidence interval [CI]: -1.76 to -1.43) per 1000 person-months, and HR was 0.69 (95%CI: 0.68 to 0.71). The results were consistent across the stratum of sex, age (<65 and ≥65 years), and calendar periods (alpha-variant predominance period and delta-variant predominance period), including the individuals who were infected prior to the index date, as well as among participants who received two doses of vaccines. There was no significant difference between BNT162b2 and ChAdOx1 vaccines for SARS-CoV-2 infection among the participants who received only one dose of the vaccine under investigation.
Fig. 2.
Cumulative incidence of SARS-CoV-2 infection between participants received BNT162b2 and ChAdOx1 nCoV-19 vaccines
Table 2.
Estimated comparative effectiveness of the BNT162b2 and ChAdOx1 vaccines for SARS-CoV-2 infection
| Population | Number | No. of Events | Mean follow up, months | Incidence ratea, per 1000 person-months | Rate differencea (95%CI), per 1000 person-months |
Hazard ratioa (95%CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| BNT162b2 | ChAdOx1 | BNT162b2 | ChAdOx1 | BNT162b2 | ChAdOx1 | BNT162b2 | ChAdOx1 | |||
| Overall | 536,371 | 774,704 | 20,070 | 31,611 | 6.65 | 7.33 | 3.65 | 5.25 | -1.60 (-1.76, -1.43) | 0.69 (0.68, 0.71) |
| Sex | ||||||||||
| Male | 252,646 | 384,903 | 8,582 | 14,666 | 6.34 | 7.25 | 3.42 | 4.85 | -1.44 (-1.67, -1.20) | 0.70 (0.68, 0.73) |
| Female | 283,725 | 389,801 | 11,488 | 16,945 | 6.93 | 7.40 | 3.85 | 5.59 | -1.74 (-1.98, -1.50) | 0.68 (0.66, 0.71) |
| Age | ||||||||||
| Age>=65 | 132,624 | 235,594 | 2,482 | 4,886 | 8.49 | 8.36 | 2.17 | 2.72 | -0.55 (-0.74, -0.36) | 0.79 (0.75, 0.84) |
| Age<65 | 403,747 | 539,110 | 17,588 | 26,725 | 6.07 | 6.89 | 5.15 | 7.62 | -2.47 (-2.76, -2.19) | 0.67 (0.65, 0.70) |
| Study period | ||||||||||
| Alpha-variant predominance | 356,679 | 739,203 | 1,252 | 1,227 | 2.61 | 2.24 | 0.84 | 0.94 | -0.10 (-0.23, 0.04) | 0.89 (0.80, 1.00) |
| Delta-variant predominance | 179,692 | 35,501 | 8,413 | 1,996 | 4.31 | 4.86 | 8.02 | 11.99 | -3.96 (-5.02, -2.90) | 0.67 (0.62, 0.72) |
| Including prior infected population | 561,140 | 803,200 | 20,398 | 32,007 | 6.63 | 7.32 | 3.65 | 5.28 | -1.62 (-1.79, -1.47) | 0.69 (0.67, 0.71) |
| Received two doses | 491,591 | 730,363 | 12,229 | 28,196 | 6.96 | 7.58 | 3.01 | 4.59 | -1.58 (-1.73, -1.42) | 0.65 (0.64, 0.67) |
| Received one dose | 29,701 | 21,870 | 2,138 | 905 | 4.26 | 5.13 | 9.07 | 8.91 | 0.16 (-1.31, 1.64) | 1.01 (0.91, 1.14) |
No Number, CI Confidence interval
aEstimates were time-stratified overlap weighted of propensity score
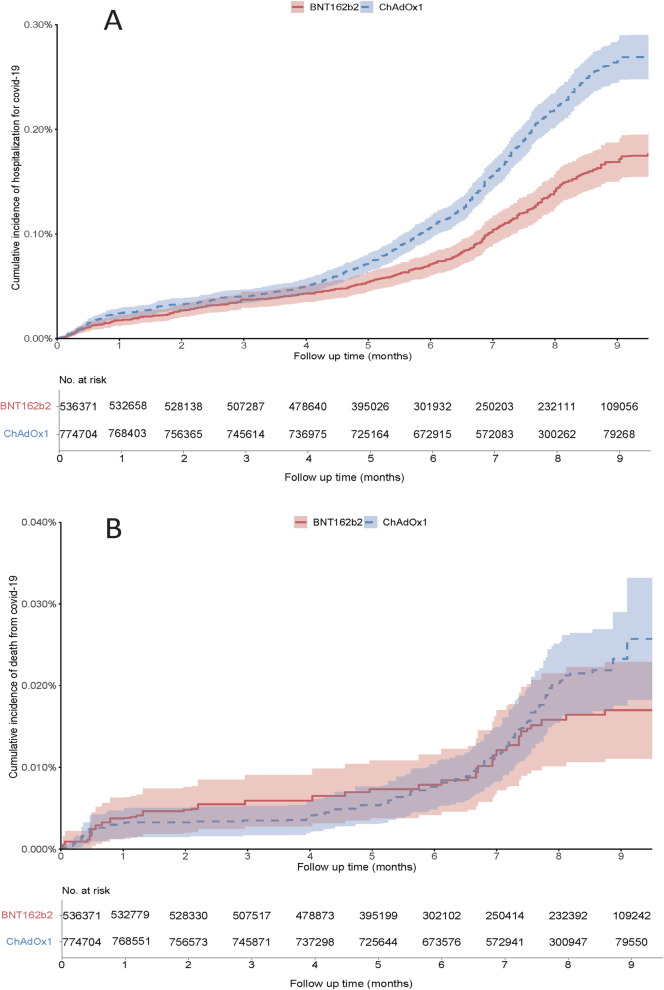
There were 603 and 1,432 hospitalisations for COVID-19 in individuals who received the BNT162b2 vaccine and in those who received the ChAdOx1 nCoV-19 vaccine, respectively. The weighted incidence of hospitalisation for COVID-19 was lower in the BNT162b2 vaccine group (0.17 per 1000 person-months) than that in the ChAdOx1 vaccine group (0.26 per 1000 person-months), and the corresponding RD and HR were -0.09 (95%CI: -0.13 to -0.05) per 1000 person-months and 0.65 (95%CI: 0.57 to 0.74), respectively. The mortality rate from COVID-19 was slightly lower, albeit non-statistically significant, in the BNT162b2 vaccine group (0.018 per 1000 person-months) than in the ChAdOx1 vaccine group (0.022 per 1000 person-months), with corresponding RD and HR being -0.005 (95%CI: -0.016 to 0.006) and 0.66 (95%CI: 0.42 to 1.04), respectively (Fig. 3 and Table 3).
Fig. 3.
Cumulative incidence of hospitalisation for COVID-19 (A) and death from COVID-19 (B) between participants received BNT162b2 and ChAdOx1 nCoV-19 vaccines
Table 3.
Comparison of the BNT162b2 and the ChAdOx1 vaccines for hospitalisation for COVID-19 and death from COVID-19
| Outcomes | BNT162b2 | ChAdOx1 |
|---|---|---|
| Number | 536,371 | 774,704 |
| Hospitalisation for COVID-19 | ||
| Event (n) | 603 | 1,432 |
| Mean follow-up (months) | 6.74 | 7.41 |
| Incidence ratea, per 1000 person-months | 0.17 | 0.26 |
| Rate difference (95%CI) a, per 1000 person-months | -0.09 (-0.13, -0.05) | 0.00 (ref) |
| Hazard ratio (95% CI) a | 0.65 (0.57, 0.74) | 1.00 (ref) |
| Death from COVID-19 | ||
| Event (n) | 42 | 112 |
| Mean follow-up (months) | 6.74 | 7.41 |
| Incidence ratea, per 1000 person-months | 0.018 | 0.022 |
| Rate difference (95%CI) a, per 1000 person-months | -0.005 (-0.016, 0.006) | 0.00 (ref) |
| Hazard ratio (95% CI) a | 0.66 (0.42, 1.04) | 1.00 (ref) |
CI Confidence interval
aEstimates were time-stratified overlap weighted of propensity score
No difference in the risk of SARS-CoV-2 infection during the first 14 days after receiving the first vaccine dose (i.e., negative control outcome) of the BNT162b2 vaccine vs. the ChAdOx1 vaccine was observed. Compared with those who received the ChAdOx1 vaccine, the RD of SARS-CoV-2 infection during the first 14 days after receiving the first vaccine dose among individuals who received the BNT162b2 was 0.20 (95% CI: -0.26 to 0.66) per 1000 person-months, and HR was 1.11 (95% CI: 0.94 to 1.30). Similar null association was observed in the risk of death from CVD after receiving the BNT162b2 vaccine vs. the ChAdOx1 vaccine (HR 0.90, 95%CI: 0.57 to 1.41).
Discussion
Our study provides timely real-world evidence of the comparative effectiveness of the BNT162b2 and the ChAdOx1 vaccines against the risk of SARS-CoV-2 infection. Individuals who received the BNT162b2 vaccine had a 30% lower risk of SARS-CoV-2 infection than those who received the ChAdOx1 vaccine over 10 months of follow-up. Our findings are consistent across sex, age, and study periods and unlikely to be explained by major residual confounders. In addition, the BNT162b2 vaccine appears to provide a better protective effect on hospitalisation for COVID-19 than the ChAdOx1 vaccine.
Comparison with previous studies
To date, results from their respective phase III clinical trials demonstrated that the BNT162b2 vaccine had a 95% efficacy in protecting against COVID-19, while the ChAdOx1 vaccine had a 70% efficacy against COVID-19. Consistent with the findings of clinical trials, results from several observational studies also indirectly showed a slightly higher effectiveness of the BNT162b2 vaccine than the ChAdOx1 vaccine when compared with unvaccinated individuals [12–16]. Meanwhile, other observational studies suggested similar effectiveness of the BNT162b2 and ChAdOx1 vaccines against SARS-CoV-2 infection, symptoms, hospital admissions, and death from SARS-CoV-2 infection when compared with unvaccinated individuals [4, 17–20] Our study demonstrated greater effectiveness of the BNT162b2 vaccine, indicated by both absolute (i.e., RD) and relative (i.e., HR) effects on the risk of SARS-CoV-2 infection and hospitalisation for COVID-19 than the ChAdOx1 vaccine over ten months follow-up. Although the infection rate was different between the SARS-CoV-2 alpha-variant predominance period and the delta-variant predominance period, which may be due to a differential vaccine waning, the comparative effectiveness of the BNT162b2 and ChAdOx1 vaccines against SARS-CoV-2 infection was consistent across these two study periods. This real-world empirical data provided critical evidence of the relative effectiveness of these two vaccines in mitigating the COVID-19 burden in the general population [33].
Potential explanations
Both the mRNA and AdV vector vaccines encode the production of the SARS-CoV-2 spike (S) protein, which is the major target for neutralizing antibodies generated from natural infection and for therapeutic monoclonal antibodies [34–36]. However, the two mechanisms leverage different aspects of the immune response, particularly the innate immune response, which may lead to differences in immunogenicity [34]. Previous studies have reported that the BNT162b2 vaccine might induce higher antibody titres than the ChAdOx1 vaccine [7–9], which may partially explain the differences in the effectiveness against COVID-19 between these two vaccines.
Strengths and limitations
There are several strengths to our study. First, we emulated a hypothetic trial using a population-based electronic database to evaluate the comparative effectiveness of two different vaccines against COVID-19. Second, the findings are consistent with the indirect comparison of the effectiveness of these two vaccines based on the results of their respective clinical trials. Third, results from the sensitivity analyses using negative control outcomes confirmed that the effect of residual confounding, if present, is likely to be minimal, supporting the validity of our main findings. Fourth, considering that the time-varying confounders (e.g., COVID-19 vaccines where rollout in the period specified started in the oldest age groups) [37] may impact the results, we divided the participants’ enrolment period into weekly time blocks when they received either the BNT162b2 or ChAdOx1 nCoV-19 vaccines. In each time block we calculated propensity score (PS) and adopted an overlap weighting approach in each weekly time block to balance the baseline characteristics.
However, our study also has some limitations. First, although we used rigorous approaches to control for confounding (i.e., demographic characteristics, medical history, comorbidities, and health care utilization), unmeasured residual confounding, such as the severity of comorbidities, cannot be ruled out. Second, owing to a lack of detailed information in IMRD, we are unable to assess the comparative effectiveness of two vaccines on several other sequelae of COVID-19, such as intensive care unit admission, mechanical ventilation, or length of hospitalisation. Future studies are needed to evaluate these additional outcomes. Third, we cannot access the data held in the hospital and were not reported back to GPs (e.g., tests were performed at the hospital and were not reported back to GPs). Thus, misclassification of the COVID-19 diagnosis could occur and bias the study findings. However, such bias, if occurred, is likely to be small and non-differential. As a result, it would bias the observed associations towards the null. Moreover, although the original PCR test results were not available and the sensitivity for capturing COVID-19 cases through the Read Code has not been validated in the IMRD, a recent study of COVID-19 codes recorded in the primary care setting suggested that clinical diagnosis of COVID-19 by physicians followed a similar trend to test positive cases confirmed by the UK national testing service [38]. Fourth, we could only access data within the GP system. Thus, we were unable to evaluate the comparative effectiveness of BNT162b2 and ChAdOx1 nCoV-19 vaccines among individuals who were not registered with a GP or whose vaccination record was not captured by their GP. Future studies are needed to assess whether the effectiveness of BNT162b2 and ChAdOx1 nCoV-19 vaccine varies among this population.
Clinical implications
This emulation of a target trial of a head-to-head comparison of two vaccines, provides evidence that the effectiveness of the BNT162b2 against COVID-19 appears better than the ChAdOx1 vaccine for both SARS-CoV-2 infection and hospitalisation for COVID-19 but not death from COVID-19. The percentage of fully vaccinated population remains low (<20%) in most countries of Africa and several countries of West Asia [6]. Although our findings suggest BNT162b2 vaccine may be preferred to reduce the SARS-CoV-2 infection rate and hospitalisation for COVID-19, the choice of vaccine type should not only be based on the potential differences in effectiveness. The decision regarding which COVID-19 vaccine to receive should consider other factors including availability, cost, and individual characteristics [39].
Conclusions
In this population-based data, the effectiveness of the BNT162b2 against COVID-19 appears better than the ChAdOx1 vaccine for SARS-CoV-2 infection and hospitalisation for COVID-19. Although our findings suggested that the BNT162b2 vaccine may have a slightly better effect on lowering the SARS-CoV-2 infection rate and hospitalisation for COVID-19 than the ChAdOx1 nCoV-19 vaccine, both vaccines are effective against COVID-19 and its severe sequelae and should be encouraged to receive whichever vaccine is available.
Supplementary Information
Additional file 1: Table S1. Read codes used for covid-19 vaccination records and diagnosed SARS-CoV-2 infection. Figure S1. A directed acyclic graph of the comparative effectiveness of BNT162b2 and ChAdOx1 nCoV-19 vaccines against COVID-19 and potential confounders.
Acknowledgments
Everyone who contributed significantly to the work has been listed.
Abbreviations
- AdV
Adenovirus
- IMRD
IQVIA Medical Research Database
- GPs
General practitioners
- PS
Propensity score
- BMI
Body mass index
- RD
Rate difference
- HR
Hazard ratio
- CI
Confidence interval
Authors’ contributions
All authors have read and approved the final manuscript. GL, CZ and YZ had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. GL, CZ and YZ are joint corresponding authors. Concept and design: JW, WZ, MD, ZSW, JAS, CZ, GL, YZ. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: JW, CZ, GL, YZ. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: JW, NL, XL, YZ. Administrative, technical, or material support: ZSW, JAS, CZ, GL, YZ. Supervision: WZ, MD, ZC, GL, YZ.
Funding
This work was supported by the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (ZSW: K23 AR073334 and R03 AR078938; JAS: R01 AR077607, P30 AR070253, and P30 AR072577), and the R. Bruce and Joan M. Mickey Research Scholar Fund (JAS). The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; praparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Availability of data and materials
Available for purchase from info@the-health-improvement-network.co.uk.
Declarations
Ethics approval and consent to participate
This study received approval from the medical ethical committee at Xiangya Hospital (2018091077), with waiver of informed consent. This study was approved by the IMRD Scientific Review Committee (22SRC008).
Consent for publication
Not applicable. This work uses de-identified data provided by patients as a part of their routine primary care.
Competing interests
Dr. Sparks has received research support from Bristol Myers Squibb and performed consultancy for AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer unrelated to this work. No conflict of interest for other authors.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chao Zeng, Email: zengchao@csu.edu.cn.
Guanghua Lei, Email: lei_guanghua@csu.edu.cn.
Yuqing Zhang, Email: yzhang108@mgh.harvard.edu.
References
- 1.Vaccine BNT162b2—Conditions of Authorisation Under Regulation 174. [https://www.gov.uk/government/publications/regulatory-approval-of-pfizer-biontechvaccine-for-covid-19/conditions-of-authorisation-for-pfizerbiontechcovid-19-vaccine.]
- 2.Regulatory Approval of COVID-19 Vaccine AstraZeneca. [https://www.gov.uk/government/publications/regulatory-approval-of-covid-19-vaccine-astrazeneca.]
- 3.Regulatory Approval of COVID-19 Vaccine Moderna. [https://www.gov.uk/government/publications/regulatory-approval-of-covid-19-vaccine-moderna.]
- 4.Pritchard E, Matthews PC, Stoesser N, Eyre DW, Gethings O, Vihta KD, Jones J, House T, VanSteenHouse H, Bell I, et al. Impact of vaccination on new SARS-CoV-2 infections in the United Kingdom. Nat Med. 2021;27:1370–1378. doi: 10.1038/s41591-021-01410-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO Coronavirus (COVID-19) Dashboard. [https://covid19.who.int/.]
- 6.Tracking Coronavirus Vaccinations Around the World [https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracker.html]
- 7.Mlcochova P, Kemp SA, Dhar MS, Papa G, Meng B, Ferreira I, Datir R, Collier DA, Albecka A, Singh S, et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature. 2021;599:114–119. doi: 10.1038/s41586-021-03944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis D. Mix-and-match COVID vaccines: the case is growing, but questions remain. Nature. 2021;595:344–345. doi: 10.1038/d41586-021-01805-2. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt T, Klemis V, Schub D, Schneitler S, Reichert MC, Wilkens H, Sester U, Sester M, Mihm J. Cellular immunity predominates over humoral immunity after homologous and heterologous mRNA and vector-based COVID-19 vaccine regimens in solid organ transplant recipients. Am J Transplant. 2021;21:3990–4002. doi: 10.1111/ajt.16818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Perez Marc G, Moreira ED, Zerbini C, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menni C, Klaser K, May A, Polidori L, Capdevila J, Louca P, Sudre CH, Nguyen LH, Drew DA, Merino J, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21:939–949. doi: 10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pouwels KB, Pritchard E, Matthews PC, Stoesser N, Eyre DW, Vihta KD, House T, Hay J, Bell JI, Newton JN, et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med. 2021;27:2127–2135. doi: 10.1038/s41591-021-01548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheikh A, McMenamin J, Taylor B, Robertson C, Public Health S, the EIIC SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397:2461–2462. doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vasileiou E, Simpson CR, Shi T, Kerr S, Agrawal U, Akbari A, Bedston S, Beggs J, Bradley D, Chuter A, et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397:1646–1657. doi: 10.1016/S0140-6736(21)00677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, Stowe J, Tessier E, Groves N, Dabrera G, et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris RJ, Hall JA, Zaidi A, Andrews NJ, Dunbar JK, Dabrera G. Effect of Vaccination on Household Transmission of SARS-CoV-2 in England. N Engl J Med. 2021;385:759–760. doi: 10.1056/NEJMc2107717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheikh A, Robertson C, Taylor B. BNT162b2 and ChAdOx1 nCoV-19 Vaccine Effectiveness against Death from the Delta Variant. N Engl J Med. 2021;385:2195–2197. doi: 10.1056/NEJMc2113864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shrotri M, Krutikov M, Palmer T, Giddings R, Azmi B, Subbarao S, Fuller C, Irwin-Singer A, Davies D, Tut G, et al. Vaccine effectiveness of the first dose of ChAdOx1 nCoV-19 and BNT162b2 against SARS-CoV-2 infection in residents of long-term care facilities in England (VIVALDI): a prospective cohort study. Lancet Infect Dis. 2021;21:1529–1538. doi: 10.1016/S1473-3099(21)00289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez Bernal J, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, Simmons R, Cottrell S, Roberts R, O'Doherty M, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373:n1088. doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chisholm J. The Read clinical classification. BMJ. 1990;300:1092. doi: 10.1136/bmj.300.6732.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.First Databank. Multilex drug data file. https://www.fdbhealth.co.uk/solutions/multilex-clinical-decision-support. Accessed 10 Feb 2022.
- 23.Lewis JD, Schinnar R, Bilker WB, Wang X, Strom BL. Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf. 2007;16:393–401. doi: 10.1002/pds.1335. [DOI] [PubMed] [Google Scholar]
- 24.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 25.Li F, Thomas LE, Li F. Addressing Extreme Propensity Scores via the Overlap Weights. Am J Epidemiol. 2019;188:250–257. doi: 10.1093/aje/kwy201. [DOI] [PubMed] [Google Scholar]
- 26.Thomas LE, Li F, Pencina MJ. Overlap Weighting: A Propensity Score Method That Mimics Attributes of a Randomized Clinical Trial. JAMA. 2020;323:2417–2418. doi: 10.1001/jama.2020.7819. [DOI] [PubMed] [Google Scholar]
- 27.Chandan JS, Zemedikun DT, Thayakaran R, Byne N, Dhalla S, Acosta-Mena D, Gokhale KM, Thomas T, Sainsbury C, Subramanian A, et al. Nonsteroidal Antiinflammatory Drugs and Susceptibility to COVID-19. Arthritis Rheumatol. 2021;73:731–739. doi: 10.1002/art.41593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, D'Silva KM, Jorge AM, Li X, Lyv H, Wei J, et al. Increased risk of COVID-19 in patients with rheumatoid arthritis: a general population-based cohort study. Arthritis Care Res (Hoboken). 2022;74:741–7 [DOI] [PMC free article] [PubMed]
- 29.Haroon S, Subramanian A, Cooper J, Anand A, Gokhale K, Byne N, Dhalla S, Acosta-Mena D, Taverner T, Okoth K, et al. Renin-angiotensin system inhibitors and susceptibility to COVID-19 in patients with hypertension: a propensity score-matched cohort study in primary care. BMC Infect Dis. 2021;21:262. doi: 10.1186/s12879-021-05951-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meropol SB, Metlay JP. Accuracy of pneumonia hospital admissions in a primary care electronic medical record database. Pharmacoepidemiol Drug Saf. 2012;21:659–665. doi: 10.1002/pds.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology (Cambridge, Mass). 2010;21:383–388. doi: 10.1097/EDE.0b013e3181d61eeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dunkler D, Ploner M, Schemper M, Heinze G. Weighted Cox Regression Using the R Package coxphw. J Stat Software. 2018;84:1–26. doi: 10.18637/jss.v084.i02. [DOI] [Google Scholar]
- 33.Rothman KJ. Epidemiology: An introduction. Springer; 2012. [Google Scholar]
- 34.Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021;21:195–197. doi: 10.1038/s41577-021-00526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heinz FX, Stiasny K. Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines. 2021;6:104. doi: 10.1038/s41541-021-00369-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Creech CB, Walker SC, Samuels RJ. SARS-CoV-2 Vaccines. JAMA. 2021;325:1318–1320. doi: 10.1001/jama.2021.3199. [DOI] [PubMed] [Google Scholar]
- 37.Patel MK, Bergeri I, Bresee JS, Cowling BJ, Crowcroft NS, Fahmy K, Hirve S, Kang G, Katz MA, Lanata CF, et al. Evaluation of post-introduction COVID-19 vaccine effectiveness: Summary of interim guidance of the World Health Organization. Vaccine. 2021;39:4013–4024. doi: 10.1016/j.vaccine.2021.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hull SA, Williams C, Ashworth M, Carvalho C, Boomla K. Prevalence of suspected COVID-19 infection in patients from ethnic minority populations: a cross-sectional study in primary care. Br J Gen Pract. 2020;70:e696–e704. doi: 10.3399/bjgp20X712601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McIntyre PB, Aggarwal R, Jani I, Jawad J, Kochhar S, MacDonald N, Madhi SA, Mohsni E, Mulholland K, Neuzil KM, et al. COVID-19 vaccine strategies must focus on severe disease and global equity. Lancet. 2022;399:406–410. doi: 10.1016/S0140-6736(21)02835-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Read codes used for covid-19 vaccination records and diagnosed SARS-CoV-2 infection. Figure S1. A directed acyclic graph of the comparative effectiveness of BNT162b2 and ChAdOx1 nCoV-19 vaccines against COVID-19 and potential confounders.
Data Availability Statement
Available for purchase from info@the-health-improvement-network.co.uk.